Members of the transforming growth factor β (TGF-β) superfamily of cytokines are important regulators of many fundamental cellular and developmental processes, including cell fate determination, proliferation, differentiation, migration, and apoptosis (1). TGF-β also harbors a paradoxical dual role in tumorigenesis. During early tumor development, TGF-β has tumor-suppressor-like activity because of its ability to inhibit cell-cycle progression and tumor growth. However, many late-stage, dedifferentiated tumor cells become refractory to the growth inhibition mediated by TGF-β, either because of genetic loss of TGF-β signaling components or downstream perturbation of the signaling pathway (2). In addition, late-stage tumors often display increased TGF-β expression (3), which is thought to enhance the motility and metastasis potential of these cells (4). Furthermore, although TGF-β is a potent growth inhibitor to most normal epithelial cells, it can also stimulate the proliferation of some fibroblastic cell lines, such as NIH 3T3 (5). The molecular mechanisms underlying the basis of these differential responses to TGF-β have not been elucidated. However, in this issue of PNAS, Bhowmick et al. (6) have now shed some light on an important clue of alternative signaling pathways involved in TGF-β-mediated growth regulation. By analyzing the paradoxical effects of TGF-β on epithelial cells versus fibroblasts, Bhowmick et al. (6) have uncovered a TGF-β growth-inhibitory pathway that links RhoA/p160ROCK to Cdc25A and at least partially explains the different response of epithelial cells and fibroblasts to TGF-β.
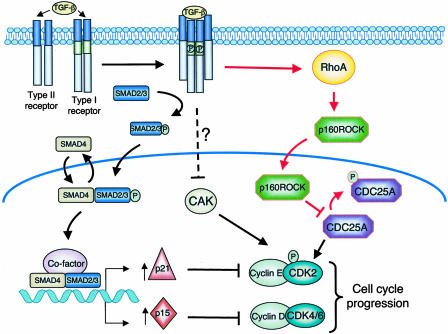
TGF-β signals through a heteromeric transmembrane complex composed of two type I and two type II receptor serine/threonine kinases (Fig. 1) (7). On TGF-β binding, the type I and type II receptors are brought together, resulting in the activation of the dormant kinase activity of the type I receptor. The activated type I receptor then propagates the signal by phosphorylation of Smad proteins. Activated Smad complexes rapidly translocate to the nucleus and regulate, in collaboration with other cofactors, the transcription of target genes. One key event leading to TGF-β-mediated growth arrest involves the inhibition of cyclin-dependent kinases (CDKs). CDKs and their binding partners, the cyclins, form an active complex that positively regulates G1 phase cell-cycle progression (8).
Fig. 1.
TGF-β signaling pathway. TGF-β binding to the type II receptor results in phosphorylation and activation of the type I receptor. The activated type I receptor then phosphorylates the receptor-associated SMADs (SMAD 2/3) that promote dimer or trimer formation with SMAD4 followed by nuclear translocation. SMAD complexes, in collaboration with cofactors, modulate transcription of TGF-β target genes. Induction of cell-cycle arrest by TGF-β involves transcription of CDK inhibitors p15INK4b and p21CIP1. p15INK4b specifically inhibits CDK4/6, whereas p21CIP1 inhibits cyclin E–CDK2 complexes. The inhibited cyclin–CDK complexes can drive progression of the cell cycle for a longer time. Activation of CDK2 requires phosphorylation of its Thr-160 residue by CAK and removal of inhibitory phosphates on Thr-14 and Tyr-15 by Cdc25A phosphatase. The new pathway proposed by Bhowmick et al. (6) involves TGF-β activation of the RhoA/p160ROCK signaling pathway. By an undefined mechanism, TGF-β activates RhoA and causes the translocation of p160ROCK to the nucleus, where it phosphorylates and inactivates Cdc25A and thereby prevents activation of CDK2.
To promote G1 phase, CDK4/6 associates with D-type cyclins, whereas CDK2 activation requires association with cyclin E. G1 phase progression is also subject to negative regulation by CDK inhibitors, CIP/KIP and INK4 family members (8). INK4 family members (p16NK4a, p15INK4b, p18INK4d, and p19INK4d) specifically bind to and inhibit the activity of CDK4 and CDK6 and thereby prevent their association with D-type cyclins. In addition, CIP/KIP family members (p21CIP1, p27KIP1, and p57KIP2) can bind directly to active cyclin D-CDK4/6 and cyclin E-CDK2 complexes to inhibit their activity by blocking the catalytic site of the CDK. In epithelial cells, the best-characterized cytostatic gene response to TGF-β involves up-regulation of both p15INK4b and p21CIP1 and repression of c-myc, an important transcriptional activator required for cell proliferation (9–12). TGF-β also modulates the posttranscriptional control of cell-cycle progression by activation of protein phosphatase 2A (PP2A), which, in turn inactivates p70s6k, a serine/threonine kinase that induces translation of mRNAs and is essential for G1/S phase progression (13). Through an as-yet-to-be-defined signaling mechanism, TGF-β signaling can also lead to the inhibition of the CDK activating kinase (CAK) (14) and down-regulation of the CDK-activating phosphatase Cdc25A (15).
The data of Bhowmick et al. (6) add a dimension to the complex network of TGF-β signaling and subsequent cell-cycle arrest. This study was initiated by a previous observation from the same group that TGF-β could rapidly activate RhoA, a small GTPase involved in actin rearrangement, in several epithelial cells but not in NIH 3T3 fibroblasts (16). Following this lead, they uncovered a pathway where RhoA and its effector kinase p160ROCK directly inhibit the activity of Cdc25A to induce cell-cycle arrest on TGF-β treatment in epithelial cells but not in fibroblasts. Cdc25A is a member of the Cdc25 family of dual-specificity protein phosphatases that function as positive regulators of cell-cycle progression. Cdc25 phosphatases activate CDKs by dephosphorylating their inhibitory threonine and tyrosine phospho residues (Thr-14-PO4 and Tyr-15-PO4 on CDK2) (17). The inhibition of Cdc25A activity observed after treatment with TGF-β contributes to the induction of a cell-cycle arrest due in part to decreased CDK2 activity.
In normal murine mammary gland NMuMG epithelial cells, the work of Bhowmick et al. (6) unequivocally demonstrates that signaling through RhoA and its effector kinase p160ROCK is a key event in TGF-β inhibition of cell proliferation. The activation of this pathway by TGF-β seems to be required for cell-cycle arrest, because inhibition of p160ROCK by the use of either a specific inhibitor or by siRNA abrogates the effect of TGF-β. Furthermore, reactivation of p160ROCK through RhoA signaling in TGF-β-treated NIH 3T3 cells reverted the response from growth stimulation to growth inhibition. TGF-β treatment of NMuMG epithelial cells induced relocalization of p160ROCK from the cytoplasm to the nucleus; this relocalization correlated with increased levels of phosphorylated Cdc25A and inhibition of Cdc25A phosphatase activity. The data presented provide evidence that p160ROCK directly interacts with and phosphorylates Cdc25A to mediate its inhibition. However, the exact target phosphorylation site on Cdc25A remains elusive and will require further investigation.
Signaling through RhoA is a key event in TGF-β inhibition of the cell cycle.
Although these findings offer a previously uninvestigated TGF-β-regulated pathway to cell-cycle arrest, how TGF-β activates RhoA in the first place and why this pathway is not activated in fibroblasts remain unclear. In addition, the mechanism by which TGF-β induces proliferation of fibroblasts remains unanswered. Cdc25A is also involved in cell-cycle arrest in response to DNA damage. The ATM and ATR protein kinases activate the checkpoint kinases Chk1 and Chk2 that lead to Cdc25A hyperphosphorylation and ubiquitin-mediated proteolysis of Cdc25A (18–20). Bhowmick et al. (6) suggest that cell-cycle arrest induced by TGF-β (similar to genotoxic stress) would occur in a “two-wave” response. The first step involves the rapid inhibition of Cdc25A by RhoA signaling and a posttranslational mechanism, and the second step would involve transcriptional modulation of important regulators of cell-cycle progression by Smad complexes.
The RhoA/p160ROCK pathway is best characterized for its role in many motile responses that involve the actin-cytoskeleton and microtubule networks (21). p160ROCK is involved in actin-cytoskeleton assembly and cell contractility by controlling stress fiber and focal adhesion complex formation (21). In previous work, Bhowmick et al. (16) showed that in NmuMG epithelial cells TGF-β activates a RhoA-dependent signaling pathway involved in the formation of stress fibers and induction of epithelial to mesenchymal transdifferentiation (EMT). EMT is a process by which a polarized epithelial cell is converted into a motile cell. This process is also involved in the dedifferentiation program that leads to progression of malignant carcinoma (22). The fact that TGF-β uses the RhoA signaling pathway to induce cell-cycle arrest and EMT at the same time is puzzling. It is possible that, depending on the epithelial cell type and context, TGF-β induction of EMT requires a cell-cycle arrest necessary for the cytoskeletal changes to occur before migration of cells. The findings of Bhowmick et al. (6) also raise an important question of whether RhoA modulates Cdc25A activity in other circumstances where TGF-β is not involved. Regardless, these observations not only represent a step forward in our understanding of the complexity of TGF-β signaling and growth arrest, but also uncover a previously uninvestigated cell-cycle regulation mechanism.
See companion article on page 15548.
References
- 1.Siegel, P. M. & Massague, J. (2003) Nat. Rev. Cancer 3, 807–820. [DOI] [PubMed] [Google Scholar]
- 2.Waite, K. A. & Eng, C. (2003) Nat. Rev. Genet. 4, 763–773. [DOI] [PubMed] [Google Scholar]
- 3.Dalal, B. I., Keown, P. A. & Greenberg, A. H. (1993) Am. J. Pathol. 143, 381–389. [PMC free article] [PubMed] [Google Scholar]
- 4.Walker, R. A. & Dearing, S. J. (1992) Eur. J. Cancer 28, 641–644. [DOI] [PubMed] [Google Scholar]
- 5.Koskinen, P. J., Sistonen, L., Bravo, R. & Alitalo, K. (1991) Growth Factors 5, 283–293. [DOI] [PubMed] [Google Scholar]
- 6.Bhowmick, N. A., Ghiassi, M., Aakre, M., Brown, K., Singh, V. & Moses, H. L. (2003) Proc. Natl. Acad. Sci. USA 100, 15548–15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi, Y. & Massague, J. (2003) Cell 113, 685–700. [DOI] [PubMed] [Google Scholar]
- 8.Sherr, C. J. & Roberts, J. M. (1999) Genes Dev. 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- 9.Datto, M. B., Li, Y., Panus, J. F., Howe, D. J., Xiong, Y. & Wang, X. F. (1995) Proc. Natl. Acad. Sci. USA 92, 5545–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannon, G. J. & Beach, D. (1994) Nature 371, 257–261. [DOI] [PubMed] [Google Scholar]
- 11.Reynisdottir, I., Polyak, K., Iavarone, A. & Massague, J. (1995) Genes Dev. 9, 1831–1845. [DOI] [PubMed] [Google Scholar]
- 12.Alexandrow, M. G. & Moses, H. L. (1995) Cancer Res. 55, 1452–1457. [PubMed] [Google Scholar]
- 13.Petritsch, C., Beug, H., Balmain, A. & Oft, M. (2000) Genes Dev. 14, 3093–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagahara, H., Ezhevsky, S. A., Vocero-Akbani, A. M., Kaldis, P., Solomon, M. J. & Dowdy, S. F. (1999) Proc. Natl. Acad. Sci. USA 96, 14961–14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iavarone, A. & Massague, J. (1997) Nature 387, 417–422. [DOI] [PubMed] [Google Scholar]
- 16.Bhowmick, N. A., Ghiassi, M., Bakin, A., Aakre, M., Lundquist, C. A., Engel, M. E., Arteaga, C. L. & Moses, H. L. (2001) Mol. Biol. Cell 12, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu, Y., Rosenblatt, J. & Morgan, D. O. (1992) EMBO J. 11, 3995–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falck, J., Mailand, N., Syljuasen, R. G., Bartek, J. & Lukas, J. (2001) Nature 410, 842–847. [DOI] [PubMed] [Google Scholar]
- 19.Mailand, N., Falck, J., Lukas, C., Syljuasen, R. G., Welcker, M., Bartek, J. & Lukas, J. (2000) Science 288, 1425–1429. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen, C. S., Syljuasen, R. G., Falck, J., Schroeder, T., Ronnstrand, L., Khanna, K. K., Zhou, B. B., Bartek, J. & Lukas, J. (2003) Cancer Cell 3, 247–258. [DOI] [PubMed] [Google Scholar]
- 21.Riento, K. & Ridley, A. J. (2003) Nat. Rev. Mol. Cell Biol. 4, 446–456. [DOI] [PubMed] [Google Scholar]
- 22.Thiery, J. P. (2002) Nat. Rev. Cancer 2, 442–454. [DOI] [PubMed] [Google Scholar]