Abstract
The post-Golgi trafficking of rhodopsin in photoreceptor cells is mediated by rhodopsin-bearing transport carriers (RTCs) and regulated by the small GTPase rab8. In this work, we took a combined pharmacological-proteomic approach to uncover new regulators of RTC trafficking toward the specialized light-sensitive organelle, the rod outer segment (ROS). We perturbed phospholipid synthesis by activating phospholipase D with sphingosine 1-phosphate (S1P) or inhibiting phosphatidic acid phosphohydrolase by propranolol (Ppl). S1P stimulated the overall rate of membrane trafficking toward the ROS. Ppl stimulated budding of RTCs, but blocked membrane delivery to the ROS. Ppl caused accumulation of RTCs in the vicinity of the fusion sites, suggesting a defect in tethering, similar to the previously described phenotype of the rab8T22N mutant. Proteomic analysis of RTCs accumulated upon Ppl treatment showed a significant decrease in phosphatidylinositol-4,5-bisphosphate–binding proteins ezrin and/or moesin. Ppl induced redistribution of moesin, actin and the small GTPase rac1 from RTCs into the cytosol. By confocal microscopy, ezrin/moesin and rac1 colocalized with rab8 on RTCs at the sites of their fusion with the plasma membrane; however, this distribution was lost upon Ppl treatment. Our data suggest that in photoreceptors phosphatidylinositol-4,5-bisphosphate, moesin, actin, and rac1 act in concert with rab8 to regulate tethering and fusion of RTCs. Consequentially, they are necessary for rhodopsin-laden membrane delivery to the ROS, thus controlling the critical steps in the biogenesis of the light-detecting organelle.
INTRODUCTION
Intracellular trafficking and maintenance of organelle integrity are highly regulated by the interplay of protein–lipid interactions. Changes in lipid composition lead to the recruitment and activation of proteins that, in turn, modify lipid composition to change these interactions (for recent reviews, see Cullen et al., 2001; Simonsen et al., 2001; De Matteis et al., 2002). Lipid microdomains, also called rafts, organize macromolecular complexes involved in membrane trafficking and signaling (Simons and Ikonen, 1997; Anderson and Jacobson, 2002). Membrane budding from the Golgi complexisregulatedthroughthesynthesisofphosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] and the activation of phospholipase D (PLD) by small GTPase ADP-ribosylation factor (ARF) (Liscovitch and Cantley, 1995). Activation of Golgi-associated PLD by ARF results in the hydrolysis of phosphatidylcholine (PC) to form phosphatidic acid (PA). PA, which is also generated by the acylation of lysophosphatidic acid (Weigert et al., 1999), is a fission-promoting lipid (Kozlov, 2001), which regulates Golgi structure and post-Golgi membrane budding from the trans-Golgi network (TGN) (Siddhanta et al., 2000). Some of the effects of PA are mediated trough the activation of type I PI(4)P(5)OH kinase and the synthesis of PI(4,5)P2 (Moritz et al., 1992).
In the Golgi, synthesis of PI(4,5)P2 and its precursor PI(4)P is regulated by ARF through the recruitment and activation of PI(4)OH kinase-β and type I PI(4)P(5)OH kinase (Godi et al., 1999; Jones et al., 2000). PI(4)P controls membrane exit from the Golgi (De Matteis et al., 2002). PI(4,5)P2 activates ARF directly (Randazzo, 1997) and acts as a PLD cofactor that potentiates the stimulatory effect of ARF on PLD (Liscovitch and Cantley, 1995). This causes a positive feedback loop that creates rapid changes in lipid composition and leads to the formation of microdomains enriched in PI(4,5)P2 and PA (Liscovitch and Cantley, 1995). These microdomains recruit regulatory proteins, such as GTPase dynamin, and through PI(4,5)P2-binding protein spectrin, cause local rearrangement of the actin-based Golgi membrane skeleton, which is believed to facilitate post-Golgi membrane budding (De Matteis and Morrow, 2000; Lorra and Huttner, 1999). Hydrolysis of PI(4,5)P2, and the consequent inhibition of PLD, may be a signal to terminate the positive feedback loop that regulates membrane budding.
Diacylglycerol (DAG), and a phosphoinositide (PI)-modifying enzyme phosphatidylinositol 3 kinase, also play a critical role in the regulation of membrane traffic (Simonsen et al., 2001; Baron and Malhotra, 2002). Availability of PI is controlled by PI/PC transfer protein, which was found to be essential for post-Golgi membrane budding (Ohashi et al., 1995; Jones et al., 1998; Simon et al., 1998).
Membrane trafficking in retinal photoreceptor cells involves synthesis, sorting, and transport, through the rod inner segments (RIS), of prodigious quantity of rhodopsinladen membranes. They continuously renew specialized organelles filled with light-sensitive disk membranes, the rod outer segments (ROS). We have characterized rhodopsin-bearing transport carriers (RTCs), and uncovered several regulators of rhodopsin trafficking through the post-Golgi compartment, including the small GTPase rab8 (Deretic and Papermaster, 1991; Deretic et al., 1995, 1998; Deretic, 1997; Moritz et al., 2001). We identified a phospholipid-binding protein that associates with RTCs, evectin-1, (Krappa et al., 1999), which contains a phosphoinositide-binding PH domain recently reported to preferentially bind PI(3,4,5)P3 and PI(3,4,)P2 (Dowler et al., 2000). These phosphoinositides are also responsible for the recruitment of multifunctional regulators of small GTPases, ARAP proteins (Miura et al., 2002), suggesting a potential involvement of evectin in protein–lipid complexes that regulate membrane trafficking.
RTCs cotransport rhodopsin and newly synthesized phospholipids, particularly PC and phosphatidylethanolamine (PE), enriched in docosahexaenoic acid (DHA, 22:6n-3) to the ROS (Rodriguez de Turco et al., 1997). DHA is taken up from the interphotoreceptor matrix by the RIS where DHA-phospholipids are synthesized and subsequently delivered to the ROS (Rodriguez de Turco et al., 1994). DHA represents ∼50% of the ROS disk membrane phospholipid acyl chains (Fliesler and Anderson, 1983). It is essential for the exquisite ROS membrane fluidity that facilitates rapid conformational changes and lateral mobility of rhodopsin upon light activation. We previously reported a relative enrichment of TGN membranes with newly synthesized DHA-PI (Rodriguez de Turco et al., 1997), which suggests an important role for DHA-phospholipids in RTC budding.
To uncover new regulators of RTC trafficking, we pharmacologically modified phospholipid biosynthesis with propranolol (Ppl), or sphingosine 1-phosphate (S1P), and monitored their effects on synthesis and trafficking of DHA-phospholipids, budding of RTCs, and membrane delivery to the ROS. Ppl increases the production of acidic phospholipids by inhibiting PA phosphohydrolase, and the subsequent production of DAG, PC, PE, and triacylglycerides (Truett et al., 1992; Bazan and Rodriguez de Turco, 1994). S1P increases PA levels by PLD activation (Desai et al., 1992). S1P is an intracellular second messenger and an extracellular ligand for the EDG family of G protein-coupled receptors (S1PRs) (Spiegel and Milstien, 2002). S1P can also be translocated across the membrane bilayer by a translocator protein, possibly an ATP-binding cassette transporter (van Meer and Lisman, 2002). Using Ppl and S1P, we found that modifications of phospholipid biosynthesis differentially affected budding and fusion of RTCs in retinal photoreceptors. These differences give indications of how these processes may be regulated.
MATERIALS AND METHODS
Pulse-Chase Labeling and Retinal Subcellular Fractionation
Southern leopard frogs, Rana berlandieri, were dark adapted for 2 h before the experiment. Isolated frog retinas were preincubated for 1 h at 22°C in oxygenated medium with 0.5 mM Ppl or with 10 μM S1P. Retinas were incubated for 1 h with [35S]-Express protein labeling mixture (25 μCi/retina) and [3H]DHA (5.7 μCi/retina, final concentration 0.24 μM) as described previously (Rodriguez de Turco et al., 1997), followed by 2.5-h chase. Ppl or S1P where present throughout the pulse and the chase.
Retinal fractionation, ROS isolation, and preparation of postnuclear supernatant (PNS) enriched in photoreceptor biosynthetic membranes were performed as described previously (Deretic and Papermaster, 1991). PNS was centrifuged at 17,500 × g in JA25.5 rotor (Beckman-Coulter, Fullerton, CA) for 10 min at 4°C to sediment large biosynthetic membranes (LBMs) (Morel et al., 2000). Supernatant (∼3 ml in 0.25 M sucrose) was loaded on the step sucrose gradient containing 2.1 M and 20% sucrose. Gradients were centrifuged for 90 min at 100,000 × gav in a SW 40 rotor (Beckman-Coulter) at 4°C. Small carrier membranes (SCMs) were collected from the 20%/2.1 M sucrose interface, diluted with 10 mM Tris acetate, pH 7.4, and centrifuged at 336,000 × gav for 30 min in a 70.1 Ti rotor (Beckman-Coulter). Pelleted LBMs and SCMs were resuspended in 10 mM Tris acetate, pH 7.4. Cytosol was recovered from the top of the step gradient in 0.25 M sucrose and clarified by centrifugation at 336,000 × gav.
In some experiments, LBMs and SCMs were further fractionated on linear 20–39% (wt/wt) sucrose gradients. For this fractionation, SCMs were collected from the step gradients in ∼1.5 ml and diluted with an equal volume of 10 mM Tris acetate, pH 7.4, as described previously (Morel et al., 2000), or with an equal volume of 0.25 M sucrose and loaded on top of linear sucrose gradients. After centrifugation at 100,000 × gav in a SW 40 rotor for 15 h at 4°C, 14 fractions were collected from the top of the gradient. Subcellular fraction pools were created as described previously (Morel et al., 2000) diluted with 10 mM Tris acetate, pH 7.4, and processed as described above.
In Vitro Labeling of Inositol Lipids and Inositol Phosphates
Isolated retinas were labeled for 3 h with myo-[2-[3H]inositol (7 μCi/retina) at 22°C in oxygenated medium. After two washes with the medium containing 5 mM inositol, one set of retinas was frozen immediately, to determine the basal levels of [3H]inositol, phosphoinositides, and inositol phosphates. The remaining retinas were incubated for 10 min in the medium containing 10 mM LiCl to inhibit dephosphorylation of inositol phosphates (Rodriguez de Turco et al., 1992). Retinas were then incubated in the presence or absence of 0.5 mM Ppl for 2 h, or for 4.5 h, in the constant presence of 10 mM LiCl. After the incubation, retinas were snap-frozen in liquid N2. The analysis of radiolabeled inositol phosphates and inositol phospholipids was performed as described previously (Rodriguez de Turco et al., 1992).
Lipid Extraction and Analysis
Lipids were extracted with hexane:isopropanol (3:2, vol/vol), dried under nitrogen, and resuspended in a small volume of hexane:isopropanol. Aliquots were taken to determine total radioactivity and to analyze and quantify total lipid-fatty acids by gas liquid chromatography (GLC) as described previously (Rodriguez de Turco et al., 1997). Individual phospholipids and neutral lipids were isolated by two-dimensional, three step thin layer chromatography in 10 × 10 cm high performance thin layer chromatographic plates (Analtech, Newark, DE) (Rodriguez de Turco et al., 1997). The plates were sprayed with primuline in acetone/water (80%), and lipid spots were visualized under UV light. To determine radioactivity, lipid spots were scraped off into vials and counted in a Beckman-Coulter scintillation counter. To determine the fatty acid content and composition of individual lipids, lipid spots were scraped off into screw-cap tubes and lipids were derivatized to fatty acid methyl esters for GLC analysis (Rodriguez de Turco et al., 1997).
SDS-PAGE and Immunoblotting
Radiolabeled proteins were analyzed by SDS-PAGE. Dried SDS gels were subjected to quantitative analysis of 35S-rhodopsin distribution among retinal subcellular fractions in a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The images of the radiolabeled proteins were generated by autoradiography at -85°C by using Kodak BioMax MR film. Gels were stained by Phast Gel Blue R (Amersham Biosciences, Piscataway, NJ) or with the silver staining kit (SilverQuest; Invitrogen, Carlsbad, CA). Imaging of stained gels was performed with a model GS-700 imaging densitometer (Bio-Rad, Hercules, CA). Gels were also blotted onto Immobilon-P membranes as described previously (Deretic et al., 1995). Blots were probed with the following antibodies: polyclonal anti-rab6 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-calnexin (StressGen Biotechnologies, San Diego, CA), and anti-actin (Sigma-Aldrich, St. Louis, MO) diluted 1:200; monoclonal anti-ARF3, anti-moesin, anti-rho-GDI, anti-rho, anti-rac1, and anti-Na,K-ATPase β2 (BD Biosciences, San Jose, CA) diluted 1:100. Bound antibodies were detected using a chemiluminescent Western lightning immunodetection system (PerkinElmer Life Sciences, Boston, MA). The distribution of detected antigens was quantified using Multianalyst software (Bio-Rad).
Protein Identification by Tandem Mass Spectrometry
Protein identification was performed at the Harvard Microchemistry Facility by microcapillary reverse-phase high-performance liquid chromatography nano-electrospray tandem mass spectrometry on a Finnigan LCQ DECA quadrupole ion trap mass spectrometer. The collision-induced dissociation spectra were correlated with known sequences by using Sequest software (Eng et al., 1994) and programs developed at Harvard Microchemistry Facility.
Electron Microscopy
After 4.5 h of incubation with S1P, Ppl, or in the control media, retinas were fixed in 4% formaldehyde and 1% glutaraldehyde in 0.12 M cacodylate buffer, pH 7.5, for 1 h at 22°C, postfixed in OsO4, and embedded in Epon. Retinas were also embedded in LR gold and labeled with anti-rhodopsin 11D5 monoclonal antibody (mAb) (Deretic and Papermaster, 1991). Bound antibody was detected by protein A Polygold (15 nm) (Polysciences, Warrington, PA). Thin sections were stained with uranyl acetate and lead citrate and examined in a Philips CM-100 transmission electron microscope. For quantitative analysis, a grid of lines was placed on the micrographs over the ellipsoid areas of RIS-containing RTCs (Figure 1), and the surface density of RTCs (SvRTC) was calculated from the number of intersections of membranes labeled with anti-rhodopsin mAb 11D5 with the index lines of known length (Weibel and Bolender, 1973).
Figure 1.
(A) Diagram of the photoreceptor cell. Biosynthetic organelles are localized in the RIS, in the myoid region. Rhodopsin-bearing post-Golgi transport carriers (RTCs) traverse the ellipsoid region filled with mitochondria and fuse with the RIS plasma membrane only in the proximity of the connecting cilium (C), in the periciliary region. Newly synthesized membranes are then delivered to the ROS. (B) Experimental scheme for retinal labeling and subcellular fractionation (Morel et al., 2000). Retinas are preincubated with S1P or Ppl and membrane trafficking toward the ROS is monitored by redistribution of radiolabeled proteins and lipids from LBMs (containing ER, Golgi, and the TGN), through SCMs (containing predominantly RTCs), to the ROS.
Confocal Microscopy
Confocal microscopy was performed on dark-adapted frog retinas as described previously (Deretic et al., 1995). Retinas fixed with 4% paraformaldehyde were embedded in 5% agarose and blocks were sectioned at 100-μm thickness on a Vibratome (Technical Products International, St. Louis, MO). Sections were labeled overnight at 4°C with monoclonal anti-moesin or anti-rac1 (dill 1:100), followed by an overnight incubation with Cy3 goat anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA) and Alexa Fluor 488-phalloidin (1:30; Molecular Probes, Eugene, OR). For colocalization experiments, sections were labeled with monoclonal anti-moesin or anti-rac1 (dill 1:100), followed by Cy2 goat anti-mouse IgG (1:200). This was followed by an overnight incubation with anti-rab8 (1:100; BD Biosciences), which was detected with Cy3 goat anti-mouse IgG (1:200). Alternatively, anti-rab8 was detected with Cy2 goat anti-mouse IgG, whereas anti-moesin or anti-rac1 was detected with Cy3 goat anti-mouse IgG. All sections were incubated for 10 min at room temperature with nuclear counterstain TO-PRO-3 (1:1000; Molecular Probes). Confocal optical sections at 0.5-μm intervals were obtained in a 510 laser scanning confocal microscope (Carl Zeiss, Jena, Germany) by using a 488-nm argon ion laser for Alexa Fluor 488 and Cy2, and 543- and 633-nm HeNe lasers for Cy3 and TO-PRO-3 excitation, respectively. Digital images were prepared using Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA).
RESULTS
S1P Stimulates the Uptake and Esterification of DHA
To reveal new regulators of rhodopsin trafficking, we increased the synthesis of acidic phospholipids in the retina with Ppl or S1P (Desai et al., 1992; Bazan and Rodriguez de Turco, 1994). The results of our preliminary experiments showed that de novo synthesis of DHA-phospholipids in the presence of phospholipid-modifying agents is an excellent indicator of total phospholipid turnover, therefore [3H]DHA could be used to monitor phospholipid metabolism.
Rhodopsin is the major protein synthesized by photoreceptor cells. After a 1-h pulse and a 2.5-h chase, 35S-rhodopsin is found in the TGN and in the RTCs, before it reaches the ROS (Deretic and Papermaster, 1991; Deretic et al., 1998; Morel et al., 2000). Therefore, after 1-h preincubation with 0.5 mM Ppl or with 10 μM S1P, we used this previously established pulse-chase labeling protocol to label lipids and proteins in transit trough the post-Golgi compartment. Isolated frog retinas were incubated with 35S-labeled amino acids and [3H]DHA, as described previously (Rodriguez de Turco et al., 1997). After the chase, retinal subcellular fractionation was performed as described previously (Morel et al., 2000). After the removal of ROS, radiolabeled retinal PNS was fractionated into LBMs and SCMs, as schematically represented in Figure 1. Detailed analysis, previously reported by Morel et al. (2000) revealed that LBM fractions contain endoplasmic reticulum (ER), Golgi, and the TGN, whereas SCM fractions contain predominantly, but not exclusively, RTCs (Morel et al., 2000). In the present study, we fractionated radiolabeled retinas into LBMs, SCMs, and ROS and monitored the effects of phospholipid-modifying drugs on the distribution of radiolabeled lipids and proteins among these fractions reflecting membrane trafficking toward the ROS.
First, we found that a significant proportion of [3H]DHA was esterified in LBMs and SCMs after a 2.5-h chase, whereas ROS contained mostly unesterified DHA at that time, consistent with the previous findings that the synthesis of DHA-phospholipids takes place in the RIS (Rodriguez de Turco et al., 1994). S1P significantly stimulated [3H]DHA uptake and esterification (286 ± 33 dpm/mmol fatty acid in control LBMs vs. 436 ± 60 with S1P; four separate experiments). Ppl had no significant effect on these processes. SCM fraction had the highest specific activity in all experimental conditions (647 ± 14 dpm/mmol fatty acid in control SCMs vs. 286 ± 33 in control LBMs, and 58 ± 6 in control ROS), indicating that RTCs, a major component of the SCM fraction, actively incorporated newly synthesized DHA-phospholipids.
Both S1P and Ppl Increase the Synthesis of Acidic Phospholipids, but Ppl Inhibits, Whereas S1P Stimulates the Delivery of Major Phospholipids to the ROS
We next examined the effects of S1P and Ppl on the biosynthesis of acidic phospholipids. S1P stimulated PA synthesis and caused increase in the PI production in LBMs, as shown in Figure 2A. The increase in PI(4)P and PI(4,5)P2 synthesis was greater than the increase in PI, suggesting that the stimulation of PLD activated the positive feedback loop that elevates local concentration of PI(4,5)P2 and PA. The increase in polyphosphoinositide production was even more prominent in the SCM fraction (Figure 2A). Ppl caused a 10-fold increase in the [3H]DHA PA content of LBMs and SCMs (Figure 2A). This was accompanied by the modest increase in PI and PI(4)P, but not in PI(4,5)P2. High level of PA in Ppl-treated retinas is expected to stimulate synthesis of PI(4,5)P2, yet no increase was observed. However, specific activity of DAG was significantly higher than in the control cells. Because Ppl inhibits direct production of DAG from PA, this suggests that DHA-DAG accumulated as a product of increased PI(4,5)P2 metabolism. This increase seems to be significant because specific activity of [3H]DHA-DAG was >10-fold higher than that of [3H]DHA-PI(4,5)P2. Therefore, our data suggest that during a 4.5-h incubation Ppl stimulated the production of PI(4,5)P2, but that concomitant stimulation of PLC led to rapid hydrolysis of the newly synthesized PI(4,5)P2. Ppl can stimulate the phospoinositide cycle by acting on phospholipase C (PLC) (Bazan et al., 1985), or by elevating PA, which also stimulates PLC isozymes (Sekiya et al., 1999; Litosch, 2000). To test whether the 4.5-h incubation with Ppl stimulated the phosphoinositide cycle, we labeled frog retinas with [3H]inositol and examined the polyphosphoinositide metabolism. As shown in Figure 2B, Ppl stimulated the production of [3H]inositol phospholipids, as already observed with [3H]DHA incorporation. During a 2-h incubation with Ppl, the production of inositol phosphates was lower than in the control retinas; however, it increased significantly over that of the control (p < 0.05) during a 4.5-h incubation (Figure 2B). These data confirm and extend the previous observation (Bazan et al., 1985) that the increased polyphosphoinositide degradation is at least partially responsible for the decreased levels of PI(4,5)P2 observed in Ppl-treated retinas.
Figure 2.
(A) S1P and Ppl differentially affect phospholipid biosynthesis and trafficking in retinal photoreceptors. Results are expressed as the ratio of the specific activity of phospholipids isolated from the drug treated retinas to that of the control retinas. Data represent the mean ± SEM of four independent experiments. S1P stimulated biosynthesis and trafficking of all phospholipids with particular increase in PI(4,5)P2 synthesis in the SCM fraction. Ppl increased PA production by 10-fold in LBM and SCM fractions and nearly completely inhibited synthesis and trafficking of PC. (B) Synthesis of phosphoinositides (PIs) and inositol phosphates (IPs) in Ppl-treated retinas was compared with the control after a 2 h, or a 4.5-h incubation. Results are expressed as in A.
As expected, Ppl profoundly inhibited synthesis of PC, PE, and triacylglycerides (Figure 2A). The production of PC was inhibited by ∼80%, whereas PE was less affected, probably due in part to its direct synthesis from phosphatidylserine (PS). It is important to note that Ppl-induced cellular effects are reversible, both in the retina and in HeLa cells (Ilincheta de Boschero and Bazan, 1983; Baron and Malhotra, 2002). We also determined that the effects of Ppl, which is a β-adrenergic receptor antagonist, were not caused by the changes in cAMP in photoreceptor cells, because control retinas contained 6.58 ± 0.69 pmol of cAMP/mg protein versus 6.97 ± 1.50 in propranolol-treated retinas (three separate experiments).
The effects of S1P and Ppl on the arrival of newly synthesized phospholipids to the ROS were distinctly different. S1P clearly increased the delivery of [3H]DHA-phospholipids to the ROS, whereas Ppl profoundly inhibited trafficking of all major phospholipids, including PC, PE, and, surprisingly, PI (Figure 2A). Although the PA content of ROS was higher in Ppl-treated retinas, frog ROS did not convert PA to PI, because they lack the necessary enzymes and depend on the RIS for the newly synthesized PI (Choe et al., 1990). PE and PS seemed to be less affected by Ppl than PC. Higher than expected specific activity of [3H]DHA PE and PS is likely due to the higher percentage of dipolyunsaturated species in these phospholipids (Choe and Anderson, 1990).
Ppl Stimulates Budding of RTCs but Inhibits Their Delivery to the ROS
To determine the effects of phospholipid modifications on rhodopsin trafficking to the ROS, we first examined the effect of the drugs on protein synthesis. The total protein labeling in the S1P-treated retinas remained the same as in control cells (352 ± 59 × 103 cpm in control vs. 349 ± 36 × 103 cpm with S1P; four separate experiments), but in Ppl-treated cells, protein synthesis was reduced by 50% (174 ± 33 × 103 cpm). Subcellular distribution of radiolabeled proteins is shown in Figure 3. Coomassie-stained SDS-PAGE indicates that LBM fractions contain the majority of proteins. SCM fractions have lower protein content, and a protein composition that greatly resembles that of isolated RTCs (Deretic and Papermaster, 1991; Morel et al., 2000). The autoradiogram of this gel (Figure 3) shows an increase in radiolabeled rhodopsin content in SCMs after S1P and Ppl treatment, suggesting that both drugs stimulated budding of RTCs from the TGN. At this time, a small amount of 35S-rhodopsin reached the ROS in control and S1P-treated cells, whereas a negligible amount of radiolabeled protein was detected in ROS from Ppl-treated retinas.
Figure 3.
The delivery of radiolabeled rhodopsin to the ROS is stimulated by S1P and blocked by Ppl. Top, fractions from control (C), S1P-(S), or Ppl (P)-treated retinas were subjected to SDS-PAGE and autoradiography. Radiolabeled membranes in LBM and SCM fractions were isolated from one retina, whereas ROS fractions were from one quarter of a retina. Rhodopsin (arrow) is the major protein of the ROS and the major newly synthesized protein in photoreceptor cells. Its specific activity after the chase is the highest in the SCM fraction containing RTCs. Bottom, effects of S1P and Ppl on rhodopsin trafficking. Subcellular distribution of radiolabeled rhodopsin was determined by PhosphorImager analysis of SDS-PAGE, and expressed as the percentage of total. Data represent the mean ± SEM of four independent experiments (2.5-h chase), or the mean ± range of two separate experiments (19-h chase).
Quantitative analysis of the distribution of newly synthesized rhodopsin among subcellular fractions confirms that in control retinas the labeling of RTCs precedes that of ROS (Deretic and Papermaster, 1991). Although they constitute only a minor fraction of the total cellular membrane, the RTCs found in the SCM fraction contained ∼30%, whereas the ROS contained ∼5% of the total radiolabeled rhodopsin after a 2.5-h chase (Figure 3). S1P and Ppl further increased radiolabeled rhodopsin content of SCMs. A fraction of radiolabeled rhodopsin that reached the ROS after a 2.5-h chase in control cells was very small as observed previously; however, S1P caused a slight increase and Ppl caused a decrease in rhodopsin delivery (Figure 3). This decrease is smaller than the increase in radiolabeled rhodopsin content of SCMs, suggesting that after a 2.5-h chase in Ppl-treated cells the major contribution in newly synthesized rhodopsin in SCM fraction comes from the increased budding of RTCs. To further define the effect of Ppl on membrane delivery to the ROS, we performed a 19-h chase to allow a larger fraction of newly synthesized rhodopsin to reach the ROS. After 19-h chase, ∼40% of radiolabeled rhodopsin arrived to the ROS in control cells. S1P stimulated, whereas Ppl nearly completely inhibited rhodopsin delivery to the ROS. The amount of radiolabeled rhodopsin present in the ROS after 19 h did not increase over that found after a 2.5-h chase, indicating that the prolonged exposure to Ppl completely blocked rhodopsin trafficking to the ROS. Rhodopsin most likely accumulated in RTCs because SCMs in Ppl-treated retinas already had almost 40% of radiolabeled rhodopsin after a 2.5-h chase, RTC budding continued throughout the 19-h chase, and the fusion with the plasma membrane did not occur (see below). We also measured the [3H]DHA-phospholipid delivery to ROS after the 19-h chase. We found that S1P stimulated the delivery of all [3H]DHA phospholipids to the ROS by >50% and [3H]PC by >80%. Ppl caused near complete inhibition of the PC delivery and further reduction, over that seen after 2.5 h, in the delivery of PE, PS, and DAG (our unpublished data). Near complete inhibition of the delivery of rhodopsin (Figure 3) and PC (Figure 2A; our unpublished data) suggests that Ppl causes a block in membrane trafficking to the ROS.
In Ppl-treated Cells, Enlarged RTCs Bud from the TGN and Accumulate at the Site of Fusion
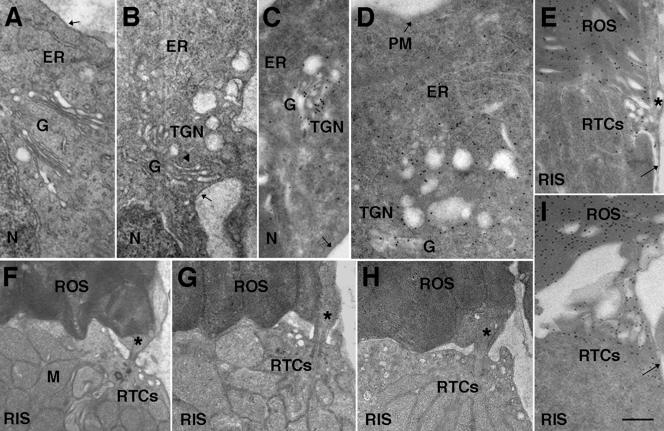
Our subcellular fractionation data indicate that Ppl stimulates budding of RTCs but inhibits membrane delivery to the ROS. To localize budded RTCs in photoreceptor cells treated with Ppl, we first examined Epon-embedded retinas by electron microscopy (Figure 4). Ppl shows dramatic effects on cell morphology. Enlarged vesicles bud from the TGN (arrowhead in Figure 4B) and accumulate in its proximity, whereas the morphology of the Golgi and the ER appears similar to that of the control cell (Figure 4A). The vesicular structures in Ppl-treated retinas are much larger than the biosynthetic organelles normally observed in the untreated RIS (Papermaster et al., 1985; Deretic and Papermaster, 1991). To determine the nature of these vesicles, we performed immunocytochemistry of LR-gold–embedded retinas with the mAb 11D5 to the C-terminal of rhodopsin (Deretic and Papermaster, 1991). Anti-rhodopsin antibody labeled Golgi, TGN, and the vesicular structures in its vicinity (Figure 4, C and D), thus establishing that Ppl-induced budded profiles are enlarged RTCs.
Figure 4.
In Ppl-treated retinas enlarged RTCs bud from the TGN and accumulate near the fusion sites. (A and B) Thin sections of Epon-embedded retinas through the myoid region of control (A) and Ppl-treated (B) photoreceptors. (C and D) Anti-rhodopsin C-terminal mAb 11D5 labeling showing the presence of rhodopsin in the Golgi (G), the TGN, and in the enlarged budded profiles, but not in the RIS plasma membrane (arrows) in Ppl-treated retinas. (E) Periciliary RTCs and ROS membranes are labeled with anti-rhodopsin in control retinas. (F–H) RTCs in control, S1P-treated, or Ppl-treated retinas, respectively. Note the accumulation of RTCs in the proximity of the cilium (*) and the RIS plasma membrane in Ppl-treated retinas. (I) RTCs accumulated in Ppl-treated retinas are labeled with anti-rhodopsin. N, nucleus, M, mitochondria, arrows denote the RIS plasma membrane. Bar, 0.5 μm.
In the frog retina, RTCs are regularly observed in the proximity of the connecting cilium, at the exclusive site where the fusion with the RIS plasma membrane occurs, before membrane delivery to the ROS (Papermaster et al., 1985; Deretic and Papermaster, 1991). We examined the periciliary region and the ellipsoid (see Figure 1), of Epon-embedded control retinas (Figure 4F), and retinas treated with S1P (Figure 4G), and found them to be indistinguishable, both displaying several vesicles consistent with RTCs. Their identity was confirmed by anti-rhodopsin immunolabeling (Figure 4E). By contrast, the number of vesicular structures in Ppl-treated retinas far exceeded that seen in control photoreceptors, or in photoreceptors treated with S1P, suggesting that these are accumulated RTCs that are unable to dock at the specialized fusion site (Figure 4H). Anti-rhodopsin immunolabeling showed that they were heavily labeled, indicating that the newly synthesized rhodopsin was accumulating in these RTCs, instead of being delivered to the ROS (Figure 4I). To evaluate this interpretation we determined the surface density (Weibel and Bolender, 1973) of RTCs (SvRTC), a measure of surface occupied by RTCs in a volume of cell cytoplasm in the ellipsoid and the periciliary region. SvRTC in Ppl-treated retinas was significantly higher than in control retinas: 0.70 ± 0.1/μm versus 0.39 ± 0.1, respectively (n = 11, p < 0.05). On longer exposure to Ppl (19 h), the number of accumulated RTCs further increased, but changes in the Golgi morphology could also be detected (our unpublished data).
Notably, the RIS plasma membrane was devoid of label both in control (Figure 4E, arrow) and in Ppl-treated retinas (Figure 4C, D, and I; arrows). Because rhodopsin is normally not found in the RIS plasma membrane (Papermaster et al., 1985; Deretic and Papermaster, 1991), this suggests that Ppl did not cause noncognate fusion of RTCs and mislocalization of rhodopsin to the plasma membrane. Accumulation of RTCs in the proximity of the RIS plasma membrane, near the fusion sites, suggests that their tethering is disrupted by Ppl treatment. This type of tethering defect and RTC accumulation has been observed only in transgenic frogs expressing a mutant of the small GTPase rab8 (rab8T22N), deficient in GTP-binding (Moritz et al., 2001).
Our biochemical and electron microscopy (EM) analysis shows that Ppl affects more than one step in photoreceptor membrane trafficking because 1) very large carriers containing rhodopsin accumulate in the vicinity of the TGN, 2) the increase in radiolabeled rhodopsin content of SCMs is larger than the decrease in the delivery to the ROS, 3) the surface density of RTCs in the vicinity of the fusion site is significantly higher than in control retinas, and 4) newly synthesized rhodopsin and newly synthesized phospholipids are not delivered to the ROS. Together, these data suggest that the most sensitive steps in rhodopsin trafficking affected by Ppl-mediated phospholipid modulation involve RTC budding, tethering, and fusion.
TGN Membranes Show the Greatest Increase in Acidic Phospholipids and the Smallest Decrease in PC and PE in Ppl-treated Cells
Enlarged RTCs seen in the vicinity of the TGN in Ppl-treated retinas suggest that the local increase in acidic phospholipids in the TGN contributes to increased membrane budding. To test whether this increase was actually in the TGN, we fractionated LBM fractions on linear sucrose gradients and determined the distribution of radiolabeled rhodopsin and [3H]DHA-phospholipids among subcellular fractions. As reported previously (Morel et al., 2000), after a 2.5-h chase, rhodopsin in LBM fractions was mostly found in the Golgi and in the TGN, enriched in rab6 and ARF3 (Figure 5). ER membranes, as revealed by anti-calnexin labeling, distributed throughout most of the LBM fractions. The greatest Ppl-mediated increase in PA, PI, and PS was observed in the TGN-enriched fraction, suggesting that it could account for the increased budding of RTCs. There was no unique increase in DAG production in the TGN compared with the other fractions, suggesting that these membranes did not preferentially recruit PLC to hydrolyze newly synthesized PI(4,5)P2. Interestingly, this fraction also showed the smallest decrease in [3H]DHA-PC and PE. These data are consistent with the specific lipid remodeling during post-Golgi membrane budding from the TGN where newly synthesized PC and PE are actively incorporated into RTCs.
Figure 5.
In Ppl-treated retinas TGN-enriched fractions show the greatest increase in PA and the smallest decrease in PC and PE. Newly synthesized rhodopsin is revealed by autoradiography, whereas ER, Golgi, and TGN markers are revealed by immunoblotting. The majority of newly synthesized rhodopsin recovered from LBM fractions after 2.5-h chase is in the Golgi and in the TGN. These fractions also show the greatest increase in newly synthesized PA and the smallest decrease in PC and PE.
Ppl Causes Subtle Changes in Total Phospholipid Composition of RTCs
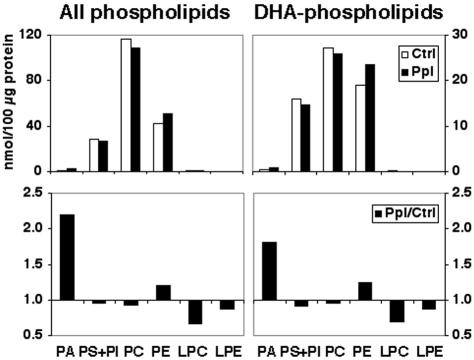
The accumulation of RTCs around the cilium in Ppl-treated retinas suggests that these membranes may have impaired capacity for fusion. A significant increase in the contribution of acidic phospholipids and the decrease in PC and PE caused by Ppl could affect the fusion capability of these RTCs. The decrease in PC synthesis may be particularly important, because one previous study reported accumulation of vesiculated membranes in photoreceptor cells after inhibition of PC synthesis in the retina (Pu and Masland, 1984). To determine the extent to which a 4.5-h treatment with Ppl modifies the phospholipid composition of SCMs, we analyzed their total lipid composition by GLC. As shown in Figure 6, Ppl caused subtle changes in the composition of major phospholipids of SCMs. In particular, the total PC content was decreased by ∼5%. DHA-phospholipids reflected similar changes (Figure 6). This suggests that the changes in PC content are unlikely to cause the formation of abnormal RTCs. Ppl caused an increase in PA, and a decrease in lysophosphatidylcholine (LPC) content of SCMs. Although the total amount of LPC in SCMs (and therefore RTCs) is low, the decrease caused by Ppl may be sufficient to alter the membrane curvature of RTCs, as was observed by the EM analysis (Figure 4, B–D). Likewise, the total content of PA is very low, but a twofold increase caused by Ppl may be sufficient to induce membrane curvature unfavorable for fusion, or changes in the recruitment and activity of regulatory proteins. We also measured total DAG levels in the control and Ppl treated retinas and found no significant difference in the total (14.9 ± 1.2 vs. 15.3 ± 0.3 nmol/100 μg of protein) or DHA-DAG content (5.6 ± 0.5 vs. 5.7 ± 0.1 nmol/100 μg of protein).
Figure 6.
Total phospholipid composition of SCMs from Ppl-treated retinas is not dramatically different from the control. GLC analysis shows that PS, PI, PC, and PE are the major phospholipids of SCMs. Ppl causes only subtle changes in their total composition, and this is true for all phospholipids as well as DHA-phospholipids. The greatest increase is seen in the amount of PA and the greatest decrease in the amount of LPC in these membranes.
Ppl Causes Redistribution of Ezrin/Moesin from the RTCs and the RIS Plasma Membrane into the Cytosol
Several components of the machinery involved in RTC trafficking have been identified so far (Deretic, 1997; Morel et al., 2000; Moritz et al., 2001). We determined their distribution in Ppl-treated cells in search for the potential fusion regulators affected by phospholipid modulation. We probed Ppl-treated RTCs for known soluble N-ethylmaleimide-sensitive factor attachment protein receptors SNARES and rab GTPases and their regulators. Syntaxin 6, rab8, and rab11 GTPases were present on control, as well as on Ppl-treated RTCs (our unpublished data). The membrane association of NSF, one of the components of the membrane-tethering complex, did not change, which suggests that the soluble N-ethylmaleimide-sensitive factor attachment protein receptor priming was unaffected (our unpublished data). Loss of fusion competence of RTCs could also be caused by the retention of, or failure to recruit coat proteins, which would be accompanied by changes in membrane association of ARF3. We found that control SCMs contained <1% of total membrane-bound ARF3, and this did not change in Ppl-treated cells. Because ARF3 is associated with the photoreceptor Golgi membranes (Figure 5; Morel et al., 2000), these data also suggest that Golgi fragmentation is not the source of the increase in the radiolabeled rhodopsin content of SCMs in Ppl-treated retinas. To confirm that the resident Golgi proteins are retained during RTC budding in Ppl-treated cells, we also measured their galactosyltransferase activity as described previously (Deretic and Papermaster, 1991). We found ∼10% of the total galactosyltransferase activity in RTCs from the control retinas, as previously (Deretic and Papermaster, 1991; Deretic et al., 1998), and this was not different in Ppl-treated retinas (12.1 ± 1.9 × 10-6 pmol UDP gal/min in control vs. 13.7 ± 1.9 × 10-6 in Ppl-treated retinas; n = 3). Therefore, a 4.5-h incubation with Ppl did not cause Golgi fragmentation, or the release of Golgi enzymes during RTC budding.
We next wanted to test whether Ppl treatment caused differential membrane recruitment of phospholipid-binding proteins. We first tested whether Ppl affected RTC recruitment of phosphoinositide-binding protein evectin-1 (Krappa et al., 1999) and found no significant effect (our unpublished data). We then carefully examined silver-stained SDS-PAGE for proteomic changes in SCMs after Ppl treatment (Figure 7A). Although proteins indicated with A and C in Figure 7A seemed to be more abundant, protein B was nearly absent from Ppl-treated membranes. Sequence analysis by mass spectrometry of protein B excised from the control sample revealed four tryptic fragments of moesin (accession no. U29763) and two of ezrin (accession no. A34400) (Figure 7A). Ezrin and moesin have high sequence homology, and together with radixin form the ezrin/radixin/moesin (ERM) family of proteins that regulate interactions of membrane proteins and the cytoskeleton (Bretscher et al., 2002). Membrane association of ERM proteins is regulated by PI(4,5)P2 through their FERM (four-point one, ezrin, radixin, moesin) phosphoinositide-binding domains (Bretscher et al., 2002). Thus, it is conceivable that Ppl-induced stimulation of PLC at specific sites could lead to rapid local hydrolysis of PI(4,5)P2, destabilize PI(4,5)P2-containing microdomains, and cause limited dissociation of ERM proteins from those membranes. To test this possibility, we immunoblotted SCMs with a mAb to the C-terminal peptide of moesin. This peptide sequence is identical in all ERM proteins; therefore, immunoblotting should reveal all three members of this family. As shown in Figure 7A, we found a significant reduction in anti-moesin immunoreactivity of Ppl-treated SCMs.
Figure 7.
Changes in the SCM proteome caused by Ppl include ezrin/moesin, actin, rho-GDI and rac1. (A) A region of silver stained SDS-PAGE showing the absence of protein B in SCMs isolated from Ppl-treated retinas. Tandem mass spectrometry analysis of this band from the control sample revealed the presence of moesin and ezrin. Immunoblotting of SCMs with an anti-moesin antibody (which also recognizes ezrin) confirmed a significant decrease in the ezrin/moesin content of Ppl-treated SCMs. B. Subfractionation of SCMs on linear sucrose gradients separated RTCs into three fractions. Although a lower density fraction also contained a small amount of vesiculated RIS plasma membrane, as detected by anti-Na, K-ATPase β2 antibody, ezrin/moesin were mostly associated with the higher density SCMs. Ppl caused redistribution of ezrin/moesin, actin, rho-GDI, and rac1 from the SCMs into the cytosol. (C) The distribution of ezrin/moesin among LBM membranes closely parallels that of the RIS plasma membrane and associated actin filaments. Immunoblot of LBM fractions, as well as that of the cytosol (B) with anti-moesin antibody clearly shows a protein doublet where the faster migrating band seems to be more affected by Ppl treatment. This band likely represents moesin, whereas the slower band is likely ezrin, which has higher molecular weight.
ERM proteins are mostly associated with the plasma membrane, so we wanted to test whether their source in the SCM fraction is vesiculated RIS plasma membrane, a minor contamination of RTCs that constitute the majority of SCMs (Morel et al., 2000). We subfractionated SCMs on linear sucrose gradients, as described by Morel et al. (2000) and found significant cofractionation of ERM proteins with radiolabeled rhodopsin (Figure 7B). A modification of this method (see MATERIALS AND METHODS) afforded even greater separation of RTCs than described previously. As shown in Figure 7B, RTCs were separated into three fractions. These subcellular fractions were analyzed by immunoblotting with a mAb to the β2 subunit of Na,K-ATPase, to demonstrate the distribution of this marker found exclusively in the RIS plasma membrane, between the base of the connecting cilium and the outer limiting membrane (OLM) (Schneider et al., 1991). A low-density fraction 1 contained the majority of the β2 subunit of Na,K-ATPase, but ezrin/moesin were completely absent from this fraction. They were found to cofractionate only with the more dense SCM fractions containing RTCs (fractions 2 and 3). This suggests that, surprisingly, in the SCM fractions ezrin/moesin are mostly associated with RTCs. On treatment with Ppl, they seem to dissociate from these membranes (Figure 7B). Immunoblots of cytosolic proteins (Figure 7B) show a significant (3-fold) increase in soluble ezrin/moesin in Ppl-treated retinas.
Only a minor portion of ezrin/moesin cofractionates with Na,K-ATPase in SCM fractions; however, the distribution of ezrin/moesin in the LBM fractions suggests that they associate with the plasma membrane containing Na,K-ATPase and membrane-bound actin (Figure 7C). It is important to note that LBM fractions contain >90% of Na,K-ATPase, but only ∼60% of the total fraction of ezrin/moesin, the rest is bound to RTCs. LBM and cytosolic fractions display a doublet of anti-moesin immunoreactive bands. Ppl treatment seems to affect the more abundant band of lower molecular weight (Figure 7). This band most likely represents moesin, which has lower molecular weight than ezrin. These data strongly suggest that the Ppl treatment causes release of ERM proteins (particularly moesin) from the RTCs, and from the RIS plasma membrane into the cytosol.
Inactivation of Ezrin/Moesin by Ppl Causes Dissociation of rho-GDI and rac1 from the Membranes
In the cytosol, ERM proteins are inactive with their membrane-binding N-terminal domain tightly associated with the C-terminal actin-binding domain (Bretscher et al., 2002). PI(4,5)P2 binds to the N-terminal (FERM) domain and activates ERM proteins (Yonemura et al., 2002), possibly by increasing their susceptibility to C-terminal phosphorylation. N-terminal domain of activated ERM proteins binds to rho-GDI, causes its dissociation from, and activation of rho-family proteins (Takahashi et al., 1997). Therefore, if Ppl causes inactivation and dissociation of ERM proteins from the membranes, this should prevent their binding to rho-GDI and downstream signaling through rho-dependent pathways. To test whether Ppl also affects rho signaling through ezrin/moesin inactivation, we determined the subcellular distribution of rho-GDI, rho, and rac1 in treated cells. Ppl treatment released rho-GDI and rac1 from SCM membrane fractions to a similar extent as ezrin/moesin (Figure 7B). Release of ezrin/moesin also caused actin dissociation from the membranes (Figure 7B). Together, data presented in Figure 7 suggest that phospholipid modifications caused by Ppl induce redistribution of a fraction of the membrane-bound ezrin/moesin (particularly moesin) into the cytosol. This is likely due to their inactivation caused by the localized reduction in PI(4,5)P2 in specific membrane compartments, including RTCs. This causes subsequent dissociation of rho-GDI from these compartments now lacking ezrin/moesin, and, presumably, inactivation of rac1. However, we could not confirm this by the direct measurement of rac1 activation, because subcellular fractionation necessary to obtain RTCs caused rapid GTP hydrolysis and rac1 inactivation in control, as well as in Ppl-treated cells.
Ezrin/Moesin and rac1 Are Localized on rab8-positive RTCs at the Sites of Their Fusion with the Plasma Membrane and Ppl Disrupts This Localization
Given the biochemical evidence that ezrin/moesin association with RTCs may also regulate rho-dependent signaling, we proceeded to determine the distribution of ezrin/moesin and rho-family proteins within photoreceptors by confocal microscopy. Ezrin/moesin immunoreactivity (Figure 8A, red) was present in punctate structures throughout the rod photoreceptor inner segments, the outer nuclear and outer plexiform layers containing photoreceptor nuclei, and the synaptic terminals, respectively, but was absent from the ROS. Ezrin/moesin was also present in the inner nuclear layer containing nuclei of the secondary retinal neurons. Localization of actin filaments was revealed by fluorescent phalloidin (Figure 8, green). Microfilament bundles encircle photoreceptor inner segments and extend from the calycal processes that surround the ROS, to the junctional complexes that form the OLM at the base of the RIS. High ezrin/moesin immunoreactivity was noted at the OLM (Figure 8A, arrow), suggesting that ezrin/moesin participate in membrane anchoring of actin cables in photoreceptor cells. Higher magnification shown in Figure 8C indicates that ezrin/moesin-positive structures are found along actin bundles in the vicinity of the connecting cilium (arrow) where RTCs are also localized (as seen in Figure 4).
Figure 8.
Confocal microscopy shows that Ppl profoundly affects the distribution of the fraction of ezrin/moesin and rac1, which is normally associated with rab8-positive RTCs. Control (A and C) or Ppl-treated retinas (B and D) labeled with anti-moesin antibody (red), phalloidin (green), and nuclear stain TO-PRO-3 (blue). Punctate anti-moesin labeling along actin filaments seen in control photoreceptors (arrows in A and C) is mostly absent in Ppl-treated retinas, except at the OLM (arrow in D). (E and F) Retinal sections labeled with anti-moesin (red, E) or anti rac1 (red, F) showing that both proteins are localized to the same punctate structures, along microfilaments (green) and in the proximity of the cilium consistent with RTCs (arrows). (G) On Ppl treatment, rac1 is diffusely distributed along the RIS/ROS border. (H and I) Higher magnification images of anti rac1 (red, H) or anti-moesin (red, I) localized to periciliary RTCs. (J and K) Control (J) or Ppl-treated retinas (K) labeled with anti-rab8 (red) and anti-moesin antibody (green). In control retinas, rab8 and moesin are colocalized (yellow) on RTCs (arrow in J) and in punctate structures along microfilaments in the calycal processes (arrowheads). In Ppl-treated retinas, accumulated RTCs are rab8 positive (arrow in K), but devoid of moesin, whereas the two proteins are still colocalized in the calycal processes (arrowheads). Bar, 10 μm (A and B); 5 μm (C–F and H); 7 μm (G), and 3 μm (I–K).
The ezrin/moesin immunoreactivity dramatically changes with Ppl treatment (Figure 8, B and D). Punctate structures in the inner segments are no longer seen, probably due to the redistribution of ezrin/moesin into the cytosol where they are no longer at high enough concentration to be detectable. However, ezrin/moesin is still localized at the OLM (Figure 8D, arrow). We compared the distribution of ezrin/moesin (Figure 8E) to that of rac1 (Figure 8F) and found a striking similarity in their intracellular localization. In Figure 8, E and F, showing tangential retinal sections, arrows point to the rod inner-outer segment junctions where ezrin/moesin and rac1 are highly concentrated in punctate structures consistent with RTCs. Ppl treatment causes complete redistribution of rac1 in photoreceptor cells (Figure 8G). Absent is the punctate staining visible in the control and instead a uniform label is observed along what seems to be the rod inner-outer segment border (arrow).
The remarkably similar rac1 (Figure 8H) and ezrin/moesin immunoreactivities (Figure 8I) in the vesicular structures along the microfilaments around the connecting cilium (arrows) closely resemble our previously reported colocalization of actin and rab8 in these structures (Deretic et al., 1995). Because rab8 regulates fusion of RTCs with the plasma membrane (Moritz et al., 2001), we proceeded to determine whether rab8, ezrin/moesin, and rac1 are colocalized on RTCs. Figure 8J shows that rab8 (red) and ezrin/moesin (green) colocalize on RTCs (yellow, arrow), as well as in the punctate structures associated with microfilaments in the calycal process (arrowhead), where rab8 has been localized previously (Deretic et al., 1995). The colocalization of rab8 and moesin, or rac1 (our unpublished data) was also observed when fluorescently conjugated secondary antibodies used for their detection were reversed (see MATERIALS AND METHODS). Remarkably, in Ppl-treated retinas accumulation of rab8-positive RTCs is seen in the vicinity of the cilium (Figure 8K, arrow) but they no longer colocalize with ezrin/moesin, which redistribute into the cytosol. However, rab8 and ezrin/moesin still colocalize on microfilaments in the calycal process (Figure 8K, arrowheads). These data support the notion that Ppl selectively affects the post-Golgi compartment before it destabilizes the structures that form the OLM (Figure 8D), or the calycal processes (Figure 8K).
Together, these data show that ezrin/moesin and rac1 are localized on rab8-positive RTCs at the sites of their fusion with the plasma membrane. Ppl profoundly affects this localization and therefore potentially disrupts the fusion sites. Our data suggest that in Ppl-treated cells the lack of PI(4,5)P2, functional ezrin/moesin and rac1 on fusion sites is the cause for the inability of RTCs to tether to and fuse with the RIS plasma membrane and deliver rhodopsin and newly synthesized phospholipids to the ROS.
DISCUSSION
In this study, we report that a key step in the intracellular transport of rhodopsin, the tethering of its transport carriers (RTCs) to the target RIS plasma membrane in preparation for fusion, is regulated by protein–lipid interactions that involve PI(4,5)P2, phosphoinositide-binding ERM proteins, particularly moesin, actin, and small GTPase rac1 and its regulator rho-GDI. These factors cooperate with the previously identified fusion regulator rab8 (Deretic et al., 1995; Moritz et al., 2001) to regulate the polarized delivery of RTCs. In photoreceptor cells, the area surrounding the cilium that connects the RIS with the ROS is a site of continuous active fusion. The specific docking and fusion site represents a minor fraction of the RIS plasma membrane and its biochemical characterization has not been possible so far. However, the molecular mechanisms involved in tethering and fusion of RTCs are beginning to be understood.
The small GTPase rab8 provides the specificity of RTC fusion (Moritz et al., 2001). The octameric membrane tethering sec6/8 complex (known as “exocyst” in yeast) (Novick and Guo, 2002), is a likely rab8 effector at the RTC docking site. Strikingly, photoreceptor cells expressing GFP-rab8T22N dominant-negative mutants show defect in tethering (Moritz et al., 2001) and accumulate RTCs in a manner similar to the cells treated with Ppl (Figure 4, H and I). This similarity strongly suggests that Ppl may interfere with the assembly of tethering complexes. In photoreceptors, both endogenous rab8 and exogenously expressed GFP-rab8 colocalize with actin filaments at the sites of fusion (Deretic et al., 1995; Moritz et al., 2001), and actin-based motors are involved in trafficking to the ROS (Liu et al., 1999). The actin network and the actin-based motors are essential for sec6/8 and the exocyst localization and for polarized exocytosis (Hsu et al., 1999). Subcellular distribution of rac1 closely parallels that of rab8. Therefore, rac1 localized on RTCs may provide access to, or organize polarized sites on the cell surface, through its dynamic control of the actin cytoskeleton. Small GTPases rho1, cdc42p, and ral interact directly with sec6/8 and the exocyst components in yeast and mammals (Novick and Guo, 2002), suggesting multiple levels of regulation of tethering complexes, which may also be cell specific. Our finding that RTC accumulation coincides with inactivation and diffuse localization of rac1 caused by Ppl, implicates rac1 as a potential regulator of the tethering complexes involved in RTCs docking and fusion.
An elegant study in Drosophila showed that transgenic expression of a dominant-active rac1 rescued photoreceptor morphogenesis in rhodopsin-null mutants, whereas expression of dominant-negative rac1 resulted in retinal degeneration, similar to that seen in rhodopsin-null mutants (Chang and Ready, 2000). This study showed the existence of an important relationship between rhodopsin and rac1. Our data support the notion that this relationship is maintained in vertebrate photoreceptors. Members of the rho family of GTPases have been implicated, although indirectly, in the maintenance of photoreceptor cytoskeleton in vertebrates (Pittler et al., 1995). Our data also suggest involvement of rho in RTC trafficking, but its role remains to be further elucidated.
Ezrin promotes membrane morphogenesis in retinal pigment epithelium (Bonilha et al., 1999), and moesin was found to be involved in rhabdomere morphogenesis in Drosophila (Kargiosis and Ready, 2002), but no role for ezrin and moesin in vertebrate photoreceptors has been reported thus far. Our present study suggests that the PI(4,5)P2-enriched microdomains may be present on the RTCs and at their docking site, where they recruit ezrin/moesin and rac1, which bring them in contact with the actin network before fusion. PI(4,5)P2- and ezrin/moesin-dependent actin assembly was also observed on latex bead phagosomes, possibly in preparation for fusion (Defacque et al., 2002). A positive feedback loop exists between ezrin/moesin and rho-pathway signaling (Mackay et al., 1997). Our data imply that Ppl disrupts PI(4,5)P2–ezrin/moesin–rho family interactions. The resulting accumulation of rab8-positive RTCs, loss of ezrin/moesin, and diffuse distribution of rac1 throughout the area of accumulation suggests that this then causes a loss of targeted membrane delivery. PI(4,5)P2 may also affect rac1 through its GEFs that contain phosphoinositide-binding domains. The punctate staining of ezrin/moesin and rac1 observed in control photoreceptors is similar to that seen in rat brain neurons, where rac1 is reported to be associated with lipid rafts (Kumanogoh et al., 2001). Cholesterol-enriched domains surround photoreceptor cilium (Andrews and Cohen, 1983), and we also see raft-like structures on isolated RTCs by freeze-fracture analysis (Defoe and Deretic, unpublished data). Recent reports that cholesterol-enriched rafts may also contain PI(4,5)P2 (Pike and Miller, 1998; Cullen et al., 2001) add support to our hypothesis that these microdomains are involved in RTC tethering and fusion.
In this study, phospholipid modifications that caused dramatic inhibition of RTC membrane fusion enhanced RTC budding from the TGN. Particular enrichment of PA at the photoreceptor TGN in propranolol treated cells (Figure 5) and increased RTC budding, suggests an important role of PA in these processes as has been previously suggested in other cells (Siddhanta et al., 2000). Our data also support the involvement of DAG and PI(4)P in membrane budding at the TGN (Baron and Malhotra, 2002; De Matteis et al., 2002). Interestingly, consistent with the time-dependent differential effects of Ppl on phosphoinositide metabolism and DAG synthesis that we observed (Figure 2B), in the study of Baron and Malhotra (2002) Ppl inhibited DAG synthesis and caused relocation of protein kinase D that regulates the fission of post-Golgi carriers from the TGN, whereas in our study which used a much longer incubation, it increased DAG synthesis and stimulated budding from the TGN. Remarkably, we also found that activation of PLD by S1P increased phospholipid biosynthesis and membrane delivery to the ROS, suggesting that photoreceptor membrane renewal may be subject to regulation by activators of PLD, such as growth factors. This is particularly intriguing given the already high rate of membrane trafficking toward the ROS in these cells.
A surprising finding was that the most sensitive steps in membrane trafficking affected by phospholipid modulation in photoreceptor cells involve budding and fusion of RTCs. Although phospholipid biosynthesis takes place in the ER, ER and Golgi seemed to be initially unaffected, because the morphology of these compartments remained unaltered in cells treated with Ppl for 4.5 h (Figure 4B). It is tempting to speculate that phospholipids needed for RTC budding are supplied to the TGN by direct membrane interactions that bypass the rough ER and the Golgi cisternae. This possibility is supported by the elegant studies of Golgi morphology that show close membrane association, at the sites that may facilitate lipid exchange between the ER and the trans-Golgi, a compartment involved in the budding of post-Golgi carriers (Ladinsky et al., 1999; Marsh et al., 2001). Interestingly, we also found that the amount of newly synthesized PC and PE was least reduced in the TGN membranes of Ppl-treated cells (Figure 5), again suggesting directed trafficking and active incorporation of these phospholipids into budding RTCs.
In this study, we found that the behavior of DHA-phospholipids greatly reflected the synthesis and trafficking of all phospholipids (as shown in Figure 6). However, our data also suggest that the compounds that increase the uptake and esterification of DHA, such as S1P, increase membrane turnover in photoreceptor cells, and are therefore important factors for the maintenance of their polarity. This is especially relevant for retinal degenerations caused by mutations in photoreceptor proteins that show metabolic defects in DHA biosynthesis (Hoffman et al., 1995), where improved membrane delivery to the ROS through increased DHA delivery may be essential for photoreceptor preservation and health.
Many aspects of protein–lipid interactions involved in rhodopsin trafficking still remain to be elucidated. However, this study provides a first glimpse into these complex processes and draws attention to a number of new regulators of RTC trafficking. Based on the results presented here, phosphoinositides, ezrin/moesin, and small GTPase rac1 and its regulator rho-GDI act on the same pathway as described previously for small GTPase rab8. Consequentially, they are essential for rhodopsin-laden membrane delivery to the ROS, a critical step in the biogenesis of the light-detecting organelle of the photoreceptor cell.
Acknowledgments
We thank Dr. Barbara G. Schneider for advice on EM morphometric analysis. This study was supported by National Institutes of Health grant EY-12421 (to D.D.). Confocal images in this article were generated in the Fluorescence Microscopy Facility, which received support from National Center for Research Resources, National Science Foundation, National Cancer Institute, and the University of New Mexico Cancer Center.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–04–0203. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-04-0203.
Abbreviations used: DAG, diacylglycerol; DHA, docosahexaenoic acid; ERM, ezrin/radixin/moesin; LBM, large biosynthetic membrane; OLM, outer limiting membrane; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PH, plekstrin homology; PI(4,5)P2, phosphatidylinositol-4,5-bisphosphate; PI, phosphatidylinositol; PLC, phospholipase C; PLD, phospholipase D; PNS, postnuclear supernatant; Ppl, propranolol; PS, phosphatidylserine; RIS, rod inner segment(s); ROS, rod outer segment(s); RTC, rhodopsin-bearing transport carrier; S1P, sphingosine 1-phosphate; SCM, small carrier membrane; TGN, trans-Golgi network.
References
- Anderson, R.G., and Jacobson, K. (2002). A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821-1825. [DOI] [PubMed] [Google Scholar]
- Andrews, L.D., and Cohen, A.I. (1983). Freeze-fracture studies of photoreceptor membranes: new observations bearing upon the distribution of cholesterol. J. Cell Biol. 97, 749-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, C.L., and Malhotra, V. (2002). Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295, 325-328. [DOI] [PubMed] [Google Scholar]
- Bazan, N.G., Roccamo de Fernandez, A.M., Giusto, N.M., and Ilincheta de Boschero, M.G. (1985). Propranolol-induced membrane perturbation and the metabolism of phosphoinositides and arachidonoyl diacylgycerols in the retina. In: Inositol and Phosphoinositides: Metabolism and Regulation, ed. J.E. Bleasdale, J. Eichberg, and G. Hauser, Clifton, NJ: Humana Press, 67-85.
- Bazan, N.G., and Rodriguez de Turco, E.B. (1994). Review: pharmacological manipulation of docosahexaenoic-phospholipid biosynthesis in photoreceptor cells: implications in retinal degeneration. J. Ocul. Pharmacol. 10, 591-604. [DOI] [PubMed] [Google Scholar]
- Bonilha, V.L., Finnemann, S.C., and Rodriguez-Boulan, E. (1999). Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J. Cell Biol. 147, 1533-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher, A., Edwards, K., and Fehon, R.G. (2002). ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell. Biol. 3, 586-599. [DOI] [PubMed] [Google Scholar]
- Chang, H.Y., and Ready, D.F. (2000). Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science 290, 1978-1980. [DOI] [PubMed] [Google Scholar]
- Choe, H.G., and Anderson, R.E. (1990). Unique molecular species composition of glycerolipids of frog rod outer segments. Exp. Eye Res. 51, 159-165. [DOI] [PubMed] [Google Scholar]
- Choe, H.G., Ghalayini, A.J., and Anderson, R.E. (1990). Phosphoinositide metabolism in frog rod outer segments. Exp. Eye Res. 51, 167-176. [DOI] [PubMed] [Google Scholar]
- Cullen, P.J., Cozier, G.E., Banting, G., and Mellor, H. (2001). Modular phosphoinositide-binding domains–their role in signalling and membrane trafficking. Curr. Biol. 11, R882-R893. [DOI] [PubMed] [Google Scholar]
- De Matteis, M., Godi, A., and Corda, D. (2002). Phosphoinositides and the Golgi complex. Curr. Opin. Cell Biol. 14, 434. [DOI] [PubMed] [Google Scholar]
- De Matteis, M.A., and Morrow, J.S. (2000). Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci. 113, 2331-2343. [DOI] [PubMed] [Google Scholar]
- Defacque, H., Bos, E., Garvalov, B., Barret, C., Roy, C., Mangeat, P., Shin, H.W., Rybin, V., and Griffiths, G. (2002). Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol. Biol. Cell 13, 1190-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic, D. (1997). Rab proteins and post-Golgi trafficking of rhodopsin in photoreceptor cells. Electrophoresis 18, 2537-2541. [DOI] [PubMed] [Google Scholar]
- Deretic, D., Huber, L.A., Ransom, N., Mancini, M., Simons, K., and Papermaster, D.S. (1995). rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J. Cell Sci. 108, 215-224. [DOI] [PubMed] [Google Scholar]
- Deretic, D., and Papermaster, D.S. (1991). Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J. Cell Biol. 113, 1281-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic, D., Schmerl, S., Hargrave, P.A., Arendt, A., and McDowell, J.H. (1998). Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc. Natl. Acad. Sci. USA 95, 10620-10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, N.N., Zhang, H., Olivera, A., Mattie, M.E., and Spiegel, S. (1992). Sphingosine-1-phosphate, a metabolite of sphingosine, increases phosphatidic acid levels by phospholipase D activation. J. Biol. Chem. 267, 23122-23128. [PubMed] [Google Scholar]
- Dowler, S., Currie, R.A., Campbell, D.G., Deak, M., Kular, G., Downes, C.P., and Alessi, D.R. (2000). Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351, 19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng, J.K., McCormack, A.L., and Yates, J.R., 3rd. (1994). An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 5, 976-989. [DOI] [PubMed] [Google Scholar]
- Fliesler, S.J., and Anderson, R.E. (1983). Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22, 79-131. [DOI] [PubMed] [Google Scholar]
- Godi, A., Pertile, P., Meyers, R., Marra, P., Di Tullio, G., Iurisci, C., Luini, A., Corda, D., and De Matteis, M.A. (1999). ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1, 280-287. [DOI] [PubMed] [Google Scholar]
- Hoffman, D.R., Uauy, R., and Birch, D.G. (1995). Metabolism of omega-3 fatty acids in patients with autosomal dominant retinitis pigmentosa. Exp. Eye Res. 60, 279-289. [DOI] [PubMed] [Google Scholar]
- Hsu, S.C., Hazuka, C.D., Foletti, D.L., and Scheller, R.H. (1999). Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex. Trends Cell Biol. 9, 150-153. [DOI] [PubMed] [Google Scholar]
- Ilincheta de Boschero, M.G., and Bazan, N.G. (1983). Reversibility of propranolol-induced changes in the biosynthesis of monoacylglycerol, diacylglycerol, triacylglycerol, and phospholipids in the retina. J. Neurochem. 40, 260-266. [DOI] [PubMed] [Google Scholar]
- Jones, D.H., Morris, J.B., Morgan, C.P., Kondo, H., Irvine, R.F., and Cockcroft, S. (2000). Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J. Biol. Chem. 275, 13962-13966. [DOI] [PubMed] [Google Scholar]
- Jones, S.M., Alb, J.G., Jr., Phillips, S.E., Bankaitis, V.A., and Howell, K.E. (1998). A phosphatidylinositol 3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J. Biol. Chem. 273, 10349-10354. [DOI] [PubMed] [Google Scholar]
- Karagiosis, S.A., and Ready, D.F. (2002). Moesin is critical for rhabdomere morphogenesis in the developing Drosophila retina [abstract]. Mol. Biol. Cell, 13S, 480a. [Google Scholar]
- Kozlov, M.M. (2001). Fission of biological membranes: interplay between dynamin and lipids. Traffic 2, 51-65. [DOI] [PubMed] [Google Scholar]
- Krappa, R., Nguyen, A., Burrola, P., Deretic, D., and Lemke, G. (1999). Evectins: vesicular proteins that carry a pleckstrin homology domain and localize to post-Golgi membranes. Proc. Natl. Acad. Sci. USA 96, 4633-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanogoh, H., Miyata, S., Sokawa, Y., and Maekawa, S. (2001). Biochemical and morphological analysis on the localization of Rac1 in neurons. Neurosci. Res. 39, 189-196. [DOI] [PubMed] [Google Scholar]
- Ladinsky, M.S., Mastronarde, D.N., McIntosh, J.R., Howell, K.E., and Staehelin, L.A. (1999). Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol. 144, 1135-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch, M., and Cantley, L.C. (1995). Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell 81, 659-662. [DOI] [PubMed] [Google Scholar]
- Litosch, I. (2000). Regulation of phospholipase C-beta(1) activity by phosphatidic acid. Biochemistry 39, 7736-7743. [DOI] [PubMed] [Google Scholar]
- Liu, X., Udovichenko, I.P., Brown, S.D., Steel, K.P., and Williams, D.S. (1999). Myosin VIIa participates in opsin transport through the photoreceptor cilium. J. Neurosci. 19, 6267-6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorra, C., and Huttner, W.B. (1999). The mesh hypothesis of Golgi dynamics. Nat. Cell Biol. 1, E113-E115. [DOI] [PubMed] [Google Scholar]
- Mackay, D.J., Esch, F., Furthmayr, H., and Hall, A. (1997). Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J. Cell Biol. 138, 927-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, B.J., Mastronarde, D.N., Buttle, K.F., Howell, K.E., and McIntosh, J.R. (2001). Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc. Natl. Acad. Sci. USA 98, 2399-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, K., Jacques, K.M., Stauffer, S., Kubosaki, A., Zhu, K., Hirsch, D.S., Resau, J., Zheng, Y., and Randazzo, P.A. (2002). ARAP 1, a point of convergence for Arf and Rho signaling. Mol. Cell 9, 109-119. [DOI] [PubMed] [Google Scholar]
- Morel, V., Poschet, R., Traverso, V., and Deretic, D. (2000). Towards the proteome of the rhodopsin-bearing post-Golgi compartment of retinal photoreceptor cells. Electrophoresis 21, 3460-3469. [DOI] [PubMed] [Google Scholar]
- Moritz, A., De Graan, P.N., Gispen, W.H., and Wirtz, K.W. (1992). Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J. Biol. Chem. 267, 7207-7210. [PubMed] [Google Scholar]
- Moritz, O.L., Tam, B.M., Hurd, L.L., Peranen, J., Deretic, D., and Papermaster, D.S. (2001). Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol. Biol. Cell 12, 2341-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick, P., and Guo, W. (2002). Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol. 12, 247-249. [DOI] [PubMed] [Google Scholar]
- Ohashi, M., Jan de Vries, K., Frank, R., Snoek, G., Bankaitis, V., Wirtz, K., and Huttner, W.B. (1995). A role for phosphatidylinositol transfer protein in secretory vesicle formation. Nature 377, 544-547. [DOI] [PubMed] [Google Scholar]
- Papermaster, D.S., Schneider, B.G., and Besharse, J.C. (1985). Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Investig. Ophthalmol. Vis. Sci. 26, 1386-1404. [PubMed] [Google Scholar]
- Pike, L.J., and Miller, J.M. (1998). Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J. Biol. Chem. 273, 22298-22304. [DOI] [PubMed] [Google Scholar]
- Pittler, S.J., Fliesler, S.J., Fisher, P.L., Keller, P.K., and Rapp, L.M. (1995). In vivo requirement of protein prenylation for maintenance of retinal cytoarchitecture and photoreceptor structure. J. Cell Biol. 130, 431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu, G.A., and Masland, R.H. (1984). Biochemical interruption of membrane phospholipid renewal in retinal photoreceptor cells. J. Neurosci. 4, 1559-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo, P.A. (1997). Functional interaction of ADP-ribosylation factor 1 with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 272, 7688-7692. [PubMed] [Google Scholar]
- Rodriguez de Turco, E.B., Deretic, D., Bazan, N.G., and Papermaster, D.S. (1997). Post-Golgi vesicles cotransport docosahexaenoyl-phospholipids and rhodopsin during frog photoreceptor membrane biogenesis. J. Biol. Chem. 272, 10491-10497. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco, E.B., Gordon, W.C., and Bazan, N.G. (1992). Light stimulates in vivo inositol lipid turnover in frog retinal pigment epithelial cells at the onset of shedding and phagocytosis of photoreceptor membranes. Exp. Eye Res. 55, 719-725. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco, E.B., Gordon, W.C., and Bazan, N.G. (1994). Docosahexaenoic acid is taken up by the inner segment of frog photoreceptors leading to an active synthesis of docosahexaenoyl-inositol lipids: similarities in metabolism in vivo and in vitro. Curr. Eye Res. 13, 21-28. [DOI] [PubMed] [Google Scholar]
- Schneider, B.G., Shyjan, A.W., and Levenson, R. (1991). Co-localization and polarized distribution of Na, K-ATPase alpha 3 and beta 2 subunits in photoreceptor cells. J. Histochem. Cytochem. 39, 507-517. [DOI] [PubMed] [Google Scholar]
- Sekiya, F., Bae, Y.S., and Rhee, S.G. (1999). Regulation of phospholipase C isozymes: activation of phospholipase C-gamma in the absence of tyrosinephosphorylation. Chem. Physiol. Lipids 98, 3-11. [DOI] [PubMed] [Google Scholar]
- Siddhanta, A., Backer, J.M., and Shields, D. (2000). Inhibition of phosphatidic acid synthesis alters the structure of the Golgi apparatus and inhibits secretion in endocrine cells. J. Biol. Chem. 275, 12023-12031. [DOI] [PubMed] [Google Scholar]
- Simon, J.P., Morimoto, T., Bankaitis, V.A., Gottlieb, T.A., Ivanov, I.E., Adesnik,M.,andSabatini,D.D.(1998).Anessentialroleforthephosphatidylinositol transfer protein in the scission of coatomer-coated vesicles from the trans-Golgi network. Proc. Natl. Acad. Sci. USA 95, 11181-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and Ikonen, E. (1997). Functional rafts in cell membranes. Nature 387, 569-572. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Wurmser, A.E., Emr, S.D., and Stenmark, H. (2001). The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13, 485-492. [DOI] [PubMed] [Google Scholar]
- Spiegel, S., and Milstien, S. (2002). Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 277, 25851-25854. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Sasaki, T., Mammoto, A., Takaishi, K., Kameyama, T., Tsukita, S., and Takai, Y. (1997). Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J. Biol. Chem. 272, 23371-23375. [DOI] [PubMed] [Google Scholar]
- Truett, A.P., 3rd, Bocckino, S.B., and Murray, J.J. (1992). Regulation of phosphatidic acid phosphohydrolase activity during stimulation of human polymorphonuclear leukocytes. FASEB J. 6, 2720-2725. [DOI] [PubMed] [Google Scholar]
- van Meer, G., and Lisman, Q. (2002). Sphingolipid transport: rafts and translocators. J. Biol. Chem. 277, 25855-25858. [DOI] [PubMed] [Google Scholar]
- Weibel, E.R., and Bolender, R.P. (1973). Stereological techniques for electron microscopic morphometry. In: Principles and Techniques of Electron Microscopy. Biological Applications, vol. 3, ed. M.A. Hayat, New York: Van Nostrand Reinhold, 237-296. [Google Scholar]
- Weigert, R., et al. (1999). CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 402, 429-433. [DOI] [PubMed] [Google Scholar]
- Yonemura, S., Matsui, T., and Tsukita, S. (2002). Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J. Cell Sci. 115, 2569-2580. [DOI] [PubMed] [Google Scholar]