Abstract
Background
Genetically-modified mice offer the unique opportunity to gain insights into the pathophysiology of pulmonary arterial hypertension (PAH). In mice, right heart catheterization is the only available technique to measure right ventricular systolic pressure (RVSP). However, it is a terminal procedure and does not allow for serial measurements. Our objective was to validate a non-invasive technique to assess RVSP in mice.
Methods and Results
Right ventricle catheterization and echocardiography (30-MHz transducer) were simultaneously performed in mice with pulmonary hypertension induced acutely by infusion of a thromboxane analogue, U-46619, or chronically by lung-specific over-expression of interleukin 6 (IL-6). Pulmonary acceleration time (PAT) and ejection time (ET) were measured in the parasternal short axis view by pulsed-wave Doppler of pulmonary artery flow. Infusion of U-46619 acutely increased RVSP, shortened PAT, and decreased PAT/ET. The pulmonary flow pattern changed from symmetric at baseline to asymmetric at higher RVSPs. In wild-type and IL-6-over-expressing mice, the PAT correlated linearly with RVSP (r2=−0.67; p<0.0001), as did PAT/ET (r2=−0.76; p<0.0001). Sensitivity and specificity for detecting high RVSP (>32 mmHg) were 100% (7/7) and 86% (6/7), respectively, for both indices (cutoff values: PAT <21 ms and PAT/ET <39%). Intra-observer and inter-observer variability of PAT and PAT/ET were less than 6%.
Conclusion
Right ventricular systolic pressure can be estimated non-invasively in mice. Echocardiography is able to detect acute and chronic increases in RVSP with high sensitivity and specificity, as well as to assess the effects of treatment on RVSP. This non-invasive technique may permit the characterization of the evolution of PAH in genetically-modified mice.
Keywords: Echocardiography, right ventricular systolic pressure, mice
INTRODUCTION
Pulmonary arterial hypertension (PAH) is characterized by pulmonary vascular remodeling and is associated with elevated right ventricular systolic pressure (RVSP) and pulmonary vascular resistance (PVR) that may result in progressive RV failure, low cardiac output, and premature death.1, 2 Despite the development of new therapeutic strategies such as endothelin-receptor antagonists, phosphodiesterase type-5 inhibitors, and prostanoids,3 the prognosis of patients with PAH remains poor.2 Advances in understanding the pathophysiological mechanisms that contribute to PAH are critical to the discovery of new therapeutic targets. Small rodent models, in particular genetically-modified mice, offer the unique opportunity to study the signaling pathways involved in PAH and to evaluate the effectiveness of therapeutic interventions.
In mice models, right heart catheterization and measurement of RVSP, which is equal to pulmonary artery systolic pressure in the absence of pulmonary stenosis, has been central in the detection and quantification of PAH.4-6 Right heart catheterization, however, is a terminal procedure in mice that does not allow longitudinal follow-up. Indirect assessment of the severity of the PAH disease by histological evaluation of post-mortem heart and lung tissue also precludes serial assessment. The absence of a non-invasive technique that would allow serial assessment of pulmonary artery pressures or PVR in mice has significantly slowed progresses in the field of PAH, preventing the rapid evaluation and development of mouse PAH models.
Although right heart catheterization is required to confirm the diagnosis of PAH, echocardiography is a pivotal screening test in humans for PAH, and the only non-invasive technique to follow the course of the disease.7 Pulmonary artery systolic pressure is usually estimated from the tricuspid regurgitation peak flow velocity. Tricuspid regurgitation is often visualized in the apical or subcostal views. In contrast, the estimation of pulmonary artery pressure in mice using echocardiography remains technically challenging. Apical and subcostal views are not reliably obtained, preventing proper flow alignment and accurate measurement of tricuspid regurgitation by Doppler in these views. Furthermore, tricuspid regurgitation appears to be uncommon in rodents except at very high pulmonary pressures.8
Nevertheless echocardiography may be a reliable tool to non-invasively assess RVSP in mice. Importantly, PVR may also be measured, as mouse cardiac output can be quantified using echocardiography.9 The pulmonary acceleration time (PAT, time from the onset of pulmonary flow to peak velocity by pulsed-wave Doppler recording) and the ratio of PAT to ejection time (ET, time interval between the onset and end of the systolic flow velocity) have been proposed as alternative indexes to estimate RVSP, when tricuspid regurgitation is insufficient to reliably measure its peak velocity. In response to an increase in pulmonary artery systolic pressure, the pulmonary valve tends to close prematurely,10 and peak flow velocity is reached earlier in systole. Therefore, PAT decreases as pulmonary pressure increases. Pulmonary acceleration time correlates inversely and linearly with mean pulmonary artery pressure in humans11-15 and with RVSP in rats.8
Pulmonary acceleration time may represent a useful index of pulmonary artery pressure in mice. A parasternal short axis view of the heart at the level of the aortic valve can easily be obtained in mice, allowing for correct alignment with the pulmonary artery flow. This view enables precise pulsed-wave Doppler recording and measurement of right ventricular systolic time intervals such as PAT and ET. Furthermore, the increased spatial resolution of recently developed high-frequency echocardiographic probes permits better visualization of the pulmonary valve and more precise sampling of the flow.
The objective of the present study was to investigate whether RVSP and thus pulmonary arterial systolic pressure could be estimated non-invasively in mice. Echocardiographic parameters of RVSP were compared to RVSP measured using a pressure catheter. Two models of pulmonary hypertension were studied: an acute model induced by infusion of the thromboxane agonist, U-46619,16, 17 and a chronic model induced by lung-specific over expression of a transgene encoding interleukin 6 (IL-6).4
METHODS
Hemodynamic measurements
Mice were anesthetized with intraperitoneal ketamine (120 mg/kg) and fentanyl (200 μg/kg), intubated, and mechanically ventilated (115 breaths per minute, FiO2=1). A PE-10 polyethylene catheter was placed in the left carotid artery for continuous heart rate and systemic arterial pressure monitoring. Another polyethylene line was placed in the left jugular vein for infusion of U-46619. A micro manometer pressure catheter (Model FTS-1211B-0018, SciSense Instruments, London, Ontario) was placed in the right jugular vein and advanced into the right ventricle. The systemic arterial pressure, heart rate and RVSP were recorded and analyzed using a data acquisition system (Chart, AD Instruments, Colorado Springs, CO). Right ventricular systolic pressure and echocardiographic parameters were simultaneously acquired.
Echocardiography
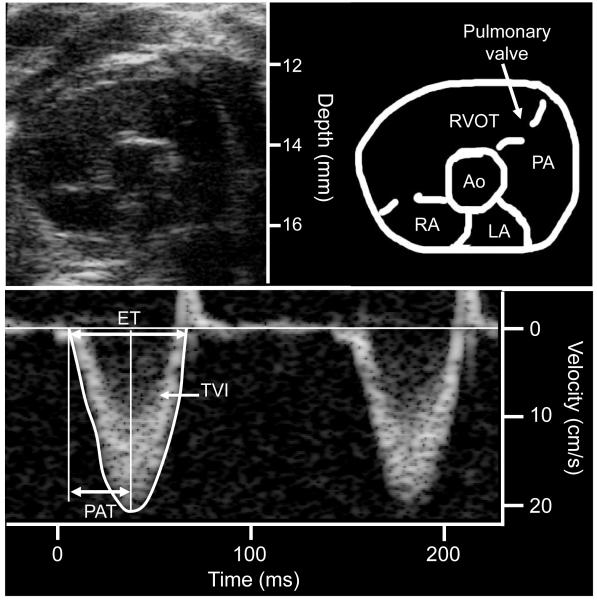
Transthoracic closed-chest echocardiography was performed using a mechanical transducer centered on 30 MHz (Vevo 770, Visualsonics, Toronto, Canada). Two-dimensional images of the pulmonary infundibulum were obtained from the parasternal short axis view at the level of the aortic valve (Figure 1, upper panel) and pulsed-wave Doppler recording of the pulmonary blood flow was obtained (Figure 1, lower panel). The Pulsed-wave Doppler sample was positioned at the tip of the pulmonary valve leaflets and aligned to maximize laminar flow. Sample volume was 0.027 mm3. Doppler tracings were recorded at a sweep speed of 400 mm/sec. Measurements were performed offline (Vevo 770 workstation) by two different readers (HT and BK for the acute study, BK for the chronic study) blinded to the condition or genotype of the mice. The following variables were measured: systolic time velocity integral of pulmonary flow (TVI, the area under the flow curve), pulmonary acceleration time (PAT, defined as the time from the onset of flow to peak velocity by pulsed-wave Doppler recording), and right ventricular ejection time (ET, the time from the onset to the termination of pulmonary flow). All measurements were averaged on 5 cardiac cycles.
Figure 1.
Measurement of pulmonary flow using pulsed-wave Doppler in a mouse. The upper panel displays a 2D parasternal short axis view obtained at the level of the aortic valve. The lower panel displays the pulsed-wave Doppler acquisition of the pulmonary flow. RA: right atrium, LA: left atrium, RVOT: right ventricular outflow tract, PA: pulmonary artery, Ao: aortic valve, PAT: pulmonary acceleration time, ET: ejection time, TVI: time velocity integral.
Experimental protocols
Acute model of pulmonary hypertension
Seven C57BL6 wild type male mice (3 months old) were studied. Right ventricular systolic pressure and echocardiographic parameters were simultaneously acquired at baseline. U-46619 was then infused at a rate of 1.5 μmole/kg/min for 5 minutes. Right ventricular systolic pressure and an echocardiogram were obtained. The infusion was discontinued for 15 minutes, and after the RVSP returned to baseline, U-46619 was infused (3 μmole/kg/min for 5 minutes) for a second set of measurements.
To test whether echocardiography could detect the effect of a treatment lowering pulmonary artery pressure, RVSP and echocardiographic parameters were simultaneously acquired in 4 C57BL6 wild type mice at baseline and after infusion of U-46619 (1.5 μmole/kg/min for 5 minutes). The infusion was continued for the entire experiment. After the RVSP had increased by 30%, 80 ppm of inhaled nitric oxide (NO) were added to the air breathed by the mouse. A set of echocardiographic and hemodynamic measurements was recorded 5 minutes after the start of inhaled NO.
Chronic model of pulmonary hypertension
CC10-IL-6 transgenic mice (IL-6 Tg+) were bred on a C57/BL6 (Tg−) background.18 In these mice, the Clara cell 10-kD promoter 5(CC10) was used to constitutively drive lung-specific expression of IL-6. Nine mice with lung-specific over expression of IL-6 (IL-6 Tg+)18 and five C57BL6 wild type (WT) mice were studied in a blinded manner. In ten mice, echocardiography was first obtained using isoflurane (2% for induction followed by 0.5 to 0.7% for anesthesia). A second echocardiogram was performed in 14 mice simultaneously with RV catheterization, as described in the acute model protocol.
Statistics
Statistical analysis was performed with the JMP statistical software package (SAS Institute, Cary, NC). Values are expressed as mean±SEM except inter-observer and intra-observer variability, which are expressed as mean±SD. We aimed to be able to detect a difference in RVSP of 6 mmHg (2SD of the baseline RVSP). Based on the SD of the difference between values of RVSP in the same mouse before and after infusion of U-46619, 6 mice were necessary to detect a difference of 6 mmHg with a power of 0.85 at a 5% significance level. Similarly, based on the SD of the difference between values of the RVSP between mice, 14 mice were necessary to detect a difference of 6 mmHg between wild-type and IL-6 Tg+ mice. Variations in echocardiographic and hemodynamic measurements between different infusions rate of U-46619 and inhaled NO were tested using an ANOVA for repeated measurements. If the overall ANOVA was significant, Student paired t-tests were used. Comparisons between mice with RVSP above and below 32 mmHg were performed by use of the Student paired t-tests. To adjust for the multiplicity of outcome variables, p values < 0.05/3=0.015 was considered as indicative of a statistically significant difference for the 3 primary outcome variables (RVSP, PAT, PAT/ET). The prediction of RVSP by echocardiography was obtained by simple regression analysis. Both intra-observer and inter-observer variability were assessed in 10 measurements. Measurements were repeated by the same observer after an interval of at least one week and by a second independent observer. The variability was then estimated by the difference between the two observations and was expressed as both absolute numbers and percentages.
RESULTS
Acute model of pulmonary hypertension
Hemodynamic effects of U-46619 infusion and nitric oxide inhalation
In the first set of experiment, infusion of 1.5 and 3.0 μmole/kg/min U-46619 into C57BL6 mice markedly increased systolic blood pressure and RVSP (Table 1). U-46619 increased RVSP in a dose-dependent manner. U-46619 did not affect the heart rate.
Table 1.
Hemodynamic and pulmonary pulsed-wave Doppler measurements obtained at baseline and during U-46619 infusion in 7 mice
| Baseline | U-46619 | ||
|---|---|---|---|
| 1.5 μmole/kg/min | 3 μmole/kg/min | ||
| Hemodynamics | |||
| HR (bpm) | 392±11 | 415±25 | 410±18 |
| SAP (mmHg) | 79±5 | 141±15* | 140±10* |
| RVSP (mmHg) | 27±1 | 34±2* | 46±3*† |
|
| |||
| Pulmonary pulsed-wave | |||
| Doppler | |||
| Pulmonary TVI (cm) | 1.6±0.1 | 1.1±0.2* | 0.8±0.1*† |
| PAT (ms) | 30±1 | 20±1* | 14±1*† |
| ET (ms) | 64±2 | 58±3* | 50±3*† |
| PAT/ET (%) | 46±2 | 35±2* | 28±2*† |
HR, heart rate; SAP, systolic arterial pressure; RVSP, right ventricular systolic pressure; TVI, time velocity integral; PAT, pulmonary acceleration time; ET, ejection time.
p<0.01 vs. baseline;
p<0.01 vs. U-46619 (1.5 μmole/kg/min)
In the second set of acute PAH model experiment, U-46619 increased RVSP by 43% (from 23±1 to 33±1 mmHg) and systolic blood pressure by 71% compared to baseline. Inhaled NO did not affect blood pressure but decreased RVSP by 24% (to 25±2 mmHg).
The U-46619-induced increase in RVSP is associated with a decrease in PAT and PAT/ET
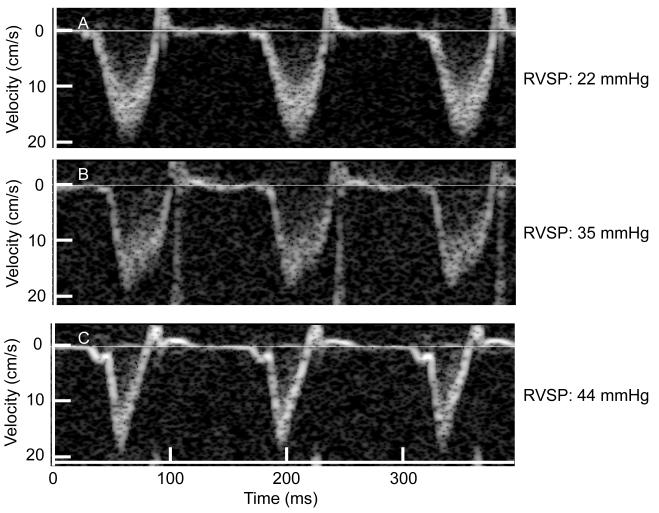
Pulmonary artery flow was obtained using pulsed-wave Doppler in all animals, and the quality of the images allowed measurements of right ventricular systolic time intervals in all cases. At baseline, the pulmonary systolic flow pattern was symmetric. Infusion of increasing doses of U-46619 progressively shifted the peak of the Doppler flow pattern towards early systole, reflecting a decrease in the acceleration time and resulting in an asymmetric pattern (Figure 2). At baseline, ET was 64±2 ms, and PAT was 30±1 ms (46±2% of the ET). U-46619 infusion decreased both the ET (by 9% at the dose of 1.5μmol/kg/min) and the PAT (by 33% at the dose of 1.5μmol/kg/min) (Table 1). At both doses of the U-46619 infusion, PAT decreased more than ET resulting in a dose-dependent decrease of PAT/ET. The pulmonary TVI also decreased during the infusion of U-46619.
Figure 2.
Representative example of the pulsed-wave Doppler of pulmonary flow recorded in the same mouse at baseline (A), after infusion of 1.5 μmole/kg/min of U-46619 for 5 minutes (B) and after infusion of 3 μmole/kg/min of U-46619 for 5 minutes (C). The right ventricular systolic pressure (RVSP) measured simultaneously is displayed next to each pulsed-wave Doppler tracing.
Inhaled NO reverses the U-46619-induced increase in RVSP and is associated with an increase in PAT and PAT/ET
U-46619 infusion decreased both the PAT (by 35%) and the PAT/ET (by 25%) to an extent similar to that observed in the first set of mice. Inhaled NO given while continuing the U-46619 infusion increased PAT (by 35%) and PAT/ET (by 19%), but had no effect on pulmonary TVI.
Chronic model of pulmonary hypertension
Both the PAT and the PAT/ET correlate closely with invasively-measured RVSP
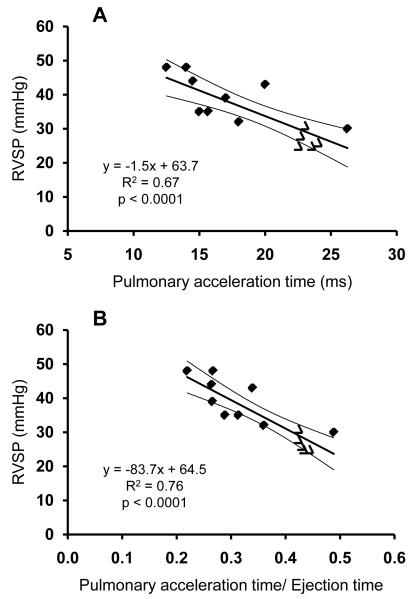
Pulmonary acceleration time correlated closely (R2=0.67; p<0.0001) with the RVSP (Figure 3A). A close correlation was also noted between PAT/ET and RVSP (R2=0.76; p<0.0001) (Figure 3B). The standard error of the estimate was 0.3 mmHg/ms for the slope and 6 mmHg for the intercept for PAT and 13.6 mmHg and 4.9 mmHg for the PAT/ET. The standard deviation of the residuals was 5 mmHg for the PAT and 4 mmHg for the PAT/ET. Neither PAT nor PAT/ET correlated with heart rate, systolic blood pressure, or TVI.
Figure 3.
Correlation between RVSP measured by invasive cardiac catheterization and PAT measured by pulsed-wave Doppler (Panel A). Correlation between RVSP measured by invasive cardiac catheterization and PAT/ET measured by pulsed-wave Doppler (Panel B). Five wild-type mice (open triangles) and nine IL-6 Tg+ mice (filled diamonds) were studied. Curved lines represent the 95% mean confidence interval.
PAT and PAT/ET detect mice with elevated RVSP
In the present study, C57BL6 wild type mice had a RVSP of 27±2.5 mmHg. Elevated RVSP was defined as RVSP above the mean +2SD of the normal values (27+5),19 yielding a threshold value for elevated RVSP of 32 mmHg. The hemodynamic and echocardiographic characteristics of the mice with normal or elevated RVSP are shown in Table 2. All mice with an elevated RVSP were IL-6 Tg+ mice. There were no differences in HR, SAP or pulmonary TVI between groups. Pulmonary acceleration time was shorter and PAT/ET was lower in the mice with elevated RVSP than in mice with RVSP within normal values (Table 2).
Table 2.
Hemodynamic and pulmonary pulsed-wave Doppler flow measurements obtained in WT and IL-6 Tg+ mice
| RVSP ≤ 32 mmHg n=7 |
RVSP > 32 mmHg n=7 |
|
|---|---|---|
| Hemodynamics | ||
| HR (bpm) | 462±20 | 433±21 |
| SAP (mmHg) | 95±6 | 91±3 |
| RVSP (mmHg) | 28±1 | 42±2* |
|
| ||
| Pulmonary pulsed-wave | ||
| Doppler | ||
| Pulmonary TVI (cm) | 2.3±0.2 | 2.2±0.2 |
| PAT (ms) | 23±1 | 16±1* |
| ET (ms) | 53±1 | 56±2 |
| PAT/ET (%) | 43±1 | 28±1* |
HR, heart rate; SAP, systolic arterial pressure; RVSP, right ventricular systolic pressure; TVI, time velocity integral; PAT, pulmonary acceleration time; ET, ejection time.
p<0.01 vs. normotensive mice
A cut-off value of 21 ms (value corresponding to a RVSP of 32 mmHg) was chosen for the PAT. Using this cut-off, echocardiographic analysis had a sensitivity of 100% (7/7) and a specificity of 86% (6/7) in the detection of RVSP higher than 32 mmHg. Similarly, using a cut off value of 39% for the PAT/ET ratio, the sensitivity in the prediction of high RVSP was 100% (7/7) and specificity is 86% (6/7).
Right ventricular systolic pressure can be measured with echocardiography using light anesthesia
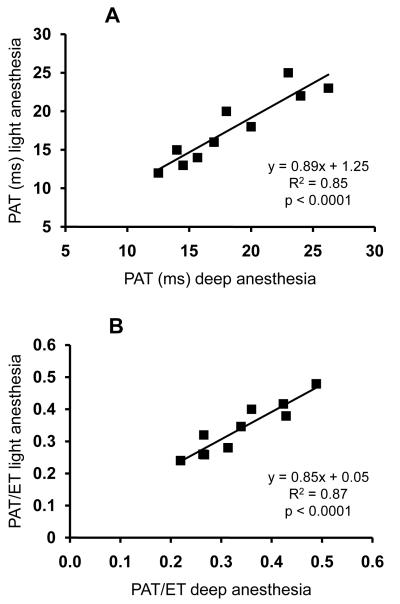
Pulmonary acceleration time measured during light (isoflurane) anesthesia correlated with PAT measured during deep anesthesia (ketamine and fentanyl used for RV catheterization) in IL-6 Tg+ and wild-type mice (r2=0.85, p<0.0001) (Figure 4A). Similarly, PAT/ET measured during light anesthesia correlated closely with the PAT/ET obtained during deep anesthesia (r2= 0.87, p<0.0001) (Figure 4B). All mice that were defined as having high RVSP using PAT or PAT/ET during deep anesthesia had also high RVSP during isoflurane anesthesia (7/7).
Figure 4.
Panel A: Correlation between PAT measured by pulsed-wave Doppler during ketamine-fentanyl anesthesia (deep anesthesia) and during isoflurane anesthesia (light anesthesia). Panel B: Correlation between PAT/ET measured by pulsed-wave Doppler during ketamine-fentanyl anesthesia and during isoflurane anesthesia.
Intra- and inter-observer variability analysis
Intra-observer and inter-observer variability of PAT were 3.9 and 6%, respectively (Table 3). Intra-observer and inter-observer variability of PAT/ET were 3.3 and 5.6%, respectively.
Table 3.
Intra- and inter-observer variability of pulsed-wave Doppler measurements
| Mean error±SD | Mean error±SD, % | |
|---|---|---|
| Intraobserver variability | ||
| PAT (ms) | 0.4±1.2 | 1.3±3.9 |
| ET (ms) | 0.1±1.1 | 0.3±1.8 |
| PAT/ET | 0.01±0.02 | 1.0±3.3 |
| Pulmonary TVI (cm) | 0.00±0.02 | −0.3±1.5 |
|
| ||
| Interobserver variability | ||
| PAT (ms) | 2.0±1.8 | 6.7±6.0 |
| ET (ms) | 1.2±1.3 | 1.8±2.0 |
| PAT/ET | 0.02±0.03 | 4.9±5.6 |
| Pulmonary TVI (cm) | 0.01±0.06 | 0.5±4.1 |
PAT, pulmonary acceleration time; ET, ejection time, TVI, time velocity integral.
DISCUSSION
This study demonstrates that non-invasive assessment of pulmonary artery systolic pressure is feasible in mice. Both the PAT and the ratio of PAT to the ejection time measured using high frequency echocardiography correlated closely with invasively measured RVSP. Pulmonary acceleration time and PAT/ET were able to detect acute and chronic increases in RVSP with high sensitivity and specificity, as well as to assess the effects of treatment on RVSP.
The pulmonary flow velocity patterns were similar in mice to those reported in humans12 in both high and normal RVSP conditions. Normotensive mice displayed a “dome like” flow velocity pattern. In mice with high RVSP the flow velocity accelerated rapidly to a peak in early systole, resulting in a shortened PAT.13 Of note, the PAT/ET ratio in mice was close to that seen in normal humans as well as in patients with PAH. In humans without PAH, the ratio is 46±3% (43±1% in control mice). In patients with a mean pulmonary artery pressure above 40 mmHg, the PAT/ET ratio is 26±2%; this value is close to the ratio found in mice with high RVSP (28±2% when the RVSP is 46±3 mm Hg).11, 20, 21
In both acute and chronic models of PAH, RVSP measured invasively and pulmonary flow velocity were recorded simultaneously, enabling the comparison of the echocardiographic parameters with the pressure measurement “gold standard”. Echocardiography allowed the non-invasive detection of acute changes in pulmonary artery pressure induced by pharmacological interventions within the same mouse. Both PAT and PAT/ET shortened with increasing doses of U-46619 and increasing RVSP. One characteristic of U-46619 is that it may decrease the cardiac output.22 The decrease in cardiac output was reflected in our acute model of PAH by the decrease in TVI. Pulmonary flow is equal to the product of pulmonary flow TVI and pulmonary artery area. As pulmonary area does not change when PAP is acutely increased,22 the decrease in TVI suggests the occurrence of a decrease in pulmonary flow and therefore of stroke volume. Such a decrease in stroke volume may by itself decrease PAT.13 To eliminate the potential effects of a decrease in stroke volume in our model, we measured RVSP both invasively and non-invasively in mice with established chronic pulmonary hypertension (lung-specific IL-6–overexpressing transgenic mice).4 The chronic mice models of PAH may also be more relevant to the human PAH condition than the acute and major increases in pulmonary pressure obtained with pharmacological interventions. The IL-6 Tg+ mice have similar pulmonary TVI to WT mice, suggesting that mice of both genotypes have similar cardiac output. The correlation of invasively determined RVSP and echocardiographic parameters is close in IL-6 Tg+ and WT mice, demonstrating that a change in cardiac output is not responsible for the decreased PAT and PAT/ET observed in mice with high RVSPs.
In the chronic PAH model, RVSP correlated closely with PAT and PAT/ET. The strength of the correlation obtained in mice between PAT or PAT/ET with RVSP is close to that described in humans. In humans, PAT correlates with HR,23 necessitating a normalization of PAT by ET. In the present studies, the range of HR was between 330 and 520 bpm. No correlation of PAT with HR was noted in that range. The ratio of PAT/ET, however, may be useful to compare mice with different cardiac outputs, as both PAT21 and ET24 decrease with a decrease in cardiac output.
Although the assessment of pulmonary arterial systolic pressure is widely used to monitor PAH, echocardiography may also provide additional information by non-invasively estimating PVR. A novel non-invasive echocardiographic index that correlates closely with invasively measured PVR has been recently described in humans.25 This index is obtained by measuring RVSP using the maximum velocity of tricuspid regurgitation and dividing the RVSP by the cardiac output estimated by the pulmonary TVI. In mice, pulmonary TVI also correlates closely with invasively measured cardiac output,9 potentially allowing the use of the echocardiographic PVR index in mice.
Anesthesia in mice can change the hemodynamic state and cardiac function.26 It is therefore important to use light anesthesia rather than deep anesthesia for cardiac evaluation. Echocardiographic assessment of RVSP obtained under deep anesthesia was reproduced in more physiological conditions using light anesthesia. Specifically, both PAT and PAT/ET measured using light anesthesia correlated closely with the same parameters measured using deep anesthesia, suggesting that echocardiographic parameters may be used to assess pulmonary artery systolic pressure under light anesthesia.
Both intra- and inter-observer variabilities were below 6% for both the PAT and the PAT/ET. These variabilities compare favorably to the variabilities observed using invasive RVSP measurements in humans.27
In conclusion, right ventricular systolic pressure can be estimated non-invasively in mice. Transthoracic echocardiography can monitor acute and chronic changes in RVSP. This non-invasive technique may permit the characterization of the evolution of PAH in genetically-modified mice.
CLINICAL PERSPECTIVE.
Despite the development of new therapeutic strategies such as endothelin-receptor antagonists, phosphodiesterase type-5 inhibitors, and prostanoid, the prognosis of patients with pulmonary arterial hypertension (PAH) remains poor. Advances in understanding the pathophysiological mechanisms that contribute to PAH are critical to the discovery of new therapeutic targets. In this setting, small rodent models, in particular genetically-modified mice, offer the unique opportunity to study the signaling pathways involved in PAH and to evaluate the effectiveness of therapeutic interventions. Indeed, the ability to study pulmonary artery pressure or right ventricular systolic pressure (RVSP) in mice underexpressing or overexpressing a gene will help to elucidate the functional role of this particular gene in the regulation of pulmonary pressure. In mice, right heart catheterization is the only available method to measure RVSP and is a terminal procedure. The absence of a non-invasive technique that would allow serial assessment of RVSP in mice has significantly undermined progress in the field of PAH, preventing the rapid evaluation and development of mouse PAH models. In the present study, pulmonary artery flow measurements obtained using transthoracic echocardiography detect acute and chronic increases in RVSP with high sensitivity and specificity and identify the effect of treatment on RVSP. Transthoracic echocardiography may allow the characterization of the evolution of PAH and the evaluation of therapeutic interventions noninvasively in mice.
ACKNOWLEDGEMENTS
We thank Paul B. Yu, MD for sharing his experience on genetically-modified mice with us.
FUNDING SOURCES: This study was supported by a Shared Equipment Grant (NIH/NHLBI 1S10RR022586-01A1, MSC) as well as by fellowship grants from the Fédération Française de Cardiologie (to HT and BK) and by the Milton Fund (to MSC).
Footnotes
DISCLOSURES: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, Olschewski H, Peacock A, Pietra G, Rubin LJ, Simonneau G, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie M, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, McGregor K, Morais J, Oto A, Smiseth OA, Barbera JA, Gibbs S, Hoeper M, Humbert M, Naeije R, Pepke-Zaba J. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J. 2004;25:2243–2278. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 4.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, Zhang YY. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol. 2008;295:H677–690. doi: 10.1152/ajpheart.91519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41:397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Moliterno DJ, Mukherjee D, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 8.Jones JE, Mendes L, Rudd MA, Russo G, Loscalzo J, Zhang YY. Serial noninvasive assessment of progressive pulmonary hypertension in a rat model. Am J Physiol Heart Circ Physiol. 2002;283:H364–371. doi: 10.1152/ajpheart.00979.2001. [DOI] [PubMed] [Google Scholar]
- 9.Tournoux F, Thibault H, Petersen B, Raher MJ, Halpern E, Picard MH, Scherrer-Crosbie M. Validation of a non-invasive method to measure cardiac output in mice. Europ Heart J. 2008;S1:4623. (abstract) [Google Scholar]
- 10.Weyman AE, Dillon JC, Feigenbaum H, Chang S. Echocardiographic patterns of pulmonic valve motion with pulmonary hypertension. Circulation. 1974;50:905–910. doi: 10.1161/01.cir.50.5.905. [DOI] [PubMed] [Google Scholar]
- 11.Beard JT, 2nd, Newman JH, Loyd JE, Byrd BF., 3rd Doppler estimation of changes in pulmonary artery pressure during hypoxic breathing. J Am Soc Echocardiogr. 1991;4:121–130. doi: 10.1016/s0894-7317(14)80523-3. [DOI] [PubMed] [Google Scholar]
- 12.Dabestani A, Mahan G, Gardin JM, Takenaka K, Burn C, Allfie A, Henry WL. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol. 1987;59:662–668. doi: 10.1016/0002-9149(87)91189-1. [DOI] [PubMed] [Google Scholar]
- 13.Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, Mishima M, Uematsu M, Shimazu T, Hori M, Abe H. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–309. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 14.Isobe M, Yazaki Y, Takaku F, Koizumi K, Hara K, Tsuneyoshi H, Yamaguchi T, Machii K. Prediction of pulmonary arterial pressure in adults by pulsed Doppler echocardiography. Am J Cardiol. 1986;57:316–321. doi: 10.1016/0002-9149(86)90911-2. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Duran R, Larman M, Trugeda A, de Prada JA Vazquez, Ruano J, Torres A, Figueroa A, Pajaron A, Nistal F. Comparison of Doppler-determined elevated pulmonary arterial pressure with pressure measured at cardiac catheterization. Am J Cardiol. 1986;57:859–863. doi: 10.1016/0002-9149(86)90627-2. [DOI] [PubMed] [Google Scholar]
- 16.Deb B, Bradford K, Pearl RG. Additive effects of inhaled nitric oxide and intravenous milrinone in experimental pulmonary hypertension. Crit Care Med. 2000;28:795–799. doi: 10.1097/00003246-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Evgenov OV, Ichinose F, Evgenov NV, Gnoth MJ, Falkowski GE, Chang Y, Bloch KD, Zapol WM. Soluble guanylate cyclase activator reverses acute pulmonary hypertension and augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs. Circulation. 2004;110:2253–2259. doi: 10.1161/01.CIR.0000144469.01521.8A. [DOI] [PubMed] [Google Scholar]
- 18.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol. 2000;22:535–542. doi: 10.1165/ajrcmb.22.5.3808. [DOI] [PubMed] [Google Scholar]
- 19.Shine B. Use of routine clinical laboratory data to define reference intervals. Ann Clin Biochem. 2008;45:467–475. doi: 10.1258/acb.2008.008028. [DOI] [PubMed] [Google Scholar]
- 20.Kosturakis D, Goldberg SJ, Allen HD, Loeber C. Doppler echocardiographic prediction of pulmonary arterial hypertension in congenital heart disease. Am J Cardiol. 1984;53:1110–1115. doi: 10.1016/0002-9149(84)90646-5. [DOI] [PubMed] [Google Scholar]
- 21.Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987;9:549–554. doi: 10.1016/s0735-1097(87)80047-5. [DOI] [PubMed] [Google Scholar]
- 22.Grignola JC, Bia D, Gines F, Armentano RL. Acute pulmonary hypertension: protective role of vascular smooth muscle activation. Rev Esp Cardiol. 2003;56:1077–1084. doi: 10.1016/s0300-8932(03)77018-3. [DOI] [PubMed] [Google Scholar]
- 23.Leighton RF, Weissler AM, Weinstein PB, Wooley CF. Right and left ventricular systolic time intervals. Effects of heart rate, respiration and atrial pacing. Am J Cardiol. 1971;27:66–72. doi: 10.1016/0002-9149(71)90084-1. [DOI] [PubMed] [Google Scholar]
- 24.Kligfield P, Okin P. Effect of ventricular function on left ventricular ejection time in aortic stenosis. Br Heart J. 1979;42:438–441. doi: 10.1136/hrt.42.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddad F, Zamanian R, Beraud AS, Schnittger I, Feinstein J, Peterson T, Yang P, Doyle R, Rosenthal D. A Novel Non-Invasive Method of Estimating Pulmonary Vascular Resistance in Patients With Pulmonary Arterial Hypertension. J Am Soc Echocardiogr. 2009;22:523–529. doi: 10.1016/j.echo.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002;282:H2134–2140. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- 27.Verdejo HE, Castro PF, Concepcion R, Ferrada MA, Alfaro MA, Alcaino ME, Deck CC, Bourge RC. Comparison of a radiofrequency-based wireless pressure sensor to swan-ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J Am Coll Cardiol. 2007;50:2375–2382. doi: 10.1016/j.jacc.2007.06.061. [DOI] [PubMed] [Google Scholar]