Abstract
Positron emission tomography (PET) is a noninvasive imaging technique that provides a functional or metabolic assessment of normal tissue or disease conditions. 18F-fluorodeoxyglucose PET imaging (FDG-PET) is widely used clinically for tumor imaging due to increased glucose metabolism in most types of tumors, and has been shown to improve the diagnosis and subsequent treatment of cancers. In this chapter, we review its use in cancer diagnosis, staging, restaging, and assessment of response to treatment. In addition, other metabolic PET imaging agents in research or clinical trial stages are discussed, including amino acid analogs based on increased protein synthesis, and choline, which is based on increased membrane lipid synthesis. Amino acid analogs and choline are more specific to tumor cells than FDG, so they play an important role in differentiating cancers from benign conditions and in the diagnosis of cancers with low FDG uptake or high background FDG uptake. For decades, researchers have shown that tumors have altered metabolic profiles and display elevated uptake of glucose, amino acids, and lipids, which can be used for cancer diagnosis and monitoring of the therapeutic response with excellent signal-to-noise ratios.
Positron Emission Tomography (PET), a noninvasive imaging technique that detects the gamma rays from positron-emitting isotopes has had a major impact on the diagnosis and treatment of disease. PET enables clinicians to view and assess the human body from a functional, biochemical perspective. As a highly sensitive and accurate nuclear medicine imaging technology based on molecular biology, PET has a unique ability to assess the functional and biochemical processes of the body's tissues, which are altered in the earliest stages of virtually all diseases. PET detects these changes — often before anatomical or structural changes have occurred and become evident on magnetic resonance imaging (MRI) or computed tomography (CT). In the 1970's and 1980's, PET was mainly used for research. During the early 1990's, the use of PET expanded into hospitals and diagnostic clinics as more and more medical communities began to realize the utility of PET in clinical applications, particularly in oncology for cancer staging, assessing treatment strategies, and monitoring the effects of therapy with appropriate radiotracers. Reimbursement of studies by Medicare and other third-party payers also contributed significantly to the growth in clinical PET imaging. However, the spatial resolution of PET is not comparable to CT or MRI, which can provide detailed anatomical information. Therefore, the fused anatomical images from CT and functional images from PET have long been appreciated, where the fusion is achieved by software methods. While generally successful for the brain, software approaches often encounter significant difficulties with the rest of the body. In 1998, the first combined PET/CT scanner was developed and installed in the University of Pittsburgh Medical Center,1 overcoming the limitations of PET and permitting the evaluation of both metabolic and anatomic characteristics of disease. Since the first commercial PET/CT scanner came into clinical practice in 2001, the combined PET/CT has proven to be a major advance for detection of primary tumors, distant metastases, recurrence after treatment, and for staging, restaging, and even monitoring therapy response in most cancers.2
The potential high sensitivity and specificity of PET are due to the high sensitivity of radioisotopes and the special biocharacter of radiotracers, which are molecularly targeted radiopharmaceuticals. Some radioisotopes that have been used for PET imaging include carbon-11 (T1/2 = 20 min), nitrogen-13 (T1/2 = 10 min), fluorine-18 (T1/2 = 110 min), copper-64 (T1/2 =12.7 h), and iodine-124 (T1/2 = 4.2 days), which are positron-emitting isotopes used to label diagnostically useful compounds and provide functional or metabolic information in PET imaging. When the positron-emitting radiotracer is administered to patients, the nucleus emits a positron which travels a short distance, up to a few millimeters, to meet an electron in tissue, resulting in the annihilation of the two particles. This annihilation event produces a pair of 511-KeV photons that are emitted in opposite directions. The resulting gamma rays are the signals detected by the PET system and converted to images. Based on its half-life, F-18 is the most practical isotope for clinical practice; C-11 is also applied in clinical research settings where there are on-site cyclotrons. Among F-18 or C-11 labeled radiotracers used in oncologic applications of PET, FDG is the most commonly used oncologic PET tracer and the only one approved by the Food and Drug Administration (FDA) for routine clinical use. More than 90% of oncologic PET imaging is performed using FDG. The uptake of FDG is substantially increased in most types of cancers due to the increased metabolism of glucose by tumor cells, as is the case in most lung, colorectal, esophageal, stomach, head and neck, cervical, ovarian, and breast cancers, as well as melanoma and most types of lymphoma.3 In addition to increased glucose uptake, increased protein synthesis in tumors induces high demand for amino acids. PET imaging using amino acids and amino acid analogs has shown significant potential for tumor detection in organ sites with an undesirable FDG-PET background or low FDG uptake tumors. Furthermore, choline, as a building block of cell membranes, is greatly consumed by rapidly proliferating tumor cells. Therefore, radiolabeled choline and its analogs are also applied in tumor PET imaging. This chapter focuses on the use of FDG and other PET tumor imaging agents under development.
FDG
FDG, an analogue of glucose, is metabolized similarly to glucose. FDG is transported across cell membranes by glucose transporters and is enzymatically phosphorylated to FDG-6-phosphate which cannot further undergo glycolysis and becomes metabolically trapped intracellularly, in contrast to glucose-6-phosphate.4 The primary exception to the metabolic trapping is in the liver, where a large concentration of phosphatase enzymes results in dephosphorylation of the FDG-6-phosphate and clearance of FDG from the liver.
Cancerous cells need to activate specific metabolic pathways to develop into solid tumors due to the significant gradients of critical factors for cell growth, such as oxygen, glucose, other nutrients, and growth factors. Hypoxia occurs in tumor cells that are 100 – 150 μm away from the nearest blood vessel and tends to be widespread in solid tumors. This tumor microenvironment forces tumor cells to rely on the anaerobic metabolism of glucose, which provides a major energy source for tumor cells in hypoxic regions. The ability of tumor cells to endure profound hypoxia indicates that their adaptation to hypoxic conditions is a crucial step in tumor progression. Hypoxia-inducible factor (HIF)-1, the pro-survival transcription factor, is involved in the response of both normal and tumor cells to hypoxia and consists of two subunits, HIF-1α and HIF-1β. The HIF-1α subunit is rapidly degraded under normoxic conditions while the β subunit is constitutively expressed. Under such low oxygen concentrations, HIF-1α is stabilized and dimerizes with HIF-1β to form an active transcription factor. The dimer then binds to the DNA sequence 5′-RCGTG-3′ (HRE), located in the promoter of target genes. This subsequently leads to up-regulation of factors that promote tumor growth and angiogenesis, including glycolytic enzymes and vascular endothelial growth factor (VEGF) 5, 6.
Strikingly, tumor cells maintain a high glycolytic rate even under aerobic conditions, which is known as aerobic glycolysis or the Warburg effect. 7-9 Even though one glucose molecule produces only two ATPs by glycolysis, which is far less efficient in producing energy than the TCA cycle (i.e., 36 ATPs per glucose), tumors that use glycolysis for their energy supply may have a growth advantage over tumors that use the TCA cycle.10 Tumors metabolize glucose by aerobic glycolysis partly through activation of oncogenes such as AKT, MYC, RAS or loss of tumor suppressors, including p53, which are then further enhanced by stabilization of the hypoxia-induced factor (HIF) via adaptive response to a hypoxic microenvironment, or through pathways that stabilize HIF under non-hypoxic conditions.6 This is the basis for detection and monitoring of human cancers by FDG-PET.
As the only FDA approved oncologic PET tracer, FDG is applied in almost all types of cancer diagnosis, staging, restaging, and in monitoring response to cancer treatment.3, 11 The stage at diagnosis is the most powerful predictor of survival, and treatments are often changed based on the tumor stage; therefore, accurate staging is critical. Restaging is performed after the treatment to detect residual tumor or suspected recurrence, and to determine the extent of a known recurrence or distant metastasis. The power of FDG-PET is fully demonstrated by detecting metastases through a single whole-body PET scan. The purpose of using PET for disease monitoring is to provide an early assessment of therapeutic response or treatment refractory disease.
Currently, the Centers for Medicare and Medicaid Services (CMS) covers FDG-PET applications in initial and subsequent treatment strategies in most of the common cancers including colorectal, esophagus, head and neck, lymphoma, non-small cell lung, and breast cancer. Other cancers are covered with exceptions, or reimbursable under CMS' coverage with evidence development paradigm (Table 1).12 In the following sections, the utility of FDG-PET in oncology will be reviewed by its role in cancer diagnosis, staging, restaging, and monitoring therapy response in the most common cancers.
Table 1. Applications of FDG-PET covered by the Centers of Medicare and Medicaid (CMS) as of April 3, 2009.
| Tumor Type | Initial Treatment Strategy * | Subsequent Treatment Strategy ** |
|---|---|---|
| Colorectal | Cover | Cover |
| Esophagus | Cover | Cover |
| Head & Neck (not Thyroid, CNS) | Cover | Cover |
| Lymphoma | Cover | Cover |
| Non-Small Cell Lung | Cover | Cover |
| Ovary | Cover | Cover |
| Brain | Cover | CED |
| Cervix | 1 or CED | Cover |
| Small Cell Lung | Cover | CED |
| Soft Tissue Sarcoma | Cover | CED |
| Pancreas | Cover | CED |
| Testes | Cover | CED |
| Breast (female and male) | 2 | Cover |
| Melanoma | 3 | Cover |
| Prostate | N/C | CED |
| Thyroid | Cover | 4 or CED |
| All Other Solid Tumors | Cover | CED |
| Myeloma | Cover | Cover |
| All other cancers not listed herein | CED | CED |
Formerly “diagnosis” and “staging”
Formerly “restaging” and “monitoring response to treatment”
CED: covered under CMS with evidence development paradigm
N/C = not covered
(1) Cervix: Covered for the detection of pre-treatment metastases (i.e., staging) in newly diagnosed cervical cancer subsequent to conventional imaging that is negative for extra-pelvic metastasis. All other initial treatment strategies are CED.
(2) Breast: Not covered for initial diagnosis and/or staging of axillary lymph nodes; covered for initial staging of metastatic disease.
(3) Melanoma: Not covered for initial staging of regional lymph nodes. All other initial staging uses are covered.
(4) Thyroid: Covered for subsequent treatment strategy of recurrent or residual thyroid cancer of follicular cell origin previously treated by thyroidectomy and radioiodine ablation and have a serum thyroglobulin >10ng/ml and have a negative I-131 whole body scan. All other uses for subsequent treatment strategy are CED.
(This National Coverage Determination was last reviewed April 2009.)
FDG-PET Lung Cancer Imaging
Lung cancer is the leading cause of cancer death in both men and women, and accounted for about 30% of cancer deaths in men and 26% of cancer deaths in women in the United States in 2009.13 Eighty percent of lung cancer is non-small cell lung cancer (NSCLC), which has the best opportunity for cure by radical surgical resection in the early stages of the disease.
FDG-PET has been widely used in the evaluation of patients with lung cancer. CMS covers the use of FDG-PET in the Initial and Subsequent Treatment Strategies (formerly diagnosis and initial staging of and the restaging, treatment monitoring, and detection of suspected recurrence) of NSCLC.
FDG-PET has been proven to be of immense value in the initial diagnosis,14 detection of recurrent tumor,15, 16 and evaluation of response to therapy.17 In the PET in Lung Cancer Staging (PLUS) Study in which NSCLC patients were randomized to staging by PET or conventional imaging, Verboom et al. demonstrated that FDG-PET provided more accurate staging than conventional workup, resulting in more appropriate management of patients with unresectable disease. Patients with locally advanced or distant metastatic disease were spared the morbidity of futile operations, and overall health care costs were reduced due to fewer operations compared with those who were staged by conventional imaging.18 In a systematic review of the literature, for the differentiation of benign and malignant pulmonary nodules in over 1200 patients, pooled sensitivity and specificity of FDG-PET was 96% and 80%, respectively; for nodal staging, the pooled sensitivity and specificity of FDG-PET was 88% and 92%.19 Another study of 70 non-small cell lung cancer patients showed that sensitivity, specificity and overall accuracy of FDG-PET was higher for the detection of residual viable primary tumor at 95%, 80%, and 91%, respectively, than for the presence of lymph node metastases at 77%, 68%, and 73%, respectively.20 FDG-PET shows promise in detecting the recurrence of lung cancers in restaging evaluations, and is ideal for differentiating recurrent or residual tumors from post-therapy changes. Both processes have identical changes on CT, while a recurrent or residual tumor has FDG uptake, and post-therapy changes typically show little or no FDG uptake.21, 22 In the aggregate, the overall sensitivity, specificity, and accuracy of FDG-PET for lung cancers are very high for primary, residual, and recurrent lung cancer diagnosis. While the combined PET/CT increased the specificity and accuracy of FDG for lung cancer imaging compared to PET alone, recently published studies of combined PET/CT showed that FDG-PET/CT had additional value in detecting extrathoracic metastasis, such as metastasis in the intestine, liver, adrenal gland, or skeleton.23, 24
Furthermore, FDG-PET has been successfully used in the early assessment of treatment response.25, 26 De Geus-Oei and colleagues27 reported a typical example of a patient with stage IV NSCLC and tumor lesions that responded to chemotherapy. FDG-PET showed a 65% decrease in standard uptake value (SUV) relative to baseline after two cycles of chemotherapy. Figure 1 is an excellent example of FDG-PET/CT use for monitoring therapeutic response. Because the changes in tumor glucose metabolism induced by chemotherapy are predictive for patient outcome, the use of FDG-PET can help to stratify patients by probability of progression-free and overall survival. While FDG-PET plays an important role in lung cancer diagnosis and treatment strategy, FDG-PET/CT can provide more specific and accurate information than FDG-PET alone. Lardinois, et al. demonstrated that FDG PET/CT imaging for staging patients with NSCLC was superior to assessment with either PET or CT alone and to visual correlation of separate PET and CT images. Integrated PET/CT allowed for more accurate nodal staging, assessment of chest wall infiltration, and differentiation between benign and malignant findings. PET/CT was also useful for the detection of unsuspected distant metastases and for the direction of biopsies for histopathologic evaluation.28
Figure 1.
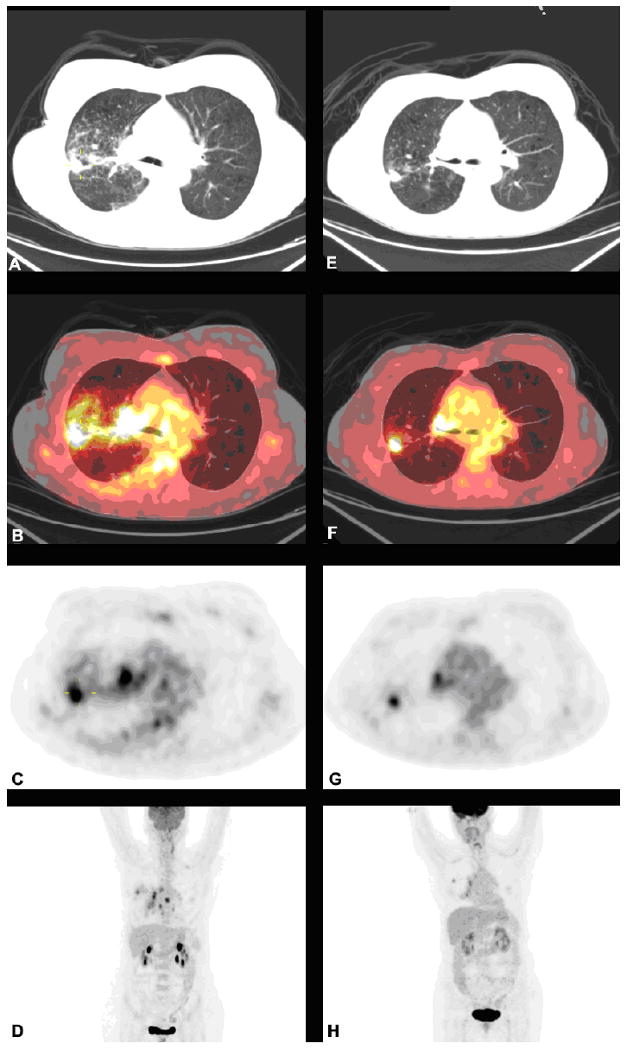
FDG-PET/CT scans of a 63 year-old female with stage IVA adenocarcinoma of the lung before and after 2 cycles of carboplatin and pemetrexed. Left panel images are pretreatment images (A, B, C and D), while the right panel are posttreatment images (E, F, G and H). The images from top to bottom are CT transaxials (A, E), CT and PET fused transaxials (B, F), PET transaxials (C, G), and maximum intensity projection images (D, H).
FDG-PET Breast Cancer Imaging
Breast cancer is the most common cancer in women, with a propensity for recurrence and metastases. It is the second leading cause of death in women and accounted for approximately 15% of cancer deaths in 2009.13 For localized cancers, the five-year survival rate was greater than 96%, but survival decreased drastically with regional or distant metastatic disease. FDG-PET has been widely used in evaluation of patients with breast cancer. CMS covers FDG-PET use in the Initial Treatment Strategy (formerly initial staging) and Subsequent Treatment Strategy (formerly restaging, monitoring of treatment response, and detection of suspected recurrence) of breast cancer, but it does not cover initial diagnosis of breast masses or the staging of axillary lymph nodes.
Potential clinical applications of FDG-PET in breast cancer include detection and differentiation of primary breast lesions, staging of axillary lymph nodes, detection of residual and metastatic disease, and monitoring of the response to chemotherapy.29-31 A systematic review and meta-analysis involving 2,460 breast cancer patients resulted in sensitivities ranging from 20 to 100%, and specificities ranging from 65 to 100%.32 Belohlavek et al. 33 pointed out that the tumor-to-background ratio of FDG uptake is too low in all breast cancers to be able to discover the smallest lesions. Furthermore, the tumor contrast is less reliable due to the breast density, as dense glandular tissue has higher FDG uptake than adipose tissue within the breast. Çermik et al. 34 have found 53% sensitivity for primary lesions < 5 mm and 92% for lesions > 20 mm. Another study reported that the sensitivity and specificity of FDG-PET/CT in large primary cancer with mean tumor size at 4.3±1.4 cm were 77% and 80%, respectively.30
Axillary lymph node status is the most powerful prognostic indicator in patients with breast cancer. Several clinical studies have been carried out to evaluate the accuracy of PET in the axillary staging of operable primary breast cancer.35-37 As for lymph node metastases, FDG-PET is not sensitive enough to detect microscopic metastases in non-pathologically enlarged lymph nodes, but for lymph node metastases larger than 3 cm, the sensitivity and specificity of FDG-PET/CT at 97% and 100% is reported.35, 38 All these studies have suggested that FDG-PET is useful in identifying tumors larger than 2 cm, but has limitations in detecting micrometastatic disease.
FDG-PET is also widely applied to monitor the breast cancer therapy response.30, 39, 40 Duch et al. 27 reported a study of neoadjuvant chemotherapy (epirubicin + cyclophosphamide + taxanes) response in large primary cancer with FDG-PET/CT with a sensitivity of 77% and a specificity of 80%. This study demonstrated a more than 40% decrease in SUVmax in responding tumors, whereas non-responding tumors showed an increase, no change, or only a small decline, as mean SUVmax declined about 24% in FDG uptake. All patients with a good prognosis had a SUVmax decrease of more than 40%.30 In a study of patients with locally advanced breast cancer, the sensitivity, specificity, and accuracy of FDG-PET/CT in detecting responders were 93%, 75%, and 87%, respectively.39 All the studies concluded that FDG-PET can predict response to chemotherapy in breast cancer early in the course of treatment. Despite limitations in identifying micrometastases, FDG-PET plays an important role in assessment of breast cancer response to chemotherapy as well as other treatments such as hormonal therapy and radiation.
FDG-PET Colorectal Cancer Imaging
Colorectal cancer is the third most frequent cancer in both men and women, and accounted for approximately 8% of cancer deaths in 2009 in the United States.13 Most individuals diagnosed with colorectal cancer will have a favorable prognosis, but 20% of patients will be found to have metastatic disease at the time of diagnosis. Metastases of colorectal cancer typically involve the lung or liver; distant metastases without lung or liver involvement are rare. A whole-body FDG-PET scan can detect cancer metastasis in a single exam setting. CMS has approved coverage of FDG-PET in the Initial Treatment Strategy (formerly diagnosis and initial staging) and Subsequent Treatment Strategy (formerly restaging, monitoring of treatment response, and detection of suspected recurrence) for colorectal cancer.
FDG-PET is rarely used for establishing a primary diagnosis of colorectal cancer due to its lack of specificity and high cost. In addition to concentrating in neoplastic disease, FDG accumulates in the normal bowel wall, in unencapsulated lymph tissue within the cecum, in premalignant colonic polyps, and in non-neoplastic inflammatory conditions affecting the bowel. However, FDG-PET is useful in identifying local recurrence and distant metastasis for preoperative evaluation.41-43 Integrated PET/ CT is an accurate modality for assessing colorectal cancer recurrence and often leads to changes in patient management. Patient-based analysis showed that the sensitivity, specificity and accuracy of FDG-PET were 80%, 69% and 75%, respectively, compared with 89%, 92%, and 90%, respectively, for PET/CT in the diagnosis of recurrent colorectal cancer and metastasis.
Finally, FDG-PET is also applied to monitor the chemotherapeutic response in advanced colorectal cancer, the effects of local ablative therapies, preoperative radiotherapy, as well as for prognosis.
FDG-PET Lymphoma Cancer Imaging
Lymphoma is the most common primary hematopoietic malignancy in the United States. Lymphoma often responds well to therapy, and the survival rate is generally 90% or higher when the disease is detected during early stages, making it one of the most curable forms of cancer.44 Lymphomas are categorized as Hodgkin's disease and non-Hodgkin's lymphoma, and broadly divided into two groups: indolent and aggressive. Indolent lymphomas tend to be slow growing, but generally incurable. Most of the aggressive lymphomas respond well to treatment and are curable; therefore, the prognosis depends on the correct classification of the disease.
The primary therapeutic modalities for lymphomas are chemotherapy and radiotherapy, as surgery is generally not utilized in this disease when it is known to be lymphoma prior to contemplation of surgery. Accurate imaging is crucial to treatment strategy, and FDG-PET has become a useful imaging modality in the staging and treatment evaluation algorithm for lymphoma by providing unique metabolic information. In addition, FDG PET may be helpful to assess for histologic transformation of indolent lymphomas into more aggressive histologies. While there may be overlap in FDG avidity of indolent and more aggressive lymphomas as indicated by SUV measurements, FDG-PET can detect metabolic changes if there is a baseline study, and FDG-PET can be used to direct biopsies for histopathologic evaluation for suspected transformation. CMS covers the use of FDG-PET imaging for the Initial Treatment Strategy (formerly diagnosis and initial staging) and Subsequent Treatment Strategy (formerly restaging, monitoring of treatment response, and detection of suspected recurrence) of both Hodgkin's and non-Hodgkin's lymphoma.
The utility of FDG-PET in the evaluation of lymphoma has been reviewed extensively.45, 46, 47 Several investigations have shown that PET is quite sensitive in detecting nodal and extranodal manifestations of Hodgkin's lymphoma prior to treatment. Currently, FDG-PET may be more accurate than anatomic imaging modalities in assessing treatment effects to correctly identify patients with residual disease and predict therapeutic outcome.46 Persistent FDG uptake during and after chemotherapy has a high sensitivity and specificity for prediction of subsequent relapse; however, after completion of chemotherapy, FDG-PET cannot detect microscopic residual disease that may lead to a subsequent relapse.
FDG-PET Head and Neck Cancer Imaging
Head and neck cancers have accounted for approximately 4-5% of all the malignant disease in the United States in recent years. Head and neck squamous cell carcinoma (HNSCC) comprises the vast majority of head and neck cancer, which often spreads to the lymph nodes of the neck; this is commonly the first manifestation of the disease at the time of diagnosis. Accurate staging is critical for selection of the appropriate treatment strategy. FDG-PET imaging is a very sensitive and valuable imaging tool for evaluation of head and neck cancer. CMS covers the use of FDG-PET for the Initial Treatment Strategy (formerly diagnosis and initial staging) and Subsequent Treatment Strategy (formerly restaging, monitoring of treatment response, and detection of suspected recurrence) of head and neck cancer.
FDG-PET plays an increasing role in diagnosis and management planning of head and neck cancer.48-50 Kyzas and colleagues51 performed a meta-analysis involving 1236 cases to address the diagnostic performance of FDG-PET in evaluating lymph node metastasis in patients with HNSCC, and to compare its performance against standard diagnostic tools. The sensitivity and specificity of FDG-PET were 80% and 86%, respectively, which was superior to conventional diagnostic tests (75% and 79%, respectively). FDG-PET has good diagnostic performance in the overall pretreatment evaluation of patients with HNSCC, but may have limitations in detecting micrometastasis, due to the low FDG uptake of small tumors without hypoxia.50 FDG-PET has become widely accepted as a standard modality for evaluating HNSCC.
The metabolic activity of pretreatment FDG-PET scans as indicated by SUV can also be used to predict prognosis. Machtay et al. 49 reported that two year disease free survival was 72% in patients whose lesions demonstrated SUV<9 × as opposed to 37% in those with lesions with SUV>9. FDG-PET is very useful in the detection of recurrent HNSCC following prior therapy, which has a high negative predictive value in the detection of recurrent HNSCC. However, FDG-PET has lower utility for identifying occult nodal metastases, and its positive predictive is somewhat lower, mainly due to false positive readings related to inflammation, infection, post-treatment effects, or biopsy trauma. However, FDG-PET/CT has proven to be a major advance for restaging, and for detection of unknown primary HNSCC, distant metastases or second primary carcinomas, and recurrent HNSCC in the post-treatment setting,48, 52-54 Post-treatment changes and physiologic structures such as muscle and brown fat are easily distinguishable from abnormalities on PET/CT images, yet are more challenging to differentiate by PET or CT alone. For the detection of recurrent or residual disease at the primary site, as well as the detection of nodal recurrence, PET/CT has been shown to be more sensitive (88-100%) and specific (75-100%) than CT (sensitivity, 38-90%; specificity, 38-85%). The significant ability of PET/CT to detect and accurately localize tumors makes it more attractive than PET alone (Figure 2).2
Figure 2.
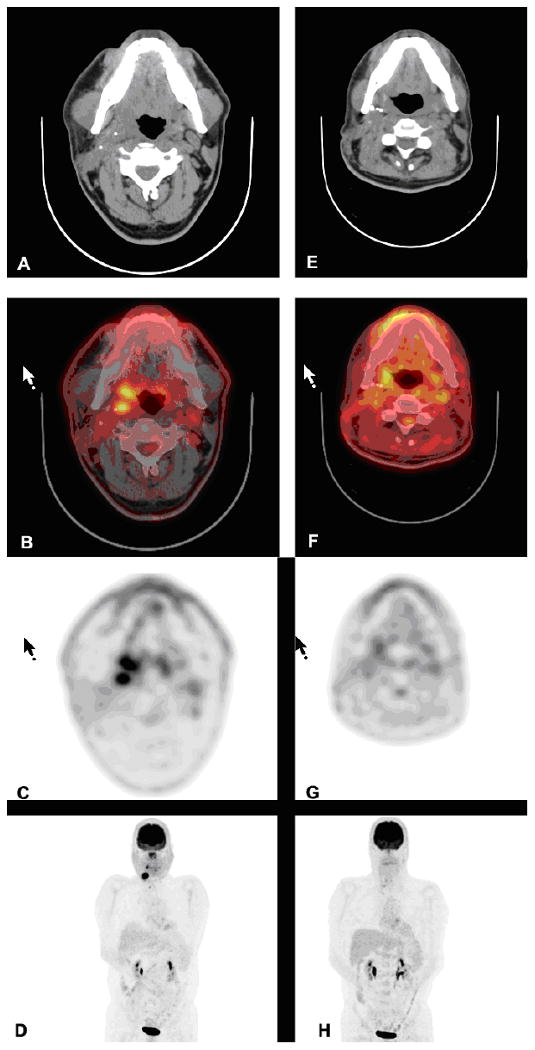
FDG-PET/CT scans of a 41 year-old male with right base of tongue squamous cell carcinoma before and after post-operative cisplatin and radiation. There is a persistent, incidental hypermetabolic lesion in the right lobe of the thyroid gland. Left panel images are pretreatment images (A, B, C and D), while the right panel are posttreatment images (E, F, G and H). The images from top to bottom are CT transaxials (A, E), CT and PET fused transaxials (B, F), PET transaxials (C, G), and maximum intensity projection images (D, H).
FDG-PET Esophageal Cancer Imaging
Esophageal cancer is one of the ten most common malignancies, with a high mortality rate worldwide. Esophageal cancers have various histologic subtypes, primarily adenocarcinoma (approx. 50-80% of all esophageal cancers) and squamous cell carcinoma. FDG-PET is extensively applied in esophageal cancer evaluation. CMS covers the use of FDG-PET for the Initial Treatment Strategy (formerly diagnosis and initial staging) and Subsequent Treatment Strategy (formerly restaging, monitoring of treatment response, and detection of suspected recurrence) of esophageal cancer.
FDG-PET is not used in initial diagnosis of esophageal cancer due to FDG uptake in infectious esophagitis, Barrett's esophagitis without malignancy, inflammatory esophagitis, and other benign diseases. FDG-PET plays a limited role in the evaluation of regional nodal disease in patients with esophageal cancer, with a low sensitivity at 22-57%, but much higher specificity at more than 90%.3 Compared to PET alone, PET/CT is highly sensitive, specific, and accurate at regional and distant sites. At local sites, sensitivity was high, but specificity was lower (50%) because of a high incidence of false-positive findings. Guo and colleagues55 reported that the overall sensitivity, specificity, and accuracy of FDG-PET/CT for detecting recurrence of squamous cell carcinoma of the esophagus of 56 patients at all sites were 93.1%, 75.7%, and 87.2%, respectively.
FDG-PET has shown promising results in assessing response to therapy and tumor control in prognosis.56-58 Krause et al. 59 compared FDG-PET and FDG-PET/CT in assessment of response after completion of therapy for esophageal cancer. Patients with a 50% or more decrease in SUVmax in the primary tumors had significantly longer disease-free survival than those with a less than 50% decrease in SUVmax. During therapy assessment, those patients with more than a 30% decrease in SUVmax were set as responders, while those patients with less than a 30% decrease in SUVmax were set as non-responders. The prognosis of the responders showed significantly improved overall survival. Therefore, assessment of response early in therapy is important in order to allow clinicians to modify treatment strategy for non-responding patients.
FDG-PET Melanoma Imaging
Once a rare tumor, malignant melanoma is increasing in incidence and has approximately doubled since 1970, with an estimated 68,720 new cases diagnosed in 2009.13 In its early stages, melanoma is curable by means of surgical excision, and up to 85% of patients are cured with treatment following diagnosis. However, the remaining 15% of patients present with locally advanced or metastatic disease at the time of diagnosis. CMS covers FDG-PET for the Initial Treatment Strategy (formerly diagnosis and initial staging) and Subsequent Treatment Strategy (formerly restaging, monitoring of treatment response, and detection of suspected recurrence) of melanoma, except for the initial staging of regional lymph nodes.
FDG-PET is applied clinically for the detection of the extent of local and regional disease spread.60-62 A meta-analysis evaluating the accuracy of FDG-PET in staging and restaging of cutaneous melanoma showed that FDG-PET is not useful in the evaluation of regional metastases as it does not detect microscopic disease. However, FDG-PET is useful in the detection of distant metastases and in the management of patients with cutaneous melanoma.63 Crippa et al. 64 reported that FDG-PET for lymph node metastases from melanoma showed a reasonable sensitivity and specificity for detecting the presence or absence of lymph node metastases in patients with melanoma. FDG-PET detected 100% of metastases ≥10 mm, 83% of metastases 6-10 mm, and 23% of metastases ≤ 5 mm. Moreover, FDG-PET had high sensitivity (> or = 93%) only for metastases with more than 50% lymph node involvement or with capsular infiltration. However, even if it were able to detect small volumes of subclinical macroscopic disease, FDG-PET cannot detect subclinical microscopic disease with acceptable sensitivity. Wagner et al.65 also reported that FDG-PET sensitivity for melanoma lymph node metastases is dependent on tumor volume.
FDG-PET Pancreatic Cancer Imaging
Pancreatic cancer, a malignant neoplasm of the pancreas, accounts for 6% of cancer deaths in the United States.13 In 2009, 42,470 individuals were diagnosed with this condition and 35,240 died, making it the fourth leading cause of death from cancer in men and women. The prognosis for pancreatic cancer is very poor because early pancreatic cancer often does not cause symptoms, and the later symptoms are usually non-specific and varied. Thus, early detection using effective diagnostic procedures is needed. CMS currently covers FDG-PET only for the Initial Treatment Strategy (formerly diagnosis and initial staging) of pancreatic cancer. FDG-PET for Subsequent Treatment Strategy (formerly restaging, monitoring of treatment response, and detection of suspected recurrence) is reimbursable by CMS under the coverage with evidence development through the National Oncologic PET Registry.
Accurate preoperative staging is one of the most useful and effective applications of FDG-PET in the management of patients with pancreatic cancer. Assessing the resectability of pancreatic cancer may avoid unnecessary surgical procedures. A comparison study of PET/contrast-enhanced CT, PET alone and PET/CT in assessing the resectability of pancreatic cancer showed a sensitivity of 96-100%, and an accuracy of 70-88%, positive predictive value of 61-82%, and negative predictive value of 96-100%. Therefore, use of FDG-PET in assessing the resectability of pancreatic cancer is feasible and accurate, and PET/contrast enhanced CT is superior to PET alone.66
Pancreatic cancer recurrence is often difficult to detect by conventional imaging. Sperti et al. 67 reported a study monitoring 72 patients after pancreatic cancer resection by FDG-PET and CT. Tumor recurrence was detected in 35 of 63 patients by CT, while FDG-PET detected 61 patients with recurrence, indicating that tumor relapse is detected by FDG-PET earlier than CT. FDG-PET is a useful tool for predicting the prognosis in pancreatic cancer, and for detection of distant metastases and hidden malignant disease.68, 69 An FDG-PET evaluation of 98 patients with primary pancreatic cancer showed that the overall survival of the group in which SUVmax was <an 7.5 was better than that of the group in which it was >7.5.70
FDG-PET is useful and cost-effective in the pre-operative staging of pancreatic cancer. FDG-PET is useful and cost effective in the pre-operative staging of pancreatic cancer. However, using FDG-PET to differentiate cancer from inflammatory lesions does have drawbacks. For instance, high FDG accumulation due to active, chronic and autoimmune pancreatitis can mimic pancreatic cancer. FDG-PET can also result in false negatives, missing lesions up to 33mm in diameter, due mainly to the low cellularity in desmoplastic tumors.71 The role of FDG-PET in pancreatic cancer is not fully explored, and because of the high lethality of pancreatic cancer, no results have yet shown that FDG-PET significantly improves patients' prognoses.
False Positive FDG-PET Findings in Normal or Benign Conditions
FDG-PET holds great promise in the diagnosis of certain malignancies. However, FDG-PET images must be reviewed carefully to avoid false-positive interpretations. A great amount of information has been gathered about potential sources of false-positive results due to normal body functions when using FDG-PET.
In addition to tumor cells, normal cells such as brain and heart cells consume large amounts of glucose due to their high metabolic demand. Furthermore, glucose is used by the body for healing and detoxification, so the kidneys and bladder both contain a byproduct of this process, which can be imaged incidentally. Large muscle groups also metabolize glucose when they are overexerted. As FDG-PET imaging is dependent on the successful uptake of FDG, patients are routinely asked to fast for several hours to inhibit the circulation of insulin, thereby minimizing the need for glycolytic absorption.
Brown adipose tissue, or brown fat, which is adrenergically innervated can also show high FDG uptake due to its high concentration of mitochondria that are activated by adrenergic outflow during physiologic and/or psychic stress. FDG uptake in brown fat tends to be bilateral, symmetric and intense in the cervical, supraclavicular, mediastinal, paravertebral, and perirenal areas. Children and teenagers have been shown to have more brown fat than adults, and young thin female patients should be given special attention in monitoring FDG uptake.72 FDG-PET/CT can be helpful in distinguishing brown fat from true pathology.
It is well known that infection and inflammation can lead to false positive FDG-PET results. Surgery and radiofrequency ablation also induce increased FDG uptake as a result of inflammatory changes by enhanced inflammatory cell infiltration.73 Therefore, for early detection of persistent or recurrent disease, a PET tracer other than FDG, with no increased uptake in inflammatory lesions, would be desirable, such as 18F-fluorothymidine (18F-FLT) which reflects cellular proliferation rather than less specific increased glucose metabolism.74 For patients receiving chemotherapy or radiotherapy, it is common to have increased FDG uptake due to the enhanced inflammatory cell infiltration, so for monitoring therapy efficacy, FDG-PET scans should be conducted generally no sooner than 4 to 6 weeks after the last cycle of therapy or radiation to avoid potential false-positive results.
Amino Acid Analogs
Due to increased proliferation requiring active protein synthesis, many types of tumors have increased amino acid uptake and upregulated amino acid transporters. Radiolabeled amino acids and their analogs are another class of tumor imaging agents. They are metabolized similarly to amino acids, which are transported across the cell membranes by amino acid transporter proteins. Sodium-independent amino acid transport system L is a major route for providing cells with large neutral amino acids, including branched and aromatic amino acids, such as leucine, isoleucine, valine, phenylalanine, tyrosine, tryptophan, methionine, and histidine.75 Studies have shown that many types of tumor cells have significantly increased L-type amino acid transporter 1 (LAT1) expression.76-78 Kaira et al. 79 also reported that metastatic tumor sites have significantly higher LAT1 expression than primary tumors. This increased LAT1 is an excellent target for tumor imaging. PET imaging using amino acids and amino acid analogs has shown significant potential for tumor detection. PET imaging with amino acids and their analogs mainly include natural amino acids and their synthetic analogs.
Natural Amino Acids
Most studies of the natural amino acids were performed using 11C-labelled amino acids. The most prominent example is 11C-labeled methionine. PET imaging with L-methyl-11C-methionine (MET-PET) shows promising results in detection and delineation of viable tumor, especially in low-grade gliomas. MET-PET has been used in clinical management of cerebral gliomas in initial diagnosis, differentiation of tumor recurrence, grading, prognostication, tumor extent delineation, biopsy planning, surgical resection, radiotherapy planning, and assessment of response to therapy.80 MET-PET is also used clinically to distinguish malignant tissue from normal tissue or benign growths in head and neck cancer, melanoma, ovarian cancer, and other tumors,81-84 but the short physical half-life of C-11 prevents its use in most nuclear medicine departments (i.e., those without an onsite cyclotron).
SyntheticAmino Acids
Due to the limitations of natural amino acids, F-18 labeled syntheticamino acids have gained great interest.85, 86 The synthetic amino acids and analogs, which are non-metabolizable compounds without any efflux of labeled metabolites or with an efflux that is highly restricted, have better imaging potential than natural amino acids due to high accumulation. The radiolabeled synthetic amino acids can be classified into two groups: aromatic amino acids and non-aromatic amino acids.
Radiolabeled aromatic amino acids are all tyrosine or phenylalanine analogs, and are primarily system L substrates, including 2-18F-fluoro-L-tyrosine (18F-TYR), L-3-18F-alpha-methyl tyrosine (18F-FMT), O-(2-18F-Fluoroethyl)-l-tyrosine (FET), and 6-18F-fluoro-L-3,4-dihydroxyphenylalanine (FDOPA).87 FET and FDOPA have been extensively evaluated in humans. FET-PET has shown to be valuable in the management of brain tumors. For differentiation of grade III and IV recurrent gliomas from grade I and II recurrences, Poperl and colleagues88 demonstrated 92% specificity and 92% sensitivity using kinetic analysis. FET demonstrates low uptake in inflammatory tissue compared to FDG and MET, suggesting that FET may be superior for distinguishing neoplasms from inflammatory lesions. FET-PET also has prognostic value in patients with resected glioblastoma multiforme.89, 90, 91
FDOPA-PET has clinical utility in brain tumor imaging.92, 93 A study comparing FDOPA and FDG for the detection of primary and recurrent brain tumors in 30 patients showed that FDOPA had a sensitivity of 96%, as opposed to 61% for FDG.94 FDOPA also may have the potential to differentiate low grade from high grade tumors.95 Additionally, FDOPA has been used to evaluate extracranial neuroendocrine tumors.96
Alicyclic amino acids, 1-Amino-cycloalkane-1-carboxylic acids, are a class of α,α-dialkyl amino acids with side chains that are covalently bonded to each other to form a cyclic ring. In general, alicyclic amino acids are neither metabolized nor readily incorporated into protein. Of this class of non-natural amino acids, 1-amino-cyclopentane-1-carboxylic acid (ACPC), 1-amino-cyclobutane-1-carboxylic acid (ACBC), and its 3-fluoro analogue anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (anti-18F-FACBC) have been evaluated in humans. Anti-18F-FACBC is a synthetic L-leucine analog that has shown promising results in a human patient with glioblastoma multiforme.97 Anti-18F-FACBC also has been demonstrated to have excellent uptake within primary and metastatic prostate carcinoma with little renal excretion compared to FDG (Figure 3).98 Anti-18F-FACBC uptake in patients with newly diagnosed renal masses was examined, and relative amino acid transport compared to the renal cortex was found to be elevated in renal papillary cell carcinoma but not in clear cell carcinoma.99 Additionally, anti-18F-FACBC is relatively unique among amino acids because it has low associated radioactivity in the urine, which simplifies the interpretation of the genitourinary tract and pelvic images.
Figure 3.
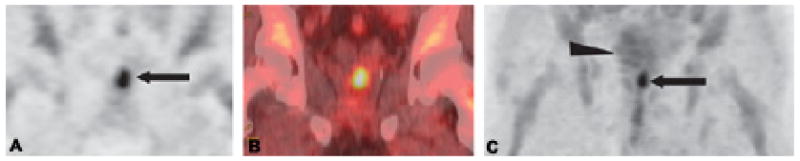
Coronal PET (A) and CT fused (B) anti-18F-FACBC images in a 71-year-old man (restaging) with biopsy-proven prostate bed recurrence extending toward the left seminal vesicle (arrow in A). Maximum-intensity-projection (MIP) image at 20 min (C) demonstrates uptake in the prostate bed (arrow) but little bladder uptake (arrowhead).
Choline
Choline is a precursor of phosphatidylcholine, which is a major constituent of membrane lipids. Membrane lipid synthesis, as well as protein synthesis, is activated during cell proliferation. Therefore, choline is consumed in large quantities by tumor cells.100 Carbon-11 choline (11C-choline) has been introduced as an oncological PET tracer for evaluation of a variety of malignant tumors such as brain tumors, lung cancer, esophageal cancer, colon cancer, bladder cancer, prostate cancer, and many other cancers.101 11C-choline uptake is significantly greater in malignant tumors than in benign tumors, correlates well with the degree of FDG accumulation within the lesion, and does not have the disadvantage of high background activity owing to urinary tract excretion which can interfere with FDG-PET evaluation.102 Most reports of 11C-choline involve prostate cancer103-105 and brain tumor106, 107 imaging. Figure 4 shows a meningioma imaged by 11C-choline with a very low background in comparison to FDG.108 Li et al.109 reported using 11C-choline PET/CT imaging for differentiating prostate cancer from benign prostate hyperplasia. 11C-choline imaging in breast cancer,110 head and neck cancer,111 and hepatocellular carcinoma112 has also been reported. 18F-choline (FCH) shows advantages in PET imaging due to F-18's much longer half-life in comparison to that of C-11. FCH's use as a therapeutic response marker for prostate cancer with bone metastasis was reported recently.103 A metastasis detected by FCH without any corresponding morphological changes on CT was also reported.113 In the same report, FCH was studied in therapy monitoring; when comparing pre- and post-therapeutic imaging, hormone treatment (HT) responders showed reduced FCH uptake. These patients' images often did not demonstrate significant morphological changes, once again reinforcing the major advantage of metabolic imaging over morphological imaging modalities (e.g., CT). Another analog of choline, Fluoroethylcholine, has been shown to be effective in detecting prostate cancer in patients.101, 114
Figure 4.
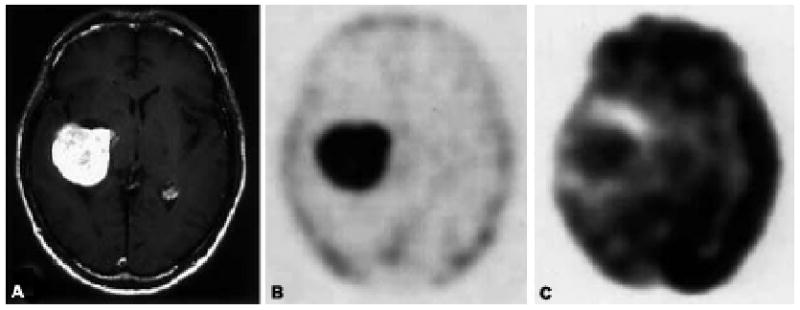
A 58-year-old man with meningioma. (A) T1-weighted gadolinium-enhanced MRI shows an enhanced lesion in the right lateral ventricle. (B) A 11C-choline PET image obtained at 5 min post injection demonstrates increased tumor uptake of 11C-choline and a tumor to white matter (T/W) ratio of 29.10. (C) An FDG-PET image obtained at 50 min post injection shows a tumor with a T/W ratio of 1.65.
Conclusion
FDG-PET is an excellent imaging tool for the diagnosis of primary tumor, recurrence, and metastasis, staging, restaging, and monitoring treatment response in almost all common tumors. Combined PET/CT, which provides functional and anatomical information together, enhances the capability of PET. This enormous capability of PET in oncology is recognized not only by research scientists and clinicians, but also by CMS which has approved coverage of FDG-PET applications in the initial and subsequent treatment strategies for most of the common cancers, such as colorectal, esophagus, head and neck, lymphoma, non-small cell lung, breast, melanoma, thyroid, myeloma, and other cancers. Recognition and understanding of the vital role of FDG-PET in management of the cancer patients greatly benefits patients as well as the whole scientific field.
Although FDG-PET has shown great success in cancer diagnosis and assessment of treatment response, FDG has the following limitations: (1) increased accumulation of FDG in some benign processes, such as infectious and inflammatory lesions that can give false positive results; (2) low sensitivity in well-differentiated low grade tumors that have relatively low glucose metabolism and are much closer to normal tissue, including most prostate cancers, carcinoid tumors, bronchoalveolar-cell carcinoma in the lung, and renal-cell carcinoma; (3) low sensitivity in micrometastases in breast cancer and melanoma, and in hypocellular cancers such as desmoplastic or mucinous tumors; and (4) increased FDG accumulation in some normal body areas such as lymphoid tissue and brown fat which could lead to false-positive results. Further, in assessing meaningful changes in FDG uptake in the therapeutic response, a major criticism of FDG-PET from oncologists is the disparate (or complete lack thereof) of quantitative systems. Positron Emission tomography Response Criteria in Solid Tumors (PERCIST) is the initial framework for developing such a system in the US.115
Other metabolic PET imaging agents, such as radiolabeled amino acids, amino acid analogs, and choline have shown promising results in imaging brain tumors, prostate cancer, and other tumors; however, compared to FDG, far less research has been done on these oncological imaging agents. In addition to the PET tracers based on increased metabolism, other tracers have been developed based on other features of tumor biology and the tumor microenvironment such as proliferation, hypoxia, angiogenesis, and apoptosis. 18F-FLT, as a proliferation indicator, has shown promising results in evaluation of tumor response to therapy, and in the prediction of early response in the course of treatment.116, 117 As a result, 18F-FLT has the potential to become the next FDA-approved PET radiotracer.
Successful PET tracers targeting specific cancer biomarkers are few in number. With the discovery of oncologic biomarkers, the development of novel PET tracers with high specificity in targeting oncologic biomarkers will be an important direction for research. Current oncologic therapy has moved forward from cytotoxic treatment to personalized therapy targeting specific molecular biomarkers which can be aided by PET imaging. With the development of highly specific molecular probes, PET will play a major and integral role in the diagnosis and monitoring treatment of cancer and other diseases.
Footnotes
Financial Disclosures: Daniel J. Lee, M.D. – Clinical Investigator for Immunomedics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Townsend DW, Beyer T. A combined PET/CT scanner: the path to true image fusion. Br J Radiol. 2002;75 doi: 10.1259/bjr.75.suppl_9.750024. Spec No:S24-30. [DOI] [PubMed] [Google Scholar]
- 2.Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology. 2007;242(2):360–85. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 3.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231(2):305–32. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- 4.Som P, Atkins HL, Bandoypadhyay D, et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med. 1980;21(7):670–5. [PubMed] [Google Scholar]
- 5.Semenza GL, Jiang BH, Leung SW, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271(51):32529–37. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269(38):23757–63. [PubMed] [Google Scholar]
- 7.Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci U S A. 1998;95(4):1511–6. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim H, Dolde C, Lewis BC, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94(13):6658–63. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osthus RC, Shim H, Kim S, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275(29):21797–800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- 11.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354(5):496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 12.Medicare National Coverage Determinations Manual C, Part 4 (Sections 200 - 310.1) Coverage Determinations 220.6 – Positron Emission Tomography (PET) Scans (Effective April 3, 2009) Available from: http://www.cms.hhs.gov/transmittals/downloads/R108NCD.pdf.
- 13.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 14.Oyen WJ, Bussink J, Verhagen AF, Corstens FH, Bootsma GP. Role of FDG-PET in the diagnosis and management of lung cancer. Expert Rev Anticancer Ther. 2004;4(4):561–7. doi: 10.1586/14737140.4.4.561. [DOI] [PubMed] [Google Scholar]
- 15.Schmid RA, Hautmann H, Poellinger B, et al. Staging of recurrent and advanced lung cancer with 18F-FDG PET in a coincidence technique (hybrid PET) Nucl Med Commun. 2003;24(1):37–45. doi: 10.1097/00006231-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Hicks RJ, Kalff V, MacManus MP, et al. The utility of 18F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med. 2001;42(11):1605–13. [PubMed] [Google Scholar]
- 17.Hicks RJ. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med. 2009;50 1:31S–42S. doi: 10.2967/jnumed.108.057216. [DOI] [PubMed] [Google Scholar]
- 18.Verboom P, van Tinteren H, Hoekstra OS, et al. Cost-effectiveness of FDG-PET in staging non-small cell lung cancer: the PLUS study. Eur J Nucl Med Mol Imaging. 2003;30(11):1444–9. doi: 10.1007/s00259-003-1199-9. [DOI] [PubMed] [Google Scholar]
- 19.Reske SN, Kotzerke J. FDG-PET for clinical use. Results of the 3rd German Interdisciplinary Consensus Conference, “Onko-PET III”, 21 July and 19 September 2000. Eur J Nucl Med. 2001;28(11):1707–23. doi: 10.1007/s002590100626. [DOI] [PubMed] [Google Scholar]
- 20.Eschmann SM, Friedel G, Paulsen F, et al. 18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radio-chemotherapy in stage III non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34(4):463–71. doi: 10.1007/s00259-006-0273-5. [DOI] [PubMed] [Google Scholar]
- 21.Gauger J, Patz EF, Jr, Coleman RE, Herndon JE., 2nd Clinical stage I non-small cell lung cancer including FDG-PET Imaging: sites and time to recurrence. J Thorac Oncol. 2007;2(6):499–505. doi: 10.1097/JTO.0b013e3180600990. [DOI] [PubMed] [Google Scholar]
- 22.Hellwig D, Groschel A, Graeter TP, et al. Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33(1):13–21. doi: 10.1007/s00259-005-1919-4. [DOI] [PubMed] [Google Scholar]
- 23.Tulchinsky M, Coquia S, Wagner H., Jr Small bowel metastasis from lung cancer detected on FDG PET/CT. Clin Nucl Med. 2009;34(7):446–8. doi: 10.1097/RLU.0b013e3181a7d1fb. [DOI] [PubMed] [Google Scholar]
- 24.Liu N, Ma L, Zhou W, et al. Bone metastasis in patients with non-small cell lung cancer: The diagnostic role of F-18 FDG PET/CT. Eur J Radiol. 2009 doi: 10.1016/j.ejrad.2009.01.036. Available from: http://dx.doi.org. [DOI] [PubMed]
- 25.Yamamoto Y, Kameyama R, Murota M, Bandoh S, Ishii T, Nishiyama Y. Early assessment of therapeutic response using FDG PET in small cell lung cancer. Mol Imaging Biol. 2009;11(6):467–72. doi: 10.1007/s11307-009-0227-y. [DOI] [PubMed] [Google Scholar]
- 26.Sunaga N, Oriuchi N, Kaira K, et al. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer. 2008;59(2):203–10. doi: 10.1016/j.lungcan.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 27.de Geus-Oei LF, van der Heijden HF, Visser EP, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48(10):1592–8. doi: 10.2967/jnumed.107.043414. [DOI] [PubMed] [Google Scholar]
- 28.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348(25):2500–7. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 29.Basely M, Jr, Bernard P, Gisserot O, Maszelin P, Bussy E, de Jaureguiberry JP. Umbilical metastasis from breast cancer detected by FDG PET. Clin Nucl Med. 2009;34(5):292–3. doi: 10.1097/RLU.0b013e31819e50cb. [DOI] [PubMed] [Google Scholar]
- 30.Duch J, Fuster D, Munoz M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36(10):1551–7. doi: 10.1007/s00259-009-1116-y. [DOI] [PubMed] [Google Scholar]
- 31.Aukema TS, Straver ME, Valdes Olmos RA, Vogel WV. A different role for FDG PET/CT in axillary lymph node staging in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36(11):1896–7. doi: 10.1007/s00259-009-1211-0. author reply 1898-9. [DOI] [PubMed] [Google Scholar]
- 32.Peare R, Staff RT, Heys SD. The use of FDG-PET in assessing axillary lymph node status in breast cancer: a systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-0771-9. Available from: http://dx.doi.org. [DOI] [PubMed]
- 33.Belohlavek O. What is the role of FDG-PET in the initial staging of breast cancer? Eur J Nucl Med Mol Imaging. 2008;35(3):472–4. doi: 10.1007/s00259-007-0643-7. [DOI] [PubMed] [Google Scholar]
- 34.Cermik TF, Mavi A, Basu S, Alavi A. Impact of FDG PET on the preoperative staging of newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging. 2008;35(3):475–83. doi: 10.1007/s00259-007-0580-5. [DOI] [PubMed] [Google Scholar]
- 35.Crippa F, Gerali A, Alessi A, Agresti R, Bombardieri E. FDG-PET for axillary lymph node staging in primary breast cancer. Eur J Nucl Med Mol Imaging. 2004;31 1:S97–102. doi: 10.1007/s00259-004-1531-z. [DOI] [PubMed] [Google Scholar]
- 36.Sloka JS, Hollett PD, Mathews M. A quantitative review of the use of FDG-PET in the axillary staging of breast cancer. Med Sci Monit. 2007;13(3):RA37–46. [PubMed] [Google Scholar]
- 37.Ueda S, Tsuda H, Asakawa H, et al. Utility of 18F-fluorodeoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer. 2008;8:165. doi: 10.1186/1471-2407-8-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Straver ME, Aukema TS, Olmos RA, et al. Feasibility of FDG PET/CT to monitor the response of axillary lymph node metastases to neoadjuvant chemotherapy in breast cancer patients. Eur J Nucl Med Mol Imaging. 2010 doi: 10.1007/s00259-009-1343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar A, Kumar R, Seenu V, et al. The role of 18F-FDG PET/CT in evaluation of early response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Eur Radiol. 2009;19(6):1347–57. doi: 10.1007/s00330-009-1303-z. [DOI] [PubMed] [Google Scholar]
- 40.Momiyama N, Ishikawa T, Ichikawa Y, Shimada H, Katayama A, Ozawa Y. Early prediction of response to primary chemotherapy by sequential FDG -PET in patients with advanced breast cancer. Nippon Rinsho. 2007;65 6:385–8. [PubMed] [Google Scholar]
- 41.Kitajima K, Murakami K, Yamasaki E, et al. Performance of integrated FDG PET/contrast-enhanced CT in the diagnosis of recurrent colorectal cancer: Comparison with integrated FDG PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging. 2009;36(9):1388–96. doi: 10.1007/s00259-009-1081-5. [DOI] [PubMed] [Google Scholar]
- 42.Shen YY, Liang JA, Chen YK, Tsai CY, Kao CH. Clinical impact of 18F-FDG-PET in the suspicion of recurrent colorectal cancer based on asymptomatically elevated serum level of carcinoembryonic antigen (CEA) in Taiwan. Hepatogastroenterology. 2006;53(69):348–50. [PubMed] [Google Scholar]
- 43.Votrubova J, Belohlavek O, Jaruskova M, et al. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2006;33(7):779–84. doi: 10.1007/s00259-006-0072-z. [DOI] [PubMed] [Google Scholar]
- 44.Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin's disease. N Engl J Med. 2007;357(19):1916–27. doi: 10.1056/NEJMoa064601. [DOI] [PubMed] [Google Scholar]
- 45.Juweid ME. Utility of positron emission tomography (PET) scanning in managing patients with Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2006:259–65. 510–1. doi: 10.1182/asheducation-2006.1.259. [DOI] [PubMed] [Google Scholar]
- 46.Kazama T, Faria SC, Varavithya V, Phongkitkarun S, Ito H, Macapinlac HA. FDG PET in the evaluation of treatment for lymphoma: clinical usefulness and pitfalls. Radiographics. 2005;25(1):191–207. doi: 10.1148/rg.251045045. [DOI] [PubMed] [Google Scholar]
- 47.Kumar R, Xiu Y, Mavi A, El-Haddad G, Zhuang H, Alavi A. FDG-PET imaging in primary bilateral adrenal lymphoma: a case report and review of the literature. Clin Nucl Med. 2005;30(4):222–30. doi: 10.1097/01.rlu.0000155983.46815.1c. [DOI] [PubMed] [Google Scholar]
- 48.Al-Ibraheem A, Buck A, Krause BJ, Scheidhauer K, Schwaiger M. Clinical Applications of FDG PET and PET/CT in Head and Neck Cancer. J Oncol. 2009;2009:208725. doi: 10.1155/2009/208725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machtay M, Natwa M, Andrel J, et al. Pretreatment FDG-PET standardized uptake value as a prognostic factor for outcome in head and neck cancer. Head Neck. 2009;31(2):195–201. doi: 10.1002/hed.20942. [DOI] [PubMed] [Google Scholar]
- 50.Wong RJ. Current status of FDG-PET for head and neck cancer. J Surg Oncol. 2008;97(8):649–52. doi: 10.1002/jso.21018. [DOI] [PubMed] [Google Scholar]
- 51.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. 2008;100(10):712–20. doi: 10.1093/jnci/djn125. [DOI] [PubMed] [Google Scholar]
- 52.Branstetter BFt, Blodgett TM, Zimmer LA, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology. 2005;235(2):580–6. doi: 10.1148/radiol.2352040134. [DOI] [PubMed] [Google Scholar]
- 53.Fukui MB, Blodgett TM, Meltzer CC. PET/CT imaging in recurrent head and neck cancer. Semin Ultrasound CT MR. 2003;24(3):157–63. doi: 10.1016/s0887-2171(03)90037-0. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen BD, Ram PC, Roarke MC. Endotracheal metastasis from squamous cell cancer of the head and neck: PET/CT imaging. Clin Nucl Med. 2008;33(5):340–1. doi: 10.1097/RLU.0b013e31816a790a. [DOI] [PubMed] [Google Scholar]
- 55.Guo H, Zhu H, Xi Y, et al. Diagnostic and prognostic value of 18F-FDG PET/CT for patients with suspected recurrence from squamous cell carcinoma of the esophagus. J Nucl Med. 2007;48(8):1251–8. doi: 10.2967/jnumed.106.036509. [DOI] [PubMed] [Google Scholar]
- 56.Klaeser B, Nitzsche E, Schuller JC, et al. Limited predictive value of FDG-PET for response assessment in the preoperative treatment of esophageal cancer: results of a prospective multi-center trial (SAKK 75/02) Onkologie. 2009;32(12):724–30. doi: 10.1159/000251842. [DOI] [PubMed] [Google Scholar]
- 57.Isa T, Kaneshiro T, Yamamoto H, et al. Case of esophageal cancer successfully performed in early response evaluation for preoperative chemotherapy by FDG-PET. Nippon Shokakibyo Gakkai Zasshi. 2008;105(8):1193–9. [PubMed] [Google Scholar]
- 58.Wieder HA, Herrmann K, Ott K, Krause BJ. 18F-FDG-PET in therapy response of esophageal cancer. Radiologe. 2007;47(2):110–4. doi: 10.1007/s00117-006-1461-9. [DOI] [PubMed] [Google Scholar]
- 59.Krause BJ, Herrmann K, Wieder H, zum Buschenfelde CM. 18F-FDG PET and 18F-FDG PET/CT for assessing response to therapy in esophageal cancer. J Nucl Med. 2009;50 1:89S–96S. doi: 10.2967/jnumed.108.057232. [DOI] [PubMed] [Google Scholar]
- 60.Mansour AA, 3rd, Kelley MC, Hatmaker AR, Holt GE, Schwartz HS. Verification of Musculoskeletal FDG-PET-CT Findings Performed for Melanoma Staging. Ann Surg Oncol. 2009 doi: 10.1245/s10434-009-0843-4. Available from: http://dx.doi.org. [DOI] [PubMed]
- 61.Strobel K, Dummer R, Husarik DB, Perez Lago M, Hany TF, Steinert HC. High-risk melanoma: accuracy of FDG PET/CT with added CT morphologic information for detection of metastases. Radiology. 2007;244(2):566–74. doi: 10.1148/radiol.2442061099. [DOI] [PubMed] [Google Scholar]
- 62.Vandewoude M, Cornelis A, Wyndaele D, Brussaard C, Kums R. 18FDG-PET-scan in staging of primary malignant melanoma of the oesophagus: a case report. Acta Gastroenterol Belg. 2006;69(1):12–4. [PubMed] [Google Scholar]
- 63.Jimenez-Requena F, Delgado-Bolton RC, Fernandez-Perez C, et al. Meta-analysis of the performance of 18F-FDG PET in cutaneous melanoma. Eur J Nucl Med Mol Imaging. 2010;37(2):284–300. doi: 10.1007/s00259-010-1476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crippa F, Leutner M, Belli F, et al. Which kinds of lymph node metastases can FDG PET detect? A clinical study in melanoma. J Nucl Med. 2000;41(9):1491–4. [PubMed] [Google Scholar]
- 65.Wagner JD, Schauwecker DS, Davidson D, Wenck S, Jung SH, Hutchins G. FDG-PET sensitivity for melanoma lymph node metastases is dependent on tumor volume. J Surg Oncol. 2001;77(4):237–42. doi: 10.1002/jso.1102. [DOI] [PubMed] [Google Scholar]
- 66.Strobel K, Heinrich S, Bhure U, et al. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med. 2008;49(9):1408–13. doi: 10.2967/jnumed.108.051466. [DOI] [PubMed] [Google Scholar]
- 67.Sperti C, Pasquali C, Bissoli S, Chierichetti F, Liessi G, Pedrazzoli S. Tumor relapse after pancreatic cancer resection is detected earlier by 18F-FDG PET than by CT. J Gastrointest Surg. 2010;14(1):131–40. doi: 10.1007/s11605-009-1010-8. [DOI] [PubMed] [Google Scholar]
- 68.Lachter J, Adler AC, Keidar Z, Haddad R. FDG-PET/CT identifies a curable pancreatic cancer surgical tract metastasis after failure by other imaging modalities. Isr Med Assoc J. 2008;10(3):243–4. [PubMed] [Google Scholar]
- 69.Nishiyama Y, Yamamoto Y, Yokoe K, et al. Contribution of whole body FDG-PET to the detection of distant metastasis in pancreatic cancer. Ann Nucl Med. 2005;19(6):491–7. doi: 10.1007/BF02985577. [DOI] [PubMed] [Google Scholar]
- 70.Mataki Y, Shinchi H, Kurahara H, et al. Clinical usefulness of FDG-PET for pancreatic cancer. Gan To Kagaku Ryoho. 2009;36(13):2516–20. [PubMed] [Google Scholar]
- 71.Higashi T, Saga T, Nakamoto Y, et al. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET) --usefulness and limitations in “clinical reality”. Ann Nucl Med. 2003;17(4):261–79. doi: 10.1007/BF02988521. [DOI] [PubMed] [Google Scholar]
- 72.Evans KD, Tulloss TA, Hall N. 18FDG uptake in brown fat: potential for false positives. Radiol Technol. 2007;78(5):361–6. [PubMed] [Google Scholar]
- 73.Okuma T, Matsuoka T, Okamura T, et al. 18F-FDG small-animal PET for monitoring the therapeutic effect of CT-guided radiofrequency ablation on implanted VX2 lung tumors in rabbits. J Nucl Med. 2006;47(8):1351–8. [PubMed] [Google Scholar]
- 74.van Waarde A, Cobben DC, Suurmeijer AJ, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med. 2004;45(4):695–700. [PubMed] [Google Scholar]
- 75.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 76.Yanagida O, Kanai Y, Chairoungdua A, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514(2):291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 77.Nakanishi K, Ogata S, Matsuo H, et al. Expression of LAT1 predicts risk of progression of transitional cell carcinoma of the upper urinary tract. Virchows Arch. 2007;451(3):681–90. doi: 10.1007/s00428-007-0457-9. [DOI] [PubMed] [Google Scholar]
- 78.Kobayashi H, Ishii Y, Takayama T. Expression of L-type amino acid transporter 1 (LAT1) in esophageal carcinoma. J Surg Oncol. 2005;90(4):233–8. doi: 10.1002/jso.20257. [DOI] [PubMed] [Google Scholar]
- 79.Kaira K, Oriuchi N, Imai H, et al. l-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008;99(12):2380–6. doi: 10.1111/j.1349-7006.2008.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol. 2008;10(1):1–18. doi: 10.1007/s11307-007-0115-2. [DOI] [PubMed] [Google Scholar]
- 81.Leskinen-Kallio S, Nagren K, Lehikoinen P, Ruotsalainen U, Teras M, Joensuu H. Carbon-11-methionine and PET is an effective method to image head and neck cancer. J Nucl Med. 1992;33(5):691–5. [PubMed] [Google Scholar]
- 82.Inoue T, Kim EE, Wong FC, et al. Comparison of fluorine-18-fluorodeoxyglucose and carbon-11-methionine PET in detection of malignant tumors. J Nucl Med. 1996;37(9):1472–6. [PubMed] [Google Scholar]
- 83.Becherer A, Karanikas G, Szabo M, et al. Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003;30(11):1561–7. doi: 10.1007/s00259-003-1259-1. [DOI] [PubMed] [Google Scholar]
- 84.Lindholm P, Leskinen S, Nagren K, et al. Carbon-11-methionine PET imaging of malignant melanoma. J Nucl Med. 1995;36(10):1806–10. [PubMed] [Google Scholar]
- 85.Couturier O, Luxen A, Chatal JF, Vuillez JP, Rigo P, Hustinx R. Fluorinated tracers for imaging cancer with positron emission tomography. Eur J Nucl Med Mol Imaging. 2004;31(8):1182–206. doi: 10.1007/s00259-004-1607-9. [DOI] [PubMed] [Google Scholar]
- 86.Laverman P, Boerman OC, Corstens FH, Oyen WJ. Fluorinated amino acids for tumour imaging with positron emission tomography. Eur J Nucl Med Mol Imaging. 2002;29(5):681–90. doi: 10.1007/s00259-001-0716-y. [DOI] [PubMed] [Google Scholar]
- 87.McConathy J, Goodman MM. Non-natural amino acids for tumor imaging using positron emission tomography and single photon emission computed tomography. Cancer Metastasis Rev. 2008;27(4):555–73. doi: 10.1007/s10555-008-9154-7. [DOI] [PubMed] [Google Scholar]
- 88.Popperl G, Kreth FW, Herms J, et al. Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J Nucl Med. 2006;47(3):393–403. [PubMed] [Google Scholar]
- 89.Thiele F, Ehmer J, Piroth MD, et al. The quantification of dynamic FET PET imaging and correlation with the clinical outcome in patients with glioblastoma. Phys Med Biol. 2009;54(18):5525–39. doi: 10.1088/0031-9155/54/18/012. [DOI] [PubMed] [Google Scholar]
- 90.Stadlbauer A, Prante O, Nimsky C, et al. Metabolic imaging of cerebral gliomas: spatial correlation of changes in O-(2-18F-fluoroethyl)-L-tyrosine PET and proton magnetic resonance spectroscopic imaging. J Nucl Med. 2008;49(5):721–9. doi: 10.2967/jnumed.107.049213. [DOI] [PubMed] [Google Scholar]
- 91.Stadlbauer A, Polking E, Prante O, et al. Detection of tumour invasion into the pyramidal tract in glioma patients with sensorimotor deficits by correlation of 18F-fluoroethyl-L: -tyrosine PET and magnetic resonance diffusion tensor imaging. Acta Neurochir (Wien) 2009;151(9):1061–9. doi: 10.1007/s00701-009-0378-2. [DOI] [PubMed] [Google Scholar]
- 92.Tripathi M, Sharma R, D'Souza M, et al. Comparative evaluation of F-18 FDOPA, F-18 FDG, and F-18 FLT-PET/CT for metabolic imaging of low grade gliomas. Clin Nucl Med. 2009;34(12):878–83. doi: 10.1097/RLU.0b013e3181becfe0. [DOI] [PubMed] [Google Scholar]
- 93.Talbot JN, Kerrou K, Montravers F, Nataf V, Chevalme Y. FDOPA PET has clinical utility in brain tumour imaging: a proposal for a revision of the recent EANM guidelines. Eur J Nucl Med Mol Imaging. 2007;34(7):1131–2. doi: 10.1007/s00259-007-0400-y. author reply 1133-4. [DOI] [PubMed] [Google Scholar]
- 94.Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47(6):904–11. [PubMed] [Google Scholar]
- 95.Schiepers C, Chen W, Cloughesy T, Dahlbom M, Huang SC. 18F-FDOPA kinetics in brain tumors. J Nucl Med. 2007;48(10):1651–61. doi: 10.2967/jnumed.106.039321. [DOI] [PubMed] [Google Scholar]
- 96.Koopmans KP, de Vries EG, Kema IP, et al. Staging of carcinoid tumours with 18F-DOPA PET: a prospective, diagnostic accuracy study. Lancet Oncol. 2006;7(9):728–34. doi: 10.1016/S1470-2045(06)70801-4. [DOI] [PubMed] [Google Scholar]
- 97.Shoup TM, Olson J, Hoffman JM, et al. Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med. 1999;40(2):331–8. [PubMed] [Google Scholar]
- 98.Schuster DM, Votaw JR, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48(1):56–63. [PubMed] [Google Scholar]
- 99.Schuster DM, Nye JA, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-[18F]Fluorocyclobutane-1-carboxylic acid (anti-[18F]FACBC) with PET in renal carcinoma. Mol Imaging Biol. 2009;11(6):434–8. doi: 10.1007/s11307-009-0220-5. [DOI] [PubMed] [Google Scholar]
- 100.Yoshimoto M, Waki A, Obata A, Furukawa T, Yonekura Y, Fujibayashi Y. Radiolabeled choline as a proliferation marker: comparison with radiolabeled acetate. Nucl Med Biol. 2004;31(7):859–65. doi: 10.1016/j.nucmedbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Hara T, Kosaka N, Kishi H. Development of 18F-fluoroethylcholine for cancer imaging with PET: synthesis, biochemistry, and prostate cancer imaging. J Nucl Med. 2002;43(2):187–99. [PubMed] [Google Scholar]
- 102.Tian M, Zhang H, Oriuchi N, Higuchi T, Endo K. Comparison of 11C-choline PET and FDG PET for the differential diagnosis of malignant tumors. Eur J Nucl Med Mol Imaging. 2004;31(8):1064–72. doi: 10.1007/s00259-004-1496-y. [DOI] [PubMed] [Google Scholar]
- 103.Kwee SA, Coel MN, Ly BH, Lim J. 18F-choline PET/CT imaging of RECIST measurable lesions in hormone refractory prostate cancer. Ann Nucl Med. 2009;23(6):541–8. doi: 10.1007/s12149-009-0273-1. [DOI] [PubMed] [Google Scholar]
- 104.Luboldt W, Kufer R, Blumstein N, et al. Prostate carcinoma: diffusion-weighted imaging as potential alternative to conventional MR and 11C-choline PET/CT for detection of bone metastases. Radiology. 2008;249(3):1017–25. doi: 10.1148/radiol.2492080038. [DOI] [PubMed] [Google Scholar]
- 105.Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon-11-choline. J Nucl Med. 1998;39(6):990–5. [PubMed] [Google Scholar]
- 106.Huang Z, Zuo C, Guan Y, et al. Misdiagnoses of 11C-choline combined with 18F-FDG PET imaging in brain tumours. Nucl Med Commun. 2008;29(4):354–8. doi: 10.1097/MNM.0b013e3282f4a21e. [DOI] [PubMed] [Google Scholar]
- 107.Hara T, Kosaka N, Shinoura N, Kondo T. PET imaging of brain tumor with [methyl-11C]choline. J Nucl Med. 1997;38(6):842–7. [PubMed] [Google Scholar]
- 108.Ohtani T, Kurihara H, Ishiuchi S, et al. Brain tumour imaging with carbon-11 choline: comparison with FDG PET and gadolinium-enhanced MR imaging. Eur J Nucl Med. 2001;28(11):1664–70. doi: 10.1007/s002590100620. [DOI] [PubMed] [Google Scholar]
- 109.Li X, Liu Q, Wang M, et al. C-11 choline PET/CT imaging for differentiating malignant from benign prostate lesions. Clin Nucl Med. 2008;33(10):671–6. doi: 10.1097/RLU.0b013e318184b3a0. [DOI] [PubMed] [Google Scholar]
- 110.Zheng QH, Stone KL, Mock BH, et al. [11C]Choline as a potential PET marker for imaging of breast cancer athymic mice. Nucl Med Biol. 2002;29(8):803–7. doi: 10.1016/s0969-8051(02)00339-6. [DOI] [PubMed] [Google Scholar]
- 111.Khan N, Oriuchi N, Ninomiya H, Higuchi T, Kamada H, Endo K. Positron emission tomographic imaging with 11C-choline in differential diagnosis of head and neck tumors: comparison with 18F-FDG PET. Ann Nucl Med. 2004;18(5):409–17. doi: 10.1007/BF02984484. [DOI] [PubMed] [Google Scholar]
- 112.Salem N, Kuang Y, Wang F, Maclennan GT, Lee Z. PET imaging of hepatocellular carcinoma with 2-deoxy-2[18F]fluoro-D-glucose, 6-deoxy-6[18F] fluoro-D-glucose, [1-11C]-acetate and [N-methyl-11C]-choline. Q J Nucl Med Mol Imaging. 2009;53(2):144–56. [PubMed] [Google Scholar]
- 113.Beheshti M, Vali R, Waldenberger P, et al. The Use of F-18 Choline PET in the Assessment of Bone Metastases in Prostate Cancer: Correlation with Morphological Changes on CT. Mol Imaging Biol. 2009 doi: 10.1007/s11307-009-0217-0. Available from: http://dx.doi.org. [DOI] [PubMed]
- 114.Pascali G, D' Antonio L, Bovone P, Gerundini P, August T. Optimization of automated large-scale production of [18F]fluoroethylcholine for PET prostate cancer imaging. Nucl Med Biol. 2009;36(5):569–74. doi: 10.1016/j.nucmedbio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 115.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 1:122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mankoff DA, Shields AF, Krohn KA. PET imaging of cellular proliferation. Radiol Clin North Am. 2005;43(1):153–67. doi: 10.1016/j.rcl.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Barwick T, Bencherif B, Mountz JM, Avril N. Molecular PET and PET/CT imaging of tumour cell proliferation using F-18 fluoro-L-thymidine: a comprehensive evaluation. Nucl Med Commun. 2009;30(12):908–17. doi: 10.1097/MNM.0b013e32832ee93b. [DOI] [PubMed] [Google Scholar]


