Abstract
Fibrillin-1, the major component of extracellular microfibrils that associate with insoluble elastin in elastic fibers, is mainly synthesized during development and postnatal growth and is believed to guide elastogenesis. Mutations in the fibrillin-1 gene cause Marfan syndrome, a multisystem disorder characterized by aortic aneurysms and dissections. The recent finding that early deficiency of elastin modifies vascular aging has raised the possibility that fibrillin-1 deficiency could also contribute to late-onset pathology of vascular remodeling. To address this question, we examined cardiovascular function in 3 week-old, 6 month-old, and 24 month-old mice that are heterozygous for a hypomorphic structural mutation of fibrillin-1 (Fbn1+/mgΔ mice). Our results indicate that Fbn1+/mgΔ mice, particularly those that are 24 month-old, are slightly more hypotensive than wild-type littermates. Additionally, aneurysm and aortic insufficiency were more frequently observed in aging Fbn1+/mgΔ mice than in the wild-type counterparts. We also noted substantial fragmentation and decreased number of elastic lamellae in the aortic wall of Fbn1+/mgΔ mice, which were correlated with an increase in aortic stiffness, a decrease in vasoreactivity, altered expressions of elastic fiber-related genes, including fibrillin-1 and elastin, and a decrease in the relative ratio between tissue elastin and collagen. Collectively, our findings suggest that the heterozygous mgΔ mutation accelerates some aspects of vascular aging and eventually lead to aortic manifestations resembling those of Marfan syndrome. Importantly, our data also indicate that vascular abnormalities in Fbn1+/mgΔ mice are opposite to those induced by elastin haploinsufficiency during aging that affect blood pressure, vascular dimensions and number of elastic lamellae.
Keywords: elastic lamellae, fibrillin-1, Marfan syndrome, arterial aging, aneurysm, transgenic mice
INTRODUCTION
Elasticity is a vital physiological property of large arteries. During systole, pressurized blood dilates large arteries while elastic components of the arterial wall store energy. During diastole, the arterial wall releases the accumulated energy by compressing the blood, therefore maintains blood pressure and flow into the arterial tree [1.]. Circumferentially-oriented elastic lamellae made of elastin and microfibrils fulfill this function in the closed circulatory system of higher vertebrates, whereas microfibrils devoid of elastin are the sole elastic components of invertebrate arteries owing to their limited stretching in response to mechanical force [2, 3.]. Microfibrils are macromolecular structures that are mainly composed of fibrillin-1 and which also include microfibril-associated glycoproteins (MAGPs), latent TGF-β-binding proteins (LTBPs) and fibulins [4.]. Despite their relatively low abundance (~10% compared to elastin), microfibrils exert a significant mechanical function in the elastic fibers of large arteries as evidenced by the vascular manifestations of fibrillin-1 mutations in Marfan syndrome (MFS), which include increased arterial stiffness and abnormal aortic wall architecture that precipitate dissecting aneurysms [5–10.].
Elastic fibers undergo a slow age-dependent degradation due to the progressive imbalance between elastolytic enzymes and their inhibitors, leading to the release of elastin peptides with vasodilatory properties [11–13.]. In accordance with these findings, genetic elastin deficiency was recently shown to modify the physiological process of arterial aging in Eln+/− mice [14.]. As already alluded, several mouse strains with discrete Fbn1 mutations have been created that replicate the clinical spectrum of MFS, including aneurysmal pathologies that manifest during neonatal or adult life [15–18.]. Amongst them is the so-called mgΔ mutation, a hypomorphic in-frame deletion of exons 19–24 of Fbn1, which in homozygosity causes dissecting aneurysm and death soon after birth but has no apparent effect on the viability and fitness of young heterozygous mutant mice [17.]. The present study employed Fbn1+/mgΔ mice to investigate whether fibrillin-1 may also contribute to cardiovascular function during aging. The results of these analyses revealed that the Fbn1 mutation promotes several negative changes in cardiovascular function as the mice ages, including an increased rate of aortic aneurysm and rupture.
METHODS
Animals
The present study employed mice heterozygous for a hypomorphic in-frame deletion in fibrillin-1 (Fbn1+/mgΔ), which were backcrossed for >5 generations into the C57Bl/6J background [17.]. The analyses were performed on male Fbn1+/mgΔ mice and WT littermates (controls) of three age-groups: 3 week-old (young animals), 5–7 month-old (adult animals, called 6 month-old) and 23–27 month-old (old animals, called 24 month-old). No change in neonatal mortality were observed in Fbn1+/mgΔ mice compared to wild-type littermates. Housing and surgical procedures were in accordance with institutional guidelines.
Blood pressure and heart weight
Mice were anesthetized using isoflurane (1.5–2%), and placed on a heating table to maintain body temperature at 37°C. Immediately after completion of anesthesia, verified by absence of response to pinching of the mouse toes, a Millar probe SPR838 was inserted into the carotid artery then moved to the ascending aorta where the blood pressure was measured. In other animals, anesthetized with pentobarbital (60mg/kg, IP), hearts and left ventricles were dissected, washed, and weighed (wet weight).
Ultrasound
Transthoracic echocardiography was performed with a Toshiba Powervision 6000, SSA 370A device equipped with an 8- to 14-MHz linear transducer, as previously described [19, 20.]. Procedures are detailed in Online Supporting Information 1.
Arterial desmosine and hydroxyproline content
Desmosine were determined by radioimmunoassay, as described [21.]. Total protein in the tissue hydrolysates was determined by a ninhydrin based method [22.]. Hydroxyproline levels were determined by amino-acid analysis using high-pressure ion-exchange chromatography on a Biochrom 30 amino acid analyzer. Norleucin was used as an internal standard. Results are expressed as amino-acid mass per vessel segment length and as amino-acid mass per vessel total protein content.
Aorta mechanics
Mechanical studies were performed on excised cannulated ascending aortae, as described [14, 23.]. Procedures are detailed in Online Supporting Information 1.
Histological analyses
Aortae were classified as aneurysmal/symptomatic when obvious dilation of a vessel section was observed by gross anatomy/visual examination. Transverse sections of the paraffin-embedded ascending aorta (pathological or non-pathological) were stained with hematoxylin-eosin for cells, Weigert for elastic fibers, and picrosirius red for collagen. Some aortae were fixed by cardiac perfusion with 2.5% glutaraldehyde in 0.1M sodium cacodylate buffer (pH 7.4), then embedded in Epon for ultrastructural analyses. Semi-thin sections were stained as described [24.], and stained with tannic acid before uranyl acetate.
RNA analyses
Total RNA was extracted from entire aortae and gene expression levels were evaluated by real-time PCR. Resulting values were normalized against the expression of the housekeeping gene Hypoxanthine guanine PhosphoRibosyl Transferase (HPRT) and total mRNA input. Amplification primers for the tested genes have been previously described [14.]. Procedures are detailed in Online Supporting Information 1.
Aorta reactivity
Variations of cannulated aorta diameters in response to 10μmol/L of the vasoconstrictor phenylephrine (PE), then 10 μmol/L PE + 10 μmol/L of the vasodilator acetylcholine (Ach) were assessed at 75 mmHg [14, 23.]. Diameters of Fbn1+/mgΔ mouse aortae were normalized against the mean diameter of WT aortae [11.].
Statistics
Two- or three-way ANOVA followed, when necessary, by Fisher’s least significant difference test (LSD) or t-test for paired value comparisons, were used to evaluate the significance of most parameters. Nonparametric Mann-Whitney U test was used to assess vessel midwall strain, stress, incremental elasticity modulus and distensibility. The results are presented as mean values ± standard error of the mean (SEM). P values ≤ 0.05 were considered as statistically significant.
RESULTS
The mgΔ allele encodes a shortened fibrillin-1 molecule that is produced at ~10% of the normal level [17.]. Previous investigations showed that newborn Fbn1mgΔ/mgΔ mice succumb to cardiovascular and/or respiratory complications, but did not determine whether seemingly normal Fbn1+/mgΔ mice display cardiovascular manifestations past their reproductive age [17, 25.]. In the present study, we noted a statistically borderline loss of Fbn1+/mgΔ viability only between 12 and 20 months of age (p=0.06; χ2 test at 18 months) in the absence of substantial changes in body weight (Fig. 1A and Table I). We also found lower blood pressures and higher pulse pressure in Fbn1+/mgΔ mice compared with WT littermates (three-way ANOVA, p≤0.05; Table I), conceivably as a result of aortic insufficiency in Fbn1+/mgΔ mice (see below). As shown in a previous study in which blood pressure was found similar in awake or anesthetized mice, an effect of anesthesia explaining this inter-group difference could not be suspected [23.]. Hence, several critical parameters of cardiovascular physiology were compared between young (3 week-old), adult (6 month-old), and old (24 month-old) Fbn1+/mgΔ and WT mice.
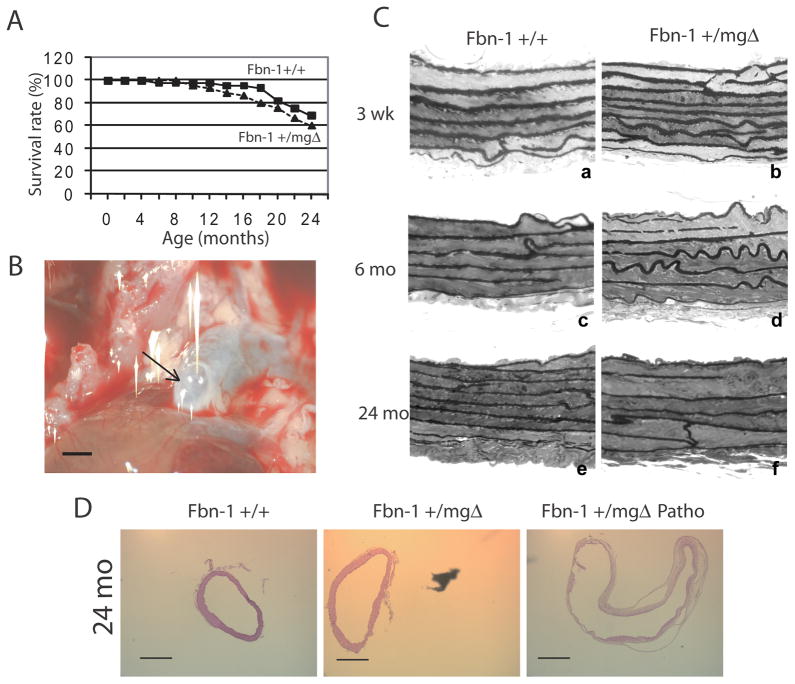
Figure 1.
Effect of aging and genotype on the survival rate (A), aneurismal ascending aorta of 24-month-old Fbn1+/mgΔ mouse (B) (the arrow indicate the saccular aneurysm at the vessel root), and histological examination of semi-thin sections of ascending aorta of 3-week-old, 6- and 24-month-old mice (C). Eosin/Hematoxylin staining of cross-sections of ascending aorta of Fbn1+/+, non-pathological Fbn1+/mgΔ, and pathological Fbn1+/mgΔ (Patho) 24-month-old mice (D). n=45 for (A). Bar sizes: 1mm (B) and 500μm (D).
Table I.
Hemodynamic and ultrasound parameters of ascending aorta and heart.
| 5–7 month-old | 23–27 month-old | |||
|---|---|---|---|---|
| Fbn1+/+ | Fbn1+/mgΔ | Fbn1+/+ | Fbn1+/mgΔ | |
| Number of Animals | 8 | 8 | 7 | 5 |
| Body Weight (g) | 29.5 ± 0.9 | 31.8 ± 0.7 * | 33.2 ±1.3 † | 31.6 ± 0.8 |
| Entire Heart weight/BW (mg/g) | 4.92 ± 0.13 | 5.99 ± 0.91 | 5.72 ± 0.35 | 6.25 ± 0.94 |
| Systolic Arterial Pressure (mmHg) | 105 ± 2 | 104 ± 3 | 110 ± 4 | 104 ± 5 |
| Mean Arterial Pressure (mmHg) | 85 ± 1 | 81 ± 2 | 84 ± 3 | 76 ± 6 |
| Diastolic Arterial Pressure (mmHg) | 75 ± 1 | 70 ± 2 | 72 ± 3 | 62 ± 6 |
| Pulse pressure (mmHg) | 30 ± 1 | 34 ± 3 | 38 ± 3 | 42 ± 2 |
| Ultrasound study | ||||
| Ascending aorta: | ||||
| Systolic Diameter (mm) | 1.70 ± 0.05 | 2.18 ± 0.17 * | 2.11 ± 0.07 † | 2.26 ± 0.19* |
| Diastolic Diameter (mm) | 1.48 ± 0.04 | 2.04 ± 0.19 * | 1.97 ± 0.08 † | 2.05 ± 0.21* |
| Surface (mm2) | 2.28 ± 0.14 | 3.90 ± 0.66 * | 3.53 ± 0.22 † | 4.09 ± 0.66 |
| Compliance (mm/mmHg) | 0.720 ± 0.137 | 0.520 ± 0.112 | 0.383 ± 0.07 ‡ | 0.579 ± 0.173 |
| Cardiac data: | ||||
| Heart Rate (bpm) | 504 ± 11 | 485 ± 25 | 475 ± 16 | 456 ± 22 |
| Left Atria Dimension (mm) | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.9 ± 0.4 |
| Left Ventricle EDD | 4.36 ± 0.18 | 5.00 ± 0.54 | 5.13 ± 0.15 † | 5.11 ± 0.26 |
| Left Ventricle EDD/BW (mm/g) | 0.148 ± 0.005 | 0.158 ± 0.017 | 0.156 ± 0.008 | 0.162 ± 0.011 |
| Fractional Shortening (%) | 40 ± 2 | 31 ± 4 | 36 ± 3 | 35 ± 4 |
| LV Weight/BW (mg/g) | 3.58 ± 0.20 | 5.40 ± 1.56 | 4.32 ± 0.24 † | 4.76 ± 0.72 |
| Vcfc (circ/s) | 2.9 ± 0.2 | 2.3 ± 0.3 | 2.5 ± 0.3 | 2.5 ± 0.2 |
| Sa (cm/s) | 2.93 ± 0.15 | 2.61 ± 0.21 | 2.84 ± 0.26 | 2.98 ± 0.50 |
| Spw (cm/s) | 2.94 ± 0.3 | 2.77 ± 0.26 | 3.22 ± 0.22 | 3.24 ± 0.22 |
| Isovolumic Relaxation Time (ms) | 17 ± 1 | 20 ± 2 | 19 ± 1 | 21 ± 1 |
| Ea (cm/s) | 4.53 ± 0.32 | 4.56 ± 0.37 | 4.74 ± 0.26 | 5.01 ± 0.77 |
| Epw (cm/s) | 4.34 ± 0.45 | 3.84 ± 0.4 | 4.97 ± 0.21 | 4.49 ± 0.49 |
| E/Ea | 20.6 ± 1.0 | 18.9 ± 2.5 | 21.6 ± 1.4 | 18.2 ± 2.4 |
| Aortic Regurgitation Frequency | 0/8 | 2/8 | 2/7 | 3/5 |
Values are mean ± sem.
Significant difference with 5–7 month-old animals of the same genotype (†: P≤0.05; ‡: P=0.056). Compliance is a local estimation calculated from the Δdiameter/Δpressure between systolic and diastolic values. LVEDD: left ventricle end diastolic diameter; Vcfc: mean shortening velocity of circumferential fibers, corrected for time (=FS/rate-corrected ejection time); Sa, Spw: maximal systolic velocity of the mitral annulus and posterior wall, respectively. Ea, Epw: maximal diastolic velocity of the mitral annulus and posterior wall, respectively. E/Ea: maximal velocity of LV mitral inflow to Ea.
Cardiovascular function
Ultrasound measurements through the cardiac cycle of ascending aortae in adult Fbn1+/mgΔ mice revealed increases of 38 % and 71 % in diastolic diameter and aortic surface, respectively (Table I and online supporting information 2A). The unusual presence of aortic aneurysm was also noted in 31% (4 out of 13) of adult Fbn1+/mgΔ mice (vs. 7% in adult Fbn1+/+ animals; 1 out of 15), which reached a significantly greater frequency than normal (67% or 8 out of 12 mice) in old Fbn1+/mgΔ mice (vs. 17% in aged Fbn1+/+ animals; 2 out of 12) (Fig. 1B). Additionally, aortic compliance (estimated from the ratio of the diameters and pressure gradients between systole and diastole) was reduced in some, not all, adult mutant animals, leading to a non-significant trend towards decreased compliance in this group (Table I). Retrograde flow (measured using color-Doppler mode) was also identified in some adult (2 out of 8) and some old (3 out of 5) Fbn1+/mgΔ mice (Table I and online supporting information 2B,C). These aortic insufficiencies (AI) were either mild with no cardiac complications or severe with a consistent pattern of left ventricle (LV) dysfunction (as evidenced by signs of LV remodeling, such as dilation and wall thickening leading to hypertrophy) affecting both ventricular contraction and relaxation. On the contrary, in non-symptomatic (= non AI/non aneurysmal) Fbn1+/mgΔ mice, cardiac dimensions and function were not modified. The variability within the Fbn1+/mgΔ mice, i.e. presence of both clearly pathological and relatively unaffected animals, leads to a trend of structural and functional cardiac alterations in this group regarding some parameters, e.g. left ventricle end diastolic diameters and left ventricle weight to body weight ratios (Table I). Collectively, these in vivo data suggested that the mgΔ mutation accelerates some aspects of the physiological process of vascular aging and also leads to pathological vascular aging to different degrees in heterozygous mutant mice. Phenotypic heterogeneity of genetically identical Fbn1+/mgΔ mice resembles the clinical variability of MFS.
Ascending aorta morphology
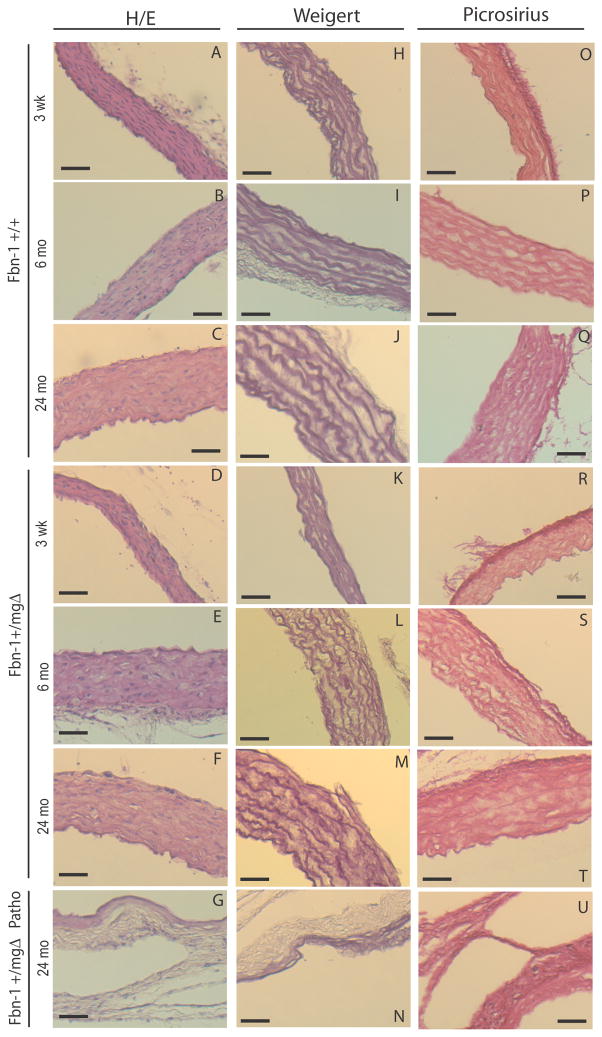
Histological examination of the ascending aortae documented significant disorganization of the medial layer in Fbn1+/mgΔ mice of all ages, which is characterized by thinner and often disrupted elastic lamellae (Figs. 1C and 2, and Table II). Interestingly, morphometric analyses also revealed that the medial layer of fibrillin-1 haploinsufficient aortae contains one less elastic lamella than normal irrespective of the age of the mice (Table II). This observation is consistent with the concept that has emerged from the analysis of elastin haploinsufficient mice that the aortic wall undergoes adaptive changes in response to changes in hemodynamic stress [23, 26.]. Additional findings in adult and old Fbn1+/mgΔ aortae included more diffused collagen staining, as well as histological evidence of dilation and rupture (Fig. 1D and 2G,N,U). Dissecting aneurysm was most frequently observed in tissue samples from old mutant mice in association with a more dramatic disorganization of the medial layer and fragmentation of elastic lamellae (Fig. 1D and 2, and Table II). These in vitro data therefore corroborated the in vivo evidence that microfibril deficiency in Fbn1+/mgΔ mice causes severe architectural changes that can predispose the aging aortic wall to structural collapse.
Figure 2.
Histological examinations of paraffin-embedded cross-sections of the ascending aorta of 3-week-old, 6- and 24-month-old Fbn1+/+ and Fbn1+/mgΔ mice, and 24-month-old pathological Fbn1+/mgΔ mice (Patho). The elastic lamellae were thinner and disorganized in adult and aged Fbn1+/mgΔ mice. H/E: Hematoxylin/Eosin staining. Bar size: 50 μm.
Table II.
Histomorphometric parameters of ascending aorta wall.
| Age | 3 weeks | 6 month | 24 months | |||
|---|---|---|---|---|---|---|
| Genotype | Fbn1+/+ | Fbn1+/mgΔ | Fbn1+/+ | Fbn1+/mgΔ | Fbn1+/+ | Fbn1+/mgΔ |
| Number of elastic lamellae (EL) | 7.1±0.3 | 6.1±0.2* | 7.7±0.5 | 7.1±0.4* | 8.4±0.3 | 6.7±0.9 * |
| Distance between EL (μm) | 0.85±0.34 | 0.70±0.27 | 0.92±0.24 | 1.18±0.37* | 0.96±0.19 | 1.23±0.6* |
| Rupture of EL (×10−4 per μm2) | 2±1 | 4±2.8* | 2±1.4 | 6±2* | 3.2±2.1 | 7.8±3.6* |
Values are mean ± SEM.
Significant difference between Fbn1+/+ and Fbn1+/mgΔ mice of matching age.
Ascending aorta biomechanics
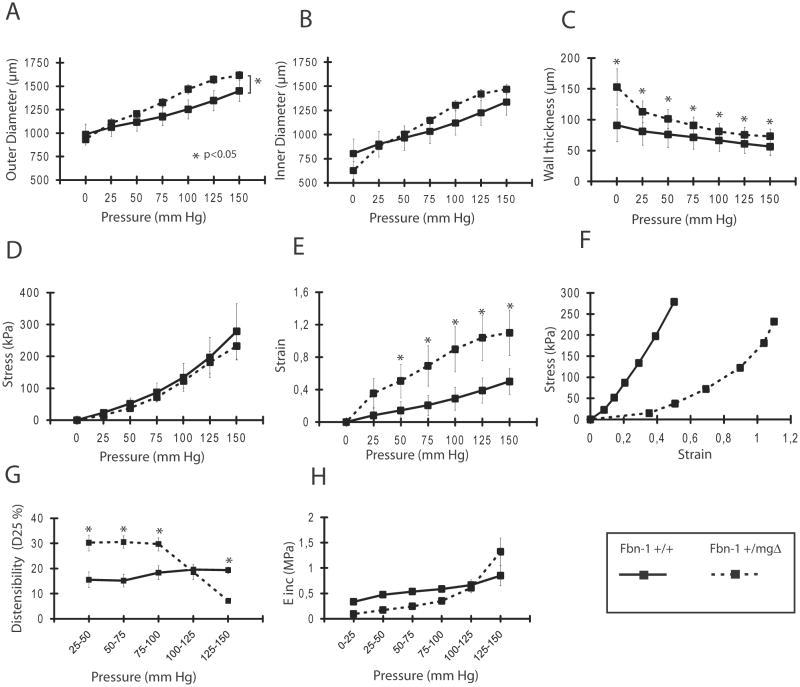
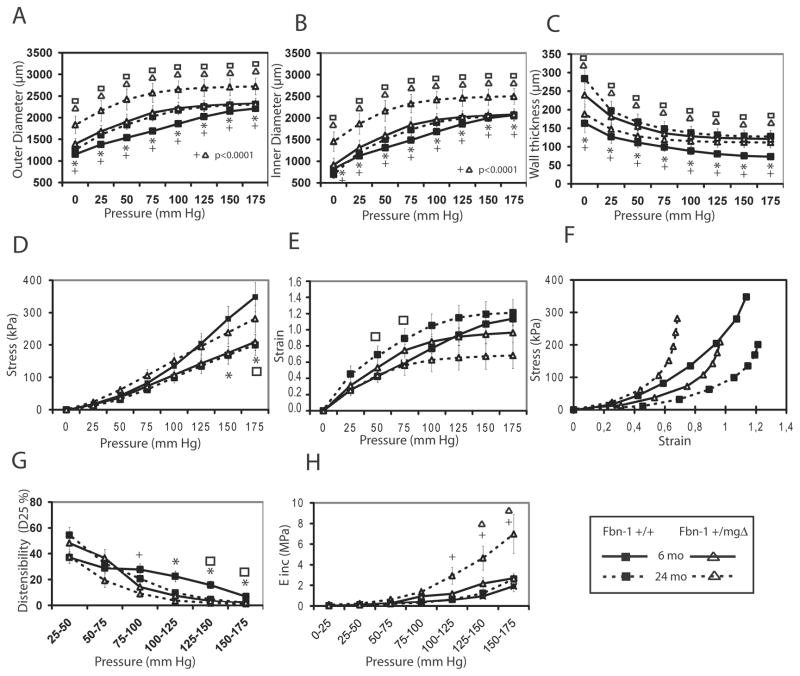
In order to compare the changed structure to the function of Fbn1+/mgΔ mouse arteries, biomechanical tests were performed on isolated aortic segments from young Fbn1+/mgΔ mice (Fig. 3). Mutant tissues displayed increased diameter, strain and wall thickness without appreciable changes in wall stress and incremental elastic modulus (Einc). They also documented that aortae from young Fbn1+/mgΔ mice are able of higher extension at imposed pressure below 100 mmHg and lower extension at imposed pressure above 125 mmHg, compared to aortae from WT mice (Fig. 3G). In adult and aged animals, the outer and inner diameters were higher and showed a greater age-related enlargement in Fbn1+/mgΔ mice, which also showed an aortic wall thickness above normal in adults and abnormally thin in aged animals (Fig. 4A–C). Similar biphasic changes from controls were observed for aortic wall stress (σ) in that this parameter calculated at pressures above 125 mmHg is decreased in adult Fbn1+/mgΔ mice and slightly increased in old mutant animals, compared to WT of matching ages (Fig. 4D). This is due to the opposite evolutions of σ, during aging, which decreased in WT because of wall thickening and increases in Fbn1+/mgΔ mice because of wall thinning (Fig. 4C). Whereas no significant variances from control were noted in the strain value (ε) of mutant aortae in adults, vascular aging was associated with a relatively lower ε in Fbn1+/mgΔ samples (Fig. 4E–F). Similarly, the results showed that the ability of mutant aortae to extend at imposed pressures above 75 mmHg was relatively lower than normal at all ages, and that the Einc value was substantially greater in old mutant animals at imposed pressure above 100 mmHg (Fig. 4G,H). Together, these results indicated that the mgΔ mutation substantially impairs the biomechanical properties of the aging aortic wall.
Figure 3.
Diameter-pressure curves and derived mechanical parameters of the ascending aorta of 3-week-old Fbn1+/+ and Fbn1+/mgΔ mice. *Significant difference between Fbn1+/+ and Fbn1+/mgΔ mice. n=4–7 per group.
Figure 4.
Diameter-pressure curves and derived mechanical parameters of the ascending aorta of 6- and 24-month-old Fbn1+/+ and Fbn1+/mgΔ mice. *,+Significant difference between 6-month-old Fbn1+/+ and Fbn1+/mgΔ mice or between 24-month-old Fbn1+/+ and Fbn1+/mgΔ mice, respectively. □,ΔSignificant difference between 6–month-old and 24-month-old values for Fbn1+/+ and Fbn1+/mgΔ, respectively. n=5–6 per group.
Ascending aorta biochemistry
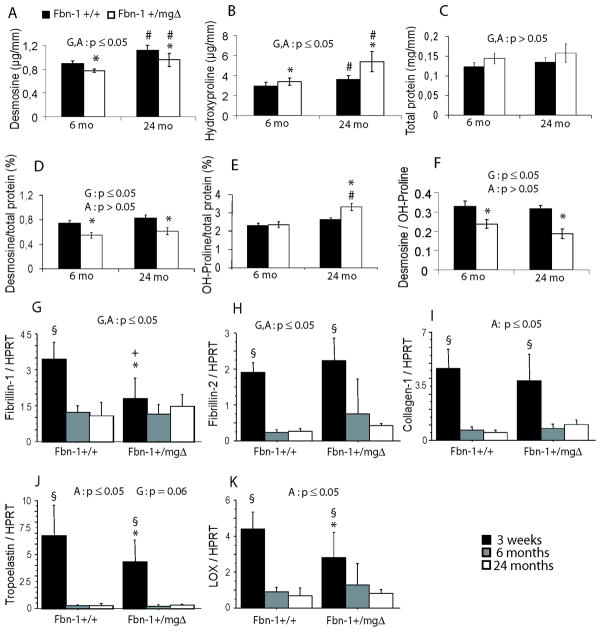
Aortic function greatly depends on the proper extracellular deposition and assembly of collagenous and elastic macroaggregates [27.]. Desmosine and hydroxyproline contents were therefore measured in Fbn1+/mgΔ aortae to quantify elastin and collagen contents, respectively [14.]. Increased hydroxyproline (collagen) levels and reduced desmosine (elastin) levels were found in mutant aortae, and both variances from normal increased with age relatively to unchanged amounts of total protein contents (Fig. 5A–E). These findings respectively corroborated the histological evidence of increased tissue remodeling and elastolysis in aging microfibril-deficient aortae. In addition, the decreased desmosine/hydroxyproline ratio (Fig. 5F) in Fbn1+/mgΔ aortae is consistent with the decreased distensibility observed in the vessels of these adult and old animals.
Figure 5.
Desmosine and hydroxyproline contents (A–F) and elastic fiber-related gene expression (G–K) of the ascending aorta of 3 weeks, 6 and 24-month-old Fbn1+/+ and Fbn1+/mgΔ mice. D,E present the desmosine and hydroxyproline ratios relative to total protein content. The general effect of genotype (G) and age (A) was assessed by two-way ANOVA separately in each condition (A to K), and followed by Fisher’s LSD test for paired comparisons: *,#Significant difference between Fbn1+/+ and Fbn1+/mgΔ mice of the same age, and between 6- and 24-months-old mice of the same genotype, respectively. §Significant difference between young (3-week-old) on one hand and 6- and 24-month old animals of the same genotype on the other hand. +Significant difference between young (3-week-old) and 6-month old animals of the same genotype on the other hand. For desmosine and hydroxyproline dosages: n=5–7 (6-month-old mice) and n=3–5 (24-month-old mice) in each group. For mRNA expression: n=6 (3-week-old), n=8 (6-month-old) and n=5–7 (24-month-old) in each group.
Ascending aorta gene expression
Expression of key extracellular gene products, ratioed to the expression level of HPRT, was examined in the ascending aortae of young, adult, and old Fbn1+/mgΔ mice to further support the above findings (Fig. 5G–K). As expected, Fbn1 transcripts were significantly lower than normal (−48%) in mutant aortae of young animals and a slightly relative increase was noted in old tissues (Fig. 5G). We also observed an increase in Fbn2 expression in Fbn1+/mgΔ animals (Fig. 5H). These increases in Fbn1 and Fbn2 gene expression in aging Fbn1+/mgΔ mice might be attributed to the unproductive tissue remodeling response that is mounted by smooth muscle cells in reaction to the formation of a structurally abnormal matrix. In accordance with this hypothesis, collagen I transcripts were also slightly elevated in the aortae of old Fbn1+/mgΔ mice (decreased in youngs) (Fig. 5I). Furthermore, elastin (Eln) transcripts were significantly lower than normal in Fbn1+/mgΔ aortae from young animals; a similar difference was noted for lysyl oxidase (Lox) mRNA (Fig. 5J,K). The same trends were confirmed when the expressions of these genes were ratioed to total mRNA (online supporting information 3). These results were consistent with the notion that Fbn1 deficiency down-regulates the expression of other major contributors to collagen and elastic fiber assembly in early stages, while up-regulating the expression of most of these genes in late-life.
Ascending aorta reactivity
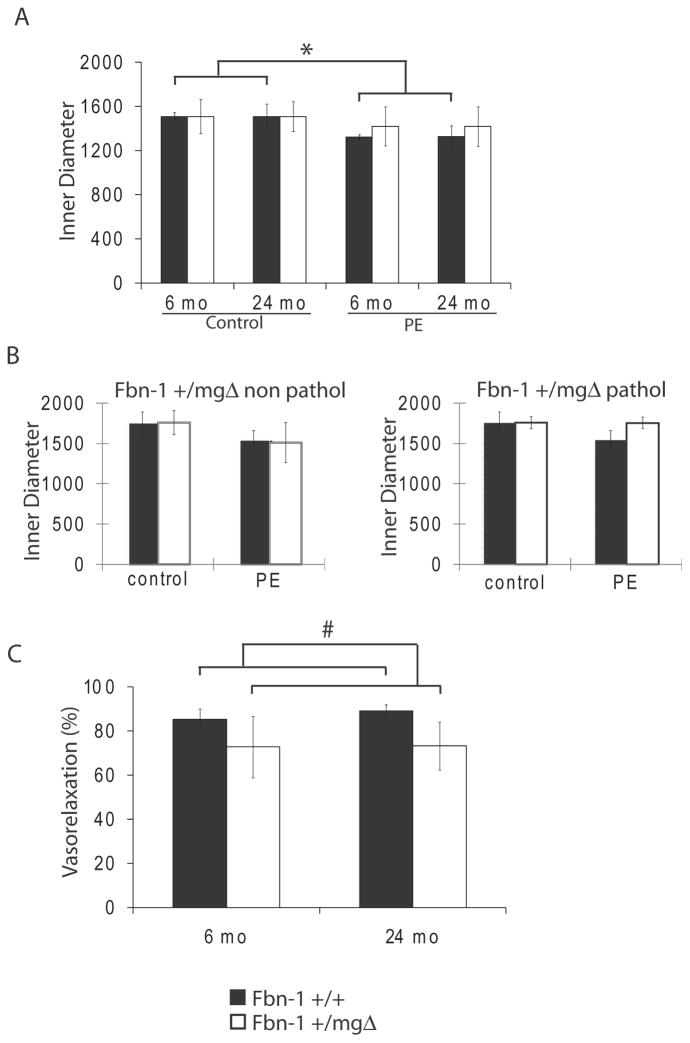
Lastly, we investigated how microfibril deficiency may affect aortic tissue ability to respond to vasoconstrictor (phenylephrine, PE) and vasodilator (acetylcholine, Ach) stimuli in adult and aged animals. Response to PE, significant in Fbn1+/+ mice independently of age, was decreased and became non-significant in Fbn1+/mgΔ aortae of all ages (Fig. 6A). When old tissues were separated into non-pathological and pathological (= aneurysmal) samples, we found that the mutant aortae of the former group reacted to PE treatment more strongly than the latter group (almost no response) and equally to WT samples (Fig. 6B). Non-pathological Fbn1+/mgΔ vessels presented a lowered response to Ach (Fig. 6C), independently of age, while pathological vessels could not be tested for vasorelaxation induction by Ach due to the absence of pre-constriction in response to PE. These results indicate that fibrillin-1 insufficiency alters the capacity of the aortic wall to respond to physiological vasoactive agonists.
Figure 6.
Reactivity to vasoactive agents of the ascending aorta of 6- and 24-month-old Fbn1+/+ and Fbn1+/mgΔ. (A,B) 10μmol/L phenylephrine (PE). 24-month-old Fbn1+/mgΔ mice were pooled (A) or separated (B) in non-pathological and pathological group. Ach-induced vasorelaxation (10μmol/L phenylephrine + 10μmol/L acetylcholine) in non-pathological vessels is represented as the reversal of the PE-induced vasoconstriction, in percent (C). *Generally significant difference with the control value, independent of age. #Generally significant difference between Fbn1+/+ and Fbn1+/mgΔ mice, independent of age. n=3–6 per group.
DISCUSSION
Elastic fiber components regulate cell proliferation, migration and arterial morphogenesis, in addition to imparting elastic properties to blood vessels [27–32.]. Interestingly, mutations in the major structural components of elastic fibers, elastin and fibrillin-1, have distinct vascular outcomes. On the one hand, elastin haploinsufficiency causes hyperproliferation of vascular smooth muscle cells and narrowing of large arteries in supravalvular aortic stenosis (SVAS) and Williams syndrome [33–38.]. Mutations in fibrillin-1, on the other hand, cause aortic aneurysms and dissections in MFS. The opposite effects of elastin and fibrillin-1 deficiencies on vascular morphogenesis and function have been replicated in genetically engineered mouse models of SVAS and MFS. Whereas the former mutant animals have provided important insights into elastin contribution to vascular aging, comparable information about fibrillin-1 is currently unavailable due to the fact that most studies have focused on homozygous mutant mice that die of dissecting aneurysm during the first 2 weeks of postnatal life or between 2 to 4 months of age. Here we elucidated the potential contribution of fibrillin-1 to vascular aging in Fbn1+/mgΔ mice that live past sexual maturity and whose adult phenotype has not been characterized. There are two major findings of our analyses.
First, aging Fbn1+/mgΔ mice are significantly more prone than wild-type littermates to develop dissecting aortic aneurysm. As shown in previous works, aneurysms appear earlier in Fbn1mgΔ/mgΔ, which only produce about 10% of the normal amount of fibrillin-1 [17.], than in Fbn1+/mgΔ animals, which contain about 60% of the normal content in fibrillin-1. Hence it is reasonable to argue that the presence of mutant and wild-type fibrillin-1 molecules in Fbn1+/mgΔ mice could have a dominant-negative effect on microfibril assembly, leading to the observed vasculature abnormalities. Contrasting this hypothetical argument, however, is the factual evidence that mice under-expressing wild-type fibrillin-1 (Fbn1mgR/mgR mice ) to nearly same extent as Fbn1+/mgΔ mice develop aortic aneurysms as well but earlier in life (2–4 months of age) [18]. Taken together, these data strongly support the original hypothesis - that the mgΔ allele has little or no dominant-negative effect on the assembly of wild-type fibrillin-1 molecules [17]. It follows that the vascular phenotype of aging Fbn1+/mgΔ mice probably reflects a gene-dosage effect that triggers aortic alterations later in life.
Aging Fbn1+/mgΔ mice are slightly hypotensive and display arteries of abnormally larger diameters and with fragmented elastic lamellae, altered mechanical properties and reactivity, as well as increased signs of cardiac hypertrophy. These observations support the notion that microfibril deficiency modifies the process of vascular aging in a manner opposite to elastin haploinsufficiency [14, 39.].
Impaired remodeling of the aortic wall in Fbn1+/mgΔ mice, as evidenced by the progressive fragmentation of elastic lamellae, is likely to reflect the modified expression of at least Fbn1, Fbn2, Eln, Lox and Col1 genes, as well as enhanced matrix degradation by matrix metalloproteases (MMPs). We rest the latter conclusion on published evidence of aortic elastic fiber degradation and disorganization as well as increased MMP activity in the aortae of MFS patients and other fibrillin-1 deficient mice, and on the ability of MMP inhibition to mitigate aneurysmal progression in mouse models of MFS [6, 40–42.]. Expression of wild-type or mutant fibrillin-1 occurs predominantly throughout the arterial media and, to a much lower extent, in the adventitia [17.]. This supports the previously articulated alternative hypothesis that increased arterial diameter and aneurysms of Fbn1+/mgΔ animals reflects in part a decreased tensile strength of the adventitia, in which fibrillin-1 microfibrils may be required to properly organize the collagenous extracellular matrix during development [17]. In this view, the mutant artery would be unable to accommodate the intraluminal blood pressure and abnormally inflate.
The fact that vascular disease in Fbn1+/mgΔ mice, our model of MFS, progresses at a slower pace than in MFS patients probably reflects the lower mechanical constraints in the aortic wall of these mammalian species. In this regard, it is worth noting that a delay in the emergence of vascular abnormalities was also observed in Eln+/− mice in which, unlike SVAS and Williams patients, there is no appreciable arterial stenosis even though they display increased number of thinner lamellar units, cardiac hypertrophy, arteries of smaller diameter and decreased elastin content, hypertension and altered mechanics [14, 23, 26.].
The above findings clearly indicate that fibrillin-1 is an important structural and instructive determinant of arterial wall morphogenesis, mechanical compliance and tissue homeostasis. It is conceivable to argue that some of these functions are accounted for by the role of microfibrils in regulating cell performance through the interaction of fibrillin-1 with cells surface receptors, such as integrins and heparan sulfate proteoglycans, and latent TGFβ complexes. For example, fibrillin-1 triggers integrin-mediated signalling may regulate adhesion and spreading of VSMC that could be required for the proper organization of aortic tissue during development [43, 44.]. Furthermore, improper activation of TGF signalling, as a result of impaired sequestration of latent complexes in the extracellular matrix, could also promote changes in VSMC phenotype. Moreover, it has recently been shown that fibrillin-1 fragments and microfibrils, which can pass through the basement membrane and anchor the endothelial cells to the internal elastic lamina [45.], trigger integrin-mediated calcium signaling in endothelial cells [46.], whose dysfunction has clearly been involved in the pathogenesis of Marfan syndrome [41,47.]. A decrease in fibrillin-1 signaling, in mutant mice or human patients, could cause abnormal endothelial function and signaling to the medial VSMCs, and subsequent arterial wall dysorganisation. Such endothelial dysfunction, i.e. impaired response to acetylcholine, was evidenced in Fbn1+/mgΔ mice in our experiments. Along the same lines, decreased number of elastic lamellae in adult Fbn1+/mgΔ aortae could be viewed as a change in the remodeling capacity of Fbn1+/mgΔ VSMC that in turn reflects changes in the biosynthetic properties of these cells due to impaired cell-matrix interactions and/or latent TGFβ sequestration.
The finding that aging Fbn1+/mgΔ mice can display either seemingly normal arteries, except for some modified parameters (diameter enlargement, larger wall thickness, higher stiffness), or enhanced aneurysms, cardiac hypertrophy and aortic insufficiency suggests that, similarly to MFS patients, fibrillin-1 mutations have varied penetrance in this mouse model. In our genetically identical animals, these phenotypic variances likely involve epigenetic or environmental factors. More generally, our data imply that accumulation of age-related environmental insults that affect the aortic wall microenvironment may disrupt cell-matrix with the consequence of accelerating the physiological process of aortic tissue degradation. In this view, mutations (even sub-clinical mutations) that affect molecules involved in elastogenesis or elastic fiber interactions with structural, cellular or soluble molecules would be expected to predispose aortic tissues to late-onset pathologies and/or premature aging.
Supplementary Material
Detailed materials and methods regarding ultrasound, aorta mechanics and RNA analyses.
A: In vivo arterial diameter at the level of the ascending aorta. Measurements of systolic (sd) and diastolic (dd) diameters are gated on the ECG signal in time-motion mode. B,C: Continuous wave Doppler images from the apical two-chamber view. B. Normal mitral diastolic inflow (M) of a 24 month-old Fbn1+/+ mouse. C. Detection of a simultaneous rapid (> 3m/s) diastolic retrograde flow from the aorta, indicative of aortic regurgitation (AR) in a 24 month-old Fbn1+/mgΔ mouse.
Specific mRNA levels relative to total mRNA.
Acknowledgments
The authors acknowledge the support of the European Commission (contracts TELASTAR, 5th PCRD, number QLK6-CT-2001-00332; and ELAST-AGE, 6th PCRD, number LSHM-CT-2005-018960), the French Ministry of Foreign Affairs-EGIDE (fellowship to B. Mariko), the Association Française contre les Myopathies, the Association “Autour des Williams”, and the National Institutes of Health (AR049698).
Footnotes
Authors declare no conflict of interest.
STATEMENT OF AUTHOR CONTRIBUTIONS
MB and FG conceived and carried out experiments, and analysed data. HP and RF conceived experiments. PM, EB, BS, AJP, SB, QD and JMP carried out experiments. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Belz GG. Elastic properties and Windkessel function of the human aorta. Cardiovasc Drugs Ther. 1995;9:73–83. doi: 10.1007/BF00877747. [DOI] [PubMed] [Google Scholar]
- 2.Faury G. Function-structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathol Biol (Paris) 2001;49:310–325. doi: 10.1016/s0369-8114(01)00147-x. [DOI] [PubMed] [Google Scholar]
- 3.Kielty CM, Baldock C, Lee D, et al. Fibrillin: from microfibril assembly to biomechanical function. Philos Trans R Soc Lond B Biol Sci. 2002;357:207–217. doi: 10.1098/rstb.2001.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 5.Adams JN, Brooks M, Redpath TW, et al. Aortic distensibility and stiffness index measured by magnetic resonance imaging in patients with Marfan’s syndrome. Br Heart J. 1995;73:265–269. doi: 10.1136/hrt.73.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahimastos AA, Aggarwal A, D’Orsa KM, et al. Effect of perindopril on large artery stiffness and aortic root diameter in patients with Marfan syndrome: a randomized controlled trial. Jama. 2007;298:1539–1547. doi: 10.1001/jama.298.13.1539. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner D, Baumgartner C, Matyas G, et al. Diagnostic power of aortic elastic properties in young patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2005;129:730–739. doi: 10.1016/j.jtcvs.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Jeremy RW, Huang H, Hwa J, et al. Relation between age, arterial distensibility, and aortic dilatation in the Marfan syndrome. Am J Cardiol. 1994;74:369–373. doi: 10.1016/0002-9149(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 9.Vitarelli A, Conde Y, Cimino E, et al. Aortic wall mechanics in the Marfan syndrome assessed by transesophageal tissue Doppler echocardiography. Am J Cardiol. 2006;97:571–577. doi: 10.1016/j.amjcard.2005.09.089. [DOI] [PubMed] [Google Scholar]
- 10.Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 11.Faury G, Chabaud A, Ristori MT, et al. Effect of age on the vasodilatory action of elastin peptides. Mech Ageing Dev. 1997;95:31–42. doi: 10.1016/s0047-6374(96)01842-8. [DOI] [PubMed] [Google Scholar]
- 12.Faury G, Garnier S, Weiss AS, et al. Action of tropoelastin and synthetic elastin sequences on vascular tone and on free Ca2+ level in human vascular endothelial cells. Circ Res. 1998;82:328–336. doi: 10.1161/01.res.82.3.328. [DOI] [PubMed] [Google Scholar]
- 13.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 14.Pezet M, Jacob MP, Escoubet B, et al. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res. 2008;11:97–112. doi: 10.1089/rej.2007.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carta L, Pereira L, Arteaga-Solis E, et al. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281:8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Judge DP, Biery NJ, Keene DR, et al. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114:172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira L, Andrikopoulos K, Tian J, et al. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nature genetics. 1997;17:218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- 18.Pereira L, Lee SY, Gayraud B, et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parlakian A, Charvet C, Escoubet B, et al. Temporally controlled onset of dilated cardiomyopathy through disruption of the SRF gene in adult heart. Circulation. 2005;112:2930–2939. doi: 10.1161/CIRCULATIONAHA.105.533778. [DOI] [PubMed] [Google Scholar]
- 20.Royer A, van Veen TA, Le Bouter S, et al. Mouse model of SCN5A-linked hereditary Lenegre’s disease: age-related conduction slowing and myocardial fibrosis. Circulation. 2005;111:1738–1746. doi: 10.1161/01.CIR.0000160853.19867.61. [DOI] [PubMed] [Google Scholar]
- 21.Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31:133–140. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
- 22.Brown-Augsburger P, Tisdale C, Broekelmann T, et al. Identification of an elastin cross-linking domain that joins three peptide chains. Possible role in nucleated assembly. J Biol Chem. 1995;270:17778–17783. doi: 10.1074/jbc.270.30.17778. [DOI] [PubMed] [Google Scholar]
- 23.Faury G, Pezet M, Knutsen RH, et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franc S, Garrone R, Bosch A, et al. A routine method for contrasting elastin at the ultrastructural level. J Histochem Cytochem. 1984;32:251–258. doi: 10.1177/32.2.6198356. [DOI] [PubMed] [Google Scholar]
- 25.Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nature genetics. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 26.Li DY, Faury G, Taylor DG, et al. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102:1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 28.Dietz HC, Mecham RP. Mouse models of genetic diseases resulting from mutations in elastic fiber proteins. Matrix Biol. 2000;19:481–488. doi: 10.1016/s0945-053x(00)00101-3. [DOI] [PubMed] [Google Scholar]
- 29.Maki JM, Rasanen J, Tikkanen H, et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Lozano PR, Ikeda Y, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 31.Urban Z, Riazi S, Seidl TL, et al. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li DY, Brooke B, Davis EC, et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 33.Curran ME, Atkinson DL, Ewart AK, et al. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell. 1993;73:159–168. doi: 10.1016/0092-8674(93)90168-p. [DOI] [PubMed] [Google Scholar]
- 34.Ewart AK, Jin W, Atkinson D, et al. Supravalvular aortic stenosis associated with a deletion disrupting the elastin gene. J Clin Invest. 1994;93:1071–1077. doi: 10.1172/JCI117057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewart AK, Morris CA, Atkinson D, et al. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nature genetics. 1993;5:11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- 36.Ewart AK, Morris CA, Ensing GJ, et al. A human vascular disorder, supravalvular aortic stenosis, maps to chromosome 7. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3226–3230. doi: 10.1073/pnas.90.8.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li DY, Toland AE, Boak BB, et al. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1021–1028. doi: 10.1093/hmg/6.7.1021. [DOI] [PubMed] [Google Scholar]
- 38.Olson TM, Michels VV, Urban Z, et al. A 30 kb deletion within the elastin gene results in familial supravalvular aortic stenosis. Hum Mol Genet. 1995;4:1677–1679. doi: 10.1093/hmg/4.9.1677. [DOI] [PubMed] [Google Scholar]
- 39.Carta L, Wagenseil JE, Knutsen RH, et al. Discrete contributions of elastic fiber components to arterial development and mechanical compliance. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:2083–2089. doi: 10.1161/ATVBAHA.109.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong W, Knispel RA, Dietz HC, et al. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg. 2008;47:166–172. doi: 10.1016/j.jvs.2007.09.016. discussion 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung AW, Au Yeung K, Cortes SF, et al. Endothelial dysfunction and compromised eNOS/Akt signaling in the thoracic aorta during the progression of Marfan syndrome. Br J Pharmacol. 2007;150:1075–1083. doi: 10.1038/sj.bjp.0707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung AW, Yang HH, Yeung KA, et al. Mechanical and pharmacological approaches to investigate the pathogenesis of Marfan syndrome in the abdominal aorta. J Vasc Res. 2008;45:314–322. doi: 10.1159/000113603. [DOI] [PubMed] [Google Scholar]
- 43.Bax DV, Bernard SE, Lomas A, et al. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J Biol Chem. 2003;278:34605–34616. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- 44.Williamson MR, Shuttleworth A, Canfield AE, et al. The role of endothelial cell attachment to elastic fibre molecules in the enhancement of monolayer formation and retention, and the inhibition of smooth muscle cell recruitment. Biomaterials. 2007;28:5307–5318. doi: 10.1016/j.biomaterials.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Davis EC. Immunolocalization of microfibril and microfibril-associated proteins in the subendothelial matrix of the developing mouse aorta. J Cell Sci. 1994;107:727–736. doi: 10.1242/jcs.107.3.727. [DOI] [PubMed] [Google Scholar]
- 46.Mariko B, Ghandour Z, Raveaud S, et al. Microfibrils and fibrillin-1 induce integrin-mediated signaling, proliferation and migration in human endothelial cells. Am J Physiol Cell Physiol. 2010;299:C977–987. doi: 10.1152/ajpcell.00377.2009. [DOI] [PubMed] [Google Scholar]
- 47.Wilson DG, Bellamy MF, Ramsey MW, et al. Endothelial function in Marfan syndrome: selective impairment of flow-mediated vasodilation. Circulation. 1999;99:909–915. doi: 10.1161/01.cir.99.7.909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed materials and methods regarding ultrasound, aorta mechanics and RNA analyses.
A: In vivo arterial diameter at the level of the ascending aorta. Measurements of systolic (sd) and diastolic (dd) diameters are gated on the ECG signal in time-motion mode. B,C: Continuous wave Doppler images from the apical two-chamber view. B. Normal mitral diastolic inflow (M) of a 24 month-old Fbn1+/+ mouse. C. Detection of a simultaneous rapid (> 3m/s) diastolic retrograde flow from the aorta, indicative of aortic regurgitation (AR) in a 24 month-old Fbn1+/mgΔ mouse.
Specific mRNA levels relative to total mRNA.