Abstract
While searching for potential candidate molecules relevant for the pathogenesis of endometriosis, we discovered a 2910-base pair cDNA encoding a novel putative 411-amino acid integral membrane protein that we called shrew-1. The putative open-reading frame was confirmed with antibodies against shrew-1 peptides that labeled a protein of ∼48 kDa in extracts of shrew-1 mRNA-positive tissue and also detected ectopically expressed shrew-1. Expression of epitope-tagged shrew-1 in epithelial cells and analysis by surface biotinylation and immunoblots demonstrated that shrew-1 is indeed a transmembrane protein. Shrew-1 is able to target to E-cadherin-mediated adherens junctions and interact with the E-cadherin–catenin complex in polarized MCF7 and Madin-Darby canine kidney cells, but not with the N-cadherin–catenin complex in nonpolarized epithelial cells. Direct interaction of shrew-1 with β-catenin in in vitro pull-down assay suggests that β-catenin might be one of the proteins that targets and/or retains shrew-1 in the adherens junctions. Interestingly, shrew-1 was partially translocated in response to scatter factor (ligand of receptor tyrosine kinase c-met) from the plasma membrane to the cytoplasm where it still colocalized with endogenous E-cadherin. In summary, we introduce shrew-1 as a novel component of adherens junctions, interacting with E-cadherin–β-catenin complexes in polarized epithelial cells.
INTRODUCTION
Epithelial cell morphology and tissue architecture depend on cell polarity that is defined by the apical side of the cell facing the lumen, the basal side, and the lateral part with different types of junctions (adherens junctions, tight junctions, desmosomes, and gap junctions (Farquhar and Palade, 1965; Burridge et al., 1988; Chrzanowska-Wodnicka and Burridge, 1996; Gumbiner, 1996). Central to the maintenance of epithelial cell polarity are the adherens junctions (zonula adherens) for which cell-cell adhesion is a coordinating force. Adherens junctions are known to play key roles in forming and maintaining tight junctions and desmosomes, in addition to concentrating many biologically active molecules such as membrane receptors, signaling molecules, and oncoproteins (Tsukita et al., 1992; Aberle et al., 1996; Anastasiadis et al., 2000; Gumbiner, 2000; Nagafuchi, 2001). An understanding of the processes and proteins leading to the formation of cell-cell junctions as well as controlling their dynamics can provide a clue to the development and function of epithelia in both physiology and pathology (Eaton and Simons, 1995; Pollack et al., 1998).
The formation of epithelial adherens junctions is mediated by the calcium-dependent cell adhesion protein E-cadherin, a so-called classical cadherin. The polarizing function of E-cadherin depends largely on its clustering and interaction with the actin-based cytoskeleton through a number of cytoplasmic components (Takeichi, 1995; Gumbiner, 1996). Important cytoplasmic scaffolding proteins of the E-cadherin complex are in particular β-catenin, α-catenin, and proteins of the p120(ctn) family, but also AF-6 (Boettner et al., 2000), ponsin (Mandai et al., 1999), erbin (Borg et al., 2000), and conceivably others. The direct or indirect interaction of these proteins with the cytoplasmic part of E-cadherin is important for the stabilization of the complexes at the adherens junctions. Crystallographic studies have revealed that the cadherin cytoplasmic domain is essentially disordered in the absence of binding partners (Huber et al., 2001). Moreover, transmembrane proteins such as nectin (Tachibana et al., 2000) and vezatin (Küssel-Andermann et al., 2000) are also found in the adherens junctions to which they are recruited, directly or indirectly, via α-catenin (Nagafuchi, 2001) and thus contribute to the interaction of E-cadherin with the cytoskeleton.
Adherens junctions are highly dynamic structures that can undergo rapid but nevertheless regulated assembly and disassembly, as in epithelial-mesenchymal transitions. Such processes are needed, for example, when cells decrease the strength of their adhesion to move. If deregulated in pathological situations these phenomena may participate in establishing invasion and metastasis of carcinomas, as has been shown in many cases (Shimoyama and Hirohashi, 1991; Shiozaki and Mori, 1991; Takeichi, 1991). The dynamics of adherens junctions is influenced by regulatory proteins such as kinases for e.g., c-src, and c-Fyn, or phosphatases known to be present in adherens junctions. Regulatory signals also include intracellular proteins such as Ras, Rac, and Cdc small GTPases or the EGF/EGF receptor signaling system (Gumbiner, 1996, 2000). Another particular example is the receptor tyrosine kinase c-met with the ligand scatter factor (SF; also known as hepatocyte growth factor, HGF). Stimulation of epithelial cells with SF/HGF induces disassembly of the adherens junctions, whereby cells loose their typical morphology and become motile and invasive (Birchmeier et al., 1993; Watabe et al., 1993; Weidner et al., 1996). Along with the disassembly of epithelial cells, E-cadherin–catenin complexes may be endocytosed and degraded or recycled, very likely in caveolin-containing vesicles (Akhtar and Hotchin, 2001). Endocytosis and degradation of E-cadherin can be controlled by HAKAI, recently identified as a novel ubiquitin E3 ligase interacting with E-cadherin, which can modulate cell adhesion and increases the response of epithelia to scatter factor (Fujita et al., 2002).
In this article, we introduce and characterize a novel membrane protein, shrew-1, that targets to epithelial adherens junctions and interacts with cadherin–catenin complexes in polarized but not in nonpolarized cells.
MATERIALS AND METHODS
Differential Display Reverse Transcriptase-Polymerase Chain Reaction (DDRT-PCR) and Rapid Amplification of cDNA Ends (RACE)
DDRT-PCR was essentially performed as described previously (Liang and Pardee, 1992) by using a commercially available kit (Genhunter, Nashville, TN). Briefly, the Genhunter kit contains four different downstream primers (T12MA, T12MG, T12MT, and T12MC) used for first-strand cDNA synthesis and 20 different upstream primers (AP-1 to AP-20) for amplification. The cDNAs amplified from poly A+ RNA from either invasive or noninvasive EEC145T cells in the presence of radioactively labeled nucleotides were separated on polyacrylamide gels, autoradiographed, and the band patterns compared. Amplification products differentially and reproducibly found in either of the EEC145T variants (invasive or noninvasive) were cut out of the gel, reamplified, and cloned into a vector. The nucleotide sequences of the cloned products were determined, and the differential expression pattern of the identified sequences was validated by RT-PCR and Northern blots. The kit for RACE (BD Biosciences Clontech, Heidelberg, Germany) was used according to the manufacturer's instructions. Briefly, PCR was performed using Marathon ready cDNA and the anchor primer AP1 provided, which annealed specifically to the linker sequence on the cDNA. The sequence of the gene specific primer from within the known 391-base pair sequence used for 3′RACE was 5′-gtgttggaagatgctacc-3′ and that of the primer used for 5′RACE was 5′-tgaactcagtctctgtgg-3′. To confirm the specificity of the product nested RACE was performed with a nested gene-specific primer for 5′RACE (5′-ggatttggcagcagctgg-3′) and a nested primer provided with the kit for 3′RACE (5′-tagacggttggtgagtgg-3′). The product was cloned into a vector and sent for sequencing.
Phage Library
The ZAP Express/EcoRI/XhoI custom cDNA phagebank was constructed by Stratagene from the poly A(+) mRNA from passage 17 of EEC145T. Screening was performed according to standard protocols (Short et al., 1988).
Plasmid Constructs and Protein Expression
Shrew-1 cDNA isolated from the epithelial endometriotic cell line EEC145T was cloned into eukaryotic expression vectors pEGFP-N3 (BD Biosciences Clontech) and into pcDNA3.1(+) with a BP tag (Kaufmann et al., 2000) by using restriction sites introduced by PCR. PCRs were performed using Platinum Pfx-DNA polymerase (Invitrogen, Karlsruhe, Germany). The primers used for cloning into pEGFP-N3 contained the restriction sites BglII and Acc651. The sequence of the forward primer was 5′-agatctgaccatgtggattcaacagc-3′ and the reverse primer 5′-ggtaccgcaggagatttcaaacc-3′. For cloning into the pcDNA 3.1(+) vector, the restriction sites HindIII and EcoRI were incorporated using the forward primer 5′-aagcttgaccatgtggattcaacagc-3′ and the reverse primer 5′-gaattccagcaggagatttcaaacc-3′.
β-Catenin cloned into pcDNA vector was a kind gift from the group of R. Kemler (MPI for Immunology, Freiburg, Germany). The cytoplasmic domain of shrew-1 was cloned using PCR amplification into the pGEX-5 × 1 vector (GST-CPD-shrew) by using primers with an EcoRI site incorporated into the forward primer and SalI into the reverse primer. The forward primer had the sequence 5′-atcgaattcatgtctgggggcaaatac-3′ and the reverse primer 5′-ggctcgagtcgacttatatttctttctg-3′. The protein was expressed in bacteria and purified as described previously (Kaufmann et al., 2000).
Antibodies
Monoclonal anti-green fluorescent protein (GFP) antibody was obtained from BD Biosciences Clontech. Monoclonal pyruvate kinase antibody was obtained form Schebotech (Wettenberg, Germany). Monoclonal E-cadherin (clone 36) and monoclonal â-catenin (clone 14) were obtained from BD PharMingen (Los Angeles, CA). Another monoclonal antibody (mAb) against E-cadherin, 5H9, and a Pan-cadherin were purchased from Sigma Chemie (Deisenhofen, Germany). Monoclonal N-cadherin antibody (clone 3B9) was purchased from Zymed Laboratories (South San Francisco, CA). DECMA-1, a rat mAb against E-cadherin, was a kind gift from R. Kemler (Max Planck Institute of Immunology, Freiburg, Germany).
Monoclonal antibodies were generated against shrew-1 in mice by using the peptide sequence NH2-ACMTLQTKGFTESLDPRRRIPGGVS-amide by Nanotools (Teningen, Germany). Polyclonal antibodies were generated against the cytoplasmic domain of shrew-1 in rats in collaboration with Genovac (Freiburg, Germany). Secondary antibodies (fluorescein isothiocyanate, Texas Red, cyanine-5, and Cy3) were from Jackson Immunochemicals (Dianova, Hamburg, Germany). Alexa Fluor 466-labeled antibodies were purchased from Molecular Probes (Leiden, The Netherlands).
Surface Biotinylation Experiment and Immunoblots
The cell surface of confluent monolayers was labeled on ice with 0.5 μg/ml membrane-impermeable EZ-Link Sulfo-NHS-Biotin (Perbio, Bonn, Germany) in phosphate-buffered saline (PBS), pH 9.0. After quenching (50 mM ammonium chloride in PBS, 0.1 mM CaCl2, 1 mM MgCl2), the cells were lysed in 0.5 ml of radioimmunoprecipitation assay buffer (150 mM NaCl, 50 mM Tris, pH 7.5, 0.25% SDS, 0.1% Nonidet P-40) containing the protein inhibitor cocktail Complete (Roche Diagnostics, Mannheim, Germany) for 10 min at 4°C. Protein of each lysate was used for precipitation (16 h at 4°C) with 30 μl of neutravidin beads (Perbio). The precipitates were washed as described previously (Rajasekaran et al., 1999) and immunoblotted. Immunoblots were performed as described previously (Kaufmann et al., 2000).
Cell Culture, Plasmid Transfection, and Generation of Stable Cell Line
The human urinary bladder cell lines RT112 and EJ28 were described previously (Gaetje et al., 1997). MCF7 cells were obtained from European Collection of Cell Cultures (Salisbury, UK). EEC145T and 12Z were endometriotic cell lines established by our group as described previously (Zeitvogel et al., 2001). Canine kidney epithelial cell line Madin-Darby canine kidney (MDCK) obtained from American Type Culture Collection (Manassas, VA) (ATCC CCL 34) was used to generate cell lines stably expressing shrew-1-GFP. All cell lines were cultured in Dulbecco's modified Eagle's medium containing antibiotics and 10% fetal calf serum. All cell culture reagents were purchased from Invitrogen. Transient transfections were performed in MCF7 by using Polyfect transfection reagent from QIAGEN (Hilden, Germany). MDCK cells were transfected using Effectene transfection reagent from QIAGEN.
Immunofluorescence, Confocal Microscopy, and Antibody Permeabilization Assay
For immunofluorescence staining, cells grown on glass coverslips were fixed in 4% paraformaldehyde and permeabilized by treatment with 0.2% Triton X-100 (both in PBS). Incubation with primary and secondary antibodies as well as visualization by immunofluorescence and CLSM was done as described previously (Kaufmann et al., 2000). Image processing of three-dimensional data sets was performed with a Silicon Graphics (Mountain View, CA) workstation (using “Imaris” Bitplane AG, Zurich, Switzerland) three-dimensional multichannel image processing software (Messerli et al., 1993). Images were further processed using Adobe Photoshop.
For the antibody permeabilization assay, MCF7 cells were transfected with shrew-1-GFP and grown to confluence. Cells were either fixed with 4% paraformaldehyde or left unfixed (living samples). Immunoflourescence was performed as described.
Coimmunoprecipitation
Cells were washed twice with ice-cold PBS and lysed for 30 min at 4°C in a buffer containing 10 mM Tris, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 60 mM n-octyl-glucoside. Samples were precleared for 1 h at 4°C by using protein G-Sepharose (20 μl, 1:1) and subjected to immunoprecipitation overnight at 4°C by using anti-GFP IgG (5 μg, mAb), anti-E-cadherin (5 μg, mAb 5H9), anti-Pan-cadherin (3 μg, followed by 2-h incubation with protein G-Sepharose (30 μl, slurry 1:1). After four to five washes with the immunoprecipitation buffer, samples were separated by SDS-PAGE (12% acrylamide) and transferred to nitrocellulose. Immunoblotting was performed as described.
In Vitro “Pull-Down” Binding Assays
β-Catenin cloned in the expression vector pcDNA3.1 was synthesized by in vitro transcription-translation in the presence of [35S]methionine by using the TNT-coupled reticulocyte lysate (Promega, Mannheim, Germany). Glutathione S-transferase cytoplasmic domain of shrew-1 fusion (GST-CPD-shrew) (see “Plasmid Constructs and Protein Expression”) was expressed in Escherichia coli BL21 pLysS. Pull-down assays were performed as described previously (Kaufmann et al., 2000).
RESULTS
Identification of shrew-1 from Endometriotic Cell Line
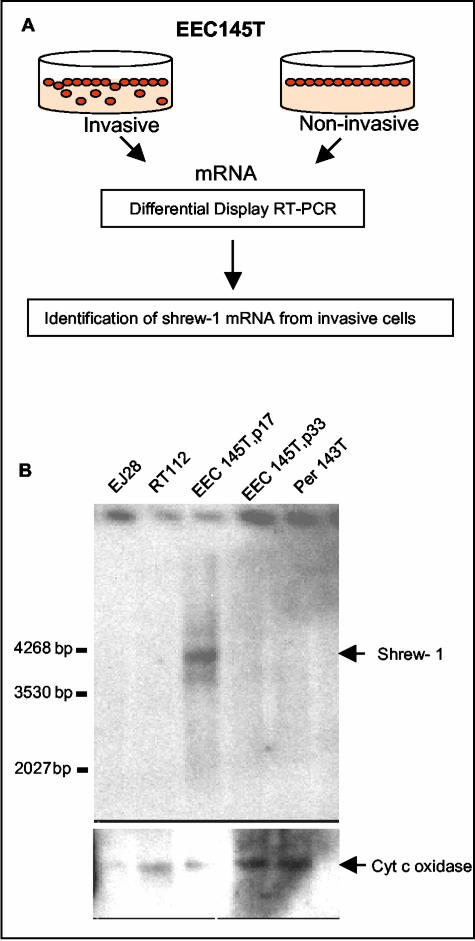
A cell line (EEC145T) from endometriosis lesions that has recently been established in our laboratory was found to be epithelial in nature (cytokeratin positive, E-cadherin negative) (Zeitvogel et al., 2001). The fact that this cell line became noninvasive after a few passages prompted us to use it as a tool for identifying markers differentially expressed during endometriosis (Figure 1A). We therefore performed DDRTPCR with the invasive (p17) and noninvasive (p33) passages of this cell line. This reproducibly resulted in the isolation of a 391-base pair DDRT-PCR fragment that was differentially expressed in the invasive EEC145T cell line. Northern blots (Figure 1B) by using the 391-base pair fragment as a probe confirmed the presence of a corresponding message in invasive EEC 145T cells and revealed an mRNA of ∼4 kb. The gene and its products (mRNA and protein) were called shrew-1.
Figure 1.
(A) Diagram depicting DDRT-PCR performed with invasive and noninvasive passages of the endometriotic cell line EEC145T, leading to the identification of shrew-1 mRNA. (B) The 391-base pair cDNA was used as a probe to test for the presence of shrew-1 mRNA in endometriotic and carcinoma cell lines. Poly A+ RNA was prepared from the cell lines EJ28 (invasive bladder carcinoma), RT112 (noninvasive bladder carcinoma), EEC145T (p17, invasive passage 17; p33, noninvasive passage 33 of the endometriotic cell line), and Per 143T (peritoneal cells immortalized with simian virus 40 T antigen). A Northern blot probed with 32P-labeled shrew-1 probe detected an mRNA of ∼4 kb in the invasive endometriotic cell line. Bottom, membrane was reprobed with cytochrome c oxidase to check the integrity and loading of the RNA samples. p17 and p33 are on the same blot at the same exposure-same as cytochrome c oxidase control.
Isolation of the cDNA and Nucleotide Sequence Analysis
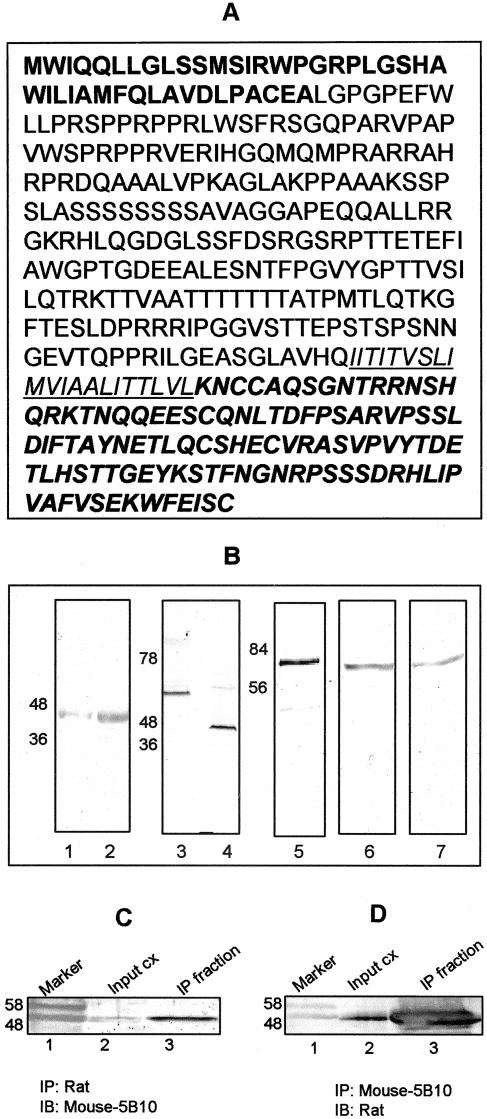
Database searches for sequences identical to shrew-1 DDRT cDNA sequences revealed expressed sequence tags (ESTs) with identical sequences from various parts of the brain, kidney, and fetus, as well as colon carcinomas. Screening of a ZAP Express/EcoRI/XhoI custom cDNA phage library constructed from RNA of invasive passage p17 of EEC145T led to the isolation of phagemid clone Q2A containing an insert of 2204 nucleotides, including the original DDRT-PCR fragment. Longer cDNA fragments could not be obtained from this library. Therefore, we isolated the rest of the cDNA by 5′ and 3′RACE experiments by using commercially available Marathon-Ready cDNA (Clontech, Heidelberg, Germany) from human brain, which is also positive for shrew-1. The cDNA finally obtained contained 2910 nucleotides and was identical to mRNA sequences in the EEC145T cells as revealed by overlapping reverse transcription (RT)-PCRs and DNA sequencing (accession no. of shrew-1, AY282806). It encodes a putative protein of 411 amino acids, sharing >90% identity with a putative mouse counterpart derived from ESTs published in the database (accession no. BI990953). The amino acid composition (Figure 2A) of shrew-1 predicts a highly alkaline protein with an isoelectric point of 9.86 and a theoretical molecular mass of 44.5 kDa. A computer search for conserved protein motifs revealed a putative signal peptide of ∼43 aa (bold in Figure 2A), a putative transmembrane domain (underlined in Figure 2A), and some potential sites for phosphorylation, glycosylation, and myristylation (our unpublished data).
Figure 2.
(A) The complete 411-amino acid sequence of the shrew-1 protein. The putative signal peptide is depicted in bold letters and the transmembrane domain is underlined. (B) Lanes 1 and 2 show the endogenous expression of shrew-1 protein in pancreas and uterus sections, respectively, as detected by immunoblotting by using the mAb against shrew-1; lanes 3 and 4 show the autoradiography of in vitro-translated luciferase control cDNA and shrew-1-BP, respectively, after separation by SDS-PAGE; lane 5 depicts shrew-1-GFP expressed in MCF7 cells, as detected by monoclonal GFP antibody, lane 6 shows shrew-1-GFP detected by the rat polyclonal antibody against shrew-1 cytoplasmic peptide, and lane 7 shows shrew-1-GFP detected by the mAb generated against shrew-1 peptide of the putative ectodomain. (C) Immunoprecipitation of endogenous shrew-1 from uterus cell extracts by using shrew-1 polyclonal antibody and detection with the mAb. Lane 2 depicts the input cell extract and lane 3 shows the immunoprecipitated protein detected with the mAb. (D) Immunoprecipitation of endogenous shrew-1 from uterus cell extracts using shrew-1 mAb and detection with the polyclonal antibody. Lane 2 depicts the input cell extract and lane 3 depicts the immunoprecipitated protein detected with the polyclonal antibody. Input is 10% of the total cell extract. Lane 1 in each C and D depicts the marker proteins.
It should be noted that the nucleotide sequence of a cDNA isolated from a fibrosarcoma (accession no. AF175409; but not described otherwise) allows the generation of an open reading frame that is completely identical to that of shrew-1. So far, there is no evidence that shrew-1 is a member of any protein family because there are no conserved domains in other proteins, either in human or in any other organism, including yeast, Drosophila, or Caenorhabditis elegans. Orthologous protein sequences of shrew-1 itself were found in mouse and zebrafish but not in Drosophila, C. elegans, or yeast, suggesting that shrew-1 might be a protein that is restricted to vertebrates.
Expression of shrew-1 Protein
Two different types of antibodies were generated to analyze expression at the protein level. First, custom-made mouse monoclonal antibodies were produced against a synthetic peptide deduced from the putative extracellular domain (for sequence, see MATERIALS AND METHODS). The resulting monoclonal antibodies were tested against protein extracts from human pancreas and uterus in an immunoblot, because both these tissues were found to contain shrew-1 mRNA as shown by Northern blot and RT-PCR analysis (our unpublished data). As shown in Figure 2B, lanes 1 and 2, both tissues were found to contain a protein of ∼48 kDa corresponding to the predicted size of the shrew-1 protein.
Second, polyclonal antibodies were generated in rats by genetic immunization against the putative cytoplasmic domain of shrew-1 (in collaboration with Genovac). This antibody also recognized endogenous protein from tissue extract (described below).
For ectopic expression, shrew-1 was cloned into two different expression vectors fused to either a 10-aa-long birch profilin tag (shrew-1-BP) or a green fluorescent protein tag (shrew-1-GFP). To check whether these vectors expressed the predicted open reading frame protein of 411 aa, we performed different kinds of experiments. First, shrew-1-BP was translated radioactively in vitro using a reticulocyte lysate kit (see MATERIALS AND METHODS). SDS-PAGE and autoradiography revealed that shrew-1 cDNA encoded indeed a protein of ∼48 kDa (Figure 2B, lane 4). The positive control used for in vitro translation was luciferase cDNA supplied by the manufacturer (Figure 2B, lane 3). In extracts of human epithelial MCF7 cells ectopically expressing shrew-1-GFP, anti-GFP antibody detected a protein of the expected size of ∼75 kDa in Western blots (Figure 2B, lane 5). Shrew-1 rat polyclonal antibody against the putative cytoplasmic polypeptide sequence gave a signal of comparable size in these extracts (Figure 2B, lane 6). The shrew-1 monoclonal antibodies that detected shrew-1 in pancreas and uterus cell extracts (Figure 2B, lanes 1 and 2) also detected the recombinant transfected shrew-1-GFP in MCF7 cell extracts (Figure 2B, lane 7). It should be noted that, although MCF7 cells do contain shrew-1 mRNA endogenous protein could not be detected possibly due to low abundance (our unpublished data).
To indicate unambiguously the authenticity of the endogenous protein band as shrew-1 protein, an immunoprecipitation (IP) experiment was performed with uterus extract by using polyclonal rat antibody and subsequent immunoblot (IB) with the mAb (Figure 2C, lanes 2 and 3). Vice versa, IP was performed with the mAb and IB with the polyclonal antibody (Figure 2D, lanes 2 and 3). Both approaches revealed a protein of the expected size as in the input cell extracts (10% of the total cell extract). That the two antibodies against peptides from two different regions in shrew-1 sequence detected apparently the same protein additionally confirmed that the predicted shrew-1 open reading frame is endogenously expressed in mammalian cells.
Membrane Localization and Orientation of shrew-1
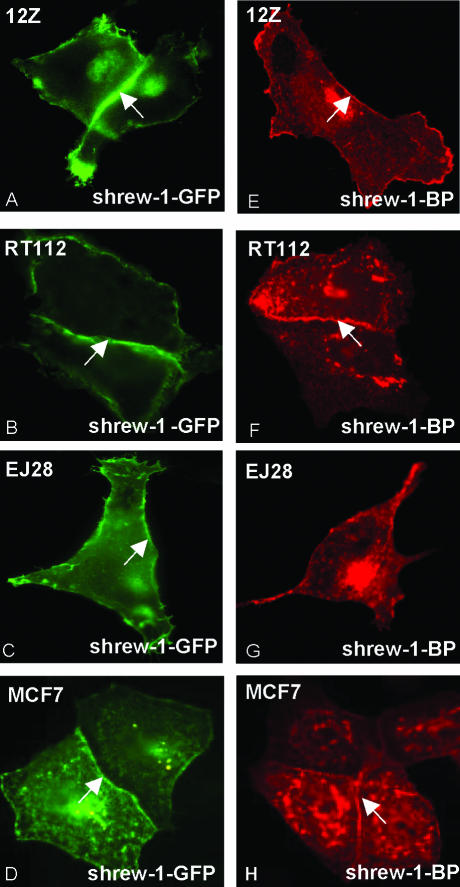
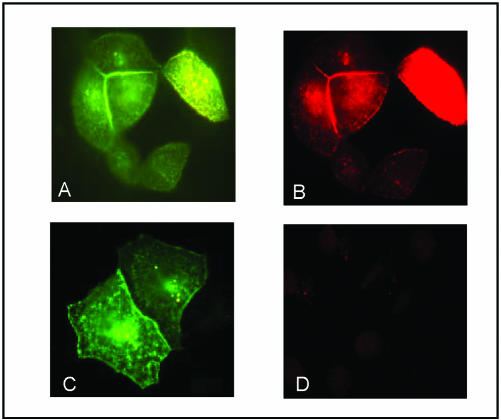
Shrew-1 fused to two different tags (to rule out the possibility that cellular localization is affected by the tags) was used to determine the cellular localization of the protein. These studies were performed in epithelial cell lines 12Z, RT112, EJ28, and MCF7 transiently transfected with shrew-1-GFP and shrew-1-BP. In all cases, major pools of shrew-1 seemed to be localized at the plasma membrane, especially at the regions of cell-cell contacts, irrespective of whether RT-PCR (our unpublished data) showed that the cell lines contained endogenous shrew-1, namely, MCF7 and 12Z (Figure 3, A, D, E, and H) or not, i.e., RT112 and EJ28 (Figure 3, B, C, F, and G). The cells shown in the pictograph are mainly shrew-1–transfected cells with a few neighboring cells.
Figure 3.
Membrane localization of shrew-1. Shrew-1 tagged with GFP (shrew-1-GFP) or BP (shrew-1-BP) was expressed in the eukaryotic epithelial cells 12Z (human invasive endometriotic cell line), RT112 (human bladder carcinoma cell line, noninvasive), EJ28 (human bladder carcinoma cell line, invasive), and MCF7 (human breast carcinoma cell line, noninvasive). A–D show shrew-1-GFP fluorescence and E–H show immunofluorescence signals by using a mouse mAb against the BP tag visualized by a mouse-specific fluorochrome-conjugated secondary antibody. The arrows indicate the expression of shrew-1 at the membrane. The arrows depict the areas of cell-cell contact where shrew-1 is concentrated. The cells shown are transfected cells which border a few nontransfected cells.
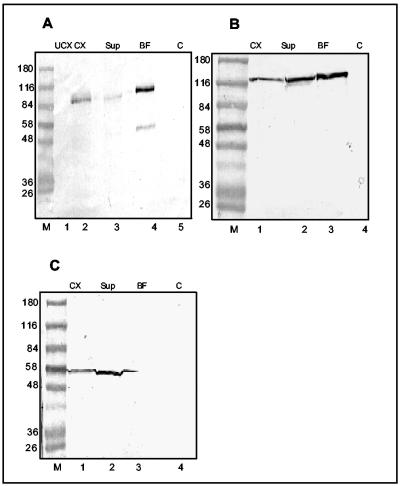
To check whether shrew-1 is exposed on the cell surface, shrew-1-GFP was transiently transfected into MCF7 cells. Surface-exposed proteins were then biotinylated using a membrane-impermeable biotin. After cell lysis and detergent solubilization, biotinylated proteins were isolated by incubation with agarose-coupled neutravidin beads. Immunoblotting using antibodies against GFP revealed that shrew-1-GFP was present in the biotinylated protein fraction (Figure 4A, lane 4), confirming that shrew-1 is an integral component of the plasma membrane. E-cadherin, a transmembrane protein (Figure 4B, lane 3), and pyruvate kinase, a cytosolic protein (Figure 4C, lane 2), were used as positive and negative controls, respectively.
Figure 4.
Cell surface biotinylation of MCF7 cells transfected with shrew-1-GFP. The biotinylated cell surface proteins were pulled down with neutravidin-coupled beads. The proteins present in various cell extract fractions were analyzed by Western blots. (A) Shrew-1-GFP was detected by anti-GFP antibody (lanes 1–5). (B) E-cadherin, a positive control membrane protein, was detected by a mAb against E-cadherin (lanes 1–4). (C) Pyruvate kinase, a negative control cytosolic protein, was detected with a specific antibody (lanes 1–4). UCX, untransfected cell extract; CX, transfected cell extract; sup, supernatant after pull down of the biotinylated fraction; BF, pulled-down biotinylated fraction; C, control of neutravidin beads bound to nonbiotinylated cell extract.
Furthermore, we tested whether the carboxy terminus of shrew-1 is cytoplasmic by performing permeabilization studies. MCF7 cells were transiently transfected with shrew-1 tagged with a C-terminal GFP tag (shrew-1-GFP). One aliquot of the transfected cells was permeabilized (Figure 5, A and B) and immunodetection was performed using GFP antibody (Figure 5, B and D), whereas the other aliquot was not permeabilized (Figure 5, C and D) and immunostaining was performed on live cells by using GFP antibody in the presence of sodium azide to prevent antibody-induced capping. The autofluorescence from shrew-1-GFP (Figure 5, A and C) could be seen in both cases, but antibody staining could only be seen with cells that were permeabilized. This clearly implies that the C terminus is indeed cytoplasmic. A comparable result was obtained when a similar experiment was performed in MDCK cells (our unpublished data).
Figure 5.
Carboxy terminus of shrew-1 is cytoplasmic. Shrew-1-GFP–transfected MCF7 cells were permeabilized (A and B) or not permeabilized (C and D), as described under MATERIALS AND METHODS and then subjected to immunofluorescence staining with anti-GFP antibody and Alexa 594-labeled secondary goat anti-mouse antibody (B and D, red fluorescence). Intrinsic GFP fluorescence is green (A and C). Shrew-1-GFP could be detected in permeabilized cells by immunostaining with anti-GFP antibody (B) but not if the cells were not permeabilized (D).
Colocalization of shrew-1 with E-Cadherin at the Adherens Junctions
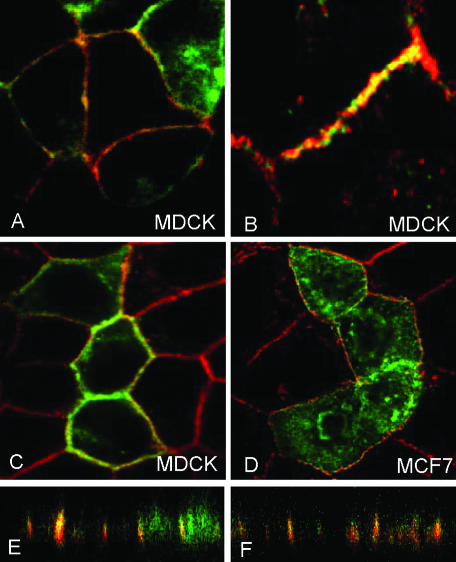
As seen in Figure 3, shrew-1-GFP seemed to be concentrated mainly at sites of cell-cell contact. This was even more evident in epithelial cells that expressed E-cadherin at the membrane such as MCF7 (Figures 3 and 6) and MDCK cells (Figure 6). We therefore asked whether shrew-1 and E-cadherin colocalize in these cells. Shrew-1-GFP was transfected into MCF7 and MDCK cells and subsequently costained for endogenous E-cadherin by indirect immunofluorescence. Optical sectioning with confocal microscopy revealed that E-cadherin colocalizes with shrew-1-GFP along the xy-axis (Figure 6, A–D). Additionally, when the sections were recorded along the xz-axis (Figure 6, E and F), shrew-1 was found to colocalize with E-cadherin at the junctions.
Figure 6.
Colocalization of shrew-1-GFP with endogenous E-cadherin (red) at the membrane in MDCK cells (A–C); and in MCF7 cells (D) along the xy-axis as seen in the confocal microscope. Colocalization at the junctions is seen along the xz-axis with the confocal microscope (E and F).
Because E-cadherin is a marker of adherens junctions, we presume that shrew-1 is also present in these junctions. Whether this colocalization was the result of shrew-1 interacting specifically with E-cadherin or just a coincidence was further investigated.
Interaction of shrew-1 with Cadherin-β–Catenin Complexes in Polarized and Nonpolarized Cells
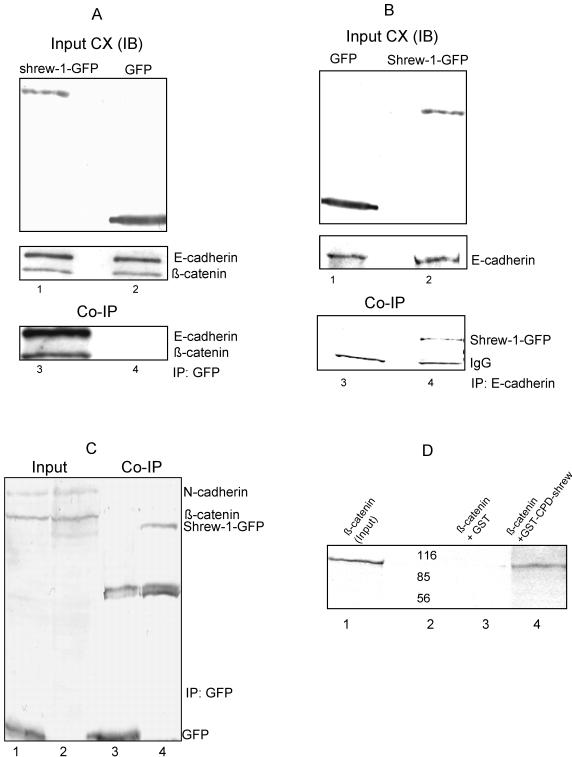
To check whether shrew-1 can complex with E-cadherin, MCF7 cells were transiently transfected with shrew-1-GFP or the vector alone and grown to confluence. Cell extracts were prepared and transfection efficiencies were monitored by immunoblotting 10% of the total cell extract by using GFP antibody (Figure 7A, input). The remaining cell extract was immunoprecipitated with GFP antibody and protein G-Sepharose beads, and then the whole complex was immunoblotted using E-cadherin antibody (Figure 7A, lanes 3 and 4). E-Cadherin could be detected in the immunocomplex pulled down by monoclonal anti-GFP antibody. Complexing of shrew-1 and E-cadherin could be observed in confluent but not in subconfluent cells where, however, colocalization of endogenous E-cadherin and shrew-1 could already be seen (for the latter, see Figure 8). This suggested that shrew-1 complexes with cadherin–catenin protein detectably only upon the formation of junctions (see DISCUSSION). β-Catenin was also detected on reprobing the same blot with the β-catenin antibody (Figure 7A). The reverse experiments confirmed the same results when IP was done with E-cadherin antibody and shrew-1-GFP could be detected in the same complex (Figure 7B).
Figure 7.
Interaction between shrew-1 and E-cadherin shown by coimmunoprecipitation. (A) MCF7 cells transfected with shrew-1-GFP (lanes 1 and 3) or with GFP (lanes 2 and 4) were subjected to immunoprecipitation with anti-GFP. To test antigen content, 10% of the total cell extract (input) was immunoblotted (IB) with anti-GFP (top) and anti-E-cadherin plus anti-β-catenin (middle) antibodies. Coimmunoprecipitations (CoIP) were performed with anti-GFP antibody and the immunoprecipitates subjected to immunoblotting with anti-E-cadherin and then anti-β-catenin antibodies (lanes 3 and 4). (B) In the reverse experiment, the cell extracts from MCF7 cells transfected with GFP (lanes 1 and 3) or shrew-1-GFP (lanes 2 and 4) were subjected to IP with anti-E-cadherin antibody. Input panels depict 10% of the cell extracts immunoblotted with anti-GFP antibody (top) or endogenous E-cadherin protein immunoblotted with anti-E-cadherin antibody (bottom). CoIP were performed with E-cadherin antibody, and shrew-1 was detected by immunoblotting with anti-GFP antibody as seen in lane 4. CX denotes the total cell extract. (C) Coimmunoprecipitation of N-cadherin and shrew-1-GFP. A, EJ28 cells were transfected with GFP (lanes 1 and 3) or shrew-1-GFP (lanes 2 and 4). Input shows 10% of the total cell extracts (lanes 1 and 2). Immunoprecipitation (CoIP) was performed with GFP-antibody (lanes 3 and 4). Immunoblotting was performed with antibodies against GFP, N-cadherin, and β-catenin. No interaction of shrew-1 with N-cadherin and β-catenin was observed. (D) Direct interaction of β-catenin with the cytoplasmic domain of shrew-1 (GST-CPD-shrew) in an in vitro pull-down assay. Full-length β-catenin was translated in vitro using [35S]methionine. GST and GST-CPD-shrew were purified on glutathione-Sepharose beads, and then incubated at RT for 1 h with radioactively labeled β-catenin. After washing the beads, samples prepared as described under MATERIALS AND METHODS, were subjected to SDS-PAGE and autoradiography. Lane 1, radioactive β-catenin as input; lane 2, the marker; lane 3; GST alone with β-catenin; and lane 4, GST-CPD-shrew with β-catenin.
Figure 8.
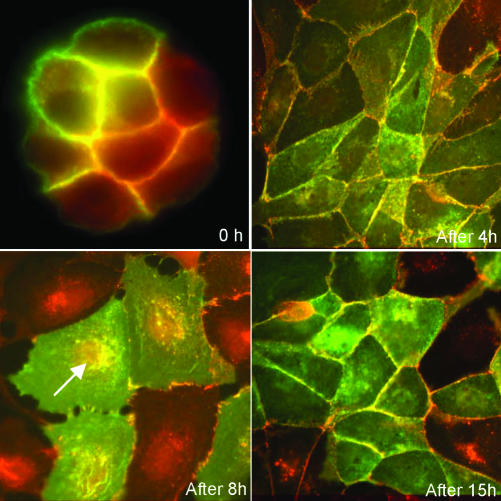
Effect of SF on MDCK cells. MDCK cells transfected with shrew-1-GFP were seeded at very low density on coverslips and were grown until formation of small colonies. SF/HGF was added at a concentration of 20 ng/ml to these cells, and the effect was monitored on coverslips from the same culture dish at 0, 4, 8, and 15 h. After 8 h, cell-cell contacts were disrupted but colocalization of shrew-1-GFP (green) and endogenous E-cadherin (red) could also be seen in cytoplasmic vesicles. After 15 h, shrew-1-GFP and endogenous E-cadherin colocalized again predominantly at the plasma membrane.
Furthermore, we analyzed the ability of shrew-1 to interact with cadherins in epithelial cell lines that are unable to form adherens junctions (for example EJ28 cells, an invasive human bladder carcinoma cell line expressing N-cadherin; Figure 7C). EJ28 cells were transfected with shrew-1-GFP and GFP alone. Monoclonal anti-GFP antibody was used for CoIP assays performed as described above. For IB detection we used anti-N-cadherin, anti-β-catenin, and anti-GFP antibodies. N-Cadherin and β-catenin could not be detected in the immunocomplex pulled down by anti-GFP antibody. These data reiterate that shrew-1 can interact with cadherin–catenin complexes in junctions of polarized epithelial cells but not with cadherin–catenin complexes (here N-cadherin) in nonpolarized cells.
The results shown so far do not indicate whether the interaction between E-cadherin and shrew-1 is due to direct binding of the proteins or is caused by an intermediate protein such as a scaffolding protein in the complex (β-catenin being a candidate). We therefore performed in vitro pull-down assays (see MATERIALS AND METHODS) between the cytoplasmic domain (CPD) of shrew-1 (used as GST fusion protein) and in vitro translated β-catenin (Figure 7D, lane 1) or full-length E-cadherin (our unpublished data). Although E-cadherin could not be pulled down by GSTCPD-shrew-1 (our unpublished data), β-catenin clearly interacted with the cytoplasmic domain of shrew-1 (Figure 7D, lane 4).
Together, these data support the idea that shrew-1 interacts with β-catenin in epithelial adherens junctions. However, this does not exclude that shrew-1 binds to other as yet unidentified components of the adherens junctions.
Effect of Addition of SF/HGF on Colocalization of shrew-1 and E-Cadherin
We decided to disrupt cellular junctions by adding SF/HGF to find out whether shrew-1 and E-cadherin still colocalize after junction disruption. SF/HGF is a cytokine that acts as a morphogen leading to epithelial-mesenchymal transitions. It is known to disrupt E-cadherin–mediated junctions in MDCK cells through activation of its receptor c-met. During disruption, E-cadherin is transiently transported into recycling vesicles reported to contain caveolin-1 (Akhtar and Hotchin, 2001).
MDCK cells transiently transfected with shrew-1-GFP were seeded at very low density on coverslips and were grown until the formation of islands. SF/HGF was added at a concentration of 20 ng/ml, and the effect was monitored on coverslips from the same culture dish at 0, 4, 8, and 15 h (Figure 8). After 8 h, a drastic change in the intracellular distribution of shrew-1 was observed. GFP fluorescence at the plasma membrane was reduced and intracellular particulate structures were labeled, which also stained for E-cadherin (marked with arrow heads). When the scattering effect of SF/HGF was gone after 15 h, GFP fluorescence and E-cadherin were again prominent at the plasma membrane. These results suggest that upon disruption of junctions by a physiological stimulus shrew-1 is translocated together with E-cadherin to intracellular vesicles. Although it seems from these experiments that shrew-1 and E-cadherin have a comparable pattern of internalization, it can however not be discriminated whether this is caused by a weak interaction not detectable by coimmunoprecipitation or simply by a similar response to stimulation by SF/HGF.
DISCUSSION
Shrew-1 in Junctional and Nonjunctional Protein Complexes
Here, we described the isolation and characterization of a novel protein, shrew-1, that is able to target to E-cadherin–mediated adherens junctions. Confocal microscopic analysis of transiently expressed shrew-1 revealed its localization at the lateral part of the cell membrane, where it colocalized and coimmunoprecipitated with endogenous E-cadherin, also present at the lateral part of the membrane in polarized MDCK cells (Le Bivic et al., 1990; Shore and Nelson, 1991). It has been shown in many studies that adhesion is mediated by the cytoplasmic domain of E-cadherin linking to the actin cytoskeleton (Takeichi et al., 1988) via associated proteins such as β-catenin and α-catenin (Aberle et al., 1994). Interestingly, direct interaction between β-catenin and shrew-1 in an in vitro pull-down assay suggested that shrew-1 might be linked to the E-cadherin–mediated junctional complex via β-catenin.
The fact that no interaction could be found between shrew-1 and N-cadherin–β-catenin complex in nonpolarized cells, however, favors the idea that β-catenin alone is not sufficient to target shrew-1 into adherens junctions, but other, as yet unidentified components might also be necessary. One component could be E-cadherin itself, although we were unable to identify a direct interaction between full-length E-cadherin and the cytoplasmic domain of shrew-1 in vitro. It is still possible that an interaction between E-cadherin and shrew-1 might be mediated via the transmembrane and/or the extracellular domains.
This interpretation also raises the possibility that shrew-1 is in fact targeted to specific cell contact sites, e.g., adherens junctions, rather than merely binding to E-cadherin per se. In line with such an idea is the observation that coimmunoprecipitation of shrew-1 with the E-cadherin–catenin complex was dependent on the formation of junctions and, in spite of colocalization in immunofluorescence between shrew-1-GFP and endogenous E-cadherin, complexing could not be observed in subconfluent cells.
An explanation for this could be that the interaction is rather weak at this stage of cell growth and only stabilizes when cells junctions mature in confluent cells. The second explanation could be that initially E-cadherin and shrew-1 are in two independent complexes that only interact in rather mature junctions. This might be a sort of anchoring requiring additional bridging protein(s).
The colocalization of shrew-1 with E-cadherin observed at the cellular junctions in MDCK cells transiently expressing shrew-1 also persisted when the junctions were disrupted by the physiological stimulus SF/HGF and during subsequent endocytosis of E-cadherin. It can, however, not be discriminated whether this is caused by a weak interaction not detectable by coimmunoprecipitation or simply by a similar response to stimulation by SF/HGF.
The vesicles in which E-cadherin and shrew-1 were endocytosed are found to contain caveolin-1 (our unpublished data), an integral membrane protein of caveolae, which is in agreement with published reports with regard to E-cadherin (Akhtar and Hotchin, 2001). It remains to be tested whether this colocalization during endocytosis is of physiological relevance particularly with regard to shrew-1–positive structures.
Shrew-1 Protein Sequence and Potential Functional Features
The deduced protein sequence of shrew-1 exhibits a number of unusual features. Computational analysis predicted a transmembrane domain and an unusual signal peptide. The functional integrity of these domains was implied by our experiments showing that the shrew-1 cDNA encodes a membrane-spanning protein with a cytoplasmic C terminus.
Surprisingly, no other structural features such as an α-helix or immunoglobulin-like domains could be predicted for the protein. Instead, we identified so-called low-complexity regions (Bork and Schneider, personal communication) for which no structure can be predicted with the algorithms available (Schultz et al., 1998). One general idea about the functional relevance of these low-complexity regions is that they only acquire a rigid structure (e.g., an α-helix) upon interaction with another protein partner or oligomerization into a protein complex (Wright and Dyson, 1999). This has been reported for soluble N-ethylmaleimide-sensitive factor attachment protein receptor, which play a role in vesicle docking and fusion, and which only form an α-helix when they bind to their interaction partner (Fasshauer et al., 1997; Jahn and Sudhof, 1999).
Further analysis of the shrew-1 protein sequence revealed a putative nuclear localization signal in the predicted extracellular domain and putative glycosylation signals in the cytoplasmic domain (our unpublished observations). Although these signals might be some artifacts of computational analysis, a functional relevance of these findings cannot be excluded. For example, it has recently been shown that the EGF receptor, an integral plasma membrane protein, can travel to the nucleus where it apparently exhibits a specific function possibly in the regulation of gene activities (Waugh and Hsuan, 2001).
In conclusion, we isolated a novel integral membrane protein, which might play a role in the function and/or regulation of E-cadherin–mediated junctional complexes. As an integral component of the adherens junctions and as a protein complexing with E-cadherin–β-catenin complex, investigation of shrew-1 gene activity and function could be important to improve our understanding of the dynamics of adherens junctions in both physiological and pathological processes, i.e., tumor progression and metastasis.
Acknowledgments
We thank Reinhard Jahn and Joachim Kirsch for critical reading of the manuscript; Peer Bork and Gisbert Schneider for valuable support with the computational analysis; Karl-Friedrich Becker for pancreas tissue extracts; Ela Frye and Alex Schreiner for helpful discussions and enthusiasm; and Viktor Jakob, Beata Krebs, and Heinz Schewe for expert technical assistance. Fellowships have been provided by the Boehringer Ingelheim Foundation to S.Z. and the FAZIT Foundation to A.Z. and S.B. This work was supported by grants of the Deutsche Forschungsgemeinschaft (Sta 187/11-1-5; SFB 628), the Stifterverband der Deutschen Wissenschaft, and the Boehringer Ingelheim Stiftung.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–05–0281. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0281.
References
- Aberle, H., Butz, S., Stappert, J., Weissig, H., Kemler, R., and Hoschuetzky, H. (1994). Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107, 3655-3663. [DOI] [PubMed] [Google Scholar]
- Aberle, H., Schwartz, H., Hoschuetzky, H., and Kemler, R. (1996). Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J. Biol. Chem. 271, 1520-1526. [DOI] [PubMed] [Google Scholar]
- Akhtar, N., and Hotchin, N.A. (2001). RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol. Biol. Cell 12, 847-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis, P.Z., Moon, S.Y., Thoreson, M.A., Mariner, D.J., Crawford, H.C., Zheng, Y., and Reynolds, A.B. (2000). Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2, 637-644. [DOI] [PubMed] [Google Scholar]
- Birchmeier, W., Weidner, K.M., and Behrens, J. (1993). Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J. Cell Sci. Suppl. 17, 159-164. [DOI] [PubMed] [Google Scholar]
- Boettner, B., Govek, E.E., Cross, J., and Van Aelst, L. (2000). The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc. Natl. Acad. Sci. USA 97, 9064-9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg, J.P., Marchetto, S., Le Bivic, A., Ollendorff, V., Jaulin-Bastard, F., Saito, H., Fournier, E., Adelaide, J., Margolis, B., and Birnbaum, D. (2000). ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat. Cell Biol. 2, 407-414. [DOI] [PubMed] [Google Scholar]
- Burridge, K., Fath, K., Kelly, T., Nuckolls, G., and Turner, C. (1988). Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 4, 487-525. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka, M., and Burridge, K. (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol., 133, 1403-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, S., and Simons, K. (1995). Apical, basal, and lateral cues for epithelial polarization. Cell 82, 5-8. [DOI] [PubMed] [Google Scholar]
- Farquhar, M.G., and Palade, G.E. (1965). Cell junctions in amphibian skin. J. Cell Biol., 26, 263-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer, D., Otto, H., Eliason, W.K., Jahn, R., and Brunger, A.T. (1997). Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem. 272, 28036-28041. [DOI] [PubMed] [Google Scholar]
- Fujita, Y., Krause, G., Scheffner, M., Zechner, D., Leddy, H.E., Behrens, J., Sommer, T., and Birchmeier, W. (2002). Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4, 222-231. [DOI] [PubMed] [Google Scholar]
- Gaetje, R., Kotzian, S., Herrmann, G., Baumann, R., and Starzinski-Powitz, A. (1997). Nonmalignant epithelial cells, potentially invasive in human endometriosis, lack the tumor suppressor molecule E-cadherin. Am. J. Pathol. 150, 461-467. [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B.M. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345-357. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. (2000). Regulation of cadherin adhesive activity. J. Cell Biol., 148, 399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, A.H., Stewart, D.B., Laurents, D.V., Nelson, W.J., and Weis, W.I. (2001). The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J. Biol. Chem. 276, 12301-12309. [DOI] [PubMed] [Google Scholar]
- Jahn, R., and Sudhof, T.C. (1999). Membrane fusion and exocytosis. Annu. Rev. Biochem. 68, 863-911. [DOI] [PubMed] [Google Scholar]
- Kaufmann, U., Zuppinger, C., Waibler, Z., Rudiger, M., Urbich, C., Martin, B., Jockusch, B.M., Eppenberger, H., and Starzinski-Powitz, A. (2000). The armadillo repeat region targets ARVCF to cadherin-based cellular junctions. J. Cell Sci. 113, 4121-4135. [DOI] [PubMed] [Google Scholar]
- Küssel-Andermann, P., El-Amraoui, A., Safieddine, S., Nouaille, S., Perfettini, I., Lecuit, M., Cossart, P., Wolfrum, U., and Petit, C. (2000). Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherins-catenin complex. EMBO J. 19, 6020-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic, A., Sambuy, Y., Mostov, K., and Rodriguez-Boulan, E. (1990). Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J. Cell Biol. 110, 1533-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, P. and Pardee, A. B. (1992). Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257, 967-971. [DOI] [PubMed] [Google Scholar]
- Mandai, K., Nakanishi, H., Satoh, A., Takahashi, K., Satoh, K., Nishioka, H., Mizoguchi, A., and Takai, Y. (1999). Ponsin/SH3P 12, an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J. Cell Biol. 144, 1001-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli, J.M., van der Voort, H.T., Rungger-Brandle, E., and Perriard, J.C. (1993). Three-dimensional visualization of multi-channel volume data: the amSFP algorithm. Cytometry 14, 725-735. [DOI] [PubMed] [Google Scholar]
- Nagafuchi, A. (2001). Molecular architecture of adherens junctions. Curr. Opin. Cell Biol. 13, 600-603. [DOI] [PubMed] [Google Scholar]
- Pollack, A.L., Runyan, R.B., and Mostov, K.E. (1998). Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol. 204, 64-79. [DOI] [PubMed] [Google Scholar]
- Rajasekaran, S.A., Ball, W.J., Jr., Bander, N.H., Liu, H., Pardee, J.D., and Rajasekaran, A.K. (1999). Reduced expression of beta-subunit of Na, K-ATPase in human clear-cell renal cell carcinoma. J. Urol. 162, 574-580. [PubMed] [Google Scholar]
- Schultz, J., Milpetz, F., Bork, P., and Ponting, C.P. (1998). SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95, 5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama, Y., and Hirohashi, S. (1991). Cadherin intercellular adhesion molecule in hepatocellular carcinomas: loss of E-cadherin expression in an undifferentiated carcinoma. Cancer Lett. 57, 131-135. [DOI] [PubMed] [Google Scholar]
- Shiozaki, H., and Mori, T. (1991). [Adhesion molecules and cancer metastasis]. Gan To Kagaku Ryoho 18, 2361-2368. [PubMed] [Google Scholar]
- Shore, E.M., and Nelson, W.J. (1991). Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 266, 19672-19680. [PubMed] [Google Scholar]
- Short, J.M., Fernandez, J.M., Sorge, J.A., and Huse, W.D. (1988). Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 16, 7583-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana, K., Nakanishi, H., Mandai, K., Ozaki, K., Ikeda, W., Yamamoto, Y., and Nagafuchi, A. (2000). Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 5, 1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi, M. (1991). Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451-1455. [DOI] [PubMed] [Google Scholar]
- Takeichi, M. (1995). Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 7, 619-627. [DOI] [PubMed] [Google Scholar]
- Takeichi, M., Hatta, K., Nose, A., and Nagafuchi, A. (1988). Identification of a gene family of cadherin cell adhesion molecules. Cell Differ. Dev. Suppl. 25, 91-4. [DOI] [PubMed] [Google Scholar]
- Tsukita, S., Nagafuchi, A., and Yonemura, S. (1992). Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr. Opin. Cell Biol. 4, 834-839. [DOI] [PubMed] [Google Scholar]
- Watabe, M., Matsumoto, K., Nakamura, T., and Takeichi, M. (1993). Effect of hepatocyte growth factor on cadherin-mediated cell-cell adhesion. Cell Struct. Funct. 18, 117-124. [DOI] [PubMed] [Google Scholar]
- Waugh, M.G., and Hsuan, J.J. (2001). EGF receptors as transcription factors: ridiculous or sublime? Nat. Cell Biol. 3, E209-E211. [DOI] [PubMed] [Google Scholar]
- Weidner, K.M., Di Cesare, S., Sachs, M., Brinkmann, V., Behrens, J., and Birchmeier, W. (1996). Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384, 173-176. [DOI] [PubMed] [Google Scholar]
- Wright, P.E., and Dyson, H.J. (1999). Intrinsically unstructured proteins: reassessing the protein structure-function paradigm. J. Mol. Biol. 293, 321-331. [DOI] [PubMed] [Google Scholar]
- Zeitvogel, A., Baumann, R., and Starzinski-Powitz, A. (2001). Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am. J. Pathol. 159, 1839-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]