Abstract
Background
Low awareness of chronic kidney disease (CKD) may reflect uncertainty about the accuracy or significance of a CKD diagnosis in individuals otherwise perceived to be low-risk. Whether reclassification of CKD severity using the CKD Epidemiology Collaboration (CKD-EPI) equation to estimate glomerular filtration rate (GFR) modifies estimates of CKD awareness is unknown.
Methods
In this cross-sectional study, we used data collected from 2000 to 2009 for 26,213 participants in the Kidney Early Evaluation Program (KEEP), a community-based screening program, with CKD based on GFR estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation and measurement of albuminuria. We assessed CKD awareness after CKD stage was reclassified using the CKD-EPI equation.
Results
Of 26,213 participants with CKD based on eGFRMDRD, 23,572 (90%) were also classified with CKD based on eGFRCKD-EPI. Based on eGFRMDRD, 9.5% of participants overall were aware of CKD, as were 4.9%, 6.3%, 9.2%, 41.9%, and 59.2% with Stages 1-5, respectively. Based on eGFRCKD-EPI, 10.0% of participants overall were aware of CKD, as were 5.1%, 6.6%, 10.0%, 39.3%, and 59.4% with Stages 1-5, respectively. Reclassification to a less advanced CKD stage with eGFRCKD-EPI was associated with lower odds for awareness (OR, 0.58; 95% CI, 0.50-0.67); reclassification to a more advanced stage was associated with higher odds for awareness (OR, 1.50; 95% CI, 1.05-2.13) after adjustment for confounding factors. Of participants unaware of CKD, 10.6% were reclassified as not having CKD using eGFRCKD-EPI.
Conclusions
Using eGFRCKD-EPI led to a modest increase in overall awareness rates, primarily due to reclassification of low-risk unaware participants.
Keywords: awareness, chronic kidney disease, CKD-EPI, estimated glomerular filtration rate
Chronic kidney disease (CKD) is common among US adults, and it contributes to increased risks for death, end-stage renal disease, and cardiovascular events.1;2 While awareness of CKD has improved modestly over time, it remains low. For example, in the 2000-2004 National Health and Nutrition Examination Survey (NHANES), 6% of individuals with CKD were aware of the condition.3 Among those with Stage 4 CKD, fewer than half were aware; among those with Stage 3 CKD, fewer than 15% were aware.3 Early detection and treatment of CKD may slow progression, prevent complications, and increase preparedness for end-stage renal disease. Thus, improving CKD awareness among patients and providers is a key step towards improving CKD care.
Low CKD awareness may reflect poor provider recognition and communication of CKD and uncertainty about the accuracy of a CKD diagnosis in certain individuals. The 4-variable Modification of Diet in Renal Disease (MDRD) Study equation used to estimate glomerular filtration rate (GFR) has gained broad acceptance in clinical care, yet controversy remains about the implications of its widespread use. In particular, because the MDRD Study equation systematically underestimates GFR, especially among individuals with GFR > 60 mL/min/1.73 m2, it may lead to false-positive diagnoses of CKD.4 The prognostic significance of mild reductions in eGFR in the absence of other CKD risk factors among older individuals has also been questioned.5;6 Concerns about these issues may lead providers to underreport CKD diagnoses to patients they consider low risk for progression or other complications.
The newly developed CKD Epidemiology Collaboration (CKD-EPI) equation is reported to have greater precision and less bias for estimating GFR.7;8 Its application has led to downwardly revised estimated US prevalence of CKD, attributable primarily to lower prevalence of Stage 3 CKD (eGFR of 30-59 mL/min/1.73m2).7 Preliminary reports suggest that the CKD-EPI equation may also be more accurate for mortality risk prediction than the MDRD Study equation.9;10 We used data collected as part of the Kidney Early Evaluation Program (KEEP), a community-based convenience health screening sample, to compare estimates of CKD awareness with the CKD-EPI and MDRD Study equations. We hypothesized that the high prevalence of CKD unawareness would be attenuated by reclassification of CKD severity using CKD-EPI estimates of GFR.
Methods
Study Population
KEEP is a free community-based voluntary screening program launched in August 2000, designed to identify individuals at increased risk of kidney disease and to encourage follow-up care.11 KEEP screenings are conducted in urban and rural locations throughout the US through each state's National Kidney Foundation affiliate. In this study, we included eligible KEEP participants screened from August 2000 through December 2009 (n = 123,704) aged at least 18 years, with a diagnosis of CKD based on National Kidney Foundation guidelines using the MDRD Study equation to estimate GFR (n = 28,109). From this sample, we excluded individuals receiving maintenance dialysis or with previous kidney transplant, leaving 27,987 individuals in the analytic cohort. We further excluded individuals with missing values for CKD awareness and other covariates, resulting in a final sample size of 26,213.
KEEP Screening Procedures
During the KEEP screening, participants complete a questionnaire to assess demographic characteristics, personal and family medical history, and health behaviors. Blood pressure, height, and weight are recorded, and blood and urine specimens are collected for determination of serum creatinine, fasting glucose, and urine albumin-creatinine ratio (ACR). KEEP laboratory procedures have been described in detail previously.12
Definitions
CKD was categorized into stages13 as follows using eGFR calculated from both the IDMS-traceable MDRD Study equation (eGFRMDRD) and the CKD-EPI equation (eGFRCKD-EPI): Stage 1, eGFR ≥ 90 mL/min/1.73 m2 with ACR ≥ 30 mg/g; Stage 2, eGFR 60-89 mL/min/1.73 m2 with ≥ ACR 30 mg/g; Stage 3, eGFR 30-59 mL/min/1.73 m2; and Stages 4-5, eGFR < 30 mL/min/1.73 m2. CKD awareness was defined as an affirmative answer to the question, “Have you ever been told by a doctor or healthcare professional you have kidney disease (do not include kidney stones, bladder infections or incontinence)?” Age was categorized as 18-30, 31-45, 46-60, 61-75, and > 75 years. Education was categorized as high school graduate versus not. Diabetes was defined as self-report, use of medications for diabetes, fasting glucose values ≥ 126 mg/dL, or nonfasting glucose values ≥ 200 mg/dL. Hypertension was defined as self-report, use of medications for hypertension, systolic blood pressure ≥ 130 mmHg, or diastolic blood pressure ≥ 80 mmHg. Cardiovascular disease was defined as self-report of heart angioplasty, heart bypass surgery, heart attack, heart failure, abnormal heart rhythm, stroke, or peripheral vascular disease (peripheral vascular disease information was collected only until May 2005).
Statistical Analysis
Participant baseline characteristics and CKD awareness are described by CKD stage and eGFR equation using proportions. We used logistic regression, expressed as odds ratio (OR) and 95% confidence interval (CI) to describe the association of CKD stage and other clinical characteristics with CKD awareness. Separate models were constructed using eGFRMDRD and eGFRCKD-EPI to categorize CKD stage. Adjusted models accounted for age, sex, race, education, and diabetes, plus all other variables significant at the P < 0.1 level in unadjusted analyses. To determine the relation between reclassification of CKD severity with eGFRCKD-EPI and CKD awareness, we first determined the reclassification rate among unaware and aware participants. Next, we classified participants into three categories as follows: unchanged CKD stage using eGFRCKD-EPI versus eGFRMDRD, less advanced CKD stage using eGFRCKD-EPI versus eGFRMDRD, and more advanced CKD stage using eGFRCKD-EPI versus eGFRMDRD. These categories were used to determine the unadjusted and multivariable adjusted associations between CKD reclassification and awareness. We further stratified the analyses by CKD stage to assess whether findings were consistent. Analyses were conducted using SAS v9.2 (www.sas.com).
Results
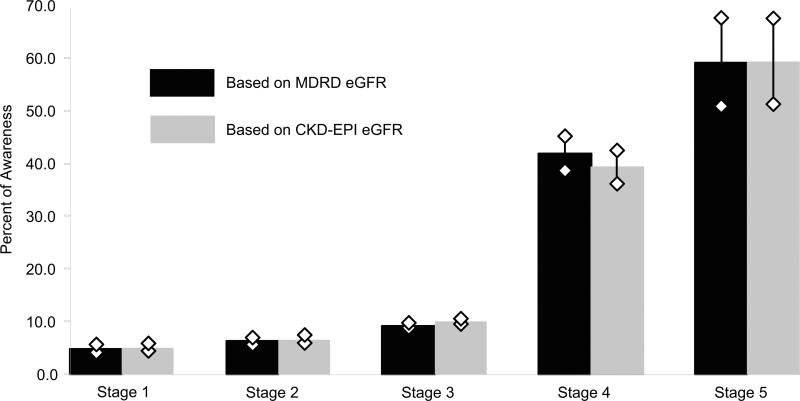
Using eGFRMDRD, 26,213 participants were classified with CKD, 8134 (31%) Stage 1-2 and 18,079 (69%) Stage 3-5 (Table 1). Using eGFRCKD-EPI, 23,572 participants were classified with CKD, 8421 (32%) Stage 1-2, and 15,151 (58%) Stage 3-5. Thus, 2641 participants (10%) were classified with CKD using eGFRMDRD but not eGFRCKD-EPI. Of participants with CKD using eGFRMDRD, 9.5% were aware of CKD; 4.9%, 6.3%, 9.2%, 41.9%, and 59.2% with Stages 1-5, respectively, were aware (Figure 1). Of participants with CKD using eGFRCKD-EPI, 10.0% were aware of CKD; 5.1%, 6.6%, 10.0%, 39.3%, and 59.4% with Stages 1-5, respectively, were aware. An association between more advanced CKD stages and higher odds for awareness remained after adjustment for clinical characteristics (Table 2). Odds for awareness were slightly higher for CKD stages based on eGFRCKD-EPI than for CKD stages based on eGFRMDRD. The association between other clinical characteristics and awareness was not substantially changed when eGFRCKD-EPI was substituted for eGFRMDRD. Among participants with eGFRCKD-EPI < 60 mL/min/1.73m2, albuminuria (ACR ≥ 30 mg/g) was associated with higher odds for awareness (OR, 1.85; 95% CI, 1.64-2.08) after adjustment for eGFR and other confounders.
Table 1.
Characteristics of KEEP Participants Classified as Having CKD by the MDRD Study Equation and Reclassification by the CKD-EPI Equation
| Characteristics | GFR Estimating Equation | ||||
|---|---|---|---|---|---|
| MDRD Study | CKD-EPI | ||||
| CKD Stages 1-2 | CKD Stages 3-5 | No CKD | CKD Stages 1-2 | CKD Stages 3-5 | |
| No. | 8134 | 18,079 | 2641 | 8421 | 15,151 |
| eGFR (mL/min/1.73 m2) | 87.3 ± 22.4 | 48.7 ± 9.7 | 62.8 ± 2.1 | 89.8 ± 18.8 | 47 ± 10.2 |
| Age Category (no(%)) | |||||
| 18-30 y | 580 (7.1) | 132 (0.7) | 49 (1.9) | 589 (7.0) | 74 (0.5) |
| 31-45 y | 1630 (20.0) | 992 (5.5) | 444 (16.8) | 1694 (20.1) | 484 (3.2) |
| 46-60 y | 2867 (35.3) | 4211 (23.3) | 1218 (46.1) | 3029 (36.0) | 2831 (18.7) |
| 61-75 y | 2305 (28.3) | 8029 (44.4) | 915 (34.6) | 2409 (28.6) | 7010 (46.3) |
| > 75 y | 752 (9.3) | 4715 (26.1) | 15 (0.6) | 700 (8.3) | 4752 (31.4) |
| Men | 2650 (32.6) | 5436 (30.1) | 588 (22.3) | 2705 (32.1) | 4793 (31.6) |
| Race | |||||
| White | 3297 (40.5) | 11,786 (65.2) | 1910 (72.3) | 3466 (41.2) | 9707 (64.1) |
| African American | 3108 (38.2) | 3970 (22.0) | 302 (11.4) | 3133 (37.2) | 3643 (24.0) |
| Other | 1729 (21.3) | 2323 (12.8) | 429 (16.2) | 1822 (21.6) | 1801 (11.9) |
| Hispanic | 1024 (12.6) | 1206 (6.7) | 256 (9.7) | 1074 (12.8) | 900 (5.9) |
| High school graduate | 6658 (81.8) | 14,871 (82.3) | 2361 (89.4) | 6911 (82.1) | 12,257 (80.9) |
| Insured | 6226 (76.5) | 16,104 (89.1) | 2198 (83.2) | 6427 (76.3) | 13,705 (90.5) |
| Access to doctor | 5498 (86.8) | 13,012 (94.3) | 1810 (89.5) | 5684 (86.7) | 11,016 (95.3) |
| Diabetes | 3701 (45.5) | 7379 (40.8) | 750 (28.4) | 3841 (45.6) | 6489 (42.8) |
| Hypertension | 7031 (86.4) | 16,424 (90.9) | 2124 (80.4) | 7275 (86.4) | 14,056 (92.8) |
| Cardiovascular disease | 2194 (27.0) | 6331 (35.0) | 618 (23.4) | 2262 (26.9) | 5645 (37.3) |
| Current tobacco use | 1087 (13.9) | 1210 (7.0) | 272 (10.8) | 1131 (14.0) | 894 (6.2) |
| Family history of kidney disease | 1580 (20.5) | 3104 (18.4) | 520 (20.8) | 1659 (20.8) | 2505 (17.7) |
Unless otherwise indicated, values shown are mean ± SD or number (percentage).
Note: CKD stages are defined as follows: Stage 1, eGFR ≥ 90 mL/min/1.73 m2 with ACR ≥ 30 mg/g; Stage 2, eGFR 60-89 mL/min/1.73 m2 with ≥ ACR 30 mg/g; Stage 3, eGFR 30-59 mL/min/1.73 m2; Stages 4-5, eGFR < 30 mL/min/1.73 m2. Values are n (%) unless otherwise indicated.
ACR, albumin-creatinine ratio; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; KEEP, Kidney Early Evaluation Program; MDRD, Modification of Diet in Renal Disease; GFR, glomerular filtration rate.
Figure 1.
Prevalence of CKD awareness by MDRD Study eGFR (n = 26,213) and CKD-EPI eGFR (n = 23,572) stages. Bars indicate 95% confidence intervals. CKD stages are defined as follows: Stage 1, eGFR ≥ 90 mL/min/1.73 m2 with ACR ≥ 30 mg/g; Stage 2, eGFR 60-89 mL/min/1.73 m2 with ≥ ACR 30 mg/g; Stage 3, eGFR 30-59 mL/min/1.73 m2; Stages 4-5, eGFR < 30 mL/min/1.73 m2. ACR, albumin-creatinine ratio; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
Table 2.
Association of CKD Stage and Other Participant Characteristics With CKD Awareness
| Characteristics | Unadjusted OR (95% CI) | Adjusted Model 1a OR (95% CI) | Adjusted Model 2a OR (95% CI) |
|---|---|---|---|
| MDRD Study CKD | |||
| Stage 1 | 1.00 (Reference) | 1.00 (Reference) | - |
| Stage 2 | 1.31 (1.07-1.60) | 1.32 (1.07-1.63) | - |
| Stage 3 | 1.99 (1.67-2.37) | 2.29 (1.89-2.78) | - |
| Stage 4 | 14.12 (11.40-17.50) | 17.32 (13.61-22.04) | - |
| Stage 5 | 28.43 (19.31-41.87) | 32.78 (21.64-49.66) | - |
| CKD-EPI CKD | |||
| None | 0.98 (0.78-1.23) | - | 1.11 (0.87-1.41) |
| Stage 1 | 1.00 (Reference) | - | 1.00 (Reference) |
| Stage 2 | 1.32 (1.10-1.59) | - | 1.53 (1.25-1.87) |
| Stage 3 | 2.07 (1.78-2.42) | - | 2.90 (2.41-3.47) |
| Stage 4 | 12.07 (9.93-14.67) | - | 18.58 (14.76-23.40) |
| Stage 5 | 27.31 (18.89-39.47) | - | 38.40 (25.69-57.41) |
| Age (per decade) | 1.05 (1.02-1.08) | 0.91 (0.87-0.94) | 0.83 (0.80-0.87) |
| Men (vs. women) | 1.33 (1.22-1.45) | 1.29 (1.18-1.42) | 1.24 (1.12-1.36) |
| White race (vs. other) | 1.30 (1.19-1.41) | 1.21 (1.09-1.33) | 1.29 (1.17-1.43) |
| High school graduate (vs. less) | 0.87 (0.79-0.97) | 0.86 (0.77-0.97) | 0.88 (0.78-0.99) |
| Insured (vs. uninsured) | 0.73 (0.66-0.81) | 0.67 (0.59-0.77) | 0.67 (0.59-0.76) |
| Access to doctor | 0.89 (0.75-1.06) | - | - |
| Diabetes | 1.22 (1.12-1.33) | 1.03 (0.94-1.14) | 1.02 (0.93-1.12) |
| Hypertension | 1.89 (1.60-2.24) | 1.59 (1.33-1.91) | 1.55 (1.29-1.86) |
| Cardiovascular disease | 1.74 (1.60-1.89) | 1.48 (1.35-1.63) | 1.48 (1.34-1.63) |
| Current Smoking | 0.97 (0.84-1.13) | - | - |
| Family history of kidney disease | 1.72 (1.56-1.90) | 1.86 (1.67-2.06) | 1.87 (1.68-2.07) |
| Screening year | |||
| 2000-2002 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 2003 | 1.26 (0.97-1.62) | 1.45 (1.10-1.91) | 1.52 (1.15-2.00) |
| 2004 | 1.10 (0.86-1.40) | 1.31 (1.01-1.70) | 1.37 (1.05-1.77) |
| 2005 | 1.66 (1.33-2.07) | 2.00 (1.58-2.53) | 2.09 (1.65-2.65) |
| 2006 | 2.15 (1.73-2.67) | 2.79 (2.20-3.53) | 2.89 (2.28-3.66) |
| 2007 | 2.57 (2.07-3.19) | 3.13 (2.48-3.96) | 3.24 (2.56-4.09) |
| 2008 | 2.55 (2.06-3.16) | 3.23 (2.56-4.08) | 3.42 (2.71-4.32) |
| 2009 | 2.87 (2.33-3.54) | 3.70 (2.94-4.65) | 3.86 (3.07-4.86) |
Note: CKD stages are defined as follows: Stage 1, eGFR ≥ 90 mL/min/1.73 m2 with ACR ≥ 30 mg/g; Stage 2, eGFR 60-89 mL/min/1.73 m2 with ≥ ACR 30 mg/g; Stage 3, eGFR 30-59 mL/min/1.73 m2; Stages 4-5, eGFR < 30 mL/min/1.73 m2.
ACR, albumin-creatinine ratio; CI, confidence interval; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; OR, odds ratio.
Adjusted models include all covariates listed.
Although prevalence estimates of awareness changed only modestly, CKD severity classification changed considerably, especially among CKD-unaware participants (Table 3). Of the 23,733 unaware participants with CKD using eGFRMDRD, 2863 (12.1%) were reclassified to a less advanced CKD stage with eGFRCKD-EPI, including 2509 (10.6%) who were reclassified with no CKD; 158 (< 1%) were reclassified to a more advanced CKD stage. Mean age of unaware participants who were reclassified with no CKD was 55 years, and mean eGFRCKD-EPI was 62 mL/min/1.73m2. All had eGFRMDRD ≥ 45 mL/min/1.73m2; 77% were women, 72% were nondiabetic, and 80% had hypertension. Of the 2480 aware participants with CKD using eGFRMDRD, 35 (1.4%) were reclassified to a more advanced stage and 188 (7.5%) to a less advanced stage.
Table 3.
Reclassification of Participants Unaware and Aware of CKD Using the CKD-EPI and MDRD Study Equations
| CKD by MDRD Study Equation | CKD-EPI Equation | Number Reclassified | |||||
|---|---|---|---|---|---|---|---|
| No CKD | Stage 1-2 | Stage 3 | Stage 4 | Stage 5 | More Advanced | Less Advanced | |
| Unaware, n = 23,733 | |||||||
| CKD Stage 1-2 | 0 | 7601 | 65 | 0 | 0 | 65 | 0 |
| CKD Stage 3 | 2509 | 322 | 12,600 | 88 | 0 | 88 | 2831 |
| CKD Stage 4 | 0 | 0 | 30 | 460 | 5 | 5 | 30 |
| CKD Stage 5 | 0 | 0 | 0 | 2 | 51 | - | 2 |
| Total No. Reclassified | 158 | 2863 | |||||
| Aware, n = 2480 | |||||||
| CKD Stage 1-2 | 0 | 463 | 5 | 0 | 0 | 5 | 0 |
| CKD Stage 3 | 132 | 35 | 1390 | 21 | 0 | 21 | 167 |
| CKD Stage 4 | 0 | 0 | 17 | 331 | 9 | 9 | 17 |
| CKD Stage 5 | 0 | 0 | 0 | 4 | 73 | - | 4 |
| Total No. reclassified | 35 | 188 | |||||
Note: CKD stages are defined as follows: Stage 1, eGFR ≥ 90 mL/min/1.73 m2 with ACR ≥ 30 mg/g; Stage 2, eGFR 60-89 mL/min/1.73 m2 with ≥ ACR 30 mg/g; Stage 3, eGFR 30-59 mL/min/1.73 m2; Stages 4-5, eGFR < 30 mL/min/1.73 m2.
ACR, albumin-creatinine ratio; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
Relative to unchanging CKD stage using eGFRMDRD and eGFRCKD-EPI, reclassification to a less advanced stage with eGFRCKD-EPI was associated with 40% lower odds for CKD awareness (OR, 0.58; 95% CI, 0.50-0.67), and reclassification to a more advanced stage with 50% higher odds for CKD awareness (OR, 1.50; 95% CI, 1.05-2.13; Table 4). These findings persisted after adjustment for age, sex, race, education, and other potential confounders. Results were consistent across all CKD stages, though most pronounced for Stages 3-5 using eGFRMDRD (Table 4).
Table 4.
Odds Ratios for CKD Awareness, KEEP Participants With Reclassified Versus Unchanged CKD Stage
| CKD Stage Reclassification from MDRD Study to CKD-EPI eGFR | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a |
|---|---|---|
| Full analytic cohort, n = 23,572 | ||
| Reclassified as less advanced | 0.58 (0.51-0.66) | 0.58 (0.50-0.67) |
| Unchanged | 1.00 (Reference) | 1.00 (Reference) |
| Reclassified as more advanced | 1.48 (1.05-2.10) | 1.50 (1.05-2.13) |
| MDRD Study Stage 1-2 CKD, n = 8134 | ||
| Reclassified as less advanced | 0.99 (0.74-1.33) | 0.92 (0.68-1.23) |
| Unchanged | 1.00 (Reference) | 1.00 (Reference) |
| Reclassified as more advanced | 0.96 (0.47-1.96) | 1.13 (0.54-2.38) |
| MDRD Study Stage 3 CKD, n = 14,456 | ||
| Reclassified as less advanced | 0.54 (0.45-0.63) | 0.45 (0.38-0.54) |
| Unchanged | 1.00 (Reference) | 1.00 (Reference) |
| Reclassified as more advanced | 2.16 (1.34-3.49) | 2.56 (1.56-4.19) |
| MDRD Study Stage 4-5 CKD, n = 982 | ||
| Reclassified as less advanced | 0.83 (0.47-1.46) | 0.56 (0.30-1.04) |
| Unchanged | 1.00 (Reference) | 1.00 (Reference) |
| Reclassified as more advanced | 2.28 (0.76-6.85) | 3.38 (1.05-10.83) |
Note: CKD stages are defined as follows: Stage 1, eGFR ≥ 90 mL/min/1.73 m2 with ACR ≥ 30 mg/g; Stage 2, eGFR 60-89 mL/min/1.73 m2 with ≥ ACR 30 mg/g; Stage 3, eGFR 30-59 mL/min/1.73 m2; Stages 4-5, eGFR < 30 mL/min/1.73 m2.
ACR, albumin-creatinine ratio; CI, confidence interval; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; OR, odds ratio.
Adjusted for age, sex, race, education, insurance, diabetes, hypertension, cardiovascular disease, and screening year.
Discussion
We found that classification of CKD severity using eGFRCKD-EPI aligned more closely with CKD awareness than classification of severity using eGFRMDRD. Application of eGFRCKD-EPI to KEEP data led to a modest increase in overall awareness rates, primarily due to reclassification of low-risk unaware participants as not having CKD. These findings suggest that eGFRCKD-EPI is a better indicator of the perceived accuracy and prognostic importance of a CKD diagnosis than eGFRMDRD.
Awareness of CKD in the US is quite low, especially compared with awareness of chronic conditions associated with CKD, such as hypertension or diabetes, where awareness rates exceed 70%.14;15 As for other chronic conditions, awareness of CKD is dependent on several patient and provider factors. Patients must have access to health care services to be tested for CKD. Providers must identify at-risk individuals, decide to evaluate kidney function, and interpret these results. KEEP screenings, promotion of CKD clinical practice guidelines, and automated eGFR reporting by laboratories aim to facilitate several of these factors. Increased CKD awareness over time in KEEP and nationally, and a recent increase in nephrology referrals suggest that these efforts may be having some impact.3;16;17
Providers must also consider the accuracy and prognostic significance of test results and communicate the findings to patients. Concern about provoking anxiety with a potentially inaccurate or inconsequential CKD diagnosis may deter provider communication.4;18 Our findings are consistent with this hypothesis. In KEEP, CKD awareness dropped off dramatically below Stage 4, rather than declining stepwise. Furthermore, 10.6% of participants labeled as CKD unaware were reclassified as not having CKD using eGFRCKD-EPI. These participants all had eGFRMDRD 45-59 mL/min/1.73 m2 and no albuminuria; most were nondiabetic. Recent findings would suggest that they compose a group at lower risk for adverse outcomes.5;6;19 In addition, the cost-effectiveness of early CKD diagnosis has been challenged, primarily due to the potential decrease in quality of life caused by a false-positive diagnosis.20;21 While the potential effects of a true-positive or false-positive diagnosis cannot be inferred from our findings, they do suggest that providers are relying on additional markers of risk beyond eGFR, such as albuminuria or family history, to communicate diagnostic and prognostic information about CKD.
These controversies should not obscure disappointingly low rates of CKD awareness among individuals with eGFR < 30 mL/min/1.73 m2, a group for whom CKD awareness is universally considered important for preventing CKD-related complications and prompting preparation for renal replacement therapy. In KEEP, only 39.3% and 59.4% of individuals with eGFRCKD-EPI of 15-30 mL/min/1.73 m2 and < 15 mL/min/1.73 m2, respectively, were aware of CKD. Correlates of CKD awareness in KEEP were similar to NHANES results; younger patients, men, whites, and patients with hypertension were more likely to be aware of CKD.3 Curiously, high school education, health insurance, and access to a doctor were associated with lower rather than higher odds for awareness, suggesting that poor health literacy or lack of access to care are not major factors preventing awareness. Additional studies are needed to understand the barriers to detection and communication of CKD in this high-risk group.
By demonstrating its relation to CKD awareness, our study also provides indirect evidence of the validity of estimating GFR using the CKD-EPI equation. Following the initial validation study, subsequent reports have confirmed that the CKD-EPI equation reduces bias across patient subgroups thought to be low risk for CKD complications, and among those with eGFR > 60 ml/min/1.73 m2, compared with the MDRD Study equation.8 Two large cohort studies have noted that eGFRCKD-EPI performs better than eGFRMDRD in predicting risk for death, cardiovascular events, and end-stage renal disease.9;10 Future studies may be able to determine whether improved accuracy and risk prognostication using eGFRCKD-EPI encourages providers to communicate a diagnosis of CKD more often.
Our study has several limitations common to large studies that use creatinine-based estimating equations for renal filtration function. First, CKD awareness (or lack of) may influence participation in a KEEP screening. Compared with the general US population, KEEP is enriched with individuals at higher risk for CKD-related morbidity.22;23 Second, because we did not have repeated assessments of eGFR, some individuals with acute changes in kidney function may have been misclassified. Finally, the questionnaire item we used to assess awareness may have been misinterpreted by participants, possibly causing underestimates of overall awareness rates. For example, participants may have been told they had “low kidney function” rather than “kidney disease.”
In summary, eGFRCKD-EPI was more strongly correlated with CKD awareness than eGFRMDRD, and its application to KEEP data led to a modest increase in CKD awareness due to upward reclassification of unaware participants with mild decrements in eGFR. Improvements in GFR estimation, such as with the creatinine-based CKD-EPI equation or other biomarkers of kidney damage, may help increase CKD awareness by reducing provider uncertainty about the accuracy and prognostic significance of a CKD diagnosis.
Acknowledgments
The authors wish to thank Shane Nygaard, BA, and Nan Booth, MSW, MPH, of the Chronic Disease Research Group, for manuscript preparation and editing, respectively.
Support: The KEEP is a program of the National Kidney Foundation, Inc., and is supported by Amgen, Abbott, Siemens, Astellas, Fresenius, Genzyme, LifeScan, Nephroceuticals, and Pfizer. Dr. Kurella Tamura receives support from the National Institute of Aging (K23AG028952). Dr. Whaley-Connell receives support from the Veteran's Affairs CDA-2. Dr. Stevens receives grant support from Gilead, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr. Norris has consulted with Amgen, King Pharmaceuticals, and Abbott. The remaining authors declare that they have no relevant financial interests.
Reference List
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Plantinga LC, Boulware LE, Coresh J, et al. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168:2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glassock RJ. Referrals for chronic kidney disease: real problem or nuisance? JAMA. 2010;303:1201–1203. doi: 10.1001/jama.2010.315. [DOI] [PubMed] [Google Scholar]
- 5.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 6.O'Hare AM, Hailpern SM, Pavkov ME, et al. Prognostic implications of the urinary albumin to creatinine ratio in veterans of different ages with diabetes. Arch Intern Med. 2010;170:930–936. doi: 10.1001/archinternmed.2010.129. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens LA, Schmid CH, Greene T, et al. Comparative Performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study Equations for Estimating GFR Levels Above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:648–659. doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–670. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Brown WW, Peters RM, Ohmit SE, et al. Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2003;42:22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 12.Stevens LA, Stoycheff N. Standardization of Serum Creatinine and Estimated Glomerular Filtration Rate in the National Kidney Foundation Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2008;51(suppl 2):S77–S82. doi: 10.1053/j.ajkd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–266. [PubMed] [Google Scholar]
- 14.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 15.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmelgarn BR, Zhang J, Manns BJ, et al. Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA. 2010;303:1151–1158. doi: 10.1001/jama.2010.303. [DOI] [PubMed] [Google Scholar]
- 17.Saab G, Whaley-Connell AT, McCullough PA, Bakris GL. CKD awareness in the United States: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2008;52:382–383. doi: 10.1053/j.ajkd.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Mann SJ. Pitfalls in diagnosing chronic kidney disease from eGFR. Arch Intern Med. 2009;169:1168–1169. doi: 10.1001/archinternmed.2009.168. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.den Hartog JR, Reese PP, Cizman B, Feldman HI. The costs and benefits of automatic estimated glomerular filtration rate reporting. Clin J Am Soc Nephrol. 2009;4:419–427. doi: 10.2215/CJN.04080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallan SI, Dahl K, Oien CM, et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ. 2006;333:1047. doi: 10.1136/bmj.39001.657755.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55(suppl 2):S23–S33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whaley-Connell AT, Sowers JR, Stevens LA, et al. CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis. 2008;51(suppl 2):S13–S20. doi: 10.1053/j.ajkd.2007.12.016. [DOI] [PubMed] [Google Scholar]