Abstract
Pneumonic plague is one of the world’s most deadly infectious diseases. The causative bacterium, Yersinia pestis, has the potential to be exploited as a biological weapon and no vaccine is available. Vaccinating B cell-deficient mice with D27-pLpxL, a live attenuated Y. pestis strain, induces cell-mediated protection against lethal pulmonary Y. pestis challenge. Here we demonstrate that prime/boost vaccination with D27-pLpxL confers better protection than prime-only vaccination. The improved survival does not result from enhanced bacterial clearance, but is associated with increased levels of IL-17 mRNA and protein in the lungs of challenged mice. The boost also increases pulmonary numbers of IL-17-producing CD4 T cells. Interestingly, the vast majority of these cells simultaneously produce canonical type 1 and type 17 cytokines; most produce IL-17 and TNFα, and many produce IL-17, TNFα and IFNγ. Neutralizing IL-17 counteracts the improved survival associated with prime/boost vaccination without significantly impacting bacterial burden. Thus, IL-17 appears to mediate the enhanced protection conferred by booster immunization. Although neutralizing IL-17 significantly reduces neutrophil recruitment to the lungs of mice challenged with Y. pestis, this impact is equally evident in mice that receive one or two immunizations with D27-pLpxL, suggesting it cannot suffice to account for the improved survival that results from booster immunization. We conclude that IL-17 plays a yet to be identified role in host defense that enhances protection against pulmonary Y. pestis challenge, and we suggest that pneumonic plague vaccines should aim to induce mixed type 1 and type 17 cellular responses.
Introduction
Yersinia pestis, the gram-negative facultative intracellular bacterium that causes plague, is one of the world’s most deadly human pathogens. Plague has killed hundreds of millions of people during three major pandemics (1–3). The disease is naturally transmitted from rodent reservoirs to humans via fleabites, which typically cause the bubonic form of plague, characterized by a painful swelling of lymph nodes draining the site of infection (2, 3). If left untreated, Y. pestis bacteria can disseminate to the blood and cause septicemic plague. If the bacteria colonize the lungs, the disease may progress to pneumonic plague, which can spread from person to person via infectious airborne droplets. Pneumonic plague is a rapidly progressing disease and nearly always fatal unless treated with antibiotics soon after symptom onset (3). However, antibiotic-resistant isolates of Y. pestis have been described (4). In addition, scientists developed techniques to effectively aerosolize Y. pestis during the Cold War (5), raising concern that antibiotic-resistant Y. pestis strains could be exploited as deadly biological weapons of terror (6).
Tremendous effort has been dedicated to the development of plague countermeasures, but safe and effective vaccines for pneumonic plague are not currently available (7). The leading candidates in development are subunit vaccines comprised of the Y. pestis F1 and LcrV proteins. It is hoped these subunit vaccines will lack the side effects of live attenuated vaccines while conferring superior protection than killed whole cell vaccines (7). Although F1/LcrV-based vaccines induce high-titer antibody responses and provide substantial protection in mouse models of pneumonic plague (7, 8), they have failed, thus far, to fully protect nonhuman primates (8, 9). The variable efficacy of these vaccines in primates raises the possibility that antibodies may not suffice to protect humans against pneumonic plague. Moreover, F1-deficient Y. pestis strains retain their virulence and pathogenic Y. pestis species can express multiple LcrV variants, some of which may evade cross-protective immunity (7, 8). Thus, the F1/LcrV subunit vaccines in development may fail to fully protect humans against weaponized plague.
Recent studies suggest that TNFα and IFNγ cytokine products of type 1 cellular immunity, also contribute to defense against Y. pestis infection (10–16). Despite inducing high-titer antibodies, F1/LcrV immunization poorly protects STAT4-deficient mice, which are impaired for type 1 immune responses (10). Moreover, wild type mice actively immunized with F1/LcrV (13), or passively immunized with F1- or LcrV-specific mAb (13,14), succumb to pulmonary Y. pestis challenge if TNFα and IFNγ are neutralized at the time of infection. TNFα and IFNγ also contribute to the T cell-mediated protection conferred by vaccinating B cell-deficient µMT mice with attenuated Y. pestis (11, 12). Together, these studies suggest that next generation plague vaccines should aim to induce both Y. pestis-specific antibody and type 1 cellular immunity (8).
The proinflammatory cytokine IL-17 (also known as IL-17A) also contributes to antibacterial defense, and CD4 T cells can produce IL-17 (17–21). These “Th17” cells also produce IL-17F, IL-21, IL-22, IFNγ, TNFα, IL-6, and GM-CSF under certain circumstances (21–24). A number of studies have established that Th17 cells contribute to host defense against bacterial pathogens by inducing expression of antimicrobial factors and recruiting neutrophils to mucosal surfaces (19–21, 25–29). Here we demonstrate that vaccination with live attenuated Y. pestis induces IL-17-producing CD4 T cells, and that IL-17 contributes to defense against pulmonary Y. pestis challenge. Interestingly, this IL-17-mediated protection does not appear to result entirely from enhanced bacterial clearance. Although the precise mechanism of protection conferred by IL-17 remains to be determined, these findings suggest that pneumonic plague vaccines should aim to induce production of mixed type 1 and type 17 cellular responses.
Materials and Methods
Mice
B cell-deficient (µMT; B6.129S2-Igh-6tml cgn) mice on the C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME) and then bred in the specific-pathogen-free Trudeau Institute Animal Breeding Facility after embryo rederivation. Experimental mice were matched for age and sex and cared for according to Trudeau Institute Animal Care and Use Committee guidelines.
Bacteria
Pigmentation-negative Y. pestis strain D27 was generously provided by Robert Brubaker (Michigan State University). Y. pestis bacilli from frozen glycerol stocks were grown overnight at 26°C with continuous shaking in Bacto heart infusion broth supplemented with 2.5 mM CaCl2. After dilution to an optical density of 0.1 at 620nm, they were regrown for 3–4 hours at 26°C, washed with saline, quantified by optical density measurement, and resuspended in saline at the desired concentration. The number of bacteria in the inoculating dose was confirmed by plating. The vaccine strain D27-pLpxL was prepared by transforming strain D27 with plasmid pLpxL (12), which was generously provided by Egil Lien (University of Massachusetts Medical School). D27-pLpxL was stored, grown and prepared as described for strain D27 in medium supplemented with 100 µg/ml ampicillin.
Vaccination and challenge
In all instances, bacteria were administered in a volume of 30 µl saline applied to the nostrils of mice lightly anesthetized by isoflurane. For the “prime-only” groups, mice were vaccinated intranasally with 2 × 106 CFU D27-pLpxL and rested for 60 days before challenge. For the “prime/boost” groups, mice were primed intranasally with 2 × 106 CFU D27-pLpxL, boosted with the same dose of D27-pLpxL 30 days later, and rested for another 60 days before challenge. For challenge, mice were infected intranasally with 20 or 200 median lethal doses (MLD) of Y. pestis strain D27. The intranasal MLD of strain D27 is approximately 1 × 104 CFU when the bacteria are grown and administered as described above (12).
Measurement of bacterial burden and survival
At the indicated days after challenge infection, mice were euthanized by carbon dioxide narcosis. Liver and lung tissues were harvested and homogenized in saline. Serial dilutions of the homogenates were plated on blood agar plates, incubated at 26°C for 48 hours, and then bacterial CFU were counted. In all survival studies, recumbent animals were considered moribund and euthanized.
Flow cytometry
Lungs were perfused with saline containing heparin, minced, and then digested with collagenase and DNase (11). Viable cells were counted using typan blue exclusion and stained immediately with anti-CD4-fluorescein isothiocyanate (clone RM4–5), anti-CD8-peridinin chlorophyll protein (clone 53-6.7), anti-CD43-phycoerythrin (clone 1B11), and anti-CD44-allophycocyanin (clone IM7). Alternatively, cells were stained with anti-CD11b-allophycocyanin (clone M1/70), anti-Ly6G-fluorescein isothiocyanate (clone 1A8), anti-CD3-peridinin chlorophyll protein (clone 145-2C11) and anti-NK1.1-phycoerythrin (clone PK136). For intracellular cytokine staining, cells were stimulated with plate-bound anti-CD3 (clone 145-2C11, 2 µg/ml) for 5 hours in medium (Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino acid, 1 mM penicillin-streptomycin and 55 µM β-mercaptoethanol) containing brefeldin A (Sigma; 12.5 µg/ml) and stained with anti-CD4-allophycocyanin (clone RM4–5) and anti-CD8-peridinin chlorophyll protein (clone 53-6.7), followed by fixation, permeabilization, and intracellular staining with anti-TNFα-fluorescein isothiocyanate (clone MP6-XT22), anti-IFNγ-phycoerythrin cyanine dye 7 (clone XMG1.2), and anti-IL-17-phycoerythrin (clone TC11-18H10). Data were collected on a Becton Dickinson FACSCanto II and analyzed using FlowJo version 8.8 software (Tree Star, Inc.). In all experiments, forward and side scatter gating was used to exclude dead cells and debris. All other gating (e.g. CD4+) is described in the figure legends. The fluorochrome-labeled anti-TNFα and anti-IFNγ mAbs were purchased from eBioscience (San Diego, CA) and all other staining mAbs were purchased from BD Bioscience (San Diego, CA).
Cytokine neutralization
To neutralize TNFα and IFNγ, mice were treated with 1 mg mAb specific for TNFα (clone XT3.11, rat IgG1) and 600 µg mAb specific for IFNγ (clone XMG1.2, rat IgG1) diluted in PBS and administered intraperitoneally on the day before challenge. Control mice received an equal quantity of isotype-matched rat IgG1 mAb (clone HRPN). To neutralize IL-17, mice were treated with 150 µg neutralizing mAb specific for IL-17 (clone 50104) diluted in PBS and administered intraperitoneally on the day before and days 1 and 3 post challenge. Control mice received an equal amount of isotype-matched control mAb (clone 2A3, rat IgG2a). The IL-17 mAb was supplied by R&D Systems, Inc. (Minneapolis, MN). All other mAb were supplied by Bio X Cell (West Lebanon, NH).
Measurement of cytokine and chemokine levels
Tissue levels of mRNA encoding cytokines and chemokines were measured by real-time PCR, normalized to levels of glyceraldehyde-3-phosphate dehydrogenase (GADPH) mRNA, and expressed as fold change relative to the average level measured in naïve uninfected mice (30). Levels of cytokine and chemokine protein in bronchoalveolar lavage fluid (BALF) were measured by Luminex technology using a MILLIPLEX MAP 32-plex kit (Millipore, Billerica, MA) according to the manufacturer’s instructions.
Statistics
Statistical analyses were performed using the program Prism 4.0 (GraphPad Software, Inc.). Survival data were analyzed by log rank tests. Flow cytometry and real-time PCR data were analyzed by parametric tests (Student’s t test or one way ANOVA, as indicated). Bacterial burden was analyzed by nonparametric tests (Mann-Whitney or Kruskal-Wallis, as indicated); CFU below the detection limit of our assay were assigned values 0.2 log below the detection limit.
Results
Prime/boost vaccination with strain D27-pLpxL provides better protection against high dose Y. pestis challenge than does a single vaccination
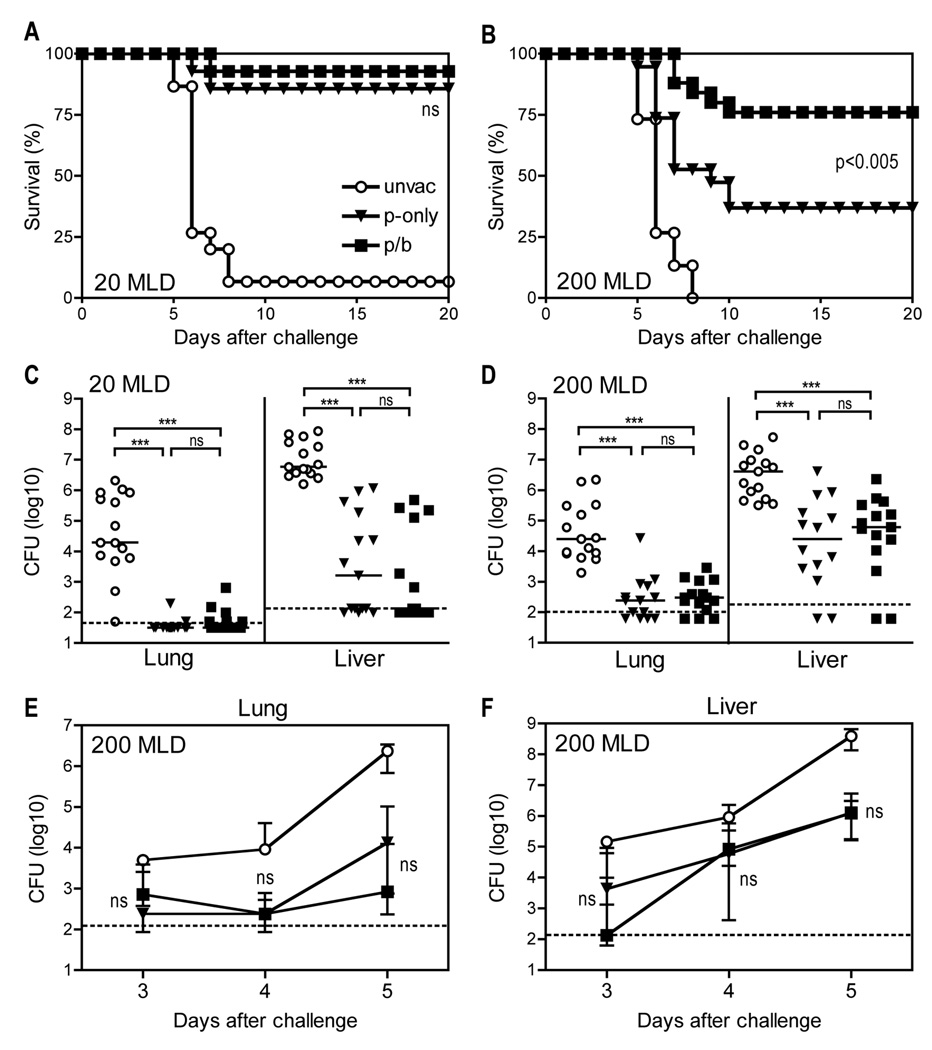
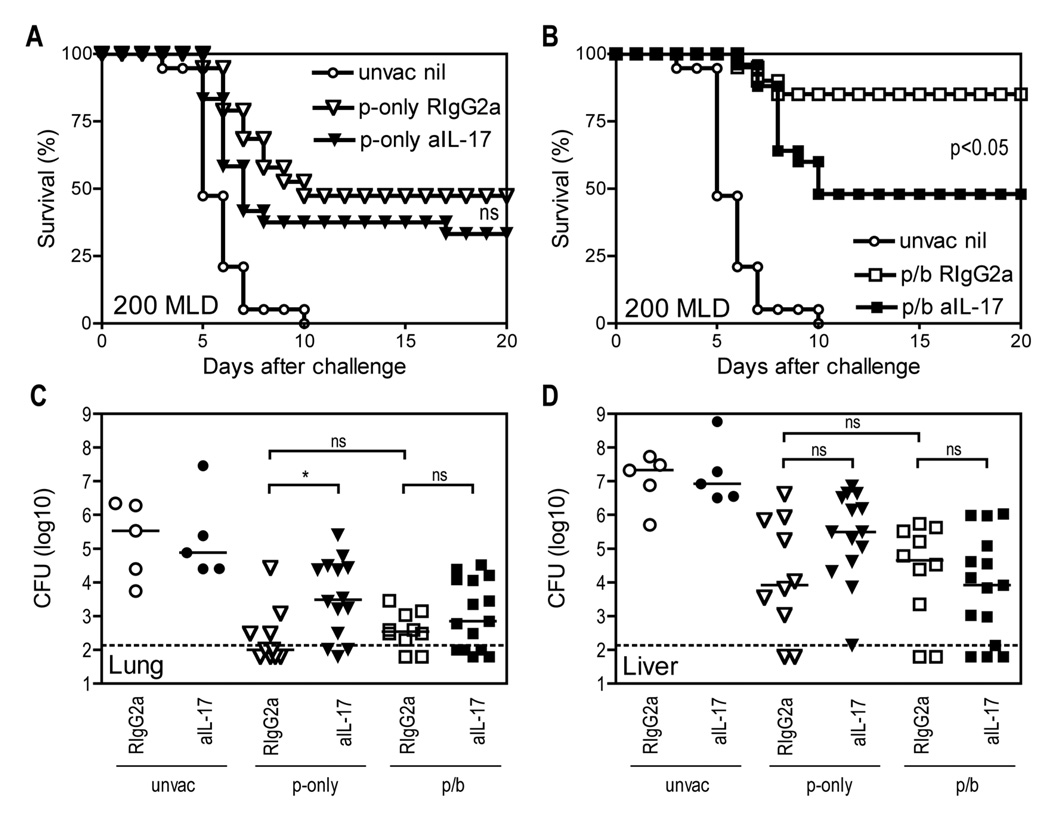
D27-pLpxL is a Y. pestis strain engineered to express E. coli LpxL (12), which promotes production of inflammatory, hexacylated forms of lipopolysaccharide, thus attenuating D27 by augmenting innate immune responses (31). B cell-deficient mice cannot produce antibodies, thus permitting specific investigation of roles for T cells in immune defense against pathogens. Consistent with prior studies (12), Fig. 1A demonstrates that immunizing B cell-deficient µMT mice with live D27-pLpxL conferred nearly full protection from lethal, low dose, intranasal challenge (20 median lethal doses; MLD) with Y. pestis strain D27 (Fig. 1A). A single immunization with D27-pLpxL also conferred significant protection from high dose challenge (200 MLD) with strain D27 (p<0.005), although more than half of the immunized mice nevertheless succumbed to high dose challenge (Fig. 1B). With the intent of further improving the protective capacity of cellular immunity, we investigated the efficacy of a prime/boost regimen. B cell-deficient µMT mice were immunized with strain D27-pLpxL, rested for 30 days, and then boosted with strain D27-pLpxL. After another 60 days, these “prime/boost” mice were challenged intranasally with either 20 or 200 MLD Y. pestis strain D27, alongside the “prime-only” mice that received one single immunization with D27-pLpxL. After 20 MLD challenge, both the prime-only and prime/boost mice exhibited more than 85% survival (Fig. 1A). After MLD challenge, only 37% of the prime-only mice survived, whereas 76% of the prime/boost mice survived (Fig. 1B). The improved protection conferred by prime/boost vaccination was highly significant (P < 0.005).
FIGURE 1. A booster vaccination with live attenuated Y. pestis improves survival after virulent Y. pestis challenge without impacting bacterial burden.
B cell–deficient µMT mice were primed (p-only; triangles) with live attenuated strain D27-pLpxL on day −60 or they were primed and boosted (p/b; squares) with D27-pLpxL on day −60 and day −90. On day 0, the vaccinated mice were challenged intranasally with Y. pestis strain D27, alongside age- and sex-matched unvaccinated controls (unvac; circles). (A and B) Survival after 20 MLD challenge (A) or 200 MLD challenge (B). In comparison with unvaccinated mice, both prime-only and prime/boost mice displayed significantly improved survival after 20 or 200 MLD challenge (all P < 0.005 by log rank test; n=14–25 mice/group; data pooled from 3 independent experiments). The difference in survival between prime-only and prime/boost mice was not significant (ns) after 20 MLD challenge, but was highly significant after 200 MLD challenge (P < 0.005 by log rank test). (C and D) Bacterial burden at day 5 after 20 MLD challenge (C) or at day 4 after 200 MLD challenge (D). In comparison with unvaccinated mice, both prime-only and prime/boost mice displayed significantly decreased CFU in lung and liver tissues after 20 or 200 MLD challenge (*** P < 0.001 by Kruskal-Wallis with Dunn’s multiple comparison test; n=15 mice/group; data is pooled from 3 independent experiments). CFU did not differ significantly between prime-only and prime/boost mice after 20 or 200 MLD challenge. The solid bar depicts the median and the broken line depicts the limit of detection. (E and F) At days 3,4, and 5 after 200 MLD challenge, bacterial burden was measured in lung (E) and liver (F) tissues. Bacterial burden did not differ significantly between prime-only and prime/boost mice at all time points (ns, not significant). Data shown are median and interquartile range of 5 mice per group. The broken line depicts the limit of detection.
The booster vaccination does not improve protection by decreasing bacterial burden
Measurements of bacterial burden after 20 MLD and 200 MLD challenge revealed that both the prime-only and prime/boost mice contained fewer bacterial CFU in lung and liver tissue, as compared with unvaccinated control mice (both P < 0.001, Fig. 1, C and D). However, the number of CFU did not differ significantly between prime-only and prime/boost mice (Fig. 1, C and D). Subsequently, we examined the kinetics of bacterial growth after 200 MLD challenge in greater detail (Fig. 1, E and F). At all time points, both the prime-only and prime/boost mice exhibited reduced bacterial burden in lung and liver tissues, as compared with unvaccinated mice. However, the prime-only and prime/boost mice showed comparable bacterial burden at days 3, 4 and 5 after challenge (Fig. 1, E and F). These data suggest that the improved survival mediated by the booster vaccination does not result from improved control of bacterial growth or dissemination.
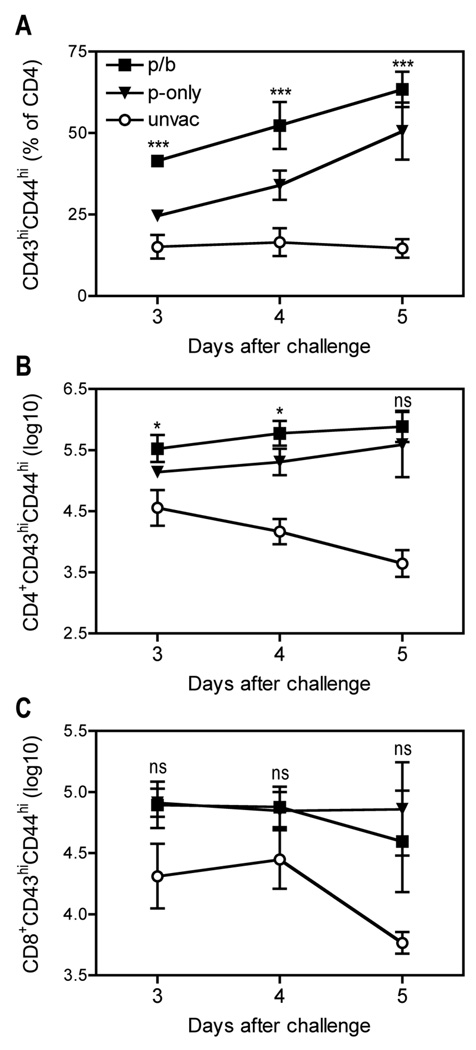
The booster vaccination increases the number of pulmonary CD4 T cells with an activated phenotype
We previously reported that a single immunization with D27-pLpxL increases the number of CD4 and CD8 T cells with an activated phenotype (CD43hiCD44hi) in the lungs of mice challenged with 20 MLD Y. pestis strain D27 (12). Given that prime/boost vaccination with D27-pLpxL improved protection, we investigated whether prime/boost vaccination further increased the number of activated T cells. After challenge with 200 MLD Y. pestis strain D27, we observed a significant increase in the frequency of CD43hiCD44hi CD4 T cells in the lungs of the prime-only mice, as compared with unvaccinated mice (P < 0.001, Fig. 2A). In comparison with the prime-only mice, the boosted mice harbored increased frequencies of CD43hiCD44hi CD4 T cells at days 3, 4 and 5 after challenge (all P < 0.001, Fig. 2A). The significant increases in the frequencies of CD43hiCD44hi CD4 T cells led to significant increases in the absolute number of these cells at days 3 and 4 after challenge (both P < 0.05, Fig. 2B). At day 5, the increase was still evident, although it did not achieve statistical significance.
FIGURE 2. A booster vaccination with live attenuated Y. pestis increases numbers of activated CD4 T cells in the lungs of mice challenged with virulent Y. pestis.
B cell–deficient µMT mice were primed (p-only; triangles) or prime/boosted (p/b; squares) with D27-pLpxL, or left unvaccinated (unvac; circles), and then challenged intranasally with 200 MLD Y. pestis strain D27 as described in Figure 1. At the indicated days after challenge, cells were isolated from lung tissues and analyzed by flow cytometry. Panels depict (A) the percentage of CD4+CD43hiCD44hi cells, (B) the number of CD4+CD43hiCD44hi cells and (C) the number of CD8+CD43hiCD44hi cells. Data depict the mean and SD of 5 mice per group. Statistical significance was measured by one-way ANOVA with Bonferroni’s multiple comparison test and results are shown for p-only versus p/b (* P < 0.05, *** P < 0.001, ns, not significant).
We also observed an increase in the number of CD43hiCD44hi CD8 T cells in the lungs of the prime-only and prime/boost mice challenged with 200 MLD Y. pestis, however, the magnitude of this increase was similar for both prime-only and prime/boost mice (Fig. 2C). Likewise, the numbers and frequencies of total T cells (CD3+), NK cells (NK1.1+CD3−), macrophages (CD11b+Ly6G−) and neutrophils (CD11b+Ly6G+) were similar in the lungs of prime-only and prime/boost mice at days 3, 4 and 5 after challenge (data not shown). Notably, none of the cell types we investigated, including activated CD4 T cells, differed significantly in frequency or number in the spleens of prime-only and prime/boost mice (data not shown). We conclude that the booster vaccination specifically increases the number of activated CD4 T cells in the lung after Y. pestis challenge.
The booster vaccination increases the number of pulmonary CD4 T cells capable of producing TNFα and IFNγ
We used an intracellular cytokine staining procedure to quantify the number of T cells with the capacity to produce cytokines upon ex vivo activation with plate-bound anti-CD3. Previously we reported that a single immunization with D27-pLpxL increases the number of CD4 and CD8 T cells capable of producing both TNFα and IFNγ after 20 MLD challenge with Y. pestis strain D27 (12). Figs 3A and 3B demonstrate that few pulmonary CD4 T cells in unvaccinated mice could be stimulated to produce both TNFα and IFNγ at day 3 after 200 MLD challenge, and the number of these cells decreased further as the infection progressed. In comparison with unvaccinated mice, the frequency and number of CD4 T cells capable of producing both TNFα and IFNγ increased dramatically in mice immunized with D27-pLpxL (Fig. 3, A and B). In comparison with the prime-only mice, prime/boost mice exhibited significantly increased frequencies (Fig. 3A) and absolute numbers (Fig. 3B) of TNFα and IFNγ-producing CD4 T cells at days 3 and 4, but not day 5, after challenge. The frequency of CD4 T cells producing TNFα, but not producing IFNγ, also increased in the boosted mice (Fig. 3C). Notably, the prime-only mice and prime/boost mice did not differ significantly with regard to the percentage or number of TNFα and IFNγ-producing CD8 T cells (data not shown).
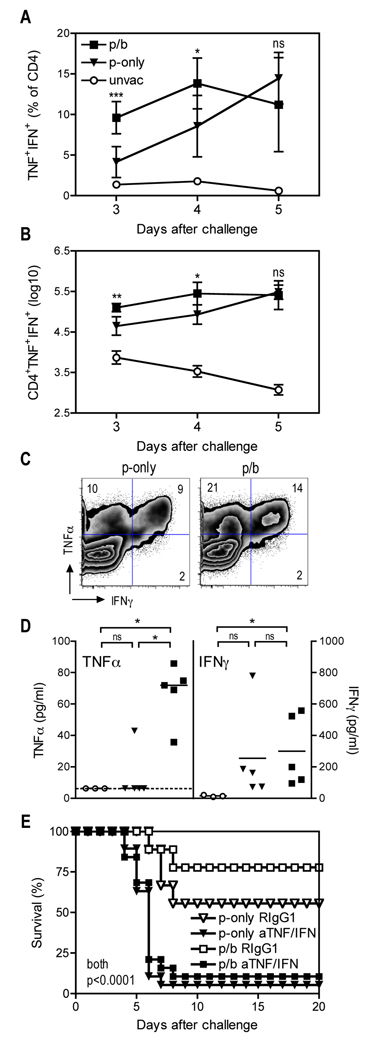
FIGURE 3. TNFα and IFNγ contribute to protection mediated by vaccination with live attenuated Y. pestis.
B cell–deficient µMT mice were primed (p-only; triangles) or prime/boosted (p/b; squares) with D27-pLpxL, or left unvaccinated (unvac; circles), and then challenged intranasally with 200 MLD Y. pestis strain D27 as described in Figure 1. (A and B) At the indicated days after challenge, cells were isolated from lung tissues, restimulated with plate-bound anti-CD3 mAb for 5h, stained for intracellular cytokines and analyzed by flow cytometry. The percentage (A) and number (B) of TNFα+IFNγ+ CD4 T cells are shown. The data depict the mean and SD of 5 mice per group and statistical significance was measured by one-way ANOVA with Bonferroni’s multiple comparison test. Results are shown for p-only versus p/b (* P < 0.05, ** P < 0.01, *** P < 0.001). (C) Flow cytometry analysis of cytokine producing CD4+ T cells at day 4 post challenge. Each plot depicts a concatenation of data from five mice per group. Data shown were gated on CD4+ T cells. The numbers depict the percentage of cells in the indicated quadrant. Similar results were observed in three independent experiments. (D) TNFα and IFNγ protein levels in BALF collected at day 4 post challenge. The broken line depicts the limit of detection (6.4 pg/ml) and the solid bar depicts the median of TNFα and the mean of IFNγ Statistical significance was measured by nonparametric Kruskal-Wallis with Dunn’s multiple comparison test (* P < 0.05; ns, not significant). (E) At the time of challenge, mice received neutralizing mAb specific for TNFα and IFNγ (aTNF/IFN) or an isotype-matched mAb (RIgG1). In comparison with mice treated with isotype-matched mAb, both prime-only and prime/boost mice treated with cytokine-neutralizing mAb exhibited significantly reduced survival (P < 0.0001 by log rank test; n=19 for aTNF/IFN-treated groups, n=9 for RIgG1-treated groups; data are pooled from 2 independent experiments).
We also measured levels of TNFα and IFNγ protein in BALF after challenge with 200 MLD Y. pestis strain D27 (Fig. 3D). While all of the unvaccinated mice and most of the prime-only mice showed undetectable levels of TNFα at day 4 after challenge, the prime/boost mice exhibited significantly increased TNFα levels. In contrast, IFNγ levels did not differ significantly between the prime-only and prime/boost mice.
To determine whether TNFα and IFNγ contribute to protection mediated by booster vaccination, we administered specific mAb that neutralize these cytokines at the time of 200 MLD Y. pestis challenge. Control mice received isotype-matched mAb of irrelevant specificity. As shown in Fig. 3E, neutralizing TNFα and IFNγ at the time of challenge almost completely abolished the protection conferred by either prime-only or prime/boost immunization. These findings complement our prior studies reporting that TNFα and IFNγ play critical roles during vaccine-mediated protection from pulmonary Y. pestis challenge (11–13). However, it is unclear that the modestly increased numbers of TNFα and IFNγ-producing CD4 T cells in the prime/boost mice suffices to account for the improved protection conferred by booster vaccination.
The booster vaccination increases IL-17 production in the lung
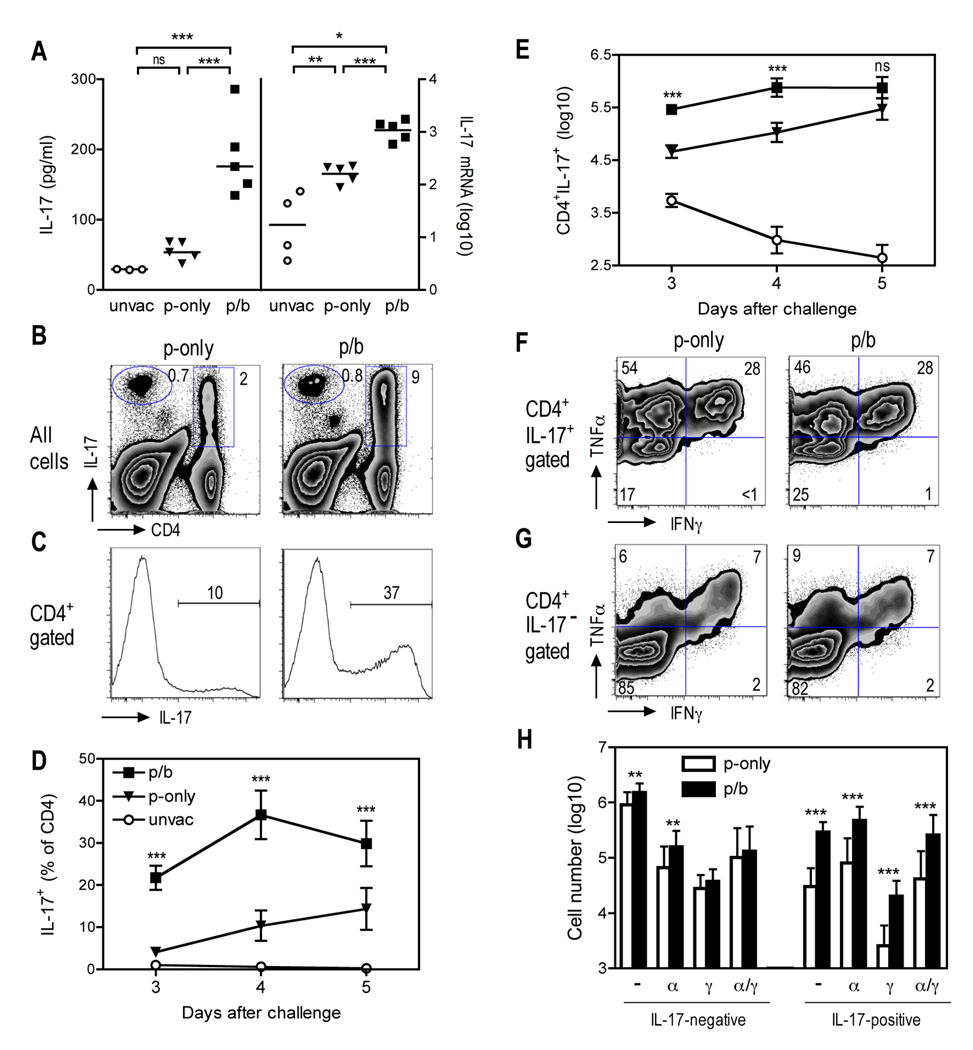
When measuring cytokine levels in mice that had been challenged with Y. pestis, we also observed a dramatic and highly significant increase in the amount of IL-17 protein in the BALF collected from the prime/boost mice, as compared with BALF from the prime-only mice (P < 0.001; Fig. 4A). Likewise, real-time PCR revealed that lung tissue collected from the boosted mice contained significantly more IL-17 mRNA than that of prime-only mice (P < 0.001; Fig. 4A).
FIGURE 4. A booster vaccination with live attenuated Y. pestis increases IL-17 production in the lung.
B cell–deficient µMT mice were primed (p-only; triangles) or prime/boosted (p/b; squares) with D27-pLpxL, or left unvaccinated (unvac; circles), and then challenged intranasally with 200 MLD Y. pestis strain D27 as described in Figure 1. (A) IL-17 protein levels in BALF (left panel) and IL-17 mRNA levels in lung tissue (right panel) were measured at day 4 after challenge by Luminex technology or real-time PCR, respectively. The solid bar depicts the mean. Statistical significance was measured by one-way ANOVA with Bonferroni’s multiple comparison test (* P < 0.05, ** P < 0.01; *** P < 0.001). (B and C) Flow cytometry analysis of IL-17-producing cells at day 4 after challenge. Each plot depicts a concatenation of data from five mice per group. The numbers depict the percentage of cells in the indicated region. Similar results were observed in three independent experiments. (D and E) Kinetic analysis of the percentage (D) and number (E) of pulmonary CD4 T cells capable of producing IL-17 as determined by intracellular cytokine staining. The data depict the mean and SD of 5 mice per group and statistical significance was measured by one-way ANOVA with Bonferroni’s multiple comparison test. Results are shown for p-only versus p/b (*** P < 0.001). (F and G) Plots of TNFα and IFNγ expression within the IL-17+ (F) or IL-17− (G) CD4 T cell subsets at day 4 after challenge. Each plot depicts a concatenation of data from five mice per group. (H) Numbers of IL-17+ or IL-17− CD4 T cells that are TNFα−IFNγ− (−), TNFα+IFNγ− (α), TNFα−IFNγ+ (γ), or TNFα+IFNγ+ (α/γ) at day 4 after challenge. Data shown are mean and SD of 15 mice pooled from 3 independent experiments. Statistical significance was measured by student t test (** P < 0.01; *** P < 0.001).
Flow cytometry revealed that most of the T cells capable of producing IL-17 in the lung at day 4 after 200 MLD challenge expressed CD4 (Fig. 4B), and the frequency of these cells increased substantially in the prime/boost mice, as compared with the prime-only mice (Fig. 4C). Specifically, 37% of the CD4 T cells produced IL-17 in the boosted mice, as compared with only 10% in prime-only mice (Fig. 4C). Kinetic analyses revealed that the prime/boost mice, in comparison with prime-only mice, harbored significantly increased frequencies of IL-17-producing CD4 T cells at days 3, 4 and 5 after challenge (P < 0.001; Fig. 4D). Absolute numbers of IL-17-producing CD4 T cells in the prime/boost mice were more than 6-fold higher than that of prime-only mice at days 3 and 4 after challenge (Fig. 4E).
Gating on IL-17-producing CD4 T cells revealed that most of these cells also produced canonical type 1 cytokines; more than 70% also produced TNFα and 28% produced both TNFα and IFNγ (Fig. 4F). Interestingly, the frequencies of IL-17-producing CD4 T cells that also produced TNFα and IFNγ (i.e. TNFα+IFNγ+ cells) did not differ between the prime-only and prime/boost mice (Fig 4F). Likewise, among the CD4 T cells that did not produce IL-17, frequencies of TNFα+IFNγ+ cells did not differ between the prime-only and prime boost mice (Fig 4G). The absolute numbers of TNFα−IFNγ− and TNFα+IFNγ− cells increased modestly among the population that did not produce IL-17, whereas the numbers of TNFα−IFNγ−, TNFα+IFNγ−, TNFα−IFNγ+, and TNFα+IFNγ+ cells all increased nearly 10-fold among the population that did produce IL-17 (Fig 4H). Together, these data indicate that a primary impact of boosting was to increase the abundance of multiple, IL-17-producing CD4 T cell subsets.
In comparison with CD4 T cells, we observed relatively small increases in the frequencies of CD8 T cells capable of producing IL-17 (data not shown). There were significant increases in the number of IL-17-producing CD8 T cells in vaccinated mice as compared with unvaccinated mice (data not shown). However, there were no significant differences between the prime-only and prime/boost mice with regard to the percentages or absolute numbers of IL-17-producing CD8 T cells.
IL-17 contributes to the protection conferred by booster vaccination
Given that prime/boost vaccination dramatically increased pulmonary IL-17 levels, we hypothesized that IL-17 may contribute to the improved protection conferred by booster vaccination. To test this hypothesis, we vaccinated mice and then administered a neutralizing mAb specific for IL-17 at the time of challenge. As shown in Fig. 5A, 47% of the prime-only mice treated with control mAb survived a 200 MLD Y. pestis challenge. Neutralizing IL-17 reduced the survival of the prime-only mice to 33%. This modest impact of IL-17 neutralization in the prime-only mice did not achieve statistical significance (Fig. 5A). In contrast, prime/boost vaccination resulted in 85% survival, and neutralization of IL-17 in the prime/boost mice reduced survival significantly (P < 0.05; Fig. 5B). Notably, the IL-17 neutralization in the prime/boost mice reduced survival to 48% (Fig. 5B), which was comparable to the level observed in the prime-only mice treated with control mAb (Fig. 5A). Despite its significant impact on survival, IL-17 neutralization did not significantly affect the bacterial burden in lung and liver tissues of prime/boost mice (Fig. 5, C and D). Altogether, these data indicate that IL-17 contributes to the improved survival mediated by booster vaccination, but suggest that IL-17 may not function protectively by reducing bacterial growth or dissemination.
FIGURE 5. IL-17 contributes to vaccine-mediated protection against pulmonary Y. pestis challenge without impacting bacterial burden.
B cell–deficient µMT mice were primed (p-only; triangles) or prime/boosted (p/b; squares) with D27-pLpxL, or left unvaccinated (unvac; circles), and then challenged intranasally with 200 MLD Y. pestis strain D27. At the time of challenge, the mice were treated with neutralizing mAb specific for IL-17 (aIL-17; solid symbols) or an isotype-matched control mAb (RIgG2a; open symbols). In parallel, sex- and age-matched naïve mice were challenged with same dose of strain D27 without mAb treatment (unvac nil). (A and B) Survival decreased significantly in prime/boost mice (B) but not in prime-only mice (A) treated with IL-17 mAb, as compared with mice treated with control mAb (P < 0.05 by log rank test; ns, not significant; n=17–25 mice per group; data pooled from 3 independent experiments). (C and D) Bacterial burden at day 4 after challenge. Neutralizing IL-17 modestly increased the bacterial burden in the lungs of prime-only mice, but did not significantly affect the pulmonary or hepatic burden in prime/boost mice (* P < 0.05 by Mann-Whitney test; ns, not significant; data are pooled from two independent experiments). The solid bar depicts the median and the broken line depicts the limit of detection.
IL-17 induces CXCL1 expression and recruits neutrophils to the lungs of vaccinated mice
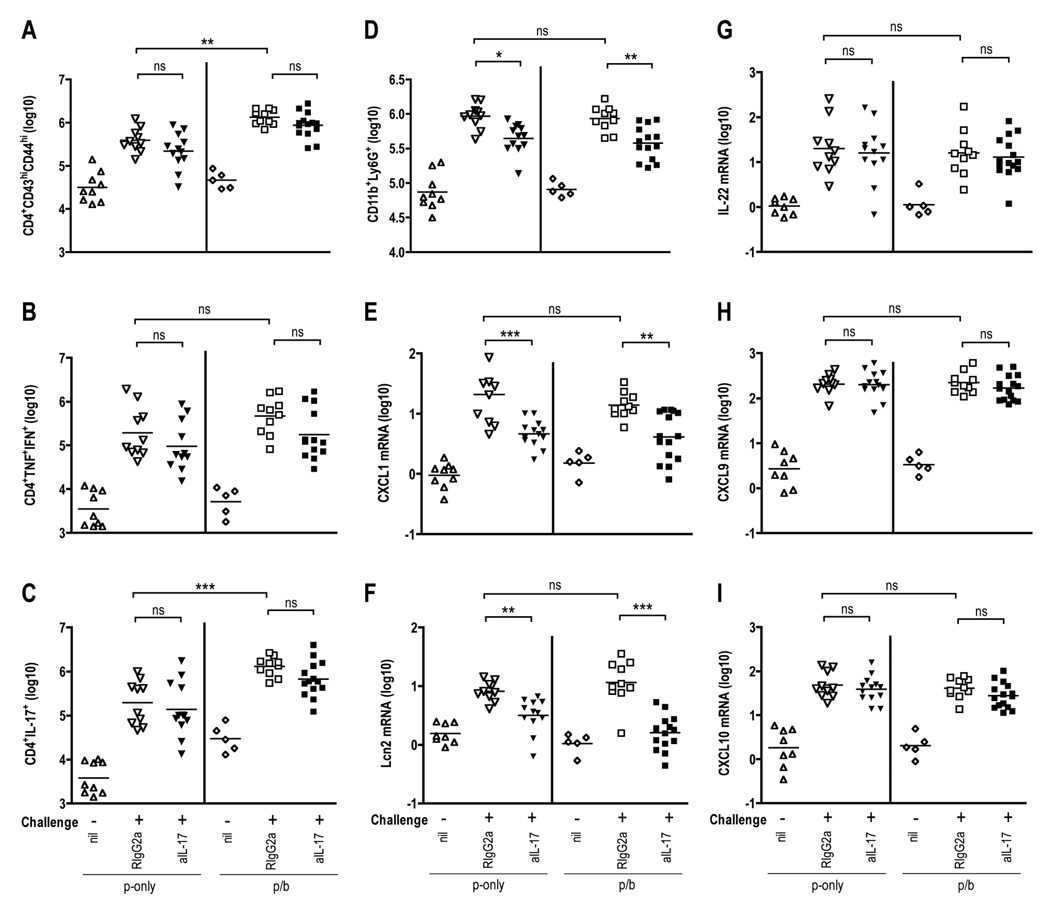
To investigate how IL-17 contributes to survival after challenge with 200 MLD Y. pestis, we examined the cellular composition in the lungs of mice treated with IL-17 neutralizing mAb. Consistent with Figures 3–5, the boosted mice harbored more CD43hiCD44hi CD4 T cells (Fig. 6A), TNFα+IFNγ+ CD4 T cells (Fig. 6B, and IL-17-producing CD4 T cells (Fig. 6C) than prime-only mice at day 4 after challenge. However, the number of these activated and cytokine-producing CD4 T cells did not differ significantly between mice treated with IL-17 mAb and control mice (Fig. 6, A–C). When examining other cell populations in mice treated with IL-17 neutralizing mAb, we found that the frequency and total number of CD4+ cells, CD8+ cells, CD3+ cells, NK cells, NKT cells and macrophages in the lungs and spleens of the prime/boost mice were comparable to those of prime-only mice, and IL-17 neutralization did not impact their numbers significantly (data not shown). We also did not observe differences in the number of splenic neutrophils in IL-17 mAb-treated mice (data not shown). However, neutralization of IL-17 did significantly, albeit only partially, reduce the number of pulmonary CD11b+Ly6G+ neutrophils in both the prime-only and prime/boost mice (P < 0.05 and P < 0.01, respectively; Fig. 6D).
FIGURE 6. Impact of neutralizing IL-17 on levels of T cells, neutrophils, and mRNA encoding inflammatory cytokines and chemokines in lungs of mice challenged with Y. pestis.
B cell–deficient µMT mice were primed (p-only) or prime/boosted (p/b) with D27-pLpxL and then challenged intranasally with 200 MLD Y. pestis strain D27. At the time of challenge, mice were treated with neutralizing mAb specific for IL-17 (aIL-17; solid symbols) or an isotype-matched control mAb (RIgG2a). Additional groups of mice were left unchallenged and did not receive mAb treatment (nil). At day 4 after challenge, flow cytometry was used to determine the number of pulmonary (A) CD4+CD43hiCD44hi cells, (B) CD4+TNFα+IFNγ+ cells, (C) CD4+IL-17+ cells, and (D) CD11b+Ly6G+ neutrophils. (E–I) At day 4 after challenge, real-time PCR was used to measure levels of pulmonary mRNA encoding (E) CXCL1, (F) lipocalin 2 (Lcn2), (G) IL-22, (H) CXCL9, and (I) CXCL10. Statistical significance was measured by one-way ANOVA followed by Bonferroni’s multiple comparison test (* P < 0.05, ** P < 0.01, *** P < 0.001; ns, not significant). Data are pooled from two independent experiments.
In parallel with studies of the cellular content of the lungs, we used real-time PCR to measure pulmonary levels of mRNA encoding proteins implicated previously in IL-17-mediated biology (IL-17, IL-22, CXCL1, CXCL9, CXCL10, CXCL11, G-CSF, CCR6, CCR4 and lipocalin 2) and generally involved in host defense against bacterial infection (IFNγ, TNFα, IL-6, IL-10, IL-1β, iNOS, IL-12p35, and IL-12p40). Levels of CXCL1, a chemokine known to attract and activate neutrophils, increased 10-fold in both the prime-only and prime/boost mice and neutralizing IL-17 decreased CXCL1 levels significantly, albeit only partially (Fig. 6E). Prime-only and prime/boost immunization also increased levels of the IL-17-inducible anti-microbial protein lipocalin 2 and neutralization of IL-17 significantly decreased lipocalin 2 levels (Fig. 6F). Prime-only and prime/boost immunization likewise increased expression of IL-17, IL-22, CXCL9, CXCL10, CXCL11, G-CSF, CCR4, IFNγ, TNFα, IL-6, IL-10, IL-1β, and IL-12p35, however, neutralizing IL-17 did not significantly impact their expression levels (Fig. 6, G–I and data not shown).
Discussion
Over the past 50 years, studies on plague vaccine development have focused largely on antibody-mediated humoral immunity and devoted relatively little attention to cellular defense. Nevertheless, we recently demonstrated that type 1 cellular immunity induced by vaccination with live attenuated strain D27-pLpxL can suffice to protect antibody-deficient mice against lethal pulmonary Y. pestis challenge (12). The present study demonstrates that a prime/boost regimen of D27-pLpxL vaccination further improves T cell-mediated defense against pulmonary Y. pestis challenge.
Boosting mice with D27-pLpxL significantly improves survival, even though the bacterial burden does not differ significantly between prime-only and prime/boost mice (Fig. 1). These observations strongly suggest that the bacterial load per se is not the only cause of mortality in this model. They also suggest that the booster vaccination improves the capacity of mice to survive in the presence of a substantial bacterial burden. We speculate that boosting mice with D27-pLpxL reduces some form of pathology induced by Y. pestis challenge that acts in concert with the bacterial burden to cause mortality.
The boosting regimen significantly increases the numbers of IL-17-producing CD4 T cells in the lung pre-challenge (Fig. 6C). Boosting also dramatically increases the numbers of IL-17-producing CD4 T cells in the lung after Y. pestis challenge (Fig. 4 and 6C), as well as the levels of IL-17 mRNA and protein (Fig. 4). Neutralizing IL-17 significantly reduces the survival of boosted mice (Fig. 5). In contrast, neutralizing IL-17 does not significantly reduce the survival of prime-only mice (Fig. 5), which produce relatively low levels of IL-17 after Y. pestis challenge (Fig. 4). Thus, IL-17 appears to be a major mediator of the improved survival that results from the boost.
In wild type mice, the protective impacts of cytokines and T cells must be dissociated from the impacts of antibodies, which protect against pneumonic plague in passive transfer experiments (13, 14). Thus, here we focused on the µMT mice, where the lack of antibody enables decisive studies of acquired defense mediated by T cells and the cytokines produced by T cells. Importantly, in addition to increasing numbers of IL-17-producing CD4 T cells in the lungs of µMT mice challenged with virulent Y. pestis (Fig. 4), boosting similarly expands populations of IL-17-producing CD4 T cells in wild type mice (data not shown).
We previously demonstrated that TNFα and IFNγ contribute to protection mediated by humoral and cellular defense against lethal pulmonary Y. pestis challenge (12–15). In all those studies, neutralizing TNFα and IFNγ significantly increased the bacterial burden. In contrast, neutralizing IL-17 does not significantly compromise the control of bacterial growth in mice challenged with Y. pestis (Fig. 5, C and D). Moreover, IL-17 neutralization selectively impacts the survival of boosted mice (Fig. 5B), whereas neutralizing TNFα and IFNγ almost completely abolishes the protection conferred by either prime-only or prime/boost vaccination (Fig. 3E). These observations further suggest that IL-17, not TNFα or IFNγ, is a primary contributor to the improved survival conferred by the booster vaccination. Importantly, these findings also indicate that type 1 cytokines and IL-17 protect mice against plague through distinct mechanisms. We suggest that the type 1 responses control bacterial growth and clear the infection, while type 17 responses provide critical additional “help” that acts in concert with bacterial control to reduce tissue damage and/or enhance tissue repair mechanisms. We envision that even a modest reduction in pathology could result in a substantial increase in survival by providing additional time for the immune system to control and clear the infection.
IL-17-mediated protection against pathogens is thought to result, in large part, from increased neutrophil recruitment (19). IL-17 stimulates granulopoiesis by increasing G-CSF production, and induces expression of the chemokines CXCL1 and CXCL2, which recruit neutrophils to sites of infection. In most of the infection models in which IL-17 plays a critical role in host defense, abrogating IL-17 signaling results in significantly reduced neutrophil recruitment, in turn reducing pathogen clearance (19). Consistent with those prior studies, our data suggest IL-17 contributes to the recruitment of neutrophils to the lungs of mice challenged with Y. pestis, apparently secondary to IL-17-mediated induction of the neutrophil chemoattractant CXCL1 (Fig. 6E). However, neutrophil recruitment, as well as CXCL1 expression, is similar in magnitude in prime-only and prime/boost mice, and the partial reductions in neutrophil numbers and CXCL1 expression that result from IL-17 neutralization are comparable in prime-only and prime/boost mice. Moreover, depleting neutrophils increases the bacterial burden in mice challenged with Y. pestis ((32–34) and unpublished data), whereas neutralizing IL-17 does not impact the bacterial burden significantly (Fig. 5). Therefore, in contrast to prior studies where neutrophils were identified as the major cells mediating IL-17-dependent host resistance, our data suggest that IL-17-driven neutrophil recruitment does not account for the improved protection observed in mice boosted with D27-pLpxL.
In addition to recruiting neutrophils, IL-17 reportedly augments recruitment of type 1 T cells during infection (27, 35). Mycobacterial-specific T cells from IL-17-deficient mice display impaired IFNγ production (35) and neutralizing IL-17 in vaccinated mice during M. tuberculosis challenge significantly reduces the frequency of antigen-specific IFNγ-producing T cells in the lungs and also inhibits expression of genes encoding the CXCR3 ligands CXCL9, CXCL10 and CXCL11, which contribute to the recruitment of Th1 cells (27). Likewise, IL-17 is required for the generation of protective IFNγ responses during pulmonary tularemia (26). While these prior studies suggest IL-17 can contribute to defense against infection, in part, by augmenting type 1 responses, we do not observe such impacts in our model of vaccine-mediated protection against pneumonic plague. Specifically, neutralizing IL-17 during Y. pestis challenge does not significantly impact the number of IFNγ-producing T cells in the lung (Fig. 6B) or the expression of CXCL9, CXCL10 and CXCL11 (Fig. 6, H–I and data not shown). Neutralizing IL-17 also does not impact the expression of two other T cell chemokines, CCL5 and CCL20 (data not shown). Therefore, our data suggest that increased recruitment of Th1 cells does not account for the IL-17-mediated improvement in protection against Y. pestis infection.
IL-17 also reportedly contributes to the formation of mature pulmonary granulomas in mycobacteria-infected mice (35, 36). Specifically, IL-17-deficient mice show normal level of nascent granuloma formation but fail to develop mature granuloma at later stages of infection. Since immunization facilitates formation of granuloma-like structures in the livers of mice challenged with virulent Y. pestis (12, 37, 38), perhaps IL-17 improves the formation of granuloma-like structures in mice immunized with D27-pLpxL. Although preliminary analyses have not revealed obvious distinctions in the hepatic histopathology of prime-only and prime/boost mice, further study is warranted to address this issue.
IL-17-producing CD4 T cells often produce IL-22, a cytokine known to act synergistically or additively with IL-17 to enhance the expression of anti-microbial peptides, promote inflammation, and protect tissue (25, 39–44). However, we could not detect IL-22-producing CD4 cells in this model (data not shown), and we do not observe differences in IL-22 mRNA levels between prime-only and prime/boost mice, or between IL-17 mAb-treated and control mice (Fig. 6G). Nevertheless, IL-17 neutralization suppressed the immunization-induced upregulation of lipocalin 2 (Fig. 6F), an anti-microbial protein whose expression is upregulated synergistically by exposure to IL-22 and IL-17 (25, 39, 45).
TNFα also synergizes with IL-17 to upregulate expression of lipocalin 2 (46). Interestingly, a large proportion of the IL-17-producing CD4 T cells induced by booster immunization simultaneously produce TNFα, and many of these cells produce IFNγ as well (Fig. 4, F and H). While TNFα-producing Th17 cells have been described before (22–24), to our knowledge this is the first report to implicate cells with a mixed type 1/type 17 in host defense against infection. We also think it notable that these “Th1-17” cells, expanded by intranasal booster immunization with D27-pLpxL, persist at significant levels for at least 60 days (Fig. 4 and 6C). In contrast, the Th17 cells induced by intranasal infection with Listeria monocytogenes are short-lived (47). The apparent difference in the longevity of IL-17-producing T cells in these two distinct models of intranasal bacterial infection may reflect the different types of bacteria used, the booster regimen, and/or the different life spans for Th17 and Th1-17 cells. Regardless, our finding that intranasal booster immunization with attenuated bacteria confers robust long-lived IL-17-associated protection warrants further studies of the underlying mechanisms and their potential for translation to other vaccine settings.
Although the precise mechanism of IL-17-mediated protection during pulmonary Y. pestis infection remains to be established, our study clearly indicates that IL-17 can contribute significantly to host defense against pneumonic plague. The protection mediated by IL-17 does not seem to result from improved bacterial clearance or neutrophil recruitment, but somehow enables mice to better withstand Y. pestis challenge in the presence of a substantial bacterial load. Regardless of the underlying mechanisms, our findings suggest that mammals with a capacity to efficiently produce and respond to IL-17 may have a greater capacity to combat pneumonic plague. Prior reports demonstrated that vaccine-mediated protection against pulmonary Y. pestis infection strongly benefits from production of TNFα and IFNγ (11–15), and this study suggests that pneumonic plague vaccines also should aim to prime production of IL-17. We anticipate that the presence of Y. pestis-specific memory Th1-17 cells with the capacity to produce TNFα, IFNγ and IL-17 will constitute an immunological correlate of protection for the efficacy of human plague vaccines.
Acknowledgments
We thank Debbie Duso for technical assistance and the employees of the Trudeau Institute animal facilities for dedicated care and breeding of the mice used in these studies. We also thank Drs. Robert Brubaker and Egil Lien for generously providing access to Y. pestis strain D27 and plasmid pLpxL, respectively
Abbreviations
- BALF
bronchoalveolar lavage fluid
- MLD
median lethal dose
Footnotes
This work was supported by funding from the Trudeau Institute and Public Health Service grants R01-AI061577 and R01-AI71295.
References
- 1.Pollitzer R. Geneva: World Health Organization; Plague. 1954
- 2.Perry RD, Fetherston JD. Yersinia pestis-etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler T. Plague and Other Yersinia Infections. New York: Plenum Press; 1983. [Google Scholar]
- 4.Galimand M, Guiyoule A, Gerbaud G, Rasoamanana B, Chanteau S, Carniel E, Courvalin P. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N Engl J Med. 1997;337:677–680. doi: 10.1056/NEJM199709043371004. [DOI] [PubMed] [Google Scholar]
- 5.Zilinskas RA. The anti-plague system and the Soviet biological warfare program. Crit Rev Microbiol. 2006;32:47–64. doi: 10.1080/10408410500496896. [DOI] [PubMed] [Google Scholar]
- 6.Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, Layton M, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Schoch-Spana M, Tonat K. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. Jama. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 7.Titball RW, Williamson ED. Yersinia pestis (plague) vaccines. Expert Opin Biol Ther. 2004;4:965–973. doi: 10.1517/14712598.4.6.965. [DOI] [PubMed] [Google Scholar]
- 8.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines. 2008;7:209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashaw J, Norris S, Weeks S, Trevino S, Adamovicz JJ, Welkos S. Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin Vaccine Immunol. 2007;14:605–616. doi: 10.1128/CVI.00398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elvin SJ, Williamson ED. Stat 4 but not Stat 6 mediated immune mechanisms are essential in protection against plague. Microb Pathog. 2004;37:177–184. doi: 10.1016/j.micpath.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Parent MA, Berggren KN, Kummer LW, Wilhelm LB, Szaba FM, Mullarky IK, Smiley ST. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun. 2005;73:7304–7310. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szaba FM, Kummer LW, Wilhelm LB, Lin JS, Parent MA, Montminy-Paquette SW, Lien E, Johnson LL, Smiley ST. D27-pLpxL, an avirulent strain of Yersinia pestis, primes T cells that protect against pneumonic plague. Infect Immun. 2009;77:4295–4304. doi: 10.1128/IAI.00273-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JS, Park S, Adamovicz JJ, Hill J, Bliska JB, Cote CK, Perlin DS, Amemiya K, Smiley ST. TNFa and IFNg contribute to F1/LcrV-targeted immune defense in mouse models of fully virulent pneumonic plague. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.08.099. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kummer LW, Szaba FM, Parent MA, Adamovicz JJ, Hill J, Johnson LL, Smiley ST. Antibodies and cytokines independently protect against pneumonic plague. Vaccine. 2008;26:6901–6907. doi: 10.1016/j.vaccine.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parent MA, Wilhelm LB, Kummer LW, Szaba FM, Mullarky IK, Smiley ST. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun. 2006;74:3381–3386. doi: 10.1128/IAI.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philipovskiy AV, Smiley ST. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect Immun. 2007;75:878–885. doi: 10.1128/IAI.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 18.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connor W, Jr, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 20.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 23.Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, Moles JP, Danger Y, Ravon E, Lesaux S, Yssel H, Gascan H. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 24.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 28.Schulz SM, Kohler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, Holscher C, Muller U, Kastelein RA, Alber G. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- 29.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- 30.Johnson LL, Berggren KN, Szaba FM, Chen W, Smiley ST. Fibrin-mediated protection against infection-stimulated immunopathology. J Exp Med. 2003;197:801–806. doi: 10.1084/jem.20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 32.Lukaszewski RA, Kenny DJ, Taylor R, Rees DG, Hartley MG, Oyston PC. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect Immun. 2005;73:7142–7150. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laws TR, Davey MS, Titball RW, Lukaszewski R. Neutrophils are important in early control of lung infection by Yersinia pestis. Microbes Infect. 2010;12:331–335. doi: 10.1016/j.micinf.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Cowan C, Philipovskiy AV, Wulff-Strobel CR, Ye Z, Straley SC. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect Immun. 2005;73:6127–6137. doi: 10.1128/IAI.73.9.6127-6137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima R, Motin VL, Brubaker RR. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Straley SC, Cibull ML. Differential clearance and host-pathogen interactions of YopE- and YopK- YopL- Yersinia pestis in BALB/c mice. Infect Immun. 1989;57:1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Holscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan YR, Liu JS, Pociask DA, Zheng M, Mietzner TA, Berger T, Mak TW, Clifton MC, Strong RK, Ray P, Kolls JK. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182:4947–4956. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlsen JR, Borregaard N, Cowland JB. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. J Biol Chem. 2010;285:14088–14100. doi: 10.1074/jbc.M109.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]