Abstract
Neuronal or photoreceptor deficit observed in uveitis and multiple sclerosis derives in part from inability to control inflammatory responses in neuroretina or brain. Recently, IL-27 was found to play a role in suppressing experimental autoimmune uveitis and experimental autoimmune encephalomyelitis, two animal models that share essential pathological features of human uveitis and multiple sclerosis, respectively. However, the mechanism by which interleukin-27 (IL-27) inhibits central nervous system (CNS) inflammation is not clear. In this study we have investigated mechanisms that mitigate or curtail intraocular inflammation (uveitis) and examined whether inhibitory effects of IL-27 are mediated locally by neuroretinal cells or by regulatory T cells. We show here that microglia cells in the neuroretina constitutively secrete IL-27 and its expression is up-regulated during uveitis. We further show that photoreceptors constitutively express IL-27 receptor and respond to IL-27 signalling by producing anti-inflammatory molecules, IL-10 and suppressor of cytokine signalling 1 (SOCS1) through signal transducer and activator of transcription 1 (STAT1) -dependent mechanisms. Moreover, STAT1-deficient mice produced reduced amounts of IL-27, IL-10 and SOCS1 and developed more severe uveitis. Surprisingly, IL-10-producing regulatory T cells had marginal roles in suppressing uveitis. These results suggest that suppression of intraocular inflammation might be mediated through endogenous production of IL-27 and IL-10 by retinal cells, whereas SOCS proteins induced by IL-27 during uveitis may function to protect the neuroretinal cells from the toxic effects of pro-inflammatory cytokines. Targeted delivery of IL-27 into immune privileged tissues of the CNS may therefore be beneficial in the treatment of CNS inflammatory diseases, such as uveitis and multiple sclerosis.
Keywords: cytokine, interleukin-10, interleukin-27, intraocular inflammatory diseases, microglia, retina, signal transducer and activator of transcription 1
Introduction
Intraocular inflammatory diseases (uveitis) are a major cause of severe visual handicap and include diverse syndromes such as Behçet disease, birdshot retinochoroidopathy, sympathetic ophthalmia and ocular sarcoidosis.1 Experimental autoimmune uveitis (EAU) is a T-cell-mediated disease that shares essential pathological features with human uveitis.2 T helper type 17 (Th17) cells have recently been implicated in the aetiology of human uveitis and amelioration of EAU by treatment with antibody to IL-17 (also produced by γδ T cells) provided validation for the involvement of Th17 cells in uveitis.3 In healthy individuals, intraocular inflammation is usually self-limiting and of short duration partly because of endogenous mechanisms in this immune privileged organ. However, uveitis patients suffer from chronic intraocular inflammation that can persist throughout life and may result in blindness.4 Steroids, cyclosporine A, rapamycin and FK506 are all effective drugs to treat uveitis; however, prolonged use is precluded because of significant renal toxicity.5 Efforts towards developing a non-steroidal therapy for uveitis have focused on identifying molecular targets that can be exploited to limit the expansion of T cells in the retina.
Interleukin-27 (IL-27) is a member of the IL-12 family and is comprised of an IL-12p40-related protein, encoded by the Epstein–Barr virus-induced gene 3 (EBI3), and a unique IL-12p35-like protein, IL-27p28.6 The IL-27 binds a receptor composed of gp130 and WSX17 and regulates pathological immune responses by inhibiting the differentiation of naive lymphocytes into Th17 cells or limiting the expansion of effector T cells.8 In recent studies of experimental autoimmune encephalomyelitis (EAE) and EAU models, IL-27 was shown to contribute to mechanisms that suppress autoimmune encephalomyelitis and uveitis, respectively, by inhibiting the development or expansion of Th17 cells.3,9 However, the role of IL-27 in the suppression of encephalitis in the EAE model is indirect and was attributed to inhibitory effects of a population of IL-10-secreting interferon-γ (IFN-γ)-expressing T cells induced by IL-27 (IFN-γ+ T-bet+ Foxp3−).10 In contrast, mitigation of intraocular inflammation in the EAU model derives, in part, from production of IL-27 by retinal cells and direct inhibition of Th17 expansion by IL-27 through a signal transducer and activator of transcription 1 (STAT1) -dependent mechanism.3 The disparate mechanisms proposed for suppression of these similar central nervous system (CNS) autoimmune inflammatory diseases has rekindled the age-long debate on whether inflammation in immune-privileged tissues is controlled locally by endogenous mechanisms in the target tissue or indirectly through the agency of T cells that regulate autoreactive T cells.
In this study, we have shown that retinal microglia and ganglion cells constitutively express IL-27 while the photoreceptor cells express IL-27 receptors and produced the anti-inflammatory cytokine, IL-10, in response to IL-27 stimulation. Production of IL-27 and IL-10 by retina cells required STAT1 and STAT1-deficient mice developed a more severe form of uveitis. We further show that suppression of inflammatory responses in the immune-privileged-retina is orchestrated in part by anti-inflammatory molecules produced by neuroretinal cells in response to IL-27 signalling while IL-10-producing T cells appear to play marginal roles in controlling severity or duration of inflammation in the CNS.
Materials and methods
Mice and cell lines
C57BL/6 mice were purchased from National Cancer Institute, Bethesda, MD or Jackson Laboratory, Bar Harbor, ME. The STAT1-deficient (STAT1KO) mice are a gift from Dr D. Levy, New York University, NY. Animal care was in compliance with National Institutes of Health guidelines. The human Müller cell line (MIO-M1) was from Astrid Limb (University College London, London, UK). MIO-M1 is a spontaneously immortalized human Müller cell line derived from an eye of a 68-year-old female donor 36 hr after death. Morphological appearance under phase-contrast and transmission electron microscopy, expression of Müller cell markers, and electrophysiological responses of the MIO-M1 cells are consistent with those reported in the literature for glial Müller cells.
Flow cytometry and intracellular cytokine analysis
CD4+ T cells were isolated from mouse blood or lymph nodes using T-cell enrichment columns and CD4+ MicroBeads (Miltenyi Biotec, Bergisch-Gladbach, Germany) and the cells were analysed directly without previous activation with antigen, anti-CD3 or anti-CD28 antibodies. For intracellular cytokine analysis, the cells were stimulated for 5 hr with PMA (20 ng/ml)/ionomycin (1 μm) in the presence of Golgi-stop as recommended (BD Pharmingen, San Diego, CA). Intracellular cytokine staining was performed using the Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA). Analysis using labelled anti-CD3, CD4, IL-17, IL-10, IFN-γ monoclonal antibodies and corresponding isotype control antibodies (PharMingen, San Diego, CA) was performed on a Becton Dickinson FACSCalibur (BD Biosciences). For FACS analysis of CD11c+, the cells were pre-incubated with purified anti-mouse CD16/CD32 Fc Block (BD PharMingen) and then stained with labelled CD11b, CD11c, I-Ab, CD40, CCR7, CD86 antibodies or corresponding isotype control antibodies (BD PharMingen). For intracellular cytokine analysis of retina cells, freshly isolated retina cells were cultured overnight in medium with or without IL-27 or IFN-γ. The cells were then stimulated for 5 hr with PMA (20 ng/ml)/ionomycin (1 μm) in the presence of Golgi-stop as recommended (BD Pharmingen). Intracellular cytokine staining was performed using the BD Biosciences Cytofix/Cytoperm kit. Flow cytometry analysis using labelled anti-CD11b, WSX-1, IL-10 or rhodopsin (Chemicon, Billerica, MA) or corresponding isotype control antibodies (BD PharMingen) was performed on a Becton Dickinson FACSCalibur (BD Biosciences).
Induction of EAU
Six- to eight-week-old STAT1KO (C57BL/6 background) or wild-type (WT) C57BL/6 mice were immunized with 150 μg interphotoreceptor retinoid-binding protein (IRBP) and 300 μg of human IRBP peptide (1–20) in 0.2 ml emulsion 1 : 1 volume/volume with complete Freund's adjuvant containing Mycobacterium tuberculosis strain H37RA (2.5 mg/ml). The mice also received Bordetella pertussis toxin (0.2 μg/mouse) concurrent with immunization and clinical disease was established by histology as described previously.11 Eyes for histological EAU evaluation were harvested 0, 14 or 21 days after immunization, fixed in 10% buffered formalin, embedded in paraffin, and stained with haematoxylin and eosin (H&E).
Isolation and stimulation of retinal cells
Retinal cells were isolated from WT C57BL6 and STAT1KO mice as previously described with modification.11 Briefly, mouse retinas were dissected free of the pigment epithelium and digested in Hanks' balanced salt solution containing 120 U papain (Worthington, Lakewood, NJ) and 2000 U of DNase (Worthington) for 20 min at 37° on a rotary platform shaker. Tissue was dissociated by gentle pipetting and passed through a 40-μm cell strainer and centrifuged for 5 min at 200 g. The pellet was resuspended in Hanks' balanced salt solution containing 1% ovalbumin (Worthington) and 500 U DNase, layered on top of a 4% ovalbumin solution (in Hanks' balanced salt solution), and centrifuged for 10 min at 500 g. The pellet was resuspended in medium [1 : 1 mixture of Dulbecco's modified Eagle's medium and F12 medium containing 2.5% heat-inactivated horse serum, B27 medium supplement (Invitrogen, Carlsbad, CA), 5 mg/ml d-glucose, 2 mm l-glutamine, 20 mm HEPES, 2.5 mg/ml bovine insulin (Sigma, ST Louis, MO), and 0.1 mg/ml human transferrin (Sigma; 15 000 cells/ml)] and the number of surviving photoreceptors was determined. Viability of the cells was consistently > 90% as assessed by Trypan blue exclusion. Retinal cells were then cultured in medium containing cytokines and analysed by flow cytometry. In some studies cells were seeded in 24-well plates at 5 × 106 cells/ml overnight, starved for 2 hr with 0.5% BSA in Dulbecco's modified Eagle's medium and then treated with IL-27 (50 ng/ml) for 90 min.
Cytokine assays
The cytokines in supernatant were assayed by Multiplex ELISA using SearchLight Arrays technology by Pierce (Pierce Woburn, MA; see http://www.SearchLightOnline.com) or assayed by ELISA with kits from R&D Systems (Minneapolis, MN) (IL-10; Cat# M1000) or eBioscience (San Diego, CA) (IL-27; Cat# 88-7274).
Generation of CD11c+ cells
CD11c+ cells were generated from bone marrow as previously described.12 Briefly, bone marrow was obtained from tibias and femurs of 8- to 12-week-old C57BL/6 WT or STAT1−/− mice. ScaI+ bone marrow cells were isolated from haematopoietic progenitor cells by labelling with a biotin antibody A cocktail consisting of biotin-conjugated monoclonal antibodies against CD5, CD45R (B220), CD11b, anti-GR-1 (Ly-6G/C), 7-4 (antigens restricted to committed progenitors and differentiated cells of the neutrophil/macrophages lineages) and Ter-119, binding to anti-biotin microbeads and passing them through Mini-MACS separation columns (Miltenyi Biotec). Lin− cells were further selected on ScaI+ beads and separation columns (Miltenyi Biotec, Auburn, CA). The Lin− ScaI+ cells were grown as suspension cultures in Teflon bags with Iscove's modified Dulbecco's medium (supplemented with 10% fetal bovine serum and the following recombinant murine cytokines: 100 ng/ml stem cell factor, 20 ng/ml IL-3, 50 ng/ml macrophage colony-stimulating factor, 5 ng/ml granulocyte–macrophage colony-stimulating factor, and 25 ng/ml FLT-3 ligand (R&D Systems). The cells were cultured in this medium for 9 days and then for 3 days in medium containing granulocyte–macrophage colony-stimulating factor (10 ng/ml) plus IL-4 (10 ng/ml). CD11c+ cells were obtained after overnight stimulation with lipopolysaccharide (Escherichia coli 026:B6; used at 2 μg/ml). The CD11C+ cells were further selected by magnetic cell sorting with anti-CD11C antibody-coupled beads.
CD4+ T-cell–CD11c+ co-cultures
Naive syngeneic CD4+ T cells (2 × 106) and WT or STAT1-deficient CD11c+ cells (0.4 × 106) were cultured in medium containing anti-CD3 antibodies (10 ng/ml) for 4 days. Exogenous IL-27 (50 ng/ml) (R&D Systems) was added to some cultures. Intracellular cytokine and four-colour FACS analyses were performed on plots gated on CD3 and/or CD4.
Confocal microscopy
Sections were blocked with 5% normal goat serum in immunolabelling buffer (PBS + 0.5% BSA + 0.2% Tween-20 + 0.05% sodium azide, pH 7.3) and then incubated overnight with the following primary antibodies: goat anti-mouse IL-27 Receptor (polyclonal, 1 : 100), goat anti-mouse IL-27p28 (1 : 100) (R&D Systems), rabbit anti-mouse IL-27p28 (1 : 100) (Imgenex, San Diego, CA), rabbit anti-mouse EBI3 (1 : 200 Santa Cruz Biotechnology, Santa Cruz, CA) or rat anti-mouse F4/80. Sections were washed in immunolabelling buffer (PBS containing 0.1% Tween-20 0.5% BSA and 0.05% sodium azide) then incubated for 1 hr in the following fluorochrome-conjugated secondary antibodies (donkey anti-goat Alexa Fluor® 488, goat anti-rabbit Alexa Fluor® 555, donkey anti-rabbit Alexa Fluor® 488 and DAPI; Molecular Probes, Eugene, OR). Primary antibodies were omitted from sections used as negative controls. Sections of labelled mouse retina were washed, mounted in Gel-Mount (Biomeda, Foster City, CA), and placed under coverslips. A Leica SP2 confocal microscope was used to take images of samples. Gain and offset (black level) values were kept constant for each set of experimental and negative control samples. To delineate regions where two antibodies co-localized, cytofluorogram scatter plots were generated using images collected in sequential scan mode. Pixels from areas of signal co-localization were identified in scatter plots and mapped back to the original image. For single cell analysis, primary retina cells were bound to slides by centrifugation on a Shandon Cytospin 4 cytocentrifuge as recommended by the manufacturer (Thermo Electron Corporation, Waltham, MA). Cells were fixed in 4% paraformaldehyde, permeabilized in 0·25% Triton X-100 and blocked in 1% BSA. Staining with primary or secondary antibodies was by standard methodology and confocal microscopy was performed as described above.
Quantitative and semi-quantitative reverse transcription-PCR analyses
Total RNA was extracted using the TriZol reagent according to the procedures recommended by the manufacturer (Life Technologies, Gaithersburg, MD). All RNA samples were digested with RNase-free DNase 1 (Life Technologies) for 30 min, purified by phenol/chloroform extractions and precipitated in 0·4 m LiCl. RNA (10 μg), SuperScript III Reverse Transcriptase (Life Technologies), and oligo-dT(12–16 mer) were used for first-strand synthesis as previously described.13 Samples were subjected to hot-start reverse transcription (RT-) PCR with gene-specific primers and AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA). Primers used for RT-PCR amplification were as follows. Mouse β-actin, 5′-GTGGGCCGCTCTAGGCACCAA-3′ and 5′-TCTTTGCCAATAGTGATGACTTGGC-3′; mouse WSX-1, 5′-CAAGAAGAGGTCCCGTGCTG-3′ and 5′-TTGAGCCCAGTCCACCACAT-3′; IL-27 receptor 5′-CAAGAAGAGGTCCCG TGCTG-3′ and 5′-TTGAGCCCAGTCCACCAC AT-3′; IL-10; 5′-CACTGCTATGCTGCCTGCTCTTAC-3′ and 5′-CTCCACTGCCTTGCTCTTA TTTC-3′; IL-27p28, 5′-TGGCATCACCTCTCTGACTC-3′ and 5′- CCTCCTCCTTTGAACATT TG-3′; EBI3, 5′-TGTGGCTGAGCGAATCATC-3′ and 5′-CTTGATGATTCGCTCAGC-3′; suppressor of cytokine signalling 1 (SOCS1), 5′-CTCGAGTAGGATGGTAGCACGCAA-3′ and 5′-CATCTTCACGCTGAGCGCGA AGAA-3′; SOCS3, 5′-TGCGCCATGGTCACCCACAGCAAGTTT-3′ and 5′-GCTCCTTAAAG TGGAGCATCATACTGA-3′. Amplification was conducted for 25–33 cycles of 30 seconds each at 95°, 60° and 72°, followed by a final 10-min extension at 72°. First-strand synthesis containing each mRNA sample but no reverse transcriptase was performed to control for possible DNA contamination of mRNAs used as target for PCR amplification; failure to obtain RT-PCR products with any of the PCR amplimers confirmed the absence of contaminating DNA templates. The PCR-amplified fragments were fractionated on agarose gels. All cDNA preparations used were suitable substrates for PCR amplification on the basis of efficient amplification of a β-actin sequence. Real-time PCR was performed on an ABI 7500 (Applied Biosystems) and PCR parameters were as recommended for the TaqMan Universal PCR kit (Applied Biosystems). For analysis of IL-10, the following primers (5′-CACAAAGCAGCCTTGCAGAA-3′; 5′-GTAA GAGCAGGCAGCATAGCAG-3′) and probe (5′-FAM-AGAGAGCTCCATCATGCCTGGCT CAG-3′ BHQ-1) were used. Primers and probes for SOCS1 and SOCS3 were as described.13
Western blot analyses
Preparation of whole cell lysates was as described.11 Blots were probed with polyclonal pSTAT1- or β-actin-specific antibodies (Santa Cruz Biotechnology). Pre-immune serum was used in parallel as controls and signals were detected with horseradish peroxidase-conjugated-secondary F(ab′)2 antibodies (Zymed Laboratories, San Francisco, CA) using the enhanced chemiluminescence system (Amersham, Arlington Heights, IL).
Statistical analysis
Experiments were repeated at least twice, and usually three or more times. For EAU, disease severity for each animal was calculated as an average of both eyes. Statistical analyses were performed by independent two-tailed Student's t-test. Probability values of P ≤ 0·05 were considered statistically significant. The data, whenever applicable, were presented as mean + SD, unless otherwise specified.
Results
Retina microglia cells constitutively secrete IL-27
Th17 cells have recently been implicated in the aetiology of human uveitis3 and EAU.14 Furthermore, retinal cells were found to produce IL-27 and to limit the expansion of the Th17 cells in the retina through IL-27-dependent mechanisms.3 However, the identity of the cell types that produce this immunosuppressive cytokine in the retina is unknown. As myeloid cells are the major producers of IL-27,6 we examined whether the macrophage-like F4/80+ retina microglia cells15 are the source of IL-27 in the retina. Indeed, immunohistochemical analysis of the mouse retina using antibodies specific to the F4/80 myeloid cell marker (red) and IL-27p28 subunit (green) revealed co-localization of IL-27 and F4/80 expression (orange colour) to the ganglion cell layer, as well as to the inner nuclear layer (Fig. 1a–e).
Figure 1.
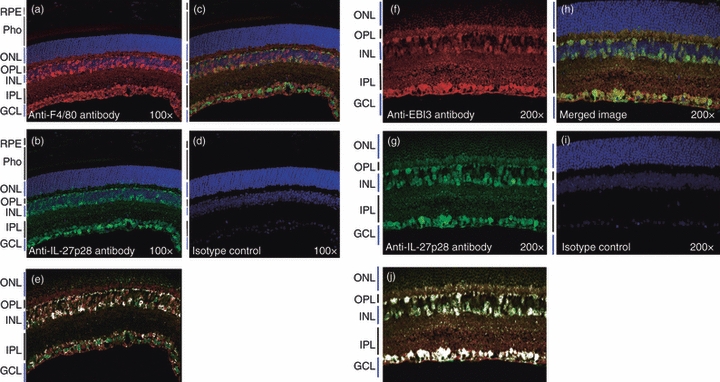
Retina microglia cells constitutively secrete interleukin-27 (IL-27). Localization of F4/80-positive cells (a; in red); IL-27p28 (b; in green) in the inner nuclear (INL) and ganglion cell (GCL) layers of the normal mouse retina. (c) Co-localization of IL-27p28 subunit of IL-27 and F4/80, a microglia cell marker in the inner nuclear and ganglion cell layers. (d, i) Isotype antibodies control. Localization of EBI3-positive cells (f; in red); IL-27p28 (g; in green) in the inner nuclear (INL) and ganglion cell (GCL) layers of the normal mouse retina. (h) Co-localization of IL-27p28 subunit of IL-27 and EBI3. Cytofluorogram delineating regions where IL-27p28- and F4/80-expressing (e) or IL-27p28- and EBI3-expressing cells co-localize in the retina (j). Retina pigmented epithelium (RPE); Photoreceptors (pho); outer nuclear layer (ONL); outer plexiform layer (OPL); inner plexiform layer (IPL). Results are representative of three independent experiments.
We also show that IL-27p28- and EBI3-expressing cells co-localize in the retina Fig. 1(f–j), suggesting that retinal microglia, as well as other cells in the retina produceIL-27. This result is in line with reports showing that brain microglial cells also produce IL-27.16,17 In view of a recent paper showing that IL-27p28 subunit binds Cytokine-Like Factor 1 (CLF-1) to produce a cytokine that is functionally similar to IL-27,18 it is possible that IL-27p28/CLF heterodimers may also contribute to the production of the IL-27-like cytokine in the retina.
Retinal cells constitutively express IL-27 receptor and IL-10
Localization of IL-27 production to F4/80+ retinal microglia led us to investigate whether retinal cells express IL-27 receptor (IL-27R). If so, to investigate whether the IL-27R is functionally responsive to IL-27 stimulation and to identify targets of IL-27 signalling in the retina. Immunohistochemical analysis of mouse eye sections revealed that the IL-27R α chain (WSX-1) is constitutively expressed in the retina and primarily localized to the photoreceptor layer (Fig. 2a). Expression of IL-27R on primary retinal cells is further confirmed by FACS analysis of freshly prepared mouse retina cells (Fig. 2b). The RT-PCR analyses further revealed that WSX-1 expression is up-regulated in the retina during EAU (Fig. 2c; top panel) and in line with our previous report,3 IL-27 expression (transcription of IL-27p28 and EBI3 genes) is up-regulated in the retina during EAU (Fig. 2c). Western blot analysis showing that STAT1 is activated by tyrosine phosphorylation in primary retinal cells in response to IL-27 (Fig. 2d) provided suggestive evidence for functional relevance of IL-27 and IL-27 receptor expression in the retina. Recent studies have shown that IL-27, through a STAT-dependent mechanism, induced IL-10-expressing T cells exhibiting IFN-γ+T-bet+Foxp3− phenotype10,19,20 and in one of these studies EAE suppression was shown to occur as a consequence of in vitro treatment of MOG-reactive cultures with IL-27 and it was suggested that the encephalitogenicity of these cells was inhibited in an IL-10-dependent manner.10 We therefore used the human retinal Müller cell line, MOI-M1, to examine whether IL-27 can also induce retinal cells to express IL-10. As indicated by the RT-PCR analysis shown in Fig. 2(e), the Müller cells expressed IL-10 in response to IL-27. As both IL-27 and IL-27R are expressed in the retina, we also examined whether IL-10 is constitutively expressed in retina. We show here by ELISA that primary retinal cells produce IL-10 in response to the inflammatory cytokine, IFN-γ (Fig. 2f). These data are in line with published data where we showed by Chip assay and RNA analysis that IFN-γ induces the expression of IL-27 in retinal cells and that Th1 cells inhibit uveitis by IFN-γ-mediated induction of IL-27 in retinal cells.3 Furthermore, immunocytochemical/confocal analysis revealed that rhodopsin-expressing primary retina cells (green) constitutively express IL-10 (red), albeit at very low levels (Fig. 2g).
Figure 2.
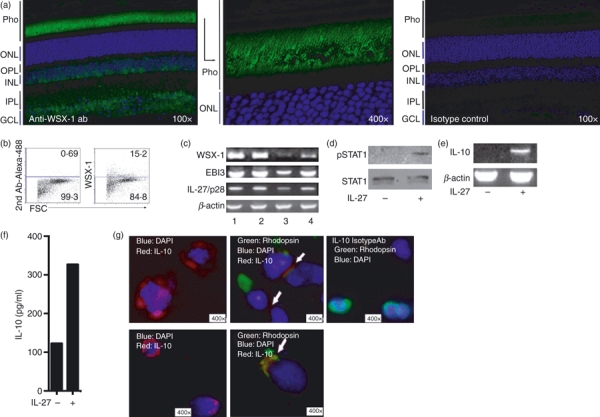
Retina cells express interleukin-27 (IL-27) receptor and produce the anti-inflammatory cytokine, IL-10, in response to IL-27. (a) Immunohistochemical localization of WSX-1 expression in mouse retina. (b) Detection of WSX-1 expression on freshly isolated mouse primary retinal cells by FACS. (c) Reverse transcription-PCR analysis of IL-27 receptor (WSX-1) and IL-27 subunits (IL-27p28 and EBI3) expression in: lymph node (lane 1); spleen (lane 2); primary retina cells (lane 3); retina cells of mouse with experimental autoimmune uveitis (EAU; lane 4). (d) Primary retina cells from WT mice were cultured for 30 min, with or without IL-27 and activation of signal transducer and activator of transcription 1 (STAT1) pathway was detected by Western blotting using pSTAT1-specific antibodies. (e) The Human Müller cell line, MOI-M1, was stimulated with IL-27 and IL-10 expression was detected by RT-PCR analysis. (f) Detection of IL-10 secretion in primary retinal cell cultures containing interferon-γ (IFN-γ). (g) Depiction of representative retinal cells expressing rhodopsin (green); DAPI (blue); IL-10 (red); white arrow indicates cells expressing both IL-10 (red) and rhodopsin (green). Results are representative of at least three independent experiments.
IL-27 up-regulates IL-10 in retina cells through activation of the STAT1 pathway
To investigate whether IL-27 regulates IL-10 expression by retinal cells through STAT1-dependent mechanisms, we cultured primary retina cells from WT and STAT1-deficient mice in medium with or without IL-27. We show here that IL-27 up-regulates expression of IL-10 in WT primary retina cells but not in retina cells from STAT1-deficient mice (Fig. 3a,b), suggesting that STAT1 is required for IL-27-induced expression of IL-10 in the retina. We further show by intracellular cytokine staining assay that primary retina cells secrete IL-10 in response to cytokines such as IL-27 and IFN-γ that activate the STAT1 pathway in retina cells (Fig. 3c). Taken together with a previous report of the requirement of STAT1 in IL-27p28 expression,3 our current finding that retina cells produce IL-10 in response to IL-27 in a STAT1-dependent manner, underscore potential involvement of Th1-induced IFN-γ/STAT1 signalling in IL-27- and IL-10-mediated immunosuppressive mechanisms in the immune-privileged retina.
Figure 3.
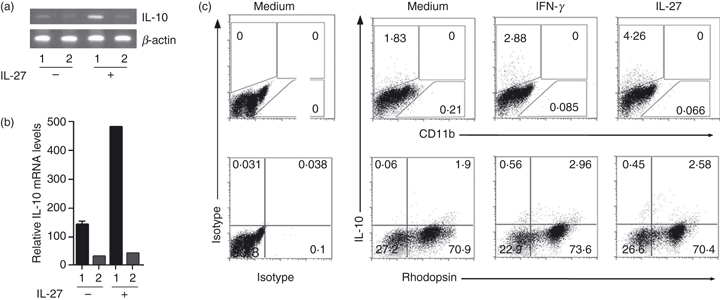
Interleukin-27 (IL-27) up-regulates IL-10 in retina cells through activation of the signal transducer and activator of transcription 1 (STAT1) pathway. Primary retina cells from wild-type (WT) (1) or STAT1-deficient (STAT1KO) (2) eyes were cultured in medium with or without IL-27 and IL-10 mRNA transcripts were detected by semi-quantitative reverse transcription-PCR (a) or real-time quantitative PCR (b). (c) Primary mouse retina cells were cultured for 16 hr in medium supplemented with interferon-γ (IFN-γ) or IL-27. FACS analysis was performed on CD11b- or rhodopsin-gated cells. Numbers in quadrants indicate percentage of retinal cells expressing IL-10 and results are representative of at least three independent experiments.
WT and STAT1KO mice produce similar levels of IL-10-secreting T cells during EAU
In our previous retina cells–T cells co-culture experiments, we showed that primary retina cells inhibited the expansion of activated Th17 through mechanisms that required both IL-27 and STAT1.3 Because the expression of IL-27 by retinal cells was also found to require STAT1,3 we hypothesized that STAT1-deficient retina cells would produce less IL-27 and IL-10 and that STAT1 knockout (STAT1KO) mice would develop a more severe EAU because of the diminution of immune protective functions normally provided by these immunosuppressive cytokines in the retina. To investigate the contribution of STAT1 in resistance or susceptibility to uveitis, we induced EAU in age-matched WT and STAT1KO mice and assessed disease severity by fundoscopy, histological analysis of the retina and flow cytometry analysis of inflammatory cells that infiltrate the retina during uveitis. Indeed, histological analysis of eye sections 14 days after immunization with IRBP revealed a more severe uveitis in STAT1KO as indicated by the massive infiltration of inflammatory cells into the STAT1KO retina compared with WT (Fig. 4a). Development of numerous massive retinal folds (indicated by arrows), a hallmark of severe uveitis, is consistent with the higher disease scores noted for the STAT1KO mice (Fig. 4b). We also observed a substantial increase of CD4+ T cells in the retina of STAT1KO compared with WT mice (Fig. 4c) and consistent with previous reports,14 Th17 cells were more numerous than Th1 in the blood and lymph nodes of STAT1KO mice (Fig. 4d). These results are consistent with reports that STAT1KO mice develop a more severe EAE compared with their WT counterparts.21,22 The recent report suggesting that IL-10-secreting T cells mediate the suppression of encephalitis in the EAE model10 persuaded us to examine the relative importance of IL-10-producing T cells in uveitis. Analysis of freshly isolated blood or LN of mice with EAU (without in vitro stimulation) revealed that WT and STAT1KO mice contained similar levels of IL-10-producing T cells (Fig. 4e). In fact, we consistently observed slight, but reproducible, increases in IL-10-producing T cells in lymph nodes of STAT1KO mice, suggesting that increased severity of EAU in the STAT1-deficient mice does not correlate with the paucity of IL-10-producing T cells (Fig. 4e).
Figure 4.
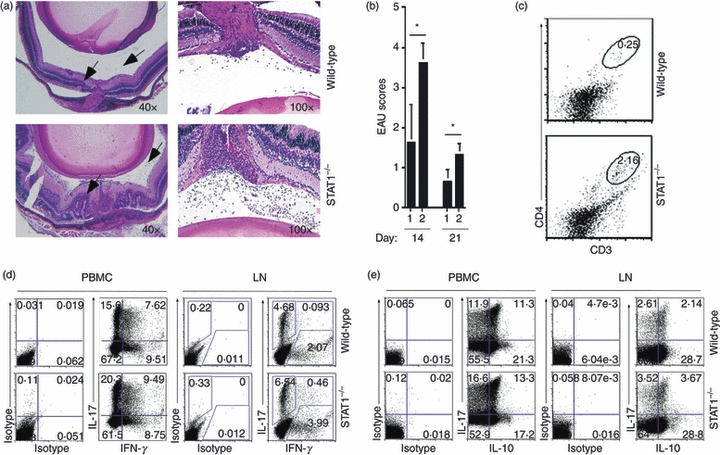
Signal transducer and activator of transcription 1 (STAT1) -deficient mice (STAT1KO) develop a more severe form of intraocular inflammation (uveitis). (a) Mice were immunized with interphotoreceptor retinoid-binding protein in complete Freund's adjuvant and their eyes were enucleated 14 or 21 days later and histological sections through the retina were stained with haematoxylin & eosin. Arrows indicate presence of inflammatory cells and depict pathological foci characterized by the presence of retinal folds and haemorrhage. (b) Clinical scores determined by fundoscopic examination and histological analysis of pathology slides of day-14 or day-21 wild-type (WT) (1) or STAT1KO (2) immunized mice. (c) CD4+ T cells present in the retina of WT or STAT1KO mice with experimental autoimmune uveitis (EAU) were detected and quantified by FACS. Numbers in the outlined areas indicate percentage of CD4+ T cells in eyes enucleated 21 days after immunization. (d, e) Freshly isolated peripheral blood mononuclear cells or lymph node cells from WT or STAT1KO mice with EAU (day-14 post-immunization) were analysed by the intracellular cytokine assay. Plots were gated on CD4+ T cells and numbers in quadrants indicate percentage of CD4+ T cells expressing interleukin-17 (IL-17) and/or interferon-γ (IFN-γ) (d) or IL-17 and/or IL-10. Results are representative of three independent experiments.
STAT1 is required for the production of IL-27 and anti-inflammatory molecules in retina
As loss of STAT1 in the CD4+ T-cell compartment had marginal effects on the level of IL-10-producing T cells, it was of interest to know whether the severe EAU in the STAT1KO mouse strain derived from defects in production of IL-27 and/or IL-10 by dendritic cell-like microglia cells that reside in the retina. In a recent study, > 50% of microglia from adult human retinal explants express CD11c.23 However, because the number of the CD11c+ cells in the mouse retina is low, we used CD11c+ cells generated from bone marrow of WT and STAT1KO mice to examine whether the secretion of IL-27 by CD11c+ microglia cells is mediated through STAT1. Phenotypic characterization of the CD11c+ cells is shown in Fig. 5(a). Analysis of cytokine production by ELISA revealed substantially reduced levels of IL-27 and IL-10 in the STAT1KO cells (Fig. 5b). We also investigated the possibility that the CD11c+ cells could directly inhibit Th17 cells without the involvement of regulatory T cells. Naive CD4+ T cells from WT mice were stimulated with anti-CD3 antibodies and their differentiation into Th1 or Th17 phenotype was evaluated in cultures that differed in the source of the co-stimulatory signals. Naive CD4+ T cells receiving co-stimulatory signals and/or soluble factors secreted by STAT1KO CD11c+ cells had a 3.9-fold increase in IL-17-expressing T cells compared with those stimulated in the presence of WT CD11+ cells (Fig. 5c). Similarly, when the WT naive CD4+ T cells were cultured with conditioned media from STAT1-deficient cells, Th17 cell differentiation was substantially elevated (compare 2.69% versus 8.53%) (Fig. 5d, left panel). In both sets of experiments, loss of STAT1 in DC affected mainly Th17 development, because equivalent percentages of Th1 cells are detected regardless of whether the T cells were primed by WT or STAT1-deficient DC (Fig. 5c) or their supernatant (Fig. 5d; left panel). These results suggest that the capacity to produce soluble factor(s) that regulate Th17 development requires STAT1. We further show that the addition of exogenous IL-27 to the conditioned medium abrogated the increase of Th17 cells induced by STAT1-deficient conditioned medium (Fig. 5d; right panel). Together, these results suggest that Th17 development and expansion in the retina or peripheral tissues may be regulated by IL-27 produced by CD11c+ innate immune cells such as microglia cells. These results are in line with a recent study showing that IL-27 inhibits the expansion of Th17 cells and required for up-regulation of IL-10 in CNS during EAE.24 The data presented here suggest that endogenous production of IL-27 and IL-10 in the retina suppresses intraocular inflammation. However, endogenous production of IL-27 and IL-10 by retinal cells may not be the sole mechanism that limits expansion of auto-aggressive T cells in this immune privileged tissue.
Figure 5.
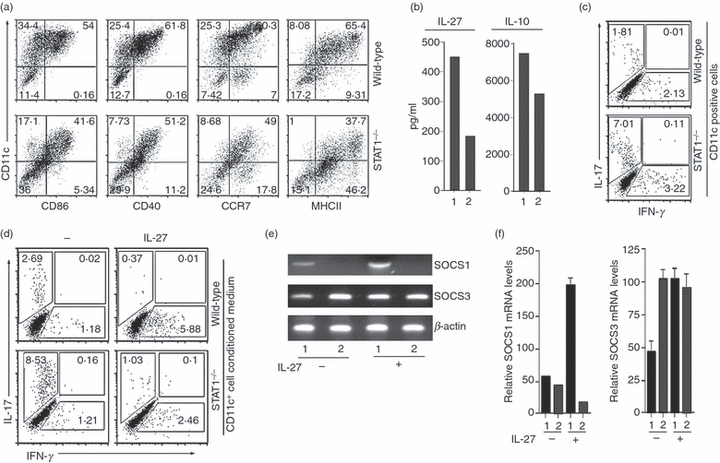
Defective production of interleukin-27 (IL-27) and IL-10 by signal transducer and activator of transcription 1 (STAT1) -deficient CD11c+ innate cells correlates with increased expansion of T helper type 17 (Th17) cells. (a) Phenotypic characterization of CD11c+ innate cells from wild-type (WT) or STAT1-deficient (STAT1KO) mice. (b) ELISA analysis of supernatant obtained from the CD11c+ innate cells of WT (1) or STAT1KO (2) mice. (c) WT naive CD4 T cells were co-cultured with CD11c+ innate cells from WT or STAT1KO mice for 4 days in medium containing anti-CD3 antibodies. (d) WT naive CD4 T cells were cultured for 4 days in medium containing anti-CD3 antibodies and supernatant from CD11c+ cells from WT or STAT1KO mice. Exogenous IL-27 was added to some cultures as indicated on the figure. IL-17- or interferon-γ (IFN-γ) -expressing T cells in the cultures were determined by intracellular cytokine staining assay and numbers in outlined areas (c, d) indicate percentage of cells expressing cell surface protein or cytokine (indicated on abscissa or ordinate). Data are representative of at least three independent experiments. (e, f) Primary retina cells from WT (1) or STAT1KO (2) retina were cultured in medium with or without IL-27. Suppressor of cytokine signalling 1 (SOCS1) or SOCS3 mRNA transcripts was detected by semi-quantitative reverse transcription-PCR (e) or real-time quantitative PCR (f). Results are representative of at least three independent experiments.
Interleukin-27 also inhibits T-cell proliferation by suppressing CD28-mediated IL-2 production through SOCS-dependent mechanisms.25 Although we previously showed that up-regulation of SOCS3 contributes to IL-27-mediated inhibition of uveitogenic T-cell expansion,3 we did not determine whether the up-regulation of SOCS expression in the retina during EAU derived from inflammatory T cells that infiltrate the retina or retinal cells. We therefore addressed this point using primary retinal cell cultures devoid of inflammatory cells. We show that primary retina cells up-regulate the expression of SOCS1 in response to IL-27 through a STAT1-dependent mechanism (Fig. 5e,f). In addition, the basal level of SOCS3 expression was higher in STAT1-deficient retina cells compared with WT cells (Fig. 5f). Elevated levels of SOCS3 in STAT1KO retina cells and the up-regulation of SOCS1 expression in response to IL-27, suggests that neuroretinal cells may contribute to mechanisms that mitigate uveitis by augmenting the levels of these anti-inflammatory molecules in the retina.
Discussion
We previously established that STAT1 is required for expression of IL-27 by retinal cells and suggested that inhibition of uveitis might be mediated, in part, through IFN-γ-induced up-regulation of IL-27 in the retina.3 In this study, we have shown that F4/80+ microglia cells distributed in the inner nuclear and ganglion cell layers of the mouse retina constitutively produce IL-27 (Fig. 1) and that expression of IL-27 is markedly elevated in the retina during intraocular inflammation or uveitis (Fig. 2c). A substantial proportion of microglia from adult human retinal explants express CD11c23 and here we provide in vivo data showing that CD11c+ cells in STAT1-deficient mice secrete markedly reduced amounts of IL-27 and this observation correlates with enhanced expansion of Th17 cells in blood and retina (Fig. 4) and development of a more severe form of uveitis by the STAT1KO mice (Fig. 4a,b). We further show that photoreceptor cells of the retina constitutively express the IL-27 receptor and respond to IL-27 signalling by up-regulating expression of the anti-inflammatory proteins, IL-10 and SOCS1. To our knowledge this is the first time that expression of the IL-27 receptor has been reported in neuronal cells. These results demonstrating a functional role of IL-27 in the neuroretina provide suggestive evidence that IL-27 may contribute to mechanisms that mitigate pathogenic autoimmune inflammation of the retina by inhibiting uveitogenic T-cell expansion in the retina.
Experimental autoimmune encephalomyelitis is another CNS inflammatory disease that shares essential immunopathogenic features with EAU and as for EAU, STAT1KO mice develop a more severe form of EAE in comparison to WT mice.21,22 A number of recent reports have shown that IL-27 induces the expansion of a population of IL-10-secreting T cells10,19,20 and this has led to the suggestion that the suppression of autoimmune inflammation of the CNS is mediated by IL-10-secreting T cells.10 However, in the EAU model, recovery from uveitis correlates with an increase in Th1 cells in the retina3,14 and endogenous systemic IFN-γ has been shown to be protective against ocular autoimmunity in mice,26,27 underscoring the potential role of Th1 cells, IFN-γ/STAT1 pathways in suppressing autoimmune inflammation in the retina. Moreover, in this study we found that STAT1KO mice develop a more severe form of EAU and contain a slightly higher level of IL-10-expressing T cells in their lymph nodes compared with WT.
Data gleaned from a study of STAT1-deficient mice and the EAU model further suggest that IFN-γ produced locally by Th1 cells may induce microglia cells to up-regulate IL-27 secretion during uveitis and IL-27-induced activation of STAT1 pathways in photoreceptor cells (possibly other retina cells) may in turn enhance the production of anti-inflammatory proteins in the retina. The data further suggest mechanisms by which the anti-inflammatory molecules might mitigate ocular inflammation and protect terminally differentiated neuronal cells from deleterious effects of pro-inflammatory cytokines produced during uveitis. First, IL-27 has also been shown to suppress the development of Th17 cells and to inhibit the expansion of effector T cells through STAT1- and SOCS3-dependent mechanisms,25,28 suggesting that the anti-inflammatory cytokines, IL-10 and IL-27, may directly inhibit the expansion of uveitogenic T cells through these mechanisms. The SOCS are a family of cytoplasmic proteins that regulate the intensity and duration of cytokine signalling and function as classical feedback regulators of cytokine activities.29,30 Interleukin-10 is a potent inducer of SOCS331–33 and SOCS proteins have recently been shown to have neuroprotective functions in the retina.34,35 Our current data showing induction of SOCS1 expression in retina cells by IL-27, suggest that in addition to inhibiting uveitogenic T-cell expansion, up-regulation of SOCS1 and SOCS3 by IL-27 and IL-10, respectively, may render retina cells unresponsive to cytokine signals, thereby conferring protection from prolonged exposure to pro-inflammatory cytokines produced in chronic intraocular inflammatory diseases.
Conclusion
In the context of treating autoimmune diseases such as uveitis, the main obstacle is that the disease usually abates after therapy but returns soon after and the patient can go through repeated cycles of remission and recurrence for many years, which may eventually result in blinding uveitis.36,37 Identifying mechanisms that can be exploited to stem the tide of new waves of Th17/Th1 cells that fuel the chronic inflammatory process is therefore of significant interest. Successful therapies for autoimmune disease must specifically inhibit pathogenic inflammation without inducing generalized immunosuppression. The over-arching conclusion drawn from this study is that inflammation in the immune-privileged retinal tissue may be controlled locally by endogenous production of IL-27 and IL-10 by retinal cells and IFN-γ/STAT1 pathways induced by Th1 cells play critical roles in enhancing IL-27 production in the retina. However, additional studies are required to firmly establish the direct in vivo role for retina-derived IL-10 in the regulation of EAU. Although production of IL-10 by T cells may also contribute to the inhibition of uveitis, it appears to have only a minor or marginal role in EAU. It is intriguing that the IL-10-producing T cells proposed to suppress encephalitis in EAE also produce IFN-γ and express T-bet but not Foxp3 (IFN-γ+ T-bet+ Foxp3−).10,19 This implies that the suppression of encephalitis may in fact derive from activation of IFN-γ/STAT1 pathways and the fact that the IFN-γ+ T-bet+ Foxp3− cells produce IL-10 may be incidental. Determination of whether inhibition of uveitis is mediated locally by cells in the retina or through the agency of infiltrating IL-10-producing T cells has important implications for therapeutic suppression of uveitis. Our data suggest that local delivery of IL-10 and/or IL-27 to retina would be more efficacious than peripheral administration of IL-10, which might risk inducing systemic immunological suppression.
Acknowledgments
We thank X. Liu, and R. M. Mahdi (Molecular Immunology Section, National Eye Institute, National Institutes of Health) for their technical assistance. We also thank R. Fariss and J. Tsai (NEI Imaging Core Unit) for assistance with confocal microscopy. This research is funded by Intramural Research Programs of the National Eye Institute and US National Institutes of Health.
Disclosures
The authors declare no financial conflicts of interests.
References
- 1.Nussenblatt RB. Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int Rev Immunol. 2002;21:273–89. doi: 10.1080/08830180212067. [DOI] [PubMed] [Google Scholar]
- 2.Nussenblatt RB. Proctor lecture. Experimental autoimmune uveitis: mechanisms of disease and clinical therapeutic indications. Invest Ophthalmol Vis Sci. 1991;32:3131–41. [PubMed] [Google Scholar]
- 3.Amadi-Obi A, Yu CR, Liu X, et al. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 4.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–8. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 5.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 6.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 7.Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–31. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 8.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev. 2005;5:521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 9.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 11.Takase H, Yu CR, Liu X, Fujimoto C, Gery I, Egwuagu CE. Induction of suppressors of cytokine signaling (SOCS) in the retina during experimental autoimmune uveitis (EAU): potential neuroprotective role of SOCS proteins. J Neuroimmunol. 2005;168:118–27. doi: 10.1016/j.jneuroim.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Jackson SH, Yu CR, Mahdi RM, Ebong S, Egwuagu CE. Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. J Immunol. 2004;172:2307–15. doi: 10.4049/jimmunol.172.4.2307. [DOI] [PubMed] [Google Scholar]
- 13.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–7. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hume DA, Perry VH, Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983;97:253–7. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–7. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Gran B, Zhang GX, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Crabe S, Guay-Giroux A, Tormo AJ, et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol. 2009;183:7692–702. doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- 19.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 20.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 21.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med. 2004;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasubramaniam B, Carter DA, Mayer EJ, Dick AD. Microglia derived IL-6 suppresses neurosphere generation from adult human retinal cell suspensions. Exp Eye Res. 2009;89:757–66. doi: 10.1016/j.exer.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–56. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 25.Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 suppresses CD28-mediated IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–80. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 26.Caspi RR, Chan CC, Grubbs BG, et al. Endogenous systemic IFN-γ has a protective role against ocular autoimmunity in mice. J Immunol. 1994;152:890–9. [PubMed] [Google Scholar]
- 27.Tarrant TK, Silver PB, Wahlsten JL, Rizzo LV, Chan CC, Wiggert B, Caspi RR. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon γ, nitric oxide, and apoptosis. J Exp Med. 1999;189:219–30. doi: 10.1084/jem.189.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–47. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 29.Hilton DJ. Negative regulators of cytokine signal transduction. Cell Mol Life Sci. 1999;55:1568–77. doi: 10.1007/s000180050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilton DJ, Richardson RT, Alexander WS, et al. Twenty proteins containing aC-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–9. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404–11. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 32.Gasperini S, Crepaldi L, Calzetti F, Gatto L, Berlato C, Bazzoni F, Yoshimura A, Cassatella MA. Interleukin-10 and cAMP-elevating agents cooperate to induce suppressor of cytokine signaling-3 via a protein kinase A-independent signal. Eur Cytokine Netw. 2002;13:47–53. [PubMed] [Google Scholar]
- 33.Matsumoto A, Seki Y, Watanabe R, et al. A role of suppressor of cytokine signaling 3 (SOCS3/CIS3/SSI3) in CD28-mediated interleukin 2 production. J Exp Med. 2003;197:425–36. doi: 10.1084/jem.20020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Mameza MG, Lee YS, Eseonu CI, Yu CR, Kang Derwent JJ, Egwuagu CE. Suppressors of cytokine-signaling proteins induce insulin resistance in the retina and promote survival of retinal cells. Diabetes. 2008;57:1651–8. doi: 10.2337/db07-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egwuagu CE, Yu CH, Mahdi RM, Mameza M, Eseonu C, Takase H, Ebong S. Cytokine-induced retinal degeneration: role of suppressors of cytokine signaling (SOCS) proteins in protection of the neuroretina. Adv Exp Med Biol. 2006;572:275–81. doi: 10.1007/0-387-32442-9_38. [DOI] [PubMed] [Google Scholar]
- 36.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–71. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 37.Nussenblatt RB, Thompson DJ, Li Z, et al. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J Autoimmun. 2003;21:283–93. doi: 10.1016/s0896-8411(03)00113-6. [DOI] [PubMed] [Google Scholar]


