Abstract
We sought to understand mechanisms underlying glucose sensing in Drosophila melanogaster. We found that adult insulin producing cells (IPCs) respond to glucose and glibenclamide with a burst-like pattern of activity. Under control conditions IPCs have a resting membrane potential of −62 ± 4 mV. In response to glucose or glibenclamide IPCs generate action potentials at a threshold of −36±1.4 mV with an amplitude of 46±4 mV and width of 9.3±1.8 ms. Real-time Ca2+ imaging confirms that IPCs respond to glucose and glibenclamide with increased intracellular Ca2+. These results provide the first detailed characterization of electrical properties of adult Drosophila IPCs and suggest that these cells sense glucose by a mechanism similar to mammalian pancreatic β cells.
Keywords: Drosophila melanogaster, KATP channels, glibenclamide, pancreatic β cells, Ca2+ imaging
INTRODUCTION
Functional similarities between flies and mammals in maintaining glucose homeostasis offer a unique opportunity for developing innovative molecular approaches for modeling type-2 diabetes in an invertebrate, genetically tractable system. Analogous to the opposing actions of insulin-secreting pancreatic β cells and glucagon-secreting pancreatic islet α cells in maintaining glucose homeostasis in mammals, insulin-like peptide-producing cells (IPCs) and adipokinetic hormone producing corpora cardiaca (CC) cells are neurosecretory cells functioning in glucose sensing in the fly [1,2]. Genetic ablation of IPCs induces hyperglycemia [1,3] whereas targeted ablation of CC cells renders larvae hypoglycemic [2]. While these genetic studies have validated the use of Drosophila as a potential model system for type-2 diabetes, the underlying mechanism responsible for glucose sensitivity remains to be determined. By measuring membrane potential of intact adult IPCs, we recently reported that similar to mammalian pancreatic β cells, membrane depolarization is recorded in response to glucose and a specific KATP channel blocker, glibenclamide, thus suggesting that KATP channels contribute to the mechanism whereby IPCs sense changes in circulating sugar [4]. Further, as demonstrated in mammalian pancreatic β cells [5], high glucose initiates a robust Ca2+ influx in adult IPCs [4]. In addition, in situ and real-time expression analysis show detectable levels the sulfonylurea receptor (Sur) subunit of the KATP channel in those cells [4].
To further establish that IPCs serve as endocrine control for insulin secretion in the adult fly, we demonstrate in this study electrophysiological properties recorded in adult IPCs are characteristic of β cells. A functional role of KATP channels in IPCs is confirmed with the use of glibenclamide. As KATP channels are recognized as a key component in regulating Ca2+ influx into β cells leading to glucose stimulated insulin secretion [6], we used real-time Ca2+ imaging techniques and recorded increased intracellular Ca2+ concentration in response to both glucose and glibenclamide in acutely dissociated adult IPCs. Additionally, we have developed patch-clamp techniques to monitor electrophysiological properties of adult Drosophila IPCs. The fact that glibenclamide elicits a robust firing rate response and increases intracellular Ca2+ suggests that adult IPCs sense and respond to glucose by a mechanism that likely involves inhibition of KATP channels.
METHODS
Whole brain preparation and electrophysiology
Whole brains were prepared from dilp2-Gal4/UAS-GFP flies and stored on poly-L-lysine coated cover slips in hemolymph-like (HL) solution as described previously [4]. Individual cover slips were transferred into recording chamber mounted on a fluorescence microscope (Zeiss Axioskop FS) and continuously perfused with a buffer solution (in mM): 3 KCl, 101 NaCl, 1 CaCl2, 4 MgCl2, 1.25 NaH2PO4, 20.7 NaHCO3, 35 sucrose, 5 glucose, equilibrated with 5% CO2 balance O2, pH 7.3 [4]. Individual IPCs were differentiated from non-IPCs by GFP fluorescence. Recordings of membrane potential were made by using an Axopatch 200B patch-clamp amplifier, digitized with a Digidata 1322A analog-to-digital converter and recorded using pCLAMP 10 software (Molecular Devices). Recordings were obtained at room temperature using electrodes with a DC resistance of 3–6 MΩ when filled with internal solution containing (mM): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 0.3 GTP-Tris, pH 7.2; electrode tips were coated with Sylgard. Firing rate was measured in the loose-patch configuration as previously described (7).
Acute dissociation preparation and real-time Ca2+ imaging
Adult IPCs were acutely dissociated from dilp2-Gal4/UAS-GFP brains in modified HL solution and plated on poly-L-lysine coated cover slips as previously described [4]. Cells were allowed to settle for 1 hour at room temperature before loading with the cell permeable Ca2+-dye rhod-3 AM according to manufacturer's instructions (Molecular Probes). Ca2+ imaging, acquisition of fluorescence images and subsequent data analysis has been described in detail previously [4]. Glibenclamide was bath applied at a concentration of 20 µM.
Data Analysis
Action potential properties were measured from the average waveform of 3–5 action potentials collected under control conditions shortly after gaining whole-cell access. As shown in Fig. 1D, several action potential properties were measured. Action potential threshold (T) was defined as the voltage at which the first time derivative of the action potential (AP’) exceeds ~1.0 V/s. Action potential amplitude (AP-H) was measured from T to peak of the depolarization while action potential width (W) was measured at 50% of AP-H. The amplitude of the afterhyperpolarization (AHP) was measured from resting membrane potential (RMP) to the most negative post-spike potential. We also used peak inflection points of the AP’ to estimate maximum rates of depolarization (MD) and repolarization (MR). Firing rate histograms were generated by integrating action potential discharge in 10-s bins and plotted using Spike 5.0 software. Instantaneous firing rate (1/time between spikes) was used to characterize time-dependent changes in neuronal activity and power spectrum analysis of instantaneous firing rate was used to detect oscillations in activity based on peaks in signal power distributed with frequency. To determine if activity oscillations were due to random changes in activity, we randomized instantaneous firing rate (15 times for each original signal), performed power spectrum analysis on each signal, and compared peak power values obtained experimentally to randomized surrogate data. Statistical significance was determined at p< 0.05 by ANOVA and Newman-Keuls multiple comparison test or t-test. Data are presented as mean ± SEM.
Figure 1. Glucose and glibenclamide stimulate activity of IPCs.
A, A diagram shows adult Drosophila brain anatomy and location of 14 IPC cells [4]. The soma of each IPC is located in the superficial layer of the pars intercerebralis and sends a unipolar projection down midline to presumably release insulin at yet undetermined locations (M. Tatar, Brown University, personal communication). B1. Firing rate trace shows that glucose (20 mM) caused a large and reversible increase in firing rate. The effects of glucose were mimicked by glibenclamide (10 µM). B2. Traces of membrane potential and instantaneous firing rate (1/time between spikes) from the same cell as in (B1) shows oscillating bursts of activity (bars above trace) during glucose exposure. B2 inset, power spectrum analysis of instantaneous firing rate during glucose exposure (original data) shows a peak power of 158 AU at ~0.1 Hz, whereas the trace of shuffled instantaneous firing rate does not exceed ~54 AU, suggesting that burst-like activity of this cell is not due to chance. C. summary data showing firing rate under control conditions (N=6) and in glucose (N=5) or glibenclamide (N=6). D, mean AP expanded in time to illustrate waveform parameters of interest (results of this analysis are summarized in table 1).
RESULTS
Electrophysiological properties of IPCs
The electrical properties of adult IPCs were measured shortly after gaining whole-cell access (summarized in Table 1). Under control conditions RMP was −62 ± 4 mV which is depolarized relative to the equilibrium potential for K+ under these conditions (−94 mV), suggesting that channels in addition to KATP contribute to RMP. These cells have an average capacitance (C) of 3.5 ± 0.7 pF and input resistance (Rin) of 1126 ± 67 MΩ, indicating they are extremely small and of low conductance. For this reason, it is likely that only small currents will be required to change membrane potential. These passive electrical properties are similar to values reported for mammalian pancreatic β cells [8–10]. Under control conditions, IPCs were not spontaneously active and did not appear to receive synaptic input as evidenced by a lack of synaptic potentials. When depolarized by DC current injection or exposure to glucose, IPCs produced action potentials at a threshold of −36 ± 1.4 mV with an amplitude of 46 ± 4 mV and width of 9.3 ± 1.8 ms (Fig. 1D). The MD was 17.9 ± 4 V/s and the MR was −7.5 ± 1 V/s. In addition, IPCs also exhibit a prominent AHP of 8.1 ± 0.2 mV (Fig. 1D) that returned to RMP within ~100 ms.
Table 1.
Electrical properties of adult IPCs.
| Resting properties | Action potential properties |
||
|---|---|---|---|
| RMP | −62 ± 4 mV (N=10) |
Threshold | −36 ± 1.4 mV (N=3) |
| Rin | 1126 ± 67 MΩ (N=13) |
Height | 46 ± 4 mV (N=3) |
| C | 3.5 ± 0.7 pF (N=5) |
Width | 9.3 ± 1.8 ms (N=3) |
|
Spontaneous activity |
None (N=10) |
AHP | 8.1 ± 0.2 mV (N=3) |
Glucose and glibenclamide have similar effects on excitability of IPCs
It is well established in mammalian pancreatic β cells that glucose-mediated ATP production inhibits KATP channels to initiate membrane depolarization and formation of Ca2+-dependent action potentials that leads to increased intracellular Ca2+ levels and insulin release [8,11]. We hypothesize that this mechanism of glucose homeostasis is highly conserved between mammals and fruit flies. This possibility is supported by our previous findings that glibenclamide mimicked effects of high glucose on membrane potential of IPCs in situ [10]. To further support the possibility that KATP channels contribute to glucose sensing in these cells, we characterized the effects of glucose and glibenclamide on the firing rate of IPCs. For these experiments we used a variant of the whole-cell patch technique known as loose-patch recording to minimize ‘washout’ of soluble intracellular molecules, including potentially vital components of the glucose signing mechanism [12]. Exposure to high glucose increased firing rate by 2.9 ± 1.2 Hz (Figs. 1B1 & 1C). The effects of glucose on excitability of IPCs were mimicked by glibenclamide which increased firing rate to 1.6 ± 0.5 Hz (Fig. 1B–1C). Interestingly, the repetitive firing response of adult Drosphila IPCs to either glucose (Fig. 1B2) or glibenclamide (data not shown) exhibited an oscillatory pattern of activity that cycled every 24.7 ± 6.6 (N=11) seconds between busts of activity (4.1 ± 1.0 Hz) followed by periods of decreased activity (1.2 ± 0.7 Hz). Power spectrum analysis of instantaneous firing rate during glucose exposure revealed an average (N=3) peak power of 153 ± 5 AU, which was greater than averaged (N=45) peak power of randomized (i.e., shuffled) instantaneous firing rate 54 ± 1 AU (inset, Fig. 1B2). This oscillatory firing rate response is reminiscent of the characteristic glucose response of mammalian pancreatic β cells [8,13,14].
Glucose and glibenclamide increase Ca2+ in IPCs
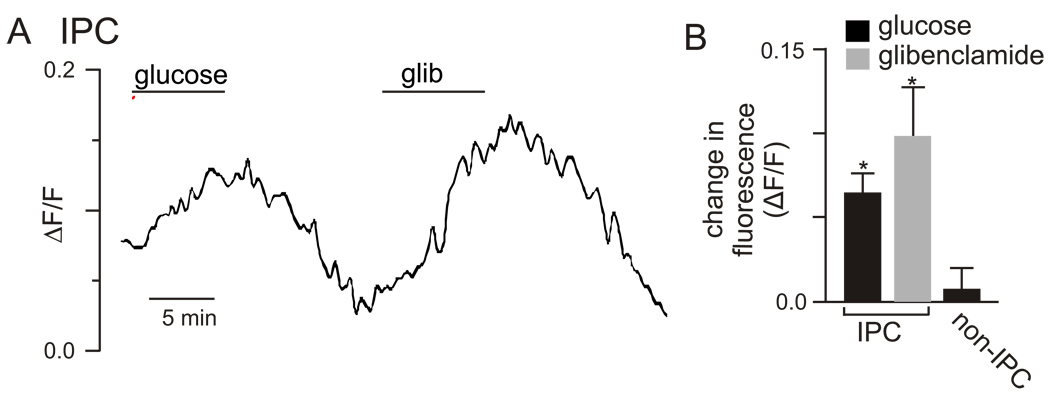
In β cells, an important link between changes in membrane potential and insulin release is Ca2+ influx resulting of the opening of voltage-gated Ca2+ channels. To extend our previous results where acutely dissociated IPCs respond to high glucose with an increase in intracellular Ca2+ concentration [4], here we demonstrate that glibenclamide elicits a similar increase in intracellular Ca2+ (Fig 2). In these experiments, acutely dissociated adult IPCs were loaded with a Ca2+-sensitive fluorescent dye (rhod-3) and Ca2+ concentration was measured during ~5 min exposure to glucose or glibenclamide. Consistent with our electrophysiological results, both glucose and glibenclamide significantly increased Ca2+ in adult IPCs (Figs. 2A–B), whereas non-IPCs did not show a Ca2+ response to either glucose or glibenclamide. We did not observe oscillations in intracellular Ca2+ concentration with kinetics that parallel burst-like changes in membrane potential, likely because our Ca2+ fluorescent sampling rate (0.03 Hz) was too slow to resolve rapid membrane oscillations.
Figure 2. Acutely dissociated IPCs respond to glucose and glibenclamide with an increase in intracellular Ca2+.
Fluorescence trace from an IPC loaded with the Ca2+-sensitive dye rhod-3 shows that exposure to glucose (80 mM) or glibenclamide (20 µM) reversibly increases intracellular Ca2+ concentration. A normalized increase in fluorescence is calculated as absolute increase in fluorescence divided by fluorescence under control conditions [4]. B. A bar graph summarizing the change in fluorescence of IPCs in response to glucose (N=8) or glibenclamide (N=5). Note that control non-IPC cells (N=3) did not respond to glucose (p<0.05, *).
DISCUSSION
It has been proposed that Drosophila IPCs are functionally analogous to pancreatic β cells in maintaining glucose homeostasis, yet little is known regarding the electrical properties of IPCs or how these cells sense changes in circulating glucose. Here, we used electrophysiological and Ca2+ imaging techniques to characterize glucose sensitivity of adult Drosophila IPCs. We show that IPCs responded to glucose and glibenclamide with a robust increase in firing rate, indicating that IPCs express functional KATP channels that likely contribute to the glucose sensing mechanism. In addition, exposure to glucose or glibenclamide increased Ca2+ in acutely dissociated IPCs. These results strongly suggest that glucose-mediated activation of IPCs involves inhibition of KATP channels, and is coupled to insulin release by a corresponding rise in intracellular Ca2+.
Our results show that electrical properties of IPCs are similar to those of mammalian β-cells. For example, β cells typically have a RMP near −70 [8] and generate action potentials at threshold potentials near −50 mV with an amplitude of ~30 mV, width >25 ms and MD of ~3 V/s [8,9,15]. In addition, when activated by glucose or glibenclamide, IPCs showed an oscillating pattern of activity similar to the characteristic response of mammalian pancreatic β cells [13,14]. In β cells, this pattern of activity has been shown to result from fluctuation in KATP channel activity [13] and intermittent activation of voltage-dependent Ca2+ channels and results in a Ca2+ influx that is required for insulin release [14]. We show in our studies that glucose- or glibenclamide-mediated activation of IPCs also resulted in increased intracellular Ca2+, suggesting that IPCs and β cells share a common glucose sensing mechanism.
Somewhat surprisingly, we found that glucose-mediated activation of IPCs did not appear to correspond with changes in whole-cell conductance. The most likely explanation for this is that only a small proportion of KATP channels are inhibited by this level of glucose. Given the very high Rin measured in IPCs, it is conceivable that only a small current is sufficient to depolarize membrane potential and increase excitability of IPCs. For example, a previous study found that glucose-mediated activation of β cells occurs with less than 10% change in KATP channel activity [16]. Likewise, a change in KATP conductance of only 0.15 nS applied with dynamic clamp was shown to have a large effect on excitability of murine β cells [9]. However, it is also possible that multiple channels contribute to glucose sensing and result in offsetting effects on conductance. Nevertheless, our finding that inhibition of KATP channels with glibenclamide mimics the effects of glucose strongly suggests that KATP channels are key mediators of glucose sensing by adult IPCs.
In conclusion, our electrophysiological and Ca2+ imaging studies in adult IPCs substantiate a role of these neurosecretory cells in glucose sensing with a mechanism that appears to involve inhibition of KATP channels as found in mammalian pancreatic β cells. This functional conservation validates adult Drosophila IPCs as a highly relevant and well-suited experimental system to rapidly test interventions modulating insulin secretion in modeling type 2 diabetes.
Acknowledgements
This work was supported by grants from the NIA to Y-W. C. F (AG21068, AG31086) and the UConn Research Foundation (DKM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 2.Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- 3.Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridell YW, Hoh M, Kreneisz O, Hosier S, Chang C, Scantling D, et al. Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging. 2009;1:699–713. doi: 10.18632/aging.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashcroft FM. K(ATP) channels and insulin secretion: a key role in health and disease. Biochem Soc Trans. 2006;34:243–246. doi: 10.1042/BST20060243. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft FM, Rorsman P. Molecular defects in insulin secretion in type-2 diabetes. Rev Endocr Metab Disord. 2004;5:135–142. doi: 10.1023/B:REMD.0000021435.87776.a7. [DOI] [PubMed] [Google Scholar]
- 7.Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 9.Kinard TA, de Vries G, Sherman A, Satin LS. Modulation of the bursting properties of single mouse pancreatic beta-cells by artificial conductances. Biophys J. 1999;76:1423–1435. doi: 10.1016/S0006-3495(99)77303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rorsman P, Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985;405:305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- 11.Henquin JC. A minimum of fuel is necessary for tolbutamide to mimic the effects of glucose on electrical activity in pancreatic beta-cells. Endocrinology. 1998;139:993–998. doi: 10.1210/endo.139.3.5783. [DOI] [PubMed] [Google Scholar]
- 12.Sala S, Parsey RV, Cohen AS, Matteson DR. Analysis and use of the perforated patch technique for recording ionic currents in pancreatic beta-cells. J Membr Biol. 1991;122:177–187. doi: 10.1007/BF01872640. [DOI] [PubMed] [Google Scholar]
- 13.Larsson O, Kindmark H, Brandstrom R, Fredholm B, Berggren PO. Oscillations in KATP channel activity promote oscillations in cytoplasmic free Ca2+ concentration in the pancreatic beta cell. Proc Natl Acad Sci. 1996;93:5161–5165. doi: 10.1073/pnas.93.10.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henquin JC, Jonas JC, Gilon P. Functional significance of Ca2+ oscillations in pancreatic beta cells. Diabetes Metab. 1998;24:30–36. [PubMed] [Google Scholar]
- 15.Matthews EK, Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol. 1975;246:421–437. doi: 10.1113/jphysiol.1975.sp010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashcroft FM, Rorsman P. ATP-sensitive K+ channels: a link between β-cell metabolism and insulin secretion. Biochem Soc Trans. 1990;18:109–111. doi: 10.1042/bst0180109. [DOI] [PubMed] [Google Scholar]