Abstract
Several studies have highlighted the importance of murine natural killer (NK) cells in the control of influenza virus infection, notably through the natural cytotoxicity receptor NKp46. However, little is known about the involvement of NK cells in human influenza infection. Here, we show that upon in vitro exposure to influenza, NKp46 expression on NK cells decreases, whereas expression of 2B4, an activating receptor that can enhance natural cytotoxicity in synergy with NKp46, is up-regulated. Consistent with these observations, NKp46dull and 2B4bright NK cells had a higher functional activity in response to influenza than NK cells expressing high levels of NKp46 or low levels of 2B4, respectively. Importantly, we assessed whether the expression of these receptors was also modified in vivo in response to influenza antigens and showed that an increase in 2B4-expressing NK cells and a decrease in NKp46+ NK cells occurred following intramuscular influenza vaccination. Altogether, our results further suggest that NKp46 may play an important role in the innate immune response to human influenza and reveal that exposure to influenza antigens is associated with a previously unrecognized increase in 2B4 expression that can impact NK cell activity against the virus.
Keywords: 2B4, influenza, innate immunity, natural killer cells, NKp46
Introduction
Seasonal influenza epidemics, which result in an average of 36 000 deaths per year in the USA,1,2 and the threat of a new pandemic represent major public-health concerns. Therefore, it is essential to understand the mechanisms underlying the establishment and the maintenance of an effective immune response during influenza virus infection to improve future vaccine strategies.
Recovery from acute influenza infection and resistance to re-infection rely both on the humoral response, mostly mediated by neutralizing antibodies targeting the haemagglutinin (HA) and neuraminidase glycoproteins, and on the cellular immune response. Influenza-specific cytotoxic CD8+ T lymphocytes play a major role in directly eliminating infected cells from the respiratory tract and mostly target conserved viral proteins such as the nucleoprotein and the matrix.3,4 In addition, several studies have highlighted the early and pivotal role of innate effector cells, including natural killer (NK) cells, in the control of influenza virus infection.5–19
Before the onset of the adaptive immune response, NK cells are not only responsible for the production of antiviral cytokines but are also directly involved in the rapid elimination of virus-infected cells without the need for previous antigen sensitization. Moreover, besides their direct antiviral activity, NK cells secrete large quantities of pro-inflammatory or anti-inflammatory cytokines that directly modulate the quality of the adaptive immune response, mainly via their interaction with dendritic cells.20 The NK cells, which initially represent a substantial proportion of the lymphocyte population that resides in the healthy lungs, are further recruited to the respiratory tract within days of influenza infection,21–24 and several studies in mice have illustrated that NK cell depletion leads to augmented morbidity and mortality from infection.15,18,19 Furthermore, impaired activity of NK cells early in the infection results in delayed clearance of the virus in the lungs of mice.25,26
The activation of NK cells is tightly regulated by a balancing of activating and inhibitory signals that are integrated from a complex network of receptors expressed on the surface of the cell. In particular, recent data show that NKp46, an activating receptor expressed specifically by NK cells, predominantly mediates the lysis of influenza virus-infected cells by directly binding to the HA proteins present at the surface of the infected cells.8,9,14 While the influence of other NK cell receptors in the recognition and killing of influenza virus-infected cells cannot be ruled out, mice lacking NCR1, the murine receptor corresponding to NKp46, fail to clear the virus and do not survive infection.11 There is compelling evidence that NK cell activation in the first hours of infection is an important determinant for the disease outcome in mice. However, little is known about the role of NK cells in human influenza infection.
2B4 (CD244) is another molecule expressed at the surface of all NK cells, but also on a subset of memory CD8+αβ T cells, γδ T cells, basophils and monocytes,27–30 and has been suggested to act as a co-receptor enhancing the effect mediated by triggering receptors such as NKp46, NKp30, NKG2D and CD16.31–34 As other activating NK cell surface molecules, 2B4 is down-regulated upon engagement with its ligand CD48.35–38
Because of its previously recognized implication in the NK cell response to influenza virus, we investigated the expression of NKp46 and that of its co-activating receptor 2B4 on human NK cells following exposure to influenza, either in vitro or in vivo in individuals receiving intramuscular influenza vaccination. We show that while Nkp46 is systematically down-regulated upon engagement and NK cell activation, 2B4 expression is increased on NK cells in the presence of influenza antigens in vitro. The later up-regulation correlated with a higher expression of CD107a on 2B4bright over 2B4dull NK cells against influenza virus. Importantly, these in vitro observations were confirmed in vivo in individuals that were vaccinated with influenza virus HA, suggesting differential pathways regulating NKp46 and 2B4 expression on NK cells in the presence of viral antigens and a potential involvement of these receptors in the human innate immune response to influenza.
Materials and methods
Study subjects
In vitro influenza infection was performed on peripheral blood mononuclear cells (PBMCs) freshly isolated from 11 healthy volunteers (six women and five men, median age 24 years, range 21–47 years). Eight of the subjects reported recent (within a year) influenza vaccination. Thirteen healthy volunteers (10 women and three men, median age 31 years, range 22–57 years) were immunized intramuscularly with 0·5 ml influenza virus vaccine (Fluarix® 2008–2009 formula; GlaxoSmithKline, Dresden, Germany) containing 15 μg purified HA from each of the following inactivated virus strains: A/Brisbane/59/2007 IVR-148 (H1N1), A/Uruguay/716/2007 NYMC X-175C (H3N2) and B/Brisbane/3/2007 (influenza B virus). Three of the subjects had never received any influenza vaccine, four were at least previously immunized with the 2007–2008 influenza vaccine and six reported at least one past influenza vaccination before the 2007–2008 season. Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples taken before vaccination and then at days 1, 4, 7, 14 and 150 post-immunization. The study was approved by the MGH Institutional Review Board and each subject gave written informed consent for participation in the study.
Flow cytometric analysis of NK cell function following influenza infection
The PBMCs were isolated by Histopaque density gradient centrifugation (Sigma, St. Louis, MO). Activation of NK cells was quantified after stimulation of fresh PBMCs either with MHC class I-devoid K562 and 221 cells (American Type Culture Collection, Manassas, VA) at an effector-to-target cell ratio of 10 : 1 as previously described39 or with the A/PR/8/34 H1N1 influenza virus (Charles River Laboratories, Wilmington, MA). Influenza infection was performed by adding 5·2 × 106 infectious viral particles to 106 cells resuspended in 0·1 ml RPMI-1640 medium without serum. After 1 hr of incubation at 37° with 5% CO2, RPMI-1640 supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin was added to a final volume of 1 ml. Then, 7 μl/ml phycoerythrin-Cy5- (-PE-Cy5) conjugated CD107a antibody (BD Biosciences, Franklin Lakes, NJ) and monensin (GolgiStop; BD Biosciences) at a final concentration of 0·3 μg/ml were added immediately to all reaction tubes and the total stimulation lasted for 2, 6, 12 and 18 hr at 37° in 5% CO2. Unstimulated PBMCs were similarly treated in parallel to define the background level of degranulation and PMA/ionomycin (2·5 and 0·5 mg/ml, respectively) served as the positive control. Unstimulated PBMCs (106 cells) were placed directly in the fridge (time 0) and subsequently analysed with samples from the other time-points. Populations of NK cells were defined as lymphocytes that were CD3-negative and were further defined by their expression of CD56 and CD16 as CD56dim (CD3− CD56+ CD16+), CD56bright (CD3− CD56+ CD16−) and CD56neg (CD3− CD56− CD16+), as described elsewhere.40 Simultaneous analysis of NK cell receptors and function was performed using CD56-PE-Cy7, CD16-allophycocyanin-Cy7 (APC-Cy7), CD3-Pac Blue, 2B4-FITC and NKp46-PE antibodies (BD Biosciences). To monitor production of interferon-γ (IFN-γ), PBMCs were stained with CD56-A700, CD16-APC-Cy7, CD3-Pac Blue, 2B4-FITC and NKp46-PE antibodies (BD Biosciences), fixed, permeabilized (Invitrogen, Carlsbad, CA) and finally stained for intracellular interferon using IFN-γ-PE-Cy7 antibody (BD Biosciences). CD48 expression on different PBMC populations was monitored using CD56-PE-Cy7, CD16-APC-Cy7, CD3-Pac Blue, CD4-QD655, CD8-A700, CD14-APC and CD48-PE antibodies (BD Biosciences). Fixed cells were analysed on an LSRII system using FacsDiva version 8.8.3 (BD Biosciences). The frequency and phenotypes of NK cells were defined using FlowJo version 7.5 (Treestar, Ashland, OR).
Flow cytometric analysis of NK cell surface receptor expression following influenza vaccination
The PBMCs were isolated by Histopaque density gradient centrifugation (Sigma) and cryopreserved (10% DMSO in fetal bovine serum). To monitor changes in NK cell receptor expression, 2 million thawed PBMCs were stained using CD56-PE-Cy7, CD16-APC-Cy7, CD3-Pac Blue, 2B4-FITC and NKp46-PE antibodies (BD Biosciences). The LIVE/DEAD® Fixable Blue Dead Cell Stain kit was used before surface staining to exclude dead cells. Fixed cells were analysed on an LSRII system using FacsDiva version 8.8.3 (BD Biosciences). The frequency and phenotypes of NK cells were defined using FlowJo version 7.5 (Treestar).
Statistical analyses
The non-parametric Wilcoxon signed-rank test was used to assess differences in phenotype frequencies and functional activities, except for differences in CD48 mean fluorescence intensities at the surface of monocytes, which were calculated using two-tailed paired t-tests. Values of P < 0·05 were considered significant.
Results
NK cells up-regulate CD107a and express IFN-γ in response to the influenza virus
Accumulating evidence suggests that human NK cells may limit early influenza virus replication and mediate the establishment of an effective adaptive immune response against the virus.8,11,13–15,17–19,41 To evaluate the NK cell response to influenza virus, PBMCs were co-cultured with H1N1 influenza virus particles and NK cell activation was assessed by monitoring the expression of the degranulation marker CD107a and the production of intracellular IFN-γ. As a control, the NK cell response to 221 cells, an MHC-devoid cell line expressing NKp46 ligands, was examined in parallel. In the presence of the influenza virus, the percentage of CD107a+ NK cells significantly increased during the 12 hr following in vitro infection (P = 0·001), although the peak of NK cell functional activity in response to 221 cells was already reached after 6 hr of stimulation, as previously demonstrated39 (P = 0·002) (Fig. 1a). Consequently, expression levels of CD107a on NK cells 12 and 18 hr after exposure to influenza virus were significantly higher than those following incubation with 221 target cells at the same time-points (P = 0·001 at 12 hr and P = 0·01 at 18 hr). CD107a expression also increased over time in unstimulated PBMCs but remained significantly lower than the expression on NK cells stimulated with either H1N1 influenza viruses or 221 cells for each time-point (P≤ 0·002). As expected, the CD56dim subset of NK cells accounted for most of the CD107a expression in response to the virus [median percentage 29·4 (range 11–40·7) at 18 hr]. However, increased proportions of CD107a+ NK cells also occurred in other NK cell subsets, with the CD56neg NK cell response being higher than that mediated by CD56bright NK cells [median percentages 20 (9·2–45·8) versus 13·2 (6·1–30·6) at 18 hr, P = 0·001 between CD56dim and CD56bright]. The NK cells, and primarily CD56bright NK cells, also produced IFN-γ upon incubation in the presence of influenza virus (Fig. 1b,c). Taken together, these data demonstrate that co-culture of PBMCs and H1N1 influenza virus triggers NK cell anti-viral functions.
Figure 1.
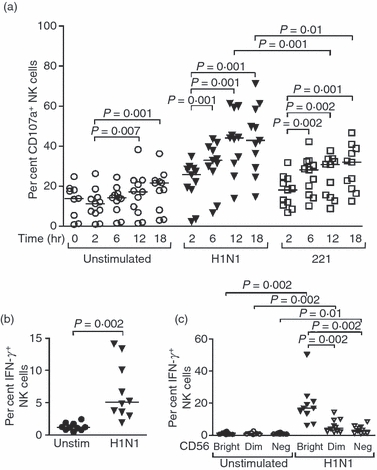
Incubation of peripheral blood mononuclear cells with the influenza virus increases natural killer (NK) cell functional activity. (a) Dot plots represent the percentage of NK cells from 11 healthy individuals expressing CD107a after 2, 6, 12 and 18 hr in the absence of stimulation or following incubation with H1N1 influenza virus or 221 cells at an effector : target ratio of 10 : 1. Displayed results were acquired from three independent experiments and pooled. (b, c) Dot plots represent the percentage of total NK cells (b) or the proportions of CD56bright, CD56dim and CD56neg NK cell subsets (c) from 10 healthy individuals producing interferon-γ (IFN-γ) after 12 hr in the absence of stimulation or following incubation with H1N1. Displayed results were acquired from four independent experiments and pooled. Horizontal lines indicate the median percentages. Differences where P < 0·05 are indicated. unstim, unstimulated.
NK cell activation involving engagement of NKp46 is associated with NKp46 down-regulation
The presence of α-2,6-linked sialic acids on the NKp46 protein allows the direct binding of the HA protein to this natural cytotoxicity receptor (NCR).8,9,14 This interaction provides a mechanism by which NK cells can specifically recognize and eliminate influenza virus-infected cells in vitro, and represents a critical antiviral process in vivo as mice lacking NKp46 are not able to control influenza infection.11,14 To further investigate the role played by this NCR in the human NK-cell response to influenza virus, we monitored changes in the expression of this particular receptor at the surface of human NK cells stimulated with the H1N1 influenza virus in vitro. Regardless of whether NK cells were activated by H1N1 or 221 cells, the increase in degranulation was associated with an overall decrease in NKp46 expression at the surface of NK cells (P = 0·001 and P = 0·01, respectively) (Fig. 2a,b). The percentages of NKp46+ NK cells also decreased following exposure to influenza virus but to a lesser extent than the mean fluorescence intensity [MFI; median differences in per cent NKp46+ NK cells: −0·36 (−6·4 to 5·1) at 2 hr, −2·05 (−7·9 to 4·4) at 6 hr, −4·75 (−16 to 5·1) at 12 hr, −3·91 (−13·9 to 2) at 18 hr; P = 0·01 at 12 hr and P = 0·02 at 18 hr]. All NK cell subsets displayed a decrease in NKp46 MFI upon influenza virus exposure [median differences in NKp46 MFI: CD56bright 540 (−1035·5 to 2304·6) at 2 hr versus −750·1 (−3387·3 to 49·3) at 18 hr, P = 0·005; CD56dim 191·9 (−162·6 to 593·9) at 2 hr versus −681·5 (−1738 to −91·5) at 18 hr, P = 0·001; CD56neg 481·4 (−27·3 to 789·3) at 2 hr versus −482·3 (−1591·5 to −96·8) at 18 hr, P = 0·001]. However, in contrast to what could be observed in the CD56dim and CD56neg NK cell subpopulations, the percentages of NKp46+ CD56bright did not decrease along with the MFI in response to the virus, consistent with the higher levels of NKp46 at the surface of unstimulated CD56bright NK cells compared with the other subsets (data not shown and Fig. 2a). To determine if different ranges of NKp46 expression led to qualitative differences in NK cell responses, we compared the functional activity of NK cells with low or no expression of NKp46 (NKp46dull) with that of NK cells expressing high amounts of NKp46 (NKp46bright) (Fig. 2c–e). Interestingly, we found that a significantly greater release of cytotoxic granules occurred in NK cells that had lost NKp46 expression, both in the presence of the influenza virus (P = 0·001, 0·001, 0·007 and 0·02 at 2, 6, 12 and 18 hr post-infection, respectively) and following incubation with 221 target cells (P = 0·02, 0·01, 0·002 and 0·002 at 2, 6, 12 and 18 hr post-infection, respectively), suggesting that NK cells that do respond to the stimulation down-modulate NKp46 (Fig. 2d). There was no significant difference in the percentages of IFN-γ+ NKp46bright and NKp46dull NK cells, but there was a trend towards higher proportions in the NKp46bright NK cell subset, which includes all the IFN-γ-producing CD56bright NK cells (Fig. 2c,e). Interestingly, NKp46 was transiently up-regulated in response to influenza virus and only started disappearing from the cell surface after 6 hr of incubation (Fig. 2a). This initial increase in NKp46 was not observed in the presence of 221 target cells, indicating that this phenomenon was specifically driven by the presence of influenza virus. The subsequent disappearance of NKp46 from the cell surface might either be triggered by a direct engagement of this receptor by influenza antigens or reflect a non-specific down-modulation of activating receptors at the surface of activated NK cells to avoid activation-induced cell death.36 To investigate whether the loss of NKp46 expression could be explained by an internalization of the receptor, we performed an intracellular staining to look at NKp46 in NK cells stimulated with H1N1 for 16 hr and found that intracellular levels of the receptor were also decreased following exposure to the virus (data not shown). Ligand-induced endocytosis of receptors is followed by recycling or degradation through the lysosomal pathway, so it cannot be ruled out that in our experimental settings, the internalized NKp46 has been degraded. Altogether, these results are in accordance with an early engagement of the NKp46 pathway upon in vitro influenza virus infection, followed by a decreased expression of this NCR at the surface of activated NK cells.
Figure 2.
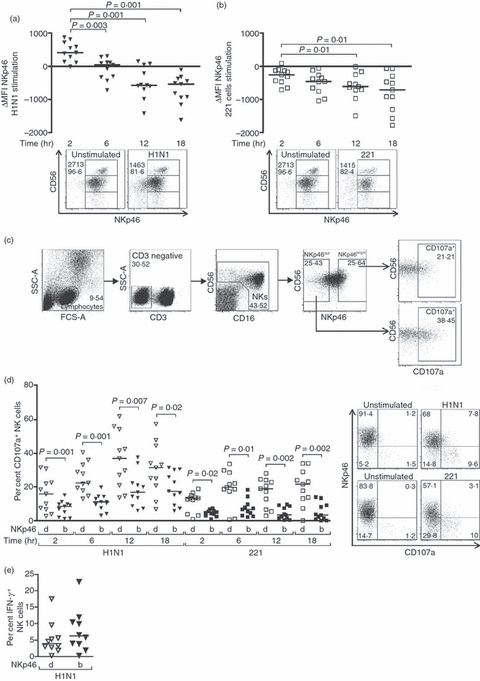
The functional activity of natural killer (NK) cells in response to influenza infection or 221 target cells is associated with a down-regulation of NKp46 surface expression. (a, b) Dot plots represent changes in NKp46 mean fluorescence intensity (MFI) on NK cells from 11 healthy donors that were stimulated with H1N1 influenza viruses (a) or 221 cells at an effector : target ratio of 10 : 1 (b) for 2, 6, 12 or 18 hr. Represented values were obtained by subtracting the NKp46 MFI of unstimulated NK cells from that of NK cells stimulated with influenza (a) or 221 cells (b). Representative primary flow panels show percentages of NKp46+ CD56bright, CD56dim and CD56neg NK cells after 12 hr in the absence of stimulation or following incubation with influenza viruses (a) or 221 cells (b). NKp46 MFI and percentages of NKp46+ bulk NK cells are indicated. (c) Gating strategy using the FlowJo software to define NKp46 negative/dull and NKp46 bright NK cells. NK cells were defined as CD3-negative lymphocytes and further characterized by their expression of CD56 and/or CD16. The gates to discriminate between NKp46dull and NKp46bright were set up so that each subpopulation consistently represented about 25% of the total NK cells and further analysed for CD107a expression, here after 12 hr of incubation with H1N1 viruses. (d) CD107a up-regulation was examined on NKp46dull and NKp46bright at 2, 6, 12 and 18 hr following incubation with the influenza virus or 221 cells. Representative primary flow panels show percentages of NKp46+ and CD107a+ NK cells in the absence of stimulation or following incubation with H1N1 viruses for 12 hr or 221 cells for 6 hr. Levels of NKp46 expression and NK cell functional activity of NK cells that were not stimulated nor incubated (time 0) were similar to those of unstimulated NK cells after 2 hr of incubation. The displayed results were acquired from three independent experiments and pooled. (e) Expression of IFN-γ in NKp46dull versus NKp46bright NK cells from 10 healthy subjects after 12 hr in the absence of stimulation or in the presence of H1N1 viruses. Horizontal lines indicate the median percentages. Differences where P < 0·05 are indicated. d, dull; b, bright.
2B4 expression at the surface of NK cells is differentially regulated upon activation with influenza virus or 221 target cells
2B4 is a member of the CD2 family expressed at the surface of all NK cells42,43 that has been proposed to function as an activating co-receptor for NKp4634 and is down-regulated upon engagement with its ligand, CD48.35,37,38,44,45 To further assess the involvement of NKp46 in the innate immune response to influenza, we checked whether the expression of 2B4 at the surface of NK cells was also affected by the virus. Surprisingly, infection with H1N1 led to an increased expression of 2B4 at the surface of NK cells (P = 0·005 and P = 0·03 at 6 and 12 hr post-infection, respectively), whereas the receptor was down-modulated upon stimulation with 221 cells as anticipated35,37,38,44,45 (P = 0·007, 0·002 and 0·002 at 6, 12 and 18 hr post-infection, respectively) (Fig. 3a,b). The percentages of 2B4+ NK cells were also affected following exposure to influenza virus [median differences in per cent 2B4+ NK cells: 1·2 (−6·3 to 13·7) at 2 hr, 2·5 (−5·1 to 8·7) at 6 hr, 5·9 (1–23·8) at 12 hr, 4·3 (−1·6 to 20·9) at 18 hr; P = 0·003 at 12 hr] or 221 cells [median differences in per cent 2B4+ NK cells: −6 (−13·8 to −0·7) at 2 hr, −22·2 (−37·8 to −6) at 6 hr, −28·7 (−41·4 to −11·2) at 12 hr, −40·9 (−55·5 to −25·7) at 18 hr; P = 0·001 at 6, 12 and 18 hr]. In the presence of influenza virus, expression of 2B4 was up-regulated on all NK cell subsets and particularly in the CD56dim and CD56neg subsets [median differences in 2B4 MFI at 12 hr: CD56bright 131·8 (42·1–1109·2); CD56dim 220·1 (93·7–392·8); CD56neg 217·8 (38·5–374·8)], whereas increases in percentages of 2B4+ NK cells were more important in the CD56bright and CD56neg subsets of NK cells [median differences in per cent 2B4+ NK cells at 12 hr: CD56bright 10·6 (1·8–31·6); CD56dim 5 (0·9–6·6), P = 0·004 versus CD56bright; CD56neg 9·9 (0·7–13·2), P = 0·03 versus CD56dim]. These observations suggest that exposure to the influenza virus may not only trigger an increase in 2B4 surface expression on NK cells but also induce the expression of this receptor on previously 2B4neg NK cells.
Figure 3.
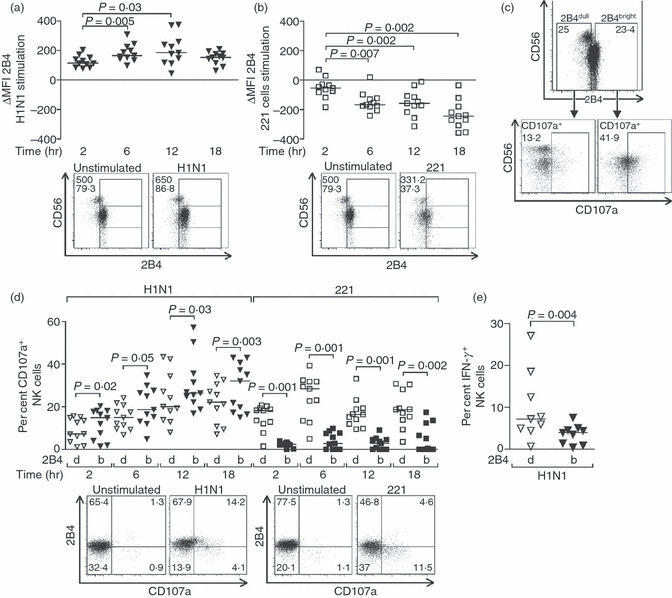
Differential regulation of 2B4 expression at the surface of natural killer (NK) cells in response to influenza and 221 target cells. (a, b) Dot plots represent the changes in 2B4 mean fluorescence intensity (MFI) on NK cells from healthy donors that were stimulated with H1N1 influenza viruses (a) or 221 cells at an effector : target ratio of 10 : 1 (b) for 2, 6, 12 or 18 hr. Represented values were obtained by subtracting the 2B4 MFI of unstimulated NK cells from the 2B4 MFI of NK cells stimulated with influenza virus (a) or 221 cells (b). Representative primary flow panels show percentages of 2B4+ CD56bright, CD56dim and CD56neg NK cells after 12 hr in the absence of stimulation or following incubation with H1N1 viruses (a) or 221 cells (b). 2B4 MFI and percentages of 2B4+ bulk NK cells are indicated. (c) NK cells were divided into 2B4dull and 2B4bright so that each subpopulation consistently represents about 25% of the total NK cells, and subsequently analysed for CD107a expression. Representative primary flow panels show CD107a up-regulation in 2B4dull and 2B4bright NK cells following 12 hr of incubation with H1N1 viruses. (d) Comparison of CD107a up-regulation in 2B4dull and 2B4bright NK cells after 2, 6, 12 and 18 hr of incubation with influenza viruses or 221 cells. Representative primary flow panels show percentages of 2B4+ and CD107a+ NK cells in the absence of stimulation or following incubation with H1N1 viruses for 12 hr or 221 cells for 6 hr. Levels of 2B4 expression and NK cell functional activity of NK cells that were not stimulated nor incubated (time 0) were similar to those of NK cells after 2 hr of incubation. Displayed results were acquired from three independent experiments and pooled. Horizontal lines indicate the median percentages. Differences where P < 0·05 are indicated. d, dull; b, bright.
We then separately analysed the expression of CD107a on NK cells bearing high (2B4bright) or low/no (2B4dull) levels of 2B4 and, in contrast to what we observed for NKp46, the functional activity of 2B4bright NK cells in response to influenza virus was more potent than that of 2B4dull (P = 0·02, 0·05, 0·03 and 0·003 at 2, 6, 12 and 18 hr post-infection, respectively), suggesting that NK cells that do respond to influenza up-regulate 2B4, whereas the killing of 221 cells was mediated by NK cells expressing low amounts of both NKp46 and 2B4 (P = 0·001, 0·001, 0·001 and 0·002 at 2, 6, 12 and 18 hr post-infection, respectively) (Fig. 3c,d). The 2B4bright NK cells produced significantly less IFN-γ than the 2B4dull NK cells (Fig. 3e). This observation is consistent with our gating strategy excluding the CD56bright NK cells (Fig. 3c), which is the subset that primarily produces IFN-γ in response to influenza virus (Fig. 1c), from the 2B4bright subpopulation.
Corroborating a function for 2B4 in the response to influenza, the expression of CD48 at the surface of monocytes, which represent the major population among PBMCs that can be infected by the virus, doubled in the presence of the virus (P = 0·02 and 0·009 at 2 and 6 hr post-infection, respectively) (Fig. 4).46–48 Interestingly, the expression of 2B4 and that of its ligand followed different kinetics as CD48 expression peaks 2 hr following infection and then decreases, whereas 2B4 MFI rose during the first 12 hr following infection. However, the decreased expression of CD48 at the surface of monocytes might have resulted from the death of influenza virus-infected cells. These data demonstrate diverging responses to influenza antigens exposure by NKp46 and 2B4, where NKp46 levels decline while 2B4 expression increases, suggesting that the expression levels of these two receptors at the surface of NK cells are differentially regulated.
Figure 4.
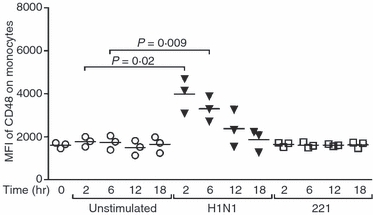
Influenza infection, but not stimulation with 221 target cells, triggers the up-regulation of CD48 at the surface of monocytes. Dot plots represent the mean fluorescence intensity (MFI) of CD48 on monocytes from three healthy donors that were unstimulated or activated with the influenza virus or 221 cells at an effector : target ratio of 10 : 1 for 2, 6, 12 and 18 hr. Horizontal lines indicate the median percentages. Differences where P < 0·05 are indicated.
NK cells expressing 2B4 expand while the proportion of NKp46+ NK cells decreases in vivo following influenza vaccination
To determine whether the influenza-driven changes in NKp46 and 2B4 expression on NK cells observed in vitro reflected in vivo changes in humans, the phenotypes of NK cells from 13 individuals were analysed immediately before intramuscular administration of the 2008–2009 seasonal influenza vaccine, composed of purified HA proteins from an H1N1, an H3N2 and an influenza B strain (Fluarix®), and then 1, 4, 7, 14 and 150 days following administration of the vaccine (Fig. 5). As shown in Fig. 5(a), there was a transient decrease in NKp46-expressing NK cells 1 day after exposure to influenza antigens (P = 0·01), with reduced proportions of NKp46+ NK cells only occurring in the CD56dim and CD56neg subsets of NK cells (Fig. 5b). In contrast, 2B4+ NK cells expanded and persisted at least throughout the first 2 weeks following influenza vaccine challenge (P = 0·0002, 0·001, 0·001, 0·004 at days 1, 4, 7 and 14 post-vaccination, respectively). The 2B4+ NK cells then significantly contracted to reach percentages that were comparable to those at baseline after 150 days (P = 0·03 and P = 0·009 compared with days 1 and 4, respectively) (Fig. 5c). Increased percentages of 2B4+ NK cells were observed in all NK cell subsets, with the CD56bright NK cells displaying the most dramatic changes compared with the other subpopulations (Fig. 5d). Hence, reduced proportions of NK cells expressing NKp46 together with an expansion of 2B4+ NK cells were observed ex vivo upon influenza vaccination, mirroring our in vitro findings. These results suggest for the first time that previous observations in the mouse model supporting a crucial and very early role of NKp46 in the response to influenza antigens might hold true in humans and reveal a previously unrecognized potential role for 2B4 in this process.
Figure 5.
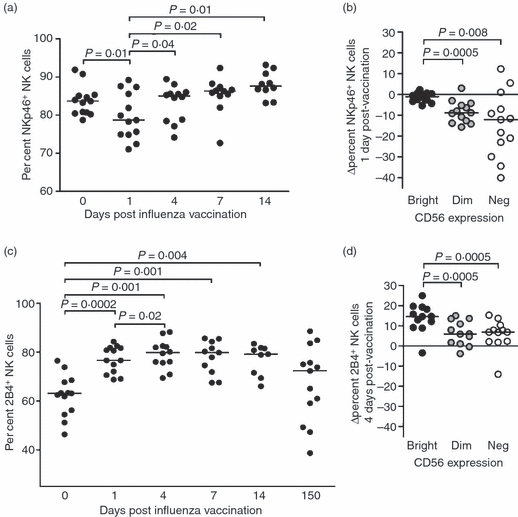
Following influenza vaccination, the proportions of NKp46+ natural killer (NK) cells temporarily decrease while NK cells expressing 2B4 expand. Thirteen healthy individuals were vaccinated intramuscularly with an inactivated influenza virus. NKp46 (a) and (b) and 2B4 (c) and (d) expression on NK cells was examined by flow cytometry using peripheral blood mononuclear cells (PBMCs) isolated and cryopreserved before vaccination and at 1, 4, 7, 14 and 150 days after vaccination. (b) Reduced percentages of NKp46+ NK cells in the CD56dim and CD56neg subsets 1 day after vaccination. (d) Increased percentages of 2B4+ NK cells in the CD56bright, CD56dim and CD56neg subsets 4 days after vaccination. Values were obtained by subtracting the percentages of NKp46+ or 2B4+ NK cells pre-vaccination from those at day 1 or 4, respectively. Horizontal lines indicate the median percentages. Differences where P < 0·05 are indicated.
Discussion
In this study, we investigated changes in the expression of two NK cell receptors in response to influenza, namely NKp46, a receptor that has been proposed to be directly involved in the elimination of influenza virus-infected cells,8,11,14 and 2B4, a receptor that efficiently co-stimulates NKp46-mediated activity.32–34 The regulation of these surface molecules was monitored over time in humans upon in vitro exposure of PBMCs to an H1N1 strain of influenza virus and following intramuscular administration of the 2008–2009 seasonal influenza vaccine. Influenza virus infection led to NK cell activation, with the functional activity being associated with a diminution of NKp46 and an increase in 2B4 surface expression. The rapid and transient decrease in NKp46+ NK cells and the long-lasting expansion of 2B4+ NK cells following in vivo exposure to influenza antigens suggest a potential involvement of these surface molecules in the innate response to influenza infection.
NKp46 has been previously identified as a key activating receptor which can interact with influenza HA, thereby triggering the NK-cell-mediated killing of influenza virus-infected cells and preventing mice from succumbing to the infection.8,11,14 Exposure of PBMCs to influenza virus first triggered an increase in NKp46 expression at the surface of human NK cells (Fig. 2a). Hence, in the earliest stages of influenza-mediated NK cell activation, the presence of the virus, and potentially a direct interaction between viral antigens and NKp46, might lead to an initial up-regulation of this NCR. Two hours following infection, the surface expression levels of NKp46 started to decline, in accordance with recently published data obtained with purified NK cells stimulated with influenza pseudotyped particles.49 The gradual decrease in NKp46 expression following in vitro exposure to the viral antigen rules out a possible blockade of NKp46 staining because of the binding of HA to the receptor, yet this possibility could account for our ex vivo observations. Upon NK cell activation, many receptors are down-regulated from the surface to prevent cell death.36 Although this process sometimes relies on a direct engagement of the receptor, internalization can also occur in a non-specific manner. The interaction between NKp46 and the influenza virus, followed by internalization of the complex might partly explain how influenza virus infects NK cells as recently reported.50,51 However, an NKp46 engagement-dependent intake into NK cells is not required for the virus to propagate because influenza virus infection of NK cells is not productive, yet activation-induced down-modulation of NKp46 might be a mechanism exploited by the virus to dampen the innate immune response by inducing NK cell death. Further experiments will have to be conducted to determine whether the internalization of NKp46 is elicited by a general NK cell activation or depends on a specific interaction with influenza antigens.
While NKp46 was down-regulated upon incubation of NK cells with either influenza virus or MHC-devoid target cells, 2B4 expression decreased in the presence of 221 cells but significantly increased in the presence of the virus. This augmentation of 2B4 expression could be the result of the release of cytokines, including interleukin-12 (IL-12), secreted by dendritic cells, and IL-2, as the production of this cytokine by influenza-specific CD4+ T cells has been shown to account for most of the NK cell response upon stimulation of PBMCs with the influenza virus.41 As a result, an elevated surface density of 2B4 might enhance NK cell-mediated killing of influenza virus-infected cells in cooperation with other activating receptors. We assessed whether the release of IL-2 and/or IL-12 upon exposure to influenza virus was responsible for the observed changes in 2B4 and NKp46 expression or NK cell activation by adding antibodies directed against IL-2 and/or IL-12. Blocking these cytokines did not significantly alter the above-mentioned changes in NKp46, 2B4, CD69 and CD107a expression on NK cells in response to influenza virus (data not shown). However, the addition of 100 U/ml of IL-2 alone triggered a slight up-regulation of 2B4 as well as a slight down-modulation of NKp46. These observations suggest that at least in our in vitro settings, IL-2 and IL-12 do not play a major role in the NK cell functional response to influenza virus. Alternatively, an excess of 2B4 at the surface might have an inhibitory effect on NK cell cytotoxic functions, as it has been suggested that 2B4 can deliver inhibitory signals when the surface density of this receptor is high.52 Influenza infection also led to an augmented expression of CD48, the ligand for 2B4, at the surface of NK cells, monocytes and lymphocytes (Fig. 4 and data not shown). A shift towards NK cells with an inhibitory phenotype triggered by influenza virus is therefore possible, as extensive engagement of 2B4 with CD48 on adjacent NK cells or other haematopoietic cells has been shown to negatively impact the nature of 2B4 signalling.52
To examine the expression of NKp46 and 2B4 at the surface of NK cells in response to influenza virus in vivo, 13 individuals vaccinated with purified HA were followed longitudinally. We first showed a transient diminution in the percentage of NKp46+ NK cells 1 day post-vaccination (Fig. 5). NKp46 down-regulation following influenza antigens exposure has been previously described in a different model of in vitro infection,49 yet this is the first time that a decrease in NKp46+ NK cells has been reported while characterizing peripheral blood NK cells from patients receiving influenza vaccination. NKp46 expression was not altered in another longitudinal study examining the phenotype of NK cells in individuals immunized against influenza.53 However, in this paper, the expression of various surface receptors was first monitored 1 week after vaccination, at a time when NKp46 levels had returned to baseline in the samples we analysed, explaining the discrepancy between our conclusions. Although it is well established that NKp46 is down-regulated from the surface of activated NK cells in vitro, the reduced percentages of peripheral blood NK cells expressing NKp46 might also reflect a recruitment of these particular cells to other compartments.
We furthermore observed increased percentages of 2B4+ NK cells persisting for at least 2 weeks following administration of the viral HA. The long-lasting presence of high percentages of NK cells expressing 2B4 is intriguing. In that respect, it would be interesting to investigate whether NK cells post-vaccination present a more potent cytotoxic response than NK cells before vaccination when re-challenged with influenza antigens. Importantly, the phenotypic changes observed in vivo may not directly relate with those occurring in vitro as many factors other than the presence of influenza antigens can impact peripheral NK cell receptor expression, including systemic cytokine secretion, recruitment of NK cells in other tissues, or selective proliferation of NK cell subpopulations. Also, in addition to the fact that in vitro NK cells are more likely to make direct contact with influenza virus-infected cells, there are substantial differences in the immune response to the live virus and to the inactivated viral antigen. Therefore, studies exploring the functional activity of NK cells exposed to the inactivated HA in vitro and in patients with acute influenza infection are warranted to further evaluate the role played by 2B4 and NKp46 in the response to the virus that is suggested by our data. NKp46 blockade has been previously shown to impair the NK cell response to influenza virus, including CD69 expression,10,49 IFN-γ production10 and lysis of influenza virus-infected cell lines,8,14 whereas 2B4 blockade alone did not alter NK cell function in one study involving NK cells and influenza virus-infected dendritic cells.10 Although these studies suggest an important role for NKp46 in the NK cell response to influenza virus, it remains to be determined whether blockade of several receptors simultaneously, including NKp46 and 2B4, is required to achieve a complete inhibition of NK cell function against influenza virus. We assessed expression of CD69, CD107a and IFN-γ by NK cells in response to influenza virus in the presence of F(ab′)2 fragments of 2B4 and/or NKp46 blocking antibodies (C1.7 and 9E2 clones, respectively) to exclude the possibility of redirected lysis mediated by intact IgG1 antibodies. CD69 expression (per cent CD69+ NK cells and CD69 MFI) was remarkably inhibited on NK cells when both receptors were blocked, whereas only a slight decrease was observed in the presence of either 2B4 or NKp46 F(ab′)2 fragments alone (data not shown). In contrast, 2B4 and/or NKp46 blockade did not significantly affect CD107a up-regulation. Altogether these results suggest that 2B4 and Nkp46 are involved in NK cell activation in response to influenza virus but that signalling through additional yet-to-be-determined receptors may be necessary to trigger NK cell antiviral functions. Production of IFN-γ was very low in this particular experiment and was not modulated upon blockade of the NK cell activating receptors. CD69 but not CD107a expression was also found to be inhibited in a previous study from Du et al.49 assessing the impact of NKp46 blockade on NK cell activation in response to influenza pseudo-particles.
Overall, influenza exposure is associated with changes in NK cell receptor expression that predominantly result in the accumulation of 2B4+ NK cells and in a transient decline in NKp46+ NK cells. While an influenza-specific effect on NKp46 expression can be questioned, as the removal of this receptor from the surface has been detected following NK cell activation with stimuli as diverse as MHC-I devoid target cell lines, hepatitis C virus, HIV or influenza virus infection (data not shown), this is the first report showing a differential regulation of 2B4 on NK cells upon stimulation with influenza virus antigens. Therefore, it will be important to elucidate whether cytokines, other viruses or other vaccines known to be immunogenic can similarly affect 2B4 expression on NK cells and to investigate the nature of the signal delivered by 2B4 on influenza virus-activated NK cells.
Finally, mechanistic studies will be required to link the observed phenotypic changes with a biological implication of 2B4 and NKp46 in the control of influenza infection. The results of such studies could contribute to the design of future therapeutic interventions that would integrate components targeting specific innate receptors to harness NK cell function and enhance immune control of influenza infection.
Acknowledgments
We are grateful to the patients and the healthy volunteers for their blood donations, and to Dr Abraham Louis Brass and Bethany Ryan for providing antibodies and technical support to determine the infectivity titers of the influenza virus stock. We thank Dr Golo Ahlenstiel for technical advice for in vitro influenza infection and Mike Waring and Andrew Cosgrove for excellent assistance with flow cytometry. This work was supported by the Doris Duke Charitable Foundation (M.A.), the Philip T. and Susan M. Ragon Foundation (M.A.), the Swiss National Science Foundation (S.J.) and the MGH ECOR Fund for Medical Discovery (S.J.).
Disclosures
The authors have no financial or conflicts of interests to disclose.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Weintraub E, Dhankhar P, Cheng PY, Brammer L, Meltzer MI, Bresee JS, Shay DK. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respi Viruses. 2009;3:37–49. doi: 10.1111/j.1750-2659.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–17. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achdout H, Arnon TI, Markel G, et al. Enhanced recognition of human NK receptors after influenza virus infection. J Immunol. 2003;171:915–23. doi: 10.4049/jimmunol.171.2.915. [DOI] [PubMed] [Google Scholar]
- 6.Achdout H, Manaster I, Mandelboim O. Influenza virus infection augments NK cell inhibition through reorganization of major histocompatibility complex class I proteins. J Virol. 2008;82:8030–7. doi: 10.1128/JVI.00870-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlenstiel G, Martin MP, Gao X, Carrington M, Rehermann B. Distinct KIR/HLA compound genotypes affect the kinetics of human antiviral natural killer cell responses. J Clin Invest. 2008;118:1017–26. doi: 10.1172/JCI32400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnon TI, Achdout H, Lieberman N, et al. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:664–72. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 9.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–9. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Draghi M, Pashine A, Sanjanwala B, et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178:2688–98. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 11.Gazit R, Gruda R, Elboim M, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–23. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 12.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- 13.Kos FJ, Engleman EG. Role of natural killer cells in the generation of influenza virus-specific cytotoxic T cells. Cell Immunol. 1996;173:1–6. doi: 10.1006/cimm.1996.0245. [DOI] [PubMed] [Google Scholar]
- 14.Mandelboim O, Lieberman N, Lev M, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–60. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 15.Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev. 2008;129:223–30. doi: 10.1016/j.mad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Owen RE, Yamada E, Thompson CI, et al. Alterations in receptor binding properties of recent human influenza H3N2 viruses are associated with reduced natural killer cell lysis of infected cells. J Virol. 2007;81:11170–8. doi: 10.1128/JVI.01217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siren J, Sareneva T, Pirhonen J, Strengell M, Veckman V, Julkunen I, Matikainen S. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J Gen Virol. 2004;85:2357–64. doi: 10.1099/vir.0.80105-0. [DOI] [PubMed] [Google Scholar]
- 18.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136:1435–41. [PubMed] [Google Scholar]
- 19.Stein-Streilein J, Guffee J, Fan W. Locally and systemically derived natural killer cells participate in defense against intranasally inoculated influenza virus. Reg Immunol. 1988;1:100–5. [PubMed] [Google Scholar]
- 20.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Ennis FA, Meager A, Beare AS, Qi YH, Riley D, Schwarz G, Schild GC, Rook AH. Interferon induction and increased natural killer-cell activity in influenza infections in man. Lancet. 1981;2:891–3. doi: 10.1016/s0140-6736(81)91390-8. [DOI] [PubMed] [Google Scholar]
- 22.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds CW, Timonen T, Herberman RB. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981;127:282–7. [PubMed] [Google Scholar]
- 24.Stein-Streilein J, Bennett M, Mann D, Kumar V. Natural killer cells in mouse lung: surface phenotype, target preference, and response to local influenza virus infection. J Immunol. 1983;131:2699–704. [PubMed] [Google Scholar]
- 25.Dong L, Mori I, Hossain MJ, Kimura Y. The senescence-accelerated mouse shows aging-related defects in cellular but not humoral immunity against influenza virus infection. J Infect Dis. 2000;182:391–6. doi: 10.1086/315727. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Mori I, Hossain MJ, Dong L, Takeda K, Kimura Y. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J Gen Virol. 2004;85:423–8. doi: 10.1099/vir.0.19596-0. [DOI] [PubMed] [Google Scholar]
- 27.Garni-Wagner BA, Purohit A, Mathew PA, Bennett M, Kumar V. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J Immunol. 1993;151:60–70. [PubMed] [Google Scholar]
- 28.Nakajima H, Cella M, Langen H, Friedlein A, Colonna M. Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur J Immunol. 1999;29:1676–83. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Schuhmachers G, Ariizumi K, Mathew PA, Bennett M, Kumar V, Takashima A. 2B4, a new member of the immunoglobulin gene superfamily, is expressed on murine dendritic epidermal T cells and plays a functional role in their killing of skin tumors. J Invest Dermatol. 1995;105:592–6. doi: 10.1111/1523-1747.ep12323533. [DOI] [PubMed] [Google Scholar]
- 30.Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178:1397–406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assarsson E, Kambayashi T, Persson CM, Chambers BJ, Ljunggren HG. 2B4/CD48-mediated regulation of lymphocyte activation and function. J Immunol. 2005;175:2045–9. doi: 10.4049/jimmunol.175.4.2045. [DOI] [PubMed] [Google Scholar]
- 32.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–66. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–66. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sivori S, Parolini S, Falco M, Marcenaro E, Biassoni R, Bottino C, Moretta L, Moretta A. 2B4 functions as a co-receptor in human NK cell activation. Eur J Immunol. 2000;30:787–93. doi: 10.1002/1521-4141(200003)30:3<787::AID-IMMU787>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 35.Chuang SS, Kim MH, Johnson LA, Albertsson P, Kitson RP, Nannmark U, Goldfarb RH, Mathew PA. 2B4 stimulation of YT cells induces natural killer cell cytolytic function and invasiveness. Immunology. 2000;100:378–83. doi: 10.1046/j.1365-2567.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev. 2008;8:259–68. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathew SO, Vaidya SV, Kim JR, Mathew PA. Human natural killer cell receptor 2B4 (CD244) down-regulates its own expression by reduced promoter activity at an Ets element. Biochem Biophys Res Commun. 2007;355:483–7. doi: 10.1016/j.bbrc.2007.01.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandusky MM, Messmer B, Watzl C. Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur J Immunol. 2006;36:3268–76. doi: 10.1002/eji.200636146. [DOI] [PubMed] [Google Scholar]
- 39.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–9. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 41.He XS, Draghi M, Mahmood K, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. 2004;114:1812–9. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boles KS, Nakajima H, Colonna M, Chuang SS, Stepp SE, Bennett M, Kumar V, Mathew PA. Molecular characterization of a novel human natural killer cell receptor homologous to mouse 2B4. Tissue Antigens. 1999;54:27–34. doi: 10.1034/j.1399-0039.1999.540103.x. [DOI] [PubMed] [Google Scholar]
- 43.Mathew PA, Garni-Wagner BA, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J Immunol. 1993;151:5328–37. [PubMed] [Google Scholar]
- 44.Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998;188:2083–90. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latchman Y, McKay PF, Reiser H. Identification of the 2B4 molecule as a counter-receptor for CD48. J Immunol. 1998;161:5809–12. [PubMed] [Google Scholar]
- 46.Bender A, Amann U, Jager R, Nain M, Gemsa D. Effect of granulocyte/macrophage colony-stimulating factor on human monocytes infected with influenza A virus. Enhancement of virus replication, cytokine release, and cytotoxicity. J Immunol. 1993;151:5416–24. [PubMed] [Google Scholar]
- 47.Fesq H, Bacher M, Nain M, Gemsa D. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology. 1994;190:175–82. doi: 10.1016/S0171-2985(11)80292-5. [DOI] [PubMed] [Google Scholar]
- 48.Nain M, Hinder F, Gong JH, Schmidt A, Bender A, Sprenger H, Gemsa D. Tumor necrosis factor-alpha production of influenza A virus-infected macrophages and potentiating effect of lipopolysaccharides. J Immunol. 1990;145:1921–8. [PubMed] [Google Scholar]
- 49.Du N, Zhou J, Lin X, Zhang Y, Yang X, Wang Y, Shu Y. Differential activation of NK cells by influenza A pseudotype H5N1 and 1918 and 2009 pandemic H1N1 viruses. J Virol. 2010;84:7822–31. doi: 10.1128/JVI.00069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo H, Kumar P, Moran TM, Garcia-Sastre A, Zhou Y, Malarkannan S. The functional impairment of natural killer cells during influenza virus infection. Immunol Cell Biol. 2009;87:579–89. doi: 10.1038/icb.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao H, Tu W, Qin G, et al. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J Virol. 2009;83:9215–22. doi: 10.1128/JVI.00805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) J Immunol. 2008;180:8159–67. doi: 10.4049/jimmunol.180.12.8159. [DOI] [PubMed] [Google Scholar]
- 53.Long BR, Michaelsson J, Loo CP, et al. Elevated frequency of gamma interferon-producing NK cells in healthy adults vaccinated against influenza virus. Clin Vaccine Immunol. 2008;15:120–30. doi: 10.1128/CVI.00357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


