Abstract
Phosphodiesterase (PDE) III is an enzyme in vascular smooth muscle that metabolizes cyclic adenosine monophosphate (cAMP). Milrinone inhibits PDE III, increasing the availability of cAMP. Cyclic guanosine monophosphate (cGMP), which is regulated by nitric oxide (NO), also inhibits PDE III. The endothelial NO component of prostacyclin (PGI2)‐mediated vasodilation is reduced in aging. This study investigated if PGI2‐mediated vasodilation during concomitant inhibition of endothelial NO and smooth muscle PDE III is affected by healthy aging. PDE III was inhibited with milrinone in 10 older subjects and 10 young matched controls while simultaneously infusing NG‐monomethyl‐l‐arginine acetate (l‐NMMA) to remove the confounding inhibitory effects of cGMP on PDE III. Incremental doses of PGI2 and sodium nitroprusside (SNP) were administered to the brachial artery during separate trials. l‐NMMA decreased baseline blood flow similarly, and the addition of milrinone increased baseline blood flow similarly in both groups. The forearm blood flow responses to PGI2 were similar between groups (younger: 7.62 ± 0.72; older: 6.88 ± 0.81 mL•dL−1 FAV•min−1 at the highest dose of PGI2). SNP responses were also similar. This study suggests that the vasodilator pathway associated with PDE III function, the bioavailability of cAMP, and the interaction with cGMP may be preserved in healthy aging. Clin Trans Sci 2010; Volume 3: 239–242.
Introduction
The vascular changes that occur with aging may contribute to the increased incidence of cardiovascular events. Physical stimuli such as increases in blood flow and pressure trigger the release of relaxing factors by the vascular endothelium. Nitric oxide (NO), prostacyclin (PGI2), and other chemicals enter smooth muscle or stimulate receptors causing an increase in second messengers cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP), leading to vasodilation ( Figure 1 ). Dysfunction in some part of this pathway is apparent in normal aging and results in a decreased capacity of vessels to respond to stimuli.
Figure 1.
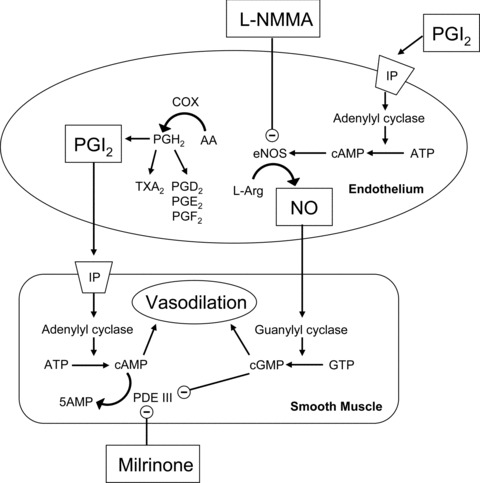
Selected mechanisms of vasodilation and the role of milrinone as discussed in the text. Prostacyclin (PGI2) via prostacyclin receptor (IP) activation causes vasodilation, secondary to an increase in cyclic adenosine monophosphate (cAMP). The cAMP is metabolized by phosphodiesterase III (PDE III). Milrinone inhibits PDE III. Increased levels of cGMP are also inhibitory of PDE III. Nitric oxide (NO) causes vasodilation secondary to an increase in cyclic guanosine monophosphate (cGMP). Endothelial NO production is inhibited by NG‐monomethyl‐ l‐arginine acetate (l‐NMMA).
Endothelial NO‐mediated vasodilation is reduced with aging. 1 Singh et al. administered NG‐monomethyl‐l‐arginine acetate (l‐NMMA) and aspirin in the brachial artery of young and older subjects and demonstrated a reduction in responsiveness to these agents in older subjects, suggesting impairment of NO and prostanoid vasodilator pathways. 2 Our laboratory recently demonstrated the role of NO in the PGI2‐mediated vasodilation in young and older adults, as dysfunction in nitric oxide synthase (eNOS)—the enzyme that catalyzes production of endothelial NO—may be responsible for the reduced functioning seen in the PGI2‐mediated vasodilatory pathway of older adults. 3 We also found that vasodilation stimulated by the endothelium‐independent vasodilator sodium nitroprusside (SNP) did not change with aging, suggesting vasodilator capacity of smooth muscle remained intact. 3
As a direct NO donor mediating smooth muscle vasodilation, SNP may augment the effect of the PGI2‐mediated pathway because NO increases levels of cGMP, which thereby inhibits the activity of phosphodiesterase III (PDE III). Reducing the activity of PDE III serves to increase levels of cAMP by inhibiting its breakdown to 5AMP. 4 The effect of aging on PDE III function and its interaction with cGMP has not been established. Therefore, the purpose of this report was to provide additional data on the smooth muscle vasodilator pathway as previously reported 3 and determine if inhibition of the NO component of the PGI2‐mediated dilation was due to an increase of PDE III in aging. We hypothesized that PDE III function is maintained in older individuals compared to the younger controls. We also determined if vasodilation by SNP during endothelial NO inhibition and PDE III inhibition was influenced by aging.
Methods
Subjects, measurements, and study drugs
This study is the final component of a previous report investigating the effect of aging on vascular responsiveness to vasoactive drugs. 3 Subjects gave written informed consent and the protocol was approved by the Mayo Clinic Institutional Review Board. As detailed previously, 10 older adults and 10 young controls were matched on sex and body mass index (BMI) ( Table 1 ). 3 Participants were lean, healthy (e.g., no cardiovascular disease or diabetes), normally active, not exercise trained, and denied medication use other than oral contraceptives at the time of the study. It was later revealed that one older participant was clinically euthyroid on thyroid supplementation. Serum cholesterol levels were not significant between groups. 3 Venous occlusion plethysmography was used to measure forearm blood flow (FBF). Heart rate, arterial pressure via brachial catheter, and FBF were collected using an automated data‐acquisition system. Forearm vascular conductance (FVC) was calculated as FVC = (FBF/MAP × 100) expressed as arbitrary units. Study drugs PGI2, SNP, and l‐NMMA were prepared as described previously. 3 Milrinone lactate (NOVAPLUS®, Irving, TX, USA) was prepared using the 1 mg•mL−1 commercial preparation and diluted with 0.9% saline to a final concentration of 10 μg•mL−1. The continuous inhibition dose was determined by application of a preliminary dose response study in five young participants. 3
Table 1.
Baseline clinical characteristics.
| Subject group | No. | M/F | Age, y | BMI | SBP | DBP | MAP | Chol |
|---|---|---|---|---|---|---|---|---|
| Young | 10 | 6/4 | 29.0 ± 2.8 | 24.0 ± 0.9 | 116 ± 5 | 61.5 ± 2.6 | 79.7 ± 2.5 | 179 ± 6 |
| Older | 10 | 6/4 | 67.8 ± 1.3 | 24.5 ± 0.7 | 122 ± 4 | 69.3 ± 3.7 | 87.0 ± 3.5 | 206 ± 16 |
Values are mean ± SEM. M/F indicates the number of males/females. BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; Chol = total cholesterol. Pressure was measured in mmHg and cholesterol in mg/dL.
Infusion protocol
As previously reported, l‐NMMA was infused (maintenance dose 1 mg•min−1) during dose responses to SNP and PGI2, followed by a 20‐minute washout period. 3 As seen in Figure 2 , milrinone infusion (0.5 μg•dL−1 of forearm volume [FAV]•min−1) was then added. Once steady‐state conditions were reached, baseline measures were obtained. Dose responses to PGI2 (2.5, 5, and 10 ng•dL−1 of FAV min−1) were generated followed by a 20‐minute washout period. Physiologically equivalent dose responses of SNP (0.25, 0.5, and 1 μg•dL−1 of FAV min−1) concluded the protocol.
Figure 2.

Protocol and timeline for administration of study medications. BL = baseline; PGI2= prostacyclin; SNP = sodium nitroprusside; l‐ NMMA = NG‐monomethyl‐ l‐ arginine acetate. *From Nicholson et al., 2009.
Statistical analysis
Changes in FBF and FVC were determined with each subject serving as their own control using a paired analysis. Repeated‐measures ANOVA were completed using SAS software version 9 (SAS Institute, Cary, NC, USA). The dependent variable was the percentage of change from baseline, and the repeated factor was dose. The independent cross‐classification variable was age group. This model also included a group‐by‐dose comparison. Because the milrinone infusion was added to a background l‐NMMA infusion, a two‐tailed paired t‐test was performed to compare baseline FBF and FVC between the l‐NMMA and l‐NMMA + milrinone conditions within each age group. Data are expressed as mean ± standard error of the mean. Statistical significance was set at p < 0.05.
Results
Hemodynamic variables were not statistically significant between younger and older groups. Table 2 shows the raw FBF and FVC data. As reported previously, the dose responses to PGI2 during l‐NMMA were similar in younger and older groups (p= 0.60, main effect of group). 3 The group‐by‐dose comparison revealed no significant interactions (p= 0.87). When milrinone infusion was added to the background l‐NMMA infusion, there was a significant increase in baseline FBF in younger (p < 0.01) and older (p < 0.03) subjects, and a significant increase in baseline FVC in younger (p < 0.02) and a tendency toward significance in older (p= 0.07) subjects. Figure 3 displays the percent increase in FBF and FVC from baseline during l‐NMMA alone and during concomitant infusions of l‐NMMA and milrinone in both groups. There was a significant dose effect of PGI2 and SNP on forearm vasodilation, but there was no evidence to suggest that PGI2‐mediated or SNP‐mediated vasodilation was different between groups (p= 0.89 and p= 0.98, main effect of group; p= 0.75 and p= 0.40, group‐by‐dose interactions, respectively).
Table 2.
FBF and FVC responses to infusion of PGI2 and SNP with co‐infusion of l‐NMMA or l‐NMMA + milrinone.
| FBF | FVC | |||
|---|---|---|---|---|
| Young | Older | Young | Older | |
| Resting baseline | 1.86 ± 0.27 | 2.12 ± 0.23 | 2.11 ± 0.30 | 2.33 ± 0.27 |
| l‐NMMA baseline | 1.17 ± 0.16 | 1.67 ± 0.13 | 1.86 ± 0.18 | 1.83 ± 0.18 |
| l‐NMMA ± milrinone | ||||
| PGI2 | ||||
| Baseline | 2.83 ± 0.41* | 2.44 ± 0.28* | 2.93 ± 0.44* | 2.60 ± 0.37† |
| 2.5 | 4.09 ± 0.45 | 3.82 ± 0.51 | 4.35 ± 0.50 | 4.06 ± 0.63 |
| 5.0 | 6.03 ± 0.54 | 5.34 ± 0.68 | 6.43 ± 0.59 | 5.74 ± 0.86 |
| 10.0 | 7.62 ± 0.72 | 6.88 ± 0.81 | 8.20 ± 0.79 | 7.36 ± 1.00 |
| SNP | ||||
| Baseline | 3.06 ± 0.46* | 3.04 ± 0.33* | 3.16 ± 0.47* | 3.18 ± 0.37* |
| 0.25 | 7.88 ± 0.99 | 7.70 ± 0.89 | 8.28 ± 1.05 | 8.26 ± 1.08 |
| 0.50 | 10.67 ± 0.76 | 10.72 ± 1.08 | 11.44 ± 0.74 | 11.11 ± 1.73 |
| 1.0 | 12.25 ± 0.85 | 13.94 ± 1.42 | 13.32 ± 0.96 | 15.48 ± 1.73 |
Values are mean ± SEM. FBF = Forearm blood flow (Ml/dL−1 FAV/min−1); FVC = forearm vascular conductance (arbitrary units); PGI2= Prostacyclin (ng/dL−1 FAV/min−1); SNP = (mcg/dL−1 FAV/min−1); l‐NMMA = NG‐monomethyl‐ l‐arginine and was infused at 1 mg/min−1, milrinone was infused at 0.5 mcg/dL−1 FAV/min−1. * indicates a significant increase in baseline FBF and FVC during l‐NMMA + milrinone when compared to baseline FBF during l‐NMMA only. † indicates p= 0.07 for similar comparison.
Figure 3.

Percent change of forearm vascular conductance (FVC) from baseline after administration of prostacyclin during continuous infusion of l‐NMMA and steady‐state milrinone in older versus younger subjects. The significant increase in FVC was similar between groups. Values are mean ± SEM.
Discussion
Our previous findings from this cohort suggested that the age‐related decrease in PGI2‐mediated vasodilation is due to a reduction in the contribution of endothelial derived NO. 3 The novelty of this report extends those findings to include an evaluation of the functionality of PDE III in younger versus older adults. When l‐NMMA was infused, baseline blood flow decreased similarly in both groups. When the PDE III inhibitor milrinone was added to the l‐NMMA infusion, baseline blood flow increased similarly in both groups. Finally during l‐NMMA and milrinone infusions, the PGI2 and SNP dose responses demonstrated significant vasodilation to a degree that was similar in younger and older adults. Taken together, these findings suggest that the vasodilator pathway associated with PDE III function, the bioavailability of cAMP, and the interaction with cGMP are relatively preserved in healthy aging. This finding also adds to evidence suggesting that the vasodilator capacity of vascular smooth muscle remains intact with healthy aging.
Endothelial contribution to vasodilation
Vascular dysfunction with aging is associated with a measurable reduction in endothelium‐dependent vasodilation. 5 The disequilibrium between vascular relaxation and contraction can prelude atherosclerotic cardiovascular disease. 6 The value in understanding age‐induced vascular degeneration is underscored by the need for an effective therapy to alter its course. 6
Growing evidence supports the central concept that NO‐ and PGI2‐mediated vasodilator pathways may be impaired with aging. 1 , 2 , 3 , 7 Taddei et al. demonstrated that the dilation of vessels in the human forearm by acetylcholine declined with increasing age, suggesting a progressive reduction in NO availability. 1 This claim was supported by our previous finding from the present cohort that when NO production was blocked by l‐NMMA, age‐dependent differences in PGI2‐mediated dilation were no longer apparent. 3
Alternatively, a study by Singh et al. suggested a less‐active, cAMP‐mediated PGI2 pathway. 2 They demonstrated that older adults show a decreased constrictor effect compared to young adults when given nonselective cyclooxygenase inhibitors, a finding that could be due to increased production of constricting prostanoids or diminished vascular responsiveness to PGI2. 2 An interesting link between the NO and PGI2 pathways was suggested by Kamper et al. 8 Using a PGI2 analog compound and l‐NMMA, they found that a portion of the PGI2‐mediated vasodilatory pathway is augmented by NO. To address this overall concept, the current protocol administered PGI2 while inhibiting NO production with l‐NMMA and inhibiting PDE III with milrinone. This demonstrated that once endothelial NO was removed by l‐NMMA, PGI2‐mediated vasodilation was preserved before and after PDE III inhibition with milrinone, an idea consistent with preserved cAMP‐mediated PGI2 function in aging.
Smooth muscle contributions
With aging, vascular smooth muscle thickens associated with an increased collagenous component amid decreased cellular density. 9 , 10 Despite these physical changes, the current study suggests that the functional vasodilatory components of cAMP, cGMP, and PDE III are largely preserved. Moreover, the preserved SNP‐evoked vasodilation is consistent with an equal SNP‐mediated vasodilation between age‐groups observed by Taddei and colleagues. 1
Phosphodiesterase III contributions
PDEs are a family of enzymes, which break down second messenger molecules such as cAMP and cGMP. The subtype PDE III degrades cAMP to its inactive form, 5AMP. 11 Drugs inhibiting this enzyme, such as milrinone and amrinone, were originally developed as a treatment for heart failure. 4 Milrinone works by inhibiting PDE III, thereby, increasing the availability of cAMP. 11 In our study, assessing the functionality of PDE III was contingent upon removing other inhibitors of the enzyme. An increase in NO raises levels of cGMP, which in turn inhibits PDE III. We used l‐NMMA to block the production of NO so that we could remove the confounding inhibitory effects of cGMP on PDE III. When PDE III and NO production were both inhibited, dilation was equal in both younger and older adults. This suggests that the functionality of PDE III in vascular smooth muscle remains intact with aging.
Limitations
One limitation of this study is that brachial artery reactivity has been evaluated in many studies, but it may not represent all vasculature in the body. Secondly, our protocol was not designed to demonstrate an age‐related effect on the response to NO inhibition with l‐NMMA administration. l‐NMMA did not produce a difference in resting flow between groups, but it deserves mention that a previous investigation generated dose‐response curves to l‐NMMA from baseline, and found that the area‐under‐the‐curve for cumulative flow responses to three doses of l‐NMMA was reduced to a greater extent in younger versus older subjects. 2
Finally, due to the aims of our previous protocol, our study did not investigate the vascular affects of milrinone administration without l‐NMMA co‐infusion. There may have been age‐related differences with PDE III inhibition alone because of the overall idea that aging impairs NO bioavailability or eNOS function. Because the NO pathway is blunted with aging, 1 , 3 it would have been interesting to compare responses to PGI2 with milrinone only between the two age groups.
Perspectives
Pharmacological effects can be altered by the aging process. 12 If age‐related changes in drug responsiveness are unaccounted for, adverse outcomes in older patients may occur. These changes may be secondary to pharmacokinetic or pharmacodynamic considerations. Our findings suggest that milrinone appears to maintain pharmacodynamic effects on vascular smooth muscle. Additional research is needed to account for pharmacokinetic or mechanistic pathological changes that may further explain populational differences that may be seen with this agent, clinically. We conclude that during inhibition of NO with l‐NMMA, the function of the enzyme PDE III in vascular smooth muscle appears to be maintained with aging.
Acknowledgments
We thank Darrell R. Schroeder (biostatistics); Christiane Hesse, Niki M. Dietz, and Timothy B. Curry (anesthesiology); and Lakshmi P.M. Somaraju, Tasha L. Pike, Brianna Vaa, Karen P. Krucker, and Pamela A. Engrav (anesthesia research) for outstanding assistance and support during this study. In addition, we thank our subjects who participated in this study. This research was supported by National Institutes of Health grant HL‐46493 (to M.J.J.), National Institutes of Health Clinical and Translational Science Award grant UL1‐RR24150 (to the Mayo Clinic), and Clinical Pharmacology Training grant GM‐08685 (to W.T.N.).
References
- 1. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age‐related reduction of NO availability and oxidative stress in humans. Hypertension. 2001; 38: 274–279. [DOI] [PubMed] [Google Scholar]
- 2. Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci (Lond). 2002; 102: 595–600. [PubMed] [Google Scholar]
- 3. Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin‐mediated dilation in the human forearm. Hypertension. 2009; 53: 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006; 58: 488–520. [DOI] [PubMed] [Google Scholar]
- 5. Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow‐induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996; 78: 1210–1214. [DOI] [PubMed] [Google Scholar]
- 6. Barac A, Panza JA. Mechanisms of decreased vascular function with aging. Hypertension. 2009; 53: 900–902. [DOI] [PubMed] [Google Scholar]
- 7. Matz RL, De Sotomayor MA, Schott C, Stoclet JC, Andriantsitohaina R. Vascular bed heterogeneity in age‐related endothelial dysfunction with respect to NO and eicosanoids. Br J Pharmacol. 2000; 131: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamper AM, Paul LC, Blauw GJ. Prostaglandins are involved in acetylcholine‐ and 5‐hydroxytryptamine‐induced, nitric oxide‐mediated vasodilatation in human forearm. J Cardiovasc Pharm. 2002; 40: 922–929. [DOI] [PubMed] [Google Scholar]
- 9. Gaballa MA, Jacob CT, Raya TE, Liu J, Simon B, Goldman S. Large artery remodeling during aging: biaxial passive and active stiffness. Hypertension. 1998; 32: 437–443. [DOI] [PubMed] [Google Scholar]
- 10. Orlandi A, Bochaton‐Piallat ML, Gabbiani G, Spagnoli LG. Aging, smooth muscle cells and vascular pathobiology: implications for atherosclerosis. Atherosclerosis. 2006; 188: 221–230. [DOI] [PubMed] [Google Scholar]
- 11. Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006; 109: 366–398. [DOI] [PubMed] [Google Scholar]
- 12. Matz RL, Andriantsitohaina R. Age‐related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging. 2003; 20: 527–550. [DOI] [PubMed] [Google Scholar]


