Abstract
The T helper type 2 (Th2) immune response, characterized by the production of interleukin-4 (IL-4), IL-5 and IL-13, is a critical immune response against helminths invading cutaneous or mucosal sites. It also plays a critical role in the pathophysiology of allergic diseases such as asthma and allergic diarrhoea. The Th2 cytokines are induced soon after helminth infection, even before a pathogen-specific adaptive immune response is established. Recent studies have shed light on such innate Th2 cytokine production by formerly uncharacterized innate immune cells such as natural helper cells capable of producing Th2 cytokines in response to IL-25 and IL-33 independently of adaptive immune responses. These cells produce large amounts of Th2 cytokines, most notably IL-5 and IL-13, leading to eosinophilia and goblet cell hyperplasia. We discuss here the mechanisms of innate production of Th2 cytokines in host immune responses against helminth infection as well as allergic immune responses and the similarities and differences between recently identified Th2-cytokine producing cells.
Keywords: adipose tissue, eosinophilia, goblet cell hyperplasia, interleukin-25, interleukin-33
Introduction
The T helper type 2 (Th2) inflammatory responses are characterized by the recruitment and activation of mast cells, basophils and eosinophils, and goblet cell hyperplasia in airway and intestinal epithelia.1,2 These immune responses are induced against helminths invading cutaneous or mucosal sites and function as protective immunity against those pathogens. Interestingly, Th2 cytokines are induced soon after helminth infection and before pathogen-specific Th2 cells are established. Although the final expulsion of helminths usually requires Th2-mediated immunity, an early Th2-type innate immune response is important in the restriction of helminth invasion before the adaptive immune response initiates. Similar Th2 responses play important roles in the pathophysiology of allergic diseases such as asthma and allergic diarrhoea.
Studies on the relatively new cytokines, thymic stromal lymphopoietin (TSLP), interleukin-25 (IL-25) and IL-33, revealed that epithelial cells play important roles in inducing Th2 immune responses against allergens and helminths.3–5 Various types of epithelial cells produce TSLP, IL-25 and IL-33 in response to allergens and helminths.6,7 For example, house dust mites stimulate lung epithelial cells through Toll-like receptor 4.8
Interleukin-4 is a key cytokine that triggers antigen-specific Th2 responses. Although the initial source of IL-4 is still unknown, CD1d-restricted natural killer T (NKT) cells,9,10 mast cells11 and basophils12,13 are able to produce IL-4 upon stimulation. Basophils produce IL-4 and accumulate in the liver and lung after Nippostrongylusbrasiliensis infection.14 Many allergens are associated with cysteine protease activities. Incubation of basophils with one of cysteine proteases, papain, leads to the production of IL-4.15 Intriguingly, basophils are transiently recruited to the draining lymph node where Th2 cells are predominantly induced after Trichurismuris and Shistosoma mansoni infection16–18 but it is controversial whether basophils present antigens to T cells.19–21
Although the antigen-specific Th2 response plays a central role in protective immunity against helminths and antigen-specific allergic responses, accumulating evidence indicates the involvement of innate immune cells in the onset of Th2 responses. Administration of IL-25 and IL-33 induces rapid production of Th2 cytokines such as IL-5 and IL-13 in mice independently of T or B cells.22–24 Recent studies identified a previously unrecognized cell population(s) capable of producing large amounts of IL-5 and IL-13 in response to IL-25 and IL-33. We discuss here the characteristics and functions of such Th2 cytokine-producing innate cells. In particular, we focus on natural helper (NH) cells found in fat-associated lymphoid clusters (FALCs) present in visceral adipose tissue.25
Epithelial cell-derived Th2-inducing cytokines
Accumulating evidence has shown the importance of epithelial cells in the production of cytokines such as TSLP, IL-25 and IL-33 in response to allergens and helminths.3–7
Thymic stromal lymphopoietin (TSLP)
Thymic stromal lymphopoietin was originally identified from thymic stromal cells and thought to support growth and differentiation of T and B cells but is now considered to be a Th2-inducing cytokine.6 It acts to induce dendritic cells (DCs) capable of differentiating naive CD4+ T cells to Th2 cells.26,27 The TSLP-activated DCs produce CXCL8 and CCL24 attracting neutrophils and eosinophils, respectively. Th2-attracting chemokines, CCL17 and CCL22, are also produced by DCs activated by TSLP. Interestingly, however, these DCs do not produce tumour necrosis factor, IL-1β, IL-6, IL-10 or IL-12. Induction of Th2 cells by TSLP-stimulated DCs depends on OX40 ligand specifically induced by TSLP.28
Interleukin-25
Interleukin-25 is a member of the IL-17 family, which is also called IL-17E.22 The IL-25 receptor (IL-25R) is composed of IL-17RA and IL-17RB whereas the receptor for other IL-17 family members is a heterodimer of IL-17RA and IL-17RC.29 Interleukin-25 is produced by Th2 cells and epithelial cells.22,23,30,31 For example, lung epithelial cells infected with Aspergillus fumigatus23 or sensitized with ovalbumin30 as well as gut epithelial cells infected with N. brasiliensis31 produce IL-25. Mast cells also produce IL-25 upon cross-linking of FcεRI.32
Unlike other family members that induce neutrophil-mediated inflammatory responses, administration of IL-25 induced eosinophilia and IgE production in mice, a typical Th2 response.22 Repeated nasal administration of IL-25 resulted in the expression of IL-5 and IL-13 in the lung and induced airway hypersensitivity.23 Similarly, transgenic mice expressing IL-25 exhibited increased levels of serum IL-5 and IL-13 and eosinophilia.33,34In vitro, IL-25 induces the production of IL-5 and IL-13 from a CD4− CD8− MHC-IIhigh CD11cdull F4/80low non-T and non-B (NTNB) population.22 Both IL-5 and IL-13 are also produced in Rag2−/− mice upon IL-25 administration, confirming the production of these cytokines by NTNB cells.23 Intriguingly, IL-25 induces the expression of IL-5 and IL-13 but not IL-4. Mice lacking the cytokine receptor common γ chain (γc) are incapable of producing IL-5 and IL-13 in response to IL-25.23
Interleukin-25-deficient mice were unable to elicit a Th2 response upon T. muris infection and are therefore unable to control the infection.35,36 Similarly, the expulsion of N. brasiliensis was significantly delayed in IL-25-deficient mice but administration of recombinant IL-25 leads to the induction of IL-5 and IL-13 from NTNB cells and to the expulsion of N. brasiliensis.23
Interleukin-33
Interleukin-33 is a member of the IL-1 family37,38 expressed in a variety of cells including fibroblasts, epithelial cells, endothelial cells and adipocytes.39–41 Surprisingly, IL-33 is localized in the nucleus and functions as a heterochromatin-associated transcriptional repressor like IL-1α.42,43 Caspases 3 and 7 cleave IL-33 at the IL-1-like domain and inactivate IL-33 during apoptosis.44,45 In contrast, full-length IL-33 is released from cells upon necrotic cell death and functions as an alarmin to stimulate various types of cells.44 Similar alarmins that are also present in the nucleus and are released upon cellular damage include high mobility group box-1,46 IL-1α47 and spliceosome associated protein 130.48
Interleukin-33R is composed of ST2 and IL-RAcP39 and is expressed on various types of cells including Th2 cells,49 mast cells50 and basophils.51–53 Interleukin-33R is also expressed on CD34+ haematopoietic progenitor cells and IL-33 induces the differentiation of mast cells from CD34+ cells.54 Interestingly, a truncated form of ST2 named soluble ST2 (sST2) is secreted and acts as a negative regulator of IL-33.55
Interleukin-33 induces the production of IL-5 and IL-13 from mast cells56,57 and CD34+ cells54in vitro. Basophils also respond to IL-33 to produce IL-4, IL-5, IL-6 and IL-13.51 Administration of IL-33 induces Th2 cytokine production and associated physiological changes in mice including IgE production, eosinophilia and goblet cell hyperplasia.24 Interleukin-33 is also involved in Th2-type inflammatory responses induced by Pseudomonas aeruginosa and respiratory syncytial virus.58,59 Mice deficient for IL-33 are resistant to allergen-induced airway hypersensitivity.60 Administration of sST2 blocks the allergic response in the allergic airway inflammation model.55,61 Many studies have shown that IL-33 is involved in allergic diseases in humans. Polymorphisms of IL-33 and ST2 are associated with asthma in humans and patients with asthma have a lower level of sST2 than healthy controls.62 The expression levels of IL-33 in epithelial cells are higher in patients with asthma than in healthy individuals63 and even higher in treatment-resistant patients.64 The levels of IL-33 were also higher in patients with allergic conjunctivitis,65 rhinitis66 and atopic dermatitis67 than in healthy individuals.
Innate cells responsible for the production of Th2 cytokines
Although administration of TSLP, IL-25 and IL-33 induces Th2 cytokine production and associated physiological changes in mice such as IgE production, eosinophilia and goblet cell hyperplasia, the identity of the cell(s) responsible for the production of Th2 cytokines has been obscure. As mentioned, mast cells, basophils and CD34+ haematopoietic progenitor cells produce Th2 cytokines. A fraction of NKT cells express IL-25R and respond to IL-25 to produce IL-13.68 However, the amounts of IL-5 and IL-13 produced by these cells were lower than those produced in vivo, suggesting the presence of yet to be discovered cell type(s). We have recently identified a previously unrecognized lymphocyte population in the visceral adipose tissue of the peritoneal cavity that we named natural helper cells.25 Cells sharing similar characteristics with NH cells have also been reported by others.69–71
Natural helper cells present in FALC
We noted a previously unrecognized γc-dependent lymphoid tissue located along the blood vessels in the mouse and human mesentery, an adipose tissue in the peritoneal cavity.25 We named these lymphoid clusters ‘fat-associated lymphoid clusters’ or FALCs (Fig. 1a). Cells in FALC are in direct contact with ambient adipocytes and no fibrous capsule is present around FALC. The FALC is also present in visceral adipose tissues around the kidney and genitalia but very few are found in the subcutaneous fat tissue or the omentum. They are structurally similar to the ‘milky spot’ in the omentum, which is considered a gateway of cells between the circulation and the peritoneal cavity.72 However, unlike the milky spot,73 T-cell and B-cell zones or germinal centre structures are not observed in FALC. Conversely, NH cells are not present in milky spots. The FALC is present in retinoic acid-related orphan receptor γ (RORγ) -deficient and aly/aly mice, suggesting that the differentiation pathway of FALC is distinct from those of lymph nodes or Peyer's patches involving lymphoid tissue inducer cells.74
Figure 1.
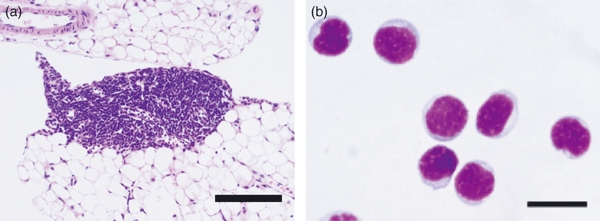
Fat-associated lymphoid clusters (FALC) and natural helper (NH) cells. (a) Haematoxylin & eosin staining of FALC. Note that cells in FALC directly attach to surrounding adipocytes. Bar indicates 200 μm. (b) Giemsa staining of isolated NH cells. The NH cells are small in size and round in shape with a dark nucleus and scanty cytoplasm. Bar indicates 20 μm. These pictures are reproduced by courtesy of Nature (ref. 22).
The FLAC contains T cells, B cells, macrophages and DCs. In addition, NH cells expressing c-Kit, Sca-1, IL-2R, IL-7R and IL-33R but no conventional lineage (Lin) markers make up 20–40% of cells in FALC.25 A cell population similar to the NH cells expressing c-Kit and IL-7Rα is also present in human FALC. Giemsa staining and electron microscopy showed that NH cells have the characteristics of lymphoid cells (Fig. 1b). The NH cells were present in Rag2−/− and nu/nu mice but absent from γc−/− and IL-7−/− mice, indicating that NHs cell are of lymphoid lineage with differentiation dependent on IL-7. Stem cell factor and IL-7 supported the survival of NH cells and IL-2 induced the proliferation of NH cells.
Natural helper cells constitutively produce IL-5 and IL-6, which are involved in antibody production.75,76 Interleukin-5 is a critical growth factor for B1 cells present in the peritoneal cavity and plays an important role in innate-type immune responses by producing natural antibodies.77,78 Indeed, NH cells support the production of IgA from B cells and self-renewal of B1 cells, indicating that NH cells have helper functions under steady-state conditions (Fig. 2a).
Figure 2.
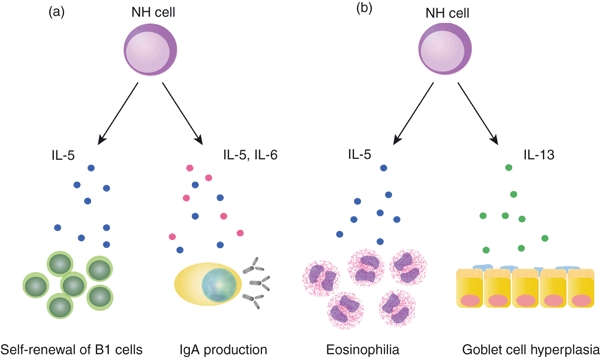
Functions of natural helper (NH) cells. (a) Interleukin-5 (IL-5) and IL-6 constitutively produced by NH cells support IgA production by B cells. Interleukin-5 supports the survival of B1 cells in the peritoneal cavity. (b) Upon helminth infection, IL-33 produced by epithelial cells, endothelial cells and adipocytes induce high level of IL-5 and IL-13 by NH cells, inducing eosinophilia and goblet cell hyperplasia, which play important roles in an early phase of anti-helminth immune responses.
The NH cells produce even larger amounts of IL-5, IL-6 and IL-13 in response to IL-33 or a combination of IL-2 and IL-25. Of note, NH cells do not respond to IL-25 without IL-2. Five thousand NH cells are able to produce microgram amounts of IL-5 and IL-13 in response to IL-33 during a 5-day culture period, which is much higher than the amounts produced by mast cells,50 basophils51–53 or polarized Th2 cells.49 Unlike basophils, NH cells do not produce IL-4 in response to IL-33 or a combination of IL-2 and IL-25. Notably, however, NH cells are capable of producing IL-4 by a combination of 12-O-tetradecanoylphorbol-13-acetate and ionomycin. So far, this is the only stimulation leading to the production of IL-4 by NH cells.
Administration of IL-33 or N. brasiliensis infection induced the production of IL-5 and IL-13 as well as goblet cell hyperplasia in the intestine of Rag2−/− but not γc−/− Rag2−/− mice devoid of NH cells. Adoptive transfer of NH cells into γc−/− Rag2−/− mice restored the production of IL-5 and IL-13 and goblet cell hyperplasia, demonstrating that NH cells indeed play an important role in the production of IL-5 and IL-13 in vivo.25 However, expulsion of helminths still requires T cells as Rag2−/− mice are not able to expel N. brasiliensis (ref. 79 and our unpublished observations) and adoptive transfer of IL-4−/− IL-13−/− CD4+ T cells resulted in the clearance of N. brasiliensis in Rag2−/− mice.79 The NH cells probably provide IL-5 and IL-13 to limit the helminth invasion before helminth-specific T cells are induced (Fig. 2b).
Multipotent progenitor type 2 cells
Using knock-in mice in which green fluorescent protein (GFP) was inserted into the IL-4 allele, termed ‘4get’ mice,80 Saenz et al. identified IL-4-producing NTNB cells (GFP+ cells) in gut-associated lymphoid tissues upon IL-25 administration.69 Adoptive transfer of GFP+ cells into IL-25-deficient mice resulted in Th2 differentiation in mesenteric lymph nodes, mucin secretion and protective immunity against T. muris. Cells induced by IL-25 administration shared cell surface markers with haematopoietic stem cells or multipotent progenitor cells and were named MPPtype2. This population seems to be heterogeneous and c-Kit+ GFP+ cells differentiate into mast cells whereas c-Kit+ GFP− cells have the potential to differentiate into basophils and macrophages.
Nuocyte
Using another knock-in mouse in which GFP was inserted into the IL-13 allele, Neill et al. reported that NTNB cells producing IL-5 and IL-13 (GFP+ cells) were induced in the mesenteric lymph nodes, spleen and small intestine after administration of IL-25 or IL-33.70 They named these GFP+ cells ‘nuocytes’, nu being the thirteenth letter of the Greek alphabet although these cells also produce IL-5. As observed for NTNB cells in an earlier report,22 nuocytes express MHC class II and respond to IL-25 alone, which are characteristics distinct from those of NH cells (Table 1). Neill et al. established mice lacking IL-17RB that could not respond to IL-25 or control N. brasiliensis infection. Adoptive transfer of wild-type nuocytes into IL-17RB-deficient mice restored their ability to expel N. brasiliensis. According to their report, 2 million/ml nuocytes produced microgram amounts of IL-5, IL-6 and IL-13 upon 1-week culture with a combination of IL-7 and IL-33.
Table 1.
Characteristics of innate T helper type 2 cytokine-producing cells as examined by surface phenotypes
| Cells | ||||
|---|---|---|---|---|
| Markers | NH cell | Nuocyte | MPPtype2 | Ih2 cell |
| c-Kit | + | ± | + | ± |
| CD45 | + | + | + | + |
| IL-7Rα | + | lo | −/lo | ? |
| Sca-1 | + | + | + | − |
| Thy-1 | + | + | ? | + |
| CD34 | − | − | −/lo | ? |
| CD25 | + | −1 | ? | +1 |
| CD44 | + | + | ? | + |
| CD69 | + | ? | − | +1 |
| CD62L | − | ? | −/lo | ? |
| FcεRI | − | − | − | ? |
| T1/ST2 | + | ± | −/lo | ? |
| MHC class II | − | + | ?2 | ? |
Based on microarray analysis.
GFP− cells expressed MHC class II after cultivation with a combination of interleukin-3 and stem cell factor.
NH, natural helper; MPP, multipotent progenitor; Ih2, innate helper type 2; IL-7R, interleukin-7 receptor.
Innate helper type 2
Price et al. recognized a Lin− c-Kit+ GFP+ population in naive 4get mice.71 It was known that eosinophils, basophils, mast cells, NKT cells and Th2 cells are GFP+ in naive 4get mice,81–83 but the Lin− c-Kit+ GFP+ population they noticed was distinct from those reported previously. Price et al. also used IL-13 reporter mice and observed that such c-Kit+ NTNB cells produce IL-5 and IL-13 in response to IL-25 or IL-33 administration and N. brasiliensis infection. Intriguingly, IL-4-GFP+ cells do not produce IL-4 at the protein level. The authors named these cells innate helper type 2 (Ih2) cells. These Ih2 cells are absent in γc−/− Rag2−/− mice incapable of controlling N. brasiliensis infection but adoptive transfer of Ih2 cells followed by administration of IL-25 produced eosinophilia and anti-helminth activity. Whether Ih2 cells are able to produce cytokines in response to IL-25 alone and the quantity of cytokines produced by Ih2 cells is currently unknown.
At the moment, the relationship between NH cells, nuocytes, MPPtype2 and Ih2 cells is unclear. There are similarities and differences, as shown in Table 1. One important difference between NH cells and the other cells is that NH cells do not respond to IL-25 alone.25,69 It is intriguing that there is no evidence of the production of IL-4 as a protein in these cells even though the il-4 gene is active as determined by microarray analyses and knock-in approaches.
Perspectives
Protective immunity against helminths
Invasion of helminths results in tissue destruction, leading to the secretion of alarmins from necrotic cells including IL-25 and IL-33. These cytokines act on innate immune cells such as NH cells to induce IL-5 and IL-13, resulting in eosinophilia and goblet cell hyperplasia, respectively (Fig. 2b). Eosinophilia is important in controlling helminths in the lung stage of N. brasiliensis and Strongyloidesvenezuelensis and goblet cell hyperplasia in the intestine is involved in the blocking of attachment of helminth during the early phase of the intestinal phase. Both IL-25 and IL-33 induce IL-4 from basophils, mast cells and possibly MPPtype2 cells that support the induction of Th2 differentiation. The Th2-mediated adaptive immune responses will eventually result in the expulsion and clearance of helminths.
These pictures are in a way similar to the Th1-type innate immune response before the differentiation of pathogen-specific Th1 cells. Natural killer cells support the phagocytes by providing interferon-γ to limit the growth of intracellular pathogens including protozoan parasites and viruses.84 The IL-22-expressing NKp46+ NK cells deal with extracellular mucosal pathogens in the intestine as well as Mycobacteriumtuberculosis in the lung until Th1 cells become ready to work.85,86 From this viewpoint, it is of interest that IL-33 induces Th1 cytokines from NK cells and NKT cells,51,87 implying that IL-33 induces a Th1-type innate immune response under certain circumstances.
Contribution to allergic responses
Given that IL-5 and IL-13 are critical factors for the induction of eosinophilia and goblet cell hyperplasia, respectively, and given that these two processes are involved in the pathogenesis of allergic diseases such as asthma and allergic diarrhoea, it stands to reason that the innate Th2 cytokine producers described here play important roles in these allergic diseases.1 Of note, FALC are present in humans and it is likely that NH cells play important roles in human allergic diseases as well.
The NH cells do not respond to Toll-like receptor ligands such as lipopolysaccharide or lectins such as concanavalin A (ref. 25 and our unpublished observations). It is unknown at the moment whether the innate Th2 cytokine producers discussed here directly respond to allergens or microbial/parasitic antigens. It remains to be shown how these Th2 producers are triggered in vivo.
Inflammatory reactions in the adipose tissues
In addition to their roles in allergic diseases and protection against helminth infection, NH cells may have additional roles in adipose tissues. Accumulating evidence demonstrates the importance of interaction between adipocytes and immune cells in the regulation of homeostasis in adipose tissues. Impaired regulation under pathogenic conditions such as obesity results in inflammatory responses in adipose tissues leading to insulin resistance.88 One subset of macrophages called M1 macrophages, is involved in the inflammation in adipose tissues.88,89 Interleukin-33 induces the alternative M2 subset of macrophages but high levels of Th2 cytokines convert them to M1 macrophages.53,90,91 Because such phase transition in macrophages plays a critical role in the course of inflammation in adipose tissues, it will be of interest and of importance to elucidate the functions of NH cells in adipose tissues.
Finally, it will be important to elucidate the developmental and functional relationship between NH cells, nuocytes and Ih2 cells in future studies.
Acknowledgments
We thank our collaborators and members of our laboratory. This work was supported by a Grant-in Aid for Young Scientist (B) (20790378 to K.M.), a Grant-in Aid for Young Scientist (A) (22689013 to K.M.), Grants-in-Aid for Scientific Research (B) (14370116, 16390146, 18390155 to S.K.), a Grant-in-Aid for Scientific Research (A) (22249013 to S.K.) and a Grant-in-Aid for Scientific Research (S) (22229004 to S.K.) from the Japan Society for the Promotion of Science. K.M. is a postdoctoral fellow of the Global COE programme supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosures
S.K. is a consultant for Medical and Biological Laboratories, Co. Ltd. The authors otherwise have no financial conflicts of interest.
References
- 1.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–84. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barret NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–37. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–68. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 5.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–6. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 8.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arase H, Arase N, Nakagawa K, Good RA, Onoé K. NK1.1+ CD4− CD8− thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307–10. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–9. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of FcεRI or to calcium ionophores. Nature. 1989;339:64–7. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 12.Piccinni MP, Macchia D, Parronchi P, et al. Human bone marrow non-B, non-T cells produce interleukin 4 in response to cross-linkage of Fcε and Fcγ receptors. Proc Natl Acad Sci USA. 1991;88:8656–60. doi: 10.1073/pnas.88.19.8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seder RA, Paul WE, Ben-Sasson SZ, LeGros GS, Kagey-Sobotka A, Finkelman FD, Pierce JH, Plaut M. Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol. 1991;94:137–40. doi: 10.1159/000235345. [DOI] [PubMed] [Google Scholar]
- 14.Min B, Prout M, Hu-Li J, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–17. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 18.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phythian-Adams AT, Cook PC, Lundie RJ, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–96. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 23.Hurst SD, Muchamuel T, Gorman DM, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 24.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–9. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 25.Moro K, Yamada T, Tanabe M, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 26.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–8. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 27.Ying S, O'Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–9. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rickel EA, Siegel LA, Yoon BR, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 30.Tamachi T, Maezawa Y, Ikeda K, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–14. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 31.Angkasekwinai P, Park H, Wang YH, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–17. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon FcεRI-mediated activation. Blood. 2003;101:3594–6. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 33.Kim MR, Manoukian R, Yeh R, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–40. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 34.Pan G, French D, Mao W, et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–67. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 35.Owyang AM, Zaph C, Wilson EH, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–9. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokine. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Oboki K, Ohno T, Kajiwara N, et al. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–60. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 39.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–49. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384:105–9. doi: 10.1016/j.bbrc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 42.Carrière V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–7. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buryskova M, Pospisek M, Grothey A, Simmet T, Burysek L. Intracellular interleukin-1α functionally interacts with histone acetyltransferase complexes. J Biol Chem. 2004;279:4017–26. doi: 10.1074/jbc.M306342200. [DOI] [PubMed] [Google Scholar]
- 44.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–6. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lüthi AU, Cullen SP, McNeela EA, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 47.Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107:2574–9. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–88. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 49.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–94. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci USA. 2007;104:18660–5. doi: 10.1073/pnas.0705939104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–30. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 52.Suzukawa M, Iikura M, Koketsu R, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–9. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 53.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–80. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 54.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–4. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 55.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–80. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 56.Iikura M, Suto H, Kajiwara N, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–8. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 57.Ho LH, Ohno T, Oboki K, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcεRI signals. J Leukoc Biol. 2007;82:1481–90. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 58.Huang X, Du W, Barrett RP, Hazlett LD. ST2 is essential for Th2 responsiveness and resistance to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007;48:4626–33. doi: 10.1167/iovs.07-0316. [DOI] [PubMed] [Google Scholar]
- 59.Walzl G, Matthews S, Kendall S, Gutierrez-Ramos JC, Coyle AJ, Openshaw PJ, Hussell T. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (Th2)- but not Th1-driven immunopathology. J Exp Med. 2001;193:785–92. doi: 10.1084/jem.193.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oboki K, Ohno T, Kajiwara N, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–6. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179:772–81. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010;40:200–8. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 63.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 64.Reh DD, Wang Y, Ramanathan M, Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy. 2010;24:105–9. doi: 10.2500/ajra.2010.24.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuda A, Okayama Y, Terai N, et al. The role of interleukin-33 in chronic allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2009;50:4646–52. doi: 10.1167/iovs.08-3365. [DOI] [PubMed] [Google Scholar]
- 66.Sakashita M, Yoshimoto T, Hirota T, et al. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin Exp Allergy. 2008;38:1875–81. doi: 10.1111/j.1365-2222.2008.03114.x. [DOI] [PubMed] [Google Scholar]
- 67.Pushparaj PN, Tay HK, H'ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci USA. 2009;106:9773–8. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Terashima A, Watarai H, Inoue S, et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–33. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saenz SA, Siracusa MC, Perrigoue JG, et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1362–6. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cranshaw ML, Leak LV. Milky spots of the omentum: a source of peritoneal cells in the normal and stimulated animal. Arch Histol Cytol. 1990;53(Suppl):165–77. doi: 10.1679/aohc.53.suppl_165. [DOI] [PubMed] [Google Scholar]
- 73.Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, Randall TD. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–43. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72–80. doi: 10.1034/j.1600-065x.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 75.Beagley KW, Eldridge JH, Lee F, et al. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–4218. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonoda E, Matsumoto R, Hitoshi Y, et al. Transforming growth factor β induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989;170:1415–20. doi: 10.1084/jem.170.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erickson LD, Foy TM, Waldschmidt TJ. Murine B1 B cells require IL-5 for optimal T cell-dependent activation. J Immunol. 2001;166:1531–9. doi: 10.4049/jimmunol.166.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev. 2000;175:70–9. [PubMed] [Google Scholar]
- 79.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–46. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 81.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–77. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 82.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–72. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 83.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korbel DS, Finney OC, Riley EM. Natural killer cells and innate immunity to protozoan pathogens. Int J Parasitol. 2004;34:1517–28. doi: 10.1016/j.ijpara.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 85.Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J Immunol. 2009;183:6634–9. doi: 10.4049/jimmunol.0901935. [DOI] [PubMed] [Google Scholar]
- 86.Satoh-Takayama N, Dumoutier L, Lesjean-Pottier S, Ribeiro VS, Mandelboim O, Renauld JC, Vosshenrich CA, Di Santo JP. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol. 2009;183:6579–87. doi: 10.4049/jimmunol.0901935. [DOI] [PubMed] [Google Scholar]
- 87.Bourgeois E, Van LP, Samson M, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-γ production. Eur J Immunol. 2009;39:1046–55. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 88.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 2010;88:33–9. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 89.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Murata Y, Yamashita A, Saito T, Sugamura K, Hamuro J. The conversion of redox status of peritoneal macrophages during pathological progression of spontaneous inflammatory bowel disease in Janus family tyrosine kinase 3−/− and IL-2 receptor γ−/− mice. Int Immunol. 2002;14:627–36. doi: 10.1093/intimm/dxf031. [DOI] [PubMed] [Google Scholar]
- 91.Hazlett LD, McClellan SA, Barrett RP, Huang X, Zhang Y, Wu M, van Rooijen N, Szliter E. IL-33 shifts macrophage polarization, promoting resistance against Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2010;51:1524–32. doi: 10.1167/iovs.09-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]


