Abstract
Systemic immunological tolerance to Ag encountered in the eye restricts the formation of potentially damaging immune responses that would otherwise be initiated at other anatomical locations. We previously demonstrated that tolerance to Ag administered via the anterior chamber (AC) of the eye required FasL-mediated apoptotic death of inflammatory cells that enter the eye in response to the antigenic challenge. Moreover, the systemic tolerance induced after AC injection of Ag was mediated by CD8+ regulatory T cells. The present study examined the mechanism by which these CD8+ regulatory T cells mediate tolerance after AC injection of Ag. AC injection of Ag did not prime CD4+ T cells, and led to increased TRAIL expression by splenic CD8+ T cells. Unlike wildtype mice, Trail−/− or Dr5−/− mice did not develop tolerance to Ag injected into the eye, even though responding lymphocytes underwent apoptosis in the AC of the eyes of these mice. CD8+ T cells from Trail−/− mice that were first injected AC with Ag were unable to transfer tolerance to naïve recipient wildtype mice, but CD8+ T cells from AC-injected wildtype or Dr5−/− mice could transfer tolerance. Importantly, the transferred wildtype (Trail+/+) CD8+ T cells were also able to decrease the number of infiltrating inflammatory cells into the eye; however, Trail−/− CD8+ T cells were unable to limit the inflammatory cell ingress. Together, our data suggest that “helpless” CD8+ regulatory T cells generated after AC injection of Ag enforce systemic tolerance in a TRAIL-dependent manner to inhibit inflammation in the eye.
INTRODUCTION
For well over 100 years, scientists interested in transplantation biology characterized immune privileged sites as areas of the body that were highly receptive of allografts. Van Dooremaal first described the concept of immune privilege (1), but it was Medawar who classically defined immune privilege in 1948 (2). A number of immune privileged sites have been identified over the years, including the hamster cheek pouch, brain, ovary, testis, pregnant uterus, placenta, and the prototypical immune privileged site – the eye. In the eye, misdirected or uncontrolled immunity, which would easily be tolerated in the skin or another large organ, can be deleterious to the ocular structures essential for vision. Thus, the eye actively pursues immune regulation to protect its visual integrity, though often at the expense of other immune effector mechanisms.
Studies examining the molecular basis of ocular immune privilege have identified multiple factors that work together to protect this vital organ from rampant inflammatory processes – including the production of immunosuppressive cytokines (3–6), localization of neuropeptides in ocular neurons (7, 8), strategic placement of specialized APC (4, 6), very low (or absent) expression of MHC I and II (4), presence of a molecule that inhibits NK function (9), and induction of systemic immune deviation (6, 10–16). Central to the principle of ocular immune privilege is the induction of apoptosis in leukocytes that enter the eye in response to Ag by Fas ligand (FasL)-expressing ocular structures (17), and our first realization that apoptosis can actively regulate immunity came from studies on the eye (18). The importance of FasL in maintaining ocular immune privilege, and its role in the induction of peripheral tolerance and survival of corneal allografts (19), has consequently become an important concept in immunology (20).
In addition to the events that help dampen immune responses within the ocular microenvironment, systemic immune responses are also affected by Ag presentation via the anterior chamber (AC) of the eye. The inability to mount systemic cell-mediated immunity (but maintain humoral immunity) after Ag presentation via the AC of the eye was first described by Kaplan and Streilein (10). Subsequent studies demonstrated a number of Ag, including haptenated spleen cells (13, 21), viruses (22), corneal allografts (15), and tumor cells (23), could induce ocular immune deviation when placed within the AC. Thus, it has been generally concluded that Ag injection into the AC induces a systemic immune deviation hallmarked by the induction of Ag-specific Ab and suppression of cell-mediated immunity, typically identified by the inhibition of delayed-type hypersensitivity (DTH) or increase in allograft survival. Interestingly, the lack of DTH results from the generation of active regulatory T cell function (6, 11–14, 16), but little is known about the molecular mechanism used by these regulatory T cells to enforce tolerance. Data presented herein suggest the essential role played by TNF-related apoptosis-inducing ligand (TRAIL)-expressing CD8+ regulatory T cells in the peripherial suppression of cell-mediated immunity to Ag injected into the AC of the eye.
Materials and Methods
Animals and reagents
Wildtype C57BL/6 (B6) mice were purchased from The National Cancer Institute. Trail−/− (24) and Dr5−/− (25) B6 mice were obtained from Amgen (Seattle, WA) and Dr. Wafik El-Deiry (University of Pennsylvania, Philadelphia, PA), respectively. Kb−/− act-mOVA mice (26) and OT-II mice were obtained from Drs. John Harty and Yi Luo, respectively (University of Iowa). All knock-out and transgenic mice were backcrossed >10 generations to the C57BL/6 background. All animal procedures were performed in accordance to National Institutes of Health guidelines, and were approved by the University of Iowa IACUC. In all in vivo experiments, groups consisted of 4 or more animals, and experiments were repeated at least 2 times with similar results before reporting. Anti-CD8 (clone 2.43) mAb for in vivo depletion was purified from hybridoma supernatants. Whole ovalbumin (OVA) and Complete Freund’s Adjuvant (CFA) were purchased from Sigma (St. Louis, MO).
HSV-1
The KOS strain of HSV-1 was propagated on Vero cells grown in RPMI-1640 supplemented with 10% FCS, antibiotic/antimycotic, glutamine, and 5 × 10−5 M β-mercaptoethanol. Virus was titrated using a standard plaque-forming assay on Vero cells and expressed as PFU/ml. UV-inactivated virus (as detected in the viral plaque assay) was prepared by exposing virus stocks to a UV lamp for 15 min at a distance of 20 cm.
Trinitrophenyl (TNP) coupling of splenocytes
Single cell suspensions of freshly isolated naïve splenocytes were coupled with TNP as previously described (27). Briefly, 108 cells were incubated in 0.5 ml PBS and 0.5 ml 10 mM 2,4,6 trinitrobenzene sulfonic acid (TNBS; Sigma, St. Louis, MO) for 5 min at room temperature. After incubation, cells were washed thrice with PBS before use. In some cases, TNP-coupled splenocytes (TNP-spl) were γ-irradiated (3000 R) to induce apoptosis.
Induction of ocular immune deviation
Ocular immune deviation was induced as previously described (13, 14, 18). Briefly, HSV-1 (2.5 × 104 pfu), act-mOVA splenocytes (5 × 105), or TNP-spl (5 × 105) were injected AC in 5 µl using a 0.25 ml Hamilton Microliter syringe (Hamilton Co., Reno, NV) fitted with a 33 ga needle. Mice were anesthetized, and injections were done under a dissecting microscope. Some mice were depleted of CD8+ cells by three daily 100 µg doses of anti-CD8 mAb (2.43) prior to AC injection. For HSV-1-induced immune deviation, mice were challenged 7 d later with 106 UV-inactivated HSV-1 in 33 µl PBS in the right and 33 µl PBS in the left footpad. For act-mOVA- or TNP-spl-induced immune deviation, mice were immunized 48 h after AC injection with 0.2 ml of 100 µg of OVA emulsified 1:1 in CFA or 0.1 ml of 10 mM TNBS s.c., respectively. The immune response to OVA or TNBS was examined after 7 d or 4 d, respectively, by challenging with 33 µl of 100 µg of heat-aggregated OVA in PBS or 10 mM TNBS in the right and 33 µl PBS in the left footpad. For all three Ag systems, the DTH response was measured 24 h later by a masked observer. Values were expressed in µm (± S.E.) and represent the difference between the right (Ag challenge) and left footpad (PBS challenge). Background values represent the difference between the challenged and unchallenged feet in naïve mice.
In vivo T cell proliferation
To measure the ability of Ag derived from AC-injected apoptotic cells to stimulate CD4+ T cell proliferation in vivo, wildtype B6 mice were seeded with 106 CFSE-labeled OT-II T cells i.v. 24 h before AC injection of 5 × 105 apoptotic Kb−/− act-mOVA splenocytes. Mice injected s.c. with CFA/OVA (0.2 ml of 100 µg of OVA emulsified 1:1 in CFA) were used as a positive control. After 5 d, splenocytes from the recipient mice were isolated, and OT-II T cell proliferation, as measured by CFSE dilution, was determined by flow cytometry.
Quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA), and 2 µg was reverse-transcribed using Superscript II. The cDNA (250 ng) was used as a template for the TaqMan assay using real-time quantitative RT-PCR primer/probe sets for mouse TRAIL and 18s rRNA (PE Applied Biosystems; Foster City, CA). The TaqMan PCR reaction was carried out as described previously (28).
Intraocular inflammatory cell apoptosis – TUNEL staining
Mice were injected with HSV-1 (2.5 × 104 PFU in 5 µl) AC. After 24 or 48 h, the eyes were removed, fixed in formalin, and processed for paraffin sectioning. Ten micron (10 µm) sections were mounted onto microscope slides and incubated overnight at 55°C. Sections were then deparaffinized in xylene (2 × 5 min), 100% EtOH (2 × 5 min), 95% EtOH (3 min), 70% EtOH (3 min), and PBS (5 min). Protein present in the sections was digested with 20 mg/ml Proteinase K for 15 min at room temperature. Following 4 washes in distilled H20, endogenous peroxidase was quenched with 2.0% H2O2 for 5 min at room temperature and sections were washed twice in PBS. Labeling of 3’-OH fragmented DNA ends was performed with an in situ apoptosis detection kit (TACS 2 TdT-DAB in situ apoptosis detection kit; R&D Systems, Minneapolis, MN) following manufacturer’s instructions.
Measurement of IFN-γ production
Wildtype and Trail−/− B6 mice were immunized AC or IP with 2.5 × 104 pfu HSV-1. After 7 d, spleens were removed and single cell suspensions (106 cells/1 ml/well) were cultured in a 24-well plate with 106 UV inactivated HSV-1 in RPMI-1640 plus 10% FCS. Samples of the culture supernatant were removed at 24 and 48 h, and tested for the presence of IFN–γ by ELISA (eBioscience, San Diego, CA).
Generation of the blood-borne TCR-related tolerogenic proteins
The basic procedure for examining the blood for TCR-related tolerogenic proteins used the following procedure. Briefly, mice were splenectomized 14 d prior to AC injection. They were then AC injected, exsanguinated 48 h later, and serum prepared from whole blood. Normal recipient mice then received 0.2 ml serum and were immunized 2–3 d later with virus to assess DTH. Serum did not contain detectable viral particles as measured by our standard plaque assay (data not shown).
CD8+ and CD4+ T cell isolation
CD8+ T cells or CD4+ T cells were purified from splenocyte single cell suspensions by negative selection using a CD8+ or CD4+ T cell isolation kit, respectively, per manufacturer’s (Miltenyi Biotec, Auburn, CA) instruction. Enriched CD8+ and CD4+ T cells were adoptively transferred i.v. via the retro-orbital plexus. Purity of the isolated cells was determined by flow cytometry using anti-CD4 or -CD8 mAb, and was >90% (data not shown).
Quantitation of intraocular leukocytes
Wildtype B6 mice were injected AC with HSV-1 as described above and removed 48 h later. Cells were dispersed by pressing the eye between the frosted ends of two glass slides. Large pieces were removed using cell strainers and the single cell suspension was washed with PBS. The cells were counted, and then stained with a PE-conjugated anti-CD45 mAb (eBioscience). The frequency of CD45+ cells within the single cell suspension was determined by flow cytometry, and the number of CD45+ cells was then calculated.
Statistical analysis
Significant differences between groups were evaluated using a two-tailed Student’s t test.
RESULTS
AC administration of Ag does not prime CD4+ T cells in wildtype B6 mice
Previous studies from our group found that apoptotic cells injected i.v. do not prime CD4+ T cell-mediated immunity (28). Due to the similarities in the lack of a cell-mediated immune response to Ag encountered via the AC of the eye to those directly injected i.v. (18, 29, 30), we initially examined CD4+ T cell priming in intact or CD8-depleted wildtype B6 mice injected with HSV-1 s.c. or AC. After 7d, splenic CD4+ T cells were isolated, and transferred to naïve B6 mice that were immediately challenged with UV-inactivated HSV-1 in the footpad. Immunity was present only in the mice that received CD4+ T cells from s.c. immunized mice (Figure 1A), and the depletion of CD8+ cells in the donor mice prior to immunization did not affect these results. These results were supported by data that directly examined CD4+ T cell priming in vivo. Specifically, wildtype B6 mice were seeded with CFSE-labeled OT-II T cells 24 h before AC injection of apoptotic Kb−/− act-mOVA splenocytes (26). After 5 d, OT-II proliferation was assessed by measuring CFSE dilution. Compared to the positive control (CFA/OVA immunization s.c.), there was minimal OT-II proliferation after AC injection of apoptotic act-mOVA cells (Figure 1B). Together, these data suggest that CD4+ T cell priming does not occur after AC injection of HSV-1.
Figure 1. CD4+ T cells are not primed for immunity in mice injected AC.
A. Wildtype B6 mice were injected s.c. or AC with 2.5 × 104 pfu HSV-1. Some groups of mice were depleted of CD8+ cells by 3 daily 100 µg doses of using anti-CD8 (mAb 2.43) prior to AC injection. After 7 d, splenic CD4+ T cells were purified and transferred to naïve B6 recipient mice that were immediately challenged with 106 pfu UV-inactivated HSV-1 in 33 µl PBS in the right and 33 µl PBS in the left footpad. Measurements (µm ± S.E.) were taken 24 h later, and represent the difference between the right (Ag challenge) and left footpad (PBS challenge). The background value represents the difference between challenged and unchallenged sites in mice that received CD4+ T cells from naïve mice. * p < 0.05 vs. s.c. B. Wildtype B6 mice received 106 CFSE-labeled OT-II T cells 24 h before AC injection of 2.5 × 104 pfu HSV-1. As a positive control, some mice were injected with CFA/OVA s.c. After 5 d, splenocytes from the recipient mice were isolated, and cell proliferation, as measured by CFSE dilution, was determined by flow cytometry. The percentage of undivided cells is indicated for each group. C. Quantitative RT-PCR analysis shows increased TRAIL mRNA expression in CD8+ T cells from AC-injected mice. Groups of wildtype B6 mice (4/group) were immunized with 0.1 ml of 10 mM TNBS s.c., but one group received 5 × 105 TNP-coupled splenocytes AC 48 h before s.c. immunization. Spenic CD8+ T cells were MACS-isolated 24 h after s.c. immunization, and TRAIL mRNA expression was measured by qRT-PCR. The relative increase was determined by comparing RNA levels to splenic CD8+ T cells obtained from naïve mice.
The pivotal role that CD4+ T cells play in the induction of CD8+ T cell responses has been highlighted in recent years (31–34), as most CD8+ T cell-mediated responses depend on concomitant CD4+ T cell priming. In contrast, CD8+ T cells activated without CD4+ T cell help express TRAIL and can undergo activation-induced cell death (AICD) after secondary Ag stimulation (34). Since the importance of TRAIL in areas outside of tumor immunology (including immune tolerance) is becoming more appreciated, we examined TRAIL expression in splenic CD8+ T cells after Ag injection in the AC. Splenic CD8+ T cells from wildtype B6 mice injected AC with haptenated splenocytes prior to s.c. immunization expressed significantly more TRAIL mRNA compared to splenic CD8+ T cells from mice only immunized s.c. (Figure 1C). These results suggest that the altered priming that occurs after AC injection of Ag results in the increased production of TRAIL by splenic CD8+ T cells.
Trail−/− mice are not tolerized to Ag injected AC
The presence of apoptotic cells favors tolerance, and previous studies from our laboratory demonstrated the importance of intraocular FasL-mediated apoptosis of infiltrating lymphocytes for the induction of ocular immune deviation (18). More recently, we described the importance of TRAIL-expressing CD8+ Treg in the induction of tolerance to Ag (specifically, haptenated apoptotic splenocytes) injected i.v. (28). As Ag injected AC directly enters the bloodstream (35), we hypothesized that the suppression of cellular immunity (by measuring DTH) during ocular immune deviation would not occur in mice with a disrupted TRAIL/DR5 pathway. Wildtype and Trail−/− B6 mice were immunized with HSV-1 via the AC or s.c. (immune control), and consistent with previous reports, AC-immunized wildtype mice failed to mount a DTH response (Figure 2A). In contrast, AC-injected Trail−/− mice displayed robust immunity – much like mice immunized with HSV-1 s.c. Similar results were obtained when a different Ag (act-mOVA splenocytes) was injected AC into mice with an intact or disrupted TRAIL/DR5 pathway (Figure 2B). Even the direct injection of apoptotic (via γ-irradiation) splenocytes into the AC of Trail−/− mice did not lead to suppressed immunity (Figure 2C), suggesting that the defect in the induction of immune deviation in Trail−/− mice was not related to the inability to induce lymphocyte apoptosis in the eye (such as seen with lpr or gld mice (18)). In support of this conclusion, there was evidence of apoptotic death of infiltrating cells in both wildtype and Trail−/− B6 mice after AC injection of HSV-1 (Figure 3). We further examined the immune response in wildtype and Trail−/− B6 mice by isolating splenocytes 7 d after AC or s.c. immunization with HSV-1 and restimulating the cells in vitro with UV-inactivated HSV-1. Analysis of IFN-γ production after 24 and 48 h found the restimulated splenocytes from AC-injected wildtype mice produced significantly less IFN-γ than the restimulated splenocytes from AC-injected Trail−/− mice (Figure 4). Together, these data suggest that the intraocular mechanisms protecting the eye after Ag challenge via the AC are intact in Trail−/− mice (i.e., induction of apoptotic death of infiltrating leukocytes), but there is an extraocular component that confers tolerance in a TRAIL-dependent manner.
Figure 2. Mice defective in the TRAIL/DR5 pathway do not develop DTH tolerance to Ag delivered through the eye.
A. Wildtype or Trail−/− B6 mice were injected s.c. or AC with 2.5 × 104 pfu HSV-1. After 7 d, the mice were challenged with 106 pfu UV-inactivated HSV-1 in 33 µl PBS in the right and 33 µl PBS in the left footpad. B. Wildtype, Trail−/−, or Dr5−/− B6 mice were injected AC with 5 × 105 Kb−/− act-mOVA splenocytes in 5 µl. The mice were then immunized with CFA/OVA (0.2 ml of 100 µg of OVA emulsified 1:1 in CFA) 48 h later. After 7 d, mice were challenged with 100 µg of heat-aggregated OVA in 33 µl of PBS in the right and 33 µl of PBS in the left footpad. C. Wildtype or Trail−/− B6 mice were injected AC with 5 × 105 TNP-coupled spleen cells (live or γ-irradiated) 48 h before immunization with 0.1 ml of 10 mM TNBS s.c. After 4 d, mice were challenged with 33 µl 10 mM TNBS in the right and 33 µl PBS in the left footpad. For A–C, measurements (µm ± S.E.) were taken 24 h after challenge, and represent the difference between the right (Ag challenge) and left footpad (PBS challenge). Immune control groups in B and C were only injected with CFA/OVA or TNBS, respectively, s.c. before footpad challenge, and background (BKG) groups were only footpad challenged. * p < 0.05 vs. s.c. group.
Figure 3. Intraocular inflammatory cell apoptosis occurs in wildtype and Trail−/− B6 mice injected with HSV-1 AC.
HSV-1 was injected into the AC (2.5 × 104 PFU in 5 µl) of wildtype or Trail−/− B6 mice. After 24 or 48 h, the eyes were removed, eyes were removed, fixed in formalin, and processed for paraffin sectioning and TUNEL staining. A) uninjected eye; B) positive control (DNase-treated section); C) wildtype 24 h; D) wildtype 48 h; E) Trail−/− 24 h; F) Trail−/− 48 h.
Figure 4. IFN-γ production in spleens of wildtype and Trail−/− mice following injection with HSV-1.
Wildtype and Trail−/− B6 mice (3/group) were injected s.c. or AC with 2.5 × 104 pfu HSV-1. After 7 d, spleens were removed and single cell suspensions were restimulated in vitro with UV-inactivated HSV-1. The amount of IFN–γ present in the culture supernatants was measured after 24 and 48 h by ELISA. * p < 0.05 vs. wildtype B6.
Suppression of DTH responses after AC-injection of Ag depends on the presence of TRAIL-expressing CD8+ T cells
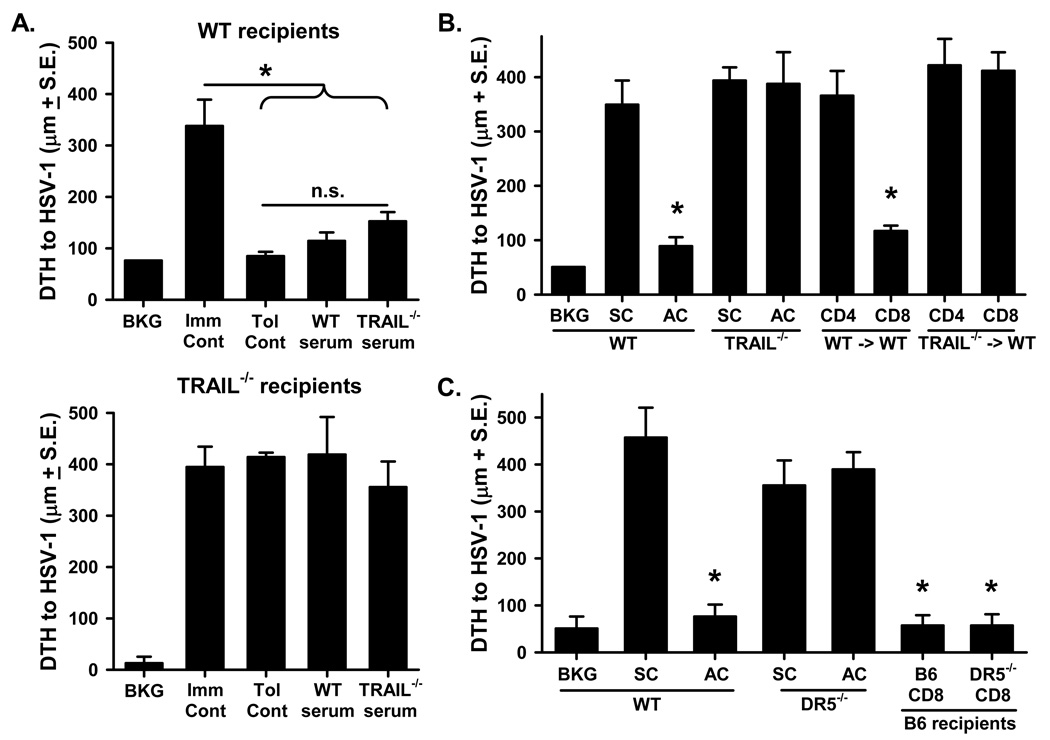
One hallmark of ocular immune deviation is the ability to transfer tolerance from AC-injected “tolerant” mice to naïve recipients using either a soluble TCR-related protein that is released directly into the blood shortly after encounter with foreign Ag in the eye or CD8+ T cells typically obtained from the spleen of AC-injected mice (13, 14, 16, 36). To investigate the potential role of TRAIL in these two aspects of ocular immune deviation, we initially tested the extent to which the failure to induce tolerance in Trail−/− mice resulted from a lack of the tolerogenic soluble TCR-related protein in the blood. In this analysis, serum from splenectomized wildtype or Trail−/− B6 mice was collected 48 h after AC injection of HSV-1, and then transferred i.v. into naïve wildtype or Trail−/− B6 recipients. After 48 h, the mice were immunized with HSV-1 s.c., and challenged 7 d later. Interestingly, the mice that received serum from wildtype or Trail−/− AC-injected mice were rendered tolerant to HSV-1, but neither serum could tolerize Trail−/− mice (Figure 5A). These data imply that the intraocular events needed to generate the “tolerance-inducing” TCR-related protein signal similarly occur in both B6 and Trail−/− mice [as suggested by the similar induction of infiltrating lymphocyte apoptosis (see Figure 3)], but the defect that prevents tolerance induction in AC-injected Trail−/− mice is subsequent to the signal entering the blood and being processed in the spleen.
Figure 5. CD8+ T cells must be able to make TRAIL to transfer tolerance after AC injection of Ag.
A. Serum from wildtype or Trail−/− mice immunized with HSV-1 AC can transfer tolerance to wildtype recipients. Wildtype or Trail−/− B6 mice were splenectomized and rested for 14 d. The mice were then injected AC with 2.5 × 104 pfu HSV-1. Blood was collected 48 h later, and serum prepared. Naïve recipient wildtype or Trail−/− B6 mice each received 0.2 ml serum i.v., followed 48 h later by s.c. immunization with 106 pfu HSV-1. After 7 d, the mice were challenged with 106 UV-inactivated HSV-1 in the right and PBS in the left footpad. B and C. CD8+ T cells from wildtype or Dr5−/− mice immunized with HSV-1 AC can transfer tolerance to wildtype recipients. Wildtype, Trail−/−, or Dr5−/− B6 mice were injected AC with 2.5 × 104 pfu HSV-1. After 7 d, spleens were removed, CD4+ and CD8+ T cells were purified, and separately transferred to naïve B6 recipient mice that were immediately immunized with 106 pfu HSV-1 s.c. After another 7 d, the mice were challenged with 106 UV-inactivated HSV-1 in the right and PBS in the left footpad. As reference, DTH responses were measured in intact wildtype, Trail−/−, or Dr5−/− B6 mice after s.c. or AC injection of HSV-1. In A–C., values are expressed in µm (± S.E.) and represent the difference between the right (Ag challenge) and left footpad (PBS challenge). The background value represents the difference between challenged and unchallenged sites in naive mice. * p < 0.05 vs. s.c. group; n.s. = not significant.
We next tested the extent to which TRAIL-sufficient or –deficient cells from AC-injected mice transferred tolerance to naïve recipient mice. Specifically, wildtype or Trail−/− B6 mice were injected with HSV-1 AC, and then splenic CD4+ and CD8+ T cells were isolated 7 d later. The cells were transferred to naïve wildtype B6 mice that were immediately immunized s.c. with HSV-1. After another 7 d, the mice were challenged with HSV-1, and DTH measurements were taken 24 h later. Tolerance could be transferred to naïve mice with CD8+ T cells obtained from AC-injected wildtype mice (Figure 5B); however, CD8+ T cells from AC-injected Trail−/− mice could not transfer tolerance. Interestingly, CD8+ T cells from Dr5−/− mice, which themselves were not tolerized by AC injection of Ag (see Figure 2B), were also able to transfer tolerance to wildtype (Dr5+/+) recipient mice (Figure 5C). These findings suggest that DR5 expression is not necessary for the induction or function of the regulatory (TRAIL-expressing) CD8+ T cells, but DR5 expression is required on the cells these regulatory CD8+ T cells act on.
We then examined the specificity of the tolerance induced after AC injection of Ag using adoptive transfer experiments similar to those in Figure 5. Wildtype B6 mice were injected AC with either HSV-1 or TNP-spl. After 7 d, splenic CD8+ T cells from these mice were isolated and transferred to naïve wildtype recipients that were immediately immunized with HSV-1 or TNBS. Data in Figure 6 show that splenic CD8+ T cells from mice injected with HSV-1 AC were able to suppress the DTH response to HSV-1, but not TNBS (and vice-versa). Together, the data in Figures 5 and 6 demonstrate injection of Ag into the AC of the eye induces Ag-specific tolerance that can be adoptively transferred by TRAIL-expressing CD8+ T cells.
Figure 6. Specificity of immunosuppressive CD8+ T cells induced by AC injection of Ag depends on the Ag initially injected.
Wildtype B6 mice were injected AC with either 2.5 × 104 pfu HSV-1 or 5 × 105 TNP-coupled spleen cells. After 7 d, spleens were removed, CD8+ T cells were purified, and transferred to naïve B6 recipient mice (4 mice/group) that were immediately immunized with 106 pfu HSV-1 or 0.1 ml of 10 mM TNBS s.c. After another 7 d, the mice were mice were challenged with 106 UV-inactivated HSV-1 (in 33 µl PBS) or 33 µl 10 mM TNBS in the right and 33 µl PBS in the left footpad. As reference, DTH responses were measured in intact wildtype B6 mice after s.c. or AC injection of HSV-1 or TNP-spl. Values are expressed in µm (± S.E.) and represent the difference between the right (Ag challenge) and left footpad (PBS challenge). The background value represents the difference between challenged and unchallenged sites in naive mice. * p < 0.05 vs. immune control (Imm Cont.) group.
TRAIL-expressing CD8+ T cells generated after AC injection limit the magnitude of inflammatory cell influx into the eye
Inflammatory responses elicited in the AC of the eye generally have a limited spread of inflammatory cells to other areas of the globe. The data presented thus far suggest TRAIL-expressing CD8+ T cells induced by AC injection of Ag play a prominent role in regulating systemic immunity. To directly assess the capability of these cells to limit the magnitude of immune responses to Ag injected into the eye, the following experiment was performed. Wildtype and Trail−/− B6 mice were injected AC with HSV-1. After 7d, the splenic CD8+ T cells were isolated and transferred to naïve wildtype B6 recipients that were immediately immunized s.c. with HSV-1. After another 7d, the mice were injected AC with HSV-1 and the number of CD45+ cells infiltrating the eyes was quantitated 48 h later by flow cytometry. Under these conditions, wildtype CD8+ T cells were able to significantly limit the ingress of inflammatory cells into the eye compared to mice that did not receive any T cells at the time of initial s.c. immunization (Figure 7). In contrast, Trail−/− cells were less capable of inhibiting the inflammatory cell recruitment into the eye. Collectively, the data presented herein suggest TRAIL-expressing CD8+ T cells as a peripheral regulatory cell component used by the immune system to systemically suppress the body’s response to Ag initially encountered in the eye.
Figure 7. Inhibition of inflammatory cell ingress into the eye by TRAIL-expressing CD8+ T cells.
Wildtype or Trail−/− B6 mice were injected AC with 2.5 × 104 pfu HSV-1. After 7 d, spleens were removed, CD8+ T cells were purified, and transferred to naïve wildtype B6 recipient mice (4 mice/group) that were immediately immunized with 106 pfu HSV-1 s.c. After another 7 d, the mice were injected AC with 2.5 × 104 pfu UV-inactivated HSV-1. Eyes were harvested after 48 h, and the number of CD45+ cells/2 eyes was quantitated by flow cytometry. p values for the different statistical comparisons are indicated.
DISCUSSION
Immune responses elicited in the AC of the eye generally have a limited spread of inflammatory cells to other areas of the globe, implying that the eye has regulatory mechanisms in place to control these reactions. Because the eye is one of several immunologically privileged sites in the body, it was initially thought the absence of immune responses was a passive process relying on physical barriers and isolation of Ag (2). As more was learned, ocular immune privilege was found to be an active process employing a number of factors to maintain organ integrity. Consequently, the objective of this study was to examine the molecular mechanism used by the regulatory cells in the periphery to control systemic immune reactions to Ag initially encountered in the eye. Our data suggest regulatory CD8+ T cells use TRAIL to mediate tolerance during ocular immune deviation, and disruption of the TRAIL/DR5 pathway inhibits the formation of the characteristic T cell tolerance seen in this model.
TRAIL has received its greatest attention in the cancer field, since it was initially shown to preferentially induce apoptosis in tumor cells and not normal cells (37). Yet, with the increased availability of mice and reagents for examining the function of TRAIL in vivo, considerable data is now in hand showing TRAIL to be an important component in a variety of physiologically-relevant systems where TRAIL-induced apoptotic death of noncancerous cells occurs (34, 38, 39). Study of autoimmune models and infectious agents found TRAIL to be important for controlling the extent of autoimmune reactions, as well as serving as an effector molecule in certain disease states (40). TRAIL deficiency exacerbates autoimmunity in an EAE model (41). TRAIL blockade similarly exacerbates type 1 diabetes onset in NOD mice (42). More recently our laboratory detailed the importance of TRAIL-expressing Ag-specific CD8+ T cells in controlling influenza virus infections (43), which was similarly suggested by Ishikawa et al. (44). TRAIL-expressing NK cells are needed to limit experimental encephalomyocarditis virus replication in vivo, and inhibition of TRAIL function in this system results in increased mortality (45). In contrast, elimination of HSV-infected cells appears to be TRAIL independent (46). Our data showing that the immune cells entering the eye after HSV-1 injection undergo apoptotic death regardless of the presence or absence of TRAIL (see Figure 3) is in agreement with the Raftery et al. publication. Thus, it is becoming more evident that TRAIL is functionally important in many immunological settings other than tumor surveillance.
Ocular immune privilege, and the associated systemic immune deviation, requires the orchestrated production and interaction of a number of molecules and cells to ensure the maintenance of ocular integrity and suppress the generation of potentially damaging immune responses in the eye. Our group has characterized several key components in the eye that are required for the maintenance of ocular immune privilege and induction of ocular immune deviation, including the wavelength of visible light entering the eye, neuropeptide and cytokine expression in the eye, FasL expression on various ocular structures, production of the “tolerance-inducing” TCR-related protein, and cells critical for controlling systemic immunity (13, 14, 17, 18, 36, 47–49). The data presented in this study calls for the addition of TRAIL to this list. While we previously defined the relationship between lymphocyte apoptosis within the eye and the induction of ocular immune deviation (18), it would appear that TRAIL expression within the eye itself not needed to induce apoptosis of immune cells entering the eye in response to Ag – even though TRAIL is expressed on a number of ocular structures (50). This conclusion is buttressed by the data in Figure 2C showing that even direct injection of apoptotic cells into the AC of Trail−/− mice did not lead to tolerance. Instead, our data suggests an extraocular role for TRAIL in ocular immune deviation, as the lack of immune deviation in AC-immunized Trail−/− mice stemmed from the inability of the circulating regulatory CD8+ T cells to exert their function on DR5-expressing target cells. At this time, we do not know which immune cell(s) the TRAIL-expressing CD8+ regulatory T cells are targeting to establish tolerance to Ag presented via the AC. However, we recently showed that CD8+ T cells from mice that experienced peptide-induced peripheral deletion mediated tolerance by deleting CD4+ T cells with the same Ag specificity in a TRAIL-dependent, TCR-specific manner (51). Based on some similarities between these two experimental models of tolerance, it is tempting to speculate that the tolerance that occurs during ocular immune deviation is similarly mediated by the cytotoxic activity of TRAIL-expressing CD8+ T cells. Future studies are warranted to investigate this.
Several studies have pointed to the spleen as the primary lymphoid organ to receive Ag after AC injection (6, 14, 23, 36), which also suggests that the spleen processes the apoptotic remnants of the lymphocytes that entered the eye after antigenic challenge. The presumption that Ag and apoptotic cells are delivered directly to the spleen is supported by the fact that: a) the AC lacks a lymphatic drainage pathway; b) aqueous humor is resorbed directly into the blood vasculature (52); and c) splenectomized mice do not develop tolerance to Ag injected into the eye (23). Previous work from our group also found the spleen is needed to take up the soluble "tolerance-inducing" TCR α-chain-related protein that directly enters the blood within 2 d after Ag presentation in the eye and is used to activate regulatory CD8+ T cells (18, 36). Data obtained in the present study adds to what we now know about this component of ocular immune deviation, which is that the soluble TCR-related protein is produced and released into the blood after AC injection of Ag via a TRAIL-independent mechanism. However, the presence of this protein leads to the induction of TRAIL-mediated immune regulation, and the defect that prevents tolerance induction in AC-injected Trail−/− mice is subsequent to the signal entering the blood and being processed in the spleen. The mechanism behind the induction of TRAIL-expressing CD8+ regulatory T cells by the soluble TCR-related protein is also unknown, but one possible scenario might be that the release TCR-related protein is taken up by APC in the spleen and presented to CD8+ T cells in the absence of CD4+ T cell help (34). Consequently, these “helpless” TRAIL-expressing CD8+ T cells would then subsequently regulate immunity through a receptor-anti-receptor process. Once again, our data from the model of tolerance induced after soluble peptide-induce peripheral T cell deletion would support this hypothesis (51).
In summary, the data presented herein suggest an essential role for TRAIL in induction of immunological unresponsiveness to Ag first encountered in the AC of the eye. Apoptotic cell death is a highly regulated process induced by specific stimuli and tends to be immunosuppressive or tolerogenic. Immunological tolerance to Ag associated with apoptotic cells has been attributed to a number of mechanisms, including the production of immunosuppressive cytokines from phagocytic cells (53), the production of inhibitors from the dead cell itself (54, 55), and effects on the maturation of the DC (33, 56). The immune system is continuously exposed to self-Ag derived from dead cells throughout an organism’s life. For example, the cells of the immune system constantly turn over, which makes them a primary source of self Ag. Depending on their activation state, dead/dying lymphocytes can cause significant, nonspecific cellular damage if their contents are released to the rest of the immune system. Thus, during an infection where significant lymphocyte apoptosis occurs, immunity can be directed away from the invader toward self, leading to autoimmunity. It seems logical, therefore, that there are mechanisms to control the induction of misdirected immunity to self. The protection of the visual axis by inhibitors of inflammation is well-documented, and several members of the TNF superfamily of cytokines (FasL, TNF, and TRAIL) are among the proteins demonstrated to be important in that protection. The ability of the eye to kill invading inflammatory cells helps maintain immune privilege (17), whereas tolerance can occur as a result of lymphocytes killing themselves or each other during an ensuing immune response. The observation showing that death and tolerance are related (18) demonstrates that these systems can also rely on each other to regulate dangerous inflammatory reactions occurring in important anatomical areas. The tolerance induced as a result of cell death in the eye is “mobile”, potentially reaching all areas of the body. Thus, the systemic tolerance (which appears to be DR5/TRAIL-dependent) induced by lymphocyte apoptosis in the eye (which appears to be Fas/FasL- and TNFR2/TNF-dependent) may be an important mechanism to suppress immunity to Ag present in anatomical sites important for survival.
Footnotes
This work was supported by the National Institutes of Health Grants AI077565 (TSG), CA109446 (TSG), EY06765 (TAF), EY015570 (TAF), and a University of Iowa Carver College of Medicine Medical Research Initiative Grant (TSG).
REFERENCES
- 1.Van Dooremaal JC. Die entwickelung der in fremden grund versetzten ledenden gewebe. Albrect von Graefe's Acrh. Ophthalmol. 1873;19:359–373. [Google Scholar]
- 2.Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 3.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest. Ophthalmol. Vis. Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- 4.Grabbe S, Bruvers S, Gallo RL, Knisely TL, Nazareno R, Granstein RD. Tumor antigen presentation by murine epidermal cells. J. Immunol. 1991;146:3656–3661. [PubMed] [Google Scholar]
- 5.Hooper P, Bora NS, Kaplan HJ, Ferguson TA. Inhibition of lymphocyte proliferation by resident ocular cells. Curr. Eye Res. 1991;10:363–372. doi: 10.3109/02713689108996342. [DOI] [PubMed] [Google Scholar]
- 6.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990;71:383–389. [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson TA, Fletcher S, Herndon J, Griffith TS. Neuropeptides modulate immune deviation induced via the anterior chamber of the eye. J. Immunol. 1995;155:1746–1756. [PubMed] [Google Scholar]
- 8.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. J. Immunol. 1996;156:2667–2673. [PubMed] [Google Scholar]
- 10.Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J. Immunol. 1977;118:809–814. [PubMed] [Google Scholar]
- 11.Wetzig RP, Foster CS, Greene MI. Ocular immune responses. I. Priming of A/J mice in the anterior chamber with azobenzenearsonate-derivatized cells induces second-order-like suppressor T cells. J. Immunol. 1982;128:1753–1757. [PubMed] [Google Scholar]
- 12.Ferguson TA, Iverson GM. Isolation and characterization of an antigen-specific suppressor inducer molecule from serum of hyperimmune mice by using a monoclonal antibody. J. Immunol. 1986;136:2896–2903. [PubMed] [Google Scholar]
- 13.Ferguson TA, Waldrep JC, Kaplan HJ. The immune response and the eye. II. The nature of T suppressor cell induction in anterior chamber-associated immune deviation (ACAID) J. Immunol. 1987;139:352–357. [PubMed] [Google Scholar]
- 14.Ferguson TA, Hayashi JD, Kaplan HJ. The immune response and the eye. III. Anterior chamber-associated immune deviation can be adoptively transferred by serum. J. Immunol. 1989;143:821–826. [PubMed] [Google Scholar]
- 15.Sonoda Y, Streilein JW. Impaired cell-mediated immunity in mice bearing healthy orthotopic corneal allografts. J. Immunol. 1993;150:1727–1734. [PubMed] [Google Scholar]
- 16.Ferguson TA, Herndon JM. The immune response and the eye: the ACAID inducing signal is dependent on the nature of the antigen. Invest. Ophthalmol. Vis. Sci. 1994;35:3085–3093. [PubMed] [Google Scholar]
- 17.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 18.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 19.Stuart PM, Griffith TS, Usui N, Pepose J, Yu X, Ferguson TA. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J. Clin. Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbas AK. Die and let live: eliminating dangerous lymphocytes. Cell. 1996;84:655–657. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson TA, Hayashi JD, Kaplan HJ. Regulation of the systemic immune response by visible light and the eye. Faseb J. 1988;2:3017–3021. doi: 10.1096/fasebj.2.14.2972579. [DOI] [PubMed] [Google Scholar]
- 22.Whittum-Hudson J, Farazdaghi M, Prendergast RA. A role for T lymphocytes in preventing experimental herpes simplex virus type 1-induced retinitis. Invest. Ophthalmol. Vis. Sci. 1985;26:1524–1532. [PubMed] [Google Scholar]
- 23.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J. Exp. Med. 1981;153:1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R, Gliniak B. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur. J. Immunol. 2002;32:2246–2254. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, Burns TF, Ajuha H, Page R, Wu GS, Chen Y, McKenna WG, Bernhard E, Lowe S, Mak T, El-Deiry WS. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol. Cell Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am. J. Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T cells produce active immune unresponsiveness. J. Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 28.Griffith TS, Kazama H, VanOosten RL, Earle JK, Jr, Herndon JM, Green DR, Ferguson TA. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J. Immunol. 2007;178:2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int. Rev. Immunol. 2002;21:153–172. doi: 10.1080/08830180212058. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson TA, Stuart PM, Herndon JM, Griffith TS. Apoptosis, tolerance, and regulatory T cells--old wine, new wineskins. Immunol. Rev. 2003;193:111–123. doi: 10.1034/j.1600-065x.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 31.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 33.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 34.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 35.Wilbanks GA, Streilein JW. The differing patterns of antigen release and local retention following anterior chamber and intravenous inoculation of soluble antigen. Evidence that the eye acts as an antigen depot. Reg. Immunol. 1989;2:390–398. [PubMed] [Google Scholar]
- 36.Griffith TS, Herndon JM, Lima J, Kahn M, Ferguson TA. The immune response and the eye. TCR alpha-chain related molecules regulate the systemic immunity to antigen presented in the eye. Int. Immunol. 1995;7:1617–1625. doi: 10.1093/intimm/7.10.1617. [DOI] [PubMed] [Google Scholar]
- 37.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 38.Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr. Opin. Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 39.Lee HO, Herndon JM, Barreiro R, Griffith TS, Ferguson TA. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J. Immunol. 2002;169:4739–4744. doi: 10.4049/jimmunol.169.9.4739. [DOI] [PubMed] [Google Scholar]
- 40.Ma H, Liu Y, Liu S, Kung HF, Sun X, Zheng D, Xu R. Recombinant adeno-associated virus-mediated TRAIL gene therapy suppresses liver metastatic tumors. Int. J. Cancer. 2005;116:314–321. doi: 10.1002/ijc.20982. [DOI] [PubMed] [Google Scholar]
- 41.Song K, Chen Y, Goke R, Wilmen A, Seidel C, Goke A, Hilliard B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J. Exp. Med. 2000;191:1095–1104. doi: 10.1084/jem.191.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mi QS, Ly D, Lamhamedi-Cherradi SE, Salojin KV, Zhou L, Grattan M, Meagher C, Zucker P, Chen YH, Nagle J, Taub D, Delovitch TL. Blockade of tumor necrosis factor-related apoptosis-inducing ligand exacerbates type 1 diabetes in NOD mice. Diabetes. 2003;52:1967–1975. doi: 10.2337/diabetes.52.8.1967. [DOI] [PubMed] [Google Scholar]
- 43.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J. Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa E, Nakazawa M, Yoshinari M, Minami M. Role of tumor necrosis factor-related apoptosis-inducing ligand in immune response to influenza virus infection in mice. J. Virol. 2005;79:7658–7663. doi: 10.1128/JVI.79.12.7658-7663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato K, Hida S, Takayanagi H, Yokochi T, Kayagaki N, Takeda K, Yagita H, Okumura K, Tanaka N, Taniguchi T, Ogasawara K. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur. J. Immunol. 2001;31:3138–3146. doi: 10.1002/1521-4141(200111)31:11<3138::aid-immu3138>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 46.Raftery MJ, Behrens CK, Muller A, Krammer PH, Walczak H, Schonrich G. Herpes simplex virus type 1 infection of activated cytotoxic T cells: Induction of fratricide as a mechanism of viral immune evasion. J. Exp. Med. 1999;190:1103–1114. doi: 10.1084/jem.190.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferguson TA, Fletcher S, Herndon J, Griffith TS. Neuropeptides modulate immune deviation induced via the anterior chamber of the eye. J. Immunol. 1995;155:1746–1756. [PubMed] [Google Scholar]
- 48.Ferguson TA, Mahendra SL, Hooper P, Kaplan HJ. The wavelength of light governing intraocular immune reactions. Invest. Ophthalmol. Vis. Sci. 1992;33:1788–1795. [PubMed] [Google Scholar]
- 49.Ferguson TA, Herndon JM, Dube P. The immune response and the eye: a role for TNF alpha in anterior chamber-associated immune deviation. Invest. Ophthalmol. Vis. Sci. 1994;35:2643–2651. [PubMed] [Google Scholar]
- 50.Lee HO, Herndon JM, Barreiro R, Griffith TS, Ferguson TA. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J. Immunol. 2002;169:4739–4744. doi: 10.4049/jimmunol.169.9.4739. [DOI] [PubMed] [Google Scholar]
- 51.Gurung P, Kucaba TA, Schoenberger SP, Ferguson TA, Griffith TS. TRAIL-expressing CD8+ T cells mediate tolerance following soluble peptide-induced peripheral T cell deletion. J. Leukoc. Biol. doi: 10.1189/jlb.0610343. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tripathi RC. Uveoscleral drainage of aqueous humour. Exp. Eye Res. 1977;(25 Suppl):305–308. doi: 10.1016/s0014-4835(77)80026-2. [DOI] [PubMed] [Google Scholar]
- 53.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J. Exp. Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 56.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]