Abstract
Background
Polyomavirus BK (BKV) infection can cause nephropathy in the allograft kidney. No well-established drug treatment is available at this time. Human intravenous immunoglobulins (IVIG) have been used as an empiric therapy without proof of effectiveness.
Methods
We tested five lots of commercially available IVIG preparations from two different suppliers for polyomavirus neutralizing activity. BKV and mouse polyomavirus were used to infect human and murine host cells, respectively, with or without prior treatment with IVIG. Neutralization activity was measured by quantitation of viral DNA after 7 days in culture.
Results
Coincubation of BKV but not mouse polyomavirus with clinically relevant concentrations of IVIG derived from healthy and hepatitis B vaccinated subjects caused more than 90% inhibition of viral DNA yield after 7 days in culture. Consistent with a direct neutralizing mechanism, this effect was significantly diminished if viral infection was performed in immunoglobulin pretreated cells or if immunoglobulin treatment was delayed 2 hr after addition of infectious virus.
Conclusion
Human IVIG preparations contain BKV neutralizing antibodies. Data on neutralizing capacity of these antibodies are presented to aid dose exploration in clinical trials seeking to validate the use of IVIG in patients with BKV infection.
Keywords: Polyomavirus BK, Treatment, Immunoglobulins, Neutralization
Polyomavirus BK (BKV) infection can cause nephropathy in the allograft kidney (1–3). No well-established drug treatment is available at this time (4). Human intravenous immunoglobulins (IVIG) have been used as an empiric therapy, but currently available studies are difficult to evaluate, as no control arm has been included. In one frequently cited study, eight renal transplant recipients received a total dose of 2 g/kg IVIG with simultaneous reduction of immunosuppression. After a mean follow-up of 11.4 months, seven patients still had functioning grafts, but simultaneous reduction of immunosuppression makes it difficult to attribute this outcome directly to IVIG (5). The rationale behind administration of IVIG is that these preparations contain antibodies that can bind several human viruses including BKV (6). It is known from experimental data that BKV can elicit antibodies that can neutralize polyomavirus capsids (7). However, whether virus neutralizing antibodies are present in commercial IVIG preparations at titers that can significantly lower BKV load is unknown. This important gap in our knowledge needs to be filled before we can justify formal evaluation of this expensive therapy in controlled clinical trials. We evaluated five different lots of two commercially available human immunoglobulin (HIG) preparations for their ability to neutralize BKV in vitro.
MATERIALS AND METHODS
BK virus, Gardner strain, was obtained from The American Type Culture Collection (ATCC, Manassas, VA; ATCC VR837) and grown in a human fetal fibroblast cell line (WI-38 cells, ATCC CCL-75) (8). For the treatment of virus with IVIG, 1E+05 viral genome equivalents in 5 µL of medium were treated with an equal volume of immunoglobulin solution diluted to the desired concentration in culture medium and incubated at 37°C for 0.5, 1.0, or 2 hr, under 5% CO2. Antibody-neutralized BKV was used to infect 96-well tissue culture plates, wherein 5,000 WI-38 cells/200 µL per well had been plated 24 hr earlier. Infection was allowed to proceed for 2 hr after which unabsorbed virus was washed off and replaced with 200-µL fresh IVIG containing medium. In some experiments, IVIG was added after infection with untreated BKV particles had been allowed to proceed for 2.0-hr intervals. In still other experiments, WI-38 cells were pretreated with immunoglobulins for 2.0 hr at 30°C and then infected with BKV for 2 hr in the absence of immunoglobulin.
Two different IVIG preparations were tested:
HIG derived from healthy human subjects was manufactured by Gammagard (Baxter Inc., Westlake village, CA). This preparation is supplied as a 10 g/100-mL solution and was tested at final concentrations ranging from 10 to 100 µg/mL.
Hepatitis B immunoglobulin (HBIG) was obtained from Biotest Pharmaceuticals Corporation (Boca Raton, FL). This preparation was tested at concentrations ranging from 0.000187 to 0.267 IU/mL.
BKV treated with bovine serum albumin (cat. A2058; Sigma, St. Louis, MO) at a concentration of 10 µg/mL was used as a control. After infection of WI-38 cells with immunoglobulin treated virus, cultures were incubated for 7 days in Dulbecco's minimum essential medium medium, supplemented by 10% fetal bovine serum, l-glutamine, and the appropriate concentration of HIG or HBIG. The cells were harvested on day 7 using 0.25% trypsin-1 mM Na-EDTA digestion at 37°C for 10 min. DNA extraction on the cell lysates was performed with a commercially available kit (QIAamp 96 DNA Blood kit, cat. 51162; Qiagen, Valencia, CA) using the manufacture's instructions. A fragment of the BKV genome encoding the viral capsid protein-1 DNA was amplified by a TaqMan quantitative polymerase chain reaction (PCR) reaction performed in an Advanced Bioystems Inc, Prism 7700 Sequence Detector (Advanced Bioystems Inc, Foster City, CA) using a previously published protocol (9). Simultaneous quantitation of a cellular housekeeping gene, aspartoacylase, permitted monitoring of host cell replication, as a measure of potential cytotoxicity of the IVIG preparations tested. Viral or cellular DNA yield at day 7 in immunoglobulin-treated cells was expressed as a percentage of the control cells to obtain percentage of DNA inhibition.
Both immunoglobulin preparations were also tested against mouse polyomavirus (MPV) using an analogous set up. For these experiments, MPV LID-1 strain, (ATCC VR-252) was grown in a hamster baby kidney cell line (Baby hamster kidney [BHK] cells, ATCC CRL10314). Infection was performed by adding 1E+05 MPV genome equivalents to 5E+03 BHK cells per well. Cultures were maintained in Dulbecco's minimum essential medium medium, supplemented by 10% fetal bovine serum and 1% l-glutamine. Primers used for amplification of MPV DNA were designed using the MPV A2 DNA sequence (10). Host housekeeping DNA replication was monitored using primers targeted at [beta] actin. PCR amplification reactions were set up using the FastStart Universal SYBR Green Master (Roche, IN, Cat. 4913850001) containing 1 ng template DNA, 0.8 µM forward primer, and 0.7 µM reverse primer. PCR conditions consisted of incubation at 50°C for 2 min, followed by a first denaturation step of 10 min at 95°C, then 40 cycles of 95°C for 15 sec (denaturation), and 60°C for 1 min (reannealing and extension). Standard curves for the real-time PCR reaction were constructed using serial 10-fold dilutions of MPV strain A2 p53.A6.6 (pPy-1) plasmid (ATCC; ATCC 45017) or mouse genomic DNA (cat. G309A; Promega, Madison, WI). Statistical analysis of data was performed using paired t-tests as implemented in SigmaStat 3.01A (Systat Software Inc, Richmond, CA).
RESULTS
Analysis of experimental data indicated that BKV neutralized by HIG incubation for 2 hr at concentrations of 10.0 to 100 µg/mL caused 95.8% to 98.76% inhibition of BKV DNA yield after 7 days in culture (Table 1 and Fig. 1). These results reflect testing of four different lots of HIG purchased from the hospital pharmacy. In the time of infection experiments, infection with native BKV (BKV not pretreated with IVIG) for 2 hr before adding IVIG resulted in reduction in therapeutic effect (26.8%–46.0% inhibition of BKV DNA). This residual inhibition likely reflects IVIG interference with uptake of BKV that remained in the ambient medium beyond the first 2 hr of the experiment. Similar reduction in efficacy was observed if the cells were pretreated with IVIG for 2 hr before infection with native BKV (41.7%–49.9% inhibition in BKV DNA yield). Again, mild residual inhibition was observed presumably reflecting IVIG that remained cell bound at the time of addition of infectious BKV.
TABLE 1.
| BK viral DNA yield (genomic equivalents) |
Inhibition (%) |
Inhibition (%) |
|||||
|---|---|---|---|---|---|---|---|
| Human W138 cells | Not treated | IVIG treated | Viral DNA | Cell DNA | Mouse BHK570 cells |
Viral DNA (IVIG treated) |
Cell DNA (IVIG treated) |
| HIG: lot 1 | |||||||
| 10 µg/mL | |||||||
| Neutralized BKV | (4.85 E+ 06)±(1.70 E+ 05) | (2.01 E+ 05)±(2.10 E+ 05) | 95.85%±4.3%c | 1.8%±1.2% | Neutralized MPV | 0 | 0 |
| Native BKV | (7.65 E+ 05)±(1.56 E+ 05) | (4.70 E+ 05)±(2.40 E+ 04) | 38.56%±3.1%c | 5.2%±1.9%c | |||
| Pretreated cells | (1.49 E+ 06)±(2.19 E+ 05) | (8.66 E+ 05)±(2.47 E+ 04) | 41.91%±1.7%c | 12.1%±1.5%c | |||
| HIG: lot 2 | |||||||
| 10 µg/mL | |||||||
| Neutralized BKV | (4.85 E+ 06)±(1.70 E+ 05) | (6.03 E+ 04)±(2.99 E+ 04) | 98.76%±0.6%c | 0 | Neutralized MPV | 34.9%±8.4%c | 0 |
| Native BKV | (7.65 E+ 05)±(1.56 E+ 05) | (5.41 E+ 05)±(2.38 E+ 05) | 29.35%±31.1% | 5.9%±5.4% | 0 | ||
| Pretreated cells | (1.49 E+ 06)±(2.19 E+ 05) | (8.10 E+ 05)±(3.82 E+ 04) | 45.64%±2.6%c | 0 | |||
| HIG: lot 3 | |||||||
| 10 µg/mL | |||||||
| Neutralized BKV | (4.85 E+ 06)±(1.70 E+ 05) | (1.57 E+ 05)±(2.62 E+ 04) | 96.77%±0.54%c | 0 | Neutralized MPV | 0 | 5.3%±2.5%c |
| Native BKV | (7.65 E+ 05)±(1.56 E+ 05) | (5.60 E+ 05)±(3.01 E+ 05) | 27.32%±38.64% | 0 | |||
| Pretreated cells | (1.49 E+ 06)±(2.19 E+ 05) | (7.43 E+ 05)±(2.93 E+ 05) | 50.14%±19.65%c | 0 | |||
| HIG: lot 4 | |||||||
| 10 µg/mL | |||||||
| Neutralized BKV | (4.85 E+ 06)±(1.70 E+ 05) | (1.55 E+ 05)±(4.81 E+ 04) | 96.81%±1.00%c | 0 | Neutralized MPV | 0 | 16.1%±20.6% |
| Native BKV | (7.65 E+ 05)±(1.56 E+ 05) | (4.13 E+ 05)±(4.45 E+ 04) | 46.08%±5.83%c | 6.6%±15.5% | |||
| Pretreated cells | (1.49 E+ 06)±(2.19 E+ 05) | (2.95 E+ 06)±(1.19 E+ 06) | 0 | 8.5%±8.6% | |||
| HBIGd | |||||||
| 0.267 IU/mL | |||||||
| Neutralized BKV | (7.44 E+ 06)±(7.28 E+ 05) | (2.20 E+ 03)±(3.75 E+ 02) | 99.97%±0%c | 12.0%±1.1%c | Neutralized MPV | 7.8%±12.7% | 5.2%±1.4% |
| Native BKV | (6.42 E+ 06)±(2.08 E+ 06) | (5.10 E+ 06)±(6.51 E+ 05) | 20.57%±10.13%c | 0 | |||
| 0.0267 IU/mL | |||||||
| Neutralized BKV | (7.44 E+ 06)±(7.28 E+ 05) | (1.56 E+ 06)±(4.24 E+ 05) | 79.03%±5.7%c | 0 | Neutralized MPV | 16.8%±21.1% | 6.7%±6.8% |
| Native BKV | (6.42 E+ 06)±(2.08 E+ 06) | (7.13 E+ 06)±(1.77 E+ 06) | 4.29%±6.06% | 1.8%±0.3% | |||
| 0.00267 IU/mL | |||||||
| Neutralized BKV | (7.44 E+ 06)±(7.28 E+ 05) | (6.41 E+ 06)±(4.88 E+ 05) | 17.54%±6.56%c | 13.1%±3%c | Neutralized MPV | 0.6%±7% | 6.8%±8.3% |
| Native BKV | (6.42 E+ 06)±(2.08 E+ 06) | (7.03 E+ 06)±(8.20 E+ 05) | 0 | 1.35%±6% | |||
Data are presented as mean±SD derived from two to three experiments. Viral DNA yield refers to BKV genomic equivalent per culture well.
Pretreated cells and neutralized BKV refer to cells or virus incubated with immunoglobulin preparations before infection of cells. Native BKV was not treated.
P<0.05 compared with no virus infection controls (paired t test).
For hepatitis B immunoglobulin 0.267 IU/mL corresponds to a protein concentration of 42.7 µg/mL.
HIG, human intravenous immunoglobulin; HBIG, hepatitis B immunoglobulin; BKV, BK virus; IVIG, intravenous immunoglobulin; MPV, mouse polyomavirus.
Figure 1.
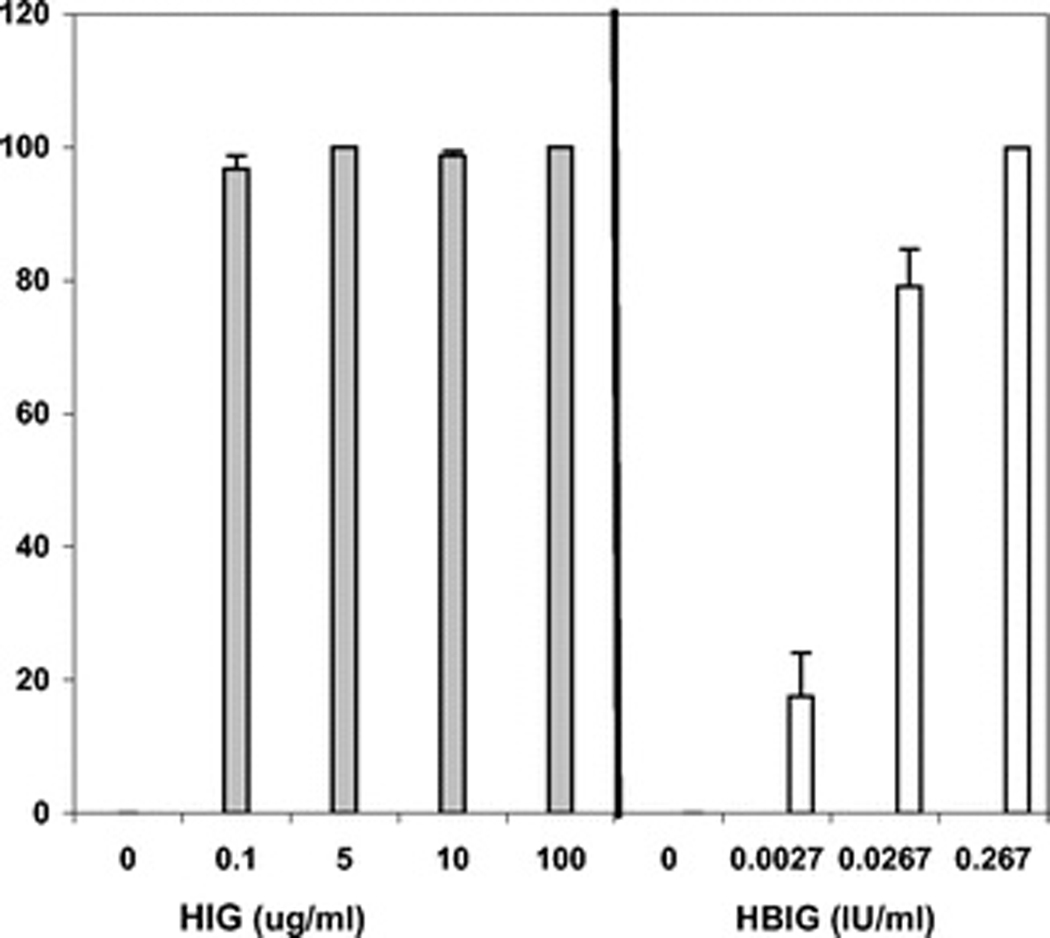
HBIG incubated with BKV for 2 hr at a final concentration 0.267 IU/mL resulted in 99% inhibition of viral DNA yield after 7 days in culture. Inhibition decreased to 79% at 0.0267 IU/mL and to 17% at a concentration of 0.00267 IU/mL. No antiviral effect was seen with bovine serum albumin run as a control at a protein concentration of 10 µg/mL. Reduction of BKV neutralization time from 2 to 0.5 hr or 1 hr resulted in less consistent effects of lower magnitude (data not shown). Inhibition of cellular DNA yield was less than 15% and possibly reflects impaired access of the cells to mitogenic stimuli in the tissue culture medium supplemented by IVIG.
In the experiments using MPV grown in BHK cells, only marginal antiviral effect was seen with HIG, which reached statistical significance only for lot 2. Inhibition in viral DNA yield varied from 0% to 34.9% at 10 µg/mL (Table 1, right side) and 17.7% to 30.7% at 100 µg/mL (data not shown in Table 1). The minor effects on viral DNA yield likely reflects partial cross reactivity between human and mouse viral epitopes recognized by antibodies in IVIG preparations. Neither HIG nor HBIG was cytotoxic to cultured mouse cells: inhibition of cellular DNA yield reached statistical significance only for HIG lot 3.
DISCUSSION
These data confirm that IVIG preparations have significant anti-BKV activity. Demonstration of this antiviral effect required that BKV virions and IVIG be first coincubated for at least 2 hr before infection. The effect was substantially reduced if infection was performed in IVIG pretreated cells or if IVIG treatment was delayed for 2 hr after addition of infectious virus. These observations are consistent with direct neutralization of BKV by virus-specific antibodies. The presence of such potent neutralizing antibodies in IVIG preparations derived from healthy subjects reflects the widespread prevalence of BKV in human populations. It is likely that these antibodies protect against viral reactivation and reinfection as long as the subject retains a healthy and functional immune system. Notably, these neutralizing antibodies were not as effective against MPV, for which man is not a normal host. It is known that MPV and BKV differ approximately 30% at the genomic level.
The experiments described also allowed us to conclude that pharmaceutical formulations of IVIG have sufficient antibody content to neutralize clinically relevant BKV loads. Thus, 50 ng (5 µL of a 10 µg/mL solution) of HIG was able to inactivate approximately 95% of a viral inoculum consisting of 1E+05 BKV virions. This implies that a single 5-g vial of HIG can neutralize a total of 9.5E+09 BKV genome equivalents or a circulating viral load of 1.9E+06 genomic copies per milliliter in a patient with a blood volume of 5000 mL. This estimate suggests that virus neutralization can be obtained with lower dose and expense than was described in a widely quoted study published by Sener et al. (5). The latter study used a dose of 2 g/kg, which would entail administration of 140 g of HIG (28 five-mL vials) in a man weighing 70 kg. A second study used a dose of 150 mg/kg, which is approximately 13 times smaller and closer to the minimal effective dose suggested by our in vitro experiments (11). An intermediate dose of 600 mg/kg for five doses given every 4 to 6 weeks has also been described (12) (13). Although determination of in vitro effective concentrations cannot be a substitute for clinical pharmacokinetic studies, experimental data can be a useful to starting point for planning exploratory clinical trials seeking to determine the optimal dose and duration of IVIG therapy for BKV viremia or nephropathy. Unfortunately, BK virus does not grow in small animal models and carefully designed direct testing in humans is the only practical option to move the field forward. Some clinical studies have found high antibody levels in patients with active BKV nephropathy (14) and question the role of these antibodies in clearing viral infection. However, it is not known whether the antibodies measured in these studies had neutralizing activity comparable with that seen in the IVIG preparations tested by us.
In the clinical setting, the efficacy of IVIG in patients with BKV would depend not only on the antibody titer but also on the ability of these antibodies to access the renal tubules, where viral replication occurs. Even if intracellular penetration does not occur, the introduction of neutralizing antibody in the peritubular capillary bed should reduce circulating viral load, limit cell to cell spread of virus, and allow the immune system to better cope with the viral infection. Proof of principle that antibodies can be useful in targeting viral infection has been obtained for other viruses (15, 16). In addition to direct neutralization of virus, other potential mechanisms by which antibodies can exert a salutary effect include steric hindrance to receptor binding, conformational change in the bound protein, complement-dependent cytotoxicity, viral agglutination, and antibody facilitated phagocytosis (17–19).
ACKNOWLEDGMENTS
The authors thank Jill March for secretarial assistance.
This work was supported by grants RO1 AI 51227 and AI 63360 and NIH contract AI 30044 (P.S.R.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Allergy and Infectious Disease.
Footnotes
The authors declare no conflicts of interest.
P.S.R., R.S., and K.S. participated in research design and participated in the writing of the manuscript; and N.F. and Y.H. participated in the performance of the research.
REFERENCES
- 1.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 2.Nickeleit V, Hirsch HH, Zeiler M, et al. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15:324. doi: 10.1093/ndt/15.3.324. [DOI] [PubMed] [Google Scholar]
- 3.Hariharan S. BK virus nephritis after renal transplantation. Kid Int. 2006;69:655. doi: 10.1038/sj.ki.5000040. [DOI] [PubMed] [Google Scholar]
- 4.Josephson MA, Williams JW, Chandraker A, et al. Polyomavirus-associated nephropathy: Update on antiviral strategies. Transpl Infect Dis. 2006;8:95. doi: 10.1111/j.1399-3062.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 5.Sener A, House AA, Jevnikar AM, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: One-year follow-up of renal allograft recipients. Transplantation. 2006;81:117. doi: 10.1097/01.tp.0000181096.14257.c2. [DOI] [PubMed] [Google Scholar]
- 6.Puliyanda D, Radha R, Amet N, et al. IVIG contains antibodies reactive with BK virus and may represent a therapeutic option for BKV nephropathy. Am J Transplant. 2003;3 suppl 5:359. [Google Scholar]
- 7.Randhawa P, Viscidi R, Carter JJ, et al. Identification of species-specific and cross-reactive epitopes in human polyomavirus capsids using monoclonal antibodies. J Gen Virol. 2009;90(pt 3):634. doi: 10.1099/vir.0.008391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farasati NA, Shapiro R, Vats A, et al. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation. 2005;79:116. doi: 10.1097/01.tp.0000149338.97084.5f. [DOI] [PubMed] [Google Scholar]
- 9.Randhawa PS, Vats A, Zygmunt D, et al. Quantitation of viral DNA in renal allograft tissue from patients with BK virus nephropathy. Transplantation. 2002;74:485. doi: 10.1097/00007890-200208270-00009. [DOI] [PubMed] [Google Scholar]
- 10.Lee EDH, Kemball CC, Wang J, et al. A mouse model for polyomavirus-associated nephropathy of kidney transplants. Am J Transplant. 2006;6:913. doi: 10.1111/j.1600-6143.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- 11.Wiseman AC. Polyomavirus nephropathy: A current perspective and clinical considerations. Am J Kidney Dis. 2009;54:131. doi: 10.1053/j.ajkd.2009.01.271. [DOI] [PubMed] [Google Scholar]
- 12.Smith JM, Jordan SC. Intravenous immunoglobulin as treatment for BK virus: Nephropathy. Pediatr Transplant. 2009;13:11. doi: 10.1111/j.1399-3046.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma AP, Moussa M, Casier S, et al. Intravenous immunoglobulin as rescue therapy for BK virus nephropathy. Pediatr Transplant. 2009;13:123. doi: 10.1111/j.1399-3046.2008.00958.x. [DOI] [PubMed] [Google Scholar]
- 14.Bohl DL, Brennan DC, Ryschkewitsch C, et al. BK virus antibody titers and intensity of infections after renal transplantation. J Clin Virol. 2008;43:184. doi: 10.1016/j.jcv.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Cleveland B, Klots I, et al. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol. 2008;82:638. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trkola A, Kuster H, Rusert P, et al. In vivo efficacy of human immunodeficiency virus neutralizing antibodies: Estimates for protective titers. J Virol. 2008;82:1591. doi: 10.1128/JVI.01792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakhashe SK, Thakar MR, Bharucha KE, et al. Quantitation of HLA proteins incorporated by human immunodeficiency virus type 1 and assessment of neutralizing activity of anti-HLA antibodies. J Virol. 2008;82:428. doi: 10.1128/JVI.00638-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konishi E, Kitai Y, Kondo T. Utilization of complement-dependent cytotoxicity to measure low levels of antibodies: Application to nonstructural protein 1 in a model of Japanese encephalitis virus. Clin Vaccine Immunol. 2008;15:88. doi: 10.1128/CVI.00347-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katpally U, Wobus CE, Dryden K, et al. Structure of antibody-neutralized murine norovirus and unexpected differences from viruslike particles. J Virol. 2008;82:2079. doi: 10.1128/JVI.02200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


