Abstract
The etiologies of the inflammatory bowel diseases (IBD; Crohn’s disease, ulcerative colitis) have not been fully elucidated. However, there is very good evidence implicating T cell and T cell trafficking to the gut and its associated lymphoid tissue as important components in disease pathogenesis. The objective of this review is to provide an overview of the mechanisms involved in naive and effector T cell trafficking to the gut-associated lymphoid tissue (GALT; Peyer’s patches, isolated lymphoid follicles), mesenteric lymph nodes and intestine in response to commensal enteric antigens under physiological conditions as well as during the induction of chronic gut inflammation. In addition, recent data suggests that the GALT may not be required for enteric antigen-driven intestinal inflammation in certain mouse models of IBD. These new data suggest a possible paradigm shift in our understanding of how and where naive T cells become activated to yield disease-producing effector cells.
Keywords: Peyer’s patches, mesenteric lymph nodes, lymphotoxin, isolated lymphoid follicles, integrins, selectins, Crohn’s disease, ulcerative colitis, inflammatory bowel disease
Introduction
The inflammatory bowel diseases (IBD; Crohn’s disease, ulcerative colitis) are chronic idiopathic inflammatory disorders of the intestine and/or colon in which patients suffer from rectal bleeding, severe diarrhea, abdominal pain, fever, and weight loss. Histologic examination of biopsies obtained from patients with active disease reveals the presence of large numbers of leukocytes such as polymorphonuclear leukocytes (PMNs), lymphocytes, and monocytes in the intestinal and/or colonic interstitium. Coincident with this inflammatory infiltrate is extensive intestinal injury including edema, loss of goblet cells, decreased mucus production, crypt cell hyperplasia, erosions, and ulcerations. Although the etiology of IBD has not been fully elucidated, there is growing clinical and experimental evidence to suggest that the initiation and perpetuation of these inflammatory disorders involves a complex interaction among genetic, immune, and environmental factors.1,2 Regardless of how these interactions converge to induce chronic intestinal inflammation, it is becoming increasingly appreciated that the adaptive immune system in general and T cells in particular play crucial roles in disease pathogenesis. Indeed, data obtained from several different laboratories over the past 15 years, using a variety of immune-manipulated and genetically engineered mouse models of IBD, suggest that chronic gut inflammation results from a dysregulated immune response to components of the commensal (nonpathogenic) flora.1–6 Thus, the ability of the host to regulate the movement of T cells into the gut-associated lymphoid tissue (GALT), mesenteric lymph nodes (MLNs), and gut is of increasingly recognized importance in the induction and perpetuation of chronic intestinal inflammation.
T cell trafficking to the GALT and gut
To mount a protective immune response to enteric pathogens, intravascular naive T cells must home to the inductive sites of the intestinal tract called the GALT[Peyer’s patches (PPs), isolated lymphoid follicles] as well as the gut-draining MLNs where they undergo antigen-driven priming/activation, polarization, and expansion to yield Th1 and/or Th17 effector cells.7–14 These effector cells then exit the lymphoid tissue via the efferent lymphatics, enter the systemic circulation, and home to the gut where they help to destroy the invading pathogens (Fig. 1). A number of different animal studies suggest that in the absence of appropriate regulatory mechanisms, this same sequence of events may occur in response to commensal (nonpathogenic) bacteria resulting in enteric antigen-dependent induction of chronic intestinal inflammation.3,4,9 Because much of the current evidence demonstrates that antigen-loaded dendritic cells (DCs) are transported to the MLNs from the intestinal lamina propria and PPs via afferent lymphatics, investigators suggest that MLNs may function as the primary gut-associated inductive site where naive T cells first encounter enteric antigens and are activated to disease-producing effector cells (Fig. 1).9,11,13–16 Thus, it has been proposed that naive T cells must first home to the MLNs where enteric antigen-loaded DCs prime, polarize, and expand these lymphocytes to yield colitogenic Th1 and/or Th17 effector cells.17,18 Trafficking of intravascular T cells to lymphoid and intestinal tissue is a complex process that is controlled by a sequence of three molecularly distinct adhesion and signaling steps that include tethering of T cells to the endothelial surface, rolling along the endothelial cell surface, and finally activation-induced firm adhesion of the lymphocytes to the endothelium.
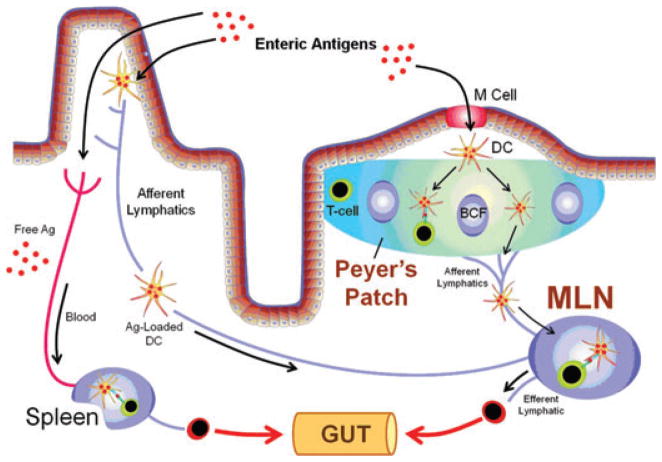
Figure 1.
Intestinal mucosal immune responses to enteric bacterial antigens. Enteric bacterial antigens may enter the Peyer’s patches (PPs) via transport by the M cells where they are endocytosed by dendritic cells (DCs) within the subepithelial dome region. Antigen-loaded DCs may then interact with T cells within the PPs to prime the lymphocytes or they may migrate from the PPs to the gut-draining mesenteric lymph nodes (MLNs) by way of the afferent lymphatics. Naive T cells that enter the MLNs may interact with these antigen-loaded DCs resulting in the priming, polarization, and expansion of the T cells to yield effector cells. These effector cells then exit the MLNs via the efferent lymphatics, return to the systemic circulation, and home to the gut lamina propria. Enteric antigens may also be endocytosed by DCs within the gut lamina propria and migrate to the MLNs via the afferent lymphatics. Finally, free enteric antigens may be absorbed by the intestinal blood supply and transported to the spleen by way of the systemic circulation. Once in the spleen, free antigen may be processed and presented by splenic DCs thereby initiating potential immune responses within the spleen. Modified from Mowat et al.,13 with permission.
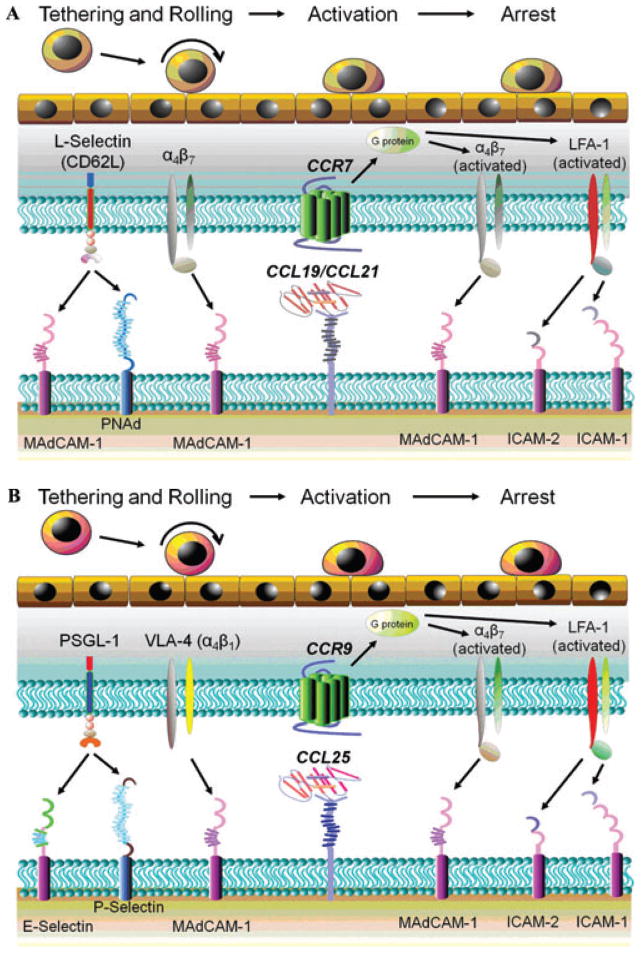
Migration of naive T cells from the blood to the secondary lymphoid tissues is thought to occur exclusively by way of high endothelial venules (HEVs) that are composed of specialized postcapillary venular endothelial cells in these tissues.7,19–22 HEVs associated with PPs contain mucosal addressin cell adhesion molecule-1 (MAdCAM-1) whereas HEVs within MLNs express both MAdCAM-1 and peripheral lymph node addressin (PNAd). It is thought that T cell–associated L-selectin binds to both MAdCAM-1 and PNAd to tether the lymphocytes to the HEVs to initiate T-cell rolling. Although the L-selectin/MAdCAM-1 and L-selectin/PNAd interactions are thought to mediate much of the T-cell tethering and rolling in the MLNs, α4β7 may be used by naive T cells to tether themselves to MAdCAM-1, thereby providing additional tethering capacity for these cells in both PPs and MLNs (Fig. 2A).21,22 Interaction of T cell–associated chemokine receptor CCR7 with secondary lymphoid tissue chemokine (SLC; CCL21) and/or Epstein Barr virus induced gene-1 ligand chemokine (ELC; CCL19) presented on the luminal surface of the HEVs activates lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) and α4β7 on the naive T cells to bind to intercellular adhesion molecule-1 (ICAM-1) and MAdCAM-1, respectively. These interactions promote firm adhesion and lymphocyte arrest, which ultimately leads to T cell extravasation into the MLNs and PPs (Fig. 2A).20–23 Once naive CD4+ T cells enter the MLNs (and PPs), they may encounter their cognate enteric antigens presented on the surface of DCs in association with major histocompatability complex class II (MHC II). In a process that is not well understood, T cells move into close proximity of the DCs, ultimately binding to the MHC-Ag complex via its T cell receptor, thereby initiating cell activation. During this initial priming/activation process the T cell will proliferate, shed its L-selectin, and enhance surface expression of specific “gut-homing” adhesion molecules such as α4β7 and CCR9 as well as LFA-1, very late antigen-4 (VLA-4; α4β1), and CD44.20–23 Data obtained from previous studies using different animal models of chronic gut inflammation suggest that a dysregulated immune response to the commensal enteric antigens results in the polarization of naive T cells to colitogenic effector cells such as Th1 and Th17 effector cells.4–6
Figure 2.
T cell trafficking to the MLNs and intestine. (A) MLNs. Naive T cell–associated l-selectin binds to both MAdCAM-1 and PNAd to tether these lymphocytes to the high endothelial venules (HEVs) to initiate rolling along the HEVs. Although the l-selectin/MAdCAM-1 and l-selectin/PNAd interactions are thought to mediate much of the T cell tethering and rolling in the MLNs, α4β7 may also be used by naive T cells to tether themselves to MAdCAM-1, thereby providing additional tethering capacity for these cells in the MLNs. Interaction of T cell–associated chemokine receptor CCR7 with secondary lymphoid tissue chemokine (SLC; CCL21) and/or Epstein Barr virus induced gene-1 ligand chemokine (ELC; CCL19) presented on the luminal surface of the HEVs activates lymphocyte function-associated antigen-1 (LFA-1) and α4β7 on the naive T cells to bind to ICAM-1 (or ICAM-2) and MAdCAM-1, respectively. These interactions promote firm adhesion and lymphocyte arrest, which ultimately leads to T cell extravasation into the MLNs. (B) Intestine. Initial tethering and rolling of effector T cells on the postcapillary venular endothelial cells in the gut begins with the interaction between lymphocyte-associated P-selectin glycoprotein ligand-1 (PSGL-1) and venular P- and/or E-selectin. In addition, there is evidence to suggest that very late antigen-4 (VLA-4; α4β1) also contributes to effector cell rolling via its interaction with MAdCAM-1. Interaction of CCR9 with venular CCL25 promotes the conformational activation of LFA-1 and α4β7, thereby enhancing effector cell binding to ICAM-1 (or ICAM-2) and MAdCAM-1, respectively, and promoting infiltration of these effector cells into the lamina propria. Part B modified from von Andrian and Mackay,19 with permission.
Following the initial priming/activation step within the GALT/MLNs, the antigen-experienced effector T cells reenter the systemic circulation via the efferent nodal lymphatics where they will home to the gut. This new pattern of homing to the small intestine is thought to be mediated by the interaction between α4β7 and CCR9 with postcapillary venular MAdCAM-1 and CCL25, respectively. However, the interactions of lymphocyte-associated P-selectin glycoprotein ligand-1 (PSGL-1), LFA-1, α4β1, and CD44 with venular P/E-selectin, ICAM-1 (and ICAM-2), VCAM-1, and hyaluronate, respectively are known to be important as well (Fig. 2B).20–23 The mechanisms responsible for “imprinting” the recruitment of T cells to the gut following antigen stimulation within the GALT are not known with certainty. However, it is thought that effector T cells are exposed to specific signals resulting from subtle differences in antigen–T cell interactions as well as the local environment of the lymphoid tissue that imprint gut-specific homing receptors.20–23 For example, it is well-appreciated that T cells activated within PPs and MLNs express high levels of α4β7 as well as the chemokine receptor CCR9 that bind to their respective ligands MAdCAM-1 and CCL25/TECK, both of which are constitutively expressed on intestinal endothelial cells and are thought to promote homing of T cells to the gut (Fig. 2B).20–23 Much of what is known regarding the mechanisms by which effector T cells traffic to intestinal tissue has come from studies focused on the small bowel. However, very little is known regarding the specific T cell and venular molecular determinants that govern T-cell trafficking to the colon. It is thought that both α4β7 and α4β1 are important for T cell migration to the inflamed large bowel; however it remains unclear whether CCR9/CCL25 interactions are important for induction and/or perpetuation of colonic inflammation.
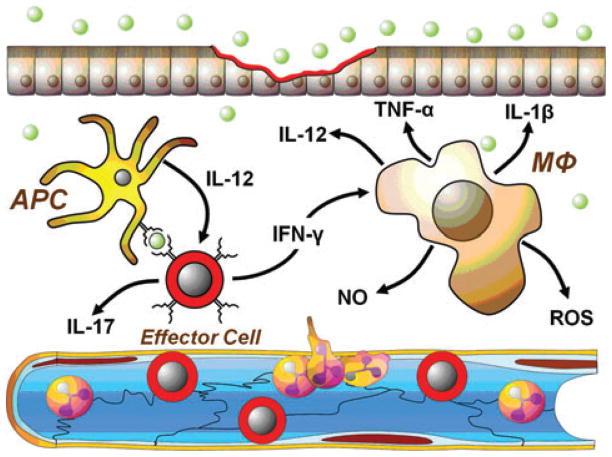
Once the effector T cells enter the gut interstitium, they re-encounter their specific antigen presented on a more diverse population of antigen presenting cells (APCs) such as macrophages and B cells as well as DCs (Fig. 3). This secondary antigen-specific interaction results in a more rapid and vigorous response of the effector T cells, thereby dramatically enhancing the production of IFN-γ, IL-17, TNF-α, lymphotoxin-α, and IL-2. IL-2 promotes the clonal expansion of T cells and enhances the function of helper T and B cells whereas IFN-γ interacts with and activates APCs and macrophages to produce additional IL-12. IFN-γ, TNF-α, and IL-17 will activate endothelial cells and enhance endothelial cell adhesion molecule expression on the postcapillary venular endothelium (Fig. 3). In addition, IFN-γ-activated macrophages produce large amounts of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8, IL-12, and IL-18 as well as reactive oxygen and nitrogen metabolites (e.g., superoxide, hydrogen peroxide, nitric oxide) (Fig. 3). The net result of this type of uncontrolled production of Th1/Th17- and macrophage-derived inflammatory mediators is the recruitment and activation of additional leukocytes (e.g., PMNs, monocytes, macrophages, and lymphocytes) in the gut tissue leading to the induction of chronic gut inflammation (Fig. 3).
Figure 3.
Effector T cells interact with a variety of antigen presenting cells (APCs), such as DCs, macrophages, and/or B cells presenting enteric antigen to initiate intestinal inflammation. APC-induced activation of effector cells promotes the production of large amounts of IFN-γ, IL-1β, IL-12, IL-17, and TNF-α. IFN-γ effectively activates macrophages to produce large amounts of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-12, and IL-17 as well as reactive oxygen species (ROS) and nitric oxide (NO). In addition, IFN-γ, TNF-α, ROS, and IL-17 activate endothelial cells and enhance endothelial cell adhesion molecule expression on the postcapillary venular endothelium. The net result of this uncontrolled production of Th1/Th17- and macrophage-derived inflammatory cytokines is the recruitment and activation of granulocytes/myeloid cells (e.g., PMNs, monocytes, and macrophages) as well as additional T and B cells into the gut tissue leading to the induction and perpetuation of chronic gut inflammation.
T cell–associated adhesion molecules and chronic gut inflammation
The obvious importance of T cell trafficking in the pathogenesis of IBD has prompted numerous studies to identify which of the many different adhesion molecules and adhesive interactions is/are important for the induction and/or perpetuation of intestinal inflammation. Several different adhesion molecules have been suggested as important cellular determinants for induction of colonic inflammation using erosive (chemically induced), self-limiting models of acute colitis. However, few if any of these molecules have been shown to be required for the induction or perpetuation of chronic gut inflammation. For example, T cell–associated CD62L and PSGL-1 have been shown to be important cellular determinants in the pathogenesis of colonic inflammation using short-term hypersensitivity or erosive self-limiting models of acute colitis.24–29 However, our laboratory has failed to demonstrate a requirement for either of these adhesion molecules in a T cell-dependent mouse model of chronic gut in-flammation.30,31 Several other T cell–associated integrins (e.g., LFA-1, α4β1, α4β7) have been suggested to be important determinants for induction of gut inflammation. We have found that LFA-1(CD11a/CD18) plays an important role in acute colitis as CD11a (LFA-1)-deficient mice develop significantly less colitis in response to short-term, oral administration of the erosive polymer dextran sulfate sodium (DSS).32 In addition, we have demonstrated a critical role for T cell–associated LFA-1 in the development of chronic colitis induced by adoptive transfer of naive T cells. We found that transfer of CD11a−/−(LFA-1−/−) CD4+CD45RBhigh T cells into immunodeficient recombinase activating gene-1 (RAG−/−) recipients induces very little colitis when compared to the robust disease produced by transfer of wild type T cells.33 Investigations into the roles of α4β1 and α4β7 have been performed in different animal models of chronic intestinal inflammation with conflicting results. For example, Sydora et al. reported that adoptive transfer of β7-deficient CD4+CD45RBhigh T cells into immunod-eficient recipient mice delayed the onset of colitis but did not attenuate the incidence and severity of the disease, suggesting that neither αEβ7 nor α4β7 were required for full expression of chronic colitis.34 This study is in agreement with Rivera-Nieves and co-workers who demonstrated that monoclonal antibody (mAb) blockade of either α4β1 or α4β7 individually did not attenuate chronic ileitis in SAMP1/Yit mice, whereas immunoneutralization of both (or their endothelial ligands) provided significant protection.35 Interestingly, it has been reported that long-term administration of anti-β4 integrin mAb actually exacerbates the colonic inflammation observed in the model of spontaneous colitis in mice.36 The reasons for these conflicting results are not clear; however, they may be explained, in part, by the fact that these studies relied on the administration of mAbs that may affect several different cell types. For example, α4β7 is found on T cells as well as natural killer cells and monocytes, whereas α4β1 is expressed by different myeloid cells (e.g., PMNs, monocytes).37–40 Although clinical studies have demonstrated the efficacy of mAbs directed against the α4 integrin (e.g., Natalizumab) in patients with CD, it is not clear whether α4β7 and/or α4β1 is/are the important molecular determinants for this protective effect or has the identity of various α4-expressing leukocyte populations involved in disease pathogenesis been identified.41,42
Role of the GALT in the pathogenesis of chronic gut inflammation
One of the major tenants highlighted in all immunology textbooks is that naive T cells continuously recirculate from the blood to the lymphoid tissue and back to the systemic circulation via the efferent lymphatics but do not normally traffic to nonlymphoid tissue. Thus, naive T cells must traffic to lymphoid tissue where they become primed/activated to memory/effector T cells that are then capable of homing to extra-lymphoid tissue such as the intestine. Indeed, there is good evidence demonstrating that MLNs play a critical role in controlling and limiting mucosal immune responses to enteric bacterial antigens; however, the evidence demonstrating a role for PPs in adaptive immune responses is somewhat vague and ill-defined.10–12,43,44 Because several studies have demonstrated that antigen-loaded DCs are continuously transported from the gut lamina propria and PPs to the MLNs, the prevailing view has been that naive T cells traffic to the MLNs where they are primed, polarized, and expanded to yield colitogenic effector (Th1/Th17) cells. These disease producing T cells are then capable of homing to the gut where they initiate disease.9,13,14,16 Although an attractive paradigm that is based upon a large body of experimental data, there have been very few attempts to directly assess the role of the GALT and/or MLNs in the induction and/or perpetuation of chronic gut inflammation. Indeed, it is not clear whether GALT and/or MLNs are even required for the generation of disease producing T cells. For example, one report suggests that MLNs and/or PPs may actually function to limit or suppress the induction of colonic inflammation45 while another study suggests that the PPs play no role in the pathogenesis of acute self-limiting colitis.46
Recent work by Makita and coworkers reported that neither the GALT, MLNs, PPs, spleen nor the peripheral lymph nodes (PLNs) are required for the induction of chronic colitis induced by adoptive transfer of enteric antigen-experienced (i.e., activated) CD4+ T cells.47 These investigators intercrossed lymphotoxin-α-deficient (LTα−/−) mice with RAG−/− animals to generate LTα−/− × RAG−/− double-deficient (DKO) offspring that are devoid of all organized lymphoid tissue including the GALT, MLNs, and all PLNs. Because enteric antigens may be transported to the spleen by way of the systemic circulation where they could conceivably influence T-cell activation and/or polarization following their presentation by splenic DCs (Fig. 1), Makita et al. splenectomized their DKO animals to generate mice devoid of all organized lymphoid tissue. Following adoptive transfer of naive or enteric antigen-activated T cells obtained from the colonic lamina propria of mice with active colitis into their splenectomized DKO recipients, these investigators observed the development of colonic inflammation that was comparable in severity but delayed in onset when compared to the disease induced by T-cell transfer into littermate controls (i.e., LTα+/+ × RAG−/− recipients).47 These data suggest that naive or activated T cells do not require the presence of GALT, MLNs, spleen, or PLNs to induce chronic gut inflammation. Does this mean that the GALT and/or MLNs are expendable for the priming/activation, polarization, and expansion of naive (antigen inexperienced) CD4+ T cells to yield disease-producing effector cells? Recent preliminary studies from our laboratory suggest that the answer may be yes. We found that adoptive transfer of naive CD4+ T cells into LTα−/− × RAG−/− recipients does indeed induce chronic colitis with very similar onset and severity as is induced in their littermate controls (LTα+/+ × RAG−/−; unpublished data). However, transfer of naive T cells into splenectomized LTα−/− × RAG−/− recipients does not induce chronic colitis, whereas adoptive transfer of naive T cell into their splenectomized littermate controls (i.e., LTα+/+ × RAG−/− mice) induces robust disease (unpublished data). Taken together, our preliminary studies suggest that the spleen may serve, in a compensatory manner, to prime/activate naive T cells in the absence of GALT, MLNs, and PLNs. These novel findings suggest a major shift in our fundamental understanding of how and where native T cells become activated to yield colitogenic (Th1/Th17) effector cells.
Conclusions
There is a large historic literature demonstrating that protection against invasion of enteric pathogens requires that intravascular T cells must traffic to the inductive sites of the intestine (GALT) and/or their draining MLNs where they undergo antigen-driven priming/activation, polarization, and expansion to yield protective effector cells that home to the gut to help destroy the invading microorganisms. If this process is not adequately regulated, enteric antigen-induced effector cells may promote chronic intestinal inflammation. New and provocative information is emerging demonstrating that in the absence of GALT, MLNs, and PLNs, certain secondary lymphoid tissue such as the spleen may function as an inductive site to prime and polarize naive T cells to yield effector T cells capable of inducing chronic inflammation. Once naive T cells have been polarized to their Th1 and/or Th17 phenotype, neither the GALT, MLNs, spleen nor PLNs are required for induction and perpetuation of disease.
Acknowledgments
Work supported by a PO1 DK43785 (Project 1, Animal Models Core and Histopathology Core).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Blumberg RS. Inflammation in the intestinal tract: pathogenesis and treatment. Dig Dis. 2009;27:455–464. doi: 10.1159/000235851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 4.Powrie F, Read S, Mottet C, et al. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–98. [PubMed] [Google Scholar]
- 5.Uhlig HH, Powrie F. The role of mucosal T lymphocytes in regulating intestinal inflammation. Springer Semin Immunopathol. 2005;27:167–180. doi: 10.1007/s00281-005-0206-6. [DOI] [PubMed] [Google Scholar]
- 6.Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 7.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29:514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 9.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwa SF, Beverley P, Smith AL. Peyer’s patches are required for the induction of rapid Th1 responses in the gut and mesenteric lymph nodes during an enteric infection. J Immunol. 2006;176:7533–7541. doi: 10.4049/jimmunol.176.12.7533. [DOI] [PubMed] [Google Scholar]
- 11.Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann NY Acad Sci. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 12.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 14.Mowat AM, Millington OR, Chirdo FG. Anatomical and cellular basis of immunity and tolerance in the intestine. J Pediatr Gastroenterol Nutr. 2004;39(Suppl 3):S723–S724. doi: 10.1097/00005176-200406003-00003. [DOI] [PubMed] [Google Scholar]
- 15.Forster R, Pabst O, Bernhardt G. Homeostatic chemokines in development, plasticity, and functional organization of the intestinal immune system. Semin Immunol. 2008;20:171–180. doi: 10.1016/j.smim.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Malmstrom V, Shipton D, Singh B, et al. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol. 2001;166:6972–6981. doi: 10.4049/jimmunol.166.11.6972. [DOI] [PubMed] [Google Scholar]
- 17.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 18.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 20.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 21.Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008;14:275–289. doi: 10.1002/ibd.20280. [DOI] [PubMed] [Google Scholar]
- 22.von Andrian UH. Adhesion and communication between lymphocytes and endothelial cells. In: Schmid-Schonbein GW, editor. Molecular Basis for Microcirculatory Disorders. Springer; Paris: 2004. pp. 101–137. [Google Scholar]
- 23.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 24.Sans M, Panes J, Ardite E, et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. 1999;116:874–883. doi: 10.1016/s0016-5085(99)70070-3. [DOI] [PubMed] [Google Scholar]
- 25.Sans M, Salas A, Soriano A, et al. Differential role of selectins in experimental colitis. Gastroenterology. 2001;120:1162–1172. doi: 10.1053/gast.2001.23252. [DOI] [PubMed] [Google Scholar]
- 26.Steeber DA, Tang ML, Zhang XQ, et al. Efficient lymphocyte migration across high endothelial venules of mouse Peyer’s patches requires overlapping expression of L-selectin and beta7 integrin. J Immunol. 1998;161:6638–6647. [PubMed] [Google Scholar]
- 27.Tang ML, Hale LP, Steeber DA, Tedder TF. L-selectin is involved in lymphocyte migration to sites of inflammation in the skin: delayed rejection of allografts in L-selectin-deficient mice. J Immunol. 1997;158:5191–5199. [PubMed] [Google Scholar]
- 28.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XW, Liu Q, Thorlacius H. Inhibition of selectin function and leukocyte rolling protects against dextran sodium sulfate-induced murine colitis. Scand J Gastroenterol. 2001;36:270–275. doi: 10.1080/003655201750074555. [DOI] [PubMed] [Google Scholar]
- 30.Ostanin DV, Furr KL, Pavlick KP, et al. T cell-associated CD18 but not CD62L, ICAM-1, or PSGL-1 is required for the induction of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1706–G1714. doi: 10.1152/ajpgi.00573.2006. [DOI] [PubMed] [Google Scholar]
- 31.Ostanin DV, Bao J, Koboziev I, Gray L, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdelbaqi M, Chidlow JH, Matthews KM, et al. Regulation of dextran sodium sulfate induced colitis by leukocyte beta 2 integrins. Lab Invest. 2006;86:380–390. doi: 10.1038/labinvest.3700398. [DOI] [PubMed] [Google Scholar]
- 33.Pavlick KP, Ostanin DV, Furr KL, et al. Role of T-cell-associated lymphocyte function-associated antigen-1 in the pathogenesis of experimental colitis. Int Immunol. 2006;18:389–398. doi: 10.1093/intimm/dxh378. [DOI] [PubMed] [Google Scholar]
- 34.Sydora BC, Wagner N, Lohler J, et al. beta7 Integrin expression is not required for the localization of T cells to the intestine and colitis pathogenesis. Clin Exp Immunol. 2002;129:35–42. doi: 10.1046/j.1365-2249.2002.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera-Nieves J, Olson T, Bamias G, et al. L-selectin, alpha 4 beta 1, and alpha 4 beta 7 integrins participate in CD4+ T cell recruitment to chronically inflamed small intestine. J Immunol. 2005;174:2343–2352. doi: 10.4049/jimmunol.174.4.2343. [DOI] [PubMed] [Google Scholar]
- 36.Bjursten M, Bland PW, Willen R, Hornquist EH. Long-term treatment with anti-alpha 4 integrin antibodies aggravates colitis in G alpha i2-deficient mice. Eur J Immunol. 2005;35:2274–2283. doi: 10.1002/eji.200526022. [DOI] [PubMed] [Google Scholar]
- 37.Bowden RA, Ding ZM, Donnachie EM, et al. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ Res. 2002;90:562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- 38.Podolsky DK, Lobb R, King N, et al. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest. 1993;92:372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podolsky DK. Selective adhesion-molecule therapy and inflammatory bowel disease–a tale of Janus? N Engl J Med. 2005;353:1965–1968. doi: 10.1056/NEJMe058212. [DOI] [PubMed] [Google Scholar]
- 40.Schneider MK, Strasser M, Gilli UO, et al. Rolling adhesion of human NK cells to porcine endothelial cells mainly relies on CD49d-CD106 interactions. Transplantation. 2002;73:789–796. doi: 10.1097/00007890-200203150-00023. [DOI] [PubMed] [Google Scholar]
- 41.Rutgeerts P, van Deventer S, Schreiber S. Review article: the expanding role of biological agents in the treatment of inflammatory bowel disease—focus on selective adhesion molecule inhibition. Aliment Pharmacol Therap. 2003;17:1435–1450. doi: 10.1046/j.1365-2036.2003.01603.x. [DOI] [PubMed] [Google Scholar]
- 42.Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136:1182–1197. doi: 10.1053/j.gastro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Spahn TW, Weiner HL, Rennert PD, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur J Immunol. 2002;32:1109–1113. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 44.Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spahn TW, Herbst H, Rennert PD, et al. Induction of colitis in mice deficient of Peyer’s patches and mesenteric lymph nodes is associated with increased disease severity and formation of colonic lymphoid patches. Am J Pathol. 2002;161:2273–2282. doi: 10.1016/S0002-9440(10)64503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dohi T, Rennert PD, Fujihashi K, et al. Elimination of colonic patches with lymphotoxin beta receptor-Ig prevents Th2 cell-type colitis. J Immunol. 2001;167:2781–2790. doi: 10.4049/jimmunol.167.5.2781. [DOI] [PubMed] [Google Scholar]
- 47.Makita S, Kanai T, Nemoto Y, et al. Intestinal lamina propria retaining CD4+CD25+ regulatory T cells is a suppressive site of intestinal inflammation. J Immunol. 2007;178:4937–4946. doi: 10.4049/jimmunol.178.8.4937. [DOI] [PubMed] [Google Scholar]