Abstract
It is rapidly emerging that the defence system of innate lymphocytes is more diverse than previously recognized. In addition to natural killer (NK) cells, lymphoid tissue inducer (LTi) cells, and natural helper cells have now been identified. LTi cells are developmentally dependent on the orphan transcription factor RORγt and instruct lymph node development during embryogenesis. More recently, it has become evident, that in addition to their role for lymph organ development, LTi cells are also potent producers of cytokines such as interleukin-22 (IL-22) and IL-17 in adult mice. In addition to LTi cells, another RORγt-dependent innate lymphocyte subset co-expressing RORγt and NK cell receptors (NKRs) has been identified. These NKR+ RORγt+ cells are also potent producers of IL-22 but it is unclear whether they are part of the NK cell or LTi cell lineage. This review will highlight recent progress in understanding development and function of innate IL-22-producing lymphocyte subsets.
Keywords: innate lymphoid cells, interleukin-22, lymphoid tissue inducer cells, natural killer cells
Introduction
The mucosal immune system faces a unique challenge. It is continuously exposed to foreign antigens, commensal bacteria and pathogens, from which it is separated only by a single layer of epithelial cells.1,2 Rather than creating an impermeable barrier, the central design principle of this system is to allow controlled interaction between microbes, food-derived antigens and the underlying immune cells. Such controlled interactions are mediated by specialized cells (e.g. M cells, transepithelial dendritic cells)3,4 and they are required to maintain tissue homeostasis. Such interactions calibrate the responsiveness of immune cells so that they do not inappropriately respond to microbes or antigens presented via these ‘secured’ pathways but retain the ability to protect the host from infections if necessary. On a cellular level, the intestinal immune system displays an unusual degree of plasticity allowing it to adapt to these highly divergent tasks.5,6 Under homeostatic conditions, the intestine can be viewed as a state of truce allowing for symbiosis between the intestinal microbiota, epithelial cells and the underlying immune system.7
It is now increasingly appreciated that intestinal epithelial cells produce cytokines and growth factors that shape the differentiation and responsiveness of the underlying immune system.2 The aggregate of these factors has been termed the ‘epimmunome’.8 However, it is less appreciated that immune cells, in addition to producing cytokines that help to fight infections or that prevent inappropriate activation of other immune cells, also produce cytokines that instruct epithelial cell function. We refer to the total of these factors as the ‘immuno-epithelome’. Research on both ‘epimmunomics’ and ‘immunoepithelomics’ is required to better understand immunity and epithelial cell biology at mucosal surfaces. Furthermore, such research will probably unravel the central checkpoints regulating the regeneration of epithelial cells and the pathogenesis of inflammatory bowel diseases such as Crohn's disease and ulcerative colitis.
Recently, various reports have identified an innate lymphocyte population residing in the intestinal lamina propria that phenotypically resembles natural killer (NK) cells and expresses the retinoic acid orphan receptor (ROR) γt.9–13 We will refer to these cells as NK cell receptor-expressing (NKR)+ RORγt+ innate lymphoid cells (ILCs). The NKR+ RORγt+ ILCs are well represented in the small intestine, colon, mesenteric lymph nodes (LNs) and liver, whereas they are absent or constitute only a small subpopulation in secondary lymphoid organs (such as spleen or peripheral LNs).9–12 The NKR+ RORγt+ ILCs are potent and constitutive producers of the cytokine interleukin-22 (IL-22). Interestingly, the IL-22 receptor (IL-22R) is not expressed by haematopoietic cells but is exclusively found on epithelial cells.14–17 It is therefore assumed that IL-22 is one of the factors that immune cells employ to modulate the function of epithelial cells. Constitutive production of IL-22 is instructed by currently undefined cues. However, the commensal microflora seems to play an important role because NKR+ RORγt+ ILCs from germ-free mice produced only low levels of IL-22.9,11 Gene array data from colon explants treated with IL-22 have provided insight into some of the IL-22-regulated genes. Intriguingly, genes involved in the antimicrobial defence of epithelial cells are up-regulated (e.g. Reg3 and S100a family of genes).16
Reg3 genes constitute a small gene family encoded on mouse chromosome 6 and human chromosome 2 with largely unknown functions.18–21Reg3 genes encode C-type lectin-like proteins that are secreted by epithelial cells and one member, RegIIIγ, displays antimicrobial activity against Gram-positive bacteria.19,20 Epithelial expression of Reg3 genes is dependent on the presence of commensal microflora as germ-free mice expressed only very low levels.20,22 It has been postulated that epithelial cell-intrinsic, TLR-dependent sensing of intestinal microbes leads to Reg3 expression.23 It is now widely accepted that Reg3 genes may be important regulators of epithelial barrier function and of the pattern of microbial colonization at epithelial surfaces. For example, Reg3 gene expression is much reduced in mice treated with antibiotics, allowing for colonization with vancomycin-resistant enterococci, which are repelled in the presence of RegIIIγ.22 Although IL-22 is constitutively expressed by NKR+ RORγt+ ILCs,9 IL-22 has also emerged as an important factor to further enhance Reg3 gene expression under inflammatory conditions.16,24 Interleukin-22-dependent Reg3 expression is required for protection against Citrobacter rodentium infection, which induces a severe form of colitis, and is a mouse model of attaching and effacing intestinal infections in humans such as those with Escherichia coli O157:H7.16 Hence, the IL-22/RegIII axis constitutes the first recognized molecular pathway of how immune cells instruct epithelial cell function.
The innate immune system is an evolutionarily ancient arm of the body's defence system. It is composed of phagocytic cells (myeloid cells) and innate lymphocytes. Until recently, NK cells were the only representative of innate lymphocyte lineages. They provide a first line of defence against virus infections and tumours. Unlike adaptive T and B lymphocytes, NK cells do not rearrange their receptor genes somatically, but rather express germ-line encoded receptors, which have inhibitory or activating qualities.25 There have been few studies of intestinal NK cells but from these, it was evident that these cells had a unique phenotype and were poor effector cells [cell-mediated cytotoxicity, interferon-γ (IFN-γ) production].26–28 Within the last couple of years, it has become obvious that in addition to NK cells there are at least two additional innate lymphocyte subsets – lymphoid tissue inducer (LTi) cells (also referred to as RORγt+ ILCs) and natural helper cells (also called nuocytes, type 2 ILCs or fat associated lymphoid clusters) all of which are well represented at mucosal surfaces.29–36 It is a rapidly emerging concept that the transcriptional and effector programme of mucosal innate lymphocytes is reminiscent of the various helper T cell (Th) effector fates (i.e. Th1, Th2, Th17, Th22). Hence, the analysis of innate lymphocytes at mucosal surfaces is of particular relevance because it may shed light on the primordial design principles of the immune system because the intestine was the first site requiring protection. Such functions preceded the emergence of adaptive immunity and formation of secondary lymphoid organs (i.e. spleen, lymph nodes).
This review will highlight the development and possible function of IL-22-producing NKR+ RORγt+ ILCs, in both human and mouse within the framework of the newly emerging innate lymphocyte populations.
Lineage relationships of IL-22-producing NKR+ RORγt+ innate lymphoid cells
Widespread interest surrounds the developmental origin and the lineage relationship of IL-22-producing NKR+ RORγt+ ILCs.37–39 Indeed, expression of the transcription factor RORγt, in combination with NKRs (e.g. NKp46/NCR1, NKG2D, NK1.1) and markers of lymphoid progenitors (e.g. CD127/IL-7Rα, CD117/c-kit), by this previously unappreciated lymphocyte subset was an unconventional feature.9–13 It remains an important and largely unresolved question where to position these cells on haematopoietic lineage maps.
Intestinal NKR+ RORγt+ ILCs are innate lymphocytes
Mice genetically deficient for the recombination activating genes (Rag) Rag1 or Rag2 lack all B and T cells in the periphery.40,41 Their immune defence comprises components of the innate immune system: myeloid cells and innate lymphocytes. Three distinct innate lymphocyte lineages can be discriminated: NK cells, LTi cells and natural helper cells, all of which are present in mice that genetically lack Rag proteins. Additional deletion of the cytokine common γ chain (i.e. Rag2−/− Il2rg−/− mice) cripples the development of innate lymphocytes and the immune system of such mice is composed of myeloid cells only.42–44 Intestinal NKR+ RORγt+ ILCs are present in Rag-deficient mice but not in Rag2−/− Il2rg−/− mice.9,11 Hence, NKR+ RORγt+ ILCs are part of the innate lymphocyte lineages.
It is an emerging picture that the development of all innate lymphocyte lineages depends on the helix-loop-helix protein inhibitor of DNA binding 2, Id2 (Fig. 1).34,45–47 Id2 dimerizes with E proteins and prevents their DNA binding.48 Mice that are Id2−/− lack all known innate lymphocyte populations (NK cells, LTi cells and natural helper cells), suggesting that these three innate lymphocyte populations share a common developmental programme and may be derived from an Id2-dependent ‘common innate lymphoid progenitor’ (Fig. 1). A common developmental programme of NK cells and LTi cells was recently further supported by the analysis of mice deficient for TOX (thymocyte selection-associated high mobility group box protein). Similar to Id2−/− mice, Tox−/− mice also lack mature NK cells and LTi cells.49 However, the dependence of LTi cells on TOX seemed to be less absolute compared with Id2 because Tox−/− mice still developed some Peyer's patches and had intestinal LTi-like cells albeit in substantially reduced numbers.49 Whereas the developmental defect of lymphocyte lineages in mice lacking Id2 is limited to innate lymphocytes, Tox−/− mice also lack CD4 T cells.50 Consistent, with their grouping within the innate lymphocyte lineages, NKR+ RORγt+ ILCs are absent in Id2-deficient mice and are substantially reduced in Tox−/− mice.46,49 Collectively, these data support the view that NKR+ RORγt+ ILCs are part of innate lymphocyte lineages. It remains to be established whether they differentiate as part of one of the three recognized innate lymphocyte lineages or define a ‘separate’ lineage (Fig. 1).
Figure 1.
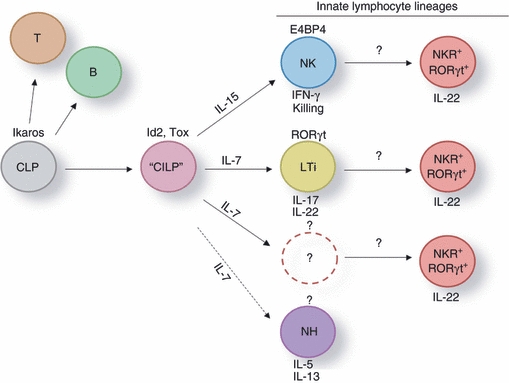
Models for the differentiation of interleukin-22 (IL-22)-producing natural killer cell receptor-positive (NKR+) retinoic acid orphan receptor (ROR) γt+ innate lymphoid cells (ILCs). All lymphocyte lineages develop from a committed common lymphoid progenitor (CLP).117,118 All of the recognized innate lymphocyte lineages [natural killer (NK) cells, lymphoid tissue inducer (LTi) cells, natural helper (NH) cells] are developmentally dependent on the transcription factor Id2.34,45,46 These data suggest that the innate lymphocyte lineages may share an Id2-dependent common innate lymphoid progenitor (CILP). Further commitment to the distinct innate lymphocyte lineages requires regulated expression of lineage-specific transcription factors (E4BP4 for the NK cell lineage, RORγt for the LTi cell lineage).31–33,74,75 A specific transcription factor determining the cell fate of NH cells is currently unknown, making it unclear whether NH cells represent a progenitor cell subset or a distinct innate lymphocyte lineage. Specific cytokines are required to further regulate differentiation and survival of the innate lymphocyte subsets. Interleukin-15 is required for NK cell lineage differentiation whereas IL-7 is required for LTi cells and NH cells.34,46,72,73,82–84 Three models for the development of NKR+ RORγt+ ILCs have been proposed. NKR+ RORγt+ ILCs are either NK cells up-regulating RORγt or LTi-like cells that up-regulate NK cell receptors. NKR+ RORγt+ cells may also represent a distinct innate lymphocyte lineage derived from a progenitor distinct from the NK or LTi cell lineage.
NKR+ RORγt+ ILCs require RORγt for their development or differentiation
Lineage specification of haematopoietic cells is defined by the temporally spaced expression of lineage-defining transcription factors and the requirement of cytokines for their differentiation and survival. Importantly, studies in mice lacking RORγt show that the population of intestinal NKR+ RORγt+ ILCs is lacking, whereas NKR+ RORγt− cells (i.e. conventional NK cells) develop normally and are even over-represented.9–11 Hence, NKR+ RORγt+ ILCs require RORγt for their development/differentiation or for assuming a specific effector fate.
RORγt plays a dual role in the immune system. It is the lineage-defining transcription factor of LTi cells (Fig. 1). Mice lacking RORγt do not develop LNs and Peyer's patches.31–33 In addition to its function as a master-regulator for the development of LTi cells, RORγt also plays an important role during T helper cell fate decisions.51 Expression of RORγt is induced when T-cell activation occurs in a certain cytokine context (IL-6 and transforming growth factor-β)51–53 and it then determines a specific functional fate for effector Th cells, driving their differentiation along the Th17 pathway. Th17 cells are characterized by the production of IL-17 family cytokines and the cytokine IL-22. They play an important disease-promoting role in autoimmune disorders such as experimental autoimmune encephalitis, a mouse model of multiple sclerosis.53 CD4 T cells from mice lacking RORγt and the related transcription factor RORα cannot be differentiated into Th17 cells, demonstrating that the differentiation of naïve T cells into Th17 cells depends on these transcription factors.51,54 This is further supported by studies with ectopic expression of RORγt in naïve T cells, which leads to the induction of the full Th17 programme.51
Given the dual function of RORγt in the immune system as (i) a lineage-defining transcription factor of LTi cells and (ii) an inducible cell-fate-determining factor in CD4 T cells, three distinct proposals for the lineage relationship of NKR+ RORγt+ ILCs are under discussion.37–39 In the following, we will review the available evidence for each of these models.
First proposal: the ‘NK lineage model’
The first proposal is that NKR+ RORγt+ ILCs are NK cells that up-regulate RORγt expression to assume a specific effector cell fate. It should be noted that this view is not invalidated by the fact that NKR+ RORγt+ ILCs are lacking in Rorc(γt)−/− mice. Similar to Th17 cells, NKR+ RORγt+ ILCs may require RORγt expression to become IL-22-producing lymphocytes. This view is supported by several lines of evidence and is largely based on studies of human NK cell subsets.
Cell surface receptors are often used to define immune cells. The activating NK cell receptor NKp46 is considered to be an almost NK cell-specific receptor.55 This is based on the finding that NKp46, in contrast to other NKRs (e.g. NK1.1, NKG2D), is not expressed by T cells, NKT cells or most γδ T cells.55–58 It is argued that the finding of a human lymphocyte subset expressing NKp46 and RORγt supports the view that these cells constitute a subpopulation of NK cells.
Human NK cells are generally defined as CD56+ CD3− lymphocytes, all of which express NKp46. Two subpopulations can be distinguished on the basis of their surface levels of CD56 and the expression of CD16 (Fcγ receptor type 3). In peripheral blood, the majority of NK cells, characterized by CD56dim CD16+ marker expression, are potent cytotoxic cells.59 The smaller CD56bright CD16− subset is not well equipped for cell-mediated cytotoxicity but is a potent source of IFN-γ.60,61 It is still not entirely clear whether there is a linear developmental relationship between these two subsets. Based on data that CD56bright NK cells express cell surface markers also found on lymphoid progenitors (e.g. CD127/IL-7Rα, CD117/c-kit), it is assumed that they are the progenitors of CD56dim cells.62,63 However, cellular activation increases CD56 expression, and in some organs such as within the tonsils, almost all NK cells express high levels of CD56.64 NKp44 is an orphan immune receptor that is not expressed by resting peripheral blood NK cells but is up-regulated upon activation (e.g. culture in IL-2 or IL-15).65,66 A murine homologue has not been identified and may be lacking.67 Interestingly, a population of NKR+ cells constitutively expressing NKp44 has been found in human tonsils and in other mucosa-associated lymphoid tissues whereas they are not well represented in human LNs.12,64 This may reflect the fact that surgically removed tonsils represent chronically inflamed tissues. Alternatively, differential expression could indicate the presence of a particular mucosa-associated innate lymphocyte subset. A fraction of NKp44+ cells in tonsils expresses CCR6, and NKp44+ CCR6+ cells are potent producers of IL-22 but they do not express perforin or granzyme B and do not produce IFN-γ.12 In addition, NKp44+ CCR6+ cells express mRNA encoding for RORγt, the aryl hydrocarbon receptor and interferon regulatory factor 4 amounting to a transcriptional and effector programme reminiscent of Th17 cells.12,13 It has been proposed that these IL-22-producing cells are NK cells that up-regulate RORγt under the influence of tissue-specific cues to assume a particular NK cell effector fate – NK-22 cells.12
Another line of evidence supporting the view that IL-22-producing CD56+ cells are an NK cell subset comes from studies that have identified a differentiation programme of NK cells from lymphoid progenitors residing within secondary lymphoid organs. These studies indicate that an NK cell-committed progenitor is present in secondary lymphoid organs and differentiates in a four-stage programme into mature NK cells.68,69 Interestingly, IL-22-producing cells are entirely contained within the population of ‘stage 3’ immature NK cells (i.e. CD34− CD117/c-kit+ CD94−) corroborating the view that IL-22-producing NKR+ cells are an immature NK cell subset.70 In contrast to mature NK cells, ‘stage 3’ NK cells expressed the IL-1R and culture in IL-1β and IL-15 led to their expansion. In vitro culture of ‘stage 3’ NK cells in the absence of IL-1 led to rapid differentiation into ‘stage 4’ mature NK cells that had lost the ability to produce IL-22 and instead expressed IFN-γ and displayed cell-mediated cytotoxicity.71
Although there is some overlap of these two distinct views concerning the differentiation of IL-22-producing NK cells, there are also dividing issues. While the data on ‘NK-22 cells’ implies that naïve NK cells up-regulate RORγt expression under tissue-specific cues to assume a specific functional fate, the data concerning ‘stage 3’ NK cells would place IL-22-producing cells within a tonsil-resident NK cell precursor population.
Conversely, there are also inconsistent data with the ‘NK lineage model’ of NKR+ RORγt+ ILCs. NK cells require IL-15 for their development and survival. As a result, both Il15−/− mice and Il15ra−/− mice lack mature NK cells.72,73 However, LTi cells and NKR+ RORγt+ ILCs are normally represented in the intestinal lamina propria of Il15−/− mice, demonstrating that NKR+ RORγt+ ILCs develop independently of IL-15.9,46 Recently, an NK cell lineage-specific transcription factor (E4BP4 or NFIL3) has been identified.74,75 E4BP4 is not required for the development of LTi cells as E4bp4−/− mice have normal development of lymph nodes (Hugh Brady, personal communication). Interestingly, intestinal NKR+ RORγt+ ILCs develop in mice genetically lacking E4BP4 demonstrating that NK cells and NKR+ RORγt+ ILCs may not share a common developmental program (Hugh Brady, personal communication).
Second proposal: the LTi lineage model (‘NKR-LTi cells’)
The second proposal is that NKR+ RORγt+ ILCs are LTi cells that up-regulate NK cell receptors. RORγt is the lineage-defining transcription factor of the LTi cell lineage and all LTi cells express RORγt.31–33Rorc(γt)−/− mice cannot differentiate LTi cells from precursors and, consequently, fail to develop peripheral lymph nodes, Peyer's patches and intestinal lymphoid follicles (e.g. cryptopatches and isolated lymphoid follicles). The LTi cells employ members of the tumour necrosis factor superfamily, in particular surface (s) lymphotoxin (sLTα1β2) for proper lymphoid organogenesis during fetal development.29,76 As an example, LTα-deficient mice have a normal LTi cell compartment but lack LNs and Peyer's patches, demonstrating the vital role of sLTα1β2 expressed by LTi cells for lymph organ development.77 The LTα1β2-expressing LTi cells interact with LTβR-expressing stromal cells and induce the expression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). This interaction subsequently triggers a complex and only partially understood cascade of events involving the production of chemokines, further up-regulation of adhesion molecules and attraction of lymphocytes to the site of the LN anlagen.13,30,78
Lymphoid tissue inducer cells were first characterized in mouse fetal lymphoid tissues as lineage marker-negative (Lin−) CD4+ CD127/IL-7Rα+ CD117/c-kit+ cells.30 The initial research already noted a potential relationship between LTi cells and NKR+ cells. Clonal culture of LTi cells isolated from mesenteric LN of newborn mice in IL-2 gave rise to NK1.1+ cells that displayed cytotoxic activity against tumour cell targets.30 However, the developmental potential of LTi cells from newborn mice was not restricted to the ‘NK cell lineage’ as culture with granulocyte–macrophage colony-stimulating factor and IL-4 resulted in the generation of antigen-presenting cells. However, genetic lineage tracing revealed that myeloid cells are negative in a RORγt-fate map demonstrating that LTi cells may not differentiate into antigen-presenting cells in vivo.79,80
In humans, the equivalent of mouse embryonic LTi cells has only recently been identified.13 A population of Lin− RORC+ CD127/IL-7Rα+ cells with LTi cell function has been isolated from first-trimester and second-trimester developing human mesenteric LN.13 Interestingly, culture of human LTi cells in the presence of IL-7, IL-15 and stem cell factor results in the up-regulation of NK cell receptors (i.e. CD56).13 Similar data have been obtained with LTi-like cells from the tonsils of adult individuals. Importantly, human LTi cells produce IL-17 and IL-22 but do not display any classical NK cell functions (cytotoxicity, IFN-γ). In contrast, CD56+ LTi-derived cells have lost the potential to produce IL-17 but still produce IL-22.13 Consistent with the data from the human system, a population of Lin− CD4+ LTi-like cells has been identified in the spleens of adult mice that also constitutes an innate source of IL-17 and IL-22.81 Collectively, these data indicate that LTi cells are potent innate producers of IL-22 and that NKR+ RORγt+ ILCs may be derived from the LTi cell lineage.
Whereas IL-15 is an important factor for NK cell development and survival, IL-7 is important for LTi cell commitment.82–84 In further support of the LTi lineage model, mice lacking IL-7 also lack NKR+ RORγt+ ILCs whereas NK cell development was largely normal.46,80 Deficiencies of IL-7R in humans leads to severe combined immunodeficiency disease (SCID) in which T-cell development is perturbed but NK cell and B-cell compartments are largely normal (T−B+NK+ SCID).85,86 Although patients with IL7R mutations have a largely normal NK cell compartment (CD56dim and CD56high NK cells), they specifically lack IL-22-producing ‘stage 3’ NK cells (‘NK-22 cells’).80 These data demonstrate that both LTi cells and ‘NK-22 cells’ require IL-7 for their development, whereas conventional NK cells can develop in the absence of IL-7.
Recently, the two prevailing models for the differentiation of NKR+ RORγt+ cells have been experimentally probed (Fig. 1).80 If NKR+ RORγt+ ILCs are derived from cNK cells, transfer of cNK cells into alymphoid mice should give rise to NKR+ RORγt+ cells. However, after transfer, cNK cells do not acquire RORγt expression and cNK cells do not turn on RORγt expression when cultured in vitro in the presence of various cytokines, including those known to induce RORγt expression in Th17 cells (i.e. IL-6 and transforming growth factor-β).80 Collectively, these data demonstrate that cNK cells are not the progenitors to NKR+ RORγt+ ILCs.
The LTi lineage model predicted that NKR+ RORγt+ ILCs are derived from LTi cell precursors. This model was recently tested in studies combining transfer of genetically tagged LTi cells into lymphopenic mice with genetic lineage tracing and in vitro differentiation assays.80 One important issue is the ‘definition’ of LTi cells. All studies to date have used cell surface markers to define the LTi cell population. This may not be appropriate because it has been shown that natural helper cells share most of these markers (CD127/IL-7Rα, CD117/c-kit).34 As natural helper cells do not express RORγt and are negative in a RORγt-fate map (A.D., unpublished data), defining LTi cells as innate RORγt-expressing lymphocytes may be more selective.34 Transfer of LTi-like cells (i.e. NKR− RORγt+ cells) into alymphoid mice demonstrated that this population contains precursors of NKR+ RORγt+ ILCs.80,87 In addition, in vitro differentiation experiments showed that NKR+ RORγt+ ILCs are derived from NKR− RORγt+ LTi-like cells.80 Collectively these data demonstrate that IL-22-producing NKR+ RORγt+ ILCs differentiate from LTi-like cells (i.e. NKR− RORγt+ ILCs) but not from cNK cells.80,87 We will refer to these cells as NKR-expressing LTi-like cells (NKR-LTi cells).
Third proposal: the NKR+ RORγt+ lineage model
Three subsets of LTi-like cells (i.e. NKR− RORγt+ ILCs) can be discriminated on the basis of CD4, CD127 (IL-7Rα) and CD117 (c-kit) expression: CD4+ CD127high CD117high, CD4− CD127high CD117high and CD4− CD127low CD117low cells (Fig. 2).87 In this study it was found that CD4+ CD127high CD117high and CD4− CD127high CD117high LTi cells up-regulate NKRs very inefficiently in vitro, whereas CD4− CD127low CD117low cells readily acquire NKR expression (Fig. 2). In addition, depletion of CD4+ cells by injection of depleting antibodies only mildly affects the numbers of NKR+ RORγt+ ILCs. On the basis of these experiments, it was then concluded that CD4− CD127low CD117low cells may constitute a committed progenitor to NKR+ RORγt+ ILCs constituting an innate lymphocyte lineage distinct from LTi cells.87 In such a model of NKR+ RORγt+ ILC differentiation its precursor does not express CD4 and NKR+ RORγt+ ILCs are also CD4– (Fig. 2). However, clonal differentiation assays of CD4+ LTi cells from newborn mice led to the efficient generation of NK1.1+ LTi-derived cells.30 In addition, a population of CD4+ NKR+ RORγt+ ILCs is clearly present in virtually all lymphoid organs and in the intestinal lamina propria.80 Hence, it will be an important avenue of future research to determine whether these subsets of RORγt+ ILCs constitute distinct innate lymphocyte lineages (Fig. 2, Model 2) or rather reflect distinct differentiation states or effector fates within the same lineage modulated by environmental factors (Fig. 2, Model 1).
Figure 2.
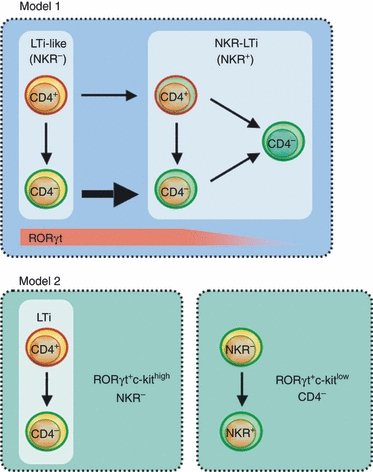
Lineage relationships between lymphoid tissue inducer (LTi) cells and natural killer cell receptor-positive (NKR+) retinoic acid orphan receptor (ROR) γt+ innate lymphoid cells (ILCs). Two different models for the development of NKR+ RORγt+ ILCs have been proposed.80,87 Model 1: LTi-like cells constitute an innate lymphocyte lineage defined by the expression of the orphan transcription factor RORγt. Genetic fate mapping for CD4 expression revealed that most NKR− LTi-like cells are derived from a CD4+ progenitor.80 Both CD4+ and CD4− LTi-like cells up-regulate NKRs differentiating into CD4+ or CD4− RORγt+ NKR-LTi cells. Dependent on the organ microenvironment NKR-LTi cells lose RORγt expression differentiating into RORγt− NKR-LTi cells that are functionally distinct from RORγt+ NKR-LTi cells.80 Model 2: The population of NKR− RORγt+ ILCs contains two distinct innate lymphocyte lineages that can be discriminated by their levels of c-kit expression. The LTi cell lineage (CD4+ or CD4−) is characterized by a c-kithigh phenotype whereas the CD4–, committed precursor to CD4− NKR+ RORγt+ ILCs is c-kitlow.87
Regulated expression of RORγt generates plasticity in the NKR-LTi population
Another important question is whether NKR-LTi cells constitute a stable cell fate (Box A). Data from CD56+ LTi cell-derived clones cultured in the presence of IL-7 indicate that the cells maintain RORγt expression.88 On the other hand, depending on the cytokine environment, NKR+ RORγt+ ILCs can switch from the production of IL-22 to the expression of IFN-γ, leukemia inhibitory factor and even IL-5 and/or IL-13.12,88–90 Using a combination of cell transfer experiments employing genetically tagged LTi-like cells with the analysis of RORγt expression in the context of a RORγt-fate map, we have recently found that LTi-like cells stably up-regulate NKRs in vivo and then progressively lose RORγt expression.80 The extent of RORγt loss depends on the organ microenvironment. Whereas NKR-LTi cells remained RORγt-positive in the lamina propria of the small intestine, the majority of NKR-LTi cells lost RORγt expression in the colon or in secondary lymphoid organs (e.g. spleen).80 Interestingly, the gradient of RORγt expression assigns distinct functional profiles to NKR-LTi cells. While RORγt+ NKR-LTi cells are a potent innate source of IL-22, RORγt− NKR-LTi cells no longer express IL-22 but instead produce IFN-γ.80
Box A: Open questions.
What is the lineage relationship between the different subsets of RORγt+ innate lymphoid cells (ILCs)?
Do the subsets of RORγt+ ILCs represent distinct differentiation states within the lineage of lymphoid tissue inducer (LTi) -like cells or do they represent distinct innate lymphocyte lineages?
Which factors stabilize or de-stabilize RORγt expression by natural killer cell receptor (NKR) -LTi cells?
What are the molecular identities of the microbiota-dependent cues promoting function of LTi-like cells and NKR-LTi cells?
Are specific microbiota required to induce the functional programme of RORγt+ ILCs?
How can LTi-like cells and NKR-LTi cells discriminate between ‘self’ and ‘non-self’?
What is the role of NKRs for the activation of NKR-LTi cells?
Which facets of epithelial cell function and renewal are regulated by RORγt+ ILCs?
The organ-specific cues that stabilize RORγt expression (in the small intestine) or accelerate RORγt loss (colon, spleen) are largely unknown. Our data demonstrate that IL-7 is one factor stabilizing RORγt expression.80 This is also supported by in vitro culture data of human LTi-like cells that stably maintained RORγt expression when cultured in the presence of IL-7.88 In contrast, IL-2, IL-12 and IL-15 promoted RORγt loss, which is in agreement with previous data showing that culture of human ‘NK-22 cells’ in IL-2 led to a switch in cytokine production from IL-22 to IFN-γ.89 In addition, clonal culture of mouse LTi cells in IL-2 generated NKR-expressing cytotoxic cells producing IFN-γ.30 Indeed, the production of IFN-γ by such NKR-LTi cells requires down-modulation of RORγt expression.80 Additional work including a complete assessment of the differentiation potential and functional profile of NKR-LTi cells in vivo is required (Box A).
Commensal microflora promotes the differentiation of NKR-LTi cells
Homeostasis of intestinal NKR-LTi cells requires signals from the commensal microflora.9,11 Studies in germ-free mice show a reduction in the relative and absolute numbers of RORγt+ NKR-LTi cells, suggesting a conditioning by the commensal microflora.9,11 In contrast, the numbers of NK cells were increased in germ-free mice whereas the number of LTi cells remained virtually unchanged. These data indicate that either the differentiation of RORγt+ NKR-LTi cells from their potential precursors is diminished or the factors stabilizing the cell fate of RORγt+ NKR-LTi cells are limited in the absence of intestinal microbiota. Microflora-dependent signals from other immune cells or from epithelial cells are potential candidates for instructing the differentiation of NKR-LTi cells (Box A). Interestingly, IL-7 production in the small intestine is partially dependent on the commensal microflora.80 These data demonstrate that the commensal microbiota plays an important role in stabilizing RORγt expression in NKR-LTi cells but it does not affect the differentiation of NKR-LTi cells from LTi-like progenitors.80
Function of innate IL-22-producing lymphocytes
Interleukin-22 is a member of the IL-10-related cytokine family.91,92 It signals through a heterodimeric receptor that consists of the IL-10Rβ and the IL-22R chain.15 Interleukin-22 signalling is mediated by janus kinase (Jak) 1 and signal transducer and activator of transcription (STAT) 1, 3 and 5.15 Interestingly, IL-22R expression is restricted to cells of the non-haematopoietic lineage.14 Hence, IL-22 is a cytokine produced by immune cells to modulate the function of epithelial cells and constitutes an important factor of the ‘immunoepithelome’.
Cellular sources of IL-22
Although IL-22 is expressed by various cell types of the adaptive immune system (Th17 cells, γδ T-cell subpopulations),93,94 here we will focus on innate sources of IL-22. In the innate immune system, two lymphocyte subsets are an important source of IL-22: LTi cells and RORγt+ NKR-LTi cells. It is emerging that the function of LTi cells is not limited to lymphoid organogenesis in the developing fetus; LTi cells are present in adult lymphoid organs (mouse and human)31,83,95 and within the intestinal lamina propria, where they are potent and constitutive producers of IL-22 and IL-17, suggesting a much broader role than previously appreciated.13,81,96 In addition to LTi cells, early reports indicated that human NKR+ cells can also produce IL-22 following in vitro stimulation with IL-12 and IL-18.14 As alluded to above, it is now increasingly obvious that human and mouse RORγt+ NKR-LTi cells, but not conventional NK cells, are an innate source of IL-22.9,10,12,13,97 In contrast to LTi cells, RORγt+ NKR-LTi cells did not appreciably express IL-17.9,13
Various reports have proposed that CD11c+ dendritic cells (DCs) can produce IL-22.16,98 This is based on data from experimental colitis models during which IL-22 producers were identified in situ by co-staining for IL-22 and various cell surface markers. As IL-22-producing cells were CD11c+, it was concluded that these cells may represent DCs. However, CD11c is not a DC-specific marker and is also expressed by other cell populations including NK cells, LTi cells and NKR-LTi cells. Additional data demonstrate that IL-22 expression is absent in mice lacking all lymphocytes (Rag2−/− Il2rg−/−).11,99 Further analyses are required to demonstrate that DCs may represent another innate source of IL-22.
Function of IL-22-producing innate lymphocytes during homeostasis
Constitutive IL-22 production by LTi cells and RORγt+ NKR-LTi cells is dependent on the presence of the commensal microbiota.9,11 Similar to findings in germ-free mice, blockade of IL-22 during steady-state or genetic deletion of all LTi and NKR-LTi cells leads to diminished expression of the antimicrobial proteins RegIIIβ and RegIIIγ by epithelial cells.9 It should be noted that epithelial Reg3 expression in Rag-deficient mice is entirely comparable to that in mice with components of the adaptive immune system, whereas expression is extinguished in alymphoid mice (Rag2−/− Il2rg−/−) (A.D. and A.M., unpublished observations).11 These data demonstrate that innate lymphocytes are required for instructing epithelial expression of tissue-protective and antimicrobial proteins during steady-state (‘immunoepithelomics’). It also raises important questions concerning the regulation of Reg3 expression in epithelial cells during homeostasis. The available data are in line with two non-exclusive models. The ‘IL-22 model’ predicts that intestinal microbiota-dependent molecular cues instruct the expression of IL-22 by innate lymphocytes and IL-22 induces Reg3 gene expression by intestinal epithelial cells (Fig. 3). This view is contrasted by an ‘epithelial cell-autonomous model’ in which MyD88-dependent sensing of intestinal microbiota by epithelial cells directly instructs Reg3 expression (Fig. 3).20,23 Future studies are required to determine the extent to which each of these processes contributes to Reg3 expression and epithelial barrier function. It is highly likely that IL-22 has a broader role in epithelial homeostasis and may, in addition to instructing the expression of Reg3 genes, regulate other aspects of epithelial function.
Figure 3.
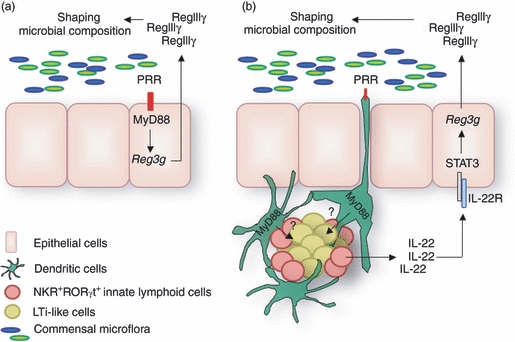
Models for interleukin-22 (IL-22) -instructed epithelial homeostasis. Lymphoid tissue inducer (LTi) -like cells and natural killer cell receptor (NKR) -LTi cells are resident within cryptopatches of the small intestine and constitutively produce IL-22 if commensal bacteria are present.9,10 The IL-22 receptor (IL-22R) is exclusively expressed by epithelial cells and IL-22 regulates the expression of Reg3 genes in intestinal epithelial cells.9,14,16 RegIII proteins have antimicrobial function and may be important regulators of the composition of the colonizing intestinal microbiota.19,20,22,23 Two non-exclusive models for the regulation of Reg3 gene expression have been proposed. The ‘epithelial cell-autonomous model’ of Reg3 gene expression (a)23 predicts that epithelial cells themselves ‘sense’ the presence of bacteria in a MyD88-dependent manner resulting in the up-regulation of Reg3 gene expression. The ‘IL-22 model’ of Reg3 gene expression (b)9 emphasizes the role of IL-22-producing innate lymphocytes in instructing Reg3 expression by epithelial cells. In this model, the microflora regulates through an unknown pathway the functionality of LTi-like cells and retinoic acid orphan receptor (ROR) γt+ NKR-LTi cells. PRR, pattern-recognition receptors.
Function of IL-22-producing innate lymphocytes during intestinal inflammation
Based on the above and the finding that IL-22 induces the expression of factors that enhance epithelial barrier function, a protective role of IL-22 during intestinal inflammation has been proposed. For the first weeks following infection, Rag-deficient mice can contain C. rodentium in an IL-22-dependent manner.16 Infection with C. rodentium leads to an accumulation of IL-22-producing NKR+ cells.11,12 In Il22−/− mice, loss of epithelial Reg3 expression increases susceptibility to C. rodentium infection and exogenous application of recombinant RegIIIγ improves survival of Il22−/− mice after C. rodentium infection.16 Expression of IL-22 is absent in alymphoid mice (Rag2−/− Il2rg−/−) and these mice quickly succumb to C. rodentium infection, which provides additional evidence that innate lymphocytes but not myeloid cells (such as DCs) are an important source of IL-22 in this model.11 The IL-22-producing NKR-LTi cells may play an important role in the intestinal innate immune defence against C. rodentium infection as depletion of NK1.1+ cells exacerbated disease.12
A recent report has re-evaluated the role of LTi-like cells and NKR-LTi cells for protection against C. rodentium infection.100 The authors found that innate lymphocytes, but not myeloid cells, are the major source of IL-22 during the first 6 days following infection, and among innate lymphocytes, CD4+ LTi-like cells are the most prominent source of the cytokine. While depletion of CD4+ LTi-like cells diminishes survival, transfer of CD4+ LTi cells but not of CD4 T cells protects Il22−/− mice against C. rodentium infection.100 Hence, CD4+ LTi-like cells are the major source of IL-22 that provides innate protection against C. rodentium infection.
A protective role of IL-22-producing NKR-LTi cells in inflammatory bowel diseases has also been illustrated in an adoptive transfer colitis model employing transfer of CD45RBhi CD25− CD4 T cells into Rag1−/− recipient hosts.99 Colitis is significantly exacerbated if CD4 T cells are transferred into Il22−/− Rag1−/− mice.99 Innate NK1.1+ CD4− cells that infiltrate the inflamed colons have been identified as IL-22 producers.99 Consistent with this, transfer of colitogenic T cells into mice lacking all IL-22-producing innate lymphocytes (Rag1−/− Il2rg−/− mice) dramatically exacerbates disease.99
Before the identification of IL-22-producing NKR-LTi cells or LTi cells, a protective role of IL-22 in acute dextran sulphate sodium (DSS)-induced colitis was observed.101 Interleukin-22 was found to regulate mucus-associated proteins and was required for the regeneration of mucus-producing goblet cells after DSS-mediated epithelial damage.101 Anti-NK1.1 treatment in DSS-treated mice results in reduced IL-22 expression in the colon, suggesting an important protective role for IL-22-producing NKR+ lymphocytes.99 Considering that IL-22 is produced at steady-state, it is conceivable that some of the effects observed in its absence reflect a pre-existing reduction of epithelial barrier function.9
Conclusions and perspectives
The identification of an IL-22-producing mucosal innate lymphoid subset co-expressing NKRs and RORγt in both mice and humans has opened a new window in the field of mucosal immunology. Two innate IL-22-producing lymphocyte susbsets have now been identified, LTi-like cells (i.e. NKR− RORγt+ ILCs)13,81,100 and RORγt+ NKR-LTi cells (also referred to as NK-22, NCR-22 or NKR+ RORγt+ ILCs).9–12,46,70,80 Recent data from genetic lineage tracing experiments have provided compelling evidence that IL-22-producing NKR+ RORγt+ ILCs are derived from NKR− RORγt+ cells.80,87 Hence, IL-22-producing ILCs are of an innate lymphocyte lineage distinct from conventional NK cells. However, because of the recently discovered phenotypic variety within the population of NKR− RORγt+ ILCs,87 it is less clear whether these various subsets represent differentiation stages or effector fates of one distinct innate lymphocyte lineage (LTi-like cells), or whether LTi-like cells and NKR+ RORγt+ ILCs, despite their shared transcriptional and developmental programme, have different precursors and may constitute separate innate lymphocyte lineages (Fig. 2 and Box A).
It has now become clear that there is far more diversity in innate lymphocyte lineages than previously appreciated. In addition to conventional NK cells, natural helper cells and RORγt+ ILCs (including LTi-like cells and NKR-LTi cells) have been identified. Their functional and transcriptional programmes strikingly resemble that of the different T helper cell effector fates (Th1, Th2, Th17 and Th22).102,103 These findings indicate that such effector profiles were already formed within the innate immune system long before adaptive immunity emerged. Although the various ILC populations obviously resemble discrete T-cell effector fates, there are also important differences that probably reflect the different design principles of innate and adaptive immunity. T lymphocytes are naïve cells and do not display effector functions before recognizing their cognate antigens. Only then, a small pool of antigen-specific T cells expands and dependent on cytokine and chemokine cues provided by innate cells, T cells assume discrete effector fates directed by specific transcriptional programmes. Hence, the vast majority of the cells within the T-lymphocyte lineage are naïve and assume distinct effector fates only when needed. In contrast, and in keeping with their innate qualities, the various innate lymphocyte lineages are already polarized towards a specific functional profile in the absence of an infection. A certain degree of plasticity and adaptation is still possible by fine-tuning the effector programme of ILCs through regulated expression of transcription factors.80,104 The various ILC populations therefore represent distinct lymphocyte lineages, each with a pre-formed and fully developed effector programme anticipating the various microbial challenges.
The intestinal microbiota has an important impact on the function and differentiation of NKR-LTi cells.9,11 While LTi cells and NK cells develop largely normally in germ-free mice, RORγt+ NKR-LTi cells are reduced in absolute and relative numbers.9,11,105 We have recently shown that the commensal microbiota controls the differentiation of NKR-LTi cells by stabilizing RORγt expression, thereby slowing their differentiation to RORγt− NKR-LTi cells.80 The organ-specific molecular cues that stabilize or de-stabilize RORγt expression are an important unresolved question (Box A). The intestinal microflora is also required for IL-22 production by intestinal LTi-like and RORγt+ NKR-LTi cells.9,11 Future research will need to clarify the question of the molecular signals induced by the microbiota instructing functionality of RORγt+ ILCs (Box A). An important role may be assigned to the DC populations surrounding lamina propria cryptopatches as the conveyors of such signals.79,106,107 Recently, specific microbiota have been identified (i.e. segmented filamentous bacteria) that are required to induce a Th17 effector programme in the intestine.108,109 Segmented filamentous bacteria are not involved in inducing functionality of LTi-like cells and NKR-LTi cells.87,108 As a result, other classes of specific microbiota may be required for the induction of IL-22 production (Box A).
How LTi-like cells and NKR-LTi cells discriminate between ‘self’ and ‘non-self’ is important (Box A). Certainly, cytokines such as IL-23 and IL-1 are shown to stimulate IL-22 expression by LTi-like cells and NKR-LTi cells9–12,71 but no data are available assigning function to the NKRs expressed by NKR-LTi cells. It is an intriguing scenario that NKRs, such as NKG2D, that recognize stress-inducible ligands, tune the function of NKR-LTi cells.110–112 The NKp46 receptor plays a redundant role in mediating innate defence against C. rodentium infection.97 Another line of evidence supports the view that ligands of pathogen (pattern)-recognition receptors (PRR) may activate LTi-like cells and NKR-LTi cells.12,81,90,96 It is controversial whether this reflects a cell-intrinsic action of such PRRs90 or is the indirect result of cytokine production by PRR-activated myeloid cells.12
Available data suggest that innate IL-22-producing cells have a largely protective function probably through their instruction of epithelial cells to express antimicrobial and tissue-protective genes. However, evidence for a disease-promoting role of LTi-like cells in Helicobacter hepaticus-induced colitis has recently been provided.113 Interestingly, IL-17 and not IL-22 is the disease-promoting factor and it may be that the balance between IL-17 and IL-22 production is decisive for a disease-promoting or protective role of these cells.113–115 Further analyses are required to understand how the balance between IL-22 and IL-17 production by LTi-like cells is regulated.
It has been difficult to devise experimental approaches that allow for selective interference with either LTi cells or NKR-LTi cells. For example, Rorc(γt)−/− mice have often been used as a model system that is devoid of both LTi-like cells and NKR-LTi cells making it impossible to assign function to a specific subset.9,11,31–33 In addition, these mice also lack LNs, Peyer's patches and intestinal lymphoid follicles, which obscures the discrimination between effects reflecting the absence of RORγt+ ILCs or those that reflect the lack of leucocyte interactions within LNs or other lymphoid clusters. Experimental systems such as the inducible ablation of LTi-like cells and NKR-LTi cells or direct targeting of NKR-LTi cells are required to more accurately assign function to these innate lymphocyte populations.
Intestinal epithelial cells have a rapid turnover and need to further adapt when additional damage occurs. The LTi-like cells and NKR-LTi cells constitutively produce IL-22 and instruct a tissue-protective programme in epithelial cells.9 Recent data from the Drosophila system demonstrate that a cytokine-mediated feedback loop instructs stem cells to generate additional progeny under conditions of infection or stress, thereby establishing epithelial homeostasis.116 The cytokine signalling pathway involved in that process is orthologous to the vertebrate Jak/Stat cascade. These data may suggest that regulation of epithelial homeostasis by cytokine cues is an evolutionarily ancient programme required for adapting epithelial renewal and maintaining barrier function at sites of direct contact with the environment. Future research into renewal of epithelial cells in the absence and presence of LTi-like cells and NKR-LTi cells is required. A more in-depth understanding of the molecular and cellular requirements for maintaining intestinal mucosal homeostasis is of considerable interest. To this end, a better understanding of both the ‘immunoepithelome’ and the ‘epimmunome’ is needed. Further research into innate, IL-22-producing lymphocyte subsets will also be an important step in understanding the pathogenesis of human inflammatory bowel disease disorders.
Acknowledgments
We are grateful to Katie Connor for critically reading the manuscript and to the members of the Diefenbach laboratory for discussions. Research in the Diefenbach laboratory is supported by grants from the Deutsche Forschungsgemeinschaft (CRC620, Di764/3, SGBM, GRK1104) and the Federal Department of Education and Research (CCI).
Disclosures
The authors have no conflicting financial interests.
References
- 1.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 2.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–20. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 3.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 4.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 5.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009;9:883–9. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 7.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 8.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11:656–65. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 11.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupedo T, Crellin NK, Papazian N, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 14.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Xie MH, Aggarwal S, Ho WH, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–9. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–9. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 18.Narushima Y, Unno M, Nakagawara K, et al. Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIII α, RegIII β, RegIII γ. Gene. 1997;185:159–68. doi: 10.1016/s0378-1119(96)00589-6. [DOI] [PubMed] [Google Scholar]
- 19.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII γ and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201:534–43. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandl K, Plitas G, Mihu CN, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–7. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host–microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–63. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 26.Tagliabue A, Befus AD, Clark DA, Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982;155:1785–96. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan PG, Hapel AJ, Doe WF. Lymphokine-activated and natural killer cell activity in human intestinal mucosa. J Immunol. 1985;135:1731–8. [PubMed] [Google Scholar]
- 28.Gibson PR, Jewell DP. The nature of the natural killer (NK) cell of human intestinal mucosa and mesenteric lymph node. Clin Exp Immunol. 1985;61:160–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 30.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+ CD3– LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 31.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–73. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 33.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci U S A. 2000;97:10132–7. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 35.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–34. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 38.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 2009;10:1103–10. doi: 10.1038/embor.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 41.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 42.DiSanto JP, Müller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA. 1995;92:377–81. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–38. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg PD, Riddell SR. Deficient cellular immunity – finding and fixing the defects. Science. 1999;285:546–51. doi: 10.1126/science.285.5427.546. [DOI] [PubMed] [Google Scholar]
- 45.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–6. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 46.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3–NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–80. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–30. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–84. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 49.Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11:945–52. doi: 10.1038/ni.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med. 2008;205:245–56. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 52.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF-β in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 54.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR α and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walzer T, Blery M, Chaix J, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–9. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–36. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188:953–60. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gazit R, Gruda R, Elboim M, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–23. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 59.Anfossi N, Andre P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 61.Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–9. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 62.Romagnani C, Juelke K, Falco M, et al. CD56brightCD16– killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–55. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 63.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–72. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cantoni C, Bottino C, Vitale M, et al. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med. 1999;189:787–96. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allcock RJ, Barrow AD, Forbes S, Beck S, Trowsdale J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol. 2003;33:567–77. doi: 10.1002/immu.200310033. [DOI] [PubMed] [Google Scholar]
- 68.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34+ subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hughes T, Becknell B, McClory S, et al. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008–10. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hughes T, Becknell B, Freud AG, et al. Interleukin-1β selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–14. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 74.Gascoyne DM, Long E, Veiga-Fernandes H, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–24. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 75.Kamizono S, Duncan GS, Seidel MG, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–86. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida H, Naito A, Inoue J, et al. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity. 2002;17:823–33. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- 77.De Togni P, Goellner J, Ruddle NH, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–7. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 78.Dejardin E, Droin NM, Delhase M, et al. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–35. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 79.Eberl G, Littman DR. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science. 2004;305:248–51. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 80.Vonarbourg C, Mortha A, Bui VL, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity. 2010;33:736–51. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–54. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Schmutz S, Bosco N, Chappaz S, Boyman O, Acha-Orbea H, Ceredig R, Rolink AG, Finke D. Cutting edge: IL-7 regulates the peripheral pool of adult RORγ+ lymphoid tissue inducer cells. J Immunol. 2009;183:2217–21. doi: 10.4049/jimmunol.0802911. [DOI] [PubMed] [Google Scholar]
- 84.Chappaz S, Finke D. The IL-7 signaling pathway regulates lymph node development independent of peripheral lymphocytes. J Immunol. 2010;184:3562–9. doi: 10.4049/jimmunol.0901647. [DOI] [PubMed] [Google Scholar]
- 85.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T–B+NK+ severe combined immunodeficiency. Nat Genet. 1998;20:394–7. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 86.Lai SY, Molden J, Goldsmith MA. Shared γ(c) subunit within the human interleukin-7 receptor complex. A molecular basis for the pathogenesis of X-linked severe combined immunodeficiency. J Clin Invest. 1997;99:169–77. doi: 10.1172/JCI119144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science. 2010;330:665–9. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 88.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+ IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207:281–90. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1β reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–6. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127+ LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–64. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 91.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–9. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 92.Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci U S A. 2000;97:10144–9. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 94.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 95.Kim MY, Rossi S, Withers D, et al. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008;124:166–74. doi: 10.1111/j.1365-2567.2007.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Maele L, Carnoy C, Cayet D, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3negCD127+ immune cells in spleen and mucosa. J Immunol. 2010;185:1177–85. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Satoh-Takayama N, Dumoutier L, Lesjean-Pottier S, Ribeiro VS, Mandelboim O, Renauld JC, Vosshenrich CA, Di SantoJP. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J Immunol. 2009;183:6579–87. doi: 10.4049/jimmunol.0901935. [DOI] [PubMed] [Google Scholar]
- 98.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–72. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–57. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis A. CD4+ lymphoid tissue inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–34. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–44. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diefenbach A, Vonarbourg C. Innate lymphocytes induce inflammatory bowel disease. Immunol Cell Biol. 2010;88:694–6. doi: 10.1038/icb.2010.82. [DOI] [PubMed] [Google Scholar]
- 103.Di Santo JP, Vosshenrich CA, Satoh-Takayama N. A ‘natural’ way to provide innate mucosal immunity. Curr Opin Immunol. 2010;22:435–41. doi: 10.1016/j.coi.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 104.Strober W. The LTi cell, an immunologic chameleon. Immunity. 2010;33:650–2. doi: 10.1016/j.immuni.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–59. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–10. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 107.Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–20. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- 108.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 110.Diefenbach A, Raulet DH. Innate immune recognition by stimulatory immunoreceptors. Curr Opin Immunol. 2003;15:37–44. doi: 10.1016/s0952-7915(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 111.Diefenbach A, Raulet DH. Strategies for target cell recognition by natural killer cells. Immunol Rev. 2001;181:170–84. doi: 10.1034/j.1600-065x.2001.1810114.x. [DOI] [PubMed] [Google Scholar]
- 112.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 113.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–5. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 118.Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S, Sharpe A. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–56. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]


