Abstract
Language experience ‘narrows’ speech perception by the end of infants’ first year, reducing discrimination of non-native phoneme contrasts while improving native-contrast discrimination. Previous research showed that declines in non-native discrimination were reversed by second-language experience provided at 9–10 months, but it is not known whether second-language experience affects first-language speech sound processing. Using event-related potentials (ERPs), we examined learning-related changes in brain activity to Spanish and English phoneme contrasts in monolingual English-learning infants pre- and post-exposure to Spanish from 9.5–10.5 months of age. Infants showed a significant discriminatory ERP response to the Spanish contrast at 11 months (post-exposure), but not at 9 months (pre-exposure). The English contrast elicited an earlier discriminatory response at 11 months than at 9 months, suggesting improvement in native-language processing. The results show that infants rapidly encode new phonetic information, and that improvement in native speech processing can occur during second-language learning in infancy.
Keywords: Event-related potentials (ERPs), infants, language development, speech perception, speech discrimination, second-language learning, mismatch negativity
Experience with language shapes infants’ abilities to process speech sounds, with early broad phonetic discrimination abilities narrowing as infants learn their native language (Best & McRoberts, 2003; Kuhl et al., 2008; Werker & Curtin, 2005). Between 6 and 12 months of age, infants’ behavioral discrimination of native-language phoneme contrasts (e.g., English “ra” vs. “la”) improves (Kuhl et al., 2006; Tsao, Liu, & Kuhl, 2006), while sensitivity to nonnative contrasts declines(Best & McRoberts, 2003; Werker & Tees, 1984). These experience-induced effects represent an important step in language development and predict subsequent language growth (Kuhl et al., 2005).
Experience-related changes in sensitivity to native and nonnative contrasts during the first year have also been observed in event-related potential (ERP) measures of brain electrical activity.In adults, a “mismatch negativity” (MMN) to a rare stimulus (deviant sound) vs. a frequent stimulus (standard sound) is elicited when a phonemic change occurs, and is believed to reflect the formation of memory traces for speech sounds over the course of language acquisition (see Näätänen et al., 1997; Näätänen, 2001). Mismatch responses to speech stimuli have also been observed in infants(Cheour, Leppannen, & Kraus, 2000; Dehaene-Lambertz & Baillet, 1998; Kuhl et al., 2008; Pang et al., 1998), and changes in these responses reveal both improvement in native-language phoneme discrimination and reductions in nonnative discrimination as infants gain experience with their native language (Cheour et al., 1998; Friederici, Friedrich, & Christophe, 2007; Rivera-Gaxiola, Silva-Pereyra, & Kuhl, 2005).1
The impact of exposure to two languages on children’s linguistic and cognitive skills is a topic of broad interest (see Bialystok, 2009). Regarding early language development, there is conflicting evidence concerning whether bilingual experience from birth alters the developmental transition in speech perception that is observed in monolingual infants during the first year. In some research, infants receiving bilingual input from birth have shown a temporary decline in perception between 6 and 12 months for contrasts that are phonemic in only one of their two native languages (Bosch & Sebastián-Gallés, 2003a, b), or phonemic in both but involving sounds that are acoustically close (Sebastián-Gallés & Bosch, 2009). However, this pattern has not been observed in all studies of speech perception in bilingual infants (Burns et al., 2007; Sundara & Polka, 2008). Moreover, other research has shown that bilingual infants achieve many developmental language milestones at the same ages and in qualitatively similar ways as monolingual infants (see review by Werker & Byers-Heinlein, 2008).
To examine the effects of dual-language experience on developmental transitions in speech perception, our laboratory’s approach has been to provide infants with short-term exposure to a second language in a laboratory setting. Previously, a language intervention, designed to examine whether 9–10-month-old infants could learn a nonnative phonetic contrast through short-term language exposure, showed that naturalistic second-language experience over a 4–6 week period induced phonetic learning as reflected in behavioral measures (Kuhl, Tsao, & Liu, 2003). In that study, discrimination of a Mandarin phoneme contrast was compared across 10.5 month-old infants who had experienced either: (1) 12 25-minute play sessions conducted in Mandarin, (2) 12 sessions of Mandarin from DVDs, (3) 12 sessions of Mandarin from audio-only sources, or (4) 12 play sessions in English (controls). Only those infants who experienced live Mandarin play sessions discriminated the Mandarin contrast, and their performance was at a level equivalent to that of Taiwanese infants who had Mandarin input from birth. The Kuhl et al. study was not designed to determine whether nonnative exposure affected native-language processing.
In the present research, we used ERPs in a pre- and post-exposure testing design to examine how second-language experience affects phonetic processing in infants’ first and second languages. Brain measures following natural second-language interventions in infants would go beyond behavioral data by demonstrating changes in the neural processes involved in phonetic learning. For example, naturalistic second language experience has been shown to produce MMNs to nonnative contrasts in adults and older children(Cheour, Shestakova, Alku, Ceponiene, & Näätänen, 2002; Peltola, Tuomainen, Koskinen, & Aaltonen, 2007; Shestakova, Huotilainen, Ceponiene, & Cheour, 2003; Winkler, et al., 1999). Measures of brain electrical activity, such as ERPs, also have excellent temporal resolution, thus providing information about the relative timing of processing across two languages in the same infants (Conboy & Mills, 2006). In the present study, we provided infants with naturalistic input to a different language, Spanish, during play sessions from 9.5 to 10.5 months; examined whether ERPs before and after this intervention reflected learning-related changes for Spanish stimuli; and also examined whether Spanish learning was accompanied by changes in native-language (English) processing.
Using a double-oddball procedure, we simultaneously recorded ERPs to Spanish and English phoneme contrasts in order to directly compare infants’ responses to each contrast. In this paradigm, a syllable that is common to Spanish and English (the voiceless unaspirated stop [ta]), serves as the background stimulus or “standard” ([t] is identified as the phoneme/t/by Spanish-speaking adults and as/d/by English-speaking adults, Rivera-Gaxiola et al., 2005). Two “deviants” (prevoiced [da], identified as the Spanish/da/, and voiceless aspirated [tha], identified as English/ta/) were randomly presented during testing so that we assessed Spanish phoneme discrimination (/ta/-/da/) and English phoneme discrimination (/da/-/ta/). Previous research showed that 11-month-old monolingual Spanish-learning infants, but not monolingual English-learning infants, produce reliable MMN-like ERP discriminatory responses (N250-550 effects) for the Spanish contrast (Rivera-Gaxiola et al., 2007). Monolingual English-learning infants show N250-550 discriminatory effects for the English contrast (Rivera-Gaxiola et al., 2005).
We predicted that infants from monolingual English-speaking homes would not show an MMN-like effect for the Spanish contrast at 9 months, but would show the effect at 11 months after experience with Spanish from 9.5 to 10.5 months. We also predicted that both 9-month-old and 11-month-old infants would show an MMN-like effect for the English contrast. Further, we predicted that infants’ continued experience with their native English outside the laboratory over the 2-month period would result in continued improvement in English processing, measured as shorter ERP latencies for the English contrast at 11 than at 9 months.
Methods
Participants
Twenty-one infants from monolingual English-speaking homes were recruited through a university-maintained list at 40 weeks of age. Infants were excluded if parents reported concerns about development or hearing, more than 3 ear infections, gestation of less than 37 weeks, birth weight of less than 6 lbs., or prior second-language experience.
Stimuli and design
Infants were pre-tested at 41 weeks using the double-oddball ERP paradigm described above. Three syllables were recorded in the same female voice and digitally manipulated (see Rivera-Gaxiola et al., 2005, for full description of stimuli and test paradigm). The standard syllable, voiceless unaspirated [ta], voice-onset time (VOT) of +12 ms, was presented on 80% of the trials. The Spanish deviant (prevoiced [da], -24 ms VOT) and the English deviant (voiceless aspirated [tha], +46 ms VOT) were each presented on 10% of the trials.
After pre-testing, infants attended twelve 25-minute play sessions conducted in Spanish between 42 and 46 weeks (Figure 1). Approximately 1–2 weeks after the 12th session, infants were post-tested on the English and Spanish contrasts.
Figure 1.
Example of a Spanish-language exposure session. Five native Spanish-speaking researchers (4 female, 1 male) served as “tutors” who used books (10 minutes) and toys (15 minutes) while talking to the infants. Each infant participated with at least 3 different tutors at least 3 times, for a total of 12 sessions. Infants’ parent/caregiver accompanied them, and either one or two parent-infant dyads were present during the sessions.
Recordings
During ERP testing, infants sat on a parent’s lap in a sound-attenuated room and listened to stimuli played at a comfortable level (69 dB SPL) from two speakers approximately 1.5 m in front of them. Infants heard 700 standards ([ta]), 100 English deviants ([tha]), and 100 Spanish deviants ([da]), in quasi-random order with at least three standards between deviants, and an interstimulus interval of 700 ms. A silent video was played and a researcher manipulated toys in front of the infants to reduce movement artifact. One-minute breaks were inserted after every two minutes of stimulus presentation to provide verbal praise and reduce possible habituation to the stimuli.
Brain electrical activity was recorded using 32-channel Electro-Caps with tin electrodes arranged according to the International 10–20 system, with all leads referenced to the left mastoid electrode (off-line re-referencing used averaged activity from left and right mastoid electrodes). Electrode impedances were kept below 10 kΩ. Signals were amplified at a gain of 20,000 within a bandpass of 0.1 to 40 Hz, using Isolated Bioelectric Amplifier System SC-32/72BA (SA Instrumentation, San Diego, CA). Signals were digitized at 250 Hz and stored on a hard disk for further analysis.
Data analyses
The electroencephalogram (EEG) was segmented into epochs that included 100 ms of pre-stimulus and 924 ms of post-stimulus activity. Vertical eye movements were detected by opposite polarity in activity at electrodes located below and just above the left eye (FP1). Trials contaminated by eye movement, excessive muscle activity, or amplifier blocking were rejected based on individualized thresholds for each test session and visual inspection of the data. The averaged data were digitally low-pass filtered at 15 Hz. Averaged ERPs were obtained for English deviants, Spanish deviants, and standards occurring immediately before each deviant. Sufficient artifact-free ERP data (at least 20 trials per stimulus type) were obtained from 17 infants at 11 months. Data were also obtained at 9 months from 14 of these infants. One infant left the study before post-exposure testing, and two would not wear the electrode cap.
Statistical analyses
To assess the effects of second-language experience on Spanish and English phonetic learning, ERP peak amplitudes at each age were measured relative to the average of a 100 ms pre-stimulus baseline for the most negative peak between 250–450 ms (N250-450) for each stimulus type (standard, English-deviant, Spanish-deviant). The N250-450 window was selected based on visual inspection of individual and grand averages to capture effects across ages and contrasts. Separate repeated measures ANOVAs were conducted for peak amplitude at 3 midline sites (FZ, CZ, PZ) or 5 lateral sites (FP1/2, F3/4, FC1/2, C3/4, CP1/2), with the 3 levels of stimulus type, and hemisphere (for the lateral analysis), as within-subjects variables, and then separate ANOVAs were conducted for each separate contrast with 2 levels of stimulus type (i.e., English-deviant vs. standard, Spanish-deviant vs. standard).
To assess the improvement that has been observed in the native language in monolingual infants, we also measured the most negative peak amplitude within an earlier (200–250 ms) window for the English deviant vs. the standard. This window was chosen based on visual inspection of the grand-averaged data that suggested that the N250-450 effect was evident by 200 ms at 11 months. Separate repeated measures ANOVAs were conducted for lateral and midline sites, with age (9 vs. 11 months), stimulus (English deviant vs. standard), electrode site (3 midline or 5 lateral), and hemisphere (for the lateral analysis) as within-subjects variables.
For each ANOVA, Huynh-Feldt sphericity corrections were applied when appropriate. Post-hoc tests were conducted for each deviant vs. standard peak amplitude comparison at each electrode site using Tukey’s HSD method. Partial-eta-squared (ηp2) effect sizes were calculated for each significant main effect and interaction in the ANOVAs, and Cohen’s d effect sizes were calculated for each paired comparison (deviant minus standard), using means and original standard deviations.
An additional correlational analysis was conducted to determine whether the N200-250 effect for the English contrast noted at 11 months corresponded more closely to the later N250-450 effect, or to the earlier P100 component that has also been shown to be sensitive to speech sounds contrasts in infants this age (Rivera-Gaxiola et al., 2007). We measured the most positive peak amplitude within an earlier (100–200 ms) window for the English deviant vs. the standard at 11 months and calculated Pearson correlations between the P100-200, N200-250 and N250-450 peak amplitudes. We also calculated difference waves for the English deviant minus standard at 11 months, and the peak amplitudes of the difference waves between 100–200, 200–250, and 250–450 ms, in order to examine correlations between the P100-200, N200-250, and N250-450 effects. We expected that if the English-contrast N200-250 ms effect at 11 months were due to smaller P100-200 amplitudes to the English deviant, then there would be a significant positive correlation between the English-deviant P100-200 and the English-deviant N200-250 peak amplitudes and a strong correlation between the English P100-200 and English N200-250 difference wave amplitudes. In contrast, if the larger amplitude for the English deviant vs. standard in the N200-250 window reflected an earlier onset of the N250-450 effect, then correlations would be stronger between the English N200-250 and English N250-450 difference wave amplitudes.
Results
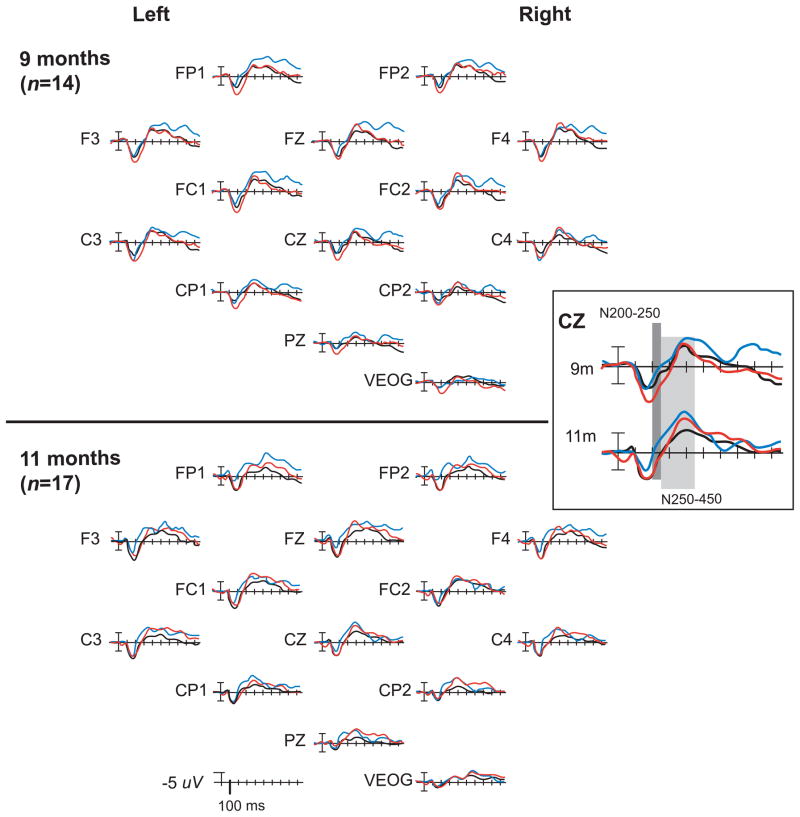
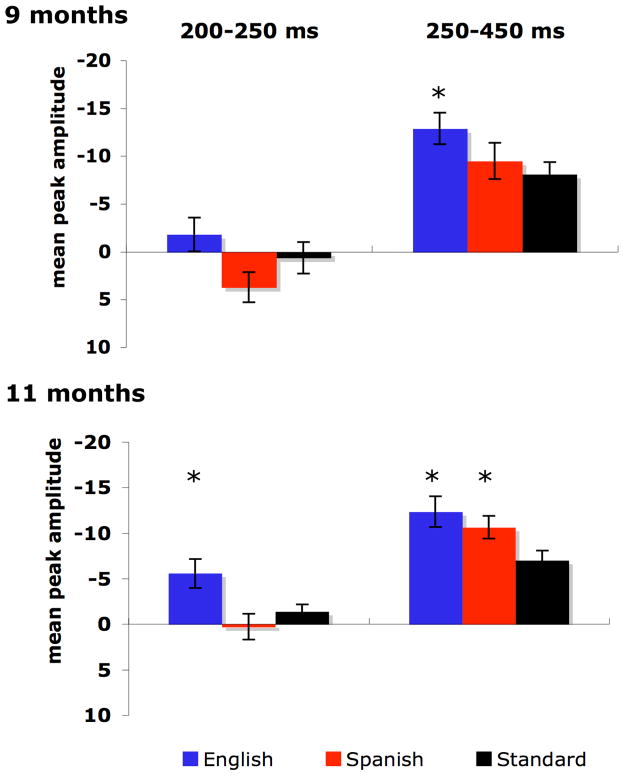
Pre- and post-exposure ERP grand-averaged results to the 3 stimuli are displayed in Figures 2 and 3. At 9 months, both lateral- and midline-site ANOVAs of N250-450 peak amplitude revealed main effects of stimulus (standard and both deviants): lateral, F(1.9, 24.73) = 5.28, p < .05, ηp2 =.29; midline, F(1.74, 22.59) = 5.97, p < .05, ηp2 =.32. A stimulus x hemisphere interaction was observed in the lateral analysis, but was significant only for the Spanish contrast, F(1, 13) = 8.11, p < .05, ηp2 =.38, and post-hoc analyses indicated that larger right-hemisphere Spanish-deviant N250-450 peak amplitudes were driving the interaction. A separate ANOVA conducted only for the Spanish-deviant vs. standard at right-hemisphere sites indicated no significant effect of stimulus, F(1, 13) = 3.052, p > .10, ηp2 = .19, and post-hoc analyses showed no significant Spanish-deviant vs. standard differences at individual electrode sites. Separate ANOVAs for each contrast indicated a significant stimulus effect only for the English contrast: lateral, F(1, 13) = 7.40, p < .05, ηp2 =.36; midline, F(1, 13) = 13.93, p < .01, ηp2 =.52. Tukey HSD tests indicated significantly larger N250-450 peak amplitudes for the English deviant compared to the standard, at 9 out of 13 electrode sites tested (FZ, CZ, PZ, FP1, FC1/2, C4, CP1/2, p < .05, d = .68–.94).
Figure 2.
Grand-averaged pre- (9-month) and post-exposure (11-month) ERPs to syllables in double-oddball paradigm. Enlarged area displays results at a representative electrode site (CZ). Infants heard 700 standard stimuli ([ta], black line), 100 English deviants ([tha], blue line), and 100 Spanish deviants ([da], red line). Negative voltages (microvolts) are plotted upward.
Figure 3.
Mean pre- and post-exposure ERP N200-250 and N250-450 peak amplitudes (microvolts) to the 3 stimuli, averaged across 13 electrode sites (5 left hemisphere, FP1, F3, FC1, C3, CP1; 5 right hemisphere, FP2, F4, FC2, C4, CP2; and 3 midline, FZ, CZ, PZ). Error bars represent + 1 SE. Asterisks represent significantly larger amplitudes to deviants vs. the corresponding standard stimuli.
At 11 months, lateral- and midline-site ANOVAs of N250-450 peak amplitude revealed main effects of stimulus: lateral, F(2, 32) = 4.83, p < .05, ηp2 =.23; midline, F(1.91, 30.55) = 6.42, p < .01, ηp2 =.29. No significant interactions were observed. As predicted, separate ANOVAs for each contrast indicated main effects of stimulus for both the English and Spanish contrasts: English lateral, F(1, 16) = 8.33, p < .05, ηp2 =.34; English midline, F(1, 16) = 9.73, p < .01, ηp2 =.38; Spanish lateral, F(1, 16) = 5.79, p < .05, ηp2 =.27; Spanish midline, F(1, 16) = 10.20, p < .01, ηp2 =.39. For the English contrast, Tukey HSD tests showed significantly larger amplitude negativity to the deviant vs. standard at 11 of the 13 electrode sites (FZ, CZ, PZ, FP1/2, F3/4, FC1, C4, CP1/2, p < .05, d = .74–1.22). For the Spanish contrast, the N250-450 amplitude was significantly larger to the deviant vs. standard at 7 of the 13 sites (FZ, PZ, FP1, FC1, C3/4, CP2, p < .05, d = .62–.95).
As seen in Figure 3, the N250-450 effect began earlier for the English contrast at 11 than at 9 months, suggesting that infants became more rapid and efficient at discriminating this native contrast with age and additional native-language experience. Repeated-measures ANOVAs for infants with data available at both ages (n=14), comparing peak amplitudes to the English deviant vs. standard between 200 and 250 ms at lateral and midline sites, showed an effect of age. The lateral analysis yielded significant effects for stimulus, F(1,13) = 5.45, p = .036, ηp2 = .30 and an age × stimulus × site interaction, F(2.79,36.27) = 4.05, p = .024, ηp2 = .24. The midline analysis showed a trend for stimulus, F(1,13) = 4.36, p = .057, ηp2 = .25. Planned paired-sample t-tests using all data available from the 17 subjects at 11 months revealed significantly larger amplitude N200-250 to the English deviant vs. standard at 10 of the 13 electrode sites (FZ, PZ, FP1/2, F3/4, C3/4, CP1/2, p < .05, d = .66–1.18), whereas available data at 9 months showed no differences.
The correlational analyses indicated that the 11-month English-deviant P100-200 and English-deviant N200-250 peak amplitudes were not significantly correlated (r = .43, p > .05), but the 11-month English-deviant N200-250 and English-deviant N250-450 peak amplitudes were positively correlated (r = .59, p < .05). There were non-significant negative associations between the English P100-200 difference wave amplitude and the N200-250 and N250-450 difference wave amplitudes (r = −.32, p > .10, and r = −.46, p < .10, respectively). Although they did not reach statistical significance, the negative polarity of these associations suggest that the N200-250 effect noted at 11 months was not due to increased negativity of the P100-200 English deviant amplitude (which would have resulted in positive correlations). Strong positive correlations between the N200-250 and N250-450 difference wave amplitudes suggest that these effects are closely related (English: r = .58, p < .05; Spanish: r = .66, p < .01).
Discussion
The data presented here show that a neural signature of second-language phonetic learning is exhibited in infants after short-term experience with the second language during a period of developmental transitions in speech perception. The brain activity of infants who experienced the Spanish language in naturalistic play interactions over approximately one month showed significant learning of a contrast that is phonemic in Spanish, the difference between the initial consonants of the words “dos” (two) and “tos” (cough). Similar neural discriminatory effects have been observed in monolingual Spanish-learning infants at 11 months, but not monolingual English-learning infants the same age (Rivera-Gaxiola et al., 2005). At 11 months, group ERP data indicated significant effects for both the English and Spanish contrasts. At 9 months, prior to Spanish exposure, the same infants did not show these effects for the Spanish contrast.
Importantly, our data also suggest that improvement of native-contrast processing continues during second-language phonetic learning. The earlier onset of the mismatch effect for English at 11 vs. 9 months may be interpreted as native-language processing becoming more efficient over the two-month period during which the infants experienced Spanish. Improvement in native speech perception between 6 and 12 months has been previously shown for monolingual infants in behavioral research in the form of a stronger effect size for native-language discrimination (Kuhl et al., 2006; Tsao et al., 2006) and in ERP studies in the form of a larger amplitude for the native-language negative mismatch effect (Cheour et al., 1998; Rivera-Gaxiola et al., 2005). In the present research, we did not find an increase in amplitude of the negative mismatch effect between 9 and 11 months. This may be due to the shorter time period between the pretest and posttest in our study compared to previous studies. However, our data suggest that enhancement may be evident in the latency of brain activity to the native contrasts, given that infants showed an earlier onset of the negative mismatch effect at 11 months (by 200 ms post stimulus onset) compared to at 9 months (by 250 ms post stimulus onset). Importantly, our data suggest that such enhancement may be seen even when infants experience a second language during this same time period. Our results do not rule out the possibility that infants who do not receive second-language exposure during the 9 to 11 month period might show even greater enhancement of native-language functioning than the infants in the present study. Similarly, our results cannot rule out the possibility that the enhancement observed in our study was due to participation in the study in addition to, or instead of, continued English exposure in the home. It is possible that the experience of learning a new language sharpened infants’ perceptual skills in a general way that benefited the native as well as the second language. If such a language-general effect were the cause of the results observed in the present study, then we might not find earlier latencies of the native-language mismatch effect in a control group. This could also explain why previous longitudinal studies of monolingual infants have not reported shortened latencies of the mismatch effect at 11–12 vs. 6–7 months (Cheour et al., 1998; Rivera-Gaxiola et al., 2005). Future studies with equivalent control groups would be needed to test these hypotheses.
Our results indicate considerable plasticity in the infant perceptual system during the developmental transition in phonetic perception that occurs throughout the first year of life. Infants who participated in 12 Spanish sessions during this period learned from that experience, and growth and facilitation in native language phonetic learning also occurred. Infants’ ERPs were recorded 1–2 weeks after their last Spanish-exposure session, suggesting that experience with a second language produced learning that was durable (see also Kuhl et al., 2003). We have argued elsewhere that naturalistic exposure in highly complex learning situations may produce more robust learning than that which occurs in less socially complex settings (Conboy & Kuhl, 2007; Kuhl, 2007). Future studies will examine effects of age and durability of phonetic learning after short-term second-language exposure.
Acknowledgments
Work was supported by the NSF Life Center (SBE-0354453), NIH F32 HD050033 to BTC, NIH HD37954 to PKK, and NIH Research Core Grant, University of Washington P30. The authors thank the infants and families who participated, Denise Padden, Alexis Bosseler, Sally Wright, and Lindsay Klarman for help with testing, Maritza-Rivera Gaxiola for providing the ERP paradigm, and Juan Silva-Pereyra for comments on a previous version of this manuscript.
Footnotes
In infants, positive as well as negative mismatch responses have been reported for speech stimuli presented in oddball paradigms (Friederici et al., 2007; Friederici, Friedrich, & Weber, 2002; Friedrich et al., 2009; Rivera-Gaxiola et al., 2005). Given that the processes underlying these polarity differences are not yet fully understood, we focused on the negative mismatch response previously linked to native-language phonetic learning in research that used identical stimuli and recording procedures with infants the same age.
References
- Best C, McRoberts G. Infant perception of non-native consonant contrasts that adults assimilate in different ways. Language & Speech. 2003;46(2–3):183–216. doi: 10.1177/00238309030460020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch L, Sebastián-Gallés N. Language experience and the perception of a voicing contrast in fricatives: infant and adult data. In: Solé MJ, Recasens D, Romero J, editors. Proceedings of the International Congress of Phonetic Sciences. Barcelona, Spain: 2003a. pp. 1987–90. [Google Scholar]
- Bosch L, Sebastián-Gallés N. Simultaneous bilingualism and the perception of a language specific vowel contrast in the first year of life. Language and Speech. 2003b;46(2–3):217–43. doi: 10.1177/00238309030460020801. [DOI] [PubMed] [Google Scholar]
- Bialystok E. Bilingualism: The good, the bad, and the indifferent. Bilingualism: Language and Cognition. 2009;12(1):3–11. [Google Scholar]
- Burns TC, Yoshida KA, Hill K, Werker JF. Bilingual and monolingual infant phonetic development. Applied Psycholinguistics. 2007;28(3):455–474. [Google Scholar]
- Cheour M, Ceponiene R, Lehtokoski A, Luuk A, Allik J, Alho K, Näätänen R. Development of language-specific phoneme representations in the infant brain. Nature Neuroscience. 1998;1(5):351–353. doi: 10.1038/1561. [DOI] [PubMed] [Google Scholar]
- Cheour M, Leppanen P, Kraus N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology. 2000;111(1):4–16. doi: 10.1016/s1388-2457(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Cheour M, Shestakova A, Alku P, Ceponiene R, Näätänen R. Mismatch negativity shows that 3–6-year-old children can learn to discriminate non-native speech sounds within two months. Neuroscience Letters. 2002;325(3):187–190. doi: 10.1016/s0304-3940(02)00269-0. [DOI] [PubMed] [Google Scholar]
- Conboy BT, Kuhl PK. Early speech perception: developing a culturally specific way of listening through social interaction. In: Bråten SL, editor. On being moved – from mirror neurons to empathy. Amsterdam/Philadelphia: John Benjamins Publishing Company; 2007. pp. 175–199. [Google Scholar]
- Conboy BT, Mills DL. Two languages, one developing brain: Effects of vocabulary size on bilingual toddlers’ event-related potentials to auditory words. Developmental Science. 2006;9(1):F1–F11. doi: 10.1111/j.1467-7687.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Baillet S. A phonological representation in the infant brain. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. 1998;9(8):1885–1888. doi: 10.1097/00001756-199806010-00040. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Friedrich M, Christophe A. Brain responses in 4-month-old infants are already language specific. Current Biology. 2007;17(14):1208–1211. doi: 10.1016/j.cub.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Friedrich M, Weber C. Neural manifestation of cognitive and precognitive mismatch detection in early infancy. Neuroreport. 2002;13(10):1251–1254. doi: 10.1097/00001756-200207190-00006. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Herold B, Friederici AD. ERP correlates of processing native and non-native language word stress in infants with different language outcomes. Cortex. 2009;45(5):662–676. doi: 10.1016/j.cortex.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Is speech learning ‘gated’ by the social brain? Developmental Science. 2007;10(1):110–120. doi: 10.1111/j.1467-7687.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Conboy BT, Coffey-Corina S, Padden D, Rivera-Gaxiola M, Nelson T. Phonetic learning as a pathway to language: New data and Native Language Magnet Theory-expanded (NLM-e) Philosophical Transactions of the Royal Society B. 2008;363(1493):979–1000. doi: 10.1098/rstb.2007.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Conboy BT, Padden D, Nelson T, Pruitt J. Early speech perception and later language development: Implications for the “critical period”. Language Learning and Development. 2005;1(3–4):237–264. [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Developmental Science. 2006;9(2):F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: Effects of short- term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Science. 2003;100(15):9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R. The perception of speech sounds by the human brain as reflected by the mismatch negativity (MMN) and its magnetic equivalent (MMNm) Psychophysiology. 2001;38(1):1–21. doi: 10.1017/s0048577201000208. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Lehtokoski A, Lennes M, Cheour M, Huotilainen M, Iivonen A, Vainio M, …Alho K. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385(6615):432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- Pang E, Edmonds G, Desjardins R, Khan S, Trainor L, Taylor M. Mismatch negativity to speech stimuli in 8-month-old infants and adults. International Journal of Psychophysiology. 1998;29(2):227–236. doi: 10.1016/s0167-8760(98)00018-x. [DOI] [PubMed] [Google Scholar]
- Peltola MS, Tuomainen O, Koskinen M, Aaltonen O. The effect of language immersion education on the preattentive perception of native and non-native vowel contrasts. Journal of Psycholinguistic Research. 2007;36(1):15–23. doi: 10.1007/s10936-006-9030-y. [DOI] [PubMed] [Google Scholar]
- Rivera-Gaxiola M, Silva-Pereyra J, Klarman L, Garcia-Sierra A, Lara-Ayala L, Cadena-Salazar C, et al. Principal component analyses and scalp distribution of the auditory P150-250 and N250-550 to speech contrasts in Mexican and American infants. Developmental Neuropsychology. 2007;31(3):363–378. doi: 10.1080/87565640701229292. [DOI] [PubMed] [Google Scholar]
- Rivera-Gaxiola M, Silva-Pereyra J, Kuhl PK. Brain potentials to native and non-native speech contrasts in 7- and 11-month-old American infants. Developmental Science. 2005;8(2):162–172. doi: 10.1111/j.1467-7687.2005.00403.x. [DOI] [PubMed] [Google Scholar]
- Sebastián-Gallés N, Bosch L. Developmental shift in the discrimination of vowel contrasts in bilingual infants: is the distributional account all there is to it? Developmental Science. 2009;12(6):874–887. doi: 10.1111/j.1467-7687.2009.00829.x. [DOI] [PubMed] [Google Scholar]
- Shestakova A, Huotilainen M, Ceponiene R, Cheour M. Event-related potentials associated with second language learning in children. Clinical Neurophysiology. 2003;114(8):1507–1512. doi: 10.1016/s1388-2457(03)00134-2. [DOI] [PubMed] [Google Scholar]
- Sundara M, Polka L, Molnar M. Development of coronal stop perception: Bilingual infants keep pace with their monolingual peers. Cognition. 2008;108(1):232–242. doi: 10.1016/j.cognition.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Tsao FM, Liu HM, Kuhl PK. Perception of native and non-native affricate-fricative contrasts: Cross-language tests on adults and infants. Journal of the Acoustical Society of America. 2006;120(4):2285–2294. doi: 10.1121/1.2338290. [DOI] [PubMed] [Google Scholar]
- Werker JF, Byers-Heinlein K. Bilingualism in infancy: first steps in perception and comprehension. Trends in Cognitive Sciences. 2008;12(4):144–151. doi: 10.1016/j.tics.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Werker JF, Curtin S. PRIMIR: A developmental model of speech processing. Language Learning and Development. 2005;1(2):197–234. [Google Scholar]
- Werker J, Tees R. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development. 1984;7(1):49–63. [Google Scholar]
- Winkler I, Kujala T, Tiitinen H, Sivonen P, Alku P, Lehtokoski A, …Näätänen R. Brain responses reveal the learning of foreign language phonemes. Psychophysiology. 1999;36(5):638–642. [PubMed] [Google Scholar]