Abstract
Although the link between high doses of ionizing radiation and damage to the heart and coronary arteries has been well established for some time, the association between lower-dose exposures and late occurring cardiovascular disease has only recently begun to emerge, and is still controversial. In this paper, we extend an earlier systematic review by Little et al. on the epidemiological evidence for associations between low and moderate doses of ionizing radiation exposure and late occurring blood circulatory system disease. Excess relative risks per unit dose in epidemiological studies vary over at least two orders of magnitude, possibly a result of confounding and effect modification by well-known (but unobserved) risk factors, and there is statistically significant (p < 0.00001) heterogeneity between the risks. This heterogeneity is reduced, but remains significant, if adjustments are made for the effects of fractionated delivery or if there is stratification by endpoint (cardiovascular disease vs. stroke, morbidity vs. mortality). One possible biological mechanism is damage to endothelial cells and subsequent induction of an inflammatory response, although it seems unlikely that this would extend to low-dose and low-dose-rate exposure. A recent paper of Little et al. proposed an arguably more plausible mechanism for fractionated low-dose effects, based on monocyte cell killing in the intima. Although the predictions of the model are consistent with the epidemiological data, the experimental predictions made have yet to be tested. Further epidemiological and biological evidence will allow a firmer conclusion to be drawn.
Introduction
It has generally been assumed that the risks of exposure to ionizing radiation at low doses and dose rates are dominated by cancer in the directly exposed individuals. The mechanisms by which low doses of ionizing radiation cause cancer are reasonably well understood, being fundamentally driven by mutational damage to DNA (UNSCEAR 2000), although a role for non-DNA targeted effects cannot be ruled out (Morgan 2003). At high radiation doses, such as would be received by patients treated with radiotherapy (RT), a variety of other (so-called deterministic or tissue reaction) effects are observed, resulting from inactivation of large numbers of cells and associated functional impairment of the affected tissue. Among such effects are direct damage to the structures of the heart—including marked diffuse fibrotic damage, especially of the pericardium and myocardium, pericardial adhesions, microvascular damage and stenosis of the valves—and to the coronary, carotid and other large arteries; these sorts of damage occur both in patients receiving RT and in experimental animals (Adams et al. 2003). Mechanisms of high-dose effects relevant to RT have been thoroughly reviewed by Schultz-Hector and Trott (2007).
However, there is emerging evidence of excess risk of blood circulatory system disease at much lower radiation doses and occurring over much longer intervals after radiation exposure in the Japanese atomic bomb survivor Life Span Study (LSS) cohort (Wong et al. 1993; Preston et al. 2003; Yamada et al. 2004) and in a few other groups (Howe et al. 2004; McGale and Darby 2005; Ivanov et al. 2006; McGeoghegan et al. 2008; Azizova and Muirhead 2009), although not in others (Vrijheid et al. 2007). In this paper, we extend previous systematic reviews by Little et al. (2008, 2009a) of the evidence for a causal interpretation of these epidemiological associations between low- and moderate-dose radiation exposure and circulatory disease. We shall also more briefly review possible biological mechanisms for the effects observed in these lower-dose epidemiological studies.
Methods
We reviewed epidemiological studies in which the mean heart or brain doses are generally in the 0–5 Gy dose range. The studies considered were more or less those documented in the systematic reviews of Little et al. (2008, 2009a), updated to consider a few more recent studies, in particular the BNFL worker study of McGeoghegan et al. (2008), the third analysis of the UK National Registry for Radiation Workers (NRRW) (Muirhead et al. 2009) and the Mayak worker study (Azizova and Muirhead 2009).
The basis of all estimations of risk was the value of the excess relative risk (ERR) coefficient (ERR Sv−1). Wherever possible this was taken directly from the relevant study or estimated from data given in the published report, using methods outlined in Little et al. (2008).
We do not present results for any cohort where the extra follow-up amounts to a year or less compared with another study that otherwise properly contains it. Therefore, the US nuclear worker study (Howe et al. 2004), which contains only one more year (1997) follow-up than the IARC 15-country study (Vrijheid et al. 2007) that otherwise subsumes it, was omitted from further consideration and likewise the studies of Johnson et al. (1999) and Atkinson et al. (2004), both subsumed within the latest NRRW analysis cohort (Muirhead et al. 2009) and with final follow-up earlier than that of this larger group (13/12/1996 and 31/12/1997, respectively, compared with 31/12/2001).
Review of the epidemiological data
Findings in the Japanese atomic bomb survivors
Excess radiation-associated mortality due to heart disease and stroke has been observed in the LSS cohort (Table 1) (Preston et al. 2003). In the latest follow-up of the Adult Health Study (AHS), Yamada et al. (2004) observed generally non-statistically significant radiation-associated excess risks for incidence of hypertension and myocardial infarction (Table 1). The study of Yamada et al. (2004) was the only epidemiological study apart from those of Ivanov et al. (2006) and Azizova and Muirhead (2009) to have assessed morbidity rather than mortality. The evidence from the Japanese atomic bomb survivors has recently been reviewed by Wakeford and Little (2009).
Table 1.
Excess relative risks (per Sv) of circulatory disease in published low/moderate dose (<5 Sv) epidemiological datasets with estimated average radiation dose to the heart/brain and for which quantitative risk assessment is possible (reproduced in part from Little et al. 2008, 2009a)
| Data | References | Average heart/brain dose (range) (Sv) |
Numbers in cohort (person years follow-up) |
Endpoint (mortality unless otherwise indicated) | Excess relative risk Sv−1 (and 95% CI) |
|---|---|---|---|---|---|
| Japanese atomic bomb survivors | |||||
| Mortality | Preston et al. (2003) | 0.1 (0–4)a | 86,572 (1,697,861) | Heart disease, 1968–1997 (ICD9 390–429) | 0.17 (0.08, 0.26)a,b |
| Stroke, 1968–1997 (ICD9 430–438) | 0.12 (0.02, 0.22)a,b | ||||
| Morbidity | Yamada et al. (2004) | 0.1 (0–4)c | 10,339 (NA) | Hypertension incidence, 1958–1998 (ICD9 401) | 0.05 (−0.01, 0.10)c |
| Hypertensive heart disease incidence, 1958–1998 (ICD9 402, 404) | −0.01 (−0.09, 0.09)c | ||||
| Ischaemic heart disease incidence, 1958–1998 (ICD9 410–414) | 0.05 (−0.05, 0.16)c | ||||
| Myocardial infarction incidence, 1964–1998 (ICD9 410) | 0.12 (−0.16, 0.60)c | ||||
| Stroke incidence, 1958–1998 (ICD9 430, 431, 433, 434, 436) | 0.07 (−0.08, 0.24)c | ||||
| Low-dose radiotherapy and medical diagnostic studies | |||||
| Peptic ulcer study | Carr et al. (2005) | 1.3 (0.0–7.6) | 3,719 (92,979) | Coronary heart disease (ICD8 410–414)d | 0.10 (−0.12, 0.33) |
| Other heart disease (ICD8 400–404, 420–429)d | −0.16 (−0.49, 0.17) | ||||
| Ankylosing spondylitis | Darby et al. (1987) | 0.14 (0.0–4.80)e | 14,106 (183,749) | Stroke (ICD7 430–434) | −2.43 (−4.29, 0.71)e |
| 2.49 (0.0–17.28)f | Other circulatory disease (ICD7 400–429, 435–468) | −0.01 (−0.12, 0.13)f | |||
| TB fluoroscopy | Davis et al. (1989) | 0.84g (NA) | 13,385 (331,006) | All circulatory disease (ICD8 390–458) | −0.11 (−0.20, −0.01)g |
| Occupational studies | |||||
| Canadian nuclear and other workers | Ashmore et al. (1998) | 0.063 (0.0 to >0.4) | 206,620 (NA) | Circulatory disease (males) (ICD9 390–459) | 2.3 (0.9, 3.7)b |
| Circulatory disease (females) (ICD9 390–459) | 12.1 (−0.4, 24.6)b | ||||
| Mayak workers | Azizova and Muirhead (2009) | 0.83 (0–5.92)i | 12,210 (205,249) | Ischaemic heart disease morbidity (ICD9 410–414) | 0.109 (0.049, 0.168)i |
| 0.52 (0–127.82)j | 12,210 (443,350) | Ischaemic heart disease mortality (ICD9 410–414) | 0.275 (0.05, 0.501)j | ||
| 0.83 (0–5.92)i | 12,210 (197,344) | Cerebrovascular disease morbidity (ICD9 430–438) | 0.464 (0.360, 0.567)i | ||
| 0.52 (0–127.82)j | 12,210 (197,344) | Cerebrovascular disease morbidity (ICD9 430–438) | 0.155 (0.075, 0.235)j | ||
| Chernobyl emergency worker morbidity | Ivanov et al. (2006) | 0.109 (0 to >0.5) | 61,017 (NA) | Hypertension (ICD10 I10–I15) | 0.26 (−0.04, 0.56) |
| Ischaemic heart disease (ICD10 I20–I25) | 0.41 (0.05, 0.78) | ||||
| Other heart disease (ICD10 I30–I52) | −0.26 (−0.81, 0.28) | ||||
| Cerebrovascular disease (ICD10 I60–I69) | 0.45 (0.11, 0.80) | ||||
| All circulatory disease (ICD10 I00–I99) | 0.18 (−0.03, 0.39) | ||||
| German uranium miner study | Kreuzer et al. (2006) | 0.041 (0 to >0.3) | 59,001 (1,801,626) | All circulatory disease (ICD10 I00–I99) | −0.26 (−0.6, 0.05)k |
| Heart disease (ICD10 I00–I52) | −0.35 (−0.7, 0.009)k | ||||
| Cerebrovascular disease (ICD10 I60–I69) | 0.09 (−0.6, 0.8)k | ||||
| BNFL workers | McGeoghegan et al. (2008) | 0.0569 (0 to >0.729) | 38,779 (1,081,570) | Ischaemic heart disease (ICD9 410–414) | 0.70 (0.37, 1.07)b |
| Cerebrovascular disease (ICD9 430–438) | 0.66 (0.17, 1.27)b | ||||
| All circulatory disease (ICD9 390–459) | 0.54 (0.30, 0.82)b | ||||
| 3rd Analysis of UK National Registry for Radiation Workers | Muirhead et al. (2009) | 0.0249 (<0.01 to >0.4) | 174,541 (3.9 × 106) | All circulatory disease (ICD9 390–459) | 0.251 (−0.01, 0.54) |
| Circulatory disease not strongly related to smoking (ICD9 390–409, 415–440, 442–459) | 0.280 (−0.19, 0.85) | ||||
| Cerebrovascular disease (ICD9 430–438) | 0.161 (−0.42, 0.91) | ||||
| US Oak Ridge workers | Richardson and Wing (1999) | NA (0 to >0.1) | 14,095 (425,486) | Ischaemic heart disease (ICD8 410–414) | −2.86 (−6.90, 1.18) |
| IARC 15-country nuclear worker study | Vrijheid et al. (2007) | 0.0207 (0.0 to >0.5) | 275,312 (4,067,861) | Circulatory disease (ICD10 I00–I99, J60–J69, O88.2, R00–R02, R57) | 0.09 (−0.43, 0.70) |
| Ischaemic heart disease (ICD10 I20–I25) | −0.01 (−0.59, 0.69) | ||||
| Heart failure (ICD10 I50) | −0.03 (<0, 4.91) | ||||
| Deep vein thrombosis and pulmonary embolism (ICD10 I26, I60–I69, I80, I82) | −0.95 (−1.00, 9.09)h | ||||
| Cerebrovascular disease (ICD10 O88.2) | 0.88 (−0.67, 3.16) | ||||
| All other circulatory disease (ICD10 R00–R02, R57, I00–I99 excluding I20–26, I50, I60–69, I80, I82) | 0.29 (<0, 2.40) | ||||
| Environmental studies | |||||
| Three Mile Island study | Talbott et al. (2003) | 0.0001 (0 to >0.00016) | 32,135 (561,063) | Heart disease (white males) | −274 (−874, 438) |
| Heart disease (white females) | −951 (−1433, −390) |
Analysis based on colon dose
90% CI
Analysis based on stomach dose, derived from Table 2 of Yamada et al. (2004)) with smoking and drinking in the stratification
Analysis excluding highest dose group (3.1–7.6 Gy)
Based on brain dose
Based on heart dose
Based on lung dose
Estimate derived from log-linear model, evaluated at 1 Sv
External γ dose (Gy)
Internal α-particle dose (Sv), applying a relative biological effectiveness (RBE) of 1
Risk estimates in relation to cumulative whole body external γ dose
Low- and moderate-dose therapeutically exposed groups
All the studies considered in this section were of patients treated for benign disease. There was a significant (two-sided p = 0.01) increasing trend of coronary heart disease mortality with radiation dose in a US cohort of persons treated for peptic ulcer (half with X-irradiation, half without), although there was no such significant trend for other cardiovascular mortality (Carr et al. 2005). In contrast, radiation-associated excess mortality from cardiovascular disease was not seen in a study of UK ankylosing spondylitis patients (Darby et al. 1987) (Table 1).
Diagnostically exposed groups
No excess circulatory disease mortality was observed in a cohort of Massachusetts tuberculosis patients receiving multiple fluoroscopic chest X-rays (Davis et al. 1989) (Table 1). Although not reported in this table, there have been a number of groups exposed to internally deposited radionuclides, in particular α-particles from the diagnostic contrast medium Thorotrast. Among the largest of these is a cohort of US, Danish and Swedish patients (Travis et al. 2001), which reported marginally significant elevations in risk from cardiac disease [for males relative risk (RR) = 1.0 (95% CI 0.8, 1.2), for females RR = 1.2 (95% CI 1.0, 1.6), total RR = 1.1 (95% CI 0.9, 1.3)] although for cerebrovascular disease there was more substantial (and statistically significant) elevations [for males RR = 1.4 (95% CI 1.0, 2.0), for females RR = 1.8 (95% CI 1.3, 2.5), total RR = 1.6 (95% CI 1.2, 2.0)]. In a somewhat smaller Portuguese series risks of circulatory disease were not significantly elevated [for males RR = 1.11 (95% CI 0.76, 1.62), for females RR = 0.97 (95% CI 0.53, 7.70), total RR = 1.08 (95% CI 0.79, 1.46)] (dos Santos Silva et al. 2003). The findings in relation to cerebrovascular disease in the international series should be treated with caution, since a frequent reason for use of Thorotrast was investigation of cerebral vascular anomalies, as pointed out by Travis et al. (2001). Thorotrast deposits α-particle dose primarily to the liver. Unfortunately, to the best of our knowledge, evaluation of these health endpoints in relation to liver dosimetry has not been performed.
Occupationally exposed groups
There were increasing trends with dose for certain circulatory disease mortality endpoints (all circulatory disease, cerebrovascular disease, other circulatory diseases), and decreasing trends for certain other endpoints (ischaemic heart disease, heart failure, deep vein thrombosis and pulmonary embolism) in the IARC 15-country study of radiation workers (Vrijheid et al. 2007) (Table 1), although none were statistically significant (1-sided p ≥ 0.20). Radiation-associated excess ischaemic heart disease and stroke morbidity was observed in excess in a group of Chernobyl recovery workers, although there was no excess morbidity due to hypertensive heart disease and other heart disease (Ivanov et al. 2006) (Table 1). There was a very strong, and highly statistically significant, increasing trend of circulatory disease mortality with dose in a Canadian cohort of nuclear workers and various other occupationally exposed groups (dentists, radiographers etc.; Ashmore et al. 1998; Table 1). However, general increases of the same sort of order were seen for a number of other diseases in the study of Ashmore et al., which implies that there may be bias. A highly statistically significant trend with dose was seen for ischaemic heart disease and cerebrovascular disease in the latest analysis of the Mayak worker data (Azizova and Muirhead 2009). As with the atomic bomb survivor data (Yamada et al. 2004) and the Chernobyl liquidators (Ivanov et al. 2006), this cohort is unusual in that morbidity as well as mortality information was available, as also information on smoking and alcohol consumption. The study is also unusual in that doses to certain internal organs, in particular the lung and liver were dominated by doses from internally deposited radionuclides, in particular the α-particle emitting radioisotopes of plutonium. Doses in this study were among the highest considered here and arguably were sufficiently high that this study should be considered outside the scope of the review: average whole body doses for external γ rays were 0.83 Gy, with a range of 0–5.92 Gy. However, unlike the doses received from radiotherapy, the external doses received by the Mayak workers were, in general, accumulated over a protracted period, so it is reasonable to include this population in the present study. Nonetheless, interpretation is complicated by the large and highly heterogeneous internal α-particle dose from plutonium. There was a significant dose response in relation to both external γ dose and internal (α-particle) dose to the liver (Azizova and Muirhead 2009; Table 1). There are few cohorts of any substance apart from this with α-particle liver dose. Groups exposed to the diagnostic contrast medium Thorotrast received a substantial α-particle liver dose, as discussed previously, and it is notable that there was little evidence of excess risk of circulatory disease risk, specifically cardiac disease in these cohorts.
A borderline significant trend with dose was seen for circulatory disease mortality in the latest analysis of the UK NRRW (Muirhead et al. 2009), an ERR of 0.25 Sv−1 (95% CI −0.01, 0.54); an increasing trend with dose of a similar magnitude was also reported for all cancer mortality, an ERR of 0.28 Sv−1 (95% CI −0.02, 0.62). In other workforces (Richardson and Wing 1999; Vrijheid et al. 2007), there were generally no statistically significant trends of circulatory disease with dose (Table 1). It should be noted that these studies overlap, and in particular there is substantial inclusion of the study populations of the studies of Richardson and Wing (1999) and Muirhead et al. (2009) within that of the IARC study (Vrijheid et al. 2007). There were no statistically significant trends of circulatory disease mortality with cumulative radon, external γ or dose from other radionuclides in a cohort of male German uranium miners (Kreuzer et al. 2006; Table 1); similar results were reported in a reanalysis of this cohort that added five more years of follow-up (1999–2003) (Kreuzer et al. 2009). There was also no trend with any measure of dose for ischaemic heart disease (Kreuzer et al. 2006), coronary heart disease or stroke (Kreuzer et al. 2009); mortality from acute myocardial infarction exhibited a borderline significant (2-sided p = 0.114) increasing trend with radon dose (Kreuzer et al. 2009), although the authors were inclined to treat this as spurious (Kreuzer et al. 2006). There was no significant trend of coronary heart disease mortality with radon dose in a cohort of Canadian fluorspar miners (Villeneuve et al. 2007).
Environmentally exposed groups
There was a decreasing trend in heart disease mortality with dose for males and females in the study of Talbott et al. (2003) of persons exposed as a result of the accident at the Three Mile Island nuclear power station. For females, the decreasing trend was significant. As with all studies of environmental exposure, exposure assessment in this study is problematic. An additional complication in relation to assessing cardiovascular endpoints is that stress would be expected to be associated with proximity to the plant, and therefore to dose; this confounding would be expected to potentially positive bias the ERR estimate. Given the very small estimated doses, and the possibility of bias, little weight should be attached to these results.
Summary of low-dose epidemiologic studies
Although the aggregate estimate of risk (see the “Metaanalysis of available epidemiological studies” section and Tables) in these studies is suggestive of a positive association, the obvious heterogeneity complicates any causal interpretation.
The variation in magnitudes of trends of circulatory disease with dose, which span at least two orders of magnitude (see Table 1), and the possibility of confounding and other sources of bias, mean that one cannot be sure that these statistical associations observed with radiation are causal in nature. The well-known independent risk factors for circulatory disease, such as cigarette smoking, diabetes, obesity, high blood pressure and high levels of blood low-density lipoprotein (LDL) (see “Discussion” section) were not available or not adjusted for in analyses of most of these study groups. This is likely to be particularly problematic in cohorts in which there was no adjustment for socioeconomic status (SES) in the analysis (all except Howe et al. 2004; Vrijheid et al. 2007; McGeoghegan et al. 2008; Muirhead et al. 2009); many of these risk factors, in particular obesity, shift work and cigarette smoking, are correlated with SES, and SES may well be associated with occupational radiation exposure.
It should be noted that in most of the studies, in particular those of the atomic bomb survivors and all occupational groups, given in Tables 1, 2, 3, 4 conventional ICRP radiation weighting factors for stochastic health effects were used, with the exception of the risks for the Mayak workers in relation to α-particle dose to the liver, for which a relative biological effectiveness (RBE) of 1 was used (Table 1). For this reason, risks are given as per Sv rather than per Gy; this may not be strictly correct, but in practice use of Gy rather than Sv would make little difference. The only cohort in which there is a substantial contribution from high linear energy transfer (LET) radiation is the Mayak workers (Azizova and Muirhead 2009). This cohort was originally analysed in relation both to external γ dose and to internal α-particle dose to the liver, although for all meta-analyses (Tables 2, 3, 4), we use only the risk estimates derived in relation to external γ dose.
Table 2.
Aggregate excess relative risks (per Sv) of circulatory disease in published low/moderate dose (<5 Sv) epidemiological datasets with estimated average radiation dose to the heart/brain and for which quantitative risk assessment is possible (using as endpoint mortality from circulatory disease unless otherwise indicated)(reproduced in part from Little et al. 2009a)
Analysis based on heart disease (males and females separately)
Analysis based on ischaemic heart disease and cerebrovascular disease morbidity, using external γ dose
Analysis including underlying and contributory causes of death
Analysis based on stroke and other circulatory disease (separately)
Analysis based on heart disease and stroke (separately)
Analysis based on morbidity from hypertension, hypertensive heart disease, ischaemic heart disease and stroke (separately)
Analysis based on coronary heart disease and other heart disease, excluding highest dose group (3.1–7.6 Gy) (separately)
p value for heterogeneity p < 0.01
p value for heterogeneity p < 0.00000001
Table 3.
Sensitivity of combined circulatory disease risk estimates to study exclusion (excess relative risk (ERR)/Sv plus 95% CI) [all assuming as baseline inclusion of Muirhead et al. (2009) and exclusion of McGeoghegan et al. (2008) (as per bottom row of Table 2)] and contribution to heterogeneity χ2 statistic (+degrees of freedom, df)
| Studies excluded | ERR /Sv (95% CI) | χ2statistic (df) |
|---|---|---|
| A-bomb (Preston et al. 2003; Yamada et al. 2004) | 0.11 (0.07, 0.14)## | 8.64 (6) |
| US peptic ulcer (Carr et al. 2005) | 0.08 (0.06, 0.11)## | 2.14 (2) |
| UK ankylosing spondylitis (Darby et al. 1987) | 0.09 (0.06, 0.11)## | 5.99 (2) |
| Massachusetts TB (Davis et al. 1989) | 0.10 (0.07, 0.13)## | 15.82 (1) |
| IARC 15-country workers (Vrijheid et al. 2007) | 0.08 (0.05, 0.11)## | 0.00 (1) |
| UK 3rd NRRW analysis (Muirhead et al. 2009) | 0.08 (0.05, 0.11)## | 1.32 (1) |
| German uranium miners (Kreuzer et al. 2006) | 0.08 (0.06, 0.11)## | 4.67 (1) |
| Chernobyl recovery workers (Ivanov et al. 2006) | 0.08 (0.05, 0.11)## | 0.84 (1) |
| Mayak workers (Azizova and Muirhead 2009) | 0.04 (0.01, 0.07)# | 53.73 (2) |
| Three Mile Island (Talbott et al. 2003) | 0.08 (0.05, 0.11)## | 11.62 (2) |
| None (All studies) | 0.08 (0.05, 0.11)## | 104.76 (18) |
p value for heterogeneity p < 0.001
p value for heterogeneity p < 0.00000001
Table 4.
Aggregate excess relative risks (per Sv) of circulatory disease by endpoint (heart vs. stroke, morbidity vs. mortality)
Analysis based on all circulatory disease mortality apart from stroke
Analysis based on all heart disease mortality
Analysis based on morbidity from hypertensive heart disease, ischaemic heart disease
Analysis based on coronary heart disease and other heart disease mortality, excluding highest dose group (3.1–7.6 Gy)
Analysis based on morbidity from hypertension, ischaemic heart disease and other heart disease
Analysis based on morbidity from ischaemic heart disease
Analysis based on stroke mortality
Analysis based on stroke morbidity
Analysis based on all circulatory disease mortality
Analysis based on hypertension morbidity
Analysis based on all circulatory disease morbidity
Meta-analysis of available epidemiological studies
In the present study, an aggregate estimate of ERR is calculated using standard statistical methodology. For those studies for which an ERR estimate together with a measure of standard deviation was available, the best linear unbiased estimate (inverse-variance weighted) of ERR was computed, given by:
| (1) |
This has standard deviation given by:
| (2) |
These formulae were used to compute aggregate measures of ERR and associated 95% confidence intervals (obtained as ERRtot ± 1.96 × sd(ERRtot)) in Tables 2, 3, 4. It should be noted that (2) is an exact estimate of the standard deviation, when the component standard deviations are known exactly. However, when the component distributions are very markedly non-normal (e.g., if they are markedly asymmetric), the resultant scaled linear sum (1) will also be non-normal (e.g., asymmetric) in general. However, as can be seen from Table 1, most estimates of ERR have approximately symmetric confidence intervals about the mean, so it is expected that the scaled sum (1) will also be approximately symmetric about its mean. The standard deviations in the individual studies are estimated from the confidence intervals given in the published papers—for example, when 95% CI are given, this is calculated from [ERR97.5 – ERR2.5]/(2 × 1.96). In many cases, these confidence intervals appear to be derived via likelihood-based methods (McCullagh and Nelder 1989), and this introduces additional approximation. We apply this formula to a subset of the studies in Table 1, selected so as to be more or less disjoint. For example, we did not include the studies of Ashmore et al. (1998) and Richardson and Wing (1999), since these were largely subsumed in the IARC 15-country study of Vrijheid et al. (2007), and likewise we did not include the study of McGeoghegan et al. (2008) whenever we included the study of Muirhead et al. (2009). However, the study of McGeoghegan et al. (2008) although partly subsumed in the study of Muirhead et al. (2009) included four more years of follow-up (2002–2005), so we chose to consider it instead of the study of Muirhead et al. (2009) at least for the purposes of certain analyses presented in Table 2. Heterogeneity was assessed via the standard χ2 statistic, which was calculated via:
| (3) |
the significance of which was assessed via comparison against centiles of the χ2 distribution with the relevant number of degrees of freedom (number of component risk estimates −1).
The results of Table 2 suggest that the aggregate estimate of ERR from all low-dose studies excluding the study of McGeoghegan et al. (2008) but including the study of Muirhead et al. (2009) is 0.08 Sv−1 (95% CI 0.05, 0.11); almost no difference is made by instead including the study of McGeoghegan et al. (2008) but excluding the study of Muirhead et al. (2009)—the aggregate estimate of ERR is 0.08 Sv−1 (95% CI 0.06, 0.11). There is significant heterogeneity (p < 0.01) in risk between studies, among all groups considered in Table 2. Further analysis in which each study is removed in turn from the “All studies excluding McGeoghegan et al.” group in Table 3 does not substantially alter the aggregate risk estimate, which increased to at most 0.11 Sv−1 (0.07, 0.14) (after exclusion of the Japanese atomic bomb survivor data of Preston et al. 2003 and Yamada et al. 2004), although removing the Mayak worker study of Azizova and Muirhead (2009) results in a substantial decrease in the best estimate of ERR, to 0.04 Sv−1 (0.01, 0.07). The results of Table 4 suggest that the heterogeneity between studies is not much diminished when the different endpoints (heart disease, stroke) are considered. However, the ERR for stroke is substantially higher 0.27 Sv−1 (0.20, 0.34) than that for heart disease, 0.07 Sv−1 (0.04, 0.11). The morbidity risk of circulatory disease appears to be somewhat higher, 0.10 Sv−1 (0.07, 0.13) than that for mortality, 0.03 Sv−1 (−0.02, 0.08).
Adjustments to dose and implications for heterogeneity in cardiovascular risk in the atomic bomb survivor and moderate-dose radiotherapy studies
Schultz-Hector and Trott (2007) imply that by adjusting doses used in certain of the medical irradiation studies that they considered, risks in these studies would become more compatible with those in the LSS. In this section, we briefly explore this question.
Dose adjustments
We calculate for the study of Carr et al. (2005) (one of those cited in Fig. 1 of Schultz-Hector and Trott 2007) the physical single acute absorbed dose D′ that would have equivalent biological effect to n equal fractions (each of D/n Gy) with total absorbed dose D Gy, assuming a total number of n = 16.5Gy/1.5Gy = 11 (see p. 843 of Carr et al. 2005), for the range of total whole heart doses D given in Table 2 of Carr et al. (2005). Additionally, a linear quadratic dose-response function f (D) = C + αD + βD2 was assumed.
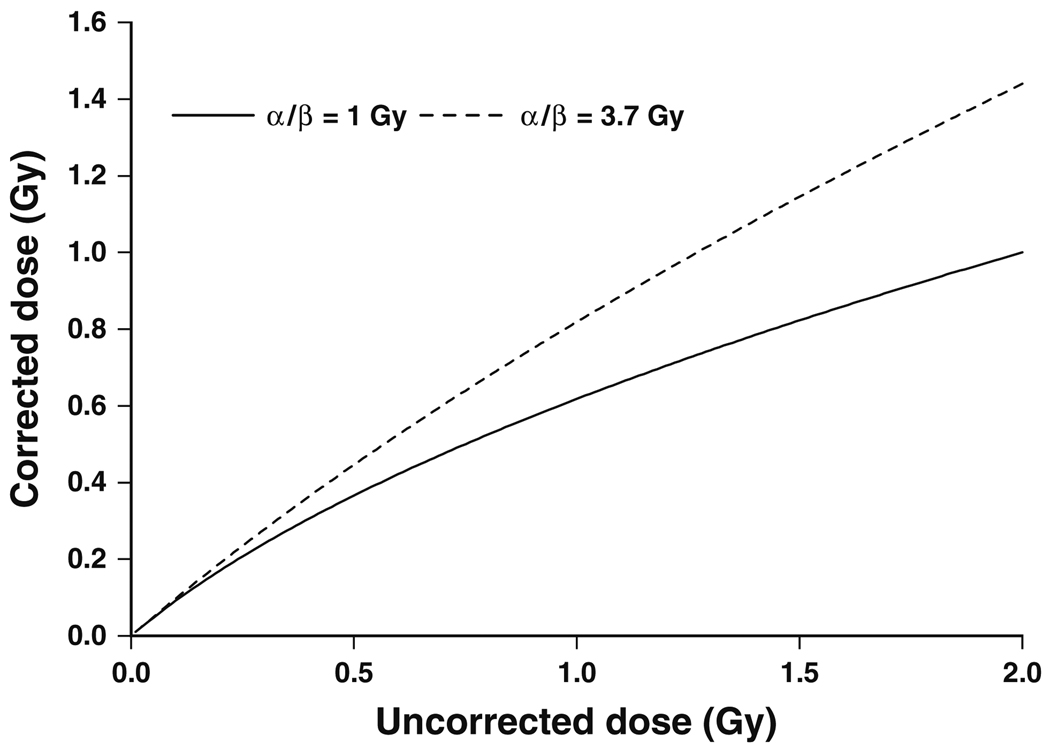
Fig. 1.
Corrected dose evaluated using expression (6) versus uncorrected dose for a variety of linear:quadratic (α/β) dose-response coefficient ratios
In this case D′ is determined by:
| (4) |
in other words by:
| (5) |
We give in Table 5 values of D′ for the range of average whole heart doses D given in Table 2 of Carr et al. (2005), assuming an α/β ratio of either 1 (Lauk et al. 1987) or 3.7 Gy (Schultz-Hector et al. 1992); these two somewhat divergent references were cited by Schultz-Hector and Trott (2007) in support of a range of α/β of between 1 and 3 Gy. Schultz-Hector and Trott (2007) used an α/β of 2 Gy in their Fig. 1.
Table 5.
The physical single absorbed dose D′ that would have equivalent biological effect to n fractions with total absorbed dose D for the whole heart doses given in Table 2 of Carr et al. (2005), based on expressions (4)–(5)
| Data description | Total dose (D) (Gy) |
α/βRatio | Number of fractions (n) |
Biologically equivalent single dose (D′) (Gy) |
|---|---|---|---|---|
| Using Lauk et al. (1987) α/β | 1.6 | 1.0 | 11 | 0.99 |
| 2.3 | 1.0 | 11 | 1.33 | |
| 2.8 | 1.0 | 11 | 1.56 | |
| 3.9 | 1.0 | 11 | 2.08 | |
| Using Schultz-Hector et al. (1992) α/β | 1.6 | 3.7 | 11 | 1.25 |
| 2.3 | 3.7 | 11 | 1.70 | |
| 2.8 | 3.7 | 11 | 1.99 | |
| 3.9 | 3.7 | 11 | 2.60 |
It should be noted that in the limit of a large number of fractions (n → ∞) formula (5) tends to:
| (6) |
which of course in the low-dose limit (D → 0) becomes D′ = D. We plot in Fig. 1, this function for the two values of α/β given earlier. Even at relatively high doses, of about 1 Gy, and assuming a low α/β of 1 Gy, the correction (6) implies a reduction of no more than 40%.
Implications for cardiovascular risk in certain medical studies
In the present study, the excess relative risks for various low-dose medical studies given in the systematic reviews of Little et al. (2008, 2009a) was estimated, with and without the acute dose corrections provided by (5) and (6). To give an upper bound on the magnitude of the correction, the smaller of the two α/β ratios employed above of 1 Gy (Lauk et al. 1987) was used. However, the central adjustment employed by Schultz-Hector and Trott (2007), an α/β ratio of 2 Gy, was also used. For the peptic ulcer study (Carr et al. 2005), we assumed 11 fractions were given, as derived previously. For the UK ankylosing spondylitis study, an estimated 8 fractions (4 Gy/0.5 Gy) derived from Trott and Kamprad (1999) was assumed, as well as the large fraction approximation, given by (6), for the study of Davis et al. (1989). The methodology used to estimate aggregate risk and heterogeneity was as described previously. As can be seen from Table 6, not much difference is made to the aggregate risk (medical + A-bomb) by use of this correction—the ERR changes from 0.03 Sv−1 (95% CI 0.00, 0.07) without correction to 0.04 Sv−1 (95% CI 0.01, 0.08) with an α/β ratio of 2 Gy, to 0.05 Sv−1 (95% CI 0.01, 0.08) with an α/β ratio of 1 Gy; in all three cases, there is statistically significant heterogeneity, p < 0.015, although the significance of the heterogeneity is reduced when corrections are made.
Table 6.
Excess relative risks (per Sv) of circulatory disease in published low/moderate dose (<5 Sv) A-bomb and medical datasets with estimated average radiation dose to the heart/brain and for which quantitative risk assessment is possible (reproduced in part from Little et al. 2008, 2009a)
| Data | References | Average heart/brain dose (range) (Sv) |
Endpoint (mortality unless otherwise indicated) |
Unadjusted excess relative risk Sv−1 (and 95% CI) |
Acute adjusted excess relative risk Sv−1 (and 95% CI)(α/β = 2 Gy) |
Acute adjusted excess relative risk Sv−1 (and 95% CI)(α/β = 1 Gy) |
|---|---|---|---|---|---|---|
| Japanese atomic bomb survivors | ||||||
| Mortality | Preston et al. (2003) | 0.1 (0–4)a | Heart disease, 1968–1997 (ICD9 390–429) | 0.17 (0.08, 0.26)a,b | – | – |
| Stroke, 1968–1997 (ICD9 430–438) | 0.12 (0.02, 0.22)a,b | – | – | |||
| Morbidity | Yamada et al. (2004) | 0.1 (0–4)c | Hypertension incidence, 1958–1998 (ICD9 401) | 0.05 (−0.01, 0.10)c | – | – |
| Hypertensive heart disease incidence, 1958–1998 (ICD9 402, 404) | −0.01 (−0.09, 0.09)c | – | – | |||
| Ischaemic heart disease incidence, 1958–1998 (ICD9 410–414) | 0.05 (−0.05, 0.16)c | – | – | |||
| Myocardial infarction incidence, 1964–1998 (ICD9 410) | 0.12 (−0.16, 0.60)c | – | – | |||
| Stroke incidence, 1958–1998 (ICD9 430, 431, 433, 434, 436) | 0.07 (−0.08, 0.24)c | – | – | |||
| Low-dose radiotherapy and medical diagnostic studies | ||||||
| Peptic ulcer study | Carr et al. (2005) | 1.3 (0.0–7.6) | Coronary heart disease (ICD8 410–414)d | 0.10 (−0.12, 0.33) | 0.15 (−0.21, 0.51) | 0.17 (−0.24, 0.58) |
| Other heart disease (ICD8 400–404, 420–429)d | −0.16 (−0.49, 0.17) | −0.25 (−0.76, 0.26) | −0.28 (−0.86, 0.30) | |||
| Ankylosing spondylitis | Darby et al. (1987) | 0.14 (0.0–4.80)e | Stroke (ICD7 430–434) | −2.43 (−4.29, 0.71)e | −2.57 (−4.53, 0.75)e | −2.68 (−4.74, 0.79)e |
| 2.49 (0.0–17.28)f | Other circulatory disease (ICD7 400–429, 435–468) | −0.01 (−0.12, 0.13)f | −0.02 (−0.18, 0.20)f | −0.02 (−0.20, 0.22)f | ||
| TB fluoroscopy | Davis et al. (1989) | 0.84g (NA) | All circulatory disease (ICD8 390–458) | −0.11 (−0.20, −0.01)g | −0.14 (−0.26, −0.01)g | −0.17 (−0.31, −0.02)g |
| Aggregate | 0.03 (0.00, 0.07) | 0.04 (0.01, 0.08) | 0.05 (0.01, 0.08) | |||
| p value for heterogeneity | p = 0.00632 | p = 0.01091 | p = 0.01364 |
Analysis based on colon dose
90% CI
Analysis based on stomach dose, derived from Table 2 of Yamada et al. (2004) with smoking and drinking in the stratification
Analysis excluding highest dose group (3.1–7.6 Gy)
Based on brain dose
Based on heart dose
Based on lung dose
In summary, using a reasonable range of adjustments similar to those of Schultz-Hector and Trott (2007) we can reduce, but not eliminate, the heterogeneity in risk between the A-bomb survivors and the medical irradiation studies.
Discussion
We have documented statistically significant heterogeneity of risks between epidemiological studies generally exposed to low and moderate doses (generally <5 Gy). The only possible exception to this inclusion criteria is the Mayak worker study (Azizova and Muirhead 2009)—the internal α-particle dose to certain tissues (e.g., liver) for some workers is considerably more than 5 Gy, although the external gamma dose is generally less than this figure (Table 1). As documented in Little et al. (2008), there are good radiobiological reasons for considering the moderate-and low-dose studies separately, since the mechanisms that operate for doses in this range are likely to be very different to those that are relevant at higher (e.g., radiotherapeutic) doses.
Given the heterogeneity in the populations and the multi-factorial disease endpoint being considered this finding is perhaps not surprising. Epidemiological research has identified specific risk factors, which include male sex, family history of heart disease, cigarette smoking, diabetes, high blood pressure, obesity, increased total and LDL cholesterol and decreased high-density lipoprotein cholesterol plasma levels (Wilson et al. 1998; Burns 2003; Stamler et al. 2005). SES and lifestyle factors (Tüchsen et al. 2006) and infections (Ridker 1998; Whincup et al. 2000; Danesh et al. 2002) are also potential risk factors for this disease independently of these other factors. Given the nature of the information contained in the published reports, none of these variables can be corrected for in the analysis conducted here; indeed there are few individual studies that adjusted for (at least some of) them—only those of Yamada et al. (2004) and Azizova and Muirhead (2009). SES may be a surrogate for some of these factors and was adjusted for in the analysis of Howe et al. (2004), Vrijheid et al. (2007), McGeoghegan et al. (2008) and Muirhead et al. (2009). Another factor that is likely to introduce heterogeneity is the diversity of endpoints. The analysis of Table 4 suggests that stroke may have a substantially larger risk than diseases specifically of the heart. Likewise, the risk in those studies that assessed circulatory disease morbidity (Yamada et al. 2004; Ivanov et al. 2006; Azizova and Muirhead 2009) was rather greater than in those that considered mortality (all other studies) (Table 4). The interactions of the previously discussed risk factors with possible radiation effects are unknown, but a role for confounding or effect modification cannot be ruled out in those studies in which no adjustment was made.
An important consideration in estimating dose to the intima, and which may have a bearing on interpretation of certain epidemiological studies, is the role of oxygen diffusion. This has been modelled by Richardson (2008a, b, c), who has highlighted the pronounced variations with oxygen concentration across the intima, which also varies with age as a result of modifications in arterial geometry (Richardson 2008b). It is well known that with decreasing oxygenation the effective dose reduces (Richardson 2008c), and this implies that the biologically effective dose per unit exposure reduces by 8–12% from age 0.5 to 70 years, whether for high-LET (222Rn, 218Po, 214Po) or for low-LET radiation (Richardson 2008c). This needs to be addressed in the dosimetry of any study, assuming that, as we argue elsewhere (Little et al. 2009b), intimal dose is of the most relevance to cardiovascular risk. Not doing so would imply a modest negative bias in modifications of the radiation response by age at exposure. Other dosimetric matters, in particular in relation to use of Sv versus Gy in the various studies were touched on in the earlier review (Little et al. 2008).
Our findings of heterogeneity of risk should be contrasted with those of Schultz-Hector and Trott (2007), who also assessed heterogeneity between studies, and who implied that there was no significant heterogeneity of doses and risks between the studies they analysed, although there was no formal statistical evaluation—the only analysis they undertook was a graphical one, using a method very close to our own (Table 6) for adjusting for dose fractionation. Schultz-Hector and Trott (2007) included two studies (Clarke et al. 2005; Darby et al. 2005) in which individual doses have not been properly evaluated, but which in any case are likely to be well above our 5 Gy exclusion limit. Schultz-Hector and Trott (2007) also did not analyse any of the occupational cohorts that we consider, nor some of the moderate- and low-dose medical studies (Darby et al. 1987; Davis et al. 1989). Given the difference in studies considered, and the fact that Schultz-Hector and Trott (2007) did not formally evaluate heterogeneity, there is not necessarily inconsistency between their findings and our own.
Based on the present state of experimental research into radiation-induced cardiovascular disease, it could be concluded that at least at high doses, radiation may cause both types of cardiovascular disease: microvascular disease, which is characterized by decrease in capillary density causing chronic ischaemic heart disease and focal myocardial degeneration; and macrovascular disease through the faster development of age-related atherosclerosis in the coronary arteries. Both macrovascular and microvascular radiation effects involve the endothelium and pro-inflammatory signalling cascades. A substantial number of studies have investigated radiation effects on endothelial cells in vitro, and a few studies have confirmed some of these observations in vivo (summarized in Schultz-Hector and Trott 2007). Whether both mechanisms, in particular microvascular damage, operate at low radiation doses is unclear.
Inflammation is believed to participate in virtually all stages of atherosclerotic disease including its inception. Epidemiological evidence for the role of inflammation in causing cardiovascular disease has come from findings that elevated levels of systemic inflammation, reflected in the increased levels of the pro-inflammatory cytokine interleukin 6 (IL-6), C-reactive protein (CRP), and a variety of cell-adhesion molecules, such as intercellular cell adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and endothelial leukocyte adhesion molecule 1 (ELAM-1; E-selectin), were associated with elevated risk of cardiovascular disease in a number of prospectively examined cohorts (Ridker et al. 2000a, b; Pai et al. 2004; Tzoulaki et al. 2005; Vasan 2006).
While the inflammatory process is recognized as an integral part of the atherosclerotic process (Hansson 2005), it does not explain the observation that the proliferation of vascular smooth muscle cells (VSMC) during atherosclerotic plaque development is monoclonal (Benditt and Benditt 1973). Clonality suggests that plaque VSMCs must have undergone multiple rounds of division, and telomere loss studies argue that this is between 7 and 13 cumulative population doublings (Matthews et al. 2006). These observations raised the possibility that spontaneous plaques (or at least their fibrous caps) arise from a subset of VSMCs that have a proliferative/survival advantage over the rest of the medial cells. This could be either a dominant transforming mutation in a single cell akin to tumorigenesis, or a more subtle mutation that alters a signalling pathway for cell division/growth arrest. Indeed, McCaffrey et al. (1997) found mutations in a microsatellite sequence in the Type II TGF-β1 receptor in plaque-derived cells, leading to a premature truncation that would cause loss of normal growth inhibition by TGF-β1. Although such mutations could indicate a mechanism by which irradiation-induced cell transformation might promote atherosclerosis, other studies have not supported these findings, particularly in regard to TGF-β1 microsatellite instability (Bobik et al. 1999; Clark et al. 2001). Furthermore, clonality itself is not synonymous with transformation of a single cell, and subsequent studies have shown that large patches in the normal vessel media are monoclonal (Chung et al. 1998). Thus, clonality is more likely to be explained by the presence of developmental clones in the normal vessel wall, rather than a mutation. Finally, in contrast to tumours, plaque VSMCs show poor proliferation, enhanced apoptosis and early senescence (Schwartz and Murry 1998). These features would not confer a proliferative or survival advantage to plaque VSMCs. Furthermore, plaque VSMC proliferation is now seen to be beneficial in atherosclerosis (Braganza and Bennett 2001), and related to this, VSMC apoptosis accelerates atherosclerotic lesion development (Clarke et al. 2008), so that the pathological consequences of a mutation promoting VSMC proliferation are unclear.
When examining possible mechanisms for the association between ionizing radiation and circulatory disease, it is important to recognize the manner in which radiation-induced cellular and molecular responses can influence the pathogenic process. At high doses (>10 Gy), there is abundant evidence from animal studies and RT patients of direct damage to the structures of the heart and large arteries resulting in predominantly early-appearing acute cardiovascular effects (e.g., acute and chronic pericarditis, accelerated atherosclerosis, conduction abnormalities, pericardial or myocardial fibrosis; Adams et al. 2003). Such effects are predominantly the consequence of microvascular injury, resulting in part from excessive cell killing (e.g., of capillaries) and the associated pro-inflammatory response to cellular damage, and leading to myocardial and other ischaemia (Adams et al. 2003). Survivors of high-dose exposures may also suffer long-term tissue damage, such as valvular changes and late pericarditis, giving rise to a variety of adverse cardiovascular effects. While studies of cellular and molecular responses following high doses may give some indications as to how low-dose radiation can influence the development of cardiac disease, it is important to evaluate these in relation to dose, dose rate and dose response. These have been reviewed in an earlier publication (Little et al. 2008).
A major challenge is the explanation of how chronically delivered radiation dose in the manner frequently encountered in occupational studies could lead to excess circulatory disease risk (Little et al. 2008). Little et al. (2009b) constructed a reaction-diffusion model of the cardiovascular system and showed that a predicted consequence of multiple small radiation doses was to cause mean chemoattractant (MCP-1) concentration to increase linearly with cumulative dose. The main driver for the increase in MCP-1 was monocyte death, and consequent reduction in MCP-1 degradation (Little et al. 2009b). The radiation-induced risks predicted by the model were quantitatively consistent with those observed in a number of occupationally exposed groups. The changes in equilibrium MCP-1 concentrations with LDL cholesterol concentration were also consistent with experimental and epidemiologic data (Little et al. 2009b). The model made a number of testable predictions, and work is underway to assess these.
Conclusions
There is no doubt that the high radiation doses to the heart, coronary, carotid and other large arteries received during certain RT procedures induce tissue damage that results in an increased risk of circulatory diseases; the underlying biological mechanism is the high level of cell killing and ensuing pro-inflammatory effects and micro-vascular damage (Schultz-Hector and Trott 2007). The central question is whether moderate and low doses can elevate the risk of these diseases, as indicated by the findings of some epidemiological studies, via a different mechanism from high-dose effects (Little et al. 2008). The epidemiological evidence for an effect of moderate and low doses remains suggestive rather than persuasive. A critical issue is the inter-study heterogeneity, although this may be in large part a result of confounding and effect modification resulting from well-known (but unobserved) risk factors. A recent paper of Little et al. (2009b) suggested a possible mechanism for fractionated low-dose effects, based on monocyte cell killing in the intima, but this has yet to be experimentally tested. As our knowledge concerning the cellular response at low doses and low-dose rates is still very limited, further research is required to better understand the nature of low/moderate dose epidemiological associations—whether they represent a direct causal relationship based upon yet to be understood biological mechanisms, or whether some other explanation is required.
Acknowledgments
The authors are grateful for the detailed and helpful comments of the editor and two referees. This work was funded partially by the European Commission under contracts FI6R-CT-2003-508842 (RISC-RAD) and FP6-036465 (NOTE). The Mayak worker analysis by Drs. Azizova and Muirhead was conducted with support from the European Commission’s Euratom Nuclear Fission and Radiation Protection Programme as part of the SOUL project; more details of this analysis can be found in separate papers by the study investigators.
Footnotes
This paper is based on a presentation given at the International Conference on Late Health Effects of Ionizing Radiation, 4–6 May 2009, Georgetown University, Washington DC, USA.
Contributor Information
M. P. Little, Department of Epidemiology and Public Health, Imperial College Faculty of Medicine, Norfolk Place, London W2 1PG, UK mark.little@imperial.ac.uk
E. J. Tawn, Westlakes Research Institute, University of Central Lancashire, Westlakes Science Park, Moor Row, Cumbria CA24 3JY, UK
I. Tzoulaki, Department of Epidemiology and Public Health, Imperial College Faculty of Medicine, Norfolk Place, London W2 1PG, UK
R. Wakeford, Dalton Nuclear Institute, University of Manchester, Pariser Building, Sackville Street, PO Box 88, Manchester M60 1QD, UK
G. Hildebrandt, Department of Radiotherapy and Radiation Oncology, University of Rostock, Suedring 75, 18059 Rostock, Germany
F. Paris, INSERM U 601, Department of Cancer Research, University of Nantes, 9 Quai Moncousu, 44093 Nantes Cedex 01, France
S. Tapio, Radiation Proteomics, Institute of Radiation Biology (ISB), Helmholtz Zentrum München, German Research Centre for Environmental Health, Ingolstädter Landstrasse 1, 85764 Oberschleissheim, Germany
P. Elliott, Department of Epidemiology and Public Health, Imperial College Faculty of Medicine, Norfolk Place, London W2 1PG, UK
References
- Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Critical Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- Ashmore JP, Krewski D, Zielinski JM, Jiang H, Semenciw R, Band PR. First analysis of mortality and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol. 1998;148:564–574. doi: 10.1093/oxfordjournals.aje.a009682. [DOI] [PubMed] [Google Scholar]
- Atkinson WD, Law DV, Bromley KJ, Inskip HM. Mortality of employees of the United Kingdom Atomic Energy Authority, 1946–97. Occup Environ Med. 2004;61:577–585. doi: 10.1136/oem.2003.012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizova TV, Muirhead CR. Epidemiological evidence for circulatory diseases—occupational exposure. EU Scientific Seminar 2008. “Emerging evidence for radiation induced circulatory diseases”. Proceedings of a scientific seminar held in Luxembourg on 25 November 2008. Radiation Protection. 2009;158:33–46. (downloadable from http://ec.europa.eu/energy/nuclear/radiation_protection/doc/publication/158.pdf) [Google Scholar]
- Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci USA. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik A, Agrotis A, Kanellakis P, Dilley R, Krushinsky A, Smirnov V, Tararak E, Condron M, Kostolias G. Distinct patterns of transforming growth factor-β isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-β in fibrofatty lesion development. Circulation. 1999;99:2883–2891. doi: 10.1161/01.cir.99.22.2883. [DOI] [PubMed] [Google Scholar]
- Braganza DM, Bennett MR. New insights into atherosclerotic plaque rupture. Postgrad Med J. 2001;77:94–98. doi: 10.1136/pmj.77.904.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Disease. 2003;46:11–29. doi: 10.1016/s0033-0620(03)00079-3. [DOI] [PubMed] [Google Scholar]
- Carr ZA, Land CE, Kleinerman RA, Weinstock RW, Stovall M, Griem ML, Mabuchi K. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- Chung I-M, Schwartz SM, Murry CE. Clonal architecture of normal and atherosclerotic aorta. Implications for atherogenesis and vascular development. Am J Pathol. 1998;152:913–923. [PMC free article] [PubMed] [Google Scholar]
- Clark KJ, Cary NR, Grace AA, Metcalfe JC. Microsatellite mutation of type II transforming growth factor-β receptor is rare in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:555–559. doi: 10.1161/01.atv.21.4.555. [DOI] [PubMed] [Google Scholar]
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Lewington S, Walker M, Lennon L, Thomson A, Wong Y-K, Zhou X, Ward M. Chlamydia pneumoniae IgA titres and coronary heart disease. Prospective study and meta-analysis. Eur Heart J. 2002;23:371–375. doi: 10.1053/euhj.2001.2801. [DOI] [PubMed] [Google Scholar]
- Darby SC, Doll R, Gill SK, Smith PG. Long term mortality after a single treatment course with X-rays in patients treated for ankylosing spondylitis. Br J Cancer. 1987;55:179–190. doi: 10.1038/bjc.1987.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby S, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- Davis FG, Boice JD, Jr, Hrubec Z, Monson RR. Cancer mortality in a radiation—exposed cohort of Massachusetts tuberculosis patients. Cancer Res. 1989;49:6130–6136. [PubMed] [Google Scholar]
- dos Santos Silva I, Malveiro F, Jones ME, Swerdlow AJ. Mortality after radiological investigation with radioactive Thorotrast: a follow-up study of up to fifty years in Portugal. Radiat Res. 2003;159:521–534. doi: 10.1667/0033-7587(2003)159[0521:mariwr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Howe GR, Zablotska LB, Fix JJ, Egel J, Buchanan J. Analysis of the mortality experience amongst U.S. nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res. 2004;162:517–526. doi: 10.1667/rr3258. [DOI] [PubMed] [Google Scholar]
- Ivanov VK, Maksioutov MA, Chekin SY, Petrov AV, Biryukov AP, Kruglova ZG, Matyash VA, Tsyb AF, Manton KG, Kravchenko JS. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- Johnson P, Atkinson WD, Nicholls JL. Updated analysis of mortality in workers at UK atomic weapons establishments. Proceedings of the SRP Sixth International Symposium: Achievements & challenges: advancing radiation protection into the 21st century.1999. [Google Scholar]
- Kreuzer M, Kreisheimer M, Kandel M, Schnelzer M, Tschense A, Grosche B. Mortality from cardiovascular diseases in the German uranium miners cohort study, 1946–1998. Radiat Environ Biophys. 2006;45:159–166. doi: 10.1007/s00411-006-0056-1. [DOI] [PubMed] [Google Scholar]
- Kreuzer M, Grosche B, Schnelzer M, Tschense A, Dufey F, Walsh L. Radon and risk of death from cancer and cardiovascular diseases in the German uranium miners cohort study—follow-up 1946–2003. Radiat Environ Biophys. 2009 doi: 10.1007/s00411-009-0249-5. [DOI] [PubMed] [Google Scholar]
- Lauk S, Rüth S, Trott K-R. The effects of dose-fractionation on radiation-induced heart disease in rats. Radiother Oncol. 1987;8:363–367. doi: 10.1016/s0167-8140(87)80187-1. [DOI] [PubMed] [Google Scholar]
- Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, Tapio S, Elliott P. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res. 2008;169:99–109. doi: 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Tapio S, Elliott P. Comments. The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005 (letter to the editor) Int J Epidemiol. 2009a;38:1159–1164. doi: 10.1093/ije/dyn122. [DOI] [PubMed] [Google Scholar]
- Little MP, Gola A, Tzoulaki I. A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose ionizing radiation exposure. PLoS Comput Biol. 2009b;5(10):e1000539. doi: 10.1371/journal.pcbi.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis. Effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Du BH, Consigli S, Szabo P, Bray PJ, Hartner L, Weksler BB, Sanborn TA, Bergman G, Bush HL. Genomic instability in the type II TGF-β1 receptor gene in atherosclerotic and restenotic vascular cells. J Clin Invest. 1997;100:2182–2188. doi: 10.1172/JCI119754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. 2nd edn. London: Chapman & Hall; 1989. [Google Scholar]
- McGale P, Darby SC. Low doses of ionizing radiation and circulatory diseases: a systematic review of the published epidemiological evidence. Radiat Res. 2005;163:247–257. doi: 10.1667/rr3314. 711. [DOI] [PubMed] [Google Scholar]
- McGeoghegan D, Binks K, Gillies M, Jones S, Whaley S. The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005. Int J Epidemiol. 2008;37:506–518. doi: 10.1093/ije/dyn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Muirhead CR, O’Hagan JA, Haylock RGE, Phillipson MA, Willcock T, Berridge GLC, Zhang W. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100:206–212. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- Richardson RB. Age-dependent changes in oxygen tension, radiation dose and sensitivity within normal and diseased coronary arteries—Part A: Dose from radon and thoron. Int J Radiat Biol. 2008a;84:838–848. doi: 10.1080/09553000802392748. [DOI] [PubMed] [Google Scholar]
- Richardson RB. Age-dependent changes in oxygen tension, radiation dose and sensitivity within normal and diseased coronary arteries—Part B: Modeling oxygen diffusion into vessel walls. Int J Radiat Biol. 2008b;84:849–857. doi: 10.1080/09553000802389645. [DOI] [PubMed] [Google Scholar]
- Richardson RB. Age-dependent changes in oxygen tension, radiation dose and sensitivity within normal and diseased coronary arteries—Part C: Oxygen effect and its implications on high- and low-LET dose. Int J Radiat Biol. 2008c;84:858–865. doi: 10.1080/09553000802389686. [DOI] [PubMed] [Google Scholar]
- Richardson DB, Wing S. Radiation and mortality of workers at Oak Ridge National Laboratory: positive associations for doses received at older ages. Environ Health Perspect. 1999;107:649–656. doi: 10.1289/ehp.99107649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. Inflammation, infection, and cardiovascular risk. How good is the clinical evidence? Circulation. 1998;97:1671–1674. doi: 10.1161/01.cir.97.17.1671. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000a;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000b;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Schultz-Hector S, Trott K-R. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- Schultz-Hector S, Sund M, Thames HD. Fractionation response and repair kinetics of radiation-induced heart failure in the rat. Radiother Oncol. 1992;23:33–40. doi: 10.1016/0167-8140(92)90303-c. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Murry CE. Proliferation and the monoclonal origins of atherosclerotic lesions. Annu Rev Med. 1998;49:437–460. doi: 10.1146/annurev.med.49.1.437. [DOI] [PubMed] [Google Scholar]
- Stamler J, Neaton JD, Garside DB, Daviglus ML. Current status: six established major risk factors—and low risk. In: Marmot M, Elliott P, editors. Coronary heart disease epidemiology: from aetiology to public health. 3rd edn. Oxford: Oxford University Press; 2005. pp. 32–70. [Google Scholar]
- Talbott EO, Youk AO, McHugh-Pemu KP, Zborowski JV. Long-term follow-up of the residents of the Three Mile Island accident area: 1979–1998. Environ Health Perspect. 2003;111:341–348. doi: 10.1289/ehp.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis LB, Land CE, Andersson M, Nyberg U, Goldman MB, Knudson Gaul L, Berger E, Storm HH, Hall P, Auvinen A, Janower ML, Holm L-E, Monson RR, Schottenfeld D, Boice JD., Jr Mortality after cerebral angiography with or without radioactive Thorotrast: an international cohort of 3,143 two-year survivors. Radiat Res. 2001;156:136–150. doi: 10.1667/0033-7587(2001)156[0136:macawo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Trott K-R, Kamprad F. Radiobiological mechanisms of anti-inflammatory radiotherapy. Radiother Oncol. 1999;51:197–203. doi: 10.1016/s0167-8140(99)00066-3. [DOI] [PubMed] [Google Scholar]
- Tüchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med. 2006;63:451–455. doi: 10.1136/oem.2006.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population. Edinburgh Artery Study. Circulation. 2005;112:976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) New York: United Nations; 2000. Sources and effects of ionizing radiation. UNSCEAR 2000 report to the general assembly, with scientific annexes. Volume II: Effects. [Google Scholar]
- Vasan RS. Biomarkers of cardiovascular disease. Molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Lane RSD, Morrison HI. Coronary heart disease mortality and radon exposure in the Newfoundland fluorspar miners’ cohort, 1950–2001. Radiat Environ Biophys. 2007;46:291–296. doi: 10.1007/s00411-007-0108-1. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Cardis E, Ashmore P, Auvinen A, Bae J-M, Engels H, Gilbert E, Gulis G, Habib RR, Howe G, Kurtinaitis J, Malker H, Muirhead CR, Richardson DB, Rodriguez-Artalejo F, Rogel A, Schubauer-Berigan M, Tardy H, Telle-Lamberton M, Usel M, Veress K. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol. 2007;36:1126–1135. doi: 10.1093/ije/dym138. [DOI] [PubMed] [Google Scholar]
- Wakeford R, Little MP. Epidemiological evidence for circulatory diseases—non-occupational exposure. EU Scientific Seminar 2008. “Emerging evidence for radiation induced circulatory diseases”. Proceedings of a scientific seminar held in Luxembourg on 25 November 2008. Radiation Protection. 2009;158:21–31. (downloadable from http://ec.europa.eu/energy/nuclear/radiation_protection/doc/publication/158.pdf) [Google Scholar]
- Whincup P, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, Hawkey C, Atherton J. Prospective study of potentially virulent strains of Helicobacter pylori and coronary heart disease in middle-aged men. Circulation. 2000;101:1647–1652. doi: 10.1161/01.cir.101.14.1647. [DOI] [PubMed] [Google Scholar]
- Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wong FL, Yamada M, Sasaki H, Kodama K, Akiba S, Shimaoka K, Hosoda Y. Noncancer disease incidence in the atomic bomb survivors: 1958–1986. Radiat Res. 1993;135:418–430. [PubMed] [Google Scholar]
- Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2004;161:622–632. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]