Abstract
Objective
To investigate the effect of VEGF stimulation and the effect of blocking VEGF with its antagonist, sFlt1, on chondrogenesis using skeletal muscle-derived stem cells (MDSCs).
Methods
The direct effect of VEGF on the in vitro chondrogenic ability of mouse MDSCs was tested using a pellet culture system followed by quantitative real time PCR and histological analyses. Next, the effect of VEGF on chondrogenesis within the synovial joint was tested using genetically engineered MDSCs implanted into the rat osteochondral defect. In this model, MDSCs, transduced with a retroviral vector to express BMP4, were co-implanted with MDSCs transduced to express either VEGF or sFlt1 (a VEGF antagonist) to provide a gain- and loss-of VEGF function experimental design. Histological scoring was used to compare cartilage formation among the treatment groups.
Results
Hyaline-like cartilage matrix production was observed in both VEGF-treated and VEGF-blocked (sFlt1-treated) pellet cultures, but real-time PCR revealed that sFlt1 treatment improved the expression of chondrogenic genes in MDSCs that were stimulated to undergo chondrogenic differentiation with BMP4 and TGF-β3. In vivo testing of articular cartilage repair showed that VEGF-transduced MDSCs caused an arthritic change in the knee joint, and sFlt1 improved the MDSC-mediated repair of articular cartilage, compared to BMP4 alone.
Conclusion
sFlt1 gene therapy improved BMP4- and TGF-β3-induced chondrogenic gene expression of MDSCs in vitro, and improved the persistence of repaired articular cartilage by preventing vascularization and bone invasion into the repaired articular cartilage.
Introduction
Articular cartilage is an avascular tissue with a limited intrinsic capacity for regeneration. For this reason, tissue engineering techniques to repair articular cartilage have been extensively studied, and chondrocyte transplantation already has become a clinical reality (1–3).
For the repair of large cartilage defects caused by osteoarthritis or rheumatoid arthritis, stem cells are more attractive than primary chondrocytes because of their superior capacity for self renewal, proliferation, and resistance to stress (4–6). Several studies have suggested that stem-like cells can undergo chondrogenesis and repair articular cartilage in experimental cartilage injury models, including studies using muscle-derived stem cells (MDSCs) (7–11). Stem cells have recently been used clinically for cartilage repair (12, 13).
However, there are still problems surrounding the use of stem cells to repair cartilage. One of the most important issues is the control of VEGF signaling during the chondrogenic differentiation of stem cells. Previous research has shown that VEGF treatment prevents condensation of chondrogenic mesenchyme during early limb bud development through abnormal vascularization (14). The expression of high levels of VEGF in the terminal stages of chondrogenesis leads to endochondral ossification through angiogenesis (15, 16). Also, VEGF has been shown to enhance endochondral bone formation elicited by BMP-transduced MDSCs in an ectopic bone formation model (17). VEGF expression by chondrocytes in osteoarthritic joints may be related to cartilage destruction (18–24). Furthermore, high doses of VEGF may induce the onset and progression of arthritis (25–27). This theory is supported by studies showing that sFlt1 (a VEGF antagonist) treatment decreased the progression of arthritis in a mouse model (28, 29). Together, these results suggest that VEGF may be a catabolic molecule for cartilage.
Contrary to this interpretation, VEGF has been shown to be necessary for chondrocyte survival during development. In VEGF conditional knock-out mice, massive cell death was observed in the joint and epiphyseal regions of cartilage during development (30, 31).
Despite these paradoxical results, the effects of VEGF on stem cell-mediated chondrogenesis in vitro and cartilage repair in vivo have not been rigorously investigated. Our current study used a gain- and loss-of-function approach based on gene therapy techniques to ascertain the role of VEGF in stem cell-mediated cartilage repair. Muscle-derived stem cells (MDSCs) were genetically engineered to express human BMP4, human VEGF165, or the VEGF antagonist, sFlt1. These cells were used to test the effect of VEGF on MDSC-mediated cartilage repair in the knee joint in an in vivo osteochondral defect model. This study was designed to test the hypothesis that increased VEGF expression within a healing osteochondral defect will inhibit the persistence of repaired cartilage, and that blocking VEGF signaling using sFlt1 will improve the quality and persistence of MDSC-mediated cartilage regeneration. The effects of VEGF stimulation and blocking VEGF signaling with sFlt1 on chondrogenesis was studied, not only with respect to the stem cells’ intrinsic chondrogenic capacity, but also within the more complex in vivo environment that includes interactions with synovia, subchondral bone, and adjacent cartilage in the knee joint.
Materials and Methods
Isolation of primary MDSCs
MDSCs were isolated from the hind-limb skeletal muscle of 3-week-old male C57BL-10J mice, (Jackson Laboratory, Bar Harbor, ME) via a modified preplate technique that has been previously described by our laboratory (5).
Retroviral transduction
Retroviral vectors expressing human BMP4 (retro-BMP4), VEGF (retro-VEGF), or sFlt1 (retro-sFl-1) were generated by replacing the U3 in the 5’ LTR with the human cytomegalovirus promoter as previously described (8, 17). MDSCs were transduced separately with these retroviral vectors at an MOI of 5 in the presence of 8 µg/ml polybrene. The transduced cells were expanded for 2 weeks before being used in experiments and conditioned media was sampled to determine transgene expression.
The level of BMP4 secreted from the transduced cells was estimated with a BMP4 bioassay as previously described (32). The levels of VEGF or sFlt1 secreted from the transduced cells were measured using ELISA kits (R&D Systems Inc. Minneapolis, Minnesota, USA).
In vitro chondrogenesis - pellet culture
Pellet culture was done as described previously (33). Cell pellets were made with the following: 1) 1.4×105 of nontransduced MDSCs and 1.4×105 of BMP4-expressing MDSCs (BMP4 group); 2) 1.4×105 VEGF-expressing MDSCs and 1.4×105 BMP4-expressing MDSCs (BMP4+VEGF group); 3) 1.4×105 sFlt1-expressing MDSCs and 1.4×105 BMP4-expressing MDSCs (BMP4+sFlt1 group); 4) 2.8×105 primary chondrocytes derived from the mouse knee (Chond group); and three more groups (5, 6, and 7) were created using 2.8×105 nontransduced MDSCs. Pellets from Groups 1–4 were cultured in 0.5 ml of chondrogenic medium (CM) that contained DMEM supplemented with 1% penicillin/streptomycin, 10−7M dexamethasone, 50 µg/ml ascorbate-2-phosphate, 40 µg/ml proline, 100 µg/ml pyruvate, and 1% BD™ ITS+Premix (Becton-Dickinson; Franklin Lakes, NJ) with 10ng/ml of transforming growth factor-beta 3 (TGF-β3; R&D Systems). Pellets in Group 5 (made with nontransduced cells) were cultured in chondrogenic medium without the TGF-β supplement (C group). Group 6 pellets were fed with chondrogenic medium without TGF-β but with 50ng/ml BMP4 added (C+B4 group). Finally, Group 7 pellets were fed with chondrogenic medium, supplemented with 10ng/ml of TGF-β (C+T group). All pellets were incubated at 37°C in 5% CO2, and the medium was changed every 2 to 3 days. Pellets were harvested after 7, 14, and 28 days in culture.
Alcian blue staining
Pellets were fixed overnight in 10% neutral buffered formalin, dehydrated, embedded in paraffin, and sectioned in 5-µm-thick slices. Pellet sections were deparaffinized, placed in 3% acetic acid for 3 minutes, and transferred into Alcian blue solution for 30 minutes. The slides were then rinsed with running tap water for 10 minutes and counterstained with nuclear fast red.
Real-time PCR analysis of pellet cultured cells
mRNA was isolated using the RNeasy Plus Kit (Qiagen, Valencia, CA), according to the manufacturers instructions. After RNA extraction, quantitative real-time PCR (qPCR) analysis was carried out as described previously (34, 35). Gene expression levels were calculated based on the ΔCT method (separate tubes). All target genes were normalized to the reference housekeeping gene, 18S. 18S primers and probes were designed by and purchased from Applied Biosystems (Foster City, CA). Primers and probes were designed for type II collagen, sox9, and type X collagen according to genbank sequence (Supplemental material). All target gene primers and probes were purchased from Integrated DNA Technologies, Inc (Coralville, IA).
Each experimental value is reported as the mean of triplicate treatments ± SEM. For qPCR assays, the coefficient of variation (COV) was calculated from three assay replicates. For all treatment groups and target genes analyzed, the COV did not exceed 3%. One-way ANOVA followed by Tukey-Kramer’s post hoc test using Stat View software was performed to determine significance among treatment groups. P values less than 0.05 were regarded as statistically significant.
Repair of osteochondral defects
The policies and procedures of our animal laboratory are in accordance with those published by the U.S. Department of Health and Human Services. The research techniques used for these experiments were approved by the Animal Research and Care Committee at Children's Hospital of Pittsburgh. Twenty-eight, 10-week-old nude rats (NIH-Whn NIHRNU-M; Taconic, NY, USA) were used in this study. The animals were anesthetized via exposure to 3% isofluorane and O2 gas (1.5 liter/minute) delivered through an inhalation mask. The knee joint was exposed by medial parapatellar approach, and the trochlear groove was exposed by lateral dislocation of the patella. A 1.8 mm outer diameter trephine drill was used to create an osteochondral defect (1.8 × 2.0 mm) in the trochlear groove of each femur. Mouse skeletal muscle-derived stem cells were mixed with fibrin glue (Tisseel VH; Baxter Healthcare Co, Hyland Immuno, Glendale, CA) before transplantation. The animals were divided into 7 treatment groups, and the defects in each group were treated as follows: 1) Group 1 (no cell control) rats were treated with acellular fibrin glue; 2) Group 2 (MDSC group) rats were treated with 500,000 MDSCs embedded in fibrin glue; 3) Group 3 (VEGF group) rats were treated with 250,000 MDSC-VEGF cells plus 250,000 MDSCs embedded in fibrin glue; 4) Group 4 (sFlt1 group) rats were treated with 250,000 MDSC-sFlt1 cells plus 250,000 MDSCs embedded in fibrin glue; 5) Group 5 (B4 group) rats were treated with 250,000 MDSC-B4 cells plus 250,000 MDSCs embedded in fibrin glue; 6) Group 6 (B4+VEGF group) rats were treated with 250,000 MDSC-B4 cells plus 250,000 MDSC-VEGF cells embedded in fibrin glue; and, 7) Group 7 (B4+sFlt1 group) rats were treated with 250,000 MDSC-B4 cells plus 250,000 MDSC-sFlt1 cells embedded in fibrin glue. Four defects (two rats) were made for each group (n=4). The rats were allowed to move freely within their cages after surgery. Rats were euthanized 8 and 16 weeks after surgery. Groups including those receiving VEGF treatment were killed 8 weeks after surgery because the deterioration of the knee joint impaired the animal’s ability to move freely.
Histological evaluation of cartilage repair
After qualitative macroscopic examination, 4 distal femora per group per time point were dissected and fixed with 10% neutral buffered formalin for 48 hours, followed by decalcification with 10% EDTA for 2 weeks and paraffin embedding. Sagittal sections, 5 µm in thickness, were obtained from the center of each defect and stained with Safranin O-fast green. The histological grading scale described by Sellers (36) was used to evaluate the quality of the repaired tissue. All data were expressed as the mean ± SD. Differences of each category and total score were analyzed by Kruskal-Wallis and Mann-Whitney U tests using SPSS software (SPSS v12.0.1). P values less than 0.05 were regarded as significant.
Results
Expression of BMP4, VEGF, and sFlt1, from transduced MDSCs
To ensure that we had successfully transduced the cells and induced protein secretion, we tested the supernatant collected from transduced or nontransduced cells using either an activity bioassay (for BMP) or ELISA (for VEGF and sFlt1). MDSCs transduced with retro-BMP4 secreted 327 ng/106cells/24hours as detected by BMP4 bioassay. MDSCs transduced with retro-VEGF secreted human VEGF at an average level of 1.14 ± 0.05 µg/106cells/24hours, compared to the nontransduced MDSCs that secreted 750 ± 12.2 pg/106cells/24hours of mouse VEGF.. Retro-sFlt1 transduced MDSCs secreted sFlt1 at a level of 2.1 ± 0.004 ng/106cells/24hours.
In a separate set of experiments, 2.5×105 transduced or nontransduced cells were cultured in 3D micromass pellet culture for 48 hours, the medium was collected, and VEGF expression was tested using ELISA. It was observed that medium from nontransduced MDSC pellets contained 409.0 ± 49.4 ng/ml of VEGF which was significantly more than primary chondrocytes (226.7 ± 53.8 ng/ml) and significantly less than cells transduced to express BMP4 (B4 cells, 592.9 ± 52 ng/ml).
Neither VEGF nor sFtl1 adversely affect the chondrogenic differentiation of MDSCs
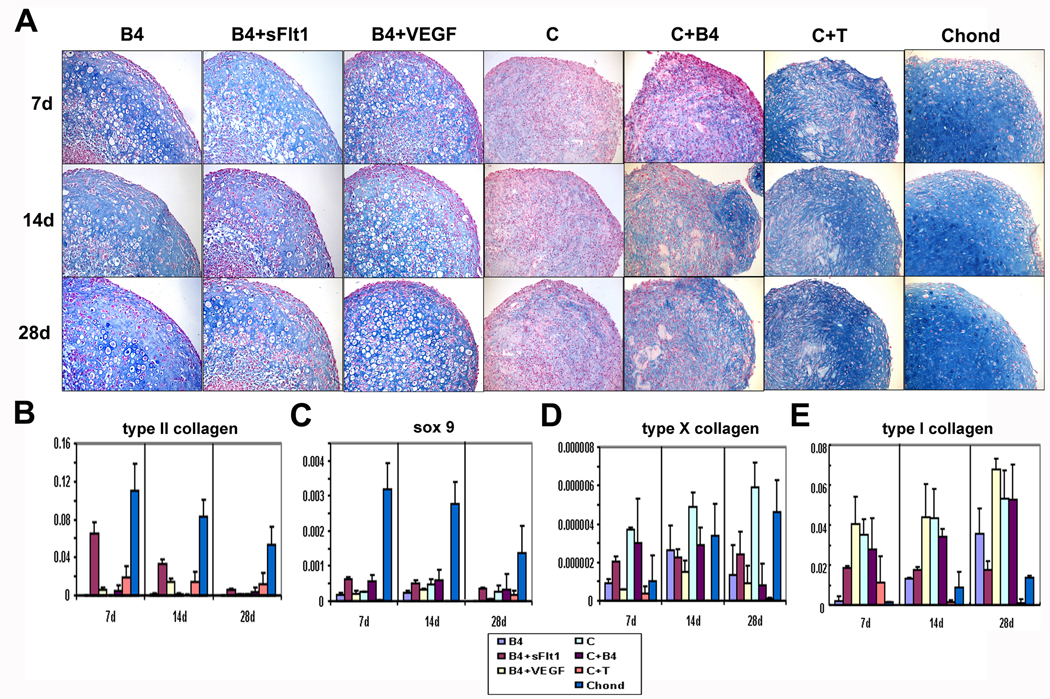
Histological analysis was used to test whether the different mixtures of transduced and nontransduced cells were capable of undergoing chondrogenic differentiation in vitro. Histological analysis of tissues stained with Alcian blue showed that all the pellets from every group contained some well-differentiated, round chondrocytic cells and showed some level of hyaline-like cartilage extracellular matrix production, evidenced by positive Alcian blue staining (Figure 1A). Nontransduced control MDSCs (C cells, Figure 1A) showed the least intense staining whereas primary chondrocytes (Chond, Figure 1A) showed the largest amount of Alcian blue-positive extracellular matrix. Among the treatment groups, the B4+VEGF pellets showed evidence of chondrocyte formation peripherally with a region of fibrous, non-cartilagenous tissue near the center of the pellet, especially after 28 days in culture (B4+VEGF, Figure 1A). Together, these results suggest that neither VEGF or sFlt1 inhibit the intrinsic chondrogenic capacity of MDSCs.
Figure 1.
(A) Alcian blue staining of pellets (100X). Hyaline cartilage-like Alcian blue-positive matrix was found in pellets created with chondrocytes (Chond) or muscle-derived stem cells transduced to express BMP4 (B4), a mixture of cells expressing BMP4 or VEGF (B4+VEGF), and in pellets with a mixture of cells expressing BMP4 or sFlt1 (B4+sFlt1). Nontransduced cells (C) with BMP4 (C+B4) or TGF-β (C+T) added to the medium did not show similar matrix formation. Chondrocyte-like, round cells were observed in all transduced groups (B4, B4 +sFlt1, B4+VEGF) and at all time points (7, 14, and 21 days) but not in control pellet cultures (C, C+B4, C+T). The B4+sFlt1 group contained more chondrocyte-like cells at all time points compared to the other groups. (B–E) Graphs showing qPCR data performed on 3D pellet cultured cells. Pellets created with a mixture of MDSCs expressing BMP4 or sFlt1 (B4+sFlt1) demonstrated significantly higher typeII collagen and sox9 gene expression than other groups at all time points in culture. There was significantly more type X collagen expression in the B4+sFlt1 group compared to the B4+VEGF group at 7 and 28 days * (P< 0.05). Type 1 collagen expression was highest in the B4+VEGF group at all time points.
In order to assess the expression of several chondrogenic marker genes under the different in vitro experimental conditions, we performed qPCR analysis. The B4+sFlt1 group showed significantly greater type II collagen and sox9 gene expression than the B4 and B4+VEGF groups at all time points and showed higher type X collagen gene expression than the B4+VEGF group at 7 and 28 days (Figure 1 B–E). The B4+VEGF group showed significantly higher type II collagen gene expression than the B4 group at 14 days (Figure 1B). It was also observed that expression of type II collagen decreased over time in the B4+sFlt1 group; however, a similar trend was observed in the primary chondrocyte control pellets (Chond, Figure 1B). Type 1 collagen expression increased over time in B4, B4+VEGF, and in Chond groups, but remained constant in B4+sFlt1 group (Figure 1E).
VEGF treatment leads to arthritic progression in knee joint
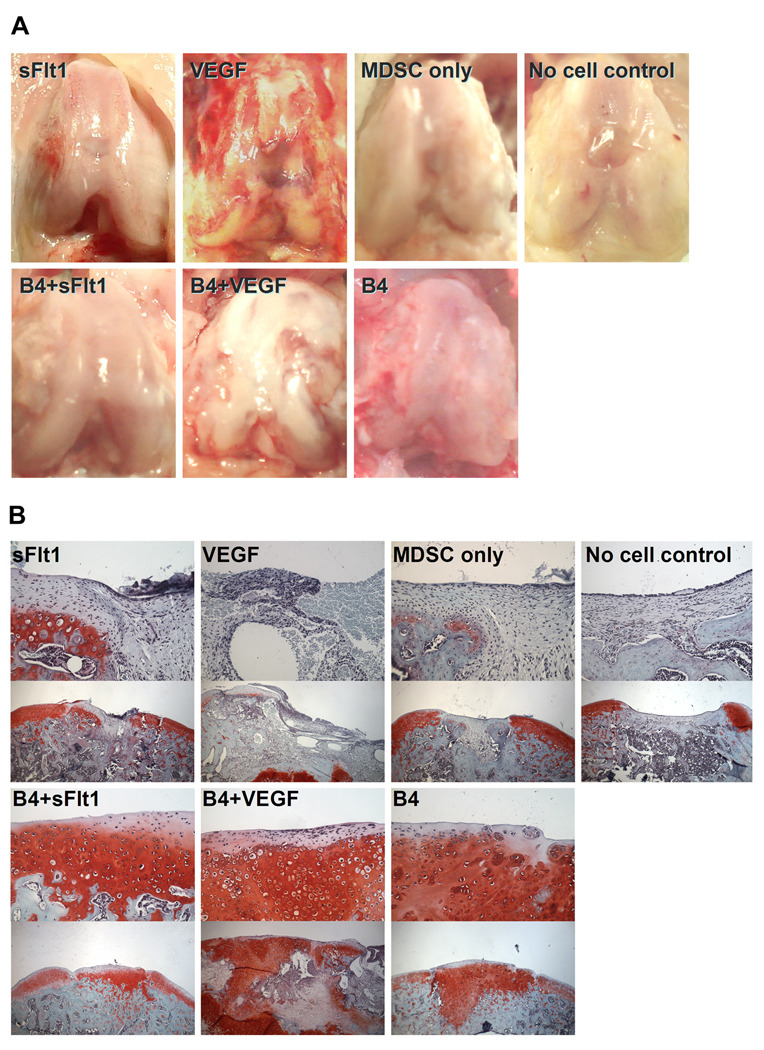
In order to generally assess the effects of our different treatments, we performed a qualitative gross analysis of the knee joint after 8 or 16 weeks postoperatively. At 8 weeks after surgery, the B4+VEGF group showed the appearance of arthritic progression (Figure 2A). In the VEGF-treated group, similar arthritic progression was observed.. Groups that were not treated with cells expressing VEGF showed no signs of arthritis 8 weeks after surgery (Figure 2A). Defects in the B4, B4+VEGF, and B4+sFlt1 groups showed good coverage of the defect by cartilaginous tissue that was well integrated with the surrounding cartilage. Defects in groups that were not treated with BMP4-expressing cells were covered by connective tissue and the surfaces of those defects were concave compared to surrounding normal convex cartilage (Figure 2A). Between 8 and 16 weeks after surgery, the knees treated with cells expressing VEGF showed high levels of joint destruction, inhibiting the animal’s normal movement. Therefore, the animals in the VEGF and B4+VEGF groups were removed from the study before the 16 week postoperative time point.
Figure 2.
(A) Macroscopic pictures of osteochondral defects 8 weeks after transplantation. Grossly, the defects treated with either BMP4 (B4) or a mixture of BMP4 and sFlt1 expressing cells (B4+sFlt1) appeared more similar to the surrounding cartilage. Also, both groups treated with VEGF (VEGF, B4+VEGF) showed evidence of cartilage destruction. (B) Histophotomicrographs showing Safranin O staining of defect areas 8 weeks postoperatively. High magnification (top, 200X) and lower magnification (bottom, 50X) images are shown for each of the treatment groups. Large areas of Safranin O-positive cartilage were noted in all defects treated with BMP4 (B4, B4+VEGF, B4+sFlt1). Although the defects treated with VEGF and BMP4 expressing cells (B4+VEGF) showed Safranin O-positive cartilage, they also showed damage in other regions of the joint.
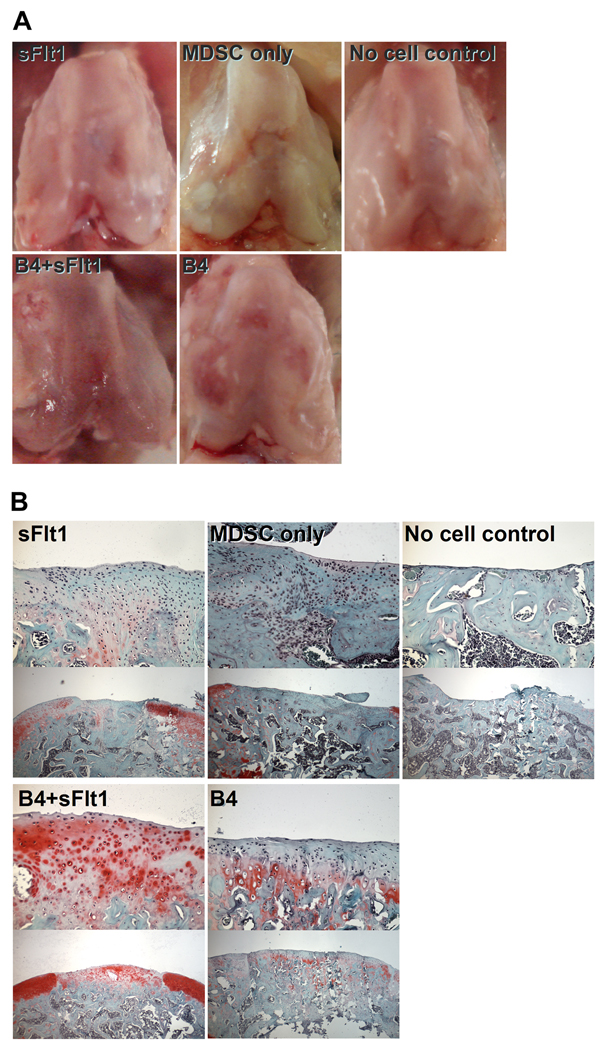
Sixteen weeks after transplantation, defects that were not treated with BMP4-expressing cells appeared to be rough, and the margin between the defects and the surrounding cartilage could be clearly identified (Figure 3A). Defects in the B4 and B4+sFlt1 groups still contained smooth repaired tissue that was well integrated with the surrounding cartilage. However, the B4 group showed some mild cartilage erosion (B4, Figure 3A, B4, white arrow).
Figure 3.
(A) Macroscopic pictures of osteochondral defects 16 weeks after transplantation. Gross inspection showed that the defects treated with BMP4 (both B4 and B4+sFlt1) appeared to be healed with cartilagenous tissue that resembled the surrounding articular cartilage. (B) Histophotomicrographs showing Safranin O staining of defect areas 16 weeks postoperatively. High magnification (top, 200X) and lower magnification (bottom, 50X) images from each of the treatment groups. Both groups treated with BMP4-expressing cells (B4 and B4+sFlt1) showed some Safranin O-positive tissue in the defect region. The defects treated with a mixture of cells expressing either BMP4 or sFlt1 (B4+sFlt1) showed the best repaired tissues based on histological analysis.
Safranin O staining reveals improved cartilage formation in sFlt1-treated group
We used Safranin O staining to highlight the glycosaminoglycan content in the repaired region as an indicator of overall cartilage health. Eight weeks after transplantation, defects in the B4 and B4+sFlt1 groups contained Safranin O-positive hyaline cartilage and the subchondral area was replaced by bone or cartilage, whereas defects in groups that were not treated with BMP4-expressing cells (no cell control, MDSC only, VEGF, and sFlt1 groups) were covered and filled with undifferentiated mesenchymal tissues or fibrous tissues (Figure 2B). Groups treated with VEGF expressing cells (VEGF and B4+VEGF groups) showed evidence of mild destructive events including pannus invasion, osteolysis, and cyst formation in subchondral bone area, although the B4+VEGF group did show Safranin O-positive hyaline cartilage repair near the articular surface.
Sixteen weeks after transplantation, defects in the no cell control group showed eburnated bone covered by thin fibrous tissue (Figure 3B). The MDSC-treated defects showed undifferentiated fibrous tissue, while the sFlt1-treated group showed the presence of cartilaginous tissue which stained slightly positive with Safranin O. The B4 and B4+sFlt1-treated defects were covered by Safranin O-positive cartilage. However, the B4 group showed less Safranin O-positive cartilage area compared to the B4+sFlt1 group.
Histological scoring supports the usefulness of blocking VEGF for cartilage repair
We evaluated articular cartilage repair semi-quantitatively using a previously described histological grading system in order to better characterize the types of tissues that formed within the defects. In this system, seven histological categories (filling of defect, integration to adjacent tissue, Safranin O staining, cellular morphology, architecture within defect, architecture of surface, subchondral bone replacement, and tidemark formation) are evaluated and scored. The total score ranges from 0 points (normal articular cartilage) to 31 points (no repair).(36)
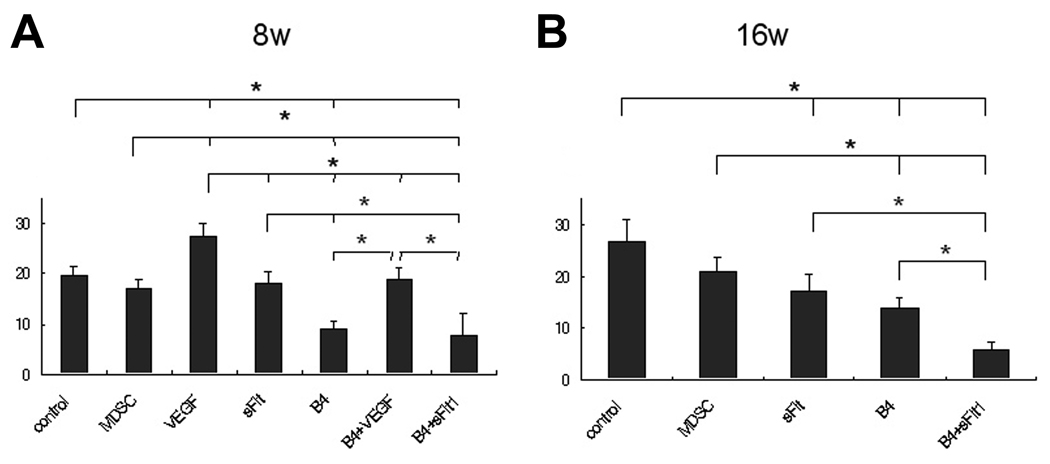
In general, 8 weeks after implantation, defects treated with BMP4 showed improved healing compared to defects that were not treated with BMP4 (Figure 4, Table). Of these groups treated with BMP4, the defects that were also treated with cells expressing sFlt1 (B4+sFlt1) showed the lowest scores, suggesting that the repaired cartilage was most histologically normal in this group.
Figure 4.
(A) Sellers Histological Score totals for samples analyzed 8 weeks after surgery. Scores were significantly lower (see Table for categorized scores) in B4 and B4+sFlt1 groups and significantly higher for B4+VEGF group (P<0.05). (B) Sellers Histological Score for samples analyzed 16 weeks after surgery. Statistical analysis of the total scores (see Table for categorized scores) showed that B4+sFlt1 had significantly lower scores than all other groups tested (P<0.05).
Table.
Sellers Histological Score of Tissue (Mean Score ± SD)
| Group | Filling of defect |
Integration | Safranin O staining |
Cellular morphology |
Architecture within defect |
Architecture of surface |
Subchondral bone replacement |
Tidemark | Total score |
|---|---|---|---|---|---|---|---|---|---|
| 8wk | |||||||||
| Control | 1.25±0.5 | 0.50±0.6 | 4.00±0.0 | 5.00±0.0 | 0.25±0.5 | 1.75±0.5 | 3.00±0.8 | 4.00±0.0 | 19.75±1.7 |
| MDSC | 0.75±1.0 | 0.00±0.0 | 3.25±0.5 | 4.50±0.6 | 0.25±0.5 | 1.00±0.8 | 3.50±0.6 | 4.00±0.0 | 17.25±1.7 |
| VEGF | 2.50±1.0 | 2.50±0.6 | 4.00±0.0 | 5.00±0.0 | 3.00±0.8 | 2.50±1.0 | 4.00±0.0 | 4.00±0.0 | 27.50±2.5 |
| sFlt1 | 1.50±0.6 | 0.00±0.0 | 3.25±0.5 | 4.75±0.5 | 0.75±1.0 | 1.25±0.5 | 3.00±1.2 | 3.50±0.6 | 18.00±2.6 |
| B4 | 0.00±0.0 | 0.00±0.0 | 0.75±0.5 | 2.00±0.0 | 0.00±0.0 | 1.00±0.0 | 1.50±1.3 | 3.75±0.5 | 9.00±1.6 |
| B4+VEGF | 0.00±0.0 | 1.25±1.0 | 1.75±1.0 | 2.50±1.3 | 3.50±1.0 | 2.50±1.0 | 4.00±0.0 | 3.50±1.0 | 19.00±2.2 |
| B4+sFlt1 | 0.00±0.0 | 0.00±0.0 | 1.75±1.3 | 1.50±1.0 | 0.00±0.0 | 0.75±1.0 | 1.75±1.3 | 2.00±0.8 | 7.75±4.4 |
| 16wk | |||||||||
| Control | 3.25±1.0 | 2.00±1.2 | 4.00±0.0 | 5.00±0.0 | 3.00±2.0 | 1.50±0.6 | 4.00±0.0 | 4.00±0.0 | 26.75±4.3 |
| MDSC | 1.75±1.0 | 0.00±0.0 | 3.50±0.6 | 5.00±0.0 | 1.25±1.9 | 1.50±0.6 | 4.00±0.0 | 4.00±0.0 | 21.00±2.7 |
| sFlt1 | 0.75±0.5 | 0.00±0.0 | 3.00±0.8 | 4.75±0.5 | 0.00±0.0 | 1.25±1.0 | 3.75±0.5 | 3.50±0.6 | 17.00±3.4 |
| B4 | 0.50±1.0 | 0.50±0.6 | 2.50±0.6 | 3.00±0.8 | 0.00±0.0 | 1.25±0.5 | 3.00±0.0 | 3.00±0.8 | 13.75±1.9 |
| B4+sFlt1 | 0.00±0.0 | 0.00±0.0 | 1.50±0.6 | 0.50±0.6 | 0.00±0.0 | 1.00±0.0 | 1.25±1.0 | 1.50±0.6 | 5.75±1.7 |
The total score of the B4+sFlt1 group was significantly lower (histologically more similar to normal, undamaged cartilage) than all the other groups (Figure 4, Table). The score of the B4 group was lower than the control, the MDSC, and the sFlt1-only groups. Also, the score of the sFlt1 group was lower than the control group.
Discussion
VEGF signaling has been shown to play important roles during endochondral bone formation, apoptosis, osteophyte formation, and cartilage destruction in the osteoarthritic joint (15, 16, 18–24). Furthermore, VEGF has been shown to be one of the most important factors that can cause arthritis (25–27). This data supports the idea that VEGF is related to cartilage destruction. However, it also has been shown that VEGF is important for stem cell and chondrocyte survival during limb bud development (30, 31) and that blocking VEGF signaling can suppress BMP4 induced endochondral bone formation by MDSCs (17). Therefore, the role of VEGF during stem cell-mediated chondrogenesis needs to be better understood.
To gain insight into the paradoxical effects of VEGF, in the current study, we first tested the in vitro chondrogenic ability of MDSCs using a 3-dimensional pellet culture system in chondrogenic medium with 10 ng/ml of TGF-β3. Using this system, it was possible to determine the direct effect of VEGF on the intrinsic capacity of MDSCs to undergo chondrogenic differentiation. Specifically, the system enabled the determination of whether VEGF secretion by differentiated chondrocytes can cause intrinsic cartilage resorption and whether inhibition of VEGF signaling by sFlt1 could prevent the chondrogenic differentiation of the MDSCs. Hyaline cartilage-like matrix production was observed through histological evaluation of the B4+VEGF pellets, as evidenced by Alcian blue-positive staining after 7, 14, and 28 days in culture (Figure 1A). Also, it was observed that co-culture of VEGF-expressing cells along with BMP4-expressing cells did not alter the expression of chondrogenic genes (type II collagen, type X collagen, and sox9) compared to BMP4 alone (Figure 1B, C). These results suggest that VEGF does not prevent the chondrogenic differentiation of BMP4-transduced MDSCs in pellet culture after stimulation with chondrogenic medium containing 10 ng/ml of TGF-β3. These results seem to be in agreement with previous research that studied the effect of VEGF on micromass culture of mesenchymal cells from the chicken limb bud (14). More importantly, it was observed that the presence of sFlt1-expressing cells within the pellets further enhanced the chondrogenic differentiation of BMP4-expressing MDSCs at all time points, both histologically (Figure 1A) and by gene expression (Figure 1B, C). These results suggest that blocking VEGF with sFlt1 does not prevent in vitro chondrogenesis of BMP4-transduced MDSCs in pellet culture, and, in fact, improves the expression of chondrogenic marker genes by MDSCs induced to undergo chondrogenic differentiation. Although sFlt1 does not block the expression of VEGF by the MDSCs, it blocks VEGF activity after it is secreted, suggesting either a potential beneficial effect of blocking VEGF activity on chondrogenesis or that sFlt1 influences chondrogenesis through an unknown mechanism independent of VEGF.
Secondly, this study employed an in vivo transplantation experiment to provide information about the effect of VEGF on the BMP4-induced chondrogenic ability of MDSCs using a gain- and loss-of VEGF function experimental design. MDSCs were exposed to the more stressful environment of the synovial joint where matrix production is normally strongly suppressed. In addition, a complete assessment of healing can be obtained in this model because the local environment in which we must elicit cartilage regeneration was preserved.
Eight weeks after surgery, the BMP4-treated groups, regardless of the presence or absence of VEGF activity, showed better cartilage repair, macroscopically, on the surface of the defect than groups not treated with BMP4 (Figure 2 A), and the repaired tissue seemed to resemble hyaline cartilage by Safranin O staining (Figure 2B; Table). The difference in the histological scores between the three BMP4-treated groups was mostly due to the pannus invasion into the subchondral region and the osteolysis of the subchondral bone which affected the histological scores of “architecture within defect” and “subchondral bone formation” (Figure 2B; Table). This result suggests that even in the synovial joint, VEGF will not prevent the BMP4-induced chondrogenesis mediated by MDSCs, but that VEGF can cause osteolysis and pannus invasion through the activation of cells related to osteolysis and synovial hypertrophy. However, the VEGF-treated groups were given markedly worse total scores than groups that had no VEGF treatment or that had sFlt1 treatment (Table). On the other hand, interestingly, when comparing the B4+VEGF group to the VEGF group, osteophyte formation was only seen in the B4+VEGF group, although both groups commonly showed arthritic development including increased joint fluid amount, synovial hypertrophy, and pannus invasion into cartilage and bone (Figures 2A, B). Regardless of the presence or absence of BMP4 treatment, the results suggest that treatments for articular cartilage repair that involve VEGF should be avoided.
Sixteen weeks after surgery, BMP4-treated groups showed improved healing compared to the groups that were not treated with BMP4 both macroscopically and microscopically (Figure 3B; Table). This result supports our previous report (8). In addition, the B4+sFlt1 group showed significantly better histological scores than that of the B4 group, which resulted from improved scores in the “cellular morphology”, “subchondral bone formation”, and “tidemark formation” categories. These scores are affected by the invasion of bone into regions that were cartilaginous 8 weeks after surgery (Figure 3A, B; Table). The improved, but sub-optimal repair of cartilage through the addition of BMP-expressing cells has been suggested in previous reports (37). The inhibition of vascular invasion by sFlt1 could be a factor that led to improved scores in these categories. Moderate osteophyte formation was observed only in the B4 group. It is possible that endogenous VEGF expression combined with BMP4 treatment might play a role in the formation of osteophytes as time progresses.
The results of the current study are consistent with previous publications that demonstrated that VEGF triggers cartilage destruction (18–24). Furthermore, this study suggests that the destruction observed following the implantation of VEGF expressing cells was caused by extrinsic environmental changes rather than through direct effects on the chondrogenic differentiation of the implanted MDSCs. This hypothesis is supported by the observation that the addition of VEGF did not inhibit MDSCs from undergoing chondrogenic differentiation in vitro (Figure 1).
On the other hand, sFlt1 improves BMP4- and TGF-β3-induced chondrogenic gene expression of MDSCs in vitro, and improves the persistence of repaired articular cartilage by preventing angiogenesis that can lead to invasion of bone into the cartilage.
However, in this study, no differences were found between any of the groups in the histological score of “architecture of surface.” This result suggests that factors other than BMP or VEGF are needed to fully repair the cartilage at the articular surface.
In conclusion, sFlt1 treatment combined with BMP4 treatment of muscle-derived stem cells is a potential therapy for cartilage repair that may improve the quality and persistence of repaired articular cartilage.
Acknowledgements
We would like to thank Dr. Ryosuke Kuroda for animal experiment guidance, and Mr. David Humiston for editing this manuscript. This work was supported in part by funding from the Henry J. Mankin Endowed Chair for Orthopaedic Research at the University of Pittsburgh, the William F. and Jean W. Donaldson Chair at Children’s Hospital of Pittsburgh, the Hirtzel Foundation, a grant from the Department of Defense (DOD W81XWH-08-0076), and a grant from the National Institutes of Health (NIH R01 DE-13420-06).
References
- 1.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2.Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84(4):571–578. doi: 10.1302/0301-620x.84b4.11947. [DOI] [PubMed] [Google Scholar]
- 3.Visna P, Pasa L, Cizmar I, Hart R, Hoch J. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques--a randomized controlled study. Acta Chir Belg. 2004;104(6):709–714. doi: 10.1080/00015458.2004.11679648. [DOI] [PubMed] [Google Scholar]
- 4.Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, et al. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12(6):1130–1141. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 5.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, et al. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16(7):3323–3333. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi N, Sato K, Usas A, Fu FH, Ochi M, Han CW, et al. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29(9):1920–1930. [PubMed] [Google Scholar]
- 8.Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433–442. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 9.Wakitani S, Yamamoto T. Response of the donor and recipient cells in mesenchymal cell transplantation to cartilage defect. Microsc Res Tech. 2002;58(1):14–18. doi: 10.1002/jemt.10111. [DOI] [PubMed] [Google Scholar]
- 10.Koga H, Muneta YJ, Ju YJ, Nagase T, Nimura A, Mochizuki T, et al. Synovial stem cells are regionally specified according to local micro environments after implantation for cartilage regeneration. Stem Cells. 2006 doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 11.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2006 doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13(5):595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 14.Yin M, Pacifici M. Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Dev Dyn. 2001;222(3):522–533. doi: 10.1002/dvdy.1212. [DOI] [PubMed] [Google Scholar]
- 15.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 16.Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113(Pt 1):59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110(6):751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41(9):1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto S, Creighton-Achermann L, Takahashi K, Amiel D, Coutts RD, Lotz M. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2002;10(3):180–187. doi: 10.1053/joca.2001.0505. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto H, Inoki I, Komiya K, Shiomi T, Ikeda E, Obata K, et al. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am J Pathol. 2003;162(1):171–181. doi: 10.1016/s0002-9440(10)63808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka E, Aoyama J, Miyauchi M, Takata T, Hanaoka K, Iwabe T, et al. Vascular endothelial growth factor plays an important autocrine/paracrine role in the progression of osteoarthritis. Histochem Cell Biol. 2005;123(3):275–281. doi: 10.1007/s00418-005-0773-6. [DOI] [PubMed] [Google Scholar]
- 22.Pufe T, Petersen W, Tillmann B, Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001;44(5):1082–1088. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Pufe T, Harde V, Petersen W, Goldring MB, Tillmann B, Mentlein R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J Pathol. 2004;202(3):367–374. doi: 10.1002/path.1527. [DOI] [PubMed] [Google Scholar]
- 24.Pufe T, Lemke A, Kurz B, Petersen W, Tillmann B, Grodzinsky AJ, et al. Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am J Pathol. 2004;164(1):185–192. doi: 10.1016/S0002-9440(10)63109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami M, Iwai S, Hiratsuka S, Yamauchi M, Nakamura K, Iwakura Y, et al. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood. 2006;108(6):1849–1856. doi: 10.1182/blood-2006-04-016030. [DOI] [PubMed] [Google Scholar]
- 26.Afuwape AO, Kiriakidis S, Paleolog EM. The role of the angiogenic molecule VEGF in the pathogenesis of rheumatoid arthritis. Histol Histopathol. 2002;17(3):961–972. doi: 10.14670/HH-17.961. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto Y, Tanaka K, Hirata G, Hanada M, Matsuda S, Shuto T, et al. Possible involvement of the vascular endothelial growth factor-Flt-1-focal adhesion kinase pathway in chemotaxis and the cell proliferation of osteoclast precursor cells in arthritic joints. J Immunol. 2002;168(11):5824–5831. doi: 10.4049/jimmunol.168.11.5824. [DOI] [PubMed] [Google Scholar]
- 28.De Bandt M, Ben Mahdi MH, Ollivier V, Grossin M, Dupuis M, Gaudry M, et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J Immunol. 2003;171(9):4853–4859. doi: 10.4049/jimmunol.171.9.4853. [DOI] [PubMed] [Google Scholar]
- 29.Afuwape AO, Feldmann M, Paleolog EM. Adenoviral delivery of soluble VEGF receptor 1 (sFlt-1) abrogates disease activity in murine collagen-induced arthritis. Gene Ther. 2003;10(23):1950–1960. doi: 10.1038/sj.gt.3302104. [DOI] [PubMed] [Google Scholar]
- 30.Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131(9):2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 31.Haigh JJ, Gerber HP, Ferrara N, Wagner EF. Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development. 2000;127(7):1445–1453. doi: 10.1242/dev.127.7.1445. [DOI] [PubMed] [Google Scholar]
- 32.Peng H, Chen ST, Wergedal JE, Polo JM, Yee JK, Lau KH, et al. Development of an MFG-based retroviral vector system for secretion of high levels of functionally active human BMP4. Mol Ther. 2001;4(2):95–104. doi: 10.1006/mthe.2001.0423. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 34.Jadlowiec J, Koch H, Zhang X, Campbell PG, Seyedain M, Sfeir C. Phosphophoryn regulates the gene expression and differentiation of NIH3T3, MC3T3-E1, and human mesenchymal stem cells via the integrin/MAPK signaling pathway. J Biol Chem. 2004;279(51):53323–53330. doi: 10.1074/jbc.M404934200. [DOI] [PubMed] [Google Scholar]
- 35.Jadlowiec JA, Zhang X, Li J, Campbell PG, Sfeir C. Extracellular matrix-mediated signaling by dentin phosphophoryn involves activation of the Smad pathway independent of bone morphogenetic protein. J Biol Chem. 2006;281(9):5341–5347. doi: 10.1074/jbc.M506158200. [DOI] [PubMed] [Google Scholar]
- 36.Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1997;79(10):1452–1463. doi: 10.2106/00004623-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Gelse K, von der Mark K, Aigner T, Park J, Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48(2):430–441. doi: 10.1002/art.10759. [DOI] [PubMed] [Google Scholar]