Abstract
Oxidation reactions are vital parts of metabolism and signal transduction. However, they also produce reactive oxygen species, which damage lipids, proteins and DNA, generating “oxidation-specific” epitopes. In this review, we will discuss the hypothesis that such common oxidation-specific epitopes are a major target of innate immunity, recognized by a variety of “pattern recognition receptors” (PRRs). By analogy with microbial “pathogen associated molecular patterns” (PAMPs), we postulate that host-derived, oxidation-specific epitopes can be considered to represent “danger (or damage) associated molecular patterns” (DAMPs). We also argue that oxidation-specific epitopes present on apoptotic cells and their cellular debris provided the primary evolutionary pressure for the selection of such PRRs. Further, because many PAMPs on microbes share molecular identity and/or mimicry with oxidation-specific epitopes, such PAMPs provided a strong secondary selecting pressure for the same set of oxidation-specific PRRs as well.
Because lipid peroxidation is ubiquitous and a major component of the inflammatory state associated with atherosclerosis, the understanding that oxidation-specific epitopes are DAMPs, and thus the target of multiple arcs of innate immunity, provides novel insights into the pathogenesis of atherosclerosis. As examples, we show that both cellular and soluble PRRs, such as CD36, toll-like receptor-4, natural antibodies, and CRP recognize common oxidation-specific DAMPs, such as oxidized phospholipids and oxidized cholesteryl esters, and mediate a variety of immune responses, from expression of proinflammatory genes to excessive intracellular lipoprotein accumulation to atheroprotective humoral immunity. These insights may lead to improved understanding of inflammation and atherogenesis and suggest new approaches to diagnosis and therapy.
Keywords: oxidation-specific epitopes, innate immunity, oxidized lipids
Introduction
The process of oxidative phosphorylation adopted by early ancestors of mitochondria has determined the evolution of Eukaryotes as organisms deriving energy from oxidation of a substrate. Our lives are clearly oxygen-centric, and both oxidative phosphorylation and non-respiratory oxygenation are vital parts of metabolism and signal transduction. However, oxidation reactions also produce reactive oxygen species, which damage, often excessively, lipids, proteins and DNA. To counteract the oxidative damage, complex anti-oxidative mechanisms reduce oxidized “bystander” molecules to their normal states. If these mechanisms fail, then oxidation-damaged molecules, molecular complexes and cells must be removed from the tissue. This task is most often performed by macrophages, professional scavenger cells, and by other components of the innate immune system.
In this review, we will discuss the hypothesis that a major mechanism by which recognition of oxidation-damaged molecular complexes occurs is via the detection of “oxidation-specific epitopes” by “pattern recognition receptors” (PRRs) of innate immunity. The concept of PRRs was first introduced to explain how a limited number of macrophage receptors were capable of binding a much larger number of bacterial ligands 1. Such PRRs were postulated to bind to structurally common pattern motifs on microbial products, termed “pathogen associated molecular patterns” (PAMPs). Thus, a limited number of PPRs could provide to the host a broad-based innate defense against a variety of microorganisms 1–3. By analogy, we postulate that host-derived, oxidation-specific epitopes, which also constitute products of diverse classes of oxidative reactions, can be considered to represent “danger (or damage) associated molecular patterns” (DAMPs) 4. DAMPs, of course, refer to a much broader group of “inadvertent” modifications of lipids, carbohydrates, proteins, and DNA, aside from oxidative events, which pose danger to the functioning of an organism. Although we will use the term DAMP to describe common oxidation-specific epitopes, as will be developed below, many such epitopes share molecular identity and/or mimicry with epitopes on microbes, thus often making the distinction between PAMPs and DAMPs a semantic issue rather than a real distinction.
Traditionally, one would argue that the need to recognize microbial PAMPs provided the evolutionary pressure for the emergence of PRRs. However, we would suggest that recognition of host-originated DAMPs, including oxidation-specific epitopes, may have provided equal if not more compelling evolutionary pressure 4. Here, we will only refer to two arguments in favor of the DAMP hypothesis in the context of oxidation-specific epitopes, and will discuss this in more detail at the end of the review. Recognition and removal of apoptotic cells is an essential and very early process in embryonic development, usually occurring in an environment protected from infectious pathogens. Macrophage PRRs, such as CD36 and SR-A, which recognize oxidation-specific epitopes on apoptotic cell surfaces, such as oxidized phospholipids (OxPL) and malondialdehyde (MDA)-modified structures, are intimately involved in the process of apoptotic cell removal 5–8. Another example is mammalian toll-like receptor-4 (TLR4) and bacterial lipopolysaccharide (LPS). Though this is considered a classic PRR/PAMP pair, which provides one of the strongest and wide-ranging innate immune responses to a microbial ligand in mammalian cells, recent evidence from our lab and others suggest that oxidized lipids, such as oxidized cholesteryl esters (OxCE) and OxPL also appear to be ligands for TLR4 9–11. Indeed, the prototypical drosophila’s Toll, although acting in a defensive role against microbes, is also a developmental gene, essential in dorsoventral axis formation 12. Further, recent studies demonstrate that both zebrafish paralogs of mammalian TLR4, TLR4a and TLR4b, do not recognize bacterial LPS 13,14, although the ligands for zebrafish TLR4 are yet unknown.
In our immediate area of research – atherogenesis – the concept that oxidation-specific epitopes form a family of DAMPs recognized by innate PRRs has led to an improved understanding of “why” innate immunity is involved in the pathophysiology of atherosclerosis. Oxidation of LDL is thought to contribute to atherogenesis by multiple mechanisms, including the formation of a myriad of oxidation-specific epitopes, which not only makes oxidized LDL a ligand for multiple PRRs, but leads to the generation of a variety of oxidized lipids that directly influence atherogenesis in multiple ways. In the first part of this article, we will briefly review potential mechanisms of LDL oxidation in vivo, and evidence that oxidation-specific epitopes mediate binding to PRRs. In the second part, we will discuss cellular and humoral PRR-mediated immune responses to oxidation-specific epitopes and consider their roles in atherogenesis. In both parts, we will limit our discussion to moieties derived from oxidation of polyunsaturated fatty acids (PUFAs) in PL or CE, as well as the moieties resulting from modification of proteins with these products of lipid oxidation. Most of these are found in various forms of oxidized LDL during the progression of atherosclerosis.
Oxidized LDL
Many lines of evidence suggest that LDL undergoes oxidation in vivo, particularly under conditions of hyperlipidemia and when retained in the vascular wall. A large body of work has defined a variety of enzymatic mechanisms that could mediate oxidation of LDL, including 15-or 12/15-lipoxygenase (LO), myeloperoxidase (MPO), and peroxidase-like activity of hemoglobin, though other modes of oxidation are important as well (reviewed 15–18). Non-enzymatic, free radical-mediated oxidation depends on the presence of superoxide, hydrogen peroxide and nitric oxide generated by NADPH oxidases and nitric oxide synthases, and the catalysis by transition metal ions, copper and iron, and hemin 18.
A widely used model of extensively oxidized LDL (OxLDL), leading to profound oxidative degradation, is LDL subjected to prolonged incubation with copper sulfate 19. This type of Cu2+-catalyzed oxidative attack on the PUFA in PL may lead to degradation of 40% of the phosphatidylcholine 20,21. The apoB also undergoes drastic alterations, partly due to direct oxidative attack and partly due to conjugation of oxidized lipid fragments with the protein. Non-enzymatic oxidation catalyzed by Cu2+ depends upon the presence of lipid hydroperoxides in the starting material 22,23, suggesting that LDL in vivo (or at least as isolated) already contains such moieties. In the presence of Cu2+ these hydroperoxides decompose to form peroxy radicals and alkoxy radicals, which turn, can initiate chain reactions that generate many more hydroperoxides. Such OxLDL are internalized by macrophages via scavenger receptors that recognize oxidation-specific epitopes, as discussed below.
In contrast to extensively oxidized OxLDL, scavenger receptors do not recognize so-called “minimally oxidized LDL” (mmLDL) 9. The reasons for this are not only a relatively low concentration of oxidation-specific epitopes, but qualitatively different types of epitopes as well. While truncated aldehydes, ketones and carboxyl products of advanced PUFA oxidation, present both as “free lipids” and covalently bound to apoB, are abundant in OxLDL, mmLDL predominantly contains hydroxides, hydroperoxides, endoperoxides and other early lipid peroxidation products of PL and CE. Such early products are formed in LDL modified by 15LO, an intracellular enzyme found in all vascular cells under inflammatory conditions 24–26. A mechanism by which cellular 15LO can lead to the oxidation of the PUFA of CE residing in the core of extracellular LDL has been suggested by Yoshimoto and co-workers 27. It involves binding of LDL to LRP-1, exchange of CE between LDL and the cellular membrane, recruitment of 15LO from the cytosol to the site of LDL binding, and then reverse transport of 15LO-oxygenated CE from the cell to the LDL. This mechanism agrees well with the known preferential oxygenation of CE by 12/15LO expressing cells 25. Human 15LO and mouse 12/15LO have been proposed to play a major role in in vivo LDL oxidation and the development of human and experimental murine atherosclerosis, as reviewed in 28.
In the absence of transition metals, CE hydroperoxides are relatively stable, particularly when shielded from water inside the lipophilic core of LDL. Accumulation of CE hydroperoxides has been documented in human atherosclerotic lesions and in the lesions of apoE−/− and LDLR−/− mice fed a high-fat diet 26,29,30. Using liquid chromatography mass spectrometry analysis of mmLDL, we demonstrated polyoxygenated CE hydroperoxides in which the cholesterol moiety was not oxidized, but the arachidonate acyl chain contained a hydroperoxide at the 15th carbon as well as 1 to 4 additional oxygen atoms. These CE hydroperoxides, which were also generated by direct incubation of cholesteryl arachidonate with 15LO (the product of this reaction is abbreviated as 15LO-CE), were demonstrated to induce a number of important biological responses in macrophages 9,26, as described below.
Innate Pattern-Recognition Receptors that Detect Oxidation-Specific Epitopes
Macrophages express the highest density of cell surface and intracellular PRRs, but other cell types express PRRs as well. In addition, there are important soluble innate PRRs including soluble variants of some cellular PRRs, pentraxins, and natural antibodies (NAbs), which are predominantly of IgM and IgA isotypes.
CD36, SR-A and other Scavenger Receptors
“Scavenger receptors” of macrophages were so named because they bound and internalized extensively oxidized LDL (OxLDL), but not native LDL 31, and to date the list includes CD36, SR-A1 and -A2, SR-BI, MARCO, LOX-1, PSOX, and others 32. Although all were originally identified for their ability to bind oxidation-specific epitopes (DAMPs) of OxLDL (or models of OxLDL), it is now apparent that they bind a wide variety of different ligands, including many microbial pathogens (PAMPs) 8,33. CD36 and SR-A have the highest affinity for OxLDL and acetylated LDL, respectively, and are responsible for up to 90% of their uptake by macrophages in vitro 34. OxPL in OxLDL have been shown to mediate CD36 binding 5,35–37. POVPC (1-palmitoyl-2-(5′-oxovaleroyl)-sn-glycero-3-phosphocholine) is an example of an OxPL that binds to CD36. There appear to be at least two different motifs on the OxPL that can confer recognition. First, we demonstrated that the non-modified phosphocholine (PC) head group of OxPL, but not of native PL, is an epitope of POVPC recognized by CD36 (and SR-B1) 36,38. This PC-dependent binding to CD36 was observed for both free POVPC, as well as for POVPC covalently linked to lysine residues of proteins (e.g. apoB in OxLDL or BSA) via Schiff base formation with the reactive sn-2 aldehyde 37,39,40. Such covalently bound OxPL explain the ability of apoB of OxLDL to bind to CD36 5,36,38,39. Experiments showing that POVPC linked to BSA also bound to CD36 strongly support the interpretation that the PC moiety of OxPL was a sufficient ligand to mediate binding to CD36. This was confirmed by demonstrating specific binding of a labeled POVPC peptide to CD36-transfected COS-7 cells, and that cleavage of the PC moiety abrogated this binding 37. We also showed that cells undergoing apoptosis contain an increased content of such OxPL, and that their binding and uptake by macrophages could be inhibited entirely both by E06, a monoclonal natural antibody that specifically recognizes the PC moiety of OxPL, as well as by POVPC-BSA 6,7. Thus, the exposed PC head group of OxPL in OxLDL and apoptotic cells is a DAMP recognized by macrophage scavenger receptors.
A second motif on OxPL was demonstrated in an elegant series of studies by Hazen, Podrez and their colleagues, who demonstrated that variousoxidized moieties on the sn-2 side chain of OxPL (in both PCand phosphoserine-containing PL) can also serve as ligands for CD36 35,41,42. These have the common motif of oxidized and truncated sn-2 fatty acids that terminate in γ-hydroxy (or oxo)-α,β-unsaturated carbonyl groups. Irrespective of the headgroup, the presence of these motifs on OxPL are also sufficient ligandsto mediate binding of both OxLDL and apoptotic cells to CD36 43–45. However, this motif could not account for the ability of modified apoB of OxLDL to bind to CD36. Indeed, we speculate that the binding of both the PC and the oxidized side chain moieties of OxPL (in both the lipid and apoB moieties) to different sites on CD36 would lead to cooperative high-affinity binding of OxLDL to CD36. These data illustrate what appears to be the common “rule” that such innate PRRs have the capacity to bind to multiple DAMPs. Another example illustrating this rule is the observation that both OxLDL and acetyl LDL bind toSR-A, but at different molecular sites 46. Because these PRRs are germline encoded, and thus limited in number, it is likely that most such conserved receptors have the capacity to bind multiple related epitopes, such as oxidation-specific epitopes, rather than one specific ligand.
Toll-like receptors
Toll-like receptors (TLRs) have been traditionally considered PRRs that sense the presence of microbial PAMPs, which initiate complex signal transduction pathways and often excessively strong inflammatory responses. TLRs often depend on binding co-receptors to mediate ligand binding and presentation. Thus, CD14 on the cell surface first binds LPS, then mediates LPS binding to TLR4/MD-2, the receptor complex that dimerizes with a different TLR4/MD-2 pair upon binding of two LPS molecules. Among these LPS-binding receptors, only TLR4 has a transmembrane domain and is capable of initiating signaling 47,48. CD14 also aids TLR2 signaling, and CD36 has been shown to associate with TLR2 and with TLR4 to provide ligands for their activation 49–51. These examples show a multifaceted, ligand-dependent architecture of PRR complexes that determines the variety of cellular responses.
Work from several laboratories have now developed the idea that TLRs also bind numerous host-derived DAMPs 52. Work from our laboratory has demonstrated that mmLDL (made by exposure of LDL to 15LO-expressing cells) binds to CD14 and activates macrophages via TLR4/MD-2 53. (Importantly, all mmLDL preparations used in these studies were shown to have negligibly low endotoxin levels). We found that 15LO-CE was responsible for many (but not all) TLR4-dependent macrophage responses to mmLDL. The presence of a hydroperoxide group in 15LO-CE is critical because a hydroperoxide-reducing agent, ebselen, abrogated the TLR-4 mediated biological activity of both mmLDL and 15LO-CE 9,26.
In addition to oxidized CE, OxPLs have been suggested to activate TLR as well. For example, oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (OxPAPC – a collection of PC-containing OxPLs) has been shown to bind membrane CD14 and an yet unidentified GPI-anchored receptor on endothelial cells (EC), leading to activation of EC via TLR4 10,54. In contrast, OxPAPC and other OxPLs binding to soluble CD14 and LPS-binding protein (LBP) prevent recognition of LPS by these proteins and impair LPS activation of TLR4 54–56.
Since CD36 was shown to bind OxLDL and OxPL 36,37,45, as well as to present diacylglycerides to the TLR2/TLR6 signaling complex 50,57, several laboratories sought experimental evidence for CD36-mediated TLR2 activation by OxLDL. However, recent results suggest that CD36 mediates OxLDL activation of the TLR4/TLR6 heterodimer, but not of TLR2. Moreover, the OxLDL-induced TLR4/TLR6 activation occurred not on the cell surface, but in the endosomal compartment 51.
Most of the studies to date have used mixtures of OxPL generated by air oxidation of PAPC, and it is likely that future studies with specific OxPLs will help to more clearly identify the exact interactions between the various OxPLs and TLRs and their co-receptors. What these studies reveal is that TLR4, TLR2 and associated binding proteins represent important PRRs for a variety of oxidized lipids, including various OxPLs and OxCEs, which constitute endogenous DAMPs.
Soluble PRRs
Many PRRs expressed on the cell surface also exist in a soluble form, including soluble CD14, MD-2, LOX-1, and CD36. Their functions have been postulated to be two-fold. Similarly to their membrane-bound form, some soluble PRRs can activate their signaling counterparts. However, at higher concentrations and when bound to different ligands, they are inhibitory, as for example, was suggested for soluble CD14 and OxPL 54.
Other circulating PRRs include LBP, various lectins, the complement family of proteins, and the pentraxins. The short pentraxin C-Reactive Protein (CRP), an acute phase protein, is a valuable biomarker of inflammation and cardiovascular disease. However, this protein was originally noted for its ability to bind to the PC adduct covalently bound to the cell wall polysaccharide of S. pneumoniae and for its ability to mediate enhanced clearance of this and other pathogens. We have shown that CRP specifically binds to the PC moiety of OxPLs, whether present on OxLDL or on apoptotic cells, but does not bind to the PC of non-oxidized phospholipids 58. Furthermore, CRP is found in atherosclerotic lesions and is colocalized with PC-containing OxPL, presumably on OxLDL and apoptotic cells 58. We hypothesize that in future studies many other highly conserved soluble PRRs will be identified with the capacity to bind oxidation-specific epitopes.
Natural antibodies
The natural antibody (NAb) response is an important arc of innate immunity. NAbs can be considered immunoglobulin PRRs, having in common with cellular and soluble PRRs a limited repertoire and yet a wide range of pattern recognition. In mice, B-2 cells of adaptive immunity secrete predominantly IgG antibodies, which have undergone affinity maturation, and represent delayed, but highly specific humoral immune responses. In contrast, a special subset of innate-like B-cells termed B-1 cells, secrete NAbs, which are predominantly IgM and IgA and represent a rapid and first-line defense against pathogens. In contrast to B-2 cells of adaptive immunity, which are negatively selectedin fetal life producing anergy, B-1 cells are positively selected, leading to the presence of NAbs at birth or shortly thereafter. Indeed, consistent with the ability to secrete NAbs in the complete absence of external antigenic stimulation, a full repertoire of such NAbs canbe found in gnotobiotic mice 59. In uninfected mice, most, if not all, IgM in plasma are of B-1 cell origin, and are predominantly secreted from the spleen even in the absence of antigen. However, established B-1 cell clones can be expanded later in life byantigen exposure leading to increased IgM levels in plasma. B-1 cells have restricted use of VH genes that are minimally edited; thus, the IgM they generate are reflective of germline usage, with minimal to no mutations. Because they are conserved by natural selection, the presumption is that, fundamentally, NAbs provide advantageous properties maintaining homeostasis, such as their crucial role in immediate host defenses against PAMPs and DAMPs 60,61, the latter include oxidation-specific epitopes.
A good example is the prototypicE06 NAb that we cloned from a panel of hybridomas from cholesterol-fed apoE-deficient mice, which had high IgM titers to OxLDL 62. E06 bound to both the lipid and apoB moiety of OxLDL and to apoptotic cells 6,62,63. We subsequently showed, by direct sequencing of the VH/VL chains and use of anti-idiotypic Abs, that E06 was100% homologous to the classic germline-encoded NAb T15, secreted by a well-characterized B-1 cell clone described more than 30years earlier 64. The T15 clone was intensively studied because it binds to PC (not as part of a PL) covalently linked to the cell wall polysaccharide of pathogens and confers optimal protection to mice from lethal infection with S. pneumoniae 65. Thus, these studies demonstrated molecular (and immunological) identity between the PC of OxPL present on OxLDL and apoptotic cells on one hand and the PC moiety present on pneumococcus and many other microbes on the other hand. This dual specificity for an endogenous self- or neo-self-antigen and an exogenous pathogen has been described as a characteristic of NAbs 61.
The PC of OxPL is only one example of what are likelyto be a wide variety of such oxidation-specific epitopes towhich NAbs bind. For example, we cloned a NAb from LDLR−/− mice, termed LRO1, which bound to oxidized cardiolipin but not native cardiolipin 66 Cardiolipin is a major phospholipid of the inner leaflet of mitochondria, which is oxidized when cells undergo mitochondrial disruption that occurs during apoptosis 67. LRO1 bound to apoptotic cells but not viable cells. In addition, LRO1 bound to OxLDL, but not native LDL – LDL is known to contain cardiolipin. As might be predicted, LRO1 immunostained atherosclerotic lesions as well.
There are many oxidation-specific epitopes. In normal mice, as much as 20% to 30% of all IgM derived from B-1 cell clones bind to oxidation-specific epitopes 59. Among these, we found ahigh prevalence of IgM to MDA and to the complex structural adducts that occur when MDA is added to proteins. Remarkably, we previously documented a high titer of IgM to MDA-LDL even in wild-typeC57BL/6 mice as well as in healthy adult humans 60,68. Remarkably, in recent studies of newborn human umbilical cord blood, we found a similar high titer of IgM to MDA epitopes, and similar to such NAbsin mice, they bind apoptotic cells and atherosclerotic tissues 59. Because IgM do not cross the placenta, such IgM are of fetal origin and considered to represent the human equivalent of innate NAbs. Thus, both in mice and humans, oxidation-specific epitopes are an important target of innate NAbs.
These data demonstrate that oxidation-specific epitopes are an important target of innate immune PRRs. Thus, PC, as presented in OxPL of OxLDL and apoptotic cells, or as component of bacterial cell wall, is a DAMP/PAMP recognized by three different groups of PRRs: scavenger receptors, CRP and NAbs. In a similar manner, we have developed data that MDA, oxidized cardiolipin and others constitute similar sets of DAMPS, recognized by their respective PRRs. We speculate that oxidized CEs, which mediate TLR4-dependent biological activity as described below, will also be shown to fall into this category.
PRR-Mediated Biological Responses to Oxidation-Specific Epitopes
Cytoskeletal rearrangements and cell migration
The actin cytoskeleton dynamics regulates cell morphology, motility and endocytosis. During the process of phagocytosis, the macrophage extends actin-driven filopodia to surround bacteria (recognized via PAMPs) or apoptotic cells (recognized via DAMPs). Macropinocytosis is similar to phagocytosis in which extensive membrane ruffling due to actin cytoskeletal rearrangements results in internalization of large volumes of extracellular liquid. Several studies have suggested that oxidation-specific epitopes regulate cytoskeletal function of macrophages.
In the filopodia of macrophages phagocytosing apoptotic cells, we observed polymerized F-actin co-localized with 12/15LO, and 12/15LO activity was required for in vitro and in vivo F-actin formation and for phagocytosis 69,70. As discussed earlier, mmLDL is in fact a carrier of the products of 12/15LO-catalyzed oxidation of lipids, and addition of mmLDL to macrophages resulted in robust actin polymerization, membrane ruffling and cell spreading 9,26,53. Such spreading would contribute to reduced migratory capacity and in turn to macrophage localization to sites of enhanced content of oxidized CE, such as in atherosclerotic lesions. While mmLDL treated macrophages had enhanced uptake of modified LDL (see below), in turn, the polydirectional actin polymerization interfered with its function of phagocytosis of apoptotic cells 53, a mechanism that may contribute to defective clearance of apoptotic cells observed in atherosclerotic lesions 71.
Active components in mmLDL responsible for macrophage cytoskeletal rearrangements were found to be polyoxygenated CE hydroperoxides, such as 15LO-CE discussed earlier, which activate a TLR4-dependent signaling cascade 9,26. This mmLDL/OxCE mediated TLR-4 dependent signaling was MyD88 independent, and mediated by spleen tyrosine kinase (Syk) recruitment to a TLR4 signaling complex. This led to TLR4 and Syk phosphorylation, downstream activation of ERK1/2 signaling cascade, phosphorylation of paxillin, activation of Rac, Cdc42 and Rho GTPases, and the formation of a N-WASP/Arp2 complex, leading to dramatic, actin-dependent membrane ruffling (Figure 1). In addition, mmLDL induced TLR4-independent activation of PI3K 70,72, which also contributes to cytoskeletal rearrangements.
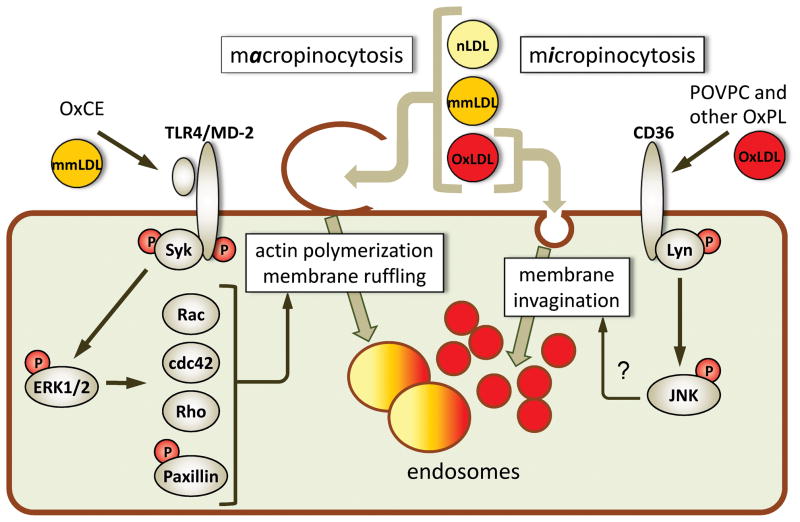
Figure 1. Oxidized lipid moieties induce lipoprotein accumulation in macrophages.
Macrophage lipoprotein uptake mechanisms can be separated into (1) macropinocytosis, when actin polymerization and extensive membrane ruffling result in the ruffles closing into large endosomes and capture of large volumes of extracellular material, including all classes of native and oxidized LDL present in the vicinity of the cell, and (2) micropinocytosis, when ligand-receptor binding leads to membrane invagination and nearly stoichiometric internalization of the ligand or the lipoprotein carrying this ligand. mmLDL and polyoxygenated CE hydroperoxides (OxCE) induce Syk recruitment to TLR4, Syk and TLR4 phosphorylation and subsequent ERK1/2-dependent activation of small GTPases Rac, cdc42 and Rho, and phosphorylation of paxillin, leading to actin reorganization and membrane ruffling. Resulting macropinocytosis promotes foam cell formation 9. Binding of OxLDL or OxPL to CD36 initiates Lyn-dependent phosphorylation of JNK, which is essential for CD36-mediated OxLDL uptake, although the mechanism linking JNK with the membrane dynamics is unclear 77. The TLR4- and CD36-mediated uptake mechanisms are only examples; there are numerous other PRRs involved in oxidation-specific epitope-stimulated lipoprotein internalization by macrophages.
Steinberg and colleagues originally showed that components of OxLDL were not only chemotactic for monocytes but also led to inhibition of migration of macrophages, which would both enhance the local accumulation of macrophages within sites of inflammation and oxidative events 73. In addition to the OxCE noted above, different oxidation-specific epitopes, formed by LDL oxidation with MPO/hydrogen peroxide/nitrite, specifically bind to CD36 and also induce macrophage spreading and reduce macrophage migration 74. The CD36-mediated actin polymerization and cell spreading involves Src-dependent activation (phosphorylation) of focal adhesion kinase (FAK) and NADPH oxidase; the latter produces reactive oxygen species, which inactivate SHP-2 protein tyrosine phosphatase, contributing to reduced dephosphorylation of FAK 74.
Lipoprotein uptake and foam cell formation
Lipoprotein uptake by macrophages within the intima and formation of foam cells is a major atherogenic process connecting lipid deposition in the vessel wall with vascular inflammation. Unregulated uptake by macrophages of OxLDL and other modified LDLs bound to the intimal matrix 75, combined with down regulated efflux of lipids, stimulates expression of pro-inflammatory cytokines, antigen presentation, secretion of matrix-degrading enzymes, and often results in cell death, thereby promoting further lesion development and its eventual rupture. The mechanisms of uptake of modified LDL by macrophages are diverse 8,32,76 and in part reflect the multitude of oxidative modifications of LDL, as well as other modifications. Depending on the prevalence of different oxidation-specific epitopes, macrophages could use different combinations of binding receptors and different uptake mechanisms to remove these DAMPs from the tissue, but at the expense of generating foam cells.
In vitro experiments in which macrophages were exposed to OxLDL and acetylated LDL only, showed that CD36 and SR-A were responsible for 75–90% of uptake of these lipoproteins 34. It has been demonstrated that the CD36-mediated OxLDL uptake depends on the activation of Src and JNK kinases 77 (Figure 1). Similarly to mouse macrophages, a comparison of monocyte/macrophages from patients with a total deficiency of CD36 with normal monocyte/macrophages suggests that about 50% of the in vitro uptake of OxLDL is attributable to this receptor, under the conditions studied 78. However, epidemiological data of CD36 deficient subjects with respect to cardiovascular disease are not yet available, and murine model studies produced mixed results. Using hypercholesterolemic CD36−/− and SR-A−/− mice, several groups suggested that SR-A and CD36 play quantitatively important roles in mediating uptake of OxLDL and promoting the development of atherosclerosis in apoE−/− mice 79–82. In contrast, a different group demonstrated that both SR-A−/−, apoE−/− and CD36−/−, apoE−/− double knockout mice, although having significant reductions in peritoneal macrophage lipid accumulation in vivo, had increased atherosclerosis or no change in the lesion size 83. A follow up study by this group confirmed that even the combined CD36/SR-A deficiency in apoE−/− mice had no effect on foam cells in atherosclerotic lesions or the lesion size, but revealed reduced complexity and size of necrotic areas in the lesions, suggesting a role for CD36 and SR-A in cell death 84. The discrepancy between the results obtained in different laboratories underscores the complexity of the mechanisms of macrophage lipid accumulation and atherogenesis 32,76.
As noted earlier, mmLDL does not bind to CD36; it binds to CD14 and induces TLR4/MD-2-dependent cytoskeletal rearrangements. Remarkably, mmLDL-induced formation of membrane ruffles results in the ruffles’ closing into large endosomal vesicles (macropinosomes). This mmLDL (and 15LO-CE)-induced process of macropinocytosis captures lipoproteins from the cellular microenvironment and results in macrophage uptake of mmLDL itself, as well as other lipoproteins in the vicinity, such as native LDL and even OxLDL (Figure 1). Knockdown of TLR4 or Syk abolishes macrophage lipoprotein accumulation stimulated by either mmLDL or 15LO-CE 9. Mouse experimental studies support a role for TLR4 in the development of diet-induced atherosclerosis. TLR4 is expressed on endothelial cells and macrophages within human and mouse atherosclerotic lesions 85,86. A deficiency in TLR4 attenuates the development of atherosclerosis in hyperlipidemic apoE−/− mice 87. Over expression of both TLR2 and TLR4 in the intima of carotid arteries of hyperlipidemic rabbits significantly augmented atherosclerosis, although transfection of only TLR4 or only TLR2 resulted in little change in atherosclerosis 88. In human epidemiologic studies, the common Asp299Gly TLR4 polymorphism (loss-of-function) was associated with a decreased risk of carotid artery and femoral artery atherosclerosis and cardiovascular cause of death 89; however subsequent studies from different laboratories that evaluated different clinical manifestations of atherosclerosis reported inconsistent results 90–95.
Unlike the defined models of OxLDL or mmLDL that we use in the laboratory, oxidized LDL in atherosclerotic lesions is likely more complex, containing not only oxidation-specific epitopes found in both OxLDL and mmLDL, but yet unidentified epitopes as well. How can one measure in vivo the quantitative importance of each individual PRR-mediated uptake mechanism? We have recently suggested to tackle this problem by comparing the kinetics of lipid accumulation in vivo by wild-type macrophages versus that by various PRR-deficient macrophages, e.g. TLR4-deficient macrophages 96. For this purpose, we used optically transparent zebrafish larvae as a model for monitoring vascular lipid accumulation in live animals. We found that feeding zebrafish a high-cholesterol diet (HCD) resulted in remarkable hypercholesterolemia and lipoprotein oxidation, accompanied by the formation of fatty streaks and myeloid cell recruitment to major blood vessels 96. Genetic homologs and/or activity of 12/15LO, MPO and Nox enzymes have been identified in zebrafish. Moreover, our recent studies found high levels of biologically active OxCE and OxPL molecular structures accumulated in hypercholesterolemic zebrafish 97. An advantage of the optically transparent zebrafish model is that unlike cell culture experiments in which lipoproteins are modified in vitro and then added at arbitrary concentrations to the cells, in our zebrafish model, macrophages are exposed to a multitude of lipoprotein modifications occurring in vivo. TLR4-deficient and wild-type mouse macrophages were injected into zebrafish larvae in which HCD has induced the formation of fatty streaks. Because zebrafish larvae are transparent and because we used fluorescently labeled cells and fluorescent dietary lipids, we were able to quantify macrophage lipid uptake in vivo, directly in the environment of a fatty streak. Using this model, we found that the rate of in vivo lipid uptake by TLR4-deficient macrophages was significantly lower compared to the uptake by wild-type macrophages, supporting results of our in vitro experiments 9,96. Similar measurements with macrophages lacking other receptors and signaling molecules could be used in the future to measure a set of rate constants to compare different mechanisms of foam cell formation and specifically, the quantitative role of different PRRs.
Proinflammatory gene expression
The first report of proinflammatory properties of OxPL was that they induced monocyte binding to vascular endothelial cells (EC) 98,99, which is a critical early step in the formation of vascular lesions. OxPAPC has been widely used as a collection of biologically active OxPL. EC stimulated with OxPAPC secrete chemoattractants MCP-1 and IL-8 and express adhesion molecules connecting segment 1 (CS1) fibronectin and P-selectin on cell surface, which lead to monocyte recruitment and binding to the EC 98. The expression of CS-1 fibronectin is mediated by cAMP-induced R-Ras and PI3K activity 100. PEIPC (1-palmitoyl-2-epoxyisoprostane E2-sn-glycero-3-phosphocholine) was identified as a highly active component of OxPAPC, and exerted its activity via binding to prostaglandin E2 receptor (EP2) 101. In addition to MCP-1 and IL-8, OxPAPC induced expression of IL-6, CCL3, CCL4, and VEGF 102,103. Although TLR4 seems to be involved in OxPAPC-induced IL-8 secretion by EC10, the NF-B pathway was not activated, and the EC response to OxPAPC was mediated by the c-Src/JAK2/STAT3, SREBP and unfolded protein response (UPR) pathways 103–106. VEGFR2 mediates OxPAPC-induced recruitment of Rac1 to the NADPH oxidase-4 complex, resulting in strong ROS generation by EC. The ROS generation was, in turn, responsible for expression of inflammatory and sterol metabolism genes, but not the genes of UPR and antioxidative responses 107 (Figure 2). It should be noted that the PC-containing OxPL present on apoptotic cells and their apoptotic bodies can also mediate such biological effects. Thus apoptotic cells and blebs can induce IL-8 release from endothelial cells, effects blocked by antibody E06 7,108.
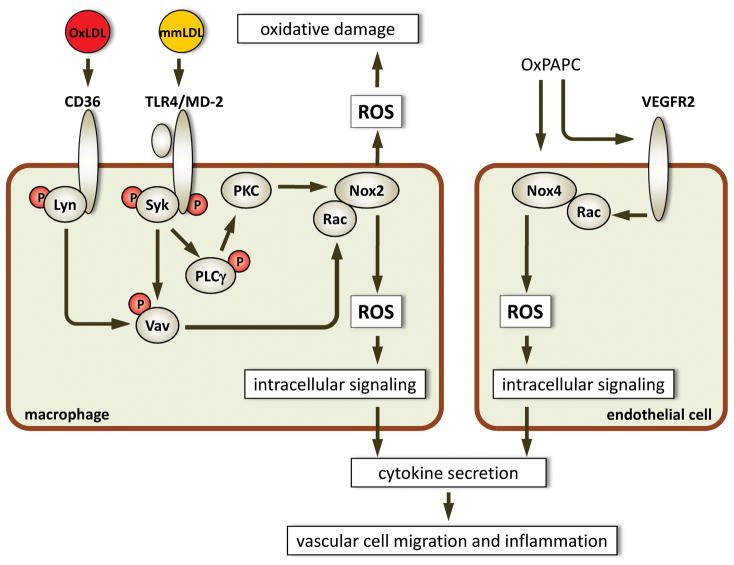
Figure 2. Oxidized LDL and OxPL induce ROS generation in vascular cells.
In macrophages, OxLDL binding to CD36 induces recruitment of Lyn, a Src kinase, its activation and phosphorylation of Vav, which in turn activates Rac 74. Unlike OxLDL, mmLDL induces recruitment of Syk to TLR4 and Syk-dependent activation of Vav and Rac 9. In addition, mmLDL induces TLR4- and Syk-dependent activation of PLCγand PKC, which induces recruitment of p47phox, and p67phox (not shown) to the Nox2 enzyme complex, and activated (GTP-bound) Rac completes the complex, leading to ROS production 9,110. High levels of extracellular ROS may exacerbate the oxidative damage, while intracellular ROS are important signaling molecules, mediating cytokine secretion. One of mmLDL-induced, Nox2-dependent chemokines, RANTES, induces VSMC migration 110. Nox4 is the predominant NADPH oxidase in endothelial cells. It is activated by OxPAPC via VEGFR2-dependent Rac recruitment 107. However, the mechanism of the OxPAPC activation is unclear. Although OxPAPC is capable of inducing VEGF production by endothelial cells 103, anti-VEGF antibodies do not block OxPAPC activation of VEGFR2 107. ROS in endothelial cells mediate secretion of MCP-1 and IL-8 and thereby promote monocyte migration.
mmLDL signals via TLR4/MD-2, but unlike LPS, the bacterial ligand for TLR4, mmLDL induces only modest levels of expression of pro-inflammatory cytokines 72. This is likely due TLR4 clustering with different cellular receptors 51,109, as well as due to the different set of adaptors recruited to the cytoplasmic domain of TLR4 in response to mmLDL as compared to LPS. mmLDL induces some MyD88-dependent effects, but a major signaling pathway for mmLDL seems to be the recruitment of Syk to TLR4, and Syk-dependent activation of ERK1/2 and PLCγ9,110. The latter activates PKC and NADPH oxidase-2 (Nox2), resulting in generation of ROS. ROS has been suggested to play a major role in regulation of many intracellular signaling pathways, including NF-κB. We have demonstrated Nox-2 dependent expression of RANTES, IL-1β, and IL-6 in macrophages stimulated with mmLDL. In turn, RANTES induced migration of vascular smooth muscle cells 110 (Figure 2).
Because of the signaling differences in the LPS- and mmLDL-induced activation of TLR4, we hypothesized that costimulation of macrophages by mmLDL and by low levels of bacterial LPS would result in cooperative effects, leading to greater activation than achieved by either stimulus alone. Indeed, we demonstrated that mmLDL and low levels of LPS cooperatively upregulated expression of a number of proinflammatory genes, including chemokines Cxcl2, Ccl3 and Ccl4, both in vivo and in vitro 111. A de novo motif analysis of promoters of cooperatively activated genes suggested involvement of AP-1 transcription factors, a finding that agrees with an ERK1/2-dependent character of mmLDL activation. In addition, mmLDL induced phosphorylation of c-Jun and the release of nuclear receptor corepressor (NCoR) from the promoter regions of Cxcl2 and Ccl3 111 (Figure 3). These molecular mechanisms of cooperative macrophage activation with mmLDL and low levels of LPS may be relevant to the increased risk of acute cardiovascular events in patients with atherosclerosis complicated by chronic infections, obesity and type 2 diabetes and other conditions associated with subclinical endotoxemia.
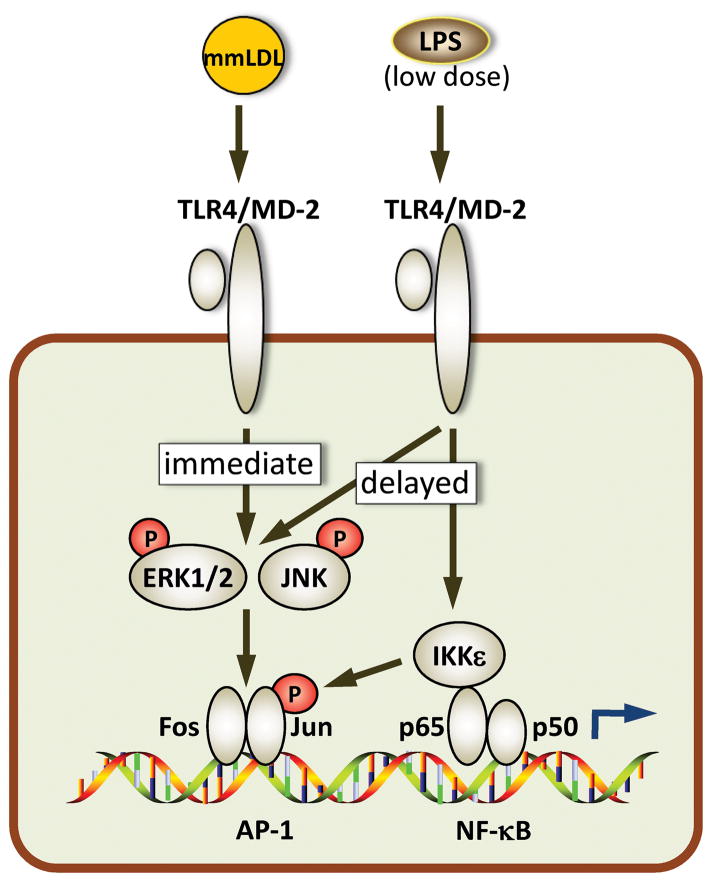
Figure 3. mmLDL and low-dose LPS induce cooperative TLR4-mediated signaling in macrophages.
mmLDL induces rapid, JNK-dependent phosphorylation of Jun, which leads to removal of NCoR and derepression of AP-1. This, together with rapid phosphorylation of ERK1/2, results in the completion of the AP-1 transcription complex, which is later strengthened by delayed ERK1/2 and Jun phosphorylation induced by LPS 111. However, LPS-induced Jun phosphorylation is mediated by IKKε, which is brought to the promoter region with the NF-κB transcription factors 127. Cooperative activation of AP-1 and NF-κB result in increased expression of proinflammatory genes induced by co-stimulation with mmLDL and LPS 111.
Although initial evidence placed pattern recognition on the cell surface, new data suggest that signaling by intracellular PRRs is as important as the ones originating from the plasma membrane. We will review two examples, both published in 2010. One demonstrates that recognition of OxLDL (and amyloid-β peptide) by CD36 triggers assembly of a heterotrimeric complex composed of CD36, TLR4 and TLR6 51. The signaling likely originates on the cell surface, where OxLDL induces CD36-mediated activation of a Src kinase Lyn, which, in turn, initiates TLR4/TLR6 dimerization. The assembly of the CD36/TLR4/TLR6 complex is completed in the endosomal compartment, where both the MyD88 and TRIF adaptors propagated the signal, leading to activation of NF-κB, generation of ROS and expression of Cxcl1, Cxcl2, Ccl9, Ccl5 and IL-1β51.
The second example involves not an oxidized lipid, but cholesterol crystals. However, lipid peroxidation is known to promote formation of crystalline structures of free cholesterol 112. Recent studies using laser reflection and fluorescent microscopy suggested that cholesterol crystals emerge at the earliest time points of diet-induced atherogenesis, together with the appearance of immune cells in the subendothelial space 113. Cholesterol crystals localized both extracellulary and inside macrophages in atherosclerotic lesions. Microcrystals in general and cholesterol crystals in particular have been shown to activate the intracellular PRR, NLRP3 (cryopyrin), the critical component in the caspase-1-mediated production of active IL-1 114. Indeed, cholesterol crystals induced IL-1βsecretion by wild type but not NLRP3−/− macrophages, and transplantation of NLR3−/− or IL-1α/β−/− bone marrow into LDLR−/− mice significantly reduced diet-induced atherosclerosis 113.
Expansion of oxidation-specific NAbs and atherosclerosis
Activation of cellular PRR by oxidation-specific epitopes results in many pro-inflammatory and pro-atherogenic effects, as summarized above. In contrast, NAbs seem to protect against atherosclerosis. In vitro, the E06 NAb, which binds the same PC-containing OxPL as does CD36, prevented OxLDL and apoptotic cell uptake by macrophages 6,63. Because immunization of mice with heat-inactivated PC-containing S. pneumoniae was known to robustly expand T15/E06 B-1 cell clones, we immunized cholesterol-fed LDLR−/− mice with heat killed pneumococci to see if this would reduce atherosclerosis. Indeed, immunization induced high titers of E06/T15 IgM and significantly reduced atherosclerosis 115. The atheroprotective property of anti-PC IgM was corroborated in a vein graft atherosclerosis model in which T15 IgM was infused intravenously 116, and by the demonstration that immunizing apoE−/− mice with PC-KLH was atheroprotective 117. Furthermore, we have shown that E06can bind to and block pro-inflammatory effects of PC-containingOxPL7,11,108, and this property undoubtedly also contributes importantly to the anti-inflammatory and anti-atherogenic properties of these oxidation-specific IgM. The overall importance of IgM to atherogenesis was demonstrated by the observation that when secretory IgM knockout mice were crossed with LDLR−/− mice, atherosclerosis was dramatically enhanced 118. As we have shown that oxidation-specific epitopes are a major target of NAbs in mice (and humans) it is very likely that IgM to such epitopes play a major role in this protective effect. We predict that similar to E06, NAbs to other oxidation-specific epitopes, such as MDA, 4-hydroxy-2-nonenal, and oxidized cardiolipin, will be anti-inflammatory and atheroprotective as well.
Because NAbs have been conserved by natural selection, it is likely that on balance they must have been beneficial to the host, aside from their role in atherogenesis. For example, we and others have shown that by binding to oxidation-specific epitopes on the surface of apoptotic cells, they facilitate complement-dependent enhanced clearance in vivo 59,119,120. Because we have shown in principle that increasing the titer of such antibodies can be anti-atherogenic, identifying the epitopes to which such NAbs bind should identify antigens that could be used to develop an atheroprotective vaccine. Inaddition, enhancing such NAb titers, possibly even passively, could be used to limit the pro-inflammatory effects of oxidized lipids in acute situations, such as in acute coronary syndromes 121, or in viral induced respiratory distress syndromes, a situation in which the oxidized lipids to which they bind are proinflammatory 11,122. Antibodies to these epitopes could be used to image not only atherosclerotic lesions 123 but a variety of inflammatory settings in which such epitopes are ubiquitously expressed, as well as to target therapeutic molecules to active sites of inflammation. It can be envisioned that a better understanding of this important compartment of innate immunity will ultimately identify novel therapeutic targets that can be exploited to interfere with atherogenesis and inflammatory states in general.
Conclusions
In this article, we summarized evidence that PRR recognition of oxidation-specific epitopes initiates many important innate immune processes, having both pro-atherogenic and anti-atherogenic consequences. To put these findings into perspective, because PRRs are germline encoded, the observation that oxidation-specific epitopes are targeted by multiple innate PRRs strongly implies that these epitopes represent important endogenous “danger signals” against which multiple defenses are selected to provide homeostasis. Moreover, endogenous oxidation-specific epitopes likely exert positive selection pressure for NAb-producing B-1 cells, which are selected in fetal life, or shortly thereafter. NAbs are present even in germ-free mice, never exposed to microbial PAMPs. The observation that similar epitopes on infectious pathogens are also recognized by NAbs suggests that such pathogens are likely to have also exerted selection pressure later in life. A similar argument may be offered that oxidation-specific epitopes are among the primary selecting antigens for other innate immune receptors as well.
We further suggest that the fundamentally important altered-self antigens leading to selection of oxidation-specific innate PRRs are apoptotic cells and the apoptotic blebs and cellular debris resulting from such programmed cell death (Figure 4). Cells undergoing apoptosis develop mitochondrial disruption, leading to generalized enhancement of oxidative events. In turn, a wide variety of lipids are oxidized, including cardiolipin, phosphatidylserine, and phosphatidylcholine 124. (Future studies will determine if OxCEs are also found on dying cells). Such epitopes are greatly enriched in the apoptotic blebs that budoff from such cells 6,108 and are present in the circulationas microparticles. Scavenger receptors participate in apoptotic cell recognition and clearance by phagocytes. We have shown that syngenic apoptotic cells are highly immunogenic and pro-inflammatory if not promptly cleared 7, and since apoptosis is a universal biological event from the earliest stages of development, we suggest that this provides a strong evolutionary pressure for innate mechanisms to efficiently clear such dying cells. Indeed, the fact that there are apparently multiple and redundant mechanisms to effect clearance of apoptotic cells indicates the biological importance of this process 125. Remarkably, nearly all of the different oxidation-specific monoclonal antibodies we have cloned to date, both from murine or human libraries, bind to apoptotic cells and apoptotic bodies, presumably to effect their removal, as noted above. Furthermore, these same oxidation-specific epitopes are ligands mediating apoptotic cell recognition by various scavenger receptors 33. Failure to adequately clear the daily burden of apoptotic cells would likely lead to inflammation, immune activation and pathology, as for example, promotion of atherogenesis 71,126.
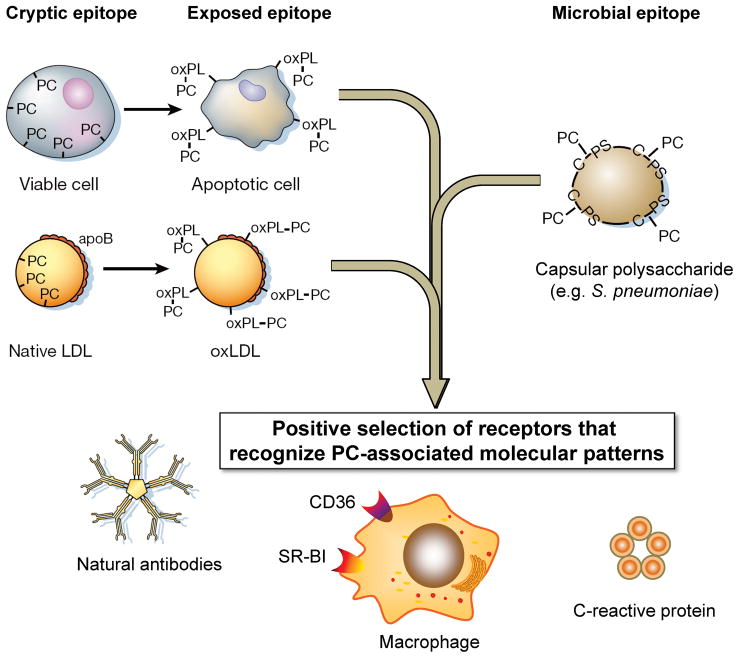
Figure 4. Pattern recognition of oxidation-specific DAMPs and microbial PAMPs.
Using the example of the phosphocholine (PC) epitope, we illustrate in this cartoon our hypothesis of the emergence and positive selection of multiple PRRs that recognize common epitopes, shared by modified self and microbial pathogens. According to this hypothesis, oxidation of plasma membrane phospholipids in apoptotic cells alters the conformation of the PC headgroup, yielding an exposed epitope, accessible to recognition by macrophage scavenger receptors, NAbs, and pentraxins, such as CRP. These PRRs were selected to clear apoptotic cells from developing or regenerating tissues. Recognition by the same receptors of the PC epitope of capsular polysaccharide in gram-positive bacteria (e.g. S. pneumoniae) strengthened positive selection of these PRRs and probably helped to select additional strong proinflammatory components to PRR-dependent responses. (Note the PC on the bacteria is not part of a phospholipid.) Finally, oxidized lipoproteins, prevalent in humans as a result of dyslipidemia and impact of environmental factors and in experimental animals, bear OxPLs with the PC epitope exposed in an analogous manner to that of apoptotic cells. This leads to OxLDL recognition by PRRs and initiation of innate immune responses. The balance between pro-inflammatory responses of cellular PRRs and atheroprotective roles of NAbs plays an important role in the development of atherosclerosis. There are likely many more oxidation-specific epitopes that represent such DAMPS and corresponding PRRs that represent respective innate responses.
In contrast to atheroprotective oxidation-specific NAbs, cellular PRR recognition of oxidation-specific epitopes have predominantly pro-atherogenic effects, at least under conditions of marked hypercholesterolemia generated in the Western diet fed murine models studied. However, such marked hypercholesterolemia is not likely to have been influential in evolutionary selection. Rather, PRRs likely evolved as protective mechanisms against the proinflammatory effects of oxidation-specific epitopes, such as those found in apoptotic cells, or even the oxidized lipids found extracellularly, such as on apoptotic blebs or lipoproteins. Because many of the same oxidation-specific PRR also recognize similar molecular patterns on pathogens, we speculate that the ability to protect against infectious pathogens provided a strong secondary selecting pressure as well. It is also plausible that these cellular PRRs developed accompanying stronger cellular responses to such infectious pathogens than those resulting from host-derived activation. This implies that the intracellular signaling pathways linking a PRR response to an endogenous DAMP may be different than those involved in the same PRR’s response to an infectious PAMP. Indeed, examples of differential responses of TLR4 to mmLDL (and its OxCE) and to LPS were discussed above. Thus, to provide a strong host response to an acute invading pathogen, TLRs initiate an “over-reactive” cellular response. In contrast, the cellular response mediated by the same PRR to an endogenous DAMP, such as OxCE, is more muted, as the OxCE is ubiquitously present, and the cellular response is aimed at maintaining homeostasis. Nevertheless, chronic exposure to increased levels of DAMPs such as oxidation-specific epitopes induced by hypercholesterolemia, induces low-grade but sustained activation of PRRs and, thus, contributes to the inflammatory state characteristic of atherogenesis.
Acknowledgments
Sources of Funding
The studies performed in the authors’ laboratories were supported by the grants HL081862, HL086559, HL088093, HL093767, and GM069338 from the NIH, and a grant from the Leducq Fondation.
Non-standard Abbreviations and Acronyms
- CE
cholesteryl ester
- DAMP
danger-associated molecular pattern
- EC
endothelial cell
- KLH
keyhole limpet hemocyanin
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide
- LO
lipoxygenase
- mmLDL
minimally oxidized LDL
- MDA
malondialdehyde
- NAb
natural antibody
- OxCE
oxidized CE
- OxLDL
oxidized LDL
- OxPAPC
oxidized PAPC
- OxPL
oxidized PL
- PAMP
pathogen-associated molecular pattern
- PAPC
1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine
- PC
phosphocholine
- PEIPC
1-palmitoyl-2-epoxyisoprostane E2-sn-glycero-3-phosphocholine
- PL
phospholipid
- POVPC
1-palmitoyl-2-(5′-oxovaleroyl)-sn-glycero-3-phosphocholine
- PRR
pattern recognition receptor
- PUFA
polyunsaturated fatty acids
- ROS
reactive oxygen species
- SR
scavenger receptor
- Syk
spleen tyrosine kinase
- TLR
toll-like receptor
Footnotes
Disclosures
Drs. Witztum, Tsimikas and Shaw are co-inventors of patents and patent applications through the University of California, San Diego (UCSD) for the use of oxidation-specific antibodies and Dr. Miller is a co-inventor of patent applications through UCSD for the use of the hypercholesterolemic zebrafish model.
References
- 1.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Approaching the Asymptote: 20 Years Later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P. The Danger Model: A Renewed Sense of Self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 5.Bird DA, Gillotte KL, Hörkkö S, Friedman P, Dennis EA, Witztum JL, Steinberg D. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc Natl Acad Sci U S A. 1999;96:6347–6352. doi: 10.1073/pnas.96.11.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci U S A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic Cells with Oxidation-specific Epitopes Are Immunogenic and Proinflammatory. J Exp Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Choi S-H, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walton KA, Hsieh X, Gharavi N, Wang S, Wang G, Yeh M, Cole AL, Berliner JA. Receptors involved in the Ox-PAPC-mediated synthesis of IL-8: A role for toll-like receptor 4 and a GPI anchored protein. J Biol Chem. 2003;278:29661–29666. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 11.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YHC, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JSM, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of Oxidative Stress and Toll-like Receptor 4 Signaling as a Key Pathway of Acute Lung Injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moussian B, Roth S. Dorsoventral Axis Formation in the Drosophila Embryo--Shaping and Transducing a Morphogen Gradient. Current Biology. 2005;15:R887–R899. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Sepulcre MP, caraz-Perez F, Lopez-Munoz A, Roca FJ, Meseguer J, Cayuela ML, Mulero V. Evolution of Lipopolysaccharide (LPS) Recognition and Signaling: Fish TLR4 Does Not Recognize LPS and Negatively Regulates NF-{kappa}B Activation. The Journal of Immunology. 2009;182:1836–1845. doi: 10.4049/jimmunol.0801755. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan C, Charette J, Catchen J, Lage CR, Giasson G, Postlethwait JH, Millard PJ, Kim CH. The Gene History of Zebrafish tlr4a and tlr4b Is Predictive of Their Divergent Functions. The Journal of Immunology. 2009;183:5896–5908. doi: 10.4049/jimmunol.0803285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radical Biology and Medicine. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 16.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radical Biology and Medicine. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 17.Shao B, Heinecke JW. HDL, lipid peroxidation, and atherosclerosis. J Lipid Res. 2009;50:599–601. doi: 10.1194/jlr.E900001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller YI, Choi S-H, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: Role of pathologic cholesterol transport in atherogenesis. Subcellular Biochemistry. 2010;51:229–251. doi: 10.1007/978-90-481-8622-8_8. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrecher UP, Parthasarathy S, Leake DS, Witztum JL, Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esterbauer H, Jurgens G, Quehenberger O, Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987;28:495–509. [PubMed] [Google Scholar]
- 21.Reaven P, Parthasarathy S, Grasse BJ, Miller E, Steinberg D, Witztum JL. Effects of oleate-rich and linoleate-rich diets on the susceptibility of low density lipoprotein to oxidative modification in mildly hypercholesterolemic subjects. J Clin Invest. 1993;91:668–676. doi: 10.1172/JCI116247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 23.Philis-Tsimikas A, Parthasarathy S, Picard S, Palinski W, Witztum JL. Aminoguanidine has both pro-oxidant and antioxidant activity toward LDL. Arterioscler Thromb Vasc Biol. 1995;15:367–376. doi: 10.1161/01.atv.15.3.367. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta. 1992;1128:117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 25.Ezaki M, Witztum JL, Steinberg D. Lipoperoxides in LDL incubated with fibroblasts that over express 15-lipoxygenase. J Lipid Res. 1995;36:1996–2004. [PubMed] [Google Scholar]
- 26.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized LDL. J Biol Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi Y, Zhu H, Xu W, Murakami T, Iwasaki T, Hattori H, Yoshimoto T. Selective uptake and efflux of cholesteryl linoleate in LDL by macrophages expressing 12/15-lipoxygenase. Biochem Biophys Res Commun. 2005;338:128–135. doi: 10.1016/j.bbrc.2005.07.182. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Funk CD. Lipoxygenase Pathways in Atherogenesis. Trends in Cardiovascular Medicine. 2004;14:191–195. doi: 10.1016/j.tcm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Upston JM, Niu X, Brown AJ, Mashima R, Wang H, Senthilmohan R, Kettle AJ, Dean RT, Stocker R. Disease Stage-Dependent Accumulation of Lipid and Protein Oxidation Products in Human Atherosclerosis. Am J Pathol. 2002;160:701–710. doi: 10.1016/S0002-9440(10)64890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitinger N. Cholesteryl ester oxidation products in atherosclerosis. Molecular Aspects of Medicine. 2003;24:239–250. doi: 10.1016/s0098-2997(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webb NR, Moore KJ. Macrophage-derived foam cells in atherosclerosis: lessons from murine models and implications for therapy. Curr Drug Targets. 2007;8:1249–1263. doi: 10.2174/138945007783220597. [DOI] [PubMed] [Google Scholar]
- 33.Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 35.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boullier A, Gillotte KL, Hörkkö S, Green SR, Friedman P, Dennis EA, Witztum JL, Steinberg D, Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 37.Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 39.Gillotte KL, Hörkkö S, Witztum JL, Steinberg D. Oxidized phospholipids, linked to apolipoprotein B of oxidized LDL, are ligands for macrophage scavenger receptors. J Lipid Res. 2000;41:824–833. [PubMed] [Google Scholar]
- 40.Friedman P, Hörkkö S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids: Importance of Schiff base formation and Aldol condensation. J Biol Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 41.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 42.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006 doi: 10.1084/jem.20060370. jem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The Lipid Whisker Model of the Structure of Oxidized Cell Membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 45.Hazen SL. Oxidized Phospholipids as Endogenous Pattern Recognition Ligands in Innate Immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman M, Ekkel Y, Rohrer L, Penman M, Freedman NJ, Chisolm GM, Krieger M. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1991;88:4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyake K. Roles for accessory molecules in microbial recognition by Toll-like receptors. J Endotoxin Res. 2006;12:195–204. doi: 10.1179/096805106X118807. [DOI] [PubMed] [Google Scholar]
- 48.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 49.Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint GI, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, Underhill DM, Kirschning CJ, Wagner H, Aderem A, Tobias PS, Ulevitch RJ. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 50.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 51.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, Khoury JE, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J Lipid Res. 2009;50:S340–S345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally Modified LDL Binds to CD14, Induces Macrophage Spreading via TLR4/MD-2, and Inhibits Phagocytosis of Apoptotic Cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 54.von Schlieffen E, Oskolkova OV, Schabbauer G, Gruber F, Bluml S, Genest M, Kadl A, Marsik C, Knapp S, Chow J, Leitinger N, Binder BR, Bochkov VN. Multi-Hit Inhibition of Circulating and Cell-Associated Components of the Toll-Like Receptor 4 Pathway by Oxidized Phospholipids. Arterioscler Thromb Vasc Biol. 2009;29:356–362. doi: 10.1161/ATVBAHA.108.173799. [DOI] [PubMed] [Google Scholar]
- 55.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 56.Walton KA, Cole AL, Yeh M, Subbanagounder G, Krutzik SR, Modlin RL, Lucas RM, Nakai J, Smart EJ, Vora DK, Berliner JA. Specific Phospholipid Oxidation Products Inhibit Ligand Activation of Toll-Like Receptors 4 and 2. Arterioscler Thromb Vasc Biol. 2003;23:1197–1203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 57.Jimenez-Dalmaroni MJ, Xiao N, Corper AL, Verdino P, Ainge GD, Larsen DS, Painter GF, Rudd PM, Dwek RA, Hoebe K, Beutler B, Wilson IA. Soluble CD36 Ectodomain Binds Negatively Charged Diacylglycerol Ligands and Acts as a Co-Receptor for TLR2. PLoS ONE. 2009;4:e7411. doi: 10.1371/journal.pone.0007411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang MK, Binder CJ, Torzewski M, Witztum JL. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci U S A. 2002;99:13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 61.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Seminars in Immunopathology. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 62.Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of Monoclonal Autoantibodies to Epitopes of Oxidized Lipoproteins from Apolipoprotein E-deficient Mice. Demonstration of Epitopes of Oxidized Low Density Lipoprotein in Human Plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hörkkö S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mi QS, Zhou L, Schulze DH, Fischer RT, Lustig A, Rezanka LJ, Donovan DM, Longo DL, Kenny JJ. Highly reduced protection against Streptococcus pneumoniae after deletion of a single heavy chain gene in mouse. Proc Natl Acad Sci U S A. 2000;97:6031–6036. doi: 10.1073/pnas.110039497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tuominen A, Miller YI, Hansen LF, Kesaniemi YA, Witztum JL, Horkko S. A Natural Antibody to Oxidized Cardiolipin Binds to Oxidized Low-Density Lipoprotein, Apoptotic Cells, and Atherosclerotic Lesions. Arterioscler Thromb Vasc Biol. 2006 doi: 10.1161/01.ATV.0000233333.07991.4a. [DOI] [PubMed] [Google Scholar]
- 67.Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radical Biology and Medicine. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 68.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res. 2007;48:425–433. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Miller YI, Chang MK, Funk CD, Feramisco JR, Witztum JL. 12/15-Lipoxygenase translocation enhances site-specific actin polymerization in macrophages phagocytosing apoptotic cells. J Biol Chem. 2001;276:19431–19439. doi: 10.1074/jbc.M011276200. [DOI] [PubMed] [Google Scholar]
- 70.Miller YI, Worrall DS, Funk CD, Feramisco JR, Witztum JL. Actin polymerization in macrophages in response to oxidized LDL and apoptotic cells: role of 12/15-lipoxygenase and phosphoinositide 3-kinase. Mol Biol Cell. 2003;14:4196–4206. doi: 10.1091/mbc.E03-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 73.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tabas I, Williams KJ, Boren J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis: Update and Therapeutic Implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 76.Witztum JL. You are right too! J Clin Invest. 2005;115:2072–2075. doi: 10.1172/JCI26130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamashita S, Hirano K, Kuwasako T, Janabi M, Toyama Y, Ishigami M, Sakai N. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol Cell Biochem. 2007;299:19–22. doi: 10.1007/s11010-005-9031-4. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Sakaguchi H, Kruijt JK, Higashi T, Suzuki T, Van Berkel TJ, Horiuchi S, Takahashi K, Yazaki Y, Kodama T. The multiple roles of macrophage scavenger receptors (MSR) in vivo: resistance to atherosclerosis and susceptibility to infection in MSR knockout mice. J Atheroscler Thromb. 1997;4:1–11. doi: 10.5551/jat1994.4.1. [DOI] [PubMed] [Google Scholar]
- 80.Sakaguchi H, Takeya M, Suzuki H, Hakamata H, Kodama T, Horiuchi S, Gordon S, van der Laan LJ, Kraal G, Ishibashi S, Kitamura N, Takahashi K. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest. 1998;78:423–434. [PubMed] [Google Scholar]
- 81.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, varez-Leite JI, de Winther MPJ, Tabas I, Freeman MW. Loss of SR-A and CD36 Activity Reduces Atherosclerotic Lesion Complexity Without Abrogating Foam Cell Formation in Hyperlipidemic Mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-Like Receptor-4 Is Expressed by Macrophages in Murine and Human Lipid-Rich Atherosclerotic Plaques and Upregulated by Oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 86.Mullick AE, Soldau K, Kiosses WB, Bell TA, III, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. PNAS. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shinohara M, Hirata Ki, Yamashita T, Takaya T, Sasaki N, Shiraki R, Ueyama T, Emoto N, Inoue N, Yokoyama M, Kawashima S. Local Overexpression of Toll-Like Receptors at the Vessel Wall Induces Atherosclerotic Lesion Formation: Synergism of TLR2 and TLR4. Arterioscler Thromb Vasc Biol. 2007;27:2384–2391. doi: 10.1161/ATVBAHA.106.139253. [DOI] [PubMed] [Google Scholar]
- 89.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 90.Boekholdt SM, Agema WR, Peters RJ, Zwinderman AH, Van Der Wall EE, Reitsma PH, Kastelein JJ, Jukema JW. Variants of Toll-Like Receptor 4 Modify the Efficacy of Statin Therapy and the Risk of Cardiovascular Events. Circulation. 2003;107:2416–2421. doi: 10.1161/01.CIR.0000068311.40161.28. [DOI] [PubMed] [Google Scholar]
- 91.Ameziane N, Beillat T, Verpillat P, Chollet-Martin S, Aumont MC, Seknadji P, Lamotte M, Lebret D, Ollivier V, de Prost D. Association of the Toll-Like Receptor 4 Gene Asp299Gly Polymorphism With Acute Coronary Events. Arterioscler Thromb Vasc Biol. 2003;23:e61–e64. doi: 10.1161/01.ATV.0000101191.92392.1D. [DOI] [PubMed] [Google Scholar]
- 92.Holloway JW, Yang IA, Ye S. Variation in the toll-like receptor 4 gene and susceptibility to myocardial infarction. Pharmacogenet Genomics. 2005;15:15–21. doi: 10.1097/01213011-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Netea MG, Hijmans A, van WS, Smilde TJ, Trip MD, Kullberg BJ, de BT, Van der Meer JW, Kastelein JJ, Stalenhoef AF. Toll-like receptor-4 Asp299Gly polymorphism does not influence progression of atherosclerosis in patients with familial hypercholesterolaemia. Eur J Clin Invest. 2004;34:94–99. doi: 10.1111/j.1365-2362.2004.01303.x. [DOI] [PubMed] [Google Scholar]
- 94.Yang IA, Holloway JW, Ye S. TLR4 Asp299Gly polymorphism is not associated with coronary artery stenosis. Atherosclerosis. 2003;170:187–190. doi: 10.1016/s0021-9150(03)00286-7. [DOI] [PubMed] [Google Scholar]
- 95.Reismann P, Lichy C, Rudofsky G, Humpert PM, Genius J, Si TD, Dorfer C, Grau AJ, Hamann A, Hacke W, Nawroth PP, Bierhaus A. Lack of association between polymorphisms of the toll-like receptor 4 gene and cerebral ischemia. J Neurol. 2004;251:853–858. doi: 10.1007/s00415-004-0447-7. [DOI] [PubMed] [Google Scholar]
- 96.Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, Crosier P, Witztum JL, Klemke RL, Miller YI. Vascular Lipid Accumulation, Lipoprotein Oxidation, and Macrophage Lipid Uptake in Hypercholesterolemic Zebrafish. Circ Res. 2009;104:952–960. doi: 10.1161/CIRCRESAHA.108.189803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, Witztum JL, Tsimikas S, Miller YI. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high-cholesterol diet: macrophage binding and activation. J Biol Chem. 2010 doi: 10.1074/jbc.M110.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson AD, Faull KF, Fogelman AM, Berliner JA. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol. 2000;20:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 100.Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized Phospholipid-Induced Endothelial Cell/Monocyte Interaction Is Mediated by a cAMP-Dependent R-Ras/PI3-Kinase Pathway. Arterioscler Thromb Vasc Biol. 2003;23:1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- 101.Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Identification of Prostaglandin E2 Receptor Subtype 2 As a Receptor Activated by OxPAPC. Circ Res. 2006;98:642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 102.Bochkov VN. Inflammatory profile of oxidized phospholipids. Thromb Haemost. 2007;97:348–354. [PubMed] [Google Scholar]
- 103.Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol. 2010;30:1007–1013. doi: 10.1161/ATVBAHA.110.204354. [DOI] [PubMed] [Google Scholar]
- 104.Yeh M, Cole AL, Choi J, Liu Y, Tulchinsky D, Qiao JH, Fishbein MC, Dooley AN, Hovnanian T, Mouilleseaux K, Vora DK, Yang WP, Gargalovic P, Kirchgessner T, Shyy JYJ, Berliner JA. Role for Sterol Regulatory Element-Binding Protein in Activation of Endothelial Cells by Phospholipid Oxidation Products. Circ Res. 2004;95:780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- 105.Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, Johnson J, Szeto WL, Hong L, Fishbein M, Wei L, Pfeffer LM, Berliner JA. Role of the JAK/STAT Pathway in the Regulation of Interleukin-8 Transcription by Oxidized Phospholipids in Vitro and in Atherosclerosis in Vivo. J Biol Chem. 2007;282:31460–31468. doi: 10.1074/jbc.M704267200. [DOI] [PubMed] [Google Scholar]
- 106.Oskolkova OV, Afonyushkin T, Leitner A, von Schlieffen E, Gargalovic PS, Lusis AJ, Binder BR, Bochkov VN. ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood. 2008;112:330–339. doi: 10.1182/blood-2007-09-112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, Li R, Zimman A, Berliner JA. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radical Biology and Medicine. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 109.Schmitz G, Orso E. CD14 signalling in lipid rafts: new ligands and co-receptors. Current Opinion in Lipidology. 2002;13:513–521. doi: 10.1097/00041433-200210000-00007. [Miscellaneous Article] [DOI] [PubMed] [Google Scholar]
- 110.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages Generate Reactive Oxygen Species in Response to Minimally Oxidized Low-Density Lipoprotein: Toll-Like Receptor 4- and Spleen Tyrosine Kinase-Dependent Activation of NADPH Oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wiesner P, Choi SH, Almazan F, Benner C, Huang W, Diehl CJ, Gonen A, Butler S, Witztum JL, Glass CK, Miller YI. Low Doses of Lipopolysaccharide and Minimally Oxidized Low-Density Lipoprotein Cooperatively Activate Macrophages via Nuclear Factor {kappa}B and Activator Protein-1. Possible Mechanism for Acceleration of Atherosclerosis by Subclinical Endotoxemia. Circ Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Self-Medlin Y, Byun J, Jacob RF, Mizuno Y, Mason RP. Glucose promotes membrane cholesterol crystalline domain formation by lipid peroxidation. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2009;1788:1398–1403. doi: 10.1016/j.bbamem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 113.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 115.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 116.Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 117.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, Kohler HV, Kaveri SV, Nicoletti A. Phosphorylcholine-Targeting Immunization Reduces Atherosclerosis. Journal of the American College of Cardiology. 2007;50:540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 118.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M Is Required for Protection Against Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y, Park YB, Patel E, Silverman GJ. IgM Antibodies to Apoptosis-Associated Determinants Recruit C1q and Enhance Dendritic Cell Phagocytosis of Apoptotic Cells. The Journal of Immunology. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsimikas S, Lau HK, Han KR, Shortal B, Miller ER, Segev A, Curtiss LK, Witztum JL, Strauss BH. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 122.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical Role of IL-17RA in Immunopathology of Influenza Infection. The Journal of Immunology. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Briley-Saebo KC, Shaw PX, Mulder WJM, Choi SH, Vucic E, Aguinaldo JG, Witztum JL, Fuster V, Tsimikas S, Fayad ZA. Targeted Molecular Probes for Imaging Atherosclerotic Lesions With Magnetic Resonance Using Antibodies That Recognize Oxidation-Specific Epitopes. Circulation. 2008;117:3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tyurin VA, Tyurina YY, Ritov VB, Lysytsya A, Amoscato AA, Kochanek PM, Hamilton R, Dekosky ST, Greenberger JS, Bayir H, Kagan VE. Oxidative lipidomics of apoptosis: quantitative assessment of phospholipid hydroperoxides in cells and tissues. Methods Mol Biol. 2010;610:353–374. doi: 10.1007/978-1-60327-029-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 126.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage Apoptosis Exerts Divergent Effects on Atherogenesis as a Function of Lesion Stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 127.Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. Transcriptional Integration of TLR2 and TLR4 Signaling at the NCoR Derepression Checkpoint. Molecular Cell. 2009;35:48–57. doi: 10.1016/j.molcel.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]