Abstract
Rationale and Objectives
Malignant pleural mesothelioma (MPM) is a neoplasm that grows circumferentially along the pleura. The tumor and concurrent pleural effusion may reduce lung function by restricting or preventing lung expansion. The purpose of this study was to provide objective evidence that pleurectomy/decortication (P/D) allows trapped lung to re-expand, quantify the re-expansion based on computed tomography (CT) scans, and investigate whether the expansion persists after surgery.
Materials and Methods
A database of 12 patients demonstrating unilateral MPM was collected. Each patient underwent a pre-surgical CT scan, surgical debulking by P/D, and two post-surgical CT scans (at one and four months). The lung volume was measured in each scan using an automated algorithm and compared for each patient across time.
Results
An increase in the ipsilateral post-surgical lung volume was observed for 10 of 12 patients (83%) one month after surgery. The median ipsilateral volume increase was 44% relative to the pre-surgical ipsilateral volume and 21% relative to the contralateral volume. A statistically significant change in ipsilateral lung volume was not observed between 1 -month and 4-month post-surgical scans, implying that the volume improvement persisted months after surgery.
Conclusions
Debulking of MPM with P/D substantially increased the ipsilateral lung volume relative to both the pre-surgical, ipsilateral volume and the contralateral lung volume. This improvement persisted months after surgery.
Rationale and Objectives
Malignant pleural mesothelioma (MPM) is a neoplasm of the mesothelial cells principally caused by asbestos exposure. MPM is a primarily unilateral disease that grows non-uniformly along the parietal and visceral pleurae and encases the lung. MPM often presents in computed tomography (CT) scans as pleural thickening growing circumferentially about the lung parenchyma with or without concurrent effusion. Pressure on the lung parenchyma from the tumor and effusion may reduce lung function by restricting or preventing lung expansion (a condition known as trapped/encased lung or restrictive pleurisy).1
Extrapleural pneumonectomy (EPP) and pleurectomy/decortication (P/D) are the principal surgical approaches for MPM. EPP is an aggressive surgical procedure with strict selection criteria that limit its applicability to the general MPM population.2 The procedure consists of removing the lung, pericardium, diaphragm, and pleura with extrapleural dissection, then reconstructing the pericardium and diaphragm.3 EPP is associated with increased patient survival, but also high morbidity and mortality rates (though recent studies suggest that these rates are less in centers specially trained for EPP surgical procedures).1, 2, 4
P/D is the resection of mesothelioma tumor with visceral and parietal pleura, while preserving the lung parenchyma. Resection and reconstruction of the pericardium and/or diaphragm3, 5 is also often performed in the affected hemithorax. Mortality and morbidity rates are substantially lower for P/D than EPP and the less extensive nature of P/D means that it is available to more MPM patients (some of which are not suitable candidates for EPP).3, 5 Although complete macroscopic resection is possible, it is often difficult to completely remove MPM (especially along the visceral pleura) and residual tumor rates in 44% to 78% of patients have been reported.6, 7 P/D is often applied for palliative effect to debulk the gross tumor and relieve symptoms such as shortness of breath and chest pains. In cases of more advanced disease where expansion of the lung is hindered by tumor and/or effusion, P/D is employed to free the lung and improve respiratory mechanics.2 In patients with chronic pleural empyema who undergo decortication,8 spirometric and scintographic measurements indicate that forced expiratory volume, vital capacity, and perfusion all improved post-surgery.
The purpose of this study was twofold. First, this study objectively measured the benefit of surgical intervention for mesothelioma patients by directly measuring the effect of P/D on the lung volume. Second, the lung volume was measured several months after surgery to determine whether surgical benefits persisted over time.
Materials and Methods
A database of 12 MPM patients (8 males and 4 females; age: 57 - 75 years, median: 69 years) who underwent surgical debulking with P/D at XXXX was reviewed retrospectively under approval by XXX Institutional Review Board (IRB #XXXX). Patient consent was not required for the retrospective analysis of anonymized patient images. Histology, tumor, node, metastasis (TNM) staging, and chemotherapeutic treatment history were recorded (Table 1). Each patient demonstrated unilateral mesothelioma (right hemithorax n = 9 and left hemithorax n = 3). A helical thoracic CT scan was acquired for each patient before surgery for surgical planning (n = 12; median 15 days) and twice after surgery at one month (n = 12, median 37 days) and four months (n = 10; median 144 days) as part of routine patient follow-up. Scans were performed on a Philips (Hartford, CT) Brilliance 16-Slice (n = 8), Brilliance 40-Channel (n = 1), Brilliance 64-Channel (n = 21), Brilliance iCT (n = 2), or Siemens (Munich, Germany) Emotion Duo (n = 1). Peak voltage was 120 kVp (n = 30), 130 kVp (n = 1), or 100 (n = 1), pixel size ranged from 0.475 - 0.977 mm and section thickness was 0.9 mm (n = 2), 1 mm (n = 26), 3 mm (n = 1), 4 mm (n = 1), or 5 mm (n = 2).
Table 1.
Patient Staging, Histology, and Chemotherapeutic Treatment History
| Patient | Staging | Histology | Pre-surgical Treatments |
Post-surgical Treatments |
Treatment Initiation (Months After Surgery) |
|---|---|---|---|---|---|
| 1 | T4 Nx Mx Stage IV |
Epithelioid | No | AZD2171 | 3 |
| 2 | T3 N0 Mx Stage III |
Mixed | No | Bevacizumab, Pemetrexate, and Cisplatin |
1 |
| 3 | T3 Nx Mx Stage III |
Epithelioid | No | Pemetrexed and Carboplatin |
3 |
| 4 | T2 N0 Mx Stage II |
Epithelioid | No | Pemetrexed and Carboplatin |
1 |
| 5 | T3 N0 Mx Stage III |
Epithelioid | No | Pemetrexed and Carboplatin |
1 |
| 6 | T3 N0 Mx Stage III |
Epithelioid | No | Pemetrexed and Carboplatin |
2 |
| 7 | T3 Nx Mx Stage III |
Mixed | No | Pemetrexed and Cisplatin |
3 |
| 8 | T3 N0 Mx Stage III |
Epithelioid | No | Pemetrexed and Carboplatin |
1 |
| 9 | T2 N1 Mx Stage III |
Mixed | No | Pemetrexed and Carboplatin |
3 |
| 10 | T2 Nx Mx Stage II/III |
Mixed | No | Pemetrexed and Carboplatin |
2 |
| 11 | T3 N0 Mx Stage III |
Epithelioid | No | None | N/A |
| 12 | T3 Nx Mx Stage III |
Epithelioid | No | Pemetrexed and Carboplatin |
1 |
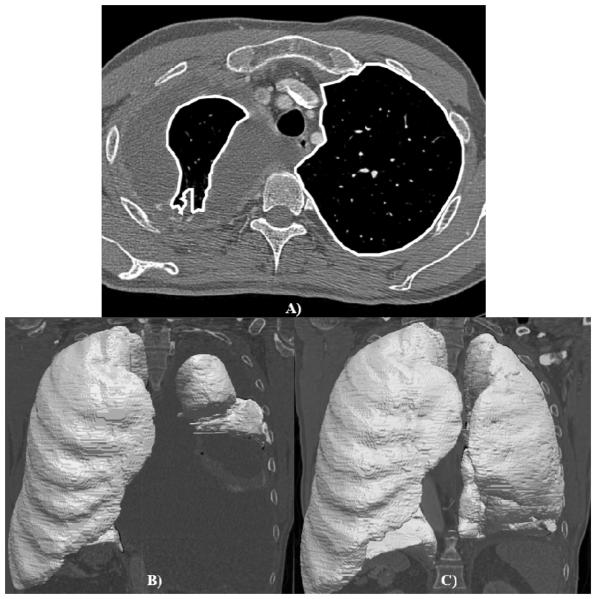
The lung volume in each scan was determined by applying a computerized segmentation algorithm to each scan (Figure 1). The computerized method uses gray-level, morphological, and texture features to segment aerated lung parenchyma from the surrounding tissue. The resulting segmentations were then reviewed by an experienced observer (WFS) to verify the accuracy of the segmentations. Once proper segmentation of the aerated lung parenchyma was confirmed, the volume of each aerated lung region was calculated from the number of voxels contained in each segmented lung multiplied by the pixel size squared and section thickness. Total lung volume was calculated for the ipsilateral and contralateral lungs in each scan by summing the volumes of the corresponding segmented regions across all sections of the scan.
Figure 1.
Segmented lung parenchyma. (A) Axial CT section with lung parenchyma segmentation (while outlines) superimposed. Segmented pre-surgical (B) and 1 -month post-surgical (C) lung volumes of the same patient.
Different levels of patient inspiration on serial CT scans may cause measurement errors when calculating the volumetric change due to surgical intervention. The right lung and left lung volumes for each patient are similar,9 thus the volume of the contralateral lung was used to normalize the volume of the ipsilateral lung and remove the effects of variable inspiration from the measurements. The normalized ipsilateral lung volume was recorded for each CT scan of each patient, and the absolute change in normalized ipsilateral lung volume between 1) pre-surgical scans and 1 -month, post-surgical scans and 2) 1 -month, post-surgical scans and 4-month, post-surgical scans was calculated according to
| (Eq. 1) |
where V is the lung volume, ips indicates the ipsilateral lung, contra indicates the contralateral lung, t indicates scan time, and ΔVabs is the change in normalized lung volume. A Wilcoxon signed-rank test (5% significance) was applied to determine whether changes in normalized lung volume were statistically significant. The normalized change of the ipsilateral lung volume one month after surgery as a proportion of the pre-surgical, ipsilateral lung volume was also calculated:
| (Eq. 2) |
Results
The average pre-surgical ipsilateral lung volume was 1259.76 ± 865.07 cm3 (range: 105.49 - 2995.12 cm3) and the average pre-surgical contralateral lung volume was 2842.44 ± 984.12 cm3 (range: 1296.62 - 5019.02 cm3). The median pre-surgical, normalized ipsilateral lung volume was 0.478 (range: 0.081 - 0.754).
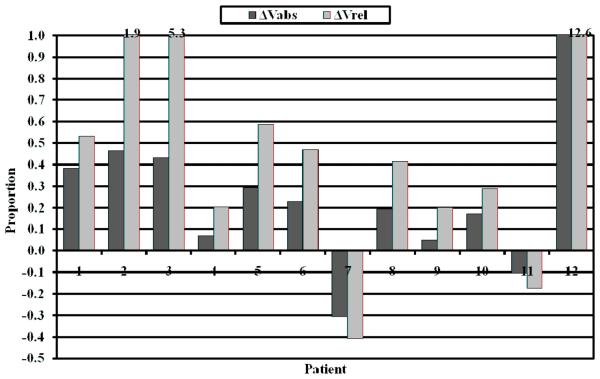
The median change in normalized ipsilateral lung volume (ΔVabs) between the 1-month post-surgical scan and the pre-surgical scan was 0.212 (range: −0.308 - 1.207) and between the 4-month post-surgical scan and the 1-month post-surgical scan was 0.044 (range: −0.338 - 0.189). The change between the pre-surgical and 1-month post-surgical scans was statistically significant (p = 0.027); however, the change between the 1-month post-surgical and the 4-month postsurgical scans was not statistically significant (p = 0.322). The median normalized change of the ipsilateral lung volume one month after surgery as a proportion of the pre-surgical lung volume (ΔVrel) was 0.443 (range: −0.408 – 12.627; p = 0.007). Figure 2 illustrates ΔVrel and ΔVabs for each patient one month after surgery. An increase in the normalized ipsilateral post-surgical lung volume was observed for 10 of 12 patients (83%) one month after surgery and 8 of 10 patients (80%) four months after surgery. All patients demonstrating increased lung volume one month after surgery continued to demonstrate increased lung volume four months after surgery. The results are summarized in Table 2. The ΔV measurements made at 4-months demonstrated no trend with the timing of chemotherapy initiation.
Figure 2.
Bar graph demonstrating the change in normalized ipsilateral lung volume one month after surgery relative to pre-surgical ipsilateral volume (ΔVrel; light gray) and contralateral volume (ΔVabs; dark gray).
Table 2.
Summary of Lung Volume Measurements
| Median | Range | P Value | |
|---|---|---|---|
| Ratio of pre-surgical ipsilateral lung volume to contralateral lung volume |
0.478 | 0.081, 0.754 | - |
| Lung expansion at 1-month relative to the pre-surgical ipsilateral volume (ΔVrel) |
44.3% | −40.8%, 1262.7% | 0.007 |
| Lung expansion at 1-month relative to contralateral volume (ΔVabs) |
21.2% | −30.8%, 120.7% | 0.027 |
| Lung volume change between 1-month and 4-months after surgery relative to 1-month volume (ΔVrel) |
7.5% | −30.5%, 36.9% | 0.232 |
| Lung volume change between 1-month and 4-months after surgery relative to the contralateral volume (ΔVabs) |
4.4% | −33.8%, 18.9% | 0.322 |
Discussion
P/D is often applied to MPM patients as a palliative treatment to free trapped lung and improve respiratory mechanics; however, previous studies have neither confirmed nor quantified lung volume improvement in this population. A computerized segmentation method was applied to a database of serial CT scans of mesothelioma patients who underwent P/D to debulk existing MPM tumor to measure lung re-expansion. A computerized method segmented the aerated lung parenchyma in each section of each scan. Lung volume was measured based on these segmentations for the ipsilateral and contralateral lungs from one pre-surgical and two postsurgical CT scans of each of 12 patients. The ipsilateral lung volume was normalized to the contralateral lung volume in each scan. This normalization was necessary for two reasons. First, the absolute lung volume is dependent on patient size (i.e., larger patients will have larger lungs). Normalization brings measurements from patients of different sizes to a common scale and thus mitigates inter-patient lung volume variability. Second, patients may exhibit different inspiration levels on serial scans. Normalization mitigates this difference by measuring the ratio of ipsilateral volume to contralateral volume.
The normalized ipsilateral lung volume change between pre-surgical and one month postsurgical scans relative to the pre-surgical ipsilateral lung volume (ΔVrel; Eq. 2) was calculated to objectively measure the re-expansion of the affected lung that results from P/D. The substantial and significant increase post-surgery indicates that P/D successfully frees the lung parenchyma for re-expansion. Although the lung may substantially re-expand relative to its pre-surgical size, it is not clear from this value whether the re-expanded lung volume is sufficient to improve a patient's respiratory mechanics. As an extreme example consider an MPM patient with only 10 cm3 of pre-surgical, ipsilateral lung volume and 2000 cm3 of pre-surgical, contralateral lung volume. If the post-surgical, ipsilateral lung volume is increased to 20 cm3 and the contralateral lung volume remains unchanged (due to consistent inspiration level), then the re-expansion relative to pre-surgical, ipsilateral volume (ΔVrel) is substantial (100% increase as calculated by Eq. 2); however, the post-surgical, ipsilateral volume is not substantial relative to the volume it would occupy in the absence of disease as represented by the pre-surgical, contralateral lung volume (0.5% as calculated by Eq. 1).
The median change in ipsilateral lung volume (ΔVabs) was calculated to objectively measure whether the ipsilateral lung volume increase is a substantial portion of the volume the lung would be in the absence of disease. The substantial and significant increase one month after surgery provides strong evidence justifying the application of P/D as a palliative treatment option to free trapped lung. The normalized ipsilateral lung volume was measured four months after surgery to investigate whether swelling and muscle weakness caused by surgery resulted in artificially low lung volume measurements. The small increase in normalized ipsilateral lung volume between the one-month and four-month post-surgical scans may indicate that postsurgical swelling and muscle weakness impact lung volume; however this increase was not statistically significant. These measurements also imply that lung volume improvement persists months after surgery and that post-surgical growth of tumor does not significantly reduce lung volume. Although the majority of patients demonstrated improvement due to P/D, two patients were observed to have decreased normalized lung volume. The decrease in one patient (Patient 7 in Figure 2) was due to the development of apical fibrosis and small loculated effusions that formed in the affected hemithorax after surgery. The decrease in the second patient (Patient 11 in Figure 2) is from superior migration of the diaphragm in the ipsilateral hemithorax due to suspected phrenic nerve paralysis.
The major limitation of this study is the small patient database. The low incidence of MPM and the still lower percentage of patients that are P/D surgical candidates who underwent both pre-surgical and post-surgical CT scans make collection of a large patient database difficult. The database was sufficient for measuring median ipsilateral lung volume improvement due to surgery and the persistence of this improvement, but we were unable to investigate covariates (such as sex, age, histology, and normalized pre-surgical ipsilateral lung volume) that could be predictive of surgical success. Furthermore, while no patient underwent chemotherapy before surgery, almost all patients began a regimen of chemotherapy between one and four months after surgery. No trend was observed between the volume measurements and the time that chemotherapy began. However, because our database did not include a control group of P/D patients without subsequent chemotherapy, it is impossible to determine if the continued improvement observed at four months was contingent on the administration of chemotherapy.
In conclusion, debulking of MPM with P/D significantly and substantially increased the ipsilateral lung volume relative to both the pre-surgical, ipsilateral volume and the contralateral lung volume. This improvement persisted months after surgery and supports the use of P/D as a palliative treatment to relieve restrictive pleurisy for MPM patients.
Acknowledgments
Author contributions: The authors would also like to thank Corson and Michael Torno for their help collecting the patient database.
This study was funded in part by National Institutes of Health (NIH) Grant: R01CA10208
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kent M, Rice D, Flores R. Diagnosis, staging, and surgical treatment of malignant pleural mesothelioma. Curr Treat Options Oncol. 2008;9:158–170. doi: 10.1007/s11864-008-0070-4. [DOI] [PubMed] [Google Scholar]
- 2.Nakas A, Trousse DS, Martin-Ucar AE, et al. Open lung-sparing surgery for malignant pleural mesothelioma: the benefits of a radical approach within multimodality therapy. Eur J Cardiothorac Surg. 2008;34:886–891. doi: 10.1016/j.ejcts.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 3.van Ruth S, Baas P, Zoetmulder FAN. Surgical treatment of malignant pleural mesothelioma. Chest. 2003;123:551–561. doi: 10.1378/chest.123.2.551. [DOI] [PubMed] [Google Scholar]
- 4.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–626. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 5.Rusch VW. Pleurectomy/decortication in the setting of multimodality treatment for diffuse malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 1997;9:367–372. [PubMed] [Google Scholar]
- 6.Hilaris BS, Nori D, Kwong E, et al. Pleurectomy and intraoperative brachytherapy and postoperative radiation in the treatment of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 1984;10:325–331. doi: 10.1016/0360-3016(84)90050-6. [DOI] [PubMed] [Google Scholar]
- 7.Soysal O, Karaoglanoglu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210–213. doi: 10.1016/s1010-7940(96)01008-1. [DOI] [PubMed] [Google Scholar]
- 8.Rzyman W, Skokowski J, Romanowicz G, et al. Decortication in chronic pleural empyema-effect on lung function. Eur J Cardiothorac Surg. 2002;21:502–507. doi: 10.1016/s1010-7940(01)01167-8. [DOI] [PubMed] [Google Scholar]
- 9.Chun EM, Suh SW, Modi HN, et al. the change in ratio of convex and concave lung volume in adolescent idiopathic scoliosis: A 3D CT scan based cross sectional study of effect of severity of curve on convex and concave lung volumes in 99 cases. Eur Spine J. 2008;17:224–22. doi: 10.1007/s00586-007-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]