Abstract
Studies on myocardial function have shown that hsp70, stimulated by an increase in temperature, leads to improved survival following ischemia reperfusion (I-R). Low frequency electromagnetic fields (EMF) also induce the stress protein hsp70, but without elevating temperature. We have examined the hemodynamic changes in concert with EMF preconditioning and the induction of hsp70 to determine whether improved myocardial function occurs following I-R injury in Sprague-Dawley rats. Animals were exposed to EMF (60Hz, 8µT) for 30 minutes prior to I-R. Ischemia was then induced by ligation of left anterior descending coronary artery (LAD) for 30 minutes, followed by 30 minutes of reperfusion. Blood and heart tissue levels for hsp70 taken at 10 minute intervals were determined by Western blot and RNA transcription by rtPCR. Significant upregulation of the HSP70 gene and increased hsp70 levels were measured in response to EMF pre-exposures. Invasive hemodynamics, as measured using a volume conductance catheter, demonstrated significant recovery of systolic contractile function after 30 minutes of reperfusion following EMF exposure. Additionally, isovolemic relaxation, a measure of ventricular diastolic function, was markedly improved in EMF-treated animals. In conclusion, noninvasive EMF induction of hsp70 preserved myocardial function and has the potential to improve tolerance to ischemic injury.
Keywords: stress response, hsp70, ischemia-reperfusion
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality in the United States, accounting for 70–80 percent of deaths in men and women over the age of 65. Furthermore, congestive heart failure is the most common cause of hospitalization of the elderly, and its incidence continues to increase (Schneider, 1999). In open and percutaneous revascularization procedures (coronary artery bypass surgery and percutaneous coronary interventions) and in the treatment of myocardial infarction and heart failure, it is essential to protect cardiomyocytes from the effects of hypoxia and ischemia (Bolli et al., 2004). Currently, myocardial protection can be accomplished by induction of the stress protein hsp70 through the use of elevated temperature (heat shock) (Currie et al., 1993; Nitta et al., 1994; Plumier and Currie, 1996; Udelsman et al., 1993). Induction of stress proteins by heat to prevent stroke and myocardial infarction during reperfusion has been shown to partially protect the myocardium under ischemic stress in a variety of models (Benjamin and McMillan, 1998; Chong et al., 1998; Cornelussen et al., 1998; Heads et al., 1995; Mestril et al., 1996; Plumier and Currie, 1996). The use of heat stress pre-treatment leads to moderate increases in hsp70 levels, but does not improve ischemia tolerance in isolated hearts (Cornelussen, et al., 1998). Moreover, heat stress pretreatment (hyperthermia) is of limited clinical utility since it requires a temperature elevation to 42°C, a level impractical for clinical use or to achieve sufficient hsp70 increases.
We have shown previously that 60 Hz electromagnetic fields upregulate the heat shock gene, HSP70 and induce elevated levels of hsp70 protein in the absence of elevated temperature (Carmody, et al., 2000; Goodman, et al., 1994; Goodman and Blank, 1998; Han et al., 1998; Lin et al., 1998; 1999; 2001). Of particular relevance, we previously elevated hsp70 levels in cultured rodent cardiomyocytes using EMF pre-treatment (Goodman and Blank, 2002). Additionally, studies from DiCarlo et al. (1999) and Shallom et al. (2002) confirmed that cardiomyocytes were protected from anoxic damage in EMF exposed chick embryos.
The induction of increased levels of hsp70 protein by low frequency EMF exposures offers multiple clinical advantages over thermal, chemical or gene-transfer methods of induction for both patient and clinician. EMF stimulation of cytoprotective proteins is a non-invasive procedure easily administered to the patient. EMF-induced hsp70 does not turn off baseline protein synthesis, in contrast to elevated temperature (Goodman et al, 1989). A significant increase in hsp70 stress protein is induced within five minutes at 14 orders of magnitude lower energy input than thermal stress. Additionally, unlike thermal stress, the induced protection can be re-stimulated even after the stress is already present, and re-stimulation with even higher hsp70 levels can be induced by a different field strength, higher (800 mG) or lower (8mG) (Blank et al., 1994; Lin et al., 1997).
Extensive reports in the literature have shown that elevated levels of hsp70 improve cardiac function after hypoxic stress and ischemia-reperfusion (I-R) (Suzuki et al, 1997; 2000; 2002). In this set of experiments, we evaluated whether pre-treatment with EMF-induced levels of hsp70 can preserve myocardial function after ischemia-reperfusion.
Methods
Animal Care
Male Sprague-Dawley rats (250–400g) were selected as the experimental species. To assure their health, the animals were examined by the animal facility veterinarian upon arrival. Animals were allowed to acclimate for at least two days and adjust to being handled before randomization into the study. Animals were housed in cages in an environmentally controlled room within the animal care facility at Columbia University. Care and management of rats was conducted according to facility standard operating procedures. At the conclusion of the acclimation period animals judged to be suitable for testing were assigned sequentially to either treatment or control. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). This study was approved by the Institutional Animal Care and Use Committee of Columbia University.
Electromagnetic field exposures
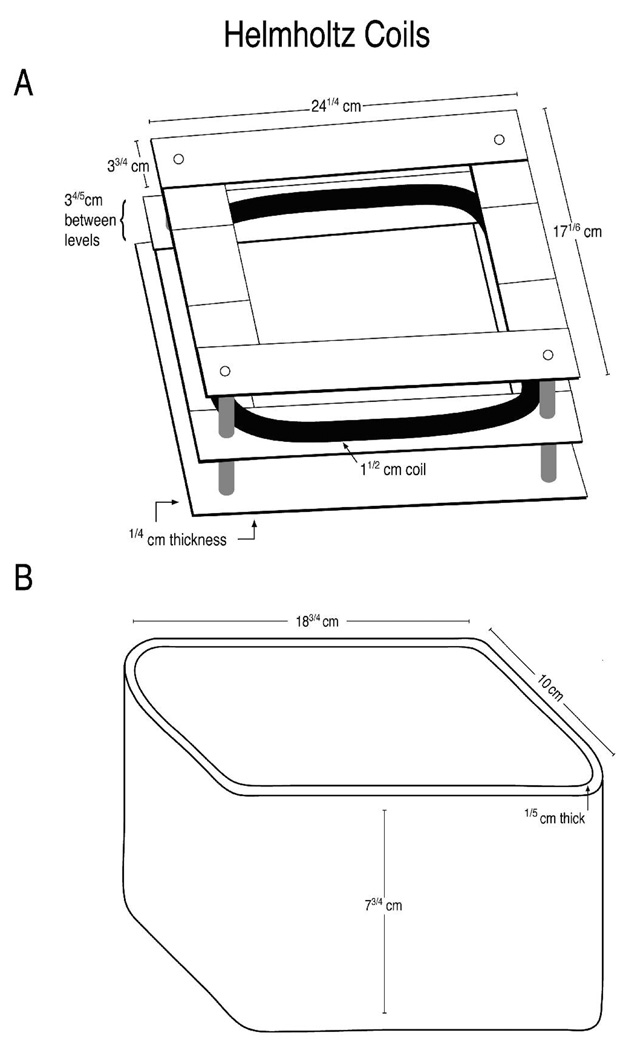
All EMF exposures were performed prior to induction of ischemia. The exposures described here are pre-treatment exposures. In previous studies, we tested a variety of field strengths and frequencies (Goodman et al., 1989; Jin et al., 1997; Wei et al., 1990), and eventually determined that a 60Hz frequency and a field strength of 8µT consistently induced the highest level of transcriptional activation of the HSP70 gene and the highest hsp70 protein levels (rev. in Goodman and Blank, 2002). In the studies reported here, animals were exposed to 60Hz/ 8µT EM fields in a plastic exposure cage (16 cm×24 cm) surrounded by Helmholtz coils (19 gauge copper wire, 164 turns, 1.5 inches thick covered with electrical tape). The system was designed and calibrated (R. Cangialosi, Electro-Biology Inc., Fairfield, NJ) to our specifications for comfortably holding a large rodent; the cage holding the rats was suspended in a plastic enclosure (Figure 1). EM field conditions were set using a function generator (BK Precision 4011A 5MHz, Yorba Linda, CA) and digital multimeter (BK Precision 2706A). Rectal temperature was continuously monitored with a thermocouple probe (+/− 0.1°C resolution; PhysiTemp., Cliffside Park, NJ). The digital multimeter was used to measure the field intensity and verify the systems operation. Field parameters were monitored with a Hitachi V-1065 100MHz oscilloscope and a calibrated inductive search coil. Exposure conditions were monitored with a Sypris triaxial magnetic field meter (Model 4080, Bell Laboratories, Orlando, FL). Experiments were carried out at room temperature (approx. 25°C).
Figure 1. EMF Exposure System.
Animals were exposed to 60Hz/ 8µT EM fields by Helmholtz coils (19 G copper wire, 164 turns, 1.5 inches thick covered with electrical tape) (Panel A) that was contained within a plastic exposure cage (Panel B). The EMF field was perpendicular to the exposure device.
Anesthesia
Rats were anesthetized with 2% isoflurane and mechanically ventilated via a tracheostomy (Harvard rodent ventilator, model 683, South Natick, MA) throughout the duration of the experiment.
Surgical Procedures
Blood collection for hsp70 determination
The left femoral vein was cannulated with polyethylene tubing (OD 0.965 mm, Becton Dickinson, Franklin Lakes, NJ) for repeated blood draws. Rats were then randomized to EMF exposure (60Hz 8µT) for 30 minutes (n=6) or Control (no EMF exposure, n=6). Blood was collected at baseline (pre-exposure) and after EMF exposure every 30 minutes up to 120 minutes. Serum was spun down at 1200 rpm for 10 minutes; packed red blood cells were flash-frozen for hsp70 protein analyses, and stored at −80°C.
Terminal tissue for HSP70 RNA determination
Heart tissue (left ventricle; LV) was flash-frozen for analysis of HSP70 RNA by reverse-transcriptase polymerase chain reaction (rtPCR). GAPDH confirmed loading concentrations.
Ischemia-Reperfusion Protocol
The hemodynamic effects of EMF exposure on myocardial function after I-R (EMF n=10, Control n=10) were measured using the following protocol. Pre-anesthetized animals were randomly assigned to EMF pre-treatment and underwent EMF exposure (60Hz, 8µT) for 30 minutes. During this time period, each individual animal was allowed to rest comfortably in the exposure device under quiet conditions. No medications were administered prior or during this time. Animals designated as controls received no exposure and their cages were maintained in an EMF-free area and monitored with a Sypris triaxial gaussmeter. At the conclusion of 30 minutes, all rats (both exposed and control) were anesthetized with 2% isoflurane and mechanically ventilated on 2% isoflurane after a tracheostomy. A 2 Fr volume conductance catheter (Millar Instruments, Houston, TX) was inserted into the left ventricle (LV) for continuous pressure-volume (PV) tracings immediately following right carotid artery cannulation. After left thoracotomy, coronary ischemia was created by ligating the left anterior descending (LAD) coronary artery with a 5-0 polypropylene suture for a total of 30 minutes before release of the suture. Reperfusion was monitored for an additional 30 minutes. Hemodynamics (Chart for Windows v5, ADI Instruments, Colorado Spring, CO) were recorded at baseline (pre-ischemia), 30 minutes of ischemia, and at 1, 10, 20, and 30 minutes after reperfusion. Upon completion of the experiment, the heart was excised, and the LV was sectioned for histology. Ischemic and non-ischemic portions of the LV were flash-frozen for extraction of protein for Western blot analysis of hsp70 levels and RNA extraction for reverse-transcriptase polymerase chain reaction (rtPCR) to determine upregulation of the HSP70 gene.
Hemodynamics and Pressure-Volume Analysis
Hemodynamic determinations were made on all rats undergoing I-R (n=20). LV end-systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), and LV volume were measured using the Millar conductance catheter placed into the LV across the aortic valve. End-systolic volume (ESV) and end-diastolic volume (EDV) were measured using standard techniques (Ito et al., 1996). Cardiac output (CO), arterial elastance (Ea), and preload-adjusted maximum power (P-A Max Power) were computed using a pressure-volume analysis program (PVAN v. 3.2, Millar Instruments, Houston, TX). The time constant of LV isovolumetric pressure relaxation, τ, was calculated using the logarithmic method described by Raff and Glantz (1981). In experiments where animals had undergone ischemia-reperfusion, the hemodynamic and pressure-volume analyses indicated no adverse events or mortality associated with EMF exposure.
Protein sample preparation
Protein was extracted from myocardial tissue and packed red blood cells (RBCs) using methods previously described (Carmody et al., 2000; Lin et al., 1998). Protein concentrations were determined by Bradford assay (Bio-Rad Laboratories).
Western blot
Equivalent (30 µg) amounts of protein were separated by gel electrophoresis on 10% polyacrylamide gels using appropriate molecular weight markers and transferred to PVDF membrane for immunoblotting. Blots were probed with anti-hsp70 antibody (1:10,000; kindly provided by Dr. Richard Morimoto, Northwestern University). The blots were then stripped and reprobed with anti-β actin (1:1000, Sigma-Aldrich, St. Louis, MO) to confirm equivalent loading. Visualization was by the ECL detection system as previously described (Lin et al., 1998).
Reverse-transcriptase polymerase chain reaction (rtPCR)
Total RNA was extracted from non-ischemic and ischemic left ventricular (LV) heart tissue using Trizol reagent (Life Technologies Inc., Rockville, MD). Total RNA (0.1 µg) was processed directly to cDNA synthesis using the TaqMan® Reverse Transcriptase Reagents kit (Applied Biosystems, Foster City, CA). All PCR primers and TaqMan probes were designed using PrimerExpress software (Applied Biosystem) and published sequence data from the NCBI database (Lin et al, 2001).
Quantification of bands on films from Western blots and rtPCR
The films from Western blots and rtPCR analyses were scanned into a computer. The density of the bands was measured using image analysis software (ImageJ v1.38, NIH).
Statistical analysis
Continuous variables are expressed as Mean ± standard error and compared using two-tailed independent t-testing with Levene’s Test for Equality of variances. Categorical variables were compared by χ2 tests. Paired t-testing was used to evaluate significance within groups at multiple time points. For all analyses, a p-value of less than 0.05 was considered statistically significant. All analysis was performed using SPSS software (v. 11.5, Chicago, Ill).
Results
EMF-Induced hsp70 levels
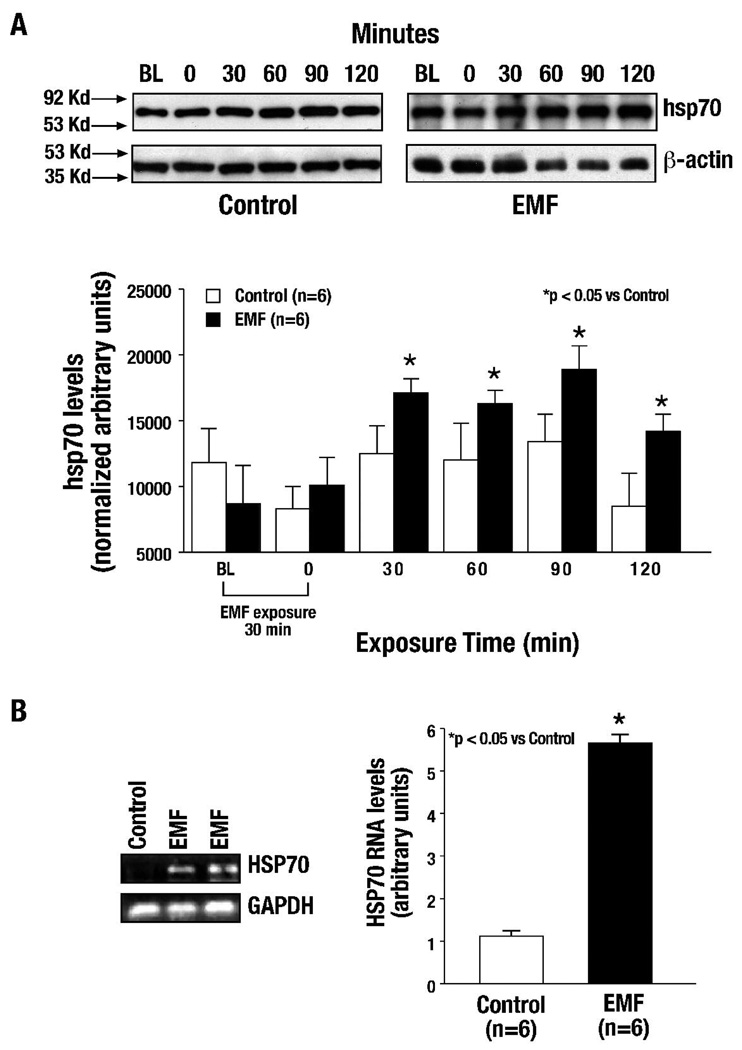
As described above, rats were exposed to EMF (60Hz, 8µT) for 30 minutes, blood samples were collected from the left femoral vein from both EMF-pre-treated and unexposed (control) rats just before (baseline) and immediately after EMF exposure (time 0) and at 30, 60, 90 and 120 minutes. Blood samples were prepared for protein extraction and subsequent Western blot analyses of hsp70. As seen in Figure 2A, the levels of hsp70 protein were significantly elevated following 30 minutes of EMF pre-exposure, and the increase in protein level was sustained for 120 minutes post-exposure (p<0.05 vs. Control, n=6 per group, Figure 2A); peak levels were reached at 30 minutes. Low baseline (constitutive) levels of hsp70 protein exist under non-stressed conditions. After EMF exposure, these levels were significantly elevated (>40%).
Figure 2. hsp70 protein levels and HSP70 RNA transcript levels are progressively elevated after a 30 minute EMF exposure.
(A) In response to 30 minutes of EMF exposure (60 Hz/8 µT), hsp70 levels in blood were significantly elevated at 30 minutes and sustained for 120 minutes as compared to unexposed control samples (*p<0.05 vs. Control). (B) HSP70 RNA transcript levels were significantly increased in terminal myocardial tissue extracts in response to EMF exposure as compared to unexposed control samples (*p<0.05 vs. Control).
EMF-Induced Transcript levels for HSP70 RNA
Terminal myocardial tissue was collected for determination of HSP70 RNA by rtPCR. Transcript levels of HSP70 RNA were significantly increased in terminal myocardial tissue extracts in response to EMF pre-exposure as compared to controls (p<0.05 vs. Control, n=6 per group), demonstrating up-regulation of the HSP70 gene (Figure 2B). There was no increase in HSP70 transcript levels in Control animals during the same time period. These results confirm previous literature that EMF induces upregulation of the HSP70 gene and increases hsp70 protein levels (Lin et al., 1997; 1998; 1999; 2001).
Ischemia-reperfusion: hsp70 levels
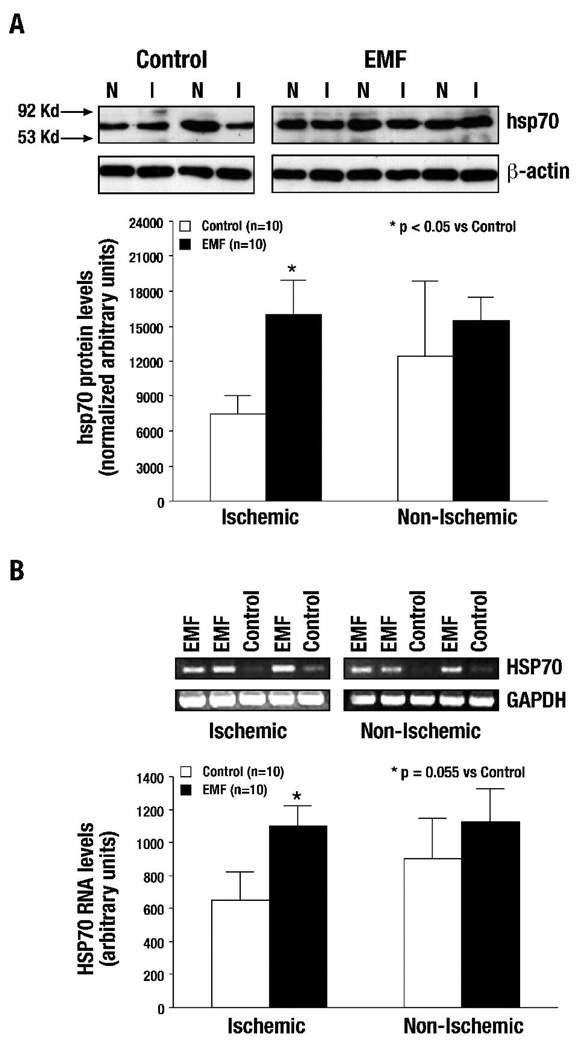
Levels of hsp70 were increased in both non-ischemic (N) and ischemic (I) left ventricle (LV) tissue after the termination of I-R in EMF-exposed samples as compared to Control animals (p<0.05 vs. Control, n=10 per group) (Figure 3A). rtPCR analysis of terminal LV tissue from ischemic (I) and non-ischemic (N), showed increased RNA transcript levels in EMF pre-exposed tissue as compared to controls (p=0.055 vs. Control, n=10 per group) (Figure 3B). The current results confirm that, in this ischemia-reperfusion model, EMF exposure upregulates the HSP70 gene and significantly increases hsp70 levels.
Figure 3. Ischemia-reperfusion: hsp70 protein levels and HSP70 RNA transcript levels were increased significantly following EMF exposure.
(A) Levels of hsp70 were increased in both non-ischemic (N) and ischemic (I) blood samples after ischemia-reperfusion. However, the hsp70 increase in ischemia samples was significant as compared with non-ischemic and control samples (*p<0.05 vs. Control). (B) A significant increase in HSP70 transcript levels was seen in the EMF pre-exposed terminal myocardium tissue from ischemia (I) left ventricular tissue as compared with non-ischemic and control samples (*p=0.055 vs. Control).
Ischemia-reperfusion: Hemodynamics
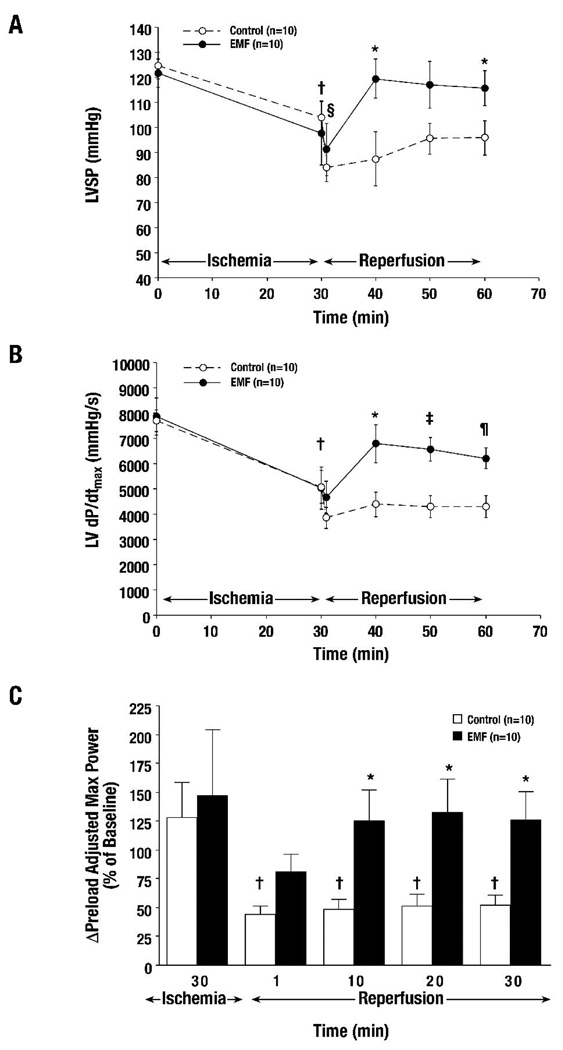
No adverse events or mortality were associated with EMF exposure. The mean temperatures before and after 30 minutes of EMF exposure were 36.1 ± 0.2°C and 36.0 ± 0.2°C, respectively, versus 36.0 ± 1.1°C in Control rats (EMF vs. Control, p=NS). The hemodynamics at baseline, after 30 minutes of coronary ischemia and after 10–30 minutes of reperfusion, are summarized in Table I. LVSP was significantly improved beginning at 10 minutes of reperfusion until 30 minutes in EMF-treated animals versus Control (Figure 4A). No significant differences were seen in LVEDP, ESV, and EDV. Coronary ischemia produced a significant reduction in LV dP/dtmax (a measure of ventricular contractility) after 30 minutes. Recovery of LV dP/dtmax was only seen in EMF-treated groups compared to Control from 10 until 30 minutes after reperfusion (Figure 4B). Pre-load adjusted maximum power, a load and heart rate independent index of systolic contractile function (the ability of the heart to eject blood), was similarly improved in EMF-treated rats after reperfusion, as shown in Figure 4C. These results, interpreted collectively, show that overall organ perfusion was increased during reperfusion (manifested by higher LVSP). The ability to increase ventricular pressure at the time of reperfusion despite ischemic injury, without changes in blood volume, ventricular dimensions, or exogenous pharmacologic agents, implicates a direct mechanism to improve cardiac myocytes, and myocardial systolic contractility. In addition, the lack of ventricular dilatation and absence of end-diastolic pressure elevation implies normal end-systolic and end-diastolic pressure volume relationships. In contrast to acutely failing hearts, which undergo pressure-volume overload, enlargement of ventricular dimensions, and elevated end-diastolic pressures.
Table 1.
Hemodynamics and Pressure-Volume Analysis
| Pre-Isch | 30 min Ischemia |
1 min Reperfusion |
10 min Reperfusion |
20 min Reperfusion |
30 min Reperfusion |
||
|---|---|---|---|---|---|---|---|
| Heart Rate (bpm) | |||||||
| Control | 275 ± 7 | 249 ± 11§ | 220 ± 10‖ | 221 ± 9‖ | 224 ± 8‖ | 226 ± 8‖ | |
| EMF | 269 ± 10 | 260 ± 9 | 246 ± 8‖ | 259 ± 10† | 254 ± 11* | 244 ± 10 | |
| LVEDP (mmHg) | |||||||
| Control | 9.9 ± 1.6 | 15.7 ± 3.1§ | 13.3 ± 2.1 | 13.2 ± 2.0 | 13.1 ± 1.9 | 13.2 ± 1.8 | |
| EMF | 9.7 ± 1.2 | 15.1 ± 2.1§ | 13.7 ± 2.1 | 15.28 ± 2.1 | 16.4 ± 2.9 | 16.3 ± 2.9 | |
| Cardiac Output (ml/s) | |||||||
| Control | 667 ± 210 | 385 ± 109 | 214 ± 42 | 286 ± 95 | 279 ± 100 | 375 ± 136 | |
| EMF | 467 ± 100 | 318 ± 60 | 224 ± 28 | 334 ± 57 | 328 ± 50 | 297 ± 47 | |
| Ea (mmHg/ml) | |||||||
| Control | 106 ± 23 | 148 ± 51 | 154 ± 40 | 151 ± 42 | 177 ± 45 | 164 ± 45 | |
| EMF | 105 ± 23 | 105 ± 29 | 108 ± 9 | 112 ± 15 | 109 ± 17 | 115 ± 17 | |
p<0.05 vs. Control,
p<0.01 vs. Control,
p<0.05 vs. Baseline,
p<0.05 vs. 30 min Ischemia
EMF-Electromagnetic Field, LVEDP-Left Ventricular End-Diastolic Pressure, Ea-Arterial Elastance
Figure 4. Ischemia-reperfusion: Contractile function was significantly improved in response to EMF exposure.
Contractile (systolic) function was significantly increased after ischemia-reperfusion in response to 30 minutes of EMF exposure, as measured by ventricular systolic pressure (LVSP) (Figure 4A), left ventricular dP/dt (LV dP/dtmax,) (Figure 4B), and preload adjusted maximum power (P-A Max Power) (Figure 4C) († p<0.05 vs. Baseline, § p<0.05 vs. Ischemia 30, * p<0.05 vs. Control, ‡ p<0.01 vs. Control, ¶ p<0.005 vs. Control).
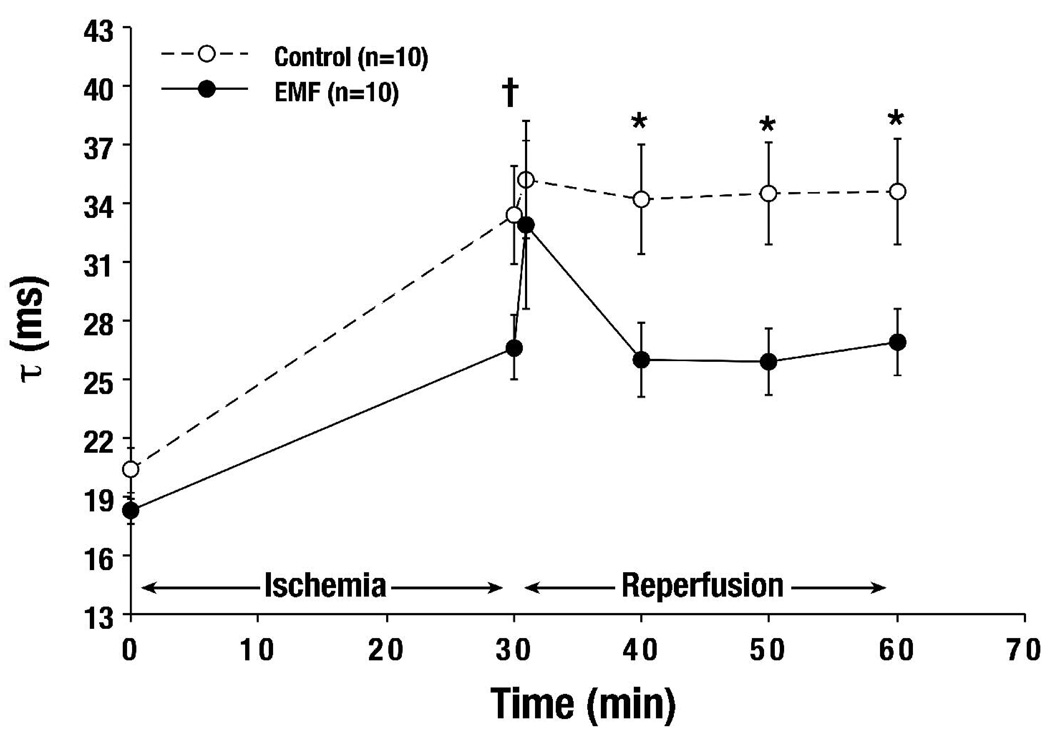
The contribution of diastolic function was also evaluated by calculating the time constant of LV isovolemic relaxation. A slight, but statistically significant decrease in τ (a measure of diastolic function) was observed throughout the course of reperfusion after EMF treatment (Figure 5), indicating improved ventricular wall compliance, allowing faster and greater diastolic filling. Greater diastolic filling and relaxation of the ventricular wall improves cardiac output, lessens myocardial oxygen consumption, and increases the overall efficiency of the heart. Although no significant changes in arterial elastance were seen, a strong recovery trend of cardiac output to 71% of baseline with EMF versus only 43% in Control was observed. However, adverse changes in arterial elastance would potentially affect long-term remodeling of central arteries, leading to multi-organ dysfunction (most notably, renal failure).
Figure 5. Ischemia-reperfusion: Diastolic function was significantly improved in response to EMF exposure.
Diastolic function, or isovolemic ventricular relaxation as measured by Tau (τ), was significantly improved throughout Reperfusion after 30 minutes of EMF exposure († p<0.05 vs. Baseline, * p<0.05 vs. Control).
In summary, a clear effect on systolic contractile function in EMF-treated animals was found after reperfusion, as shown by increases in global indices of contractile function (LV dP/dtmax and pre-load adjusted maximum power). These changes occurred without evidence of concurrent LV hypertrophy or at the expense of reduced diastolic function, and without increased ventricular dimensions, which would have been expected with pressure-volume overload seen after ischemic injury. The changes in diastolic function may also reflect an effect of hsp70 on stabilization of cellular structure.
Discussion
The cytoprotective effect of stress response proteins, specifically hsp70, as a cellular defense mechanism, has been well described in the literature (Brochierri et al., 2008; Liu et al., 2007; Westerheide and Morimoto, 2005; Zhao et al., 2007). Myocardial preservation after HSP70 gene transfection was first reported by Suzuki et al. (1997) who showed that increased hsp70 protein resulted in improved recovery of coronary flow, maximum LV dP/dt, and LV developed pressures. In isolated rat hearts undergoing 30 minutes of ischemia and 30 minutes of reperfusion, post-ischemic mitochondrial respiratory control indices (NAD+- and FAD-linked respiration) were improved in HSP70-transfected animals (Jayakumar et al., 2001). In a similar study, HSP70 gene transfection attenuated creatinine kinase release and preserved coronary endothelial response to vasodilatory agents (Jayakumar et al., 2000). Finally, infarct reduction of almost 50% was demonstrated in HSP70-transfected rabbit hearts after ischemia-reperfusion (Okubo et al., 2001).
In the experiments reported here, we have applied 60Hz, 8µT electromagnetic fields (EMF) to elevate hsp70 levels. Based on prior studies, we hypothesized that EMF exposures would increase levels of hsp70 and be cytoprotective after ischemia-reperfusion. Following ischemia-reperfusion after EMF exposure, we found significantly increased expression of HSP70 gene and elevated hsp70 protein levels, and myocardial function (both systolic and diastolic) was significantly improved.
EMF-Exposure Improves Contractile Function and Reduces Reperfusion Injury
The hemodynamic changes due to EMF exposure prior to ischemia are slight. However, our hemodynamic findings after ischemia-reperfusion demonstrate that EMF-induction of elevated hsp70 levels significantly reduced cardiac injury. Systolic contractile function, as measured by LV dP/dtmax and left ventricular systolic pressure (LVSP), was significantly increased in exposed animals compared to control animals, despite reductions in all groups after 30 minutes of ischemia. Pre-load adjusted power, a pre-load and rate-independent measure of systolic function, was also improved throughout the reperfusion period. Although LVEDP did not change, isovolemic relaxation was substantially improved after EMF exposure, suggesting that diastolic function was not adversely affected in order to maintain hemodynamics.
The changes in ventricular function appear to relate primarily to the ability of the myocyte to tolerate ischemia and prevent injury after reperfusion., After reperfusion, in addition to molecular mechanisms that permanently and directly cause cell death, cardiomyocytes face secondary insults in the form of inadequate coronary perfusion as a result of impaired hemodynamics (i.e. lower systolic pressures, decreased cardiac output, reduced contractility). The degree of ischemic tolerance displayed by EMF-exposed animals to resist the usual hemodynamic derangement after reperfusion is a likely factor in both cell survival and augmentation of existing cell functional units. Subsequently, reduction of infarction, salvage of underperfused myocytes at risk for necrosis, and increased myocardial perfusion are all expected cellular changes (Benjamin and McMillan, 1998; Chong et al., 1998; Cornelussen et al., 1998; Heads et al., 1995; Jayakumar et al., 2000, 2001; Mestril et al., 1996; Okubo et al., 2001; Plumier and Currie, 1996; Suzuki et al., 1997). Improvements in systolic function can serve to interrupt the cycle of death which normally follows impaired contractility and prolonged hypoperfusion. Finally, subtle changes in ventricular compliance (i.e., a reduction in τ) may be critical for diastolic filling after reperfusion, especially in the setting of ischemia and increased myocardial oxygen consumption/wall stress.
Possible Mechanisms for Improved Myocardial Function by EMF
Recent studies have shown strong correlation between myocardial calcium handling to the function of hsp70 – for example, deleting the inducible 70kDa heat shock genes impairs cardiac contractile function and calcium handling associated with hypertrophy (Kim et al., 2006; Okubo et al., 2001). The Na/Ca2+ exchanger, known to play an important determinant of myocardial contractility in heart failure, is acted on by hsp70, leading to desensitization by a reduction in Vmax (Kiang et al., 1998). Hsp70 may also alter protein kinase A, protein kinase C, and phospholipase A2 (Ding et al., 1998); it is conceivable that hsp70 also modulates other components of calcium-handling, such as SERCA2a and phosphorylation of the ryanodine receptor (Marx et al., 2000). These results suggest interaction of hsp70 with elements of the calcium release mechanism that result in enhanced myocardial contractility.
Induction of hsp70 levels by rapid elevation of temperature also improves cytoskeletal-based cell survival pathways (Wei et al., 2006). Maintenance of cell architecture has been reported in the literature previously (Wei et al., 2006) associated with hsp70, and anti-apoptotic mechanisms, such as attenutation of the ASK-JNK/p38 signaling cascades (Fan et al., 2005) of stress response proteins has been documented. There is also evidence that hsp70 has a direct effect on apoptosis by preventing caspase-3 activation, PARP cleavage, DNA laddering, and cell death in vitro, but its actions appear to be downstream of cytchrome c release (Westerheide and Morimoto, 2005). The ATP-binding domain of HSP70 suppresses SAPK/JNK activation, either through suppression of phosphorylation and activation of the upstream kinase SEK, or by inhibition of stress-induced suppression of JNK dephosphorylation (Meriin et al., 1999; Park et al., 2001; Yaglom et al., 1999). A secondary effect of HSP70 gene upregulation is increased manganese superoxide dismutase activity, which serves to limit mitochondrial apoptosis in ischemia-reperfusion (Suzuki et al., 2002). Interestingly, MDA and LDH levels, markers of cardiac injury, were unchanged in our experiments (data not shown); this suggests that EMF does not diminish the extent of cardiac injury but rather enhances the contractile function of remaining myocytes.
EMF and Biological Interactions
EMF interaction with cells and tissues has been extensively studied in vivo and in vitro (rev in Goodman and Blank, 2002; Blank and Goodman, 2004; 2007; Jin et al, 2000). EMF is known to induce elevated DNA transcript levels of several genes including HSP70 as well as elevated levels of hsp70 protein in the absence of increased temperature (reviewed in Goodman and Blank, 1998). The interaction mechanism of EMF with DNA in cells and tissues to stimulate protein synthesis remains unknown. Several theoretical approaches to EMF mechanism have been proposed including cyclotron resonance (Lednev, 1991; Liboff, 1985) and forced vibration of ions (Panagopoulos et al., 2002). There evidence from biochemical reactions that EM fields can accelerate electron transfer and move within DNA (Blank and Soo, 2001; Goodman and Blank, 2002; Blank and Goodman; 2004; 2007).
An important clue to EMF stimulation of biosynthesis comes from identification of a specific EMF-sensitive DNA sequence on both the c-myc and the HSP70 gene promoters (Lin et al., 1999; 2001). The HSP70 promoter has three nCTCTn recognition motifs/response elements (−158 to −203 relative to the transcription initiation site) that are EM field-sensitive (Lin et al., 1999). The heat shock element (HSE), lying between −180 and −203, is required for induction of HSP70 gene expression by EM fields (Lin et al., 1999; 2001). Mutation of the nCTCTn sequences (EMRE, electromagnetic response elements) eliminates the EMF sensitivity of the HSP70 promoter (Lin et al., 2001). The EM field domain and the heat shock domain function independently. This is an HSF-1 dependent process (Lin et al.,1998; Lin et al., 1999; Lin et al., 2001). Furthermore, the HSE in the heat shock domain is not interchangeable with the HSE in the EM field domain (Lin et al., 2001). nCTCTn sequences, placed upstream of CAT or luciferase reporter constructs (that were otherwise unresponsive to EM fields) were transfected into HeLa cells and exposed to EM fields. Protein extracts from EM field-exposed transfectants had significant increases in both CAT and luciferase activity, as compared with identical transfectants that were sham exposed (Lin et al., 2001).
Interaction with electrons could account for activation of DNA by both low and high frequency EM fields. An EM field sensitive DNA sequence suggests that EM fields may interact both directly and indirectly with DNA. The initial interaction could involve the displacement of electrons in the H-bonds that hold DNA together, thereby causing chain separation and initiating transcription and translation. Blank and Goodman (2007), using experimentally observed processes as links in a causal chain, have proposed that DNA activation of transcription is based on EM field’s displacement of electrons in DNA by the EMF and that this causes transient charging of small groups of base pairs (e.g., nCTCTn). At the charged sites disaggregation forces overcome the H-bonds, and this disaggregation of the two chains at those sites permits transcription.
Clinical Use of EMF Technology
An abundance of hsp70 is clearly important to limit myocardial injury, to improve recovery by reducing infarct size, increasing contractile function and limiting myocardial injury following coronary occlusion. Modulation of hsp70 levels in the heart, using heat stimulation, is currently problematic from a temporal standpoint. It is known that a two-fold induction of hsp70 improves heart muscle cell resistance to oxidation, ischemia and hypoxia (Chong et al., 1998; Mestril et al., 1996; Heads et al., 1995). Endogenous hsp70 doubles after only one hour following coronary artery occlusion (Loncar et al., 1998). It may take up to 24 hours for hsp70 levels to reach a four- to five-fold level. The four- to five-fold level has historically been required, at a minimum, to provide improved ischemic tolerance. This is outside the “golden window of opportunity” to protect the myocardium, which is at the greatest risk in the first six hours after artery occlusion. Lower levels of hsp70 have not provided sufficient ischemic tolerance to prevent permanent myocardial damage. In our experiments, augmented hsp70 levels occur as early as 30 minutes after exposure, and last up to 3 hours. This is well-within an average door-to-intervention period for an acute coronary syndrome. The importance of this specificity is that EMF exposure produces hsp70 without the untoward downstream effects of ubiquitous stress response activation.
It is clear that significant potential exists for this technology. It is most applicable to the coronary revascularization population, consisting of patients undergoing coronary artery bypass grafting and percutaneous coronary interventions (PCI). With open-heart surgeries and PCI being performed for expanding indications of revascularization, the need for protective strategies is even more pressing. Both therapies aim to restore coronary flow to underperfused myocardium after periods of critical or sub-acute hypoperfusion. Any portion of myocardium that faces hypoperfusion followed by reperfusion faces the hazards of reperfusion injury (i.e., hemodynamic dysfunction, infarct expansion, or arrhythmias).
In the controlled setting of cardiopulmonary bypass in the operating room or in the angiography suite, electromagnetic field exposure can easily be incorporated into the clinical care protocols. Exposure times of 30 minutes prior to intervention can easily be coordinated with ischemia and timed reperfusion. An EMF device for use in the operating room or the emergency room would be uncomplicated. For example, current EMF devices used for bone non-union and wound healing, coils are within lightweight binding strap that are placed on the patient before, during and/or following surgery. The exposure technology is entirely non-invasive. This device is portable, weighs about one pound, and can be easily applied by any technician.
The use of EM fields for the induction of hsp70 for post-ischemia reperfusion treatment has clear advantages over the invasive elevated temperature treatment efforts tested to date. Non-ionizing EMF induction of hsp70 is safe, efficient and practical. These methods can be administered and re-administered prior to and during coronary interventions. Furthermore, hsp70 levels can be increased repeatedly with EMF as against the limited single use of the thermal method.
Summary and conclusion
In these experiments, we report a novel non-invasive technique to increase hsp70 levels using exposure to low energy, low frequency EMF. While stress proteins in cells and tissues have been previously utilized as diagnostic markers and prognostic indicators, a safe, noninvasive method of augmenting endogenous defense mechanisms as a therapeutic tool, such as EMF exposure, has significant clinical potential. Our data indicate that pre-exposure with EMF prior to ischemia and reperfusion, in a mammalian model, induces upregulation of the HSP70 gene, subsequently increased levels of hsp70 protein, and, most importantly, improved ventricular function after ischemia-reperfusion.
Acknowledgements
This work was partially supported by National Institute of Health Grant T32-HL07854 (I.G.) and the Robert I. Goodman Fund (R.G.). We would also like to acknowledge Eve Vagg for her invaluable assistance with the figures.
Abbreviations
- LVSP
Left ventricular systolic pressure
- LV
Left ventricular
- P-A Max Power
dP/dtmax, preload Adjusted Maximum Power
- G
Gauss
- rtPCR
Reverse-transcriptase polymerase chain reaction
- µT
MicroTesla
- Hz
Hertz
References
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: Molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- Blank M, Khorkova O, Goodman R. Changes in polypeptide distribution stimulated by different levels of electromagnetic and thermal stress. Bioelectrochem Bioenerg. 1994;33:109–114. [Google Scholar]
- Blank M, Goodman R. Initial interaction in electromagnetic field-induced biosynthesis. J Cell Physiol. 2004;199:359–363. doi: 10.1002/jcp.20004. [DOI] [PubMed] [Google Scholar]
- Blank M, Goodman R. A mechanism for stimulation of biosynthesis by electromagnetic fields: charge transfers in DNA and base pair separation. J Cell Physiol. 2007;214:20–26. doi: 10.1002/jcp.21198. [DOI] [PubMed] [Google Scholar]
- Blank M, Soo L. Electromagnetic acceleration of electron transfer reactions. J Cell Biochem. 2001;81:278–283. [PubMed] [Google Scholar]
- Bolli R, Becker L, Gross G, Mentzer R, Balshaw D, Lathrop D. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- Brocchieri L, Conway de Marcario E, Marcario AJ. hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008 Jan 23;8(1):19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody S, Wu XL, Lin H, Blank M, Goodman R. Cytoprotection by electromagnetic field-induced hsp70: a model for clinical application. J Cell Biochem. 2000;79:453–459. doi: 10.1002/1097-4644(20001201)79:3<453::aid-jcb100>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Chong KY, Lai CC, Lille S, Chang C, Su C. Stable overexpression of the constitutive form of heat shock protein 70 confers oxidative protection. J Mol Cell Cardiol. 1998;30:599–608. doi: 10.1006/jmcc.1997.0623. [DOI] [PubMed] [Google Scholar]
- Cornelussen RN, Garnier AV, van der Vusse GJ, Reneman RS, Snoeckx LHEH. Biphasic effect of heat stress pretreatment on ischemic tolerance of isolated rat hearts. J Mol Cell Cardiol. 1998;30:365–372. doi: 10.1006/jmcc.1997.0606. [DOI] [PubMed] [Google Scholar]
- Currie RW, Tanguay R, Klingma JG. Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993;87:863–871. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Di Carlo AL, Farrell JM, Litovitz TA. Mycardial protection conferred by electromagnetic fields. Circulation. 1999;99:813–816. doi: 10.1161/01.cir.99.6.813. [DOI] [PubMed] [Google Scholar]
- Ding XZ, Tsokos GC, Kiang JG. Overexpression of HSP-70 inhibits the phosphorylation of HSF1 by activating protein phosphotase and inhibiting protein kinase C activity. FASEB J. 1998;12:451–459. doi: 10.1096/fasebj.12.6.451. [DOI] [PubMed] [Google Scholar]
- Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG. Novel Cardioprotective Role of a Small Heat-Shock Protein, Hsp20, Against Ischemia/Reperfusion Injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- Goodman R, Wei L-X, Xu J-C, Henderson A. Exposure of human cells to low-frequency electromagnetic fields results in quantitative changes in transcripts. Biochim Biophys Acta. 1989;1009:216–220. doi: 10.1016/0167-4781(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Goodman R, Blank M, Lin H, Khorkova O, Soo L, Weisbrot D, Henderson A. Increased levels of hsp transcripts are induced when cells are exposed to low frequency electromagnetic fields. Bioelectrochem Bioenerg. 1994;33:115–120. [Google Scholar]
- Goodman R, Blank M. Magnetic field stress induces expression of hsp70. Cell Stress & Chaperones. 1998;3:79–88. doi: 10.1379/1466-1268(1998)003<0079:mfsieo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Blank M. Insights into electromagnetic interaction mechanisms. J Cell Physiol. 2002;192:16–22. doi: 10.1002/jcp.10098. [DOI] [PubMed] [Google Scholar]
- Han L, Lin H, Jin M, Blank M, Goodman R. Application of magnetic field-induced heat shock protein 70 for presurgical cytoprotection. J Cell Biochem. 1998;71:577–583. doi: 10.1002/(sici)1097-4644(19981215)71:4<577::aid-jcb12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Heads RJ, Yellon DM, Latchman DS. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol. 1995;27:1669–1678. doi: 10.1016/s0022-2828(95)90722-x. [DOI] [PubMed] [Google Scholar]
- Ito H, Bassett CAL. Effect of weak pulsing electromagnetic fields on neural regeneration in the rat. Clin Orthop. 1983;181:283–290. [PubMed] [Google Scholar]
- Jayakumar J, Suzuki K, Khan M, Smolenski RT, Farrell A, Latif N, Raisky O, Abunasra H, Sammut IA, Murtuza B, Amrani M, Yacoub M. Gene therapy for myocardial protection: transfection of donor hearts with heat shock protein 70 gene protects cardiac function against ischemia-reperfusion injury. Circulation. 2000;102:302–306. doi: 10.1161/01.cir.102.suppl_3.iii-302. [DOI] [PubMed] [Google Scholar]
- Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Najma L, Abusnasra H, Murtuza B, Amrani M, Yacoub M. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation. 2001;104 Suppl 12:I-303–I-307. doi: 10.1161/hc37t1.094932. [DOI] [PubMed] [Google Scholar]
- Jin M, Lin H, Han L, Opler M, Maurer S, Blank M, Goodman R. Biological and technical variables in myc expression in HL60 cells exposed to 60Hz electromagnetic fields. Bioelectrochem. Bioenerg. 1997;44:111–120. [Google Scholar]
- Kiang JG, Ding XZ, McClain DE. Overexpression of HSP-70 attenuates increases in Ca2+ and protects human epidermoid A-431 after chemical hypoxia. Toxicol Appl Pharmacol. 1998;149:185–194. doi: 10.1006/taap.1997.8364. [DOI] [PubMed] [Google Scholar]
- Kim YK, Suarez J, Hu Y, McDonough PM, Boer C, Dix DJ, Dillmann WH. Deletion of the inducible 70-kDa heat shock protein genes in mice impairs cardiac contractile function and calcium handling associated with hypertrophy. Circulation. 2006;113:2589–2597. doi: 10.1161/CIRCULATIONAHA.105.598409. [DOI] [PubMed] [Google Scholar]
- Lednev VV. Possible mechanism for influence of magnetic fields on biological systems. Bioelectromagnetics. 1991;12:71–75. doi: 10.1002/bem.2250120202. [DOI] [PubMed] [Google Scholar]
- Liboff AR. Geomagnetic cyclotron resonance in membrane transport. J Biol Physics. 1985;13:99–102. [Google Scholar]
- Lin H, Opler M, Head M, Blank M, Goodman R. Electromagnetic field exposure induces rapid transitory heat shock factor activation in human cells. J Cell Biochem. 1997;66:482–488. doi: 10.1002/(sici)1097-4644(19970915)66:4<482::aid-jcb7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lin H, Head M, Blank M, Jin M, Goodman R. Myc-mediated transactivation of HSP70 expression following exposure to magnetic fields. J Cell Biochem. 1998;69:181–188. doi: 10.1002/(sici)1097-4644(19980501)69:2<181::aid-jcb8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Lin H, Blank M, Goodman R. Magnetic field-responsive domain in human HSP70 promoter. J Cell Biochem. 1999;75:170–176. doi: 10.1002/(sici)1097-4644(19991001)75:1<170::aid-jcb17>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lin H, Blank M, Goodman R. Regulating genes with electromagnetic response elements. J Cell Biochem. 2001;81:143–148. doi: 10.1002/1097-4644(20010401)81:1<143::aid-jcb1030>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Liu JC, He M, Wan L, Cheng XS. Heat shock protein gene transfection protects rat myocardium cell against anoxia-reoxygenation injury. Chin Med J (Engl) 2007;120:578–583. [PubMed] [Google Scholar]
- Loncar R, Flesche CW, Deussen A. Regional myocardial heat-shock protein (HSP70) concentrations under different blood flow conditions. Pflugers Arch. 1998;437:98–103. doi: 10.1007/s004240050753. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu T, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Meriin AB, Yaglom JA, Gabai VL, Zon L, Ganiatsas S, Mosser DD, Zon L, Sherman MY. Protein-damaging stresses activate c-Jun terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol Cell Biol. 1999;19:2547–2555. doi: 10.1128/mcb.19.4.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R, Giordano FJ, Conde AG, Dillmann WH. Adenovirus-mediated gene transfer of a heat shock protein 70 (hsp) protects against stimulated ischemia. J Mol Cell Cardiol. 1996;26:2351–2358. doi: 10.1006/jmcc.1996.0228. [DOI] [PubMed] [Google Scholar]
- Nitta Y, Abe K, Aoki M, Ohno I, Isoyama S. Diminished heat shock protein 70 mRNA induction in aged rat hearts after ischemia. Am J Phyisol. 1994;267:H1795–H1803. doi: 10.1152/ajpheart.1994.267.5.H1795. [DOI] [PubMed] [Google Scholar]
- Okubo S, Wildner O, Shah MR, Chelliah JC, Hess ML, Kukreja RC. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation. 2001;103:877–881. doi: 10.1161/01.cir.103.6.877. [DOI] [PubMed] [Google Scholar]
- Panagopoulos DJ, Karabarbounis A, Margaritis LH. Mechanism of action of electromagnetic fields on cells. Biochem Biophys Res Com. 2002;298:95–102. doi: 10.1016/s0006-291x(02)02393-8. [DOI] [PubMed] [Google Scholar]
- Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001;20:446–456. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier JCL, Currie RW. Heat shock -induced myocardial protection against ischemic injury: a role for Hsp70? Cell Stress & Chaperones. 1996;1:13–17. doi: 10.1379/1466-1268(1996)001<0013:hsimpa>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff GL, Glantz SA. Volume loading slows left ventricular isovolemic relaxation rate. Evidence of load-dependent relaxation in the intact dog heart. Circ Res. 1981;48:813–824. doi: 10.1161/01.res.48.6.813. [DOI] [PubMed] [Google Scholar]
- Schneider EL. Aging in the third millenium. Science. 1999;283:796–797. doi: 10.1126/science.283.5403.796. [DOI] [PubMed] [Google Scholar]
- Shallom JM, DiCarlo AL, Ko D, Penafiel LM, Nakai A. Microwave exposure induces hsp70 and confers protection against hypoxia in chick embryos. J Cell Biochem. 2002;86:490–496. doi: 10.1002/jcb.10243. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sawa Y, Kaneda Y, Ichikawa H, Shirakura R, Matsuda H. In vivo gene transfection with heat shock protein 70 enhances myocardial tolerance to ischemia-reperfusion injury in rat. J Clin Invest. 1997;99:1645–1650. doi: 10.1172/JCI119327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Sawa Y, Kagisaki K, Taketani S, Ichikawa H, Kaneda Y, Matsuda H. Reduction in myocardial apoptosis associated with overexpression of heat shock protein 70. Basic Res Cardiol. 2000;95:397–403. doi: 10.1007/s003950070039. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenkski RT, Kaneda Y, Sawa Y, Matsuda H, Yacoub MH. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106 Suppl I:I-270–I-276. [PubMed] [Google Scholar]
- Udelsman R, Blake MJ, Stagg CA, Li D-G, Putney D, Holbrook NJ. Vascular heat shock protein expression in response to stress. J Clin Invest. 1993;91:465–473. doi: 10.1172/JCI116224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Campbell W, Vander-Heide RS. Heat shock-induced cardioprotection activates cytoskeletal-based cell survival pathways. Am J Physiol Heart Circ Physiol. 2006;291:H638–H647. doi: 10.1152/ajpheart.00144.2006. [DOI] [PubMed] [Google Scholar]
- Wei L-X, Goodman R, Henderson A. Changes in levels of c-myc and histone H2B following exposure of cells to low frequency sinusoidal electromagnetic fields: evidence for a window effect. Bioelectromagnetics. 1990;11:269–272. doi: 10.1002/bem.2250110403. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280(39):33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Yaglom JA, Gabai VL, Meriin AB, Mosser DD, Sherman MY. The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J Biol Chem. 1999;274(29):20223–20228. doi: 10.1074/jbc.274.29.20223. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang W, Qian L. Hsp70 may protect cardiomyocytes from stress-induced FAS-mediated apoptosis. Cell Stress & Chaperones. 2007;12:83–95. doi: 10.1379/CSC-231R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]