Abstract
High respiratory quotient (RQ) has been associated with fat mass gain in some, but not all studies. Variability among results may reflect differences in the RQ variable measured (fasting vs. 24-hour) or may be due to differences in control for factors that affect RQ, such as diet, energy balance, circulating insulin, and insulin sensitivity. The objective of this study was to determine whether different RQ values (fasting, sleeping, non-sleeping, and 24-hour) would predict change in fat mass over 2 years in obesity-prone women, controlling for diet and adjusting for energy balance, circulating insulin, and insulin sensitivity.
Participants were 33 previously-overweight premenopausal women. Fasting, sleeping, non-sleeping, and 24-hour RQ values were measured during controlled-diet conditions by respiratory chamber calorimetry. Intravenous glucose tolerance tests were also performed to adjust for fasting insulin, acute insulin response to glucose, and insulin sensitivity. Over the following 2 years, changes in fat mass were tracked annually by dual energy X-ray absorptiometry.
High non-sleeping RQ (NSRQ) predicted 2-year change in fat mass independently of energy balance, circulating insulin, and insulin sensitivity. This observation suggests that low postprandial fat oxidation may uniquely predispose obesity-prone individuals to accrual of adipose tissue.
Introduction
More than two-thirds of American adults are classified as overweight or obese (1). Although various diet interventions can facilitate intentional weight loss, most dieters regain weight within 3–5 years (2). Identifying inherent metabolic characteristics that predispose obesity-prone individuals to weight regain presents an important public health challenge.
Inherent variability in substrate oxidation may be one such mechanism that affects weight regain and fat storage. Substrate oxidation can be assessed clinically by measuring respiratory quotient (RQ), the ratio of carbon dioxide expired to oxygen consumed during indirect calorimetry. High RQ values are indicative of low fat oxidation and high carbohydrate oxidation (3). Previous studies relating RQ to weight gain have yielded inconsistent results. While some investigators have associated high RQ with weight gain (4–9), other studies demonstrated no association (10–12).
Equivocal reports relating RQ and fat mass may be due to population differences and variable control for dietary confounders in different study designs. For example, differences in macronutrient intake between individuals may influence RQ measurements, and fasting RQ will be elevated if an individual is in positive energy balance during the days preceding the measurement (3). Individual differences in circulating insulin or insulin sensitivity may also confound reported associations between RQ and fat mass gain. Insulin is an anabolic hormone that acts to decrease fat oxidation and increase lipid storage (13). Accordingly, fasting serum insulin has been positively correlated with weight (10) and RQ (14). Moreover, high tissue sensitivity to insulin has also been associated with fat accumulation (15–17). Thus, when studying the relationship between RQ and change in fat mass, it is important to carefully control for energy balance and macronutrient intake and to consider the influence of circulating insulin and insulin sensitivity.
Discrepant results may also suggest that RQ values measured across specific periods of the day differentially predict fat mass gain. Most studies to date have focused exclusively on either fasting or 24-hour RQ. However, these values may not reflect postprandial fat oxidation. Rather, RQ measured during fasting or sleeping would reflect periods of high reliance on endogenous free fatty acids for fuel. Alternatively, non-sleeping RQ (NSRQ) measured during waking hours in the respiratory chamber would specifically correspond to an individual’s ability oxidize lipid and carbohydrate across typical physiological postprandial periods of the day.
In a previous analysis of weight-reduced African-American and Caucasian women, we reported that fasting, sleeping, and 24-hour RQ were not predictive of long-term weight change (12). In the present study, we examined the potential for NSRQ to predict change in fat mass among this cohort of previously-overweight premenopausal women, controlling for diet and adjusting for circulating insulin, insulin sensitivity, and energy balance. The specific aim of this study was to determine whether RQ measured during specific time periods (fasting, sleeping, non-sleeping, and 24-hour) would uniquely predict change in fat mass2 years following weight loss.
Methods and Procedures
Participants
Participants were 33 healthy, premenopausal women evaluated after a dietary intervention for weight loss to BMI < 25 kg/m2 (11). Forty-nine women were originally recruited for evaluation, but only those participants with all data available for RQ, insulin measures, and 2-year body composition were included in this analysis. The final study cohort included 20 African Americans and 13 Caucasians with normal menstrual cycles and normal glucose tolerance verified by oral glucose tolerance tests. Participants were nonsmokers and sedentary (self report of exercising less than one time per week over the previous year), and none were taking any medications known to affect energy expenditure or substrate oxidation. All subjects reported a family history of overweight or obesity (BMI >27 kg/m2) in a first degree relative. Participants provided written informed consent, and the study protocol was approved by the Institutional Review Board at the University of Alabama at Birmingham.
Study design
Baseline measurements were obtained after successful completion of a structured dietary weight loss intervention (11) during which time the women lost an average of 12.1 ± 3.5 kg. For 4 weeks prior to testing, food was provided to maintain each participant in a weight-stable state, and weight stability of <1% variation was documented. After 4 weeks of the weight-maintenance diet, participants were admitted to the General Clinical Research Center (GCRC) for 4 days, during the follicular phase of the menstrual cycle. Baseline testing included respiratory chamber calorimetry for fasting RQ, 24-hour RQ, sleeping RQ, and non-sleeping RQ (NSRQ) and Intravenous Glucose Tolerance Tests (IVGTT) for fasting insulin, acute insulin response to glucose, and insulin sensitivity. Body composition testing by dual energy X-ray absorptiometry (DXA) was performed at baseline and annually over the following two years. During this time, the controlled diet protocol was repeated with 4 weeks of weight maintenance on the diet followed by body composition testing.
Outcome measures
RQ values were measured by indirect calorimetry as previously described (18). Briefly, subjects were transported from the GCRC to the respiratory chamber of the Energy Metabolism Research Unit at 0700 h, after a 12-hour fast. The chamber had a volume of 17,500 liters (3.4m long, 2.1m wide, and 2.6m high). Oxygen consumption (VO2) and carbon dioxide production (VCO2) were continuously measured over 24 hours by an O2 analyzer (Magnos 4G) and a CO2 analyzer (Uras 3G, Hartmann & Braun, Germany). Substrate oxidation was calculated from CO2 production and O2 consumption rates, and this ratio was expressed as respiratory quotient (RQ). In addition to 24-hour RQ, RQ values were quantified for sleeping and non-sleeping periods in the chamber. Sleeping RQ was averaged from RQ values measured during the sleeping period. The sleeping period began when lights were turned off (between 9:30 and 11:00 PM) and ended when each participant was awakened at 6:30 AM. Radar motion sensors were used to ensure that participants were inactive during the sleeping period. Non-sleeping RQ (NSRQ) was calculated for the period of the day when participants were awake. NSRQ encompassed the portion of the day when each participant was in a postprandial state, beginning with breakfast and continuing through the period following dinner. Fasting RQ was also measured and averaged over 30 minutes for each participant in the chamber upon waking at 6:30 AM.
Energy balance was calculated as total energy expenditure in the respiratory chamber subtracted from total energy intake of food consumed during that time. While in the chamber, participants consumed 3 scheduled meals with a macronutrient distribution of 20–23% energy from fat, 16–23% as protein, and 55–64% as carbohydrate. Total kilocalories were calculated from the Harris-Benedict Equation (19) multiplied by an activity factor of 1.2, and participants were required to consume all food provided.
Fasting insulin, acute insulin response to glucose (AIRg), and insulin sensitivity were assessed by a tolbutamide-modified, frequently sampled intravenous glucose tolerance test (IVGTT) with minimal modeling (MINMOD version 3.0; c Richard N Bergman) (20). Details of this test have been previously described (21). Briefly, intravenous catheters were placed in both arms. Three blood samples were drawn over 20 min for fasting glucose and insulin measurements. At time0 min, a bolus of 50% dextrose was administered intravenously at a dose of 11.4 g/m2. A series of 29 blood samples were obtained over the following 3 hours for measurements of serum glucose and insulin. At time20 min, a bolus of Tolbutamide (125 mg/m2) was injected intravenously. Insulin sensitivity is expressed as the insulin sensitivity index (SI) derived from the model. AIRg represents the integrated incremental area under the curve for insulin during the first 10 minutes of the test. Glucose and insulin were analyzed in the Core Laboratory of UAB’s GCRC and Clinical Nutrition Research Center. Glucose was measured in 10 μl sera using an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics). In the Core Laboratory, this analysis has a mean intra-assay coefficient of variation (c.v.) of 0.61%, and a mean inter-assay c.v. of 1.45%. Insulin was assayed in duplicate200 μl aliquots with Diagnostic Products Corporation (Los Angeles, CA) “Coat-A-Count” kits. This assay has a sensitivity of 1.9 μIU/ml, a mean intra-assay c.v. of 5%, and a mean inter-assay c.v. of 6%. Commercial quality control sera of low, medium, and high insulin concentration (“Lyphochek”, Bio-Rad, Anaheim, CA) were included in every assay to monitor variation over time.
Fat mass (FM) and fat-free mass (FFM) were determined by dual-energy X-ray absorptiometry (DXA; DPX-L; Lunar Radiation Corp, Madison, WI), using ADULT software, version 1.33 (Lunar Radiation Corp). Change in fat mass (ΔFM) was calculated as the difference in fat mass between the baseline and the annual follow-up evaluations. Height (stretch stature to the nearest 0.1 cm) was measured by a stadiometer, and body weight (to the nearest 5g) was recorded on a digital scale following an overnight fast. Body mass index (BMI, kg/m2) was calculated from weight and height.
Statistical Analysis
Descriptive statistics were calculated for all variables of interest. Results are presented as means ± standard deviations (SD). Fasting insulin, AIRg, and SI were log-10 transformed for normality prior to statistical analyses. Pearson correlations were used to examine associations between each RQ measure and ΔFM. Because NSRQ was the only RQ value correlated with ΔFM, multiple linear regression analysis was used to further examine the relationship between baseline NSRQ and subsequent ΔFM over the following 2 years. To ensure that the relationship between NSRQ and ΔFM was independent of individual differences in insulin action, regression models were adjusted for energy balance and either circulating insulin (fasting insulin or AIRg) or SI. All statistical tests were two-sided with a Type I error rate of 0.05 and were performed using SPSS software, version 15.0 (Chicago, IL 2006).
Results
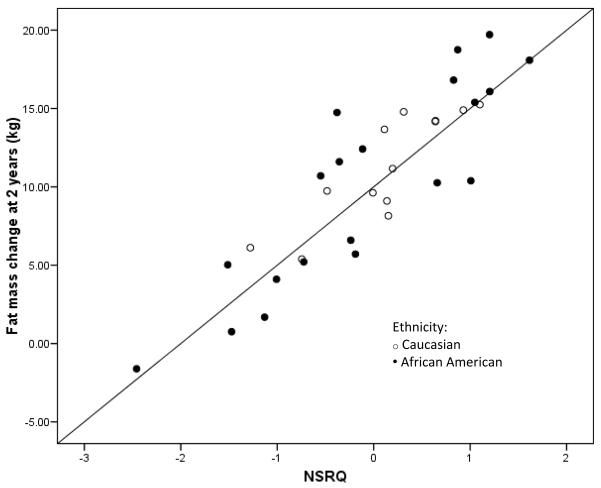
Participant characteristics are displayed in Table 1. By study design, all participants were normal weight (BMI < 25kg/m2) at the baseline evaluation. With the exception of one woman, all participants gained weight and fat mass over the 2-year follow-up period. No significant differences between ethnic groups were observed for any variable (data not shown). Of all RQ values examined, only NSRQ correlated with ΔFM (r = 0.388, p = 0.026 at 1 y). Multiple linear regression analysis revealed that NSRQ predicted ΔFM at both 1 and 2 years after adjusting for energy balance and either circulating insulin or insulin sensitivity. Final models that explained the most variance in ΔFM included NSRQ, energy balance, and SI (Table 2 and Figure 1). Similar trends were observed when statistical analyses were performed with Δweight (β for NSRQ = 63.99 ± 34.87, p = 0.08) and percent ΔFM (β for NSRQ = 43.18 ± 23.02, p = 0.07) as dependent variables in place of ΔFM.
Table 1.
Subject characteristics (mean ± SD and range)
| Baseline characteristics*: | Age(y) | 37.1 ± 5.6 (22.9 to 47.3) |
| African American/Caucasian | 20/13 | |
| Body Weight (kg) | 65.3 ± 6.9 (50.5 to 78.0) | |
| BMI (kg/m2) | 24.1 ± 1.1 (21.6 to 25.8) | |
| Fat Mass (kg) | 21.3 ± 4.2 (15.4 to 29.7) | |
| Fat-free Mass (kg) | 40.6 ± 4.4 (30.9 to 49.4) | |
| Fasting Insulin (μIU/ml) | 7.3 ± 2.4 (3.0 to 14.0) | |
| AIRg [(μIU/ml) × 10min] | 570.9 ± 362.0 (95.0 to 1689.5) | |
| SI [× 10−4 min−1/(μIU/ml)] | 6.5 ± 3.3 (2.22 to 16.80) | |
| 24-h Energy Expenditure (kcals) | 1620.9 ± 207.1 (1138.3 to 2038.5) | |
| Energy Balance (kcals) | −200.7 ± 171.6 (−571.0to 163.3) | |
| 24-h RQ | 0.88 ± 0.03 (0.81 to 0.94) | |
| Fasting RQ | 0.86 ± 0.04 (0.77 to 0.94) | |
| Sleeping RQ | 0.84 ± 0.04 (0.75 to 0.92) | |
| NSRQ | 0.89 ± 0.03 (0.82 to 0.96) | |
| Measurements at 1-y: | Δ Weight (kg) | + 7.2 ± 4.5 (−3.2 to 14.2) |
| Δ Fat Mass (kg) | + 6.9 ± 3.8 (−1.8 to 12.8) | |
| % Weight Δ | +11.1 ± 7.3 (−4.0 to 24.0) | |
| % Fat Mass Δ | + 6.5 ± 3.2 (−0.1 to 12.1) | |
| Measurements from 2-y: | Δ Weight (kg) | + 11.1 ± 6.1 (−3.6 to 19.4) |
| % Weight Δ | +17.2 ± 9.7 (−5.0 to 33.0) | |
| Δ Fat Mass (kg) | + 10.6 ± 5.4 (−1.6 to 19.7) | |
| % Fat Mass Δ | + 9.2 ± 4.4 (−0.8 to 17.1) |
Recorded after 4 weeks of a controlled diet for weight maintenance following successful completion of a weight loss intervention
Table 2.
Multiple linear regression models
| y = ΔFM at 1 y (F = 1.83, p = 0.16) | |||
|---|---|---|---|
| Independent variable | Parameter Estimate ± SEE | P | Model R2 |
| Intercept | −38.953 ± 19.767 | 0.058 | 0.159 |
| NSRQ | 50.160 ± 21.454 | 0.026 | |
| Energy balance | <−0.001 ± 0.004 | 0.985 | |
| Log10 SI | 1.708 ± 3.627 | 0.641 | |
| y = ΔFM at 2 y (F = 2.90, p = 0.05) | |||
|---|---|---|---|
| Independent variable | Parameter Estimate ± SEE | P | Model R2 |
| Intercept | −54.055 ± 26.951 | 0.054 | 0.231 |
| NSRQ | 64.018 ± 29.251 | 0.037 | |
| Energy balance | −0.003 ± 0.006 | 0.554 | |
| Log10 SI | 9.255 ± 4.945 | 0.071 | |
Figure 1.
Standardized residual plot of NSRQ vs. ΔFM adjusted for energy balance and SI
Discussion
Inherent individual variability in substrate oxidation may predispose to obesity. In this study, we found that among premenopausal obesity-prone women, high NSRQ predicted ΔFM independent of energy balance, circulating insulin, and insulin sensitivity. No association was observed between fat accumulation and other RQ values, including fasting, sleeping, and 24-hour RQ. These results suggest that low postprandial fat oxidation and/or elevated post-prandial carbohydrate oxidation may predispose certain women to weight gain and excess adiposity.
Results of this study advance existing evidence about the relationship between RQ and weight gain. Although some previous studies have reported positive associations between weight gain and either fasting or 24-hour RQ (4–8, 22), we have reported no correlation (10–12). Incongruent reports may reflect population-specific differences in energy metabolism. Notably, several studies supporting an association between weight gain and high 24-hour RQ were conducted among Pima Indians (5, 6, 22), and recent genetic studies have identified Single Nucleotide Polymorphisms specific to this population that may underlie these findings (23). Discrepancies in the literature are also likely due to variable control for dietary factors known to acutely influence RQ. For example, it is well-established that either positive energy balance or high carbohydrate intake can transiently elevate RQ (3). However, few studies relating RQ to weight gain have accounted for differences in macronutrient intake and/or energy balance between subjects. In the present study, all participants were provided with food for 4 weeks prior to RQ measurement in order to ensure uniform macronutrient intake and stable body weight. During their stay in the respiratory chamber, diet was controlled to provide a standard macronutrient profile and kilocalories for energy balance. Additionally, we used statistical methods to adjust for energy balance inside the respiratory chamber.
Individual differences in circulating insulin and/or insulin sensitivity may also confound reported relationships between weight gain and RQ. Circulating insulin suppresses lipid oxidation and facilitates fat storage (13). Among a cohort of 293 Caucasian women, Nagy et al. demonstrated that fasting insulin was inversely associated with fat oxidation (14). Similarly, Weinsier et al. reported that fasting insulin predicted 4-year weight gain among Caucasian, postmenopausal, normoglycemic women (10). Insulin sensitivity has been associated with fat accumulation in some (15–17), but not all (24) studies. Consistent with these findings, SI among our sample showed a trend toward positive association with ΔFM after 2 years (r = 0.316, p = 0.073). By statistically adjusting for fasting insulin, AIRg, and SI, we assured that the observed relationship between ΔFM and NSRQ was not influenced by confounding relationships between insulin and RQ or FM. The final multiple linear regression model that included NSRQ, energy balance, and SI explained ~23% of the variance in ΔFM after 2 years.
Our previous analyses among African American and Caucasian women with tight dietary control also identified no relationship between weight gain and other RQ measures, including fasting, 24-hour, or sleeping RQ (10–12). These three RQ measures encompass periods of the day when RQ should signify fat oxidation of endogenous free fatty acids. NSRQ, however, is measured specifically during the non-sleeping period in the respiratory chamber and would correspond to the typical, physiological postprandial period. Sources of variability in NSRQ may include endocrine factors such as concentrations of estrogen (25) or thyroid function (26). In the present study, NSRQ distinctively and independently predicted fat mass accrual. High NSRQ may represent suppressed oxidation of dietary lipid and/or an enhanced ability to switch to carbohydrate oxidation in response to a mixed substrate meal. If lipid oxidation is suppressed, these individuals may be less able to increase fat oxidation after dietary fat intake, and they may therefore be prone to store fat as adipose leading to a partitioning of energy towards fat mass at the expense of lean mass. Moreover, previous studies have suggested that greater carbohydrate oxidation and lesser fat oxidation may predispose certain individuals to store less glycogen and therefore experience more hunger (22, 27). This increased hunger may lead to chronic positive energy imbalance and gain in body fat.
The study was strengthened by robust measures for all variables, including IVGTT for insulin sensitivity, chamber calorimetry for RQ assessments, and DXA for body composition. Strengths of this study also included tight control of dietary intake prior to and during RQ measurements. Despite attempts to achieve zero energy balance within the chamber, variability in energy balance may present a potential confounder. Therefore, all models included statistical adjustment for energy balance. Efforts were made to minimize confounders by the homogeneity of the cohort. However, because we examined a very specific subject population of normal glucose tolerant, premenopausal women of only African American and European American ethnicity, it is impossible to extrapolate these findings to other ethnicities, men, older women, never-overweight individuals, or those with impaired glucose tolerance. Given the limited sample size and overall significance of the models, results should be interpreted with caution and repeated in larger samples.
In conclusion, the current study revealed that among obesity-prone premenopausal women, NSRQ uniquely predicted ΔFM independent of energy balance and insulin sensitivity. Because all of the women in our cohort reported a family history of overweight or obesity in a first degree relative, high NSRQ may be a marker for non-modifiable genetic factors affecting their capacity to oxidize lipid and carbohydrate. Studies are needed to determine whether these women may experience more success with weight maintenance by modifying their food choices to reduce fat intake or by modifying their physical activity to enhance fat oxidation.
Acknowledgments
This work was supported byR01DK49779, M01-RR-00032, and P30-DK56336. Stouffer’s Lean Cuisine provided food used prior to metabolic testing. David Bryan and Robert Petri provided technical assistance; Maryellen Williams and Kangmei Ren conducted laboratory analyses; Susan Davies and Paul Zuckerman served as project coordinators.
Footnotes
Disclosure: No conflict of interest.
References
- 1.National Center for Health Statistics. Obesity and Overweight: Data and Statistics. 2008 http://www.cdc.gov/obesity/data/index.html.
- 2.Weiss E, Galuska D, Kettel Khan L, Gillespie C, Serdula M. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007 Jul;33(1):34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Schutz Y. Abnormalities of fuel utilization as predisposing to the development of obesity in humans. Obes Res. 1995 Sep;3( Suppl 2):173S–8S. doi: 10.1002/j.1550-8528.1995.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 4.Hainer V, Kunesová M, Parizková J, Stich V, Mikulová R, Slabá S. Respiratory quotient in obesity: its association with an ability to retain weight loss and with parental obesity. Sb Lek. 2000;101(1):99–104. [PubMed] [Google Scholar]
- 5.Weyer C, Pratley R, Salbe A, Bogardus C, Ravussin E, Tataranni P. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000 Mar;85(3):1087–94. doi: 10.1210/jcem.85.3.6447. [DOI] [PubMed] [Google Scholar]
- 6.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba B, Raz I, Saad M, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990 Nov;259(5 Pt 1):E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 7.Marra M, Scalfi L, Covino A, Esposito-Del Puente A, Contaldo F. Fasting respiratory quotient as a predictor of weight changes in non-obese women. Int J Obes Relat Metab Disord. 1998 Jun;22(6):601–3. doi: 10.1038/sj.ijo.0800612. [DOI] [PubMed] [Google Scholar]
- 8.Marra M, Scalfi L, Contaldo F, Pasanisi F. Fasting respiratory quotient as a predictor of long-term weight changes in non-obese women. Ann Nutr Metab. 2004;48(3):189–92. doi: 10.1159/000079556. [DOI] [PubMed] [Google Scholar]
- 9.Seidell J, Muller D, Sorkin J, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1992 Sep;16(9):667–74. [PubMed] [Google Scholar]
- 10.Weinsier R, Nelson K, Hensrud D, Darnell B, Hunter G, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women. J Clin Invest. 1995 Mar;95(3):980–5. doi: 10.1172/JCI117807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinsier R, Hunter G, Desmond R, Byrne N, Zuckerman P, Darnell B. Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight. Am J Clin Nutr. 2002 Mar;75(3):499–504. doi: 10.1093/ajcn/75.3.499. [DOI] [PubMed] [Google Scholar]
- 12.Weinsier R, Hunter G, Zuckerman P, Darnell B. Low resting and sleeping energy expenditure and fat use do not contribute to obesity in women. Obes Res. 2003 Aug;11(8):937–44. doi: 10.1038/oby.2003.129. [DOI] [PubMed] [Google Scholar]
- 13.Galgani J, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008 Nov;295(5):E1009–17. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagy T, Goran M, Weinsier R, Toth M, Schutz Y, Poehlman E. Determinants of basal fat oxidation in healthy Caucasians. J Appl Physiol. 1996 May;80(5):1743–8. doi: 10.1152/jappl.1996.80.5.1743. [DOI] [PubMed] [Google Scholar]
- 15.Travers S, Jeffers B, Eckel R. Insulin resistance during puberty and future fat accumulation. J Clin Endocrinol Metab. 2002 Aug;87(8):3814–8. doi: 10.1210/jcem.87.8.8765. [DOI] [PubMed] [Google Scholar]
- 16.Maffeis C, Moghetti P, Grezzani A, Clementi M, Gaudino R, Tatò L. Insulin resistance and the persistence of obesity from childhood into adulthood. J Clin Endocrinol Metab. 2002 Jan;87(1):71–6. doi: 10.1210/jcem.87.1.8130. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman R, Stumbo P, Janz K, Nielsen D. Altered insulin resistance is associated with increased dietary weight loss in obese children. Horm Res. 1995;44(1):17–22. doi: 10.1159/000184584. [DOI] [PubMed] [Google Scholar]
- 18.Ravussin E, Lillioja S, Anderson T, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986 Dec;78(6):1568–78. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris J, Benedict F. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918 Dec;4(12):370–3. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergman R, Cobelli C. Minimal modeling, partition analysis, and the estimation of insulin sensitivity. Fed Proc. 1980 Jan;39(1):110–5. [PubMed] [Google Scholar]
- 21.Gower B, Weinsier R, Jordan J, Hunter G, Desmond R. Effects of weight loss on changes in insulin sensitivity and lipid concentrations in premenopausal African American and white women. Am J Clin Nutr. 2002 Nov;76(5):923–7. doi: 10.1093/ajcn/76.5.923. [DOI] [PubMed] [Google Scholar]
- 22.Pannacciulli N, Salbe A, Ortega E, Venti C, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007 Sep;86(3):625–32. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chamala S, Beckstead W, Rowe M, McClellan D. Evolutionary selective pressure on three mitochondrial SNPs is consistent with their influence on metabolic efficiency in Pima Indians. Int J Bioinform Res Appl. 2007;3(4):504–22. doi: 10.1504/IJBRA.2007.015418. [DOI] [PubMed] [Google Scholar]
- 24.Johnson M, Figueroa-Colon R, Huang T, Dwyer J, Goran M. Longitudinal changes in body fat in African American and Caucasian children: influence of fasting insulin and insulin sensitivity. J Clin Endocrinol Metab. 2001 Jul;86(7):3182–7. doi: 10.1210/jcem.86.7.7665. [DOI] [PubMed] [Google Scholar]
- 25.Lwin R, Darnell B, Oster R, Lawrence J, Foster J, Azziz R, et al. Effect of oral estrogen on substrate utilization in postmenopausal women. Fertil Steril. 2008 Oct;90(4):1275–8. doi: 10.1016/j.fertnstert.2007.07.1317. [DOI] [PubMed] [Google Scholar]
- 26.Klieverik L, Coomans C, Endert E, Sauerwein H, Havekes L, Voshol P, et al. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 2009 Dec;150(12):5639–48. doi: 10.1210/en.2009-0297. [DOI] [PubMed] [Google Scholar]
- 27.Eckel R, Hernandez T, Bell M, Weil K, Shepard T, Grunwald G, et al. Carbohydrate balance predicts weight and fat gain in adults. Am J Clin Nutr. 2006 Apr;83(4):803–8. doi: 10.1093/ajcn/83.4.803. [DOI] [PubMed] [Google Scholar]