Abstract
The C5a anaphylatoxin receptor (C5aR; CD88) is activated as part of the complement cascade and exerts important inflammatory, antimicrobial and regulatory functions, at least in part, via crosstalk with TLRs. However, the periodontal pathogen Porphyromonas gingivalis can control C5aR activation by generating C5a through its own C5 convertase-like enzymatic activity. Here we show that P. gingivalis uses this mechanism to proactively and selectively inhibit TLR2-induced IL-12p70, whereas the same pathogen-instigated C5aR-TLR2 crosstalk upregulates other inflammatory and bone-resorptive cytokines (IL-1β, IL-6, and TNF-α). In vivo, the ability of P. gingivalis to manipulate TLR2 activation via the C5a-C5aR axis allowed it to escape IL-12p70-dependent immune clearance and to cause inflammatory bone loss in a murine model of experimental periodontitis. In the latter regard, C5aR-deficient or TLR2-deficient mice were both resistant to periodontal bone loss, in stark contrast to wild-type controls, which is consistent with the interdependent interactions of C5aR and TLR2 in P. gingivalis immune evasion and induction of bone-resorptive cytokines. In conclusion, P. gingivalis targets C5aR to promote its adaptive fitness and cause periodontal disease. Given the current availability of safe and effective C5aR antagonists, pharmacological blockade of C5aR could act therapeutically in human periodontitis and reduce associated systemic risks.
Keywords: rodent (models), bacterial (infections), monocytes/macrophages, complement, inflammation
Introduction
In addition to its role in pathogen recognition and elimination, the complement network regulates immune and inflammatory responses through direct effects on immune cells or via crosstalk with TLR and other signaling pathways (1). Both complement and TLRs are rapidly activated in response to infection and their crosstalk may serve to coordinate the host response through synergistic or antagonistic interactions. These interactions may respectively enhance host defense or control it to prevent immunopathology. However, the propensity of complement and TLRs for communication may be exploited by microbial pathogens to manipulate the host response in ways that promote their adaptive fitness (2).
In this context, we have recently shown that the periodontal pathogen Porphyromonas gingivalis induces a subversive crosstalk between the complement C5a receptor (C5aR)6 and TLR2 that impairs nitric oxide–dependent intracellular killing in macrophages (3). Interestingly, P. gingivalis can control both receptors: it can directly engage TLR2 through cell-surface ligands (4), whereas it can activate C5aR (CD88) through local conversion of C5 to C5a using its own enzymes (3). Indeed, this bacterium does not have to rely on immunological mechanisms for C5a generation, but rather expresses extracellular cysteine proteinases (gingipains) which function as C5 convertase–like enzymes (3, 5).
C5aR activation was also shown to downregulate TLR4-induced production of IL-12 in vitro and in vivo (6–8). This effect is exerted at the transcriptional level since C5aR signaling in macrophages inhibits TLR4-induced mRNA expression of the IL-12p35 and IL-12/IL-23p40 subunits. Since IL-12 is a key cytokine in Th1 differentiation and cell-mediated immunity, this C5aR-TLR4 crosstalk may represent a regulatory mechanism to control IL-12 production and thereby prevent or attenuate possible immunopathology (2). However, undesirable outcomes could arise if C5a is produced at excessively high levels, as may happen in sepsis. Under such conditions, the crosstalk between C5a-activated C5aR and TLR4 could severely suppress IL-12 and interfere with protective Th1 immunity (2, 6).
High levels of C5a can be generated also through the uncontrolled action of C5-convertase-like microbial enzymes like the P. gingivalis gingipains (3). We thus hypothesized that P. gingivalis may take advantage of C5a-induced signaling to suppress biologically active IL-12 (IL-12p70). Given that IL-12p70 induces IFN-γ and mediates bacterial clearance through activated phagocytes (9), possible inhibition of this cytokine by P. gingivalis through C5aR exploitation could contribute to its ability to evade immune control. In this paper, we show that C5a (and, to a lesser extent, its desarginated derivative C5adesArg) inhibits TLR2-dependent induction of IL-12p70, but enhances induction of proinflammatory and bone-resorptive cytokines (IL-1β, IL-6, and TNF-α), in response to P. gingivalis. These in vitro observations were confirmed by in vivo studies, which additionally showed that C5aR-dependent inhibition of IL-12p70 promotes the survival of this pathogen. Moreover, C5aR signaling was required for the ability of P. gingivalis to induce periodontal bone loss in a mouse model of experimental periodontitis. Therefore, P. gingivalis exploits C5aR to promote its adaptive fitness and cause periodontal disease. This immune subversion mechanism has important therapeutic implications given the current availability of safe, selective, and potent C5aR antagonists.
Materials and Methods
Reagents, bacteria, and mice
Mouse C5a and C5adesArg were purchased from Cell Sciences or the R&D Systems. Mouse rIFN-γ, goat polyclonal anti-mouse IL-12 IgG, and anti-mouse IL-23 (p19) IgG were from R&D Systems. U0126 and wortmannin were purchased from the Cell Signaling Technology. The cyclic hexapeptide Ac-F[OP-dCha-WR] (acetylated phenylalanine–[ornithine-proline-D-cyclohexylalanine- tryptophan-arginine]), a specific and potent C5aR antagonist (also known as PMX-53) and the C3aR antagonist SB290157 were synthesized as previously described (10–12). A8Δ71–73, a dual antagonist of C5aR and C5a-like receptor-2, was expressed essentially as previously described (13). Specifically, the A8Δ71–73 sequence (13) was created by three cycles of mutagenesis of the original human C5a construct (14), using the QuickChange XL Site-Directed Mutagenesis Kit from Stratagene. The three pairs of complementary primers used for mutagenesis are as follows (forward sequences given):
5’-GTTACGATGGAGCCGCCGTTAATAATGATG-3’,
5’- CCGTGCTAATATCTCTTTTAAACGCATGCAATTGGGAAGG-3’,
5’-CTCTTTTAAACGCTCGTGAAAGCTTAATTAGC-3’,
corresponding to mutations 1) C27A, 2) H67F and D69R, and 3) M70S and Δ(71–74), respectively. The protein was then expressed and purified as previously described (14). All reagents were used at optimal concentrations determined in preliminary or published studies by our laboratories (3, 11, 14–16). When appropriate, DMSO was included in medium controls and its final concentration was ≤ 0.2 %.
P. gingivalis ATCC 33277 and its isogenic KDP128 mutant, which is deficient in all three gingipain genes (rgpA, rgpB, and kgp) (17) (kindly provided by Dr. K. Nakayama, Nagasaki University, Japan), were grown anaerobically from frozen stocks on modified Gifu anaerobic medium-based blood agar plates for 5–6 days at 37°C, followed by anaerobic subculturing for 18–24 hours at 37°C in modified Gifu anaerobic medium broth (Nissui Pharmaceutical).
Thioglycollate-elicited macrophages were isolated from the peritoneal cavity of wild-type or mice deficient in TLR2 or C5aR (4, 8), in compliance with established institutional policies and federal guidelines. We used both BALB/c and C57BL/6 C5aR-deficient mice (with their respective wild-type controls): The BALB/c were obtained from The Jackson Laboratory. The C57BL/6 C5aR-deficient mice were originally provided by Dr. Craig Gerard (Harvard Medical School) and are now housed at The Jackson Laboratory. The TLR2-deficient mice were originally C57BL/6 (The Jackson Laboratory) and we backcrossed them for nine generations onto a BALB/c genetic background before their use in these studies. The macrophages were cultured at 37°C and 5% CO2 in RPMI 1640 (InVitrogen) supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 units/ml penicillin G, 100 µg/ml streptomycin, and 0.05 mM 2-ME. None of the experimental treatments affected cell viability (monitored by the CellTiter-Blue™ assay; Promega) compared to medium-only treatments.
Cell activation assays
Induction of nitric oxide production was assessed by measuring the amount of NO2− (stable metabolite of nitric oxide) in stimulated culture supernatants using a Griess reaction-based assay kit (R&D Systems), as previously performed (15). Levels of cAMP in activated cell extracts were measured using a cAMP enzyme immunoassay kit (Cayman Chemical) (18). C5a-induced intracellular calcium mobilization was monitored in cells (4 × 106) loaded with 1 µM Indo 1-AM in the presence of 1 µM pluronic acid, as previously described (19). Calcium traces were recorded in a Perkin-Elmer fluorescence spectrometer (model 650-19) with an excitation wavelength of 355 nm and an emission wavelength of 405 nm. Induction of cytokine production in activated cell culture supernatants or in the peritoneal fluid of infected mice was determined by ELISA using kits from eBioscience or Cell Sciences. C5a levels were measured by sandwich ELISA, employing a pair of capture and biotinylated anti-C5a mAbs (BD Pharmingen), according to the manufacturer’s protocol.
Intracellular killing assay
The viability of phagocytosed P. gingivalis was monitored by an antibiotic protection-based intracellular survival assay, as previously described (20). Briefly, mouse peritoneal macrophages were allowed to phagocytose P. gingivalis (MOI = 10:1; 5×106 bacteria and 5×105 macrophages) for 30 min at 37°C. This was followed by washing to remove extracellular nonadherent bacteria and 1-hour treatment with antibiotics (300 µg/ml gentamicin and 200 µg/ml metronidazole) to eliminate residual or extracellular adherent bacteria. The macrophages were subsequently cultured overnight for a total of 24 hours. Immediately after, the macrophages were washed and lysed in sterile distilled water and viable counts of internalized P. gingivalis were determined by plating serial dilutions of macrophage lysates on blood agar plates subjected to anaerobic culture (20).
In vivo mouse studies
I.p. challenge model
10–12 week-old mice were infected i.p. with P. gingivalis (5×107 CFU) and sampled by peritoneal lavage to measure production of cytokines and enumerate recovered CFU (following anaerobic growth on blood agar plates) (15), as detailed in the corresponding figure legends.
P. gingivalis-induced bone loss
The P. gingivalis-induced periodontal bone loss model was used essentially as originally developed by Baker (21) with slight modifications as we previously described (20). Briefly, upon suppression of the normal oral flora with antibiotics, 10–12 week-old wild-type mice or mice deficient in C5aR or TLR2 were infected by oral gavage five times at 2-day intervals with 109 CFU P. gingivalis suspended in 2% carboxymethylcellulose. Sham-infected controls received 2% carboxymethylcellulose alone. The mice were euthanized six weeks later and assessment of periodontal bone loss in defleshed maxillae was performed under a dissecting microscope (×40) fitted with a video image marker measurement system (VIA-170K; Fryer). Specifically, the distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured on 14 predetermined points on the buccal surfaces of the maxillary molars. To calculate bone loss, the 14-site total CEJ-ABC distance for each mouse was subtracted from the mean CEJ-ABC distance of sham-infected mice. The results were expressed in mm and negative values indicate bone loss relative to sham-infected controls.
Age-associated periodontal bone loss
Aging mice develop naturally occurring inflammatory periodontal bone loss, which becomes quite dramatic after 9 months of age (22, 23). To determine the role of C5aR in periodontal bone loss in this chronic model, we raised C5aR-deficient and wild-type controls in parallel and bone loss was assessed as described above when the mice became 16-month old.
All animal procedures were approved by the Institutional Animal Care and Use Committee, in compliance with established federal and state policies.
Statistical analysis
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test using the InStat program (GraphPad Software, San Diego, CA). Where appropriate (comparison of two groups only), two-tailed t tests were performed. P < 0.05 was taken as the level of significance.
Results
P. gingivalis proactively and selectively inhibits IL-12p70 production via C5aR-TLR2 crosstalk
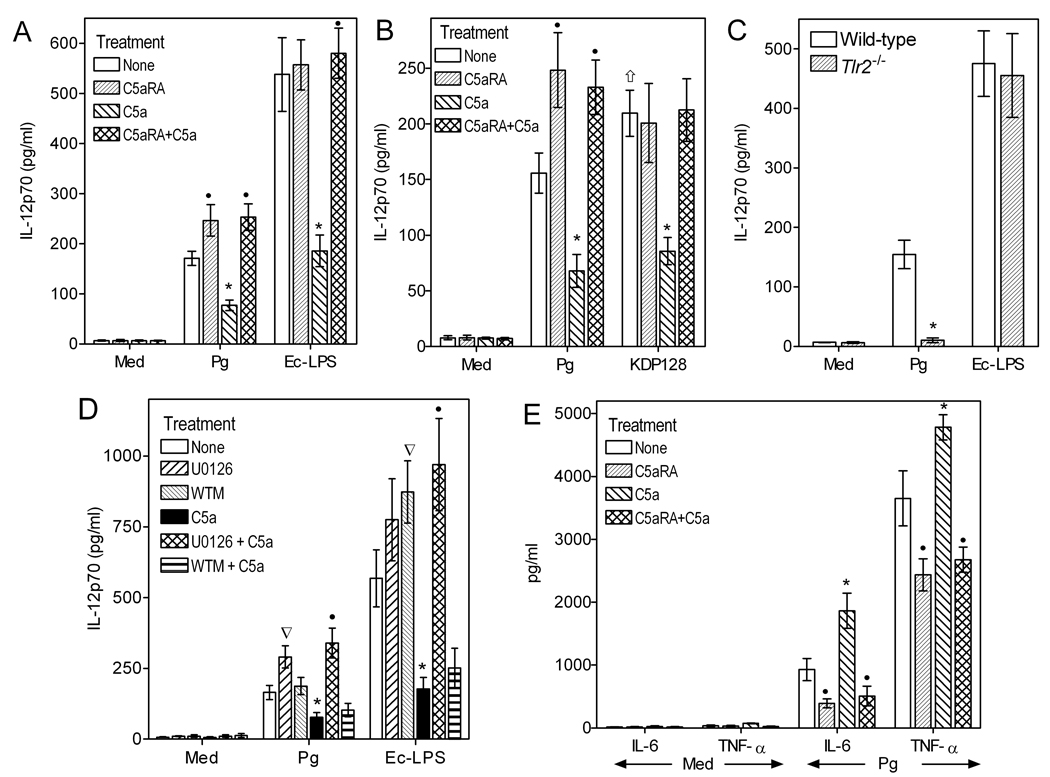
We investigated whether C5a inhibits P. gingivalis-induced IL-12p70 in peritoneal macrophages. Since macrophages are generally poor producers of IL-12p70 in vitro unless primed with IFN-γ, macrophages used in these experiments were primed with IFN-γ (0.1 µg/ml). E. coli LPS-stimulated macrophages were used as a control since C5a has been shown to inhibit IL-12p70 through a C5a/C5aR–LPS/TLR4 crosstalk (6). The host TLR response against P. gingivalis is predominantly mediated by TLR2 both in vitro and in vivo (4, 24, 25). Therefore, we additionally examined whether possible C5a-mediated inhibition of P. gingivalis-induced IL-12p70 could involve a C5aR-TLR2 crosstalk. We found that the abilities of both P. gingivalis and LPS to induce IL-12p70 production were significantly inhibited by C5a (p < 0.01; Fig. 1A). These inhibitory effects were specifically mediated by C5aR signaling since they were completely reversed by a specific C5aR antagonist (C5aRA) (p < 0.01; Fig. 1A).
Figure 1. C5aR signaling inhibits TLR2-dependent IL-12p70 induction in P. gingivalis-activated macrophages.
Mouse peritoneal macrophages were primed with IFN-γ (0.1 µg/ml) and stimulated with medium only (Med), P. gingivalis (MOI 10:1), or E. coli LPS (Ec-LPS; 0.1 µg/ml), as indicated. IFN-γ priming was performed in those experiments (A–D) investigating IL-12p70 regulation. Wild-type P. gingivalis (Pg) was used in all experiments but panel B additionally includes the use of an isogenic mutant (KDP128) which is deficient in all three gingipain genes. In A and B, the macrophages were additionally treated or not with C5a (50 nM), in the absence or presence of C5aRA (1 µM). In C, the macrophages were from wild-type or TLR2-deficient (Tlr2−/−) mice. In D, the macrophages were pretreated with U0126 (10 µM) or wortmannin (WTM; 100 nM) for 1h prior to treatments with C5a, P. gingivalis, or Ec-LPS. In E, the macrophages were stimulated with P. gingivalis as in panel A, but without IFN-γ priming, to measure levels of cytokines other than IL-12p70. Culture supernatants were assayed for induction of the indicated cytokines after 24h of incubation. Data are means ± SD (n = 3 sets of macrophages) from typical experiments performed three (A) or two (B–E) times yielding consistent results. Asterisks show statistically significant (p < 0.01) inhibition (A–D; IL-12p70) or enhancement (E; IL-6 and TNF-α) of cytokine production, whereas black circles indicate statistically significant (p < 0.01) reversal of these modulatory effects. In B, the upward arrow shows a significant difference (p < 0.05) between KDP128 and Pg under no-treatment conditions. In D, inverse triangles show significant (p < 0.01) U0126 or WTM effects on P. gingivalis- or LPS-induced IL-12p70.
Intriguingly, however, C5aRA significantly enhanced the induction of IL-12p70 production even in P. gingivalis-stimulated macrophages that were not treated with exogenous C5a (p < 0.01; Fig. 1A); this upregulatory effect was not seen in C5a-untreated and LPS-stimulated macrophages (Fig. 1A). We previously showed that P. gingivalis generates C5a in complement-inactivated serum (3) and have now confirmed the presence of C5a in the supernatants of wild-type P. gingivalis-treated cells (1460 ± 246 pg/ml, n =3); in contrast, C5a in the in the supernatants of KDP128-treated cells was below the assay detection limit (< 39 pg/ml). Therefore, endogenously generated C5a limits P. gingivalis-induced IL-12p70 production, which is thus enhanced in the presence of C5aRA. This notion was substantiated by the finding that KDP128 failed to regulate IL-12p70, unless exogenous C5a was added in the cell cultures (Fig. 1B). Indeed, C5aRA had no effect on KDP128-induced IL-12p70 in the absence of exogenously added C5a (Fig. 1B). Interestingly, in the absence of exogenous treatments with C5a or C5aRA, KDP128 induced significantly higher levels of IL-12p70 than wild-type P. gingivalis (p < 0.05; Fig. 1B). This is attributed to the inability of KDP128 to generate C5a in the culture supernatants that would limit IL-12p70 production. The ability of P. gingivalis to induce IL-12p70 was completely abrogated in TLR2-deficient macrophages, whereas, as expected, LPS-induced IL-12p70 was unaffected (Fig. 1C). Taken together, these data indicate that P. gingivalis activates a C5aR-TLR2 crosstalk which inhibits IL-12p70 production in macrophages.
The C5aR crosstalk pathways with TLR2 or TLR4 for IL-12p70 regulation appear to be similar, since the inhibitory effects of C5a were abrogated by treatment with the MEK1/2-specific inhibitor U0126 but not by the PI3K inhibitor wortmannin (p < 0.01; Fig. 1D). This implicates the MEK-ERK1/2 pathway in C5aR-mediated regulation of both TLR2- and TLR4- induced IL-12p70. On the other hand, the PI3K pathway is minimally involved, if at all (6). In the absence of C5a, however, wortmannin upregulated LPS-induced IL-12p70 (p < 0.01; Fig. 1D), suggesting that under these conditions (lack of C5aR activation) PI3K can inhibit IL-12p70 as previously shown by others (26). The finding that wortmannin failed to regulate P. gingivalis-induced IL-12p70 (Fig. 1D) is likely attributed to the presence of endogenously produced C5a in the culture supernatants. On the other hand, U0126 appeared to upregulate both LPS– and P. gingivalis–induced IL-12p70 but this difference reached statistical significance only for the latter (p < 0.01; Fig. 1D). In summary, C5a-induced inhibition of IL-12p70 by P. gingivalis or LPS is mediated by ERK1/2 but not PI3K signaling, although PI3K can regulate LPS-induced IL-12p70 in the absence of C5aR activation.
The C5aR-dependent inhibition of IL-12p70 in P. gingivalis-stimulated macrophages was selective for this cytokine, since other proinflammatory cytokines (IL-6 and TNF-α) were actually upregulated (p < 0.01; Fig. 1E). In conclusion, P. gingivalis proactively and selectively inhibits IL-12p70 production by activating a C5aR-TLR2 crosstalk without requirement for immunological mechanisms of complement activation.
C5aR signaling in vivo differentially regulates P. gingivalis-induced cytokine responses
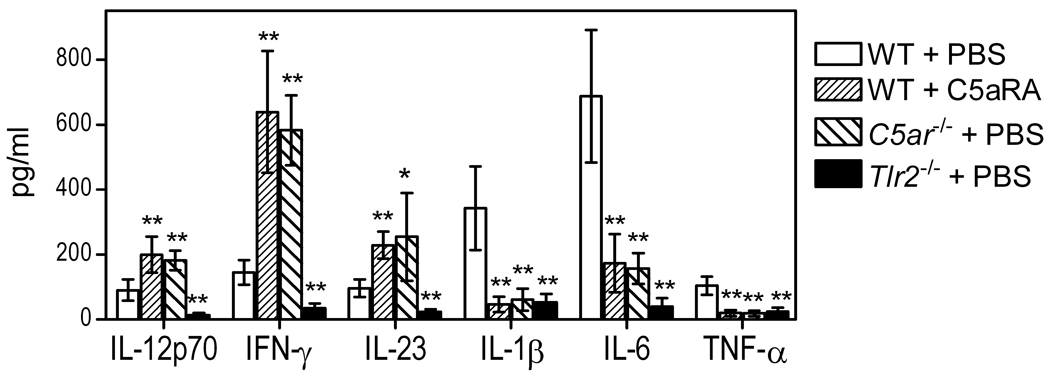
We next investigated the biological significance of the C5aR-mediated inhibition of IL-12p70 production. First, it was essential to determine whether C5aR signaling can regulate P. gingivalis-induced IL-12p70 production also in vivo. For this purpose, wild-type mice were i.p. administered C5aRA followed by i.p. challenge with P. gingivalis. Mice deficient in C5aR or TLR2 were similarly challenged with P. gingivalis, and all mice were sampled 5h postinfection by peritoneal lavage. In addition to IL-12p70, we determined production of IFN-γ (which is positively regulated by IL-12p70 (9)), IL-23 (an IL-12 family cytokine which shares a common IL-12/IL-23p40 subunit with IL-12p70 (27)), as well as proinflammatory cytokines that have been implicated in inflammatory bone resorption in periodontitis (IL-1β, IL-6, and TNF-α) (28). C5aRA-treated wild-type mice and C5aR-deficient mice elicited significantly higher levels of IL-12p70, IFN-γ, and IL-23 compared to PBS-treated wild-type controls (p < 0.01–0.05; Fig. 2). In contrast, the induction of IL-1β, IL-6, and TNF-α production was inhibited by C5aR blockade or C5aR deficiency (p < 0.01; Fig. 2). On the other hand, the induction of all tested cytokines was abrogated in TLR2-deficient mice (p < 0.01; Fig. 2). None of these cytokines was detectable in the peritoneal fluid of mice not challenged with P. gingivalis (not shown). These data show that C5aR signaling in vivo selectively inhibits the ability of P. gingivalis to induce TLR2-dependent IL-12 family cytokines (IL-12p70 and IL-23). The observed downregulation of IFN-γ is most likely secondary to inhibition of IL-12p70 production. On the other hand, maximal induction of IL-1β, IL-6, and TNF-α requires intact signaling by both C5aR and TLR2.
Figure 2. C5aR signaling regulates P. gingivalis-induced and TLR2-dependent cytokine production in vivo.
10–12 week-old wild-type (WT) mice, which were pretreated or not with C5aRA (i.p.; 25 µg/mouse), as well as mice deficient in C5aR (C5ar−/−) or TLR2 (Tlr2−/−), were i.p. infected with P. gingivalis (5×107 CFU). Peritoneal lavage was performed 5h postinfection and the peritoneal fluid was used to measure the levels of the indicated cytokines. Mice not infected with P. gingivalis had undetectable levels of the cytokines investigated. Data are means ± SD (n = 5 mice). *, p < 0.01 and **, p < 0.01 vs. WT+PBS control.
C5aR-mediated inhibition of IL-12p70 promotes P. gingivalis survival in vivo
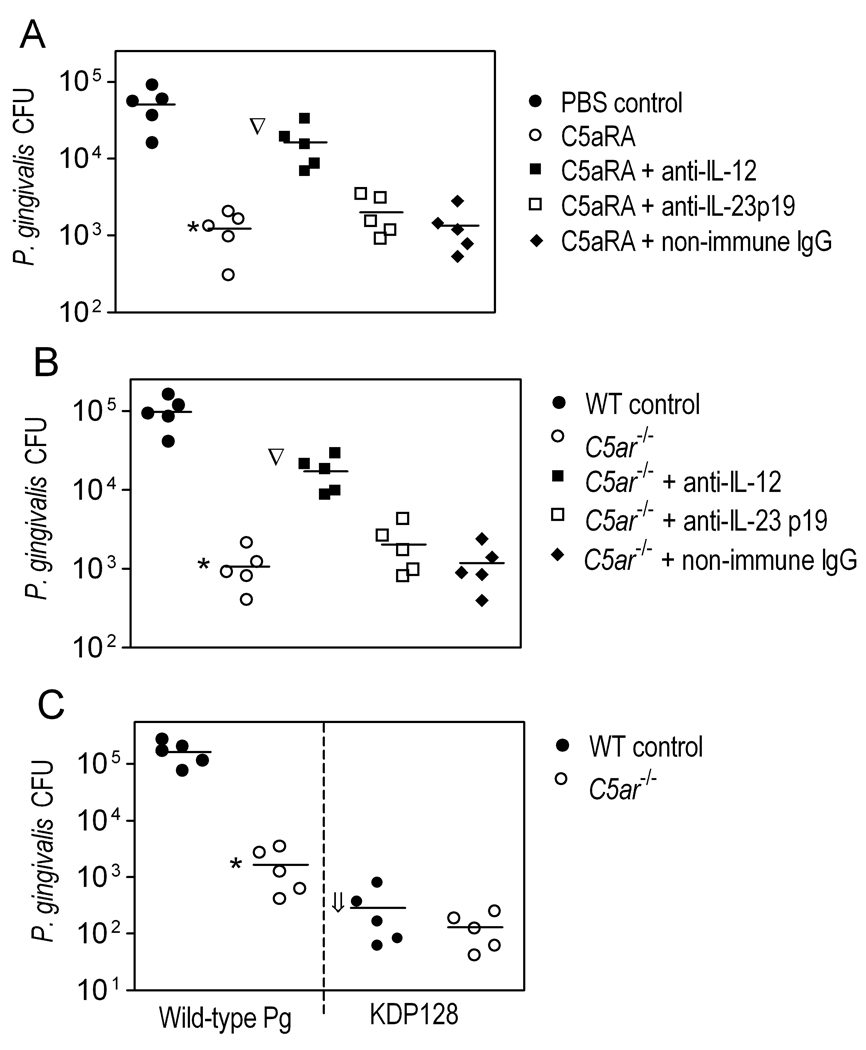
Whether the C5aR-mediated inhibitory effect on IL-12p70 production (Fig. 2) is exploited by P. gingivalis was addressed in subsequent experiments. Wild-type mice were i.p. treated with C5aRA (or PBS control) and infected with P. gingivalis by the same route. The C5aRA-treated mice comprised several groups, including mice given anti-IL-12 IgG, anti-IL-23p19 IgG, or non-immune IgG control. Treatment with anti-IL-23p19 was included because the anti-IL-12 Ab reacts with both IL-12p70 subunits, p35 and p40, the latter of which is shared by the heterodimeric IL-23 (IL-12/IL-23p40 and IL-23p19 (27)). Thus, the experiment was designed in a way that would allow specific implication of IL-12p70 or both IL-12p70 and IL-23 (on none) in P. gingivalis immune clearance. At 24h postinfection, the peritoneal lavage fluid from C5aRA-treated mice contained about 2 log10 units less P. gingivalis CFU compared to mice pretreated with PBS control (p < 0.01; Fig. 3A). However, the enhanced ability of C5aRA-treated mice to clear P. gingivalis was significantly (p < 0.01) counteracted by anti-IL-12 treatment, though not by anti-IL-23p19 or non-immune IgG (Fig. 3A). Viable P. gingivalis CFU counts were not detected in the blood or in homogenates of several organs examined (spleen, kidney, liver, and lungs) from any of the mouse groups. Taken together with the Fig. 2 findings, these data show that C5aR signaling inhibits IL-12p70 production and this inhibitory effect is exploited by P. gingivalis to resist immune clearance. This conclusion was further substantiated by similar findings from a related experiment in which C5aRA-treated mice were replaced by C5aR-deficient mice (Fig. 2B).
Figure 3. Inhibition of C5aR signaling promotes the in vivo clearance of P. gingivalis by augmenting IL-12.
(A) Wild-type (WT) mice were pretreated or not with C5aRA (i.p.; 25 µg/mouse), in the presence or absence of goat polyclonal anti-mouse IL-12 IgG, anti-mouse IL-23p19 IgG, or equal amount of non-immune IgG (i.p.; 0.1 mg/mouse). The mice were then infected i.p. with P. gingivalis (5×107 CFU). (B) Similar experiment in which C5aRA-treated mice were replaced by C5aR-deficient (C5ar−/−) mice. (C) WT and C5ar−/− mice were infected i.p. with wild-type P. gingivalis or the isogenic KDP128 mutant (both at 5×107 CFU). Peritoneal lavage was performed 24h postinfection and the peritoneal fluid was used to determine viable P. gingivalis CFU counts. Data are shown for each individual mouse with horizontal lines indicating mean values. *, p < 0.01 vs. controls. The inverted triangles indicate significant (p < 0.01) reversal of the effects of C5aRA or C5aR deficiency by anti-IL-12. In C, the downward arrow shows significant (p < 0.01) difference between KDP128 and the wild-type organism.
In a side-by-side comparison of the in vivo survival capacities of wild-type P. gingivalis and KDP128, the mutant was recovered at significantly lower levels (>500-fold difference compared to wild-type P. gingivalis) from the peritoneal cavity of wild-type mice (p < 0.01; Fig. 3C). This difference in survival capacity may be related, at least in part, to the inability of KDP128 to generate C5a, as shown in vitro. Even in vivo, where physiological mechanisms (e.g., activation of the complement cascade) could contribute to C5a generation, the peritoneal fluid of KDP128-infected mice contained significantly lower levels of C5a (374 ± 93 pg/ml) than that of wild-type P. gingivalis-infected mice (2174 ± 513 pg/ml) (p < 0.01; n = 5 mice per group); C5a levels at baseline (uninfected mice) were 101 ± 47 pg/ml. Consistent with these considerations, the survival of KDP128 was not significantly affected by C5aR deficiency (Fig. 3C), suggesting that the mutant cannot productively exploit C5aR to promote its survival, as the wild-type organism does. In conclusion, a great part of in vivo generated C5a can be attributed to the enzymatic action of P. gingivalis which thereby can efficiently manipulate IL-12p70 production and promote its survival.
Comparison of C5a and C5adesArg in regulating IL-12p70 and other macrophage activities
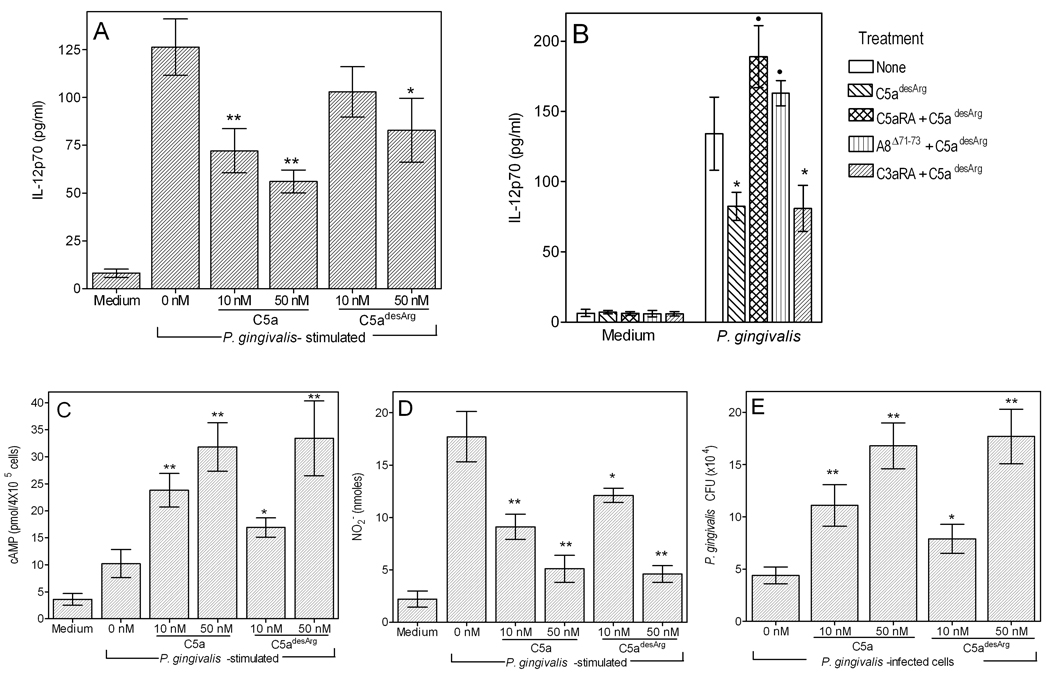
C5a is relatively unstable in biological fluids and is rapidly converted to its desarginated form (C5adesArg). In fact, a large part of in vivo detected C5a (see above) may represent C5adesArg since the capturing antibody used in the sandwich ELISA (BD Pharmingen) recognizes a neoepitope exposed in both C5a or C5adesArg (though not in intact C5). C5adesArg does not have anaphylactic action but retains a number of other biological activities (29–31). We thus investigated whether it shares the capacity of C5a to regulate IL-12p70. We found that C5adesArg can also inhibit P. gingivalis-induced IL-12p70 production, though not as strongly as C5a. Specifically, C5adesArg mediated significant (p < 0.05) inhibition of IL-12p70 at 50 nM but not at 10 nM, at which concentration C5a was already effective (Fig. 4A). However, the increased stability and thus higher prevalence of C5adesArg compared to intact C5a suggests a possible significant role for the desarginated molecule in IL-12p70 regulation.
Figure 4. Comparative modulatory effects of C5a and C5adesArg on IL-12p70 production and antimicrobial activities in P. gingivalis-challenged macrophages.
Groups of mouse peritoneal macrophages were incubated with P. gingivalis (Pg; MOI = 10:1) in the absence or presence of C5a or C5adesArg (at 10 or 50 nM) and assayed for (A) induction of IL-12p70 (after 24h), (C) generation of cAMP (1h), (D) NO2− (24h), and (E) viable counts (CFU) of internalized bacteria (24h). In panel B, the macrophages were pretreated with C5aRA (1 µM), the dual C5aR/C5a-like receptor-2 antagonist A8Δ71–73 (1 µM), or the C3aR antagonist SB290157 (5 µM) to determine the receptor by which C5adesArg (50 nM) inhibits IL-12p70 production. Data are means ± SD (n = 3 sets of macrophages) from one of two independent sets of experiments yielding consistent results. *, p < 0.05 and **, p < 0.01 compared to no C5a or C5adesArg (0 nM). In B, black circles indicate statistically significant (p < 0.01) reversal of the inhibitory effect of C5adesArg. In panels C–E, no significant differences were found between C5a and C5adesArg when tested at 50 nM.
Although C5adesArg binds also to the C5a-like receptor-2 (GPR77) with high affinity (1, 13), its observed modulatory effect on IL-12p70 production was likely mediated via the C5aR (CD88). In this regard, C5aRA by itself caused full reversal of the inhibitory effect of C5adesArg, whereas a dual C5aR/C5a-like receptor-2 antagonist (A8Δ71–73) (13, 32) had a comparable effect (Fig. 4B). In contrast, the C3aR antagonist SB290157 (control) did not influence the ability of C5adesArg to inhibit induction of IL-12p70 by P. gingivalis (Fig. 4B).
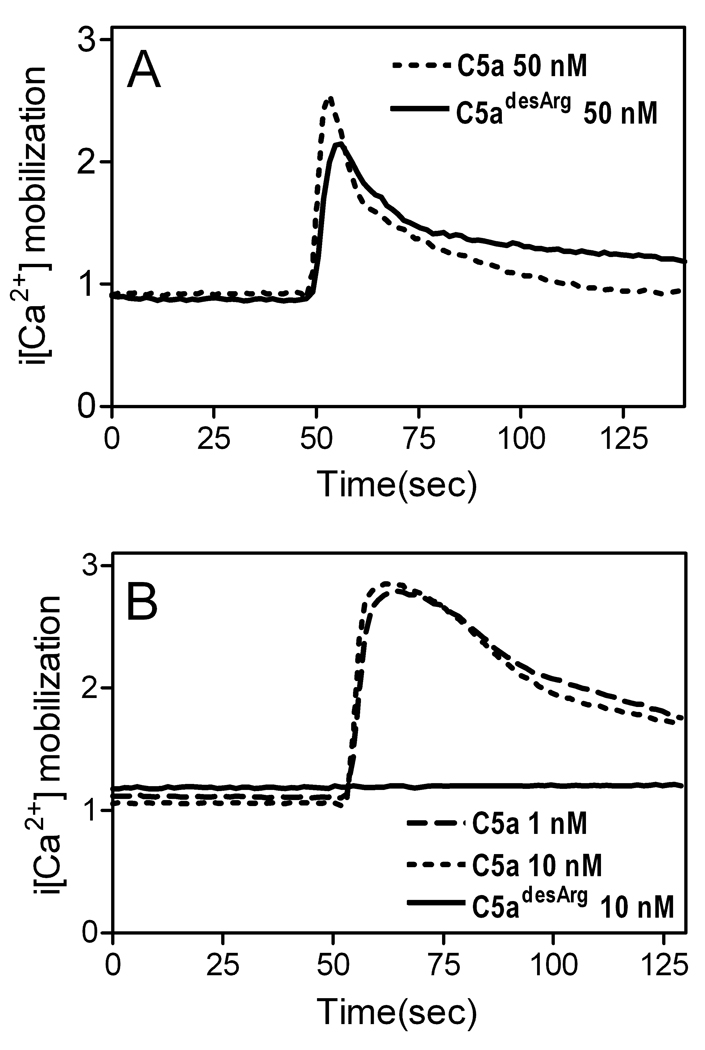
We previously implicated C5a in synergistic interactions with P. gingivalis that elevate cAMP in macrophages, leading to inhibition of nitric oxide production and of intracellular killing (3). We investigated whether these evasion mechanisms can also be activated by C5adesArg. Side-by-side comparison revealed no significant differences between C5a and C5adesArg when tested at 50 nM in elevating cAMP, inhibiting nitric oxide, and promoting its intracellular survival (Fig. 4, C–E). However, when the compounds were tested at 10 nM, C5a exhibited stronger effects than C5adesArg (Fig. 4, C–E). In view of the strict dependence of C5a on intracellular Ca2+ mobilization to synergistically elevate cAMP (3), we hypothesized that C5adesArg could similarly induce intracellular Ca2+ responses. Indeed, at 50 nM, C5a and C5adesArg induced comparable intracellular Ca2+ mobilization in macrophages (Fig. 5A), whereas only C5a was active in that regard in neutrophils (Fig. 5B). Taken together, the data from Figs. 4 and 5 indicate that P. gingivalis can exploit C5a even after its conversion to C5adesArg to undermine macrophage defense functions (induction of IL-12p70, activation of intracellular killing).
Figure 5. Comparison of C5a and C5adesArg in intracellular Ca2+ mobilization.
Mouse peritoneal macrophages (A) or neutrophils (B) were loaded with the ratiometric calcium indicator Indo-1 AM and stimulated with C5a or C5adesArg at the indicated concentrations (lower concentrations were used for neutrophils since they are more sensitive to C5a than macrophages (50)). Ca2+ mobilization was measured in a spectrofluorometer and the traces are representative of three experiments.
C5aR mediates periodontal bone loss
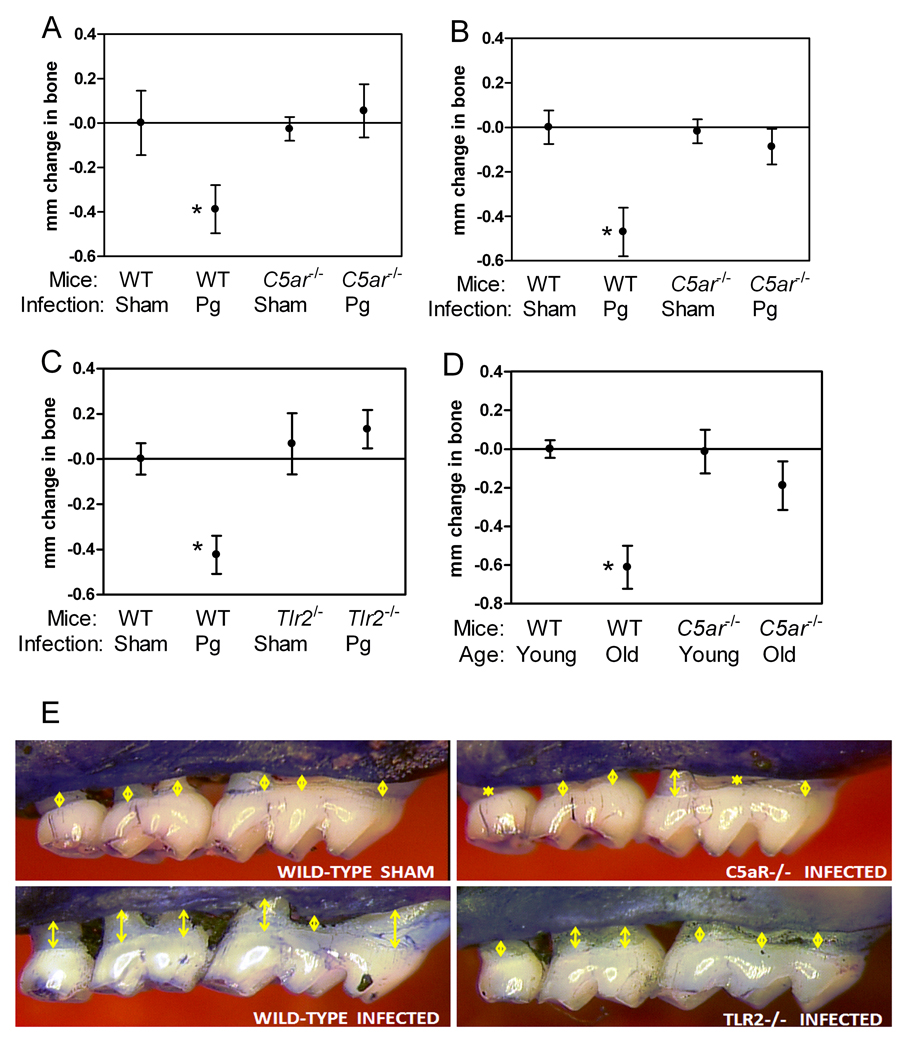
The involvement of C5aR signaling in P. gingivalis immune evasion and in the induction of proinflammatory cytokines (Figs. 1–4), such as IL-1β, IL-6, and TNF-α that mediate periodontal bone resorption (28), suggested that C5aR may play an important role in P. gingivalis-induced periodontitis. Indeed, P. gingivalis failed to induce significant periodontal bone loss in C5aR-deficient BALB/c or C57BL/6 mice, in stark contrast to corresponding wild-type mice which developed significant bone loss relative to sham-infected controls (p < 0.01; Fig. 6 A, B, and E). TLR2 participates in crosstalk interactions with C5aR that a) promote mechanisms of P. gingivalis immune evasion (ref. (3) and this study) and b) induce production of bone-resorptive cytokines (Fig. 2). Sensibly, therefore, TLR2-deficient BALB/c mice were similarly shown to be resistant to P. gingivalis-induced periodontal bone loss (Fig. 6 C and E).
Figure 6. C5aR and TLR2 deficiencies protect against periodontal bone loss.
Mice deficient in C5aR [C5ar−/−] (A, BALB/c; B, C57BL/6) or TLR2 [Tlr2−/−] (C; BALB/c) and appropriate wild-type controls were orally infected or not with P. gingivalis and assessed for induction of periodontal bone loss six weeks later. Mice used in these experiments were 10–12 week-old. (D) Induction of naturally occurring periodontal bone loss in 16-month-old wild-type or C5ar−/− BALB/c mice relative to their young counterparts (≤ 12 weeks of age). (E) Representative images of P. gingivalis-induced bone loss under wild-type or C5aR- or TLR2-deficient conditions: P. gingivalis-infected C5ar−/− or Tlr2−/− mice display considerably smaller CEJ-ABC distances (yellow arrows) compared to infected wild-type mice, but quite comparable to those of sham-infected wild-type mice. Data are means ± SD (n = 5 mice). *, p < 0.01 compared to corresponding sham-infected controls (A and B) or young counterparts (C).
Mice used for P. gingivalis-induced periodontitis studies are usually 8–12 week-old and sham-infected controls do not develop appreciable bone loss (33). However, aging mice, like aging humans, gradually develop naturally-occurring inflammatory periodontal bone loss (due to chronic exposure to indigenous periodontal bacteria), which becomes quite dramatic after 9 months of age (22, 23). To determine the role of C5aR in the age-associated periodontitis model, we raised C5aR-deficient BALB/c mice and wild-type controls until the age of 16 months. We found that old C5aR-deficient mice are significantly protected against age-associated periodontitis relative to similarly aged wild-type controls (p < 0.01; Fig. 6D). Therefore, C5aR is involved in chronic, age-associated periodontal bone loss. Although it is currently uncertain whether C5aR is exploited by mouse periodontal bacteria as shown for P. gingivalis, the ability of C5aR to amplify TLR-induced bone resorptive cytokines (and inflammation in general) render it a major mediator of periodontal bone destruction.
Discussion
Clinical and histological observations implicate complement in periodontal inflammation and pathogenesis, although the precise mechanisms or pathways involved have remained largely undefined (reviewed in ref.(34). However, our findings clearly implicate at least the C5a-C5aR axis in periodontal tissue destruction and in immune evasion of periodontal bacteria.
Our present data suggest that P. gingivalis may exploit C5aR to promote its adaptive fitness in diverse ways. On the one hand, C5aR signaling inhibits TLR2-dependent IL-12p70 induction and interferes with immune clearance of P. gingivalis. On the other hand, the P. gingivalis-instigated C5aR-TLR2 crosstalk leads to upregulation of other proinflammatory cytokines (IL-1β, IL-6, and TNF-α). Therefore, this pathogen does not appear to cause a generalized immunosuppression but, rather, has evolved the ability to selectively target pathways that could result in its elimination. In fact, non-selective immunosuppression would not be advantageous for P. gingivalis; whereas such strategy could certainly protect P. gingivalis against host immunity, at the same time the pathogen would be condemned to starvation. Indeed, P. gingivalis is an asaccharolytic organism with a strict requirement for peptides and hemin, and thus depends on the continuous flow of inflammatory serum exudate (gingival crevicular fluid) to obtain these essential nutrients and survive in its periodontal niche (35). Therefore, the proactive release of C5a by P. gingivalis and the ensuing C5a-induced inflammation (increased vascular permeability and proinflammatory synergy with TLRs) can contribute to nutrient procurement. Moreover, the ability of P. gingivalis to induce C5aR-dependent periodontal bone loss expands the useful space for increased niche for the pathogen.
On the basis of the above discussion, it becomes apparent that P. gingivalis uses a quite antithetical strategy relative to Staphylococcus aureus which promotes its survival by actually blocking C5a binding and C5aR activation, via a secreted protein known as CHIPS (chemotaxis inhibitory protein of S. aureus) (36). This mechanism inhibits C5a-induced inflammation and phagocytic cell chemotaxis and protects S. aureus from neutrophils and macrophages (36). On the other hand, the protozoan parasite Leishmania major also exploits C5aR for evading host immunity, which is restored in C5aR-deficient mice that consequently do not develop necrotizing dermal lesions as wild-type animals do (6). However, unlike P. gingivalis, L. major has to rely on C5a generation by the physiological complement cascade to become able to exploit C5aR.
P. gingivalis-induced inflammation via the C5aR-TLR2 crosstalk may have important implications from a clinical perspective, since it is likely to cause collateral tissue damage (inflammatory periodontal bone destruction). This notion is supported by our findings that mice deficient in C5aR or TLR2 are both resistant to P. gingivalis-induced periodontitis. The fact that induction of bone loss is essentially absent in the absence of either C5aR or TLR2 signaling, argues against the possibility that C5aR and TLR2 contribute to periodontal pathogenesis through independent effector mechanisms. In this regard, both receptors are under P. gingivalis control and are induced to crosstalk, while in physical proximity (3), cooperatively leading to immune evasion and induction of inflammatory/bone-resorptive cytokines.
Both the C5a and C3a anaphylatoxins are readily metabolized in serum and lose their C-terminal Arg due to carboxypeptidase activity (29). The resulting C3a fragment (C3adesArg) is biologically inert in terms of C3a receptor-dependent functions, but retains antimicrobial activity which is exerted independently of the receptor (37). On the other hand, C5adesArg can still bind C5aR, albeit with a lower affinity and a different mode of interaction relative to intact C5a (29, 38). Although C5adesArg is devoid of C5a spasmogenic (anaphylactic) activity, it retains other C5a activities to varying degrees depending on function and cell type involved. For example, monocytes/macrophages, but not neutrophils, do not appear to distinguish between C5a and C5adesArg in terms of induction of chemotaxis or lysosomal enzyme release (30, 31, 39). Our findings that C5adesArg retains the ability to inhibit P. gingivalis-induced IL-12p70 and nitric oxide production has important implications: Being considerably more stable than C5a (29), C5adesArg may provide a persisting stimulus for sustained manipulation of the antimicrobial response and destructive inflammation, properties that characterize chronic conditions like periodontitis. Intriguingly, whereas P. gingivalis attacks C5 and generates biologically active C5a/C5adesArg, it extensively degrades C3 and C3a which thus do not retain biological activity (40). Since C3a (but not C5a) exerts direct antimicrobial effects and readily kills both gram-negative and gram-positive bacteria (37), it is possible that degradation and inactivation of C3a by P. gingivalis may serve to protect this pathogen.
The data from this study collectively suggest that P. gingivalis has evolved to not only endure the host response (by selectively suppressing critical ‘killing’ pathways, such as IL-12–dependent clearance), but also to benefit from the inflammatory response, while at the same time contributing to periodontal pathogenesis. The ability of P. gingivalis to inhibit innate immune functions via C5aR exploitation may also allow bystander bacteria, i.e., co-habiting the same niche, to evade immune control. In this context, P. gingivalis is thought of as a keystone periodontal species that could promote the survival and virulence of the entire microbial community (35, 41–43).
In addition to being a prevalent and costly chronic condition that destroys tooth-supporting tissues, severe periodontitis exerts a systemic impact on health and the patients run increased risk for diseases such as atherosclerosis, diabetes, and perhaps rheumatoid arthritis (44–47). Therefore, it becomes important to identify promising therapeutics for the treatment of this oral disease. Since C5aR- or TLR2-deficient mice are both resistant to periodontal bone loss, at least in principle, pharmacological blockade of either C5aR or TLR2 could inhibit periodontitis. However, the availability of highly effective and safe C5aR antagonists, some of which have completed phase II trials (for rheumatoid arthritis and psoriasis) (48, 49), and the relative paucity of effective TLR2 antagonists, suggest that C5aR is a preferential and promising target of local therapeutic intervention to treat human periodontitis. From a mechanistic viewpoint, C5aR blockade may counteract the ability of periodontal bacteria to evade critical antimicrobial responses or to stimulate non-resolving/destructive inflammation, and thus should be capable of both controlling the infection and inhibiting periodontal bone loss.
Footnotes
This work was supported by U.S. Public Health Service R01 Grants GM-62134 and AI-068730 (to J.D.L.), and DE015254, DE021580, DE017138, and DE018292 (to G.H.).
Abbreviations used in this paper: C5aR, C5a receptor; C5aRA, C5aR antagonist; C5adesArg desarginated C5a.
References
- 1.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. Microbial hijacking of complement-Toll-like receptor crosstalk. Sci. Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajishengallis G, Tapping RI, Harokopakis E, Nishiyama S-I, Ratti P, Schifferle RE, Lyle EA, Triantafilou M, Triantafilou K, Yoshimura F. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell. Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 5.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J. Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 6.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 7.la Sala A, Gadina M, Kelsall BL. G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and JNK. J Immunol. 2005;175:2994–2999. doi: 10.4049/jimmunol.175.5.2994. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 10.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 11.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, Settmacher B, Klos A, Erhard KF, Cousins RD, Sulpizio AC, Hieble JP, McCafferty G, Ward KW, Adams JL, Bondinell WE, Underwood DC, Osborn RR, Badger AM, Sarau HM. Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol. 2001;166:6341–6348. doi: 10.4049/jimmunol.166.10.6341. [DOI] [PubMed] [Google Scholar]
- 13.Otto M, Hawlisch H, Monk PN, Muller M, Klos A, Karp CL, Kohl J. C5a mutants are potent antagonists of the C5a receptor (CD88) and of C5L2: position 69 is the locus that determines agonism or antagonism. J Biol Chem. 2004;279:142–151. doi: 10.1074/jbc.M310078200. [DOI] [PubMed] [Google Scholar]
- 14.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 15.Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc. Natl. Acad. Sci. U S A. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajishengallis G, Shakhatreh M-AK, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J. Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- 17.Grenier D, Roy S, Chandad F, Plamondon P, Yoshioka M, Nakayama K, Mayrand D. Effect of inactivation of the Arg- and/or Lys-gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis. Infect Immun. 2003;71:4742–4748. doi: 10.1128/IAI.71.8.4742-4748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang S, Wang M, Triantafilou K, Triantafilou M, Nawar HF, Russell MW, Connell TD, Hajishengallis G. The A subunit of Type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J. Immunol. 2007;178:4811–4819. doi: 10.4049/jimmunol.178.8.4811. [DOI] [PubMed] [Google Scholar]
- 19.Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–24254. [PubMed] [Google Scholar]
- 20.Wang M, Shakhatreh M-AK, James D, Liang S, Nishiyama S-i, Yoshimura F, Demuth DR, Hajishengallis G. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 21.Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect. Immun. 2000;68:5864–5868. doi: 10.1128/iai.68.10.5864-5868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang S, Hosur KB, Domon H, Hajishengallis G. Periodontal inflammation and bone loss in aged mice. J. Periodont. Res. 2010;45:574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol. Oral Microbiol. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 25.Hajishengallis G, Wang M, Bagby GJ, Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J. Immunol. 2008;181:4141–4149. doi: 10.4049/jimmunol.181.6.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 27.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 28.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 29.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marder SR, Chenoweth DE, Goldstein IM, Perez HD. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985;134:3325–3331. [PubMed] [Google Scholar]
- 31.McCarthy K, Henson PM. Induction of lysosomal enzyme secretion by alveolar macrophages in response to the purified complement fragments C5a and C5a des-arg. J Immunol. 1979;123:2511–2517. [PubMed] [Google Scholar]
- 32.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graves DT, Fine D, Teng Y-TA, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajishengallis G. Complement and periodontitis. iochem Pharmacol. 2010;80:1992–2001. doi: 10.1016/j.bcp.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krauss JL, Potempa J, Lambris JD, Hajishengallis G. Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol 2000. 2010;52:141–162. doi: 10.1111/j.1600-0757.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Haas CJC, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJB, Heezius ECJM, Poppelier MJJG, Van Kessel KPM, van Strijp JAG. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 2004;199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nordahl EA, Rydengard V, Nyberg P, Nitsche DP, Morgelin M, Malmsten M, Bjorck L, Schmidtchen A. Activation of the complement system generates antibacterial peptides. Proc Natl Acad Sci U S A. 2004;101:16879–16884. doi: 10.1073/pnas.0406678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crass T, Bautsch W, Cain SA, Pease JE, Monk PN. Receptor activation by human C5a des Arg74 but not intact C5a is dependent on an interaction between Glu199 of the receptor and Lys68 of the ligand. Biochemistry. 1999;38:9712–9717. doi: 10.1021/bi990139q. [DOI] [PubMed] [Google Scholar]
- 39.Chenoweth DE, Goodman MG, Weigle WO. Demonstration of a specific receptor for human C5a anaphylatoxin on murine macrophages. J Exp Med. 1982;156:68–78. doi: 10.1084/jem.156.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J. Biol. Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- 41.Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009;11:637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darveau RP. The oral microbial consortium's interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–395. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 44.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 45.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Sacco RL, Papapanou PN. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Circulation. 2005;111:576–582. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 47.de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 48.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov. 2010;9:43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- 50.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]