Abstract
A subset of human papillomavirus (HPV) genotypes is responsible for ~5% of all cancer deaths globally, and uterine cervical carcinoma accounts for the majority of these cases. Their impact is greatest for women who do not have access to effective secondary preventive measures, and consequently over 80% of cervical cancer deaths worldwide occur in Developing nations. The understanding that persistent infection by this ‘oncogenic’ subset of HPV genotypes is necessary for the development of cervical carcinoma has driven the development of preventive vaccines. Two preventive vaccines comprising recombinant HPV L1 virus-like particles (VLPs) have been licensed. However, the current cost of these vaccines precludes global delivery, and they target only two of the ~15 known oncogenic HPV types, although ~70% of cervical cancer cases are attributed to these two types and there is evidence for some degree of cross-protection against other closely related types. A possible approach to broader immunity at lower cost is to consider vaccination against L2. L2 vaccines can be produced inexpensively and they also have the promise of conferring much broader cross-type protective immunity than observed with L1 VLP immunization. However, L2 vaccine development lags behind L1 VLP vaccines and several technical hurdles remain.
THE CASE FOR PROPHYLACTIC VACCINATION AGAINST HPV
The precursor of cervical cancer is high grade cervical intraepithelial neoplasia (CIN, also known as squamous intraepithelial lesion SIL). Ablation or cold knife excision of these precursors is generally curative, thereby halting progression to invasive cervical cancer. Since the ‘Pap’ smear was introduced by Dr. Georgios N. Papanicolaou in the 1940s to detect high grade CIN, numerous studies have demonstrated higher mortality rates in unscreened or under-screened women and the efficacy of secondary prevention for the reduction of cervical cancer incidence. Unfortunately, cervical cancer therefore disproportionately impacts under-developed nations that lack such cytologic screeing programs wherein 83% of cervical cancer cases occur. National screening programs have shown that Pap screening for high grade CIN decreases the occurrence of cervical carcinoma by over 60%. The effectiveness of such programs could be further increased by reducing the testing interval and improve the performance characteristics of testing. The Pap test has remained virtually unchanged for 50 years as part of national screening programs until the advent of molecular testing for HPV. Molecular testing for HPV significantly improves the performance of cytologic screening and full implementation of such a combined screening approach can further reduce rates of cervical cancer. However, such programs are expensive and impractical in large parts of the world. Furthermore, HPV infection causes significant morbidity in some patients including other anogenital and some head and neck cancers. Therefore prevention of HPV infection with a low cost vaccine is preferable to large screening programs, particularly in low resource settings. Maximum benefit is obtained by combining both interventions, but this has significant cost implications and reduces the performance of screening, necessitating careful consideration of the optimal approaches to implementation.
The strength and diversity of evidence has led to general recognition of oncogenic HPV infection as the necessary cause of cervical cancer. Dr. Harald zur Hausen had first postulated this link during the 1970s, and in the early 1980s his group identified the two most important oncogenic HPV genotypes, HPV16 and HPV18 (1, 2). For this seminal work and his continued contributions, Dr zur Hausen was awarded the 2008 Nobel prize in physiology or medicine. Building of the case for the full recognition of oncogenic HPV as the central etiologic agent for cervical cancer took many years of effort by researchers worldwide. The evidence implicating oncogenic HPV infection includes basic laboratory studies of viral pathogenesis, large epidemiologic studies, and successful screening and preventive vaccine interventions.
It is important to recognize that the majority of HPV infections do not lead to cervical cancer, i.e. HPV infection per se is not a sufficient cause and that additional changes and co-factors contribute to carcinogenesis. HPV is a small, nonenveloped DNA virus that induces generally benign epithelial tumors (warts) of skin or mucosa. Most such HPV-induced lesions show limited growth and often regress spontaneously. Of the nearly 200 genotypes of HPV that have been identified, more than fifteen have been shown to induce tumors that may eventually progress to carcinomas (3). In particular, 53.5%, 17.2%, and 6.7% of cervical cancer cases worldwide are attributed to the genital types HPV16, HPV18 and HPV45 respectively (4). The remaining 12+ oncogenic genotypes (HPV31, HPV33, HPV52, HPV58, HPV35, HPV59, HPV56, HPV51, HPV39, HPV68, HPV73, and, HPV82 in descending order of importance, and probably additional less well characterized types) are considered to cause the remaining 30% of cervical cancer cases in total, with less than 3% for any one of these minor types (4). It should be noted that this type distribution does not hold for all HPV-associated cancers. For example the contribution of HPV16 is much greater for head and neck cancer (5), as well as vaginal or vulval cancers (6), whereas HPV18 and HPV45 show greater importance in cervical adenocarcinoma (7). Papillomaviruses exhibit strict tropism, and therefore preclinical studies of vaccines have been limited mainly to a few animal papillomavirus models, notably bovine papillomavirus (BPV) infection of cows, cottontail rabbit papillomavirus (CRPV) or rabbit oral papillomavirus (ROPV) infection of rabbits and canine oral papillomavirus (COPV) infection of dogs.
Over 99% of cervical cancers and their precursor lesions CIN, contain human papillomavirus (HPV) DNA (8, 9). The HPV genotypes found in cancer cells have immortalizing activity for primary human keratinocytes (10), and the viral oncoproteins, E6 and E7, are consistently expressed both in cervical cancer cell lines (11) and in HPV-associated cancers (12). Knockdown of E6 and E7 expression in cervical cancer cells triggers cell death, indicating addiction to their expression. These properties make E6 and E7 ideal tumor-associated antigens to target for therapeutic vaccination. The HPV genome frequently integrates into the host genome during the cervical carcinogenesis. This integration frequently results in the loss of particular regions of viral DNA and is correlated with the upregulation of E6 and E7 expression. E6 and E7 expression overcomes the regulation of cell cycle and apoptotic signals, to drive proliferation. E6 causes the degradation of p53, PDZ proteins and activates c-myc and hTERT expression whereas E7 inactivates Rb, events that synergize to drive malignant transformation (13, 14). Late genes encoding the capsid proteins (L1 and L2) and some early genes are commonly lost, notably E2 which de-represses E6 and E7 expression. Therefore, in contrast to E6 and E7, vaccination against the capsid proteins L1 and L2 is unlikely to confer therapeutic benefit (14).
Formalin-inactivated BPV has been successfully used in cattle to protect herds from bovine papillomas, whereas denatured BPV was not, and formalin-inactivated COPV is also protective for dogs against oral papillomas (15–17). The inability to grow large quantities of HPV in culture and its oncogenic genome precluded the development of an inactivated virus as a preventive vaccine for human use (18). However, the success of the inactivated virus vaccines in animals suggested that the capsid conferred protection if its structure was maintained.
In the early 1990’s several groups demonstrated that empty capsids, termed virus-like particles (VLPs), were formed when L1 was over-expressed in insect cells, mammalian cells and yeast (19). Significantly vaccination with L1 VLP, but not denatured L1, induced high serum titers of type-specific neutralizing antibodies, similar to those induced by vaccination with native virions (20). Studies in several animal models demonstrated that vaccination with L1 VLPs was highly protective, albeit only for the type used to derive the vaccine (21–24).
Despite the successful induction of immunity in animal models by L1 VLP vaccination, it was not clear that this could be translated into patients because they do not mimic sexual transmission of HPV. Early phase trails demonstrated that as in the animal models, vaccination with L1 VLP induced high titers of neutralizing antibody, even without the use of adjuvant, with no apparent significant side effects (25, 26). The first evidence that L1 VLP vaccines are highly protective against sexual transmission of an oncogenic HPV was a landmark efficacy trial of HPV16 L1 VLPs (27), in which 100% (95% confidence interval, 90–100; P<0.0001) protection against acquisition of persistent HPV16 infection was reported (Table 1). Furthermore, the women vaccinated with placebo acquired HPV16+ CIN, while those vaccinated with HPV16 VLPs did not. However, both groups acquired equal numbers of CIN containing genotypes other than HPV16 (Table 1). This suggests that HPV L1 VLP vaccines provide protection against infection by the homologous papillomavirus type and is consistent with the type-restricted in vitro neutralization by sera of vaccinated animals and patients. These early findings have been extended in several Phase III efficacy trials (6, 28–31). The data indicate near complete protection for more than five years against the acquisition of infection by the HPV types used to develop the vaccine. Furthermore, there is evidence of partial protection for the types most closely related to those used to generate the L1 VLP vaccine, e.g. partial protection against HPV31 is observed in those vaccinated with HPV16 L1 VLPs, and protection against HPV45 is also observed in those vaccinated with HPV18 L1 VLPs. Interesting evidence of cross-neutralization is also observed for these types in vitro, although at titers 10–100-fold lower than observed for homologous type neutralization (32–35).
Table 1.
Summary of the data from a landmark efficacy trial of an HPV16 L1 VLP vaccine (27).
| Vaccine | Placebo | |
|---|---|---|
| Women enrolled | 768 | 765 |
| Persistent HPV16 infections | 0 | 41* |
| Incident CINI HPV16+ | 0 | 5 |
| Incident CINII HPV16+ | 0 | 4 |
| Incident CIN attributed to a genotype other than HPV16 | 22 | 22 |
In this prophylactic vaccination trial to assess the safety and efficacy of HPV16 L1 VLPs, women without evidence of HPV16 infection were vaccinated three times with HPV16 L1 VLPs or placebo and followed for new persistent HPV16 infection or development of CIN over a median of 17.4 months. Vaccination provided 100% (95% confidence interval, 90–100; P<0.0001) protection against acquisition of persistent HPV16 infection, although 41 incident persistent HPV16 infections and HPV16 DNA+ 9 Cervical Intraepithelial Neoplasias (grades I or II) occurred in the placebo recipients. However, the same number of cases of non-HPV16 CIN occurred in women who received placebo and those who received the HPV16 L1 VLP vaccine (27).
Such type-restricted (not type-specific) immunity complicates comprehensive vaccination against all oncogenic HPV types. One approach is to increase the number of L1 VLP types utilized, but highly multivalent vaccines are likely to be more expensive than the currently licensed vaccines (32, 33, 36). Current formulations of both licensed L1 VLP vaccines, called CERVARIX® (GSK) and GARDASIL® (MERCK & Co), contain two oncogenic HPV genotypes, HPV16 and HPV18 which together account for ~70% of cervical cancers (28, 30). Merck includes HPV6 and HPV11 L1 VLPs in their licensed vaccine GARDASIL®, to protect against benign genital warts (30) and provide additional rationale for vaccination of men. Efforts to produce octa- or nona-valent L1 VLP vaccines are underway to extend protection to more HPV types and thus potential protect against at least 90% of all cervical cancer cases based upon the current contribution of each oncogenic HPV type (4, 7).
WHY DO WE NEED SECOND GENERATION HPV VACCINES?
The current HPV vaccines targeting HPV16 and HPV18 have the potential to produce a dramatic drop in cervical cancer rates if they are widely used (4). Nevertheless, the licensed vaccines are currently too costly for global implementation and thus reach those populations that would benefit most from such a vaccine (37). It is important to note that over 80% of cervical cancer cases occur in Developing nations (38). This reflects the lack of cytologic screening programs and intervention in these countries. Furthermore, as a significant fraction of cervical cancer (~30%) is caused by oncogenic HPV types not included in current HPV vaccine formulations, the type-restricted protection induced by the L1 VLP vaccines means that cervical cancer screening must remain in place. In these settings, therefore, the cost of HPV vaccination must be borne in addition to that of screening. In this situation cytologic screening will become much less cost effective, although cost savings might be achieved by altering the interval and/or modality (i.e. using HPV testing as a first line approach) by which vaccinated women are screened (39). Thus it would be highly desirable to develop a more affordable vaccine for the Developing world as well as to broaden protection against oncogenic HPV infections to a degree that would permit a major reduction in screening in the industrialized world. While increasing the valency of the current L1 VLP vaccines represents a logical approach to overcome the latter problem (51), it seems likely that such a second generation vaccine would, for many years, continue to be too expensive for widespread use in low resource settings. Furthermore, the development of an alternative inexpensive approach to HPV vaccination could drive down the cost of all HPV vaccines and increase their global penetration.
WHY L2?
Papillomaviruses have one other capsid protein beside L1, which is called L2. L2 is a minor component of the viral capsid (Figure 1), but it is necessary for papillomavirus infection, possibly by binding to a secondary viral receptor to facilitate exit from the endosomes and delivery of the viral genome to appropriate domains within the nucleus (40–43). Studies in animal papillomavirus models haved indicated that L2, like L1, is a promising antigen for preventive vaccination. These pioneering studies examined immunization of cows or rabbits with the L2 polypeptides produced in bacteria for protection from experimental papillomavirus challenge at both mucosal and cutaneous sites respectively (Table 2). Several studies from Campo’s group showed that vaccination with full length and polypeptides of BPV4 L2 were protective against experimental viral challenge (Figure 2) (44–47). They demonstrated that vaccination with a polypeptide comprising residues 11-200 of BPV4 L2, but not a C-terminal peptide thereof, protects cattle against papillomas resulting from experimental challenge with BPV4 virions (Figure 2). Near complete protection against warts after BPV4 challenge was obtained by vaccination with either BPV4 L2 11-200 and full length BPV4 L2, similar to that obtained after vaccination with BPV4 L1 VLP (23, 46, 48). While the correct conformation of L1 VLPs is critical for the induction of neutralizing antibodies and protection after vaccination, immunization with L2 purified under denaturing conditions both induced neutralizing antibodies and protected. The L2-vaccinated cattle were challenged shortly after completion of the vaccination regimen (Figure 1), and additional studies to address the long term efficacy of L2 vaccination suggested that protection lasts at least one year (49). However, important caveats remain; the titers of neutralizing antibodies induced by vaccination with L2 are much lower than those produced by L1 VLP vaccination (50–53), and that the cattle vaccinated with BPV4 L2 in these studies were not tested for cross-protection against warts produced by a different papillomavirus type. Furthermore, it should be noted that vaccination with BPV2 L2 did not effectively protect against BPV2 challenge, but rather induced rapid clearance of papillomas that was associated with massive T cell infiltration (54). Interestingly, vaccination with BPV2 L2 did not induce detectable neutralizing antibodies in these animals (54), which contrasts the findings for BPV1 and BPV4 (50, 51, 55), and may explain the discrepancy in protective efficacy in these studies. It is possible that the data reflect a potent T cell response to L2 vaccination that triggered rejection, but we favor the notion that a weak neutralizing antibody response triggered by BPV2 L2 vaccination reduced the size of the inoculum and thereby facilitated spontaneous clearance of the BPV2 papillomas in this study (54).
Figure 1. Image reconstruction of bovine papillomavirus type 1 alone or bound to neutralizing L1-specific monoclonal antibody.

A. An 18 Å resolution image reconstruction of a BPV1 virion purified from a bovine papilloma and viewed down the 5-fold axis of symmetry. The structure was obtained using image reconstruction of cryoelectron micrographs (70). Note that the capsid has T=7 icosahedral symmetry. It comprises 72 capsomers, each containing 5 L1 molecules and up to one L2 molecule (124). 60 capsomers are coordinated with 6 adjacent capsomers (hexavalent) and 12 with five neighboring capsomers (pentavalent). A pentavalent capsomer can be seen in the center of this image. The button of density present in the center of the pentavalent capsomers likely corresponds to the minor capsid protein L2 (124), a small portion of which is exposed on the capsid surface and available for binding by neutralizing antibodies. Image reconstructions of HPV1, HPV16 and CRPV suggest that all papillomaviruses have a very similar structure (92, 124–126). The viral genome forms a nucleohistone core with no detectible icosahedral symmetry. B. An image reconstruction of a BPV1 virion (blue) bound to either neutralizing monoclonal antibody MAb#9 (red) or 5B6 (green) (91) and viewed alone the 3-fold access of symmetry. Mab#9 covers the surface of the virion and binds to all of the capsomers. 5B6 covers the hexavalent capsomers, but not the pentavalent capsomers along the 5-fold axes of symmetry. Both images were generated by Benes Trus (CIT, NIH).
Table 2.
Summary of published L2 vaccine studies in animal models.
| Study (et al), date | Virus - Host | Antigen | Dose-Adjuvant | Vaccination Schedule | Challenge | Neutralization Peak Titer | Protected |
|---|---|---|---|---|---|---|---|
| Jarret, 1991 | BPV2-Cattle | BPV2 L2 full length | 2× 500 μg-IFA | 0, 2 wks | +2 wk | Below detection | Enhanced clearance1 |
| Campo, 1993; Chandrachud, 1995; Gaukroger, 1996; McGarvie, 1994 | BPV4- Cattle | BPV4 L2 full length or 11-200 | 2× 100 μg-Alum 2× 50 μg-None 2× 1 mg-Alum |
0, 4 wks 0, 4 wks 0, 4 wks |
+2 wk +2 wk +2 wk +1 year |
5 | Yes Yes Yes Yes |
| Christensen, 1991 | CRPV-Rabbit | CRPV L2 | 4× gelpure-CFA | 0, 4, 8, 12 wks | +2wk | 32 | Yes |
| Lin, 1992 | CRPV-Rabbit | CRPV L2 | 4× 250 μg-RIBI | 0,3,6,9 wks | +2 wk | 10 | Yes |
| Embers, 2002 | ROPV, CRPV-Rabbit | CRPV/ROPV L2 peptide | 3× 500 μg peptide/KLH-CFA/IFA | 0, 4, 8 wks | +4–12 wks | 10 | Yes |
| Gambhira, 2007 | ROPV, CRPV-Rabbit | L2 1-88 or 11-200 | 3× 500 μg –RIBI | 0, 4, 8 wks | +4 wks | 8000 | Yes, Cross-type |
| Palmer, 2006 | ROPV, CRPV-Rabbit | TMV-L2 recombinant particles | 3× 200 μg-RIBI | 0, 33, 63 days | +1 wk | 500 | Yes, Cross-type |
| Karanam, 2009 | HPV16-mouse | TA-CIN (full length HPV16 L2) | 3× 20 μg-GPI-0100 | 0, 2, 4 wks | +2 wk | 400,000 | Yes |
| Jagu, 2009 | HPV16-mouse | Polymeric L2 | 3× 25 μg-GPI-0100, or alum, or ISS 1018 or adjuvant combinations | 0, 2, 4 wks | +4 mo | 100,000 | Yes |
| Alphs, 2008 | HPV16/45-mouse | HPV16 L2 17-36 lipopeptide | 3× 20 nmol, self- adjuvanting | 0, 4, 8 wks | +2 wk | 10,000 | Yes, Cross-type |
Cattle were not protected, but warts were rapidly cleared in the vaccine group. This may reflect reduced inoculum due to partial neutralization or clearance, perhaps as a bystander effect. This enhanced clearance was not described in any other L2 vaccine study.
Figure 2. Vaccination of calves with BPV4 L2 or polypeptide comprising residues 11-200 of BPV4 L2 protects against experimental challenge at a mucosal site with BPV4.
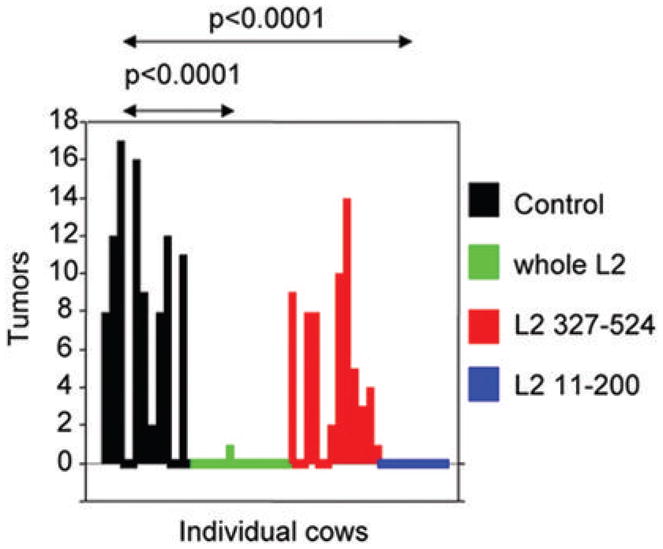
The data is taken from (46) and redrawn. Recombinant polypeptides comprising the full length BPV4 L2 or residues 11-200 or 327-524 were expressed in E. coli, prepared as inclusion bodies and formulated in alum. Each animal (12/group) was inoculated twice at monthly intervals with either 50 μg of polypeptides encompassing full length L2, or 330 μg of L2 residues 11-200 or 330 μg of residues 327-524 and the control group was not vaccinated. The cattle generated antibody responses to each antigen. BPV4 challenge was performed in the upper alimentary tract two weeks after completion of vaccination. Only the control group and the group vaccinated with the C-terminal L2 peptide developed tumors (papillomas).
Vaccination with L2 polypeptide has also conferred immunity to challenge in the rabbit model (56), although the C-terminus was also protective in this model (52). Vaccination with full length L2 or its polypeptides provided near complete protection of rabbits from cutaneous challenge with the cognate virus type, CRPV. The N-terminal region of L2 that confers protection exhibits considerable amino acid sequence conservation among the medically significant HPV genotypes, suggesting the potential for cross-reactive immune responses to vaccination with this polypeptide (Figure 3). Indeed, animals immunized with HPV L2 produce antibodies that cross-neutralize a broad range of HPV genotypes (53, 57–60) and cross-neutralizing L2-specific monoclonal antibodies have been generated (59, 61, 62), and mapped (Figure 4). This contrasts the type-restricted neutralizing antibodies and protection induced by L1 VLP vacciantion. This important distinction suggests the possibility of a simple pan-HPV prophylactic vaccine derived from L2 sequences.
Figure 3.

Amino acid sequence conservation at the N-terminus of L2.
Figure 4.
Diagram of the known functions and monoclonal antibodies binding sites in HPV16 L2.
Much of the early work on L2 vaccine development utilized animal papillomaviruses as models because of an inability to readily propagate HPV in tissue culture, the lack of in vitro HPV neutralization assays, and the strict tropism of papillomaviruses, i.e. HPV did not produce disease in animals (18). However, the value of this approach has been borne out during the development of L1 VLP vaccines which utilized the same animal papillomavirus models, notably BPV1 or BPV4 infection of cattle, CRPV or ROPV infection of rabbits and COPV infection of dogs. Unfortunately animal papillomavirus models of infection are of limited value in testing L2 vaccines for their ability to induce immunity to a broad range of HPV types as they do not involve genital challenge and the L2 sequences of animal papillomaviruses are evolutionarily distant from the genital HPV types. Many of the technical limitations in production of HPV and testing for in vitro neutralization have been overcome by the development of HPV pseudovirion technologies (32, 63, 64). These HPV pseudovirions can also be used in challenge models in which mice are infected intra vaginally or subcutaneously using pseudovirions carrying markers such as RFP or luciferase (62, 65–67). These HPV pseudovirion challenge models are consistent with the animal papillomavirus models in indicating that L2 vaccines do induce more broadly cross-protective immune responses than observed for L1 VLP (66).
One possible approach to extend protection to more genotypes might be to vaccinate with L1/L2 VLP rather than L1 only VLPs, since L2 co-assembles with L1 into VLPs when co-expressed. Unfortunately, L2 is immunologically subdominant to L1 in the context of an L1/L2 VLP or even virion vaccine as well as in serologic studies of natural infection (53), and consequently L1-specific antibodies result almost exclusively. Thus, no more cross-protection is seen in animals vaccinated with L1/L2 VLPs as compared to L1 only VLPs because they generate only L1-specific neutralizing antibodies (Figure 5) (21). This probably reflects the close packed, regular array of L1 (68, 69), whereas L2 present at a least a five-fold lower level, and more widely spaced in the capsid (Figure 1A) (70). In addition there is evidence that the principle neutralizing epitopes on L2 may be partially buried and only revealed during infection (71). It is likely that the virus evolved such that L2 is poorly immunogenic given its critical and conserved role in infection, otherwise papillomavirus types would have remained a single serotype.
Figure 5. Vaccination with L1/L2 VLPs induces type specific neutralizing antibodies, vaccination with L2 protein alone generates a lower titer, but broadly neutralizing antibody response.
Rabbits were immunized with L1/L2 VLPs or L2 protein alone derived from HPV6, HPV16 or HPV18. Sera were tested for neutralizing titer against HPV6, HPV16 or HPV18 pseudovirions (53). These HPV types represent prototypic members of three distinct evolutionary species (107).
These studies, when taken together, suggest that L2 contains protective epitopes mostly near its N-terminus, and that not all regions of L2 confer immunity to infection. Immunization with some of these protective L2 polypeptides can trigger the production of broadly neutralizing antibodies which likely mediate protection against both the papillomavirus type from which the L2 vaccine was derived, as well as challenge with divergent papillomavirus types (72). However, the L2 polypeptide vaccine must be presented to the immune system in the appropriate context to trigger immunity because it is subdominant to L1 within the capsid (53). These principles have driven studies to better define the neutralizing epitopes within L2, to understand how L2-specific antibodies prevent infection, and efforts to optimize the approach for L2 vaccine delivery through multimerization, epitope display and/or adjuvant technologies.
Mechanism of vaccine-induced protection from new HPV infection
Vaccines have had a profound impact on infectious diseases, yet much remains to be learned about how they work. T cells and antibodies are typically central to protection, and immunological memory is necessary, but there is much controversy over the details. The relative importance of cell-mediated and antibody responses in preventing viral infection is hotly debated. Opinions range from the notion that antibodies are required primarily to control bacterial, rather than viral, infection and are dispensable for the control of some viral infections, to the polar opposite view that antibodies are the only identified agent of successful vaccine protection (73, 74). Study of the unique biology of papillomavirus and the remarkable efficacy of HPV vaccines, as detailed below, can instruct this debate and further the development of second generation HPV vaccines, and approaches to vaccination against other pathogens.
Passive transfer of L1 VLP or L2-specific neutralizing antibodies is sufficient to confer protection to naïve animals suggesting that cell-mediated immunity to capsid proteins is not required for protection (21, 22, 62). However, these studies do not exclude a contribution of cellular immunity in protecting from natural infection, and indeed there is evidence of L1 and L2-specific cytotoxic T cell responses do occur in response to vaccination with capsid antigen (54, 75–77). There have been some reports that vaccination with either L1 VLP or L2 can enhance clearance of pre-existing disease (54, 75, 78), indicating a possible contribution of capsid protein-specific cytotoxic T cells in controlling infection. However, these effects have not been demonstrated in any randomized and controlled clinical trial to date (79–82). In addition, vaccination with L2 peptides protects rabbits against challenge with CRPV virions but not CRPV viral DNA. Furthermore, papillomas generated by inoculation with wild type CRPV DNA and CRPV DNA defective for L2 expression grow at the same rate in L2 vaccinated animals that are immune to challenge with CRPV virions (72, 83).
Neither the L1 nor the L2 capsid proteins are detectably expressed by the basal epithelial cells that harbor the viral infection, but only by terminally differentiated epithelial cells about to slough off (84). Probably as a consequence of this unusual biology, protective immunity upon vaccination with papillomavirus capsid protein is likely mediated primarily by neutralizing antibodies. This might occur by sterilizing neutralization at the site of infection via antibody in secretions and/or exudates at the site of microtrauma that is typically associated with transmission, e.g. during intercourse. Alternatively, as indicated for immunity to Hepatitis B after vaccination, the rapid induction of immune memory may play a key role in limiting the very early spread and establishment of disease, particularly when neutralizing titers have waned (85, 86). Patients vaccinated with GARDASIL® remain protected against HPV18 despite loss of detectable HPV18-specific serum antibody in 40% of patients, as measured by a competitive Luminex-based immune assay (86). However, it is unclear if this reflects the ability to rapidly induce immune memory, or the insensitivity of the antibody assay (85). For example, the competitive Luminex-based immune assay used to detect the HPV18 antibody response might fail to detect all neutralizing antibodies because it is based on blockade of binding of a single monoclonal antibody to the capsid, or the cutpoint may be set too high. Further studies using sensitive in vitro neutralization assays are needed to address this issue.
Serum neutralizing antibody titer is the most robust immune correlate of protection, both for papillomavirus and numerous other human infectious diseases (25, 64, 73, 87). Importantly, the degree of cross-protection that occurs in patients receiving the HPV vaccines inversely correlates with the cross-neutralization data from in vitro assays (33, 88). Taken together, the current findings suggest that neutralizing antibodies, not cytotoxic T cell responses, are the principle effectors of protection of patients from acquisition of new HPV infection after vaccination, and this is consistent with the failure of infected basal epithelial cells to express capsid antigens and therefore to be recognized by capsid antigen-specific cytotoxic T cells.
Mechanisms of viral neutralization
The complex events of HPV infection have recently been reviewed elsewhere (89). How papillomavirus infects and how antibodies block the infectious process have been the subject of much debate (71, 74). Leading hypotheses are that viruses are aggregated and/or neutralized by preventing their interaction with the cell upon the binding of capsid to one or many antibody molecules; or alternatively that antibody binding prevents necessary conformational changes in the capsid or induces inappropriate conformational states; or that the infectious process is halted by antibody after virion entry to infected cells by, for example, blocking its release of nucleic acid or re-routing the virions along a non-infectious pathway (55, 90). The simple occupancy model proposes that neutralization occurs when a significant proportion of available epitopes on the virion are bound by antibody, thereby preventing the attachment of virus to host cells or interfering with the process of entry (74). Indeed in three dimensional reconstruction of the L1-specific neutralizing monoclonal antibody (Mab)9 bound under saturating conditions to BPV1 reveals that the antibody completely coats the surface of the virion (Figure 1B) (91). Consistent with this occupancy model, Mab9 prevents binding of BPV1 to cell monolayers (55). By contrast, the L1-specific antibody 5B6 neutralizes but does not block binding to cell monolayers. Structural analysis reveals that the 5B6 antibody does not cover the pentavalent capsomers on the 5-fold axes, suggesting that they might be sufficient to mediate surface binding (91). Importantly, no evidence of structural changes in L1 was apparent in virions coated with antibody in these moderate resolution reconstructions (Figure 1B). However, there is clear evidence of different states of capsid assembly including closed/mature and open/immature forms that have been associated with differing degrees of intra- and inter- L1 subunit disulphide bond formation (92, 93). Structural analysis reveals that the 5B6 antibody bridges the capsomers (91), and between 14 to 72 antibodies per virion were necessary for neutralization, suggesting that 5B6 antibody might block virus uncoating or conformational changes in the capsid (55, 94).
The HPV16 L1-specific neutralizing Mabs H16.V5 and H16.E70 prevent endocytosis of virions but not the ability of the virions to bind to the surface of HaCaT cells (71, 95). Interestingly they do block binding of HPV16 particles to erythrocytes and to extracellular matrix (33, 95, 96), suggesting two distinct modes of binding to cell surfaces. These two antibodies also prevent a conformational change in the virion which allows during cleavage of approximately 13 amino acids from the amino terminus of L2 by furin (71). This cleavage of L2 by furin occurs on the cell surface and is critical to infection (97). Neutralizing antibodies to L2, including Mab RG-1 that recognizes residues 17-36 of HPV16 L2, do not prevent the virus from binding to the cell surface (71, 94, 98). Furin cleavage enhances the exposure of L2 on the virion surface, notably for the residue 17-36 epitope bound by RG-1 (71). Residues within the RG-1 epitope are critical for viral infection (41). Since L2 plays a necessary role for intracellular trafficking in infection and several groups have identified numerous non-neutralizing L2 Mabs of the same isotype that bind to the virus surface (62, 99), these findings suggest L2 antibody-dependent neutralization occurs by preventing conformational changes and/or interaction between L2 and key cellular proteins that mediate the complex events of uptake and intracellular papillomavirus trafficking.
Neutralization and protective epitope identification in L2
Many studies have attempted to define the relevant epitopes using vaccination with synthetic peptides or by production of neutralizing monoclonal antibodies. Vaccination with the N-terminus of L2, notably residues 11-200 or 1-88, is protective in multiple animal models (45, 46, 50, 72), and much effort to identify neutralizing epitopes has focus on these regions. Vaccination of rabbits with BPV1 L2 1-88 or 45-173 induces cross-neutralizing antibodies (55, 57). Vaccination with L2 residues 94-122 provides relatively type specific protection in rabbits (83, 100), and studies in cattle suggest the value of L2 residues 101-120, 131-151 and 151-170 for protection against BPV4 (47). Other studies support residues 108-120 as a cross-neutralizing eptiope (58-61). We recently defined L2 residues 17-36 as an important protective epitope (62, 66), and others have defined neutralizing epitopes between residues 36-49 (101) and 69-81 (59). Studies examining the remainder of L2 for neutralizing epitopes have generally been negative, although one study demonstrated protection of rabbits after vaccination with the C-terminus (52). There is also evidence for critical functions of L2 during infection within this region (102). Denatured L2 was utilized to elicit neutralizing antibodies in all of the above studies, and this clearly contrasts the conformational dependence of the L1-specific neutralizing antibodies (20). These findings are summarized in Figure 4.
PROOF OF CONCEPT
Synthetic Peptide-based vaccines
Immunization with short synthetic L2 peptides coupled to Keyhole Limpet Hemocyanin (KLH) and administered in adjuvant to animal models protects them from experimental papillomavirus infection at both mucosal and cutaneous sites (83). Embers et al used two overlapping peptides within L2 residues 94-122 coupled to KLH and administered in Freund’s adjuvant to protect rabbits from experimental challenge with CRPV and ROPV. Campo’s laboratory observed the best protection from BPV4 challenge after vaccination of cattle with three immunodominant L2 peptides comprising residues 101-120, 131-151 and 151-170 together in alum, although the 131-151 peptide was effective alone (47). Kawana et al found that intra-nasal vaccination with HPV16 L2 108-120 induced IgG and IgA antibodies that neutralized HPV16 and several other HPV genotypes without the need to couple the peptide to KLH (58). This effect was observed in Balb/c but not C57BL/10 mice, suggesting the importance of recognition of an MHC class II epitope within this peptide (58). No challenge studies were performed, although this epitope overlaps the protective epitopes described by Embers et al for the CRPV and ROPV rabbit challenge models (83).
Passive transfer of monoclonal antibody RG-1, which is specific for L2 residues 17-36 and neutralizes both HPV16 and HPV18, protects naïve mice from cutaneous challenge with HPV16 pseudovirions carrying a luciferase reporter gene (62). This suggests that the 17-36 region is a promising candidate for a preventive vaccine. Although coupling of this peptide to KLH to provide T-help, and using an adjuvant to enhance the immune response was effective at inducing cross-neutralizing antibodies (62), Alphs et al sought to assemble a totally synthetic and self-adjuvanting vaccine candidate containing the target epitope (HPV16 L2 17-36) together with the TLR2 ligand Pam2Cys (P2C) and a promiscuous T helper epitope (P25) in a simple branched configuration (66). This approach has demonstrated promise as a vaccine strategy even for poorly immunogenic self-epitopes vaccine(103). Direct chemical synthesis of vaccines has potential advantages over current biological systems for the preparation of antigen, in aspects relating to scale-up of manufacture, purification, reproducibility, stability and regulatory requirements (104). The lipopeptide is able to act as its own adjuvant for the neutralizing epitope, facilitating the generation of an antibody response by triggering dendritic cell maturation via the TLR2-MyD88 pathway and enhancing the class II MHC epitope presentation to and activation of CD4 T cells (66).
HPV L1 VLP vaccines are injected i.m. and efforts at needle-free intra-nasal immunization have resulted in variable immune responses and require larger doses (105). Furthermore, licensed HPV L1 VLP vaccines utilize an adjuvant (either alum or alum+ monophosphoryl lipid A), although this may not be required (25). In contrast, s.c. and i.n. administration of the self-adjuvanting P25-P2C-HPV vaccine each generate similarly potent and consistent serum neutralizing antibody responses in mice suggesting that this approach could be tested in patients. Activation of the antibody response is dependent upon CD40 signaling, consistent with the requirement for class II MHC signaling and a CD4 T cell-dependent humoral response. Vaccination of mice with the lipopeptide construct derived from HPV16 L2 provided effective protection against HPV16 and cross-protection against HPV45, and protected against cutaneous or intra-vaginal challenge. Thus this lipopeptide approach has advantages of direct chemical synthesis, stability and potential to be delivered without needles and generate cross-protection which would facilitate delivery in resource limited settings. The complication for clinical translation of this approach is the need for a class II epitope in the lipopeptide that is broadly recognized in the diverse human population.
Recombinant polypeptide-based vaccines
Immunization with N-terminal L2 polypeptides comprising residues 11-200 protects cattle and rabbits from experimental viral challenge at mucosal or cutaneous sites (46, 50, 72). Antisera raised to these L2 polypeptides neutralize not only the same type but also the diverse range of heterologous papillomavirus types (50, 57, 72). However, neutralizing antibody titers against the cognate type are higher than against heterologous types, and there is no clear relationship between titers and evolutionary distance of the heterologous types from the type used to derive the L2 vaccinogen (57). Vaccination of rabbits with L2 1-88 or 11-200 protected rabbits from challenge with even high divergent papillomaviruses, although protection against the homologous type tended to be better. Remarkably, vaccination with HPV16 L2 11-200 provided effective protection from CRPV challenge (72).
As an alternative to vaccination with a mixture of L2 polypeptides derived from multiple types, which would greatly increase the cost and complexity of vaccine development, Jagu et al examined concatenated fusion proteins, consisting of several homologous L2 peptides derived from different medically-significant HPV genotypes, as candidates for a broadly protective HPV vaccine (106). For example, L2 polypeptides corresponding to HPV L2 17-36, 11-88, and 11-200 were chosen for fusion constructs. Three multi-type constructs were tested: one comprising 3 copies of 11-200 (termed 11-200x3, with L2 peptides from HPV6, 16 and 18), one with 5 copies of 11-88 (termed 11-88x5, from HPV1, 5, 6, 16, and 18), and one with 22 copies of 17-36 (termed 17-36x22), with each L2 peptide being derived from different HPV types (107). The proteins were expressed in bacteria and purified under denaturing conditions, and used to immunize rabbits and mice.
The L2 11-88x5 construct generated higher HPV16 neutralizing antibody titers than the HPV16 L2 1-88 peptide, but 11-200x3 did not induce a greater response than HPV16 L2 11-200. The 17-36x22 was even less effective, although strong responses were seen in rabbits, consistent with weak murine T helper epitopes within this peptide (66). The cross-neutralizing antibody responses observed in mice vaccinated with HPV16 L2 polypeptides were consistently weaker than those generated in rabbits receiving the same vaccines, possibly reflecting their differing mechanisms to generate antibody diversity. However, when comparing the multi-type L2 versus the mono-type HPV16 L2 1-88 and 11-200 constructs, immunization with 11-200x3 and 11-88x5 was far more effective in inducing cross-neutralizing antibodies against HPV45, and HPV58, although the fusion proteins do not contain sequences from either of these HPV types, in addition to inducing neutralizing antibodies against HPV6 and HPV18, which are represented in the fusion proteins.
Mice were challenged with HPV16 pseudovirions at 4 months after vaccination with the L2 11-200x3 in a variety of different adjuvants, including alum, immunostimulatory sequence (ISS) 1018, and the modified saponin GPI-0100. Vaccination with 11-200x3 in any of the adjuvants tested induced significant protection. In particular, immunization with 11-200x3 formulated in alum+ISS1018 was more effective than 11-200x3 alone. All of the GPI-0100 adjuvant formulations tested with 11-200x3 were more effective than 11-200x3 alone or 11-200x3 formulated in alum. These findings suggest the need for a potent adjuvant to maintain long-term protection after vaccination with multi-type L2, although this may confer an increased risk for unwanted reactogenicity.
Capsid display vaccines
The VLP vaccines induce very high titer neutralizing antibody that persists but the responses to denatured L2 antigen are much weaker (108). This may partially reflect the ability of L1, but not L2-specific antibody to bind bivalently to virions providing a very high avidity, although very low avidity antibodies are protective (109). Consequently, linear L2 protein-based vaccines may require the use of a potent adjuvant, increasing the possibility of unwanted reactogenicity. Alternatively, it might be possible to confer the immunogenicity of a VLP upon L2-based epitopes by capsid display (69, 110). The immunogenicity of L1 VLPs is mediated by the close-packed array of the neutralizing epitopes on the virion surface that potentiates B cell activation (68). L1 VLPs are also able to directly activate dendritic cells and this may also contribute to their immunogenicity (111, 112). Since L1 VLP are clearly potent immunogens, several groups have replaced the surface loops comprising the L1 immunodominant neutralizing epitopes with L2 epitopes, including HPV16 L2 65-81 and 108-120 (113). However, the L1 structure is very sensitive to alteration, and this also disrupts some of the major L1 immunodominant epitopes. Varsani et al were able to insert HPV16 L2 108-120 at several sites, and vaccination with these chimeras produced detectable L2-specific antibody, although in vitro neutralization titers were not measured (113). Insertion of either HPV16 L2 65-81 and 108-120 into BPV1 L1 VLP between residues 133 and 134 produced chimeric particles (114). These chimeras were immunogenic and triggered low, but measurable L2-specific neutralizing antibody titers. Thus it is challenging to display a linear L2 neutralizing epitope by replacing or inserting into a conformation L1 neutralizing epitope and render it immunodominant (114).
Others have examined different viral particles whose structure is well characterized for display of L2 epitopes. Palmer et al utilized recombinant tobacco mosaic virus (TMV) to display the L2 94-122 epitope of ROPV and CRPV by insertion between residues 155 and 156 of the U1 coat protein (100). Vaccination of rabbits with these constructs in RIBI adjuvant triggered low titers of CRPV neutralizing antibodies and afforded protection against experimental CRPV challenge (100). These studies demonstrate the potential of this approach, but it is not clear that the optimal selection and placement of the L2 protective epitope has been achieved.
CLINICAL TRIAL DATA
Several L2-based vaccines have been tested in patients. The promising immunogenicity of the HPV16 L2 108-120 peptide vaccine upon intranasal administration in pre-clinical studies led to a placebo-controlled phase I trial (58). Kawana et al. tested intranasal inoculation with placebo (n=3), 0.1 mg (n=5) or 0.5 mg (n=5) of HPV16 L2 108-120 peptide at weeks 0, 4, and 12 (60). The immunization caused no serious local or systemic reactions, but the serum antibody response was very weak. Four of the five patients vaccinated with 0.5 mg of peptide generated detectable HPV16 and HPV52 neutralizing antibody titers, whereas this did not occur in the low dose or placebo cohorts (60). It is likely that the immune response to such L2 protective epitopes after intra-nasal inoculation would be greatly improved by coupling to KLH or using the synthetic lipopeptide approach with an appropriately broadly reactive HLA class II epitope (66, 115, 116).
Two vaccines comprising L2 fused to early viral oncogenes and expressed in bacteria have been tested in patients; TA-CIN is a single fusion protein comprising full length HPV16 L2, E7 and E6, and TA-GW is a single fusion protein comprising HPV6 L2 and E7. Each has potential as a candidate preventive and therapeutic HPV vaccine as they contain capsid antigen and the early viral oncogenes respectively. Both TA-CIN and TA-GW were produced by expression in bacteria and purified under denaturing conditions. A placebo-controlled, double-blinded phase I dose escalation study provided preliminary evidence that three immunizations with 533 μg of TA-CIN without an adjuvant is well-tolerated and immunogenic in healthy volunteers (117). However, this vaccination regimen produced only low titers of L2-specific cross-neutralizing antibodies and weak E6/E7-specific interferon γ and proliferative T cell responses and required high doses of antigen (117, 118). In phase II studies in 29 predominantly HPV16+ high grade vaginal or vulval intraepithelial neoplasia (VAIN or VIN) patients, vaccination with TA-CIN followed by a boost with recombinant vaccinia virus expressing E6 and E7 of both HPV16 and HPV18 (TA-HPV) failed to induce an improved rate of lesion regression as compared to earlier studies utilizing TA-HPV alone in 30 VIN patients (82, 119). Switching the order of vaccination did not provide improved responses (81, 82). The weak antibody and T cell responses to vaccination with TA-CIN protein alone strongly suggested the need to formulate TA-CIN with a potent adjuvant. Studies in primates and mice suggest that the saponin-based adjuvant GPI-0100 greatly enhances the responses to TA-CIN (67). In mice this combination of TA-CIN in GPI-0100 protects against experimental skin infection with HPV16 pseudovirions and challenge with HPV16 E6/E7-transformed tumor challenge (67).
TA-GW was administered at 0, 3, 30 or 300 μg in 1.2 mg of alum to healthy males in a phase I, randomized placebo controlled dose escalation trial (120) and the vaccination was well tolerated. Robust antibody responses were consistently triggered with only the 30 and 300 ug doses. Antigen-specific T cell proliferation and the production of IL5 and interferon gamma were also observed (120). This led to a Phase IIa open-label study in which 27 genital wart patients received 3 immunizations with TA-GW in alum over 4 weeks (121). The TA-GW vaccine was again well-tolerated. All of the patients generated specific serum IgG antibody responses, predominantly IgG1, against L2E7 after vaccination, and 19/25 made L2E7-specific T cell proliferative responses, producing both interferon gamma and IL5. Five of the 27 subjects completely cleared warts within 8 weeks, and the remainder were offered conventional therapy (121). Recurrence of warts was not seen in any of the 13 persons whose warts cleared by vaccine alone or with conventional therapy. These findings led to a large randomized controlled efficacy trial in patients with genital warts. In this trial the L2-E7 antigen was formulated in the AS02A adjuvant which contains the TLR4 agonist monophosphoryl lipid A, the saponin-based adjuvant QS21, and an oil-in-water emulsion (80). AS02A has been shown to induce high antibody titers, a strong CD4+ cell response, and cytotoxic T lymphocyte activity in humans and has an acceptable safety profile. Two placebo-controlled substudies were also performed to evaluate the value of the TA-GW in AS02A adjuvant when administered in addition to standard therapy. Unfortunately, there was no evidence for benefit of vaccination upon time to recurrence (80). There have been no studies addressing the value of vaccination with TA-GW for prevention of acquisition of HPV6 or HPV11 infection or genital warts to date.
Studies to date generally suggest that vaccination with L1 VLPs or L2 does not influence pre-existing infection (23, 72, 79, 80, 83), although there are exceptions (54, 78, 122). The lack of an obvious therapeutic effect likely reflects the absence of detectable capsid gene expression in HPV-infected basal cells. However, these cells do express E6 and E7 and thus E6 and E7-specific T cell responses could potentially eliminate HPV infection and disease. However, vaccination of women with persistent HPV16+ high grade VIN/VAIN with TA-CIN (without adjuvant) in prime boost combinations with recombinant vaccinia virus expressing HPV16/18 E6 and E7 (TA-HPV) failed to enhance the rate of disease clearance (81, 82, 119). Similarly, vaccination with HPV6 L2-E7 in ASO2 adjuvant had no impact on pre-existing genital warts (80). These outcomes may reflect induction of an ineffective type of immune response, a failure of the observed E6/E7-specific T cell responses to traffic to the site of the lesion, or their suppression within the lesion microenvironment, or some combination of these issues (118, 123).
Vaccination using TA-CIN without adjuvant has not been demonstrated to impact the clinical course of pre-existing lesions in randomized trials. However, it is possible that the induction of E6/E7-specific T cells responses prior to HPV infection could prevent the onset of disease from new infections or possibly the reactivation of previously subclinical disease. In the prophylactic setting, issues of tolerance and immune suppression within the lesion microenvironment might be reduced or negated. Indeed, preventive vaccination with TA-CIN+GPI-0100 completely protected mice from TC-1 tumor challenge, whereas vaccination with TA-CIN alone was poorly effective (67). Furthermore, the GPI-0100 adjuvant dramatically boosted the E7-specific IFN-γ secreting CD8+ T cell response in mice as compared to vaccination with TA-CIN alone (67). Vaccination of macaques induced systemic E6 and E7-specific CD4+ and CD8+ T cell responses. Thus it is possible that any protective efficacy against HPV16-related disease by TA-CIN vaccination via the induction of L2-specific neutralizing antibody could potentially be augmented by HPV16 E6/E7-specific T cell responses.
DISCUSSION
Here we have attempted to lay out the case that second generation preventive HPV vaccines are needed. The principle arguments are that the licensed HPV vaccines are too expensive for global implementation and that they are not targeted against all medically relevant HPV genotypes. Cost is particularly significant since over 80% of cervical cancer cases occur in Developing countries that currently also lack comprehensive cytologic screening and secondary prevention programs (38). In Developed nations, expensive national screening programs have impacted the incidence of cervical cancer. Unfortunately, despite these efforts significant numbers of cases still occur, particularly in poor and marginalized populations who may not access the costly licensed vaccines. In addition, because the licensed vaccines only target a subset of oncogenic HPV genotypes, it is likely that the current screening programs will remain in place. Thus the cost of vaccination will be borne in addition to the costs of the screening programs. Further, the reduction in disease burden by implementation of the licensed HPV vaccines will dramatically reduce the predictive value and cost effectiveness of cytologic screening.
How might these issues be addressed? It is unlikely that the licensed HPV vaccines can be produced sustainably at the costs required for global implementation without a switch to local production, and this has intellectual property issues. Local production by companies in Developing countries can achieve dramatic cost savings following the model of numerous childhood immunizations, e.g. the robust biotechnology sectors in India and China. Furthermore, new vaccines would increase the competition and help drive down costs. The production of multiple L1 VLP types in insect cells or even yeast, and formulation of all of these components is complex. It is likely that the production in bacteria of a single polypeptide, as with an L2 fusion protein, could be substantially less expensive to manufacture than a polyvalent L1 VLP vaccine prepared in eukaryotic cells. Indeed, licensed vaccines contain two or four L1 VLP types and eight or nine type vaccines are being tested. Thus if an L2 vaccine were proven effective in people for broad protection against oncogenic and other HPV types, its simpler manufacturing process could make local production of such a vaccine highly feasible. This might achieve the goal of producing an HPV vaccine at sustainable prices in emerging countries and lead to more global implementation of cervical cancer prevention. Broad and long lasting protection against all oncogenic types could also eliminate the need for cytologic screening programs in Developed nations, and L2 vaccines might also prevent the morbidity and costs associated with other non-malignant HPV disease. Unlike genital HPV infection, non-genital HPV infection is typically not transmitted sexually and frequently occurs in children. If future clinical testing of an L2-based vaccine demonstrated protection against infection at non-genital cutaneous sites, in addition to protection against genital HPV infection, it might provide a rationale for administration with other childhood vaccines. This could remove both the logistical challenges of vaccinating adolescents and the theoretical issue that vaccination of adolescents against HPV might influence their sexual behavior.
Table 3.
Summary of published studies in patients utilizing vaccines that contain L2.
| Study (et al), date | Virus - Disease | Dose-Adjuvant | Vaccination Schedule | Trial type |
|---|---|---|---|---|
| Thompson, 1999 | Healthy volunteer | 3× 0,3,30,300 μg HPV6 L2-E7 in alum |
0, 1, 4 wks 0, 4, 8 wks |
Dose escalation (randomized, controlled) |
| Lacey, 1999 | HPV6/11+ Genital warts |
3× 300 μg HPV6 L2-E7 in alum |
0, 1, 4 wks | Therapeutic trial (open label) |
| Vandepapiliere, 2005 | HPV6/11+ Genital warts |
3× ? μg HPV6 L2-E7 in ASO2A |
0, 2, 4 wks | Therapeutic trial (randomized, controlled) |
| De Jong, 2002 | Healthy volunteer | 3× HPV16 L2-E7E6 26, 128, 533 μg, no adjuvant | 0, 4, 8 wks | Dose escalation |
| Smyth, 2004 | HPV16+ AGIN |
3× 533 μg HPV16 L2-E7E6 no adjuvant | Vaccinia E6E7 then at 0, 4, 8 wks | Therapeutic trial |
| Davidson, 2004 | HPV16+ AGIN |
3× 533 μg HPV16 L2-E7E6 no adjuvant | 0, 4, 8 wks then Vaccinia E6E7 boost | Therapeutic trial |
| Fiander, 2006 | HPV16+ AGIN |
3× 533 μg HPV16 L2-E7E6 no adjuvant | 0, 4, 8 wks then Vaccinia E6E7 boost | Therapeutic trial |
| Kawana, 2003 | Healthy volunteer | HPV16 L2 108-120 peptide at 500 μg, 100 μg, no adjuvant | 0, 4, 12 wks | Dose escalation |
Acknowledgments
The authors gratefully acknowledge the support of grants to RBSR from the National Institutes of Health (National Cancer Institute, SPORE in Cervical Cancer, P50 CA098252 and RO1 CA118790), WKH (RO1 CA133749) and to SJ with a fellowship from the Prevent Cancer Foundation, Alexandria, VA.
Footnotes
Conflict of Interest Statement: RBSR is a paid consultant of Merck & Co, and Knobbe, Martens, Olson and Bear LLC. SJ and RBSR have received unrestricted educational grant funding from GSK. RBSR is an inventor on L2 patents licensed to PaxVax, Inc. and Acambis, Inc. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. WKH is a paid consultant and speaker for Merck Inc and a paid consultant for GlaxoSmithKline. He has received research support from both companies.
BIBLIOGRAPHY
- 1.Durst M, Croce CM, Gissmann L, Schwarz E, Huebner K. Papillomavirus sequences integrate near cellular oncogenes in some cervical carcinomas. Proc Natl Acad Sci U S A. 1987;84(4):1070–4. doi: 10.1073/pnas.84.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheurlen W, zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. Embo J. 1984;3(5):1151–7. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003 Feb 6;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004 Aug 20;111(2):278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers [see comments] J Natl Cancer Inst. 2000;92(9):709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 6.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007 May 10;356(19):1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 7.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007 Aug 1;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 8.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group [see comments] J Natl Cancer Inst. 1995;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 9.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. Embo J. 1989;8(12):3905–10. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasseri M, Gage JR, Lorincz A, Wettstein FO. Human papillomavirus type 16 immortalized cervical keratinocytes contain transcripts encoding E6, E7, and E2 initiated at the P97 promoter and express high levels of E7. Virology. 1991;184(1):131–40. doi: 10.1016/0042-6822(91)90829-z. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314(6006):111–4. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 13.Howley PM. Role of the human papillomaviruses in human cancer. Cancer Res. 1991;51(18 Suppl):5019s–22s. [PubMed] [Google Scholar]
- 14.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nature Rev Cancer. 2002 May;2(5):342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 15.Olson C, Segre D, Skidmore LV. Immunity to bovine cutaneous papillomatosis produced by vaccine homologous to the challenge agent. J Am Vet Med Assoc. 1959 Nov 15;135:499–502. [PubMed] [Google Scholar]
- 16.Olson C, Luedke AJ, Brobst DF. Induced immunity of skin, vagina, and urinary bladder to bovine papillomatosis. Cancer Res. 1962 May;22:463–8. [PubMed] [Google Scholar]
- 17.Bell JA, Sundberg JP, Ghim SJ, Newsome J, Jenson AB, Schlegel R. A formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology. 1994;62(4):194–8. doi: 10.1159/000163910. [DOI] [PubMed] [Google Scholar]
- 18.Galloway DA. Human papillomavirus vaccines: a warty problem. Infect Agents Dis. 1994;3(4):187–93. [PubMed] [Google Scholar]
- 19.Lowy DR, Schiller JT. Papillomaviruses and cervical cancer: pathogenesis and vaccine development. J Natl Cancer Inst Monogr. 1998;23:27–30. doi: 10.1093/oxfordjournals.jncimonographs.a024169. [DOI] [PubMed] [Google Scholar]
- 20.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89(24):12180–4. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69(6):3959–63. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92(25):11553–7. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirnbauer R, Chandrachud LM, O’Neil BW, Wagner ER, Grindlay GJ, Armstrong A, et al. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219(1):37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- 24.Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol. 1996;70(2):960–5. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93(4):284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 26.Evans TG, Bonnez W, Rose RC, Koenig S, Demeter L, Suzich JA, et al. A Phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J Infect Dis. 2001;183(10):1485–93. doi: 10.1086/320190. [DOI] [PubMed] [Google Scholar]
- 27.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002 Nov 21;347(21):1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 28.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004 Nov 13;364(9447):1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 29.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006 Apr 15;367(9518):1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 30.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005 May;6(5):271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 31.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007 Jun 30;369(9580):2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 32.Roden RB, Greenstone HL, Kirnbauer R, Booy FP, Jessie J, Lowy DR, et al. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996;70(9):5875–83. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roden RB, Hubbert NL, Kirnbauer R, Christensen ND, Lowy DR, Schiller JT. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J Virol. 1996;70(5):3298–301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giroglou T, Sapp M, Lane C, Fligge C, Christensen ND, Streeck RE, et al. Immunological analyses of human papillomavirus capsids. Vaccine. 2001 Feb 8;19(13–14):1783–93. doi: 10.1016/s0264-410x(00)00370-4. [DOI] [PubMed] [Google Scholar]
- 35.White WI, Wilson SD, Bonnez W, Rose RC, Koenig S, Suzich JA. In vitro infection and type-restricted antibody-mediated neutralization of authentic human papillomavirus type 16. J Virol. 1998;72(2):959–64. doi: 10.1128/jvi.72.2.959-964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roden RB, Ling M, Wu TC. Vaccination to prevent and treat cervical cancer. Hum Pathol. 2004 Aug;35(8):971–82. doi: 10.1016/j.humpath.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006 Oct;6(10):753–63. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006 Aug 21;24(Suppl 3):S11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 39.Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer. 2007 Jun 25;111(3):145–53. doi: 10.1002/cncr.22751. [DOI] [PubMed] [Google Scholar]
- 40.Roden RB, Day PM, Bronzo BK, Yutzy WHt, Yang Y, Lowy DR, et al. Positively charged termini of the L2 minor capsid protein are necessary for papillomavirus infection. J Virol. 2001;75(21):10493–7. doi: 10.1128/JVI.75.21.10493-10497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang R, Day PM, Yutzy WHt, Lin KY, Hung CF, Roden RB. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J Virol. 2003 Mar;77(6):3531–41. doi: 10.1128/JVI.77.6.3531-3541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang R, Yutzy WHt, Viscidi RP, Roden RB. Interaction of L2 with beta-actin directs intracellular transport of papillomavirus and infection. J Biol Chem. 2003 Apr 4;278(14):12546–53. doi: 10.1074/jbc.M208691200. [DOI] [PubMed] [Google Scholar]
- 43.Bossis I, Roden RB, Gambhira R, Yang R, Tagaya M, Howley PM, et al. Interaction of tSNARE syntaxin 18 with the papillomavirus minor capsid protein mediates infection. J Virol. 2005 Jun;79(11):6723–31. doi: 10.1128/JVI.79.11.6723-6731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campo MS. Vaccination against papillomavirus. Cancer Cells. 1991;3(11):421–6. [PubMed] [Google Scholar]
- 45.Campo MS. Vaccination against papillomavirus in cattle. Curr Top Microbiol Immunol. 1994;186:255–66. doi: 10.1007/978-3-642-78487-3_13. [DOI] [PubMed] [Google Scholar]
- 46.Chandrachud LM, Grindlay GJ, McGarvie GM, O’Neil BW, Wagner ER, Jarrett WF, et al. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211(1):204–8. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- 47.Campo MS, O’Neil BW, Grindlay GJ, Curtis F, Knowles G, Chandrachud L. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology. 1997;234(2):261–6. doi: 10.1006/viro.1997.8649. [DOI] [PubMed] [Google Scholar]
- 48.Campo MS. Vaccination against papillomavirus in cattle. Clin Dermatol. 1997;15(2):275–83. doi: 10.1016/s0738-081x(96)00165-4. [DOI] [PubMed] [Google Scholar]
- 49.McGarvie GM, Chandrachud L, Gaukroger JM, Grindlay GJ, O’Neil BW, Baird JW, et al. Vaccination of cattle with L2 protein prevents BPV-4 infection. In: Stanley MA, editor. Immunology of Human Papillomaviruses. New York: Plenum Press; 1994. pp. 283–90. [Google Scholar]
- 50.Gaukroger JM, Chandrachud LM, O’Neil BW, Grindlay GJ, Knowles G, Campo MS. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J Gen Virol. 1996;77(Pt 7):1577–83. doi: 10.1099/0022-1317-77-7-1577. [DOI] [PubMed] [Google Scholar]
- 51.Knowles G, Grindlay GJ, Campo MS, Chandrachud LM, O’Neil BW. Linear B-cell epitopes in the N-terminus of L2 of bovine papillomavirus type 4. Res Vet Sci. 1997;62(3):289–91. doi: 10.1016/s0034-5288(97)90207-1. [DOI] [PubMed] [Google Scholar]
- 52.Christensen ND, Kreider JW, Kan NC, DiAngelo SL. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181(2):572–9. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- 53.Roden RB, Yutzy WI, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 54.Jarrett WF, Smith KT, O’Neil BW, Gaukroger JM, Chandrachud LM, Grindlay GJ, et al. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology. 1991;184(1):33–42. doi: 10.1016/0042-6822(91)90819-w. [DOI] [PubMed] [Google Scholar]
- 55.Roden RB, Weissinger EM, Henderson DW, Booy F, Kirnbauer R, Mushinski JF, et al. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J Virol. 1994;68(11):7570–4. doi: 10.1128/jvi.68.11.7570-7574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin YL, Borenstein LA, Selvakumar R, Ahmed R, Wettstein FO. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992;187(2):612–9. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- 57.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005 Jul 5;337(2):365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Kawana K, Kawana Y, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Nasal immunization of mice with peptide having a cross-neutralization epitope on minor capsid protein L2 of human papillomavirus type 16 elicit systemic and mucosal antibodies. Vaccine. 2001;19(11–12):1496–502. doi: 10.1016/s0264-410x(00)00367-4. [DOI] [PubMed] [Google Scholar]
- 59.Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J Virol. 1999;73(7):6188–90. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawana K, Yasugi T, Kanda T, Kino N, Oda K, Okada S, et al. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine. 2003 Oct 1;21(27–30):4256–60. doi: 10.1016/s0264-410x(03)00454-7. [DOI] [PubMed] [Google Scholar]
- 61.Kawana K, Matsumoto K, Yoshikawa H, Taketani Y, Kawana T, Yoshiike K, et al. A surface immunodeterminant of human papillomavirus type 16 minor capsid protein L2. Virology. 1998;245(2):353–9. doi: 10.1006/viro.1998.9168. [DOI] [PubMed] [Google Scholar]
- 62.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, et al. A protective and broadly cross-neutralizing epitope of Human Papillomavirus L2. J Virol. 2007 Dec;81(24):13927–31. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004 Jan;78(2):751–7. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004 Apr 10;321(2):205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 65.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007 Jul;13(7):857–61. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 66.Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci U S A. 2008 Apr 15;105(15):5850–5. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karanam B, Gambhira R, Peng S, Jagu S, Kim DJ, Ketner GW, et al. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine. 2008 Dec 16; doi: 10.1016/j.vaccine.2008.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262(5138):1448–51. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 69.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–70. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 70.Trus BL, Roden RB, Greenstone HL, Vrhel M, Schiller JT, Booy FP. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 A resolution. Nat Struct Biol. 1997;4(5):413–20. doi: 10.1038/nsb0597-413. [DOI] [PubMed] [Google Scholar]
- 71.Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. J Virol. 2008 May;82(9):4638–46. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N-terminus of HPV16 minor capsid antigen L2. J Virol. 2007 Aug 22;81(21):13927–31. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171(6):1387–98. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 74.Burton DR. Antibodies, viruses and vaccines. Nature Rev Immunol. 2002;2(Sept):706–13. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 75.Rudolf MP, Nieland JD, DaSilva DM, Velders MP, Muller M, Greenstone HL, et al. Induction of HPV16 capsid protein-specific human T cell responses by virus-like particles. Biol Chem. 1999;380(3):335–40. doi: 10.1515/BC.1999.045. [DOI] [PubMed] [Google Scholar]
- 76.Ohlschlager P, Osen W, Dell K, Faath S, Garcea RL, Jochmus I, et al. Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J Virol. 2003 Apr;77(8):4635–45. doi: 10.1128/JVI.77.8.4635-4645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinto LA, Viscidi R, Harro CD, Kemp TJ, Garcia-Pineres AJ, Trivett M, et al. Cellular immune responses to HPV-18, -31, and -53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006 Sep 30;353(2):451–62. doi: 10.1016/j.virol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 78.Zhang LF, Zhou J, Chen S, Cai LL, Bao QY, Zheng FY, et al. HPV6b virus like particles are potent immunogens without adjuvant in man. Vaccine. 2000;18(11–12):1051–8. doi: 10.1016/s0264-410x(99)00351-5. [DOI] [PubMed] [Google Scholar]
- 79.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. Jama. 2007 Aug 15;298(7):743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 80.Vandepapeliere P, Barrasso R, Meijer CJ, Walboomers JM, Wettendorff M, Stanberry LR, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005 Dec 15;192(12):2099–107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- 81.Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004 May 1;10(9):2954–61. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 82.Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006 May-Jun;16(3):1075–81. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 83.Embers ME, Budgeon LR, Pickel M, Christensen ND. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of l2, the minor capsid protein. J Virol. 2002 Oct 1;76(19):9798–805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin Z, Yemelyanova AV, Gambhira R, Jagu S, Meyers C, Kirnbauer R, et al. Expression pattern and subcellular localization of human papillomavirus minor capsid protein L2. Am J Pathol. 2009 Jan;174(1):136–43. doi: 10.2353/ajpath.2009.080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007 Jun 21;25(26):4931–9. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 86.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008 Dec 9;26(52):6844–51. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 87.Kemp TJ, Garcia-Pineres A, Falk RT, Poncelet S, Dessy F, Giannini SL, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008 Jul 4;26(29–30):3608–16. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pastrana DV, Vass WC, Lowy DR, Schiller JT. NHPV16 VLP vaccine induces human antibodies that neutralize divergent variants of HPV16. Virology. 2001;279(1):361–9. doi: 10.1006/viro.2000.0702. [DOI] [PubMed] [Google Scholar]
- 89.Sapp M, Day PM. Structure, attachment and entry of polyoma- and papillomaviruses. Virology. 2009 Jan 20; doi: 10.1016/j.virol.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 90.Dimmock NJ. Neutralization of animal viruses. Curr Top Microbiol Immunol. 1993;183:1–149. doi: 10.1007/978-3-642-77849-0. [DOI] [PubMed] [Google Scholar]
- 91.Booy FP, Roden RB, Greenstone HL, Schiller JT, Trus BL. Two antibodies that neutralize papillomavirus by different mechanisms show distinct binding patterns at 13 A resolution. J Mol Biol. 1998;281(1):95–106. doi: 10.1006/jmbi.1998.1920. [DOI] [PubMed] [Google Scholar]
- 92.Belnap DM, Olson NH, Cladel NM, Newcomb WW, Brown JC, Kreider JW, et al. Conserved features in papillomavirus and polyomavirus capsids. J Mol Biol. 1996;259(2):249–63. doi: 10.1006/jmbi.1996.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005 Mar;79(5):2839–46. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roden RB, Kirnbauer R, Jenson AB, Lowy DR, Schiller JT. Interaction of papillomaviruses with the cell surface. J Virol. 1994;68(11):7260–6. doi: 10.1128/jvi.68.11.7260-7266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Day PM, Thompson CD, Buck CB, Pang YY, Lowy DR, Schiller JT. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J Virol. 2007 Aug;81(16):8784–92. doi: 10.1128/JVI.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roden RB, Armstrong A, Haderer P, Christensen ND, Hubbert NL, Lowy DR, et al. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. J Virol. 1997;71(8):6247–52. doi: 10.1128/jvi.71.8.6247-6252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richards RM, Lowy DR, Schiller JT, Day PM. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1522–7. doi: 10.1073/pnas.0508815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roden RB, Hubbert NL, Kirnbauer R, Breitburd F, Lowy DR, Schiller JT. Papillomavirus L1 capsids agglutinate mouse erythrocytes through a proteinaceous receptor. J Virol. 1995;69(8):5147–51. doi: 10.1128/jvi.69.8.5147-5151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu WJ, Gissmann L, Sun XY, Kanjanahaluethai A, Muller M, Doorbar J, et al. Sequence close to the N-terminus of L2 protein is displayed on the surface of bovine papillomavirus type 1 virions. Virology. 1997;227(2):474–83. doi: 10.1006/viro.1996.8348. [DOI] [PubMed] [Google Scholar]
- 100.Palmer KE, Benko A, Doucette SA, Cameron TI, Foster T, Hanley KM, et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006 Jun 29;24(26):5516–25. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 101.Laniosz V, Nguyen KC, Meneses PI. Bovine papillomavirus type 1 infection is mediated by SNARE syntaxin 18. J Virol. 2007 Jul;81(14):7435–48. doi: 10.1128/JVI.00571-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamper N, Day PM, Nowak T, Selinka HC, Florin L, Bolscher J, et al. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. J Virol. 2006 Jan;80(2):759–68. doi: 10.1128/JVI.80.2.759-768.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jackson DC, Lau YF, Le T, Suhrbier A, Deliyannis G, Cheers C, et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004 Oct 26;101(43):15440–5. doi: 10.1073/pnas.0406740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jackson DC, Purcell AW, Fitzmaurice CJ, Zeng W, Hart DN. The central role played by peptides in the immune response and the design of peptide-based vaccines against infectious diseases and cancer. Curr Drug Targets. 2002 Apr;3(2):175–96. doi: 10.2174/1389450024605436. [DOI] [PubMed] [Google Scholar]
- 105.Nardelli-Haefliger D, Lurati F, Wirthner D, Spertini F, Schiller JT, Lowy DR, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005 May 25;23(28):3634–41. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 106.Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, et al. Concatenated Multi-type L2 Fusion Proteins as Candidate Prophylactic Pan-Human Papillomavirus Vaccines. 2009 doi: 10.1093/jnci/djp106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004 Jun 20;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 108.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006 Mar;6(3):231–43. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 109.Bachmann MF, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, et al. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276(5321):2024–7. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 110.Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol. 2002 Dec 1;169(11):6120–6. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- 111.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, et al. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166(9):5346–55. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 112.Lenz P, Thompson CD, Day PM, Bacot SM, Lowy DR, Schiller JT. Interaction of papillomavirus virus-like particles with human myeloid antigen-presenting cells. Clin Immunol. 2003 Mar;106(3):231–7. doi: 10.1016/s1521-6616(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 113.Varsani A, Williamson AL, de Villiers D, Becker I, Christensen ND, Rybicki EP. Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16. J Virol. 2003 Aug;77(15):8386–93. doi: 10.1128/JVI.77.15.8386-8393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Slupetzky K, Gambhira R, Culp TD, Shafti-Keramat S, Schellenbacher C, Christensen ND, et al. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2007 Mar 1;25(11):2001–10. doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ragupathi G, Gathuru J, Livingston P. Antibody inducing polyvalent cancer vaccines. Cancer Treat Res. 2005;123:157–80. doi: 10.1007/0-387-27545-2_7. [DOI] [PubMed] [Google Scholar]
- 116.Ragupathi G, Koide F, Sathyan N, Kagan E, Spassova M, Bornmann W, et al. A preclinical study comparing approaches for augmenting the immunogenicity of a heptavalent KLH-conjugate vaccine against epithelial cancers. Cancer Immunol Immunother. 2003 Oct;52(10):608–16. doi: 10.1007/s00262-003-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Jong A, O’Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002 Oct 4;20(29–30):3456–64. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 118.Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RB. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res. 2006 Dec 1;66(23):11120–4. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]
- 119.Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003 Sep 15;63(18):6032–41. [PubMed] [Google Scholar]
- 120.Thompson HS, Davies ML, Holding FP, Fallon RE, Mann AE, O’Neill T, et al. Phase I safety and antigenicity of TA-GW: a recombinant HPV6 L2E7 vaccine for the treatment of genital warts. Vaccine. 1999;17(1):40–9. doi: 10.1016/s0264-410x(98)00146-7. [DOI] [PubMed] [Google Scholar]
- 121.Lacey CJ, Thompson HS, Monteiro EF, O’Neill T, Davies ML, Holding FP, et al. Phase IIa safety and immunogenicity of a therapeutic vaccine, TA-GW, in persons with genital warts. J Infect Dis. 1999;179(3):612–8. doi: 10.1086/314616. [DOI] [PubMed] [Google Scholar]
- 122.Govan VA, Rybicki EP, Williamson AL. Therapeutic immunisation of rabbits with cottontail rabbit papillomavirus (CRPV) virus-like particles (VLP) induces regression of established papillomas. Virol J. 2008;5:45. doi: 10.1186/1743-422X-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A. 2007 Jul 17;104(29):12087–92. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Buck CB, Cheng N, Thompson CD, Lowy DR, Steven AC, Schiller JT, et al. Arrangement of L2 within the papillomavirus capsid. J Virol. 2008 Jun;82(11):5190–7. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baker TS, Newcomb WW, Olson NH, Cowsert LM, Olson C, Brown JC. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991;60(6):1445–56. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hagensee ME, Olson NH, Baker TS, Galloway DA. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J Virol. 1994;68(7):4503–5. doi: 10.1128/jvi.68.7.4503-4505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]