Abstract
Plasma amyloid-β (Aβ) level could be useful as a non-invasive biomarker in Alzheimer’s disease research. We compared a multiplex electrochemiluminescence detection method with a well established ELISA method for plasma Aβ quantification. Compared to the ELISA method, the electrochemiluminescence detection method demonstrates a statistically significant, but modest correlation. The reasons for this may include the differences in the affinities of antibodies, and purity and source of Aβ peptides used as standards. However, the advantages of electrochemiluminescence detection technology include short processing time and small sample volume. This comparison demonstrates the need for a further study in optimizing this system.
Keywords: Amyloid-β protein, biological markers, enzyme-linked immunosorbent assay, plasma
INTRODUCTION
Since many potential treatments for Alzheimer’s disease (AD) are being developed, much effort has been dedicated to identifying biomarkers that would be useful for clinical investigation. One of the biomarkers that has received much attention is amyloid-β (Aβ) peptide. Aβ peptides are derived from β- and γ-secretase cleavages of the amyloid-β protein precursor and are the major structural components of the amyloid plaques, which are a hallmark of AD [1]. Although many cross-sectional studies have not shown significant differences in plasma Aβ levels of AD subjects compared to controls [2], a few studies have shown that plasma Aβ42 levels may be associated with disease state or prognosis. For example, plasma Aβ42 was elevated in the extended family members of subjects with sporadic AD (sAD) [3], and longitudinal studies have shown changes in plasma Aβ42 levels and Aβ42/Aβ40 ratios in those who go on to develop sAD [4,5]. Therefore, it is important to examine the utility of plasma Aβ as a biomarker for AD that might be more widely applicable than more invasive and expensive cerebrospinal fluid (CSF)-based or brain imaging biomarkers. Although Enzyme-Linked Immunosorbent Assay (ELISA) has been the standard for measuring biomarkers in the past [6], new technologies that allow simultaneous analyses of multiple biomarkers have emerged. Flow cytometric multiplex assays, also known as bead-based multiplex assays, have been previously investigated in plasma Aβ quantification [7–9]. In these assays, Aβ peptides are bound to the capture antibodies coupled to the beads and detected with biotinylated antibodies. A different multiplex assay which employs electrochemiluminescence method is more similar to the traditional ELISA. In this plate-based method, specific capture antibodies that are coated on discrete target areas in a well within a microplate bind to the protein of interest. Bound protein is detected by a second antibody that is conjugated to a label that emits light when it is excited by the electricity from carbon-coated electrodes interwoven in the base of the plate [10]. Similar to the flow cytometric multiplex assay, this method allows high throughput analyses of different markers of interests. The aim of this study was to compare this new method to a well-validated ELISA method [4,11] using the same pool of plasma samples.
Plasma samples from patients with mild to moderate AD (n = 33), amnestic mild cognitive impairment (aMCI; n = 27), and cognitively normal controls (n = 29) were collected from a consecutive series of Johns Hopkins Alzheimer’s Disease Research Center (JHADRC) participants between 2006 and 2009. Annual cognitive diagnoses for JHADRC participants are derived from consensus conferences based on review of history, examination, and neuropsychological testing. Diagnosis of probable AD was based on NINCDS-ADRDA criteria [12]. Participants with aMCI met Petersen criteria [13] and also had a Clinical Dementia Rating (CDR) = 0.5 and Mini-Mental Status Exam (MMSE) score ≥ 24. Cognitively normal controls were age-matched participants with CDR of 0.0, MMSE > 28, and with no reported memory impairments by history. All participants gave informed consent, which was approved by the Johns Hopkins Institutional Review Board.
All blood draw and processing followed established JHADRC protocols using standard venipuncture procedures. Blood was collected in EDTA polypropylene tubes for plasma, centrifuged at 3000 rpm for 15 min at 4°C, and immediately divided into 0.5 ml aliquots and later stored in −80°C freezers until analysis. Previously unthawed aliquots from the same collection time points were analyzed by the two methods.
ELISA Aβ40 and Aβ42 levels were measured in plasma using a combination of mouse monoclonal antibody 6E10 (specific to an epitope present on 3–11 amino acid residues of Aβ) as capture antibody and rabbit polyclonal antibodies specific for Aβ40 and Aβ42 as detection antibodies in a double-antibody sandwich ELISA as described previously [11]. MSD electrochemiluminescence assay (Meso Scale Discovery, Gaithersburg, MD) was performed according to the manufacturer’s specifications. Briefly, Multi-Spot® Triplex 96 well plates precoated with anti-Aβ38, Aβ40, and Aβ42 antibodies were incubated with 1% Blocker A solution for 1 h. After the plates were washed with 1X Tris buffer, 25 μl of SULFO-TAG 6E10 detection antibody solution was added with 25 μl of calibrators (standards) and plasma samples for 2 h. After the plates were washed with 1XTris buffer, 150 μl 2XMSD Read Buffer T was added to the plates and read immediately on the Meso Scale Discovery (MSD) Sector Imager 2400 at (620 nm). ELISA and electrochemiluminescence assays were independently performed on 2 separate plasma aliquots from the same collection time point. Each sample was analyzed in duplicates and then averaged for a mean value. Aβ40 and Aβ42 values are reported in this article.
Inter-assay coefficients of variation (CV) for each Aβ measure were calculated at multiple concentrations. Spearman rank correlation coefficients were estimated to compare the Aβ40/42 values and Aβ42/40 ratio from the different assays. The level of significance was a priori defined as p < 0.05. Bland-Altman curves were constructed for comparison of MSD and ELISA assays [14]. Statistical analyses were conducted using STATA Version 9.0 (StataCorp, College Station, TX).
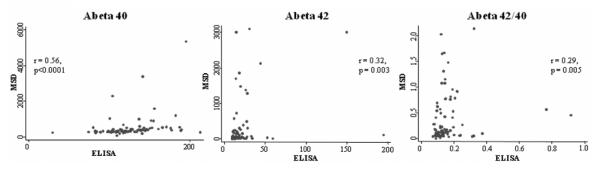
Absolute concentrations of Aβ peptides varied considerably between the ELISA and the MSD electrochemiluminescence assays. Correlations between the two methods were modest, albeit statistically significant. The correlation coefficient for plasma Aβ40 values was r = 0.56 (p < 0.0001), for Aβ42 r = 0.32 (p = 0.003), and for Aβ42/40 ratio r = 0.29 (p = 0.005) (Fig. 1). Bland-Altman analyses for Aβ40 and Aβ42 showed that approximately 95% of the measurements fell within 2 SDs of the mean difference. However, the differences within the mean difference ± 2 SDs were large for all measures; thus, the measurements from the two methods are likely not directly interchangeable (data not shown). Interassay CVs for Aβ40 and Aβ42 were 5.1 and 0.9% respectively for the MSD electrochemiluminescence assay, and 10 and 14.2% for the ELISA. There was much wider dynamic range in the measurements of Aβ levels in the MSD electrochemiluminescence assay compared to the ELISA. The ELISA Aβ40 levels ranged from 29.6 to 213 pg/ml compared to the MSD electrochemiluminescence assay values of 74.9 to 5344.4 pg/ml. Similarly, Aβ42 values ranged from 9.5 to 219 pg/ml on the ELISA, and 10 to 3000 pg/ml on the MSD electrochemiluminescence assay. Mean levels of plasma Aβ40 did not differ by diagnostic group using either ELISA (NC = 120.8 [29.8], MCI = 129.3 [33.4], AD = 127.8 [28.8], F[2,85] = 0.64, p = 0.532) or MSD electrochemiluminescence assays (NC = 521.7 [602.9], MCI = 444.1 [384.5], AD = 556.1 [893.9], F[2,86] = 0.24, p = 0.786). Similarly, plasma Aβ42 levels also did not differ by group using ELISA (NC = 24.2 [34.3], MCI = 17.5 [9.9], AD = 20.5 [24.2], F[2,86] = 0.50, p = 0.611) or MSD electrochemiluminescence assays (NC = 379.6 [746.2], MCI = 181.0 [568.7], AD = 270.7 [597.0], F[2,86] = 0.66, p = 0.519). MSD electrochemiluminescence assay was also compared with the ELISA in terms of the sample volume used, time and cost in processing plasma Aβ (Table 1).
Fig. 1.
Correlation between ELISA and MSD electrochemiluminescence assays of plasma (A) Aβ40, (B) Aβ42, and (C) Aβ42/40 ratio. Spearman rank correlation coefficients (A) Aβ40 r = 0.56, p < 0.0001; Aβ42 r = 0.32, p = 0.003; Aβ42/40 ratio r = 0.29, p = 0.005.
Table 1.
Comparison of ELISA with electrochemiluminescence assay
| ELISA | Multiplex electrochemiluminescence assay |
|
|---|---|---|
| Laboratory/Company | Mehta laboratory | Meso Scale Discovery |
| Assay | ELISA Aβ40,42 | Multi-Spot® Triplex Human (6E10) Aβ38,40,42 |
| Sample Size | 100 μl/well | 25 μl/well |
| duplicates for Aβ40 and Aβ42 | (400 μl total) | (50 μl total) |
| Time | 18 hours | 3.5 hours |
| Cost† | $20 per sample in duplicates* |
$16 per sample in duplicates** |
Equipment cost has not been factored into the cost analysis.
Since the ELISA used in this experiment is an inhouse ELISA, it is difficult to estimate the cost. $20 per sample cost estimation was based on the commercially available Invitrogen Aβ40 and Aβ42 ELISA kits (2 plate sets) (Invitrogen, CA, USA) purchased individually. These kits utilize 50 μl sample per well, and therefore total of 200 μl would be required for duplicates of Aβ40 and Aβ42. Time required would be approximately 45 h per assay (8–10 h for Aβ40 and Aβ42).
Cost per sample varies depending on how many plates are purchased. This cost was estimated from a 5 plate set.
Recently, different multiplex assays have emerged in AD biomarker research. They offer several advantages over traditional ELISAs including markedly shortened processing time, smaller sample volume requirements, and simultaneous processing of multiple biomarkers [10]. Among its many applications, electrochemiluminescence detection method has been used for quantification of Aβ levels in brain homogenates [15] and has been directly compared to SDS-PAGE/immunoblot for quantification of CSF Aβ levels [16].
In biomarker studies, many variables may influence the outcome. These include differences in the characteristics of the study population, as well as sample collection, processing, and storage methods. In this study, previously unthawed plasma samples from a well-characterized cohort of subjects whose plasma samples have been collected following a standardized protocol were used for assays. We demonstrate here that an electrochemiluminescence multiplex assay showed a large dynamic range in the detection of Aβ40 and Aβ42 levels in plasma. It required significantly less volume of plasma and a short processing time. However, absolute concentrations of Aβ peptides varied considerably when compared to the more widely used ELISA assay.
In addition to the previously mentioned factors, additional factors may contribute to the observed differences between the assays. One of the most important factors may be the differences in the mouse monoclonal or rabbit polyclonal antibodies that are used. Most commercially available ELISAs use capture antibodies that recognize the amino (N)-terminus of the Aβ peptide such as 6E10, as was in this study. In the MSD electrochemiluminescence multiplex assay, the capture antibodies recognize the carboxyl-terminus. As some of the Aβ species in AD brain are N-terminally truncated, this may create some problems for ELISA assays with N-terminal capture antibodies [17,18], possibly resulting in overall lower amplification of the bound proteins. It has also been shown that 6E10, a common antibody used in Aβ-specific ELISA assays, binds fulllength amyloid-β protein precursor, which could potentially interfere with the ability of 6E10 to sensitively detect Aβ species in plasma [19]. Additional factors may be differences in the affinity of the capture and detection antibodies, purity of the standards that are used as calibrators, as well as the temperature of the experiment, diluents buffer and other reagents that are used in each assay. There is also concern of cross-reactivity with different capture antibodies in a multiplex assay, however most commercial assays have been designed to minimize artifacts from multiplexing [5].
This study, to our knowledge, is the first study to directly compare a well established conventional ELISA to the MSD electrochemiluminescence multiplex assay for plasma Aβ quantification. While the correlation was modest, variability may be reduced by using identical antibodies as well as using similar reagents in each experiment. Currently, its uses are limited due to the considerable start up cost in acquiring the equipment, however, due to the significant advantages of the multiplex systems, they will most likely become more widely used in the academic and commercial laboratories in the future [6].
ACKNOWLEDGMENTS
National Institutes of Health Grant P50 AG005146, 1R21AG028754,R21NS060271-01,The Rosalinde and Arthur Gilbert Foundation/AFAR New Investigator Award in Alzheimer’s disease; Institute for Clinical and Translational Research; and the Hobson Gift Fund. We would like to thank Dr. Anita Taylor from the Meso Scale Discovery for technical assistance.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=448).
REFERENCES
- [1].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- [2].Oh ES, Troncoso JC, Tucker SM Fangmark. Maximizing the potential of plasma amyloid-beta as a diagnostic biomarker for Alzheimer’s disease. Neuromolecular Med. 2008;10:195–207. doi: 10.1007/s12017-008-8035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ertekin-Taner N, Younkin LH, Yager DM, Parfitt F, Baker MC, Asthana S, Hutton ML, Younkin SG, Graff-Radford NR. Plasma amyloid beta protein is elevated in late-onset Alzheimer disease families. Neurology. 2008;70:596–606. doi: 10.1212/01.WNL.0000278386.00035.21. [DOI] [PubMed] [Google Scholar]
- [4].Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, Small SA, Stern Y, Wisniewski HM, Mehta PD. Plasma amyloid beta-peptide 1-42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46:412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [5].Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- [6].Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lewczuk P, Kornhuber J, Vanmechelen E, Peters O, Heuser I, Maier W, Jessen F, Burger K, Hampel H, Frolich L, Henn F, Falkai P, Ruther E, Jahn H, Luckhaus C, Perneczky R, Schmidtke K, Schroder J, Kessler H, Pantel J, Gertz HJ, Van-derstichele H, de Meyer G, Shapiro F, Wolf S, Bibl M, Wiltfang J. Amyloid beta peptides in plasma in early diagnosis of Alzheimer’s disease: A multicenter study with multiplexing. Exp Neurol. 2010;223:366–370. doi: 10.1016/j.expneurol.2009.07.024. [DOI] [PubMed] [Google Scholar]
- [8].Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, Wallin A, Minthon L, Blennow K. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- [9].Lambert JC, Schraen-Maschke S, Richard F, Fievet N, Rouaud O, Berr C, Dartigues JF, Tzourio C, Alperovitch A, Buee L, Amouyel P. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology. 2009;73:847–853. doi: 10.1212/WNL.0b013e3181b78448. [DOI] [PubMed] [Google Scholar]
- [10].Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879–884. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mehta PD, Capone G, Jewell A, Freedland RL. Increased amyloid beta protein levels in children and adolescents with Down syndrome. J Neurol Sci. 2007;254:22–27. doi: 10.1016/j.jns.2006.12.010. [DOI] [PubMed] [Google Scholar]
- [12].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [13].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- [14].Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;327:307–310. [PubMed] [Google Scholar]
- [15].Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, Aguzzi A, Staufenbiel M, Mathews PM, Wolburg H, Heppner FL, Jucker M. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bibl M, Mollenhauer B, Lewczuk P, Esselmann H, Wolf S, Trenkwalder C, Otto M, Stiens G, Ruther E, Kornhuber J, Wiltfang J. Validation of amyloid-beta peptides in CSF diagnosis of neurodegenerative dementias. Mol Psychiatry. 2007;12:671–680. doi: 10.1038/sj.mp.4001967. [DOI] [PubMed] [Google Scholar]
- [17].Naslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, Silberring J, Gandy SE, Winblad B, Greengard P, et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci U S A. 1994;91:8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ida N, Hartmann T, Pantel J, Schroder J, Zerfass R, Forstl H, Sandbrink R, Masters CL, Beyreuther K. Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- [19].Englund H, Sehlin D, Johansson AS, Nilsson LN, Gellerfors P, Paulie S, Lannfelt L, Pettersson FE. Sensitive ELISA detection of amyloid-beta protofibrils in biological samples. J Neurochem. 2007;103:334–345. doi: 10.1111/j.1471-4159.2007.04759.x. [DOI] [PubMed] [Google Scholar]