Abstract
Objectives
Genitourinary tract samples are required to investigate male HIV-1 infectivity. Because semen collection is often impractical, we evaluated the acceptability, feasibility, and validity of post-prostatic massage fluid/urine (post-PMF/U) for studying male genitourinary HIV-1 shedding.
Methods
HIV-1-seropositive men were evaluated after 48 hours of sexual abstinence. At each visit, a clinician performed prostatic massage, then post-PMF/U and blood were collected. Participants provided semen specimens one week later. An audio computer-assisted self-interview (ACASI) administered after each specimen collection evaluated acceptability, adherence to instructions, and recent genitourinary symptoms. HIV-1 RNA was quantified using a real-time PCR assay. Detection and quantitation of HIV-1 RNA and stability over visits were compared for semen, post-PMF/U, and blood.
Results
Post-PMF/U was successfully obtained at 106 visits (64%) and semen at 136 visits (81%, p<0.001). In ACASI, discomfort was rated higher for post-PMF/U collection (p=0.003), but there was no significant difference in acceptability. Detection of HIV-1 RNA in post-PMF/U was associated with detection in semen (p=0.02). Semen and post-PMF/U HIV-1 RNA levels were correlated (ρ=0.657, p<0.001). Concordance of results at repeat visits was 78.9% for post-PMF/U (κ = 0.519, p = 0.02), and 89.5% for both blood and semen (κ = 0.774, p = 0.001).
Conclusions
Although semen collections were more successful, both post-PMF/U and semen collections were acceptable to many participants. HIV-1 RNA detection and levels were closely associated in semen and post-PMF/U, and results were relatively stable across visits. To assess male HIV-1 infectivity, post-PMF/U may represent a valid alternative when semen cannot be obtained.
Keywords: HIV-1, semen, prostate, infectivity, validation
Introduction
Sex partners of seropositive men acquire HIV-1 infection via direct contact with semen.1 Studies in varied settings have identified cofactors that increase seminal HIV-1 shedding, including acute HIV-1 infection,2 co-infection with other sexually transmitted infections (STIs),3-5 and lack of suppressive antiretroviral therapy (ART).6,7 Important gaps in our knowledge of male infectivity limit our ability to optimize ART and other interventions to decrease HIV-1 transmission.8 Assaying semen represents the optimal way to evaluate male HIV-1 shedding, but studies have proven challenging due to difficulties collecting semen by masturbation in many populations and individuals.9 Logistical and transportation issues can increase time between semen collection and testing, thereby decreasing test result accuracy. Therefore, alternative sampling methods are needed to define the determinants of male HIV-1 shedding and to improve strategies to reduce transmission.
Optimal methods to sample the male genital tract remain unclear, especially for prospective studies requiring regular specimen collections. We have determined that distal genitourinary structures represent the major sources of male genital HIV-1 shedding, and found that HIV-1 RNA levels in combined urine/prostatic fluid collected after prostate massage, but not in pre-massage urine or other genitourinary fluids, independently predicted seminal HIV-1 RNA levels.10 Therefore, we hypothesized that post-prostate massage fluid/urine (post-PMF/U) might represent a useful proxy for direct measurement of HIV-1 in semen if further studies confirmed its utility. The goals of the present study were to evaluate the acceptability, feasibility, and validity of post-PMF/U collection as a proxy for measuring male genital tract HIV-1 shedding in place of semen.
Materials and Methods
Clinical
HIV-1-seropositive Kenyan men were recruited from a high-risk cohort.11 Participants were asked to provide paired genitourinary samples at up to five quarterly visits and to observe 48 hours of sexual abstinence before each collection. At post-PMF/U visits, participants were interviewed about recent sexual behaviors and health status. Blood was collected for CD4 count and plasma viral load testing. A standardized examination was performed, including urethral swab collection. After the examination, a male or female clinician performed prostatic massage until expressed prostatic secretions (EPS) were visualized at the urethral meatus or for up to 5 minutes. Approximately 5 mL of first-void post-PMF/U was collected in a sterile, wide-mouth, 50-cc conical tube. Two study clinicians (PG, LW) were trained on-site by our consultant urologist (JNK).
One week after each post-PMF/U visit, participants were asked to submit semen specimens. As recommended by Price et al,9 participants could provide semen in clinic or at home. Specimens were collected in sterile urine specimen cups or non-reactive condoms (Durex® Avanti, SSL International, Anderson, SC, USA). Participants were instructed to submit specimens to the laboratory within 2 hours of ejaculation.
Participants completed an audio computer-assisted self-interview (ACASI) in Kiswahili after each sample collection to evaluate acceptability, comfort, adherence to the prescribed abstinence period, and genitourinary symptoms in the past week. Reasons for collection failures were recorded using ACASI to ensure privacy.
Laboratory
Post-PMF/U was centrifuged at 500 rpm for 5 minutes. Supernatant aliquots were transferred into labelled 1.8-mL cryovials and stored at −70 °C until shipment to Seattle. Screening for infections and inflammation included leukocyte esterase dipstick (Multistix 10SG, Bayer Diagnostics Mfg., Ltd, Bridgend, UK), Gram stain (urethritis defined as >5 leukocytes per 100x field), Trichomonas vaginalis culture (In-Pouch TV®, BioMed Diagnostics, White City, Oregon), and Chlamydia trachomatis and Neisseria gonorrhoeae testing (Aptima GC/CT Detection System®, GenProbe Inc., San Diego, California).
Semen was processed immediately after submission. Volume, appearance and degree of liquefaction were noted, and the specimen was warmed in an incubator until complete liquefaction before processing. A wet-mount was examined under 40x power to estimate sperm motility. Aliquots were diluted in distilled water for haemocytometer sperm and round cell counts. Giemsa-stained slides were prepared for a round cell differential (% leukocytes / 100 cells).12,13 Remaining aliquots were stored at −70 °C until shipment to Seattle. Laboratory procedures were developed by a qualified andrologist (CHM), and specimen cell-counting quality assured by the University of Washington Male Fertility Laboratory.
CD4 counts were determined on-site using an automated method (FACS Count, Becton Dickinson, Forest Lakes, NJ). HIV-1 RNA was quantified in the University of Washington’s Retrovirology Laboratory using an independently validated real-time PCR assay. Lower limits of HIV-1 RNA quantification were 150 copies/mL in seminal plasma (semen) and 30 copies/mL in post-PMF/U and blood plasma (blood). If two complete sample sets were available for a participant, both sets were tested to evaluate stability of results over time.
Statistical
Characteristics of participants versus non-participants were compared using Pearson’s Chi-square, Fisher’s exact, or the Mann-Whitney U test. Acceptability and discomfort scores were compared using the Wilcoxon signed ranks test, and success rates with McNemar’s test. Factors associated with success of each sample collection type were evaluated by logistic regression using generalized estimating equations (GEE) with an exchangeable correlation matrix. Factors associated with success at p<0.10 were entered into a multivariate model.
Associations between HIV-1 RNA detection in semen, post-PMF/U, and blood were evaluated by Fisher’s exact test. Cohen’s kappa was used to measure stability of HIV-1 RNA detection across visits for 19 participants who had sample sets from repeat visits. Exact binomial methods were used to calculate sensitivity and specificity, including joint 95% confidence intervals (CI), for predicting seminal HIV-1 RNA detection (the “gold standard” for male HIV-1 infectivity).14 Potential factors affecting sensitivity and specificity of HIV-1 detection in post-PMF/U were analyzed using GEE and a method proposed by Pepe.14
Values below the quantification limit were set to half the lower limit for that specimen type. To assess within-subject variability, the standard deviation of the difference in log10 HIV-1 RNA was calculated across repeated visits for each sample type; this analysis was restricted to samples with detectable HIV-1 RNA at one or both visits. Despite log10-transformation, quantitative data on HIV-1 RNA levels were not distributed normally. Spearman’s rho was calculated to compare HIV-1 RNA levels in semen, post-PMF/U, and blood. Analyses employed Stata version 11.0 (StataCorp, College Station, Texas).
Ethical
Participants gave informed consent, following procedures approved by committees of the Kenya Medical Research Institute and University of Washington.
Results
Population
Between November 2007 and July 2009, 52 men enrolled (Table 1). Participants were older than the 43 men who attended clinic during the study period but did not enroll (median age 31.3 vs. 28.9 years, p=0.02). Participants were less likely to be single, although this difference was borderline (69.2% vs. 86.0%, p=0.06). There were no differences in education, sexual orientation, ethnicity, or religion between groups.
Table 1.
Participant Characteristics (N = 52)
| Characteristic | Median, range or N (%) |
|---|---|
| Age, years | 31, 19 – 55 |
| Education, years | 8, 0 – 16 |
| Marital status | |
| Single | 36 (69) |
| Married | 14 (27) |
| Divorced | 2 (4) |
| Religion | |
| Christian | 36 (69) |
| Muslim | 14 (27) |
| None | 2 (4) |
| Sexual orientation | |
| Heterosexual | 13 (25) |
| Bisexual | 28 (54) |
| Homosexual | 11 (21) |
| Transactional sex worker | 34 (65) |
Participants attended quarterly visits until July 31, 2009, completion of five visits, or enrolment in a sub-study of men initiating ART. Forty-seven men had visits contributing to the present analysis; the other five participated only in the sub-study. Participants attended a median of 4 visits (inter-quartile range [IQR], 2 – 5 visits). Overall, 167 of 208 (80.3%) expected visits were completed, and 29 men (61.7%) missed no visits. Nine participants (19.1%) were lost to follow-up and two (4.3%) withdrew due to relocation outside the clinic area.
Adherence and acceptability
Complete ACASI information was available for 128 of 167 full visits (76.6%) and for 111 of 136 semen collections (81.6%), and was missing in the remaining cases due to technical failures or non-completion of the ACASI questionnaire by participants. Reported adherence to the prescribed abstinence period was 79.5% at quarterly post-PMF/U collections and 96.4% at semen collections 1 week later. Median acceptability for post-PMF/U was 10 (IQR 10 – 10, range 0 – 10), with reservations reported at 8.4% of collections. For semen collections, median acceptability was 10 (IQR 10 – 10, range 0 – 10), with reservations reported at 6.4% of collections. There was no difference in acceptability by collection method (p=0.8). Median discomfort reported with post-PMF/U collection was 0 (IQR 0 – 0, range 0 – 9), with at least some discomfort reported at 20.5% of collections. For semen collections, median discomfort was 0 (IQR 0 – 0, range 0 – 7), with some discomfort reported at 3.7% of collections. The level of discomfort reported at paired visits was higher for post-PMF/U than for semen collection (p=0.003).
All men with successful collections stated that they would be willing to provide post-PMF/U again. Reasons documented for 46 post-PMF/U refusals (75.4% of unsuccessful collections) included discomfort (12), pain (6), embarrassment (6), and other (22). Difficulty producing semen was reported at two collections (1.8%), and all men who submitted semen said they would do so again. Reasons documented for 13 missed semen collections (41.9% of unsuccessful collections) included being too busy (2), forgetting the appointment (2), having penile pain (1), being unable to ejaculate (1), and other (7).
Success rate
Successful post-PMF/U collections occurred at 106 of 167 visits (63.5%), with EPS obtained during 62 collections (58.5%). At 4 visits, EPS was obtained but the participant was subsequently unable to provide post-PMF/U, possibly due to dehydration. Semen was collected after 136 of 167 visits (81.4%), a higher success rate than for post-PMF/U (p<0.001). Most semen samples were provided in clinic (85.3%) using sterile urine containers (67.0%). Both specimens were collected at least once from 35 men (74.5%), only semen from 8 men (17.0%) and only post-PMF/U from 3 men (6.4%). One man (2.1%) provided neither specimen. Semen processing took approximately 3 hours, and post-PMF/U processing <1 hour on average.
In univariate analysis, MSM and transactional sex workers were more likely to have successful post-PMF/U collections (Table 2). Success was less likely with the female clinician and for repeat visits. A multivariate GEE model showed that female clinician and repeat visits were associated with lower odds of successful post-PMF/U collection. In contrast, unsuccessful semen collection was associated with having a CD4 count ≤200 cells/μL (OR 0.35, 95% CI 0.14 – 0.88, p=0.03).
Table 2.
Factors Associated with Successful Post-Prostate Massage Fluid/Urine (Post-PMF/U) Sample Collection
| Factor | Univariate Modeling | Multivariate Modeling | ||
|---|---|---|---|---|
| Odd Ratio (95% CI) |
P Value | Odd Ratio (95% CI) |
P Value |
|
| Age, years | 0.95 (0.88 – 1.03) | 0.2 | ||
| Education, years | 0.94 (0.82 – 1.08) | 0.4 | ||
| Ever married | 0.68 (0.25 – 1.88) | 0.5 | ||
| Muslim religion | 0.97 (0.37 – 2.58) | 1.0 | ||
| Sex with men | 4.14 (1.27 – 13.46) | 0.02 | 3.67 (0.88 – 15.30) | 0.07 |
| Transactional sex | 2.29 (0.89 – 5.93) | 0.09 | ||
| Adherence to 48-hour abstinence* |
0.24 (0.01 – 4.19) | 0.3 | ||
| Female clinician† | 0.41 (0.21 – 0.82) | 0.012 | 0.43 (0.22 – 0.84) | 0.014 |
| Genitourinary symptoms‡ | 0.97 (0.44 – 2.17) | 0.9 | ||
| CD4 count ≤ 200** | 0.97 (0.44 – 2.13) | 0.9 | ||
| Visit number > 1 | 0.37 (0.20 – 0.70) | 0.002 | 0.36 (0.17 – 0.75) | 0.006 |
Generalized estimating equations (GEE) analysis, with factors associated with success at p<0.10 entered into multivariate modeling. Transactional sex was collinear with sex with men, and is omitted.
Missing data for 79 visits.
Missing data for 12 visits.
Defined as any report of genital discharge, pain, urinary frequency, dysuria, genital sores or growths. Missing data for 2 visits.
Missing data for 8 visits.
Technical Validation
HIV-1 RNA levels were compared for post-PMF/U and semen samples from 29 men who had provided samples through June 8, 2008 (Table 3). Of eight participants for whom paired samples were unavailable, six provided only post-PMF/U, one provided only semen, and one was unable to provide either sample. Ten participants were taking ART, including nine who received a standard first-line regimen of stavudine, lamivudine, and efavirenz and one who received a second-line regimen of tenofovir, abacavir, and boosted lopinavir. Additional sample sets were collected at repeat visits from 19 men, for a total of 48 paired sample sets.
Table 3.
Characteristics of 29 Men Providing Paired Genitourinary and Blood Samples
| Characteristic | Median (range) or N (%) | Reference or Expected Value |
|---|---|---|
| Age, years | 29 (18 – 53) | |
| Participants Not Taking ART | 19 (65) | |
| Blood HIV-1 RNA, copies/mL | 2,433 (15 – 29,847) | |
| Semen HIV-1 RNA, copies/mL | 737 (75 – 121,633) | |
| CD4 count, cells/μL | 491 (128 – 1,195) | |
| Participants Taking ART | 10 (35) | |
| Blood HIV-1 RNA, copies/mL | 15 (15 – 697) | |
| Semen HIV-1 RNA, copies/mL | 75 (75 – 75) | |
| CD4 count, cells/μL | 251 (163 – 962) | |
| Days between genitourinary samples | 7 (4 – 13) | 7 |
| Semen volume, mL | 1.5 (0.4 – 6.5) | ≥2.0* |
| Semen cell count × 106 cells/mL | ||
| Leukocytes | 0.01 (0 – 10.9) | ≤1* |
| Sperm | 44.3 (0.6 – 449.4) | ≥20* |
| Leukocytospermia† | 2 (7) | 0 |
| Motile sperm present | 28 (97) | 100% |
| EPS obtained during prostate massage | 18 (62) | 80% |
| Post-PMF/U volume, mL | 7.0 (1.2 – 26.0) | 5.0 |
| Leukocyte esterase positive | 1 (3) | 0 |
| STI diagnosed** | 0 | 0 |
Normal references ranges for semen adapted from the WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, Fourth Edition (1999). Cambridge University Press, New York.
Leukocytospermia was defined as >1 × 106 leukocytes/mL of semen, per the above reference.
See methods for details of STI screening performed.
ART = antiretroviral therapy
EPS = expressed prostatic secretions
post-PMF/U = post-prostate massage fluid/urine
RNA = ribonucleic acid
STI = sexually transmitted infections
HIV-1 RNA Detection
At initial visits, HIV-1 RNA was detected in post-PMF/U alone from two men (7%), semen alone from five men (17%), and both specimens from eight men (28%). In contrast, HIV-1 RNA was detected in blood only from six men (21%), semen only from no man (0%), and both specimens from thirteen men (45%). HIV-1 RNA was undetectable in post-PMF/U and semen in all ten men taking ART, but was detectable in three blood samples (30%). HIV-1 RNA detection in post-PMF/U was associated with HIV-1 RNA in semen (p=0.02).
Stability of HIV-1 RNA Detection
Concordance of test results at 19 repeat visits was 78.9% for post-PMF/U (κ = 0.519, p = 0.02), and 89.5% for both blood and semen (κ = 0.774, p = 0.001). For the nine men taking ART who had repeat visits, concordance was 100% for both semen and post-PMF/U, and 90% for blood (κ = 0.727, p = 0.02).
Specimen Performance
Post-PMF/U sensitivity for predicting seminal HIV-1 was 61.5%, with 87.5% specificity (joint 95% CI, [28.3% – 88.3%] × [58.0% – 98.9%]). The sensitivity of blood for predicting seminal HIV-1 was 100%, with 62.5% specificity (joint 95% CI, [71.4% – 100%] × [32.3% – 86.9%]). The full dataset of 48 sample sets was explored for factors associated with post-PMF/U sensitivity and specificity, including disease stage (i.e., HIV-1 RNA level in blood, CD4 cell count, ART status) and collection technique (i.e., time since last ejaculation, whether EPS was obtained, volume of post-PMF/U collected, and clinician obtaining the sample). Relative post-PMF/U sensitivity increased by 1.35 (95% CI, 1.04-1.75) for each log10 increase in blood HIV-1 RNA level. No other factor was found to influence the sensitivity or specificity of HIV-1 detection in post-PMF/U.
HIV-1 RNA Quantitation
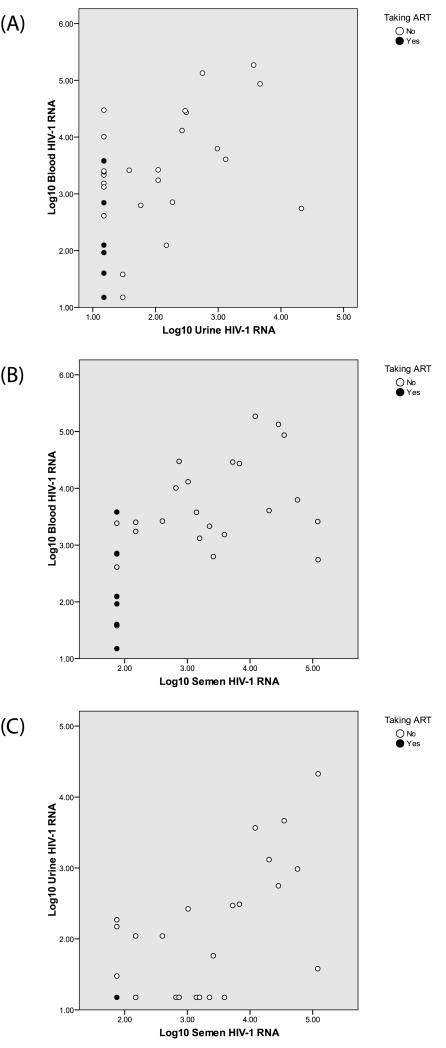
At the initial visit, 10 of 29 men (34.5%) had detectable post-PMF/U RNA with a median level of 222.5 copies/mL (range, 30–21,221 copies/mL). Thirteen men (44.8%) had detectable semen RNA with a median level of 3,901 copies/mL (range, 150–121,633 copies/mL). Figure 1 presents log10 HIV-1 RNA levels for the 48 sample sets. Correlations between semen and post-PMF/U RNA level (ρ = 0.657, p <0.001), post-PMF/U and blood RNA level (ρ = 0.502, p = 0.006), and semen and blood RNA level (ρ = 0.792, p <0.001) were all highly significant.
Figure 1.
Comparison of Log10-Transformed HIV-1 RNA Levels in (A) Blood and Urine, (B) Blood and Semen, and (C) Urine and Semen
Stability of HIV-1 RNA level
Among 19 participants with repeat visits, the standard deviation of differences in log10 HIV-1 RNA were 1.00 for post-PMF/U, 0.88 for semen, and 1.29 for blood when restricted to paired samples in which HIV-1 RNA was detected in both samples. When restricted to paired samples in which HIV-1 RNA was detected in either sample, the standard deviations of the differences in log10 HIV-1 RNA were 1.05 for post-PMF/U, 0.99 for semen, and 1.25 for blood. Results were similar (1.05 for post-PMF/U, 0.99 for semen, and 1.36 for blood) when restricted to men not taking ART.
Discussion
We validated HIV-1 RNA level in post-PMF/U as a novel proxy for direct measurement of HIV-1 RNA in semen and showed that the male genital tract can be sampled without collecting semen specimens by masturbation. Both post-PMF/U and semen collections proved acceptable to many of the Kenyan men who participated. There was good retention in this protocol requiring multiple visits, with 80% of expected visits completed. Post-PMF/U collection ensured successful sampling from certain participants who could not provide semen. Most participants rated discomfort as low for both sampling methods. However, participants expressed more discomfort with post-PMF/U, and discomfort, pain, and embarrassment were causes of post-PMF/U refusal. Success was less likely on repeat visits or when the clinician was female. There was also a suggestion that heterosexual men were less likely to provide post-PMF/U, although the small number of heterosexual participants limited our evaluation of this factor.
Despite initial concerns that Kenyan men would not find semen collection by masturbation acceptable, successful semen collection in this study compared favorably with a previous study of semen collection in Malawi, in which 145 of 212 men (68.4%) asked successfully provided samples.9 In that population of mostly heterosexual men attending STI and dermatology clinics, single men were more successful at providing samples, and having a genital ulcer was associated with failure. In contrast, our population included mostly MSM and transactional sex workers, with lower CD4 count proving the only factor related to unsuccessful semen collection. Decreased semen quality has been noted among HIV-1-seropositive men with advanced immunosuppression.15, 16 It is possible that poor overall health, decreased libido, lower testosterone levels, or neurological dysfunction associated with more advanced HIV infection impaired ejaculation in some men. Our study likely underestimates the extent of this problem, as men with a history of ejaculatory difficulties are unlikely to enroll in studies requiring provision of semen samples.
For men who cannot provide or are unwilling to provide semen specimens, post-PMF/U represents an alternative to semen for sexual transmission studies requiring large sample size or repeated evaluations. To evaluate this approach, we developed an algorithm to obtain post-PMF/U under standardized conditions, then successfully implemented this algorithm in an African clinical research setting. Specimen collection, storage, transportation, and laboratory testing of post-PMF/U all proved substantially less complicated and time consuming than direct semen evaluation. Because our initial study of post-PMF/U HIV-1 RNA measurement included only 10 men who provided paired samples at one visit and was restricted to North American men with demonstrated seminal HIV-1 shedding,10 the current study provided critical data validating post-PMF/U as a promising proxy in a different population.
Participants represented a broad range of disease status, including some with undetectable blood or seminal HIV-1 RNA due to ART. Blood plasma HIV-1 RNA levels were relatively low even in untreated men, who were healthy volunteers able to provide semen. When men with very low genital HIV-1 levels are studied, results are influenced by the HIV-1 RNA quantification limits in semen as compared to other fluids such as blood or urine.17 Dilution of HIV-1 RNA in urine samples led to lower absolute values of HIV-1 RNA, but the volume of urine collected did not affect sensitivity, specificity, or quantification despite variation above and below the requested sample volume.
Although some men experienced discomfort, embarrassment, or pain during post-PMF/U collection, many participants who provided post-PMF/U samples stated they would do so again, and most provided multiple samples. Three men who were unable to provide semen samples only provided post-PMF/U. Training of clinicians was completed in one day, and both clinicians felt comfortable with the procedure after performing massage on two or three volunteer patients. Post-PMF/U processing was simpler technically, and detection of inflammation was much more straightforward than for semen. Processing time for post-PMF/U was also much shorter. Therefore, for certain men and in certain circumstances (e.g., during wound healing after male circumcision, when a painful ulcer is present, in men with lower CD4 counts, in men with difficulty providing semen), post-PMF/U may represent a valuable alternative to semen collection in research settings requiring genitourinary sampling.
Studies of genital HIV-1 shedding are limited by a lack of definitive data on the minimal level of seminal HIV-1 RNA required for transmission. We do not know if seminal HIV-1 RNA level represents an independent predictor of transmission at a given blood plasma viral load. However, HIV-1 RNA levels above 104 copies/mL in semen have been associated with the ability to culture virus from this compartment,18 and seminal HIV-1 RNA level has been associated with HIV-1 transmission in at least one prospective trial.19 Defining the precise relationship between seminal HIV-1 RNA levels and sexual transmission will require frequent sampling of seminal fluid close to the time of sexual transmission. Our results suggest that the post-PMF/U method may help overcome this sampling hurdle by providing a valid alternative when semen cannot be obtained.
Other limitations of this study include limited sample size, uniqueness of the population studied, incomplete ACASI data, and lack of randomization of collection method order, which might have caused minor differences in HIV-1 RNA levels, particularly if adherence to the prescribed abstinence period differed. Time since last ejaculation did not independently influence HIV-1 RNA levels in post-PMF/U. This observation supports the hypothesis that most HIV-1 replication occurs in periurethral tissue rather than in glandular structures contributing to the ejaculate.10 The consistency of our results supports the usefulness of post-PMF/U as a proxy for semen.
In conclusion, post-PMF/U represents a valid alternative specimen to assess HIV-1 RNA shedding in semen. If this approach proves acceptable in other populations, then post-PMF/U collection could provide a valuable tool to investigate male genital tract HIV-1 infectivity when semen cannot be obtained.
Key messages.
Our objective was to evaluate post-prostatic massage fluid/urine (post-PMF/U) as an alternative to semen for studying male genitourinary HIV-1 shedding.
Although semen collections were more successful, both post-PMF/U and semen collections proved acceptable to many participants.
Semen and post-PMF/U detection and levels were closely associated, and results were relatively stable across visits.
To assess male HIV-1 infectivity, post-PMF/U may represent a valid alternative when semen cannot be obtained.
Acknowledgements
We thank the research staff in Kenya for their dedication, the Kenya Medical Research Institute (KEMRI) and the International AIDS Vaccine Initiative for provision of clinic and laboratory space, Coast Provincial General Hospital for provision of laboratory space, and the Director, KEMRI, for permission to publish this research. We are indebted to the men who participated in this study.
Funding. Supported by from the National Institutes of Health, grants R21 HD055864 and K23 AI69990 (SMG). Additional support was received from the University of Washington Center for AIDS Research (CFAR), an NIH-funded program (P30-AI027757) which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NCCAM). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Competing interests. None declared for all authors.
Licence agreement. The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in STI and any other BMJPGL products and sub-licences such use and exploit all subsidiary rights, as set out in our licence: http://group.bmj.com/products/journals/instructions-for-authors/licence-forms.
These data were presented in part at the 5th IAS Conference on HIV Pathogenesis, Treatment, and Prevention, Cape Town, South Africa, July 2009.
References
- 1.Ho DD, Schooley RT, Rota TR, et al. HTLV-III in the semen and blood of a healthy homosexual man. Science. 1984;226:451–453. doi: 10.1126/science.6208608. [DOI] [PubMed] [Google Scholar]
- 2.Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–845. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 4.Hobbs MM, Kazembe P, Reed AW, et al. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex Transm Dis. 1999;26:381–387. doi: 10.1097/00007435-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Price MA, Zimba D, Hoffman IF, et al. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial. Sex Transm Dis. 2003;30:516–522. doi: 10.1097/00007435-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Gupta P, Mellors J, Kingsley L, et al. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernazza PL, Gilliam BL, Flepp M, et al. Effect of antiviral treatment on the shedding of HIV-1 in semen. AIDS. 1997;11:1249–1254. doi: 10.1097/00002030-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17:455–480. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 9.Price MA, Cohen MS, Hoffman IF, et al. Collecting the essence of man: semen collection for HIV transmission studies in sub-Saharan Africa. Sex Transm Infect. 2005;81:185–186. doi: 10.1136/sti.2004.012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coombs RW, Lockhart D, Ross SO, et al. Lower genitourinary tract sources of seminal HIV. J Acquir Immune Defic Syndr. 2006;41:430–438. doi: 10.1097/01.qai.0000209895.82255.08. [DOI] [PubMed] [Google Scholar]
- 11.Sanders EJ, Graham SM, Okuku HS, et al. HIV-1 infection in high risk men who have sex with men in Mombasa, Kenya. AIDS. 2007;21:2513–2520. doi: 10.1097/QAD.0b013e3282f2704a. [DOI] [PubMed] [Google Scholar]
- 12.Muller CH, Coombs RW, Krieger JN. Effects of clinical stage and immunological status on semen analysis results in human immunodeficiency virus type 1-seropositive men. Andrologia. 1998;30(Suppl 1):15–22. doi: 10.1111/j.1439-0272.1998.tb02821.x. [DOI] [PubMed] [Google Scholar]
- 13.Krieger JN, Coombs RW, Collier AC, et al. Seminal shedding of human immunodeficiency virus type 1 and human cytomegalovirus: evidence for different immunologic controls. J Infect Dis. 1995;171:1018–1022. doi: 10.1093/infdis/171.4.1018. [DOI] [PubMed] [Google Scholar]
- 14.Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford University Press; New York, NY: 2003. [Google Scholar]
- 15.Crittenden JA, Handelsman DJ, Stewart GJ. Semen analysis in human immunodeficiency virus infection. Fertil Steril. 1992;57:1294–1299. [PubMed] [Google Scholar]
- 16.Politch JA, Mayer KH, Abbott AF, et al. The effects of disease progression and zidovudine therapy on semen quality in human immunodeficiency virus type 1 seropositive men. Fertil Steril. 1994;61:922–928. doi: 10.1016/s0015-0282(16)56707-7. [DOI] [PubMed] [Google Scholar]
- 17.Loftis A, Kshatriya R, McCall-Culbreath K, et al. Optimization of Abbott m2000 RealTime HIV-1 viral load assay on breastmilk, dried blood spots, seminal plasma, and cerebrospinal fluid [MOPDB103]. Poster presented at: 5th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; Cape Town, South Africa. July 19–22, 2009. [Google Scholar]
- 18.Coombs RW, Speck CE, Hughes JP, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 19.Baeten J, Kahle E, Lingappa J, et al. Genital HIV-1 RNA concentrations and heterosexual HIV-1 transmission risk [LBPEA07]. Poster presented at: 5th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; Cape Town, South Africa. July 19–22, 2009. [Google Scholar]