Abstract
Membrane-bound fatty acid desaturases and related enzymes play a pivotal role in the biosynthesis of unsaturated and various unusual fatty acids. Structural insights into the remarkable catalytic diversity and wide range of substrate specificities of this class of enzymes remain limited due to the lack of a crystal structure. To investigate the structural basis of the double bond positioning (regioselectivity) of the desaturation reaction in more detail, we relied on a combination of directed evolution in vitro and a powerful yeast complementation assay to screen for Δx regioselectivity. After two selection rounds, variants of the bifunctional Δ12/Δ9-desaturase from the house cricket (Acheta domesticus) exhibited increased Δ9-desaturation activity on shorter chain fatty acids. This change in specificity was the result of as few as three mutations, some of them near the putative active site. Subsequent analysis of individual substitutions revealed an important role of residue Phe-52 in facilitating Δ9-desaturation of shorter chain acyl substrates and allowed for the redesign of the cricket Δ12/Δ9-desaturase into a 16:0-specific Δ9-desaturase. Our results demonstrate that a minimal number of mutations can have a profound impact on the regioselectivity of acyl-CoA fatty acid desaturases and include the first biochemical data supporting the acyl-CoA acyl carrier specificity of a desaturase able to carry out Δ12-desaturation.
Keywords: Enzyme Mechanisms, Fatty Acid, Lipid, Lipid Synthesis, Metalloenzymes, Site-directed Mutagenesis, Fatty Acid Desaturation, Regioselectivity
Introduction
Fatty acid desaturases are nonheme diiron-containing enzymes that introduce double bonds within fatty acyl chains and belong to two main groups. The soluble desaturases are localized in the stroma of plastids where they typically introduce the first double bond in saturated fatty acids esterified to an acyl carrier protein. In contrast, the majority of the fatty acid desaturases are integral membrane proteins recognizing acyl chains esterified to either CoA or phospholipids. Although a similar diiron active site is present in membrane-bound desaturases, they are unrelated to soluble desaturases and are believed to have evolved independently (1). Although crystal structures for soluble desaturases from both castor (Ricinus communis) and the English Ivy (Hedera helix) (2, 3) have paved the way for engineered variants displaying intriguing novel catalytic activities and specificities (4–6), our understanding of the structure-function relationship of membrane-bound desaturases remains limited and scattered at best. Consequently, engineering of this class of desaturase enzymes has lagged behind that of the soluble desaturases.
Historically, three main types of regioselectivity have been distinguished (7, 8). Desaturases with Δx regioselectivity introduce the first double bond x carbon atoms away from the carboxyl end. A second group comprises ωy-desaturases that insert a double bond at position y referenced from the methyl end. Finally, v+z-desaturases require a preexisting double bond as a reference point (v) and generate the new double bond z carbon atoms further along the acyl chain. These modes of regioselectivity are not mutually exclusive, as illustrated by several bifunctional Δ12/ω3 fungal desaturases (9–12).
Thus far, membrane-bound fatty acid desaturases have been the subject of only a limited number of regioselectivity studies. Site-directed mutagenesis and the exchange of topological features between homologous fungal v+3-, bifunctional v+3/ω3-, and ω3-desaturases have pointed to the C-terminal end and residues in the neighborhood of and in between the first two conserved histidine boxes as important structural determinants for v+3 versus ω3 regioselectivity (9, 10, 13). Although such experiments have their merit, they rely heavily on sequence information. Typically, sets of homologous desaturases displaying different regioselectivities are required to identify potential amino acids or regions that might be involved in the regioselectivity outcome.
In an attempt to further increase our understanding of the structural principles that determine the regioselectivity of membrane-bound fatty acid desaturases, we relied on directed evolution in vitro rather than on more rational protein design principles that formed the basis of previous studies. Because directed evolution mimics Darwinian evolution in vitro, protein engineering puzzles can be tackled without the need for detailed structure-function information (14, 15). The recently cloned bifunctional Δ12/Δ9-desaturase from the house cricket (Acheta domesticus) (16) was chosen as a target because of the high amino acid sequence identity (65%) it shares with the Δ9-desaturase from the same insect (17). Because the former enzyme probably evolved from a Δ9-desaturase, it provides a unique opportunity to study for the first time the structure-function relationship behind Δx and v+3 types of regioselectivity. Two cycles of random mutagenesis and selection for Δx regioselectivity by genetic complementation of an ole1Δ::kanMX4 yeast deletion strain yielded three desaturase mutants exhibiting significant changes in their chain length specificity and regioselectivity. Analysis of individual contributions of each selected substitution allowed for the subsequent design of a highly specific Δ9-desaturase mutant that only differed in 3 amino acids from the wild type enzyme. In addition, we provide biochemical evidence for the acyl-CoA specificity of the wild type cricket Δ12/Δ9-desaturase and the most active of the evolved mutants, setting these enzymes apart from other Δ12-desaturases that typically recognize membrane phospholipids.
EXPERIMENTAL PROCEDURES
Yeast Strains and Expression Vectors
Saccharomyces cerevisiae strains used for the expression of wild type and mutant desaturase genes included S288C (Matα, SUC2, gal2, mal, mel, flo1, flo8-1, hap1) and an ole1 deletion mutant (his3Δ1, leu2Δ0, ura3Δ0, ole1Δ::kanMX4) (ATCC 4024422) (18), designated hereupon as ole1Δ. The pYES2-derived yeast expression vectors pXZP279, pXZP277, and pXZP282 contained the genes of the Arabidopsis thaliana FAD2 Δ12-desaturase, the cricket Δ9-desaturase, and the cricket Δ12/Δ9-desaturase, respectively (courtesy of Dr. Xue-Rong Zhou, CSIRO Plant Industry). The pYES2-TD10 vector containing the gene of the Tribolium castaneum Δ12-desaturase was obtained from Dr. Victoria Haritos (CSIRO Ecosystem Sciences). A pYES3 yeast expression vector containing the gene coding for the Δ9-elongase from Isochrysis galbana was provided by Dr. James Petrie (CSIRO Plant Industry).
Functional Expression in Yeast
Yeast transformations were carried out using the yeast-1 transformation kit (Sigma-Aldrich, Castle Hill, New South Wales, Australia) and 1 μg of plasmid DNA according to the manufacturer's instructions. Transformants were selected on drop-out agar plates containing 2% glucose and lacking uracil (SC-Ura;2 for selection of pYES2-derived vectors) or tryptophan (SC-Trp; for selection of pYES3-derived vectors). For transformations involving the ole1Δ strain, plates were supplemented with 0.5 mm 16:1Δ9, 0.5 mm 18:1Δ9, and 1% Tergitol. Colonies that developed after 3–5 days at 30 °C were tested for the presence of the appropriate plasmid DNA in a colony PCR.
Precultures of ole1Δ transformants were set up in 5 ml of SC-Ura medium supplemented with 2% glucose, 1% Tergitol, and any exogenous fatty acid required. After incubation overnight at 28 °C, yeast cells were washed with 5 ml of water and resuspended in 5 ml of SC-Ura induction medium containing 2% galactose, 1% Tergitol, and the appropriate fatty acid. When a different exogenous fatty acid was used during induction, two extra washing steps using 5 ml of 1% Tergitol and 5 ml of 0.5% Tergitol were included. Desaturation of 16:0 and 18:0 was assessed by adding 0.5 mm 17:1Δ10-cis to preculture and induction medium. Conversion of 18:1Δ9 was determined by growing cultures in the presence of 0.5 mm 18:1Δ9-cis during preculture and induction steps. For yeast feeding experiments involving 16:1Δ6-cis or 18:1Δ9-trans substrates, 0.5 mm of either fatty acid was added to the induction medium, whereas precultures were supplied with 0.25 mm 17:1Δ10-cis. Induction cultures were incubated for 3 days at 28 °C and washed with equal volumes of 1% Tergitol, 0.5% Tergitol, and water to remove any unincorporated fatty acids. Final yeast pellets were freeze-dried and stored at −20 °C until further analysis.
Random, Targeted, and Saturation Mutagenesis
Random mutagenesis of the cricket Δ12/Δ9-desaturase gene was carried out as described previously (19) except for some minor modifications. The error-prone PCR mixture consisted of 100 ng of pXZP282 plasmid DNA, 0.2 mm each dNTP, 5 units of Taq DNA polymerase (Stratagene, Jolla, CA), and 0.15 mm MnCl2. Primers (10 pmol of each) flanking the desaturase gene were chosen to avoid mutations in the GAL1 promotor and the CYC1 transcriptional terminator. The PCR program consisted of 94 °C for 2 min, 33 cycles of 94 °C for 20 s, 63 °C for 30 s, and 72 °C for 1 min 30 s and a final extension step of 72 °C for 10 min. Amplified products were purified using the QIAquik gel extraction kit (Qiagen, Doncaster, Victoria, Australia) and subsequently used as megaprimers to amplify the entire pXZP282 vector backbone with the high fidelity PfuUltra DNA polymerase (Stratagene). The PCR mixture contained 100 ng of pXZP282 template, 500 ng of the purified error-prone PCR product, 2.5 units of PfuUltra DNA polymerase, 1× PfuUltra HF reaction buffer, and 0.2 mm of each dNTP in a final volume of 50 μl. The thermocycling program included an initial denaturation step at 95 °C for 2 min followed by 30 cycles of 95 °C for 50 s, 60 °C for 50 s, and 72 °C for 10 min. Prior to transformation, each PCR mixture was digested with 20 units of DpnI (New England Biolabs, Ipswich, MA) for 2 h at 37 °C to remove the original pXZP282 template DNA.
The library diversity was assessed by sequencing five randomly selected clones after transforming an aliquot into Escherichia coli DH5α. On average, 5.8 missense mutations were found in each random mutant, and the mutation frequency was estimated at 0.75%.
Single amino acid substitutions were introduced into the cricket Δ9- and Δ12/Δ9-desaturases using the GeneTailor site-directed mutagenesis system (Invitrogen, Mulgrave, Victoria, Australia). Transformation of the cricket Δ9-desaturase mutant A100V in ole1Δ was unsuccessful, and its activity could therefore not be determined. Triple mutants of the cricket Δ12/Δ9-desaturase (E22K/A98V/K136R and F52Y/I89V/A98V) were constructed by GENEART (Regensburg, Germany). Saturation mutagenesis of the cricket Δ9-desaturase (residue Phe-54) and Δ12/Δ9-desaturase (residue Phe-52) was also performed by GENEART.
ole1Δ Complementation Assay
The desaturase library was divided in 10-μl aliquots, transformed into ole1Δ using the yeast-1 transformation kit (Sigma-Aldrich), and plated on SC-Ura medium containing 2% glucose, 0.5 mm 16:1Δ9, and 1% Tergitol. Plates were kept for 5 days at 30 °C, after which ole1Δ transformants were collected in 20 ml of water. A 500-μl aliquot of the resuspended library was diluted until an A600 of 0.5 was reached and plated on SC-Ura selection medium containing 2% galactose (3 ml/150-mm Petri dish for optimal plating density). To confirm the increased level of complementation when compared with the parent of the respective selection round, individual colonies that developed after 6–7 days at 30 °C were tested in an ole1Δ complementation assay as described below prior to lipid analysis. In addition, the integrity of the GAL1 promotor of each selected desaturase variant was verified by sequencing to rule out increased expression due to improved promotor efficiency.
Assaying single clones for ole1Δ complementation was done by resuspending cells in 100 μl of water. After adjusting the A600 to 1, each sample was further diluted 3 × 0.1, and 10 μl of each dilution was spotted on SC-Ura agar plates containing 2% glucose or 2% galactose. Cell growth was scored after 4–5 days at 30 °C. The absence of growth on the glucose plates confirmed that complementation was not a result of adaptation of the ole1Δ host itself.
Desaturase-Elongase Coexpression in Yeast
S288C precultures were grown overnight at 28 °C in 15 ml of SC-Ura-Trp medium containing 2% glucose and resuspended the following day in 45 ml of fresh medium. After a second overnight incubation, cell pellets were washed with an equal volume of water and resuspended in 45 ml of SC-Ura-Trp induction medium containing 2% galactose. At three different time points (6, 12, and 24 h), separate cell cultures were washed with an equal volume of water. A 5-ml aliquot of each sample was used for total lipid analysis. Pellets obtained from the remaining 40 ml of cell suspensions were resuspended in 1 ml of water, centrifuged, and used for lipid fractionation.
Lipid Analysis
Fatty acid methyl esters were prepared from total yeast lipids by transesterification with 0.75 ml of 1 n methanolic-HCl (Supelco, Bellefonte, PA) for 3 h at 80 °C. After adding 0.5 ml of 0.9% NaCl to each sample, methylated fatty acids were extracted with 0.5 ml of hexane and analyzed by GC as described previously (16). The proportion of each fatty acid as a weight percentage of total fatty acids was based on the integrated peak area as calculated by the Agilent Chemstation software (Revision B.02.01.SR1 (260), Agilent, Palo Alto, CA) and with 17:1Δ10-cis response based on the 16:1Δ9-cis standard. To confirm the position of the introduced double bond, fatty acid methyl esters were derivatized with 4,4-dimethyloxazoline and analyzed by GC-MS as described previously (16).
For analysis of different lipid classes, total lipids were extracted from yeast pellets using the method described by Bligh and Dyer (20). Neutral lipids (NL) and polar lipids including phosphatidylcholine (PC) were fractionated by TLC using precoated silica gel plates (Silica Gel 60; Merck) and a solvent system comprising chloroform/methanol/acetic acid/water (85/15/10/3.5, v/v). The lipid spots impregnated in silica were visualized using iodine vapor and identified according to the authentic standards run alongside. After isolating lipid spots from the TLC plates, fatty acid methyl esters were prepared and analyzed by GC.
Topology Prediction
Topology predictions for the cricket Δ9- and Δ12/Δ9-desaturases were based on the TMPRED (21) and TMHMM version 2.0 (22) algorithms and Kyte and Doolittle (23) hydropathy profiles using a window size of 19 amino acids. Both models were in close agreement with predicted and validated topologies of the yeast and mouse Δ9 acyl-CoA desaturases (24, 25).
Statistical Analysis
One-way analysis of variance and pairwise two-tailed t tests (Holm-Bonferroni) were done with R-2.11.1 for Windows.
RESULTS
Substrate Specificity of the Cricket Δ12/Δ9-desaturase
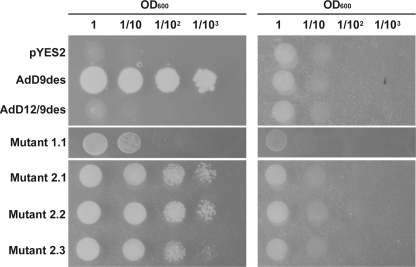
Based on the phylogenetic analysis of several insect desaturases, Zhou et al. (16) postulated that the cricket bifunctional Δ12/Δ9-desaturase most likely evolved from an ancestral Δ9-desaturase. Functional expression in yeast not only revealed Δ12-desaturation of 18:1Δ9 and to a much lesser extent of 16:1Δ9 but also Δ9-desaturation activity on 14:0 and 16:0.3 However, the Δ9-desaturation activity proved to be too low to allow for genetic complementation of the ole1Δ yeast strain (Fig. 1), a Δ9-desaturase yeast deletion mutant.
FIGURE 1.
Complementation assay of the wild type cricket Δ9-desaturase (AdD9des), wild type cricket Δ12/Δ9-desaturase (AdD12/9des), and cricket Δ12/Δ9-desaturase mutants 1.1, 2.1, 2.2, and 2.3 in ole1Δ. A dilution series of each sample was spotted on SC-Ura agar plates containing galactose (left) or glucose (right). Yeast cells transformed with the pYES2 vector served as a negative control. OD, optical density.
To obtain a better understanding of possible constraints imposed by the shape of the binding pocket of the cricket Δ12/Δ9-desaturase onto the various C16 and C18 substrate molecules, we performed two additional ole1Δ yeast feeding experiments. In the presence of the 18:1Δ9-trans, no further desaturation was observed (data not shown). The cricket Δ12/Δ9-desaturase therefore requires a preexisting double bond in 18:1Δ9 in the cis-conformation in accordance to other typical v+3-desaturases. Because 18:1Δ9-cis serves as a substrate for Δ12-desaturation, we next investigated whether 16:1Δ6-cis might also be accommodated into the binding pocket and undergo Δ9-desaturation. Again no desaturation product was observed (data not shown), implying that the Δ9-desaturation of 16:0 as catalyzed by the cricket Δ12/Δ9-desaturase is an example of Δx regioselectivity.
Random Mutagenesis of the Cricket Δ12/Δ9-desaturase
The low Δ9-desaturation activity on 16:0 led us to investigate whether the cricket Δ12/Δ9-desaturase could be “evolved back” to the proposed ancestral Δ9-desaturase form. Because ole1 gene-disrupted yeast strains require unsaturated fatty acids or the heterologous expression of a Δ9-desaturase for growth on minimal medium (18), they are an ideal screening tool for desaturase variants that display changes in regioselectivity. Because unsaturated fatty acids other than 16:1Δ9 and 18:1Δ9 have also been reported to restore the growth of ole1 yeast cells (26), such a complementation assay would select for Δx regioselectivity in general rather than for double bonds exclusively at the Δ9 position.
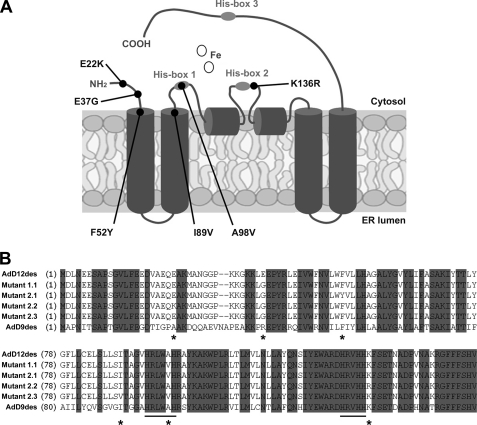
Random mutagenesis of the cricket Δ12/Δ9-desaturase followed by screening for ole1Δ complementation yielded a single desaturase mutant that allowed for minimal growth restoration of ole1Δ yeast cells on minimal medium (Fig. 1). The isolated desaturase variant 1.1 contained two amino acid substitutions (E37G and A98V) (Fig. 2B and Table 1) and was used as a parent for a subsequent similar round of directed evolution. Three second generation mutants 2.1–2.3 were able to complement for the ole1Δ deletion in yeast (Fig. 1). Sequencing revealed four new mutations (E22K, F52Y, I89V, and K136R) (Fig. 2B and Table 1). When mapped upon a predicted topological model of the cricket Δ12/Δ9-desaturase, all targeted residues appeared to be either exposed into the cytosol or situated near the membrane surface (Fig. 2A). Two substitutions, A98V and K136R, are located close to the active site within the first and near the second conserved histidine box, respectively. Remarkably, the original mutation A98V selected during the first round was absent in mutant 2.2.
FIGURE 2.
Amino acid substitutions identified in 3 selected random mutants of the cricket Δ12/Δ9-desaturase. A, proposed topology model of the cricket acyl-CoA Δ12/Δ9 fatty acid desaturase. The positions of selected random mutations are indicated by black circles. ER lumen, endoplasmic reticulum lumen. B, partial ClustalW alignment of the cricket Δ9-desaturase (AdD9des), cricket Δ12/Δ9-desaturase (AdD12/9des), and cricket Δ12/Δ9-desaturase mutants 1.1, 2.1, 2.2 and 2.3. Identical residues are shaded, conserved histidine clusters 1 and 2 are underlined, and asterisks below the alignment indicate selected random mutations.
TABLE 1.
Amino acid substitutions identified in the cricket Δ12/Δ9-desaturase mutants 1.1, 2.1, 2.2, and 2.3 and targeted mutations introduced into the Δ12/Δ9-desaturase (AdD12/9des) and at homologous positions within the cricket Δ9-desaturase (AdD9des)
| Amino acid substitution | ||||||
|---|---|---|---|---|---|---|
| Mutant 1.1 | E37G | A98V | ||||
| Mutant 2.1 | E37G | F52Y | A98V | |||
| Mutant 2.2 | E22K | E37G | K136R | |||
| Mutant 2.3 | E37G | I89V | A98V | |||
| AdD9des targeted mutations | A22K, A22E | R39G, R39E | F54Y | I91V | A100Va | K138R |
| AdD12/9des targeted mutations | E22K, E22A | E37G, E37R | F52Y | I89V | A98V | K136R |
a No data.
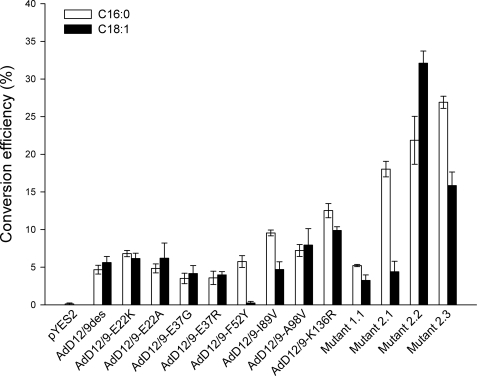
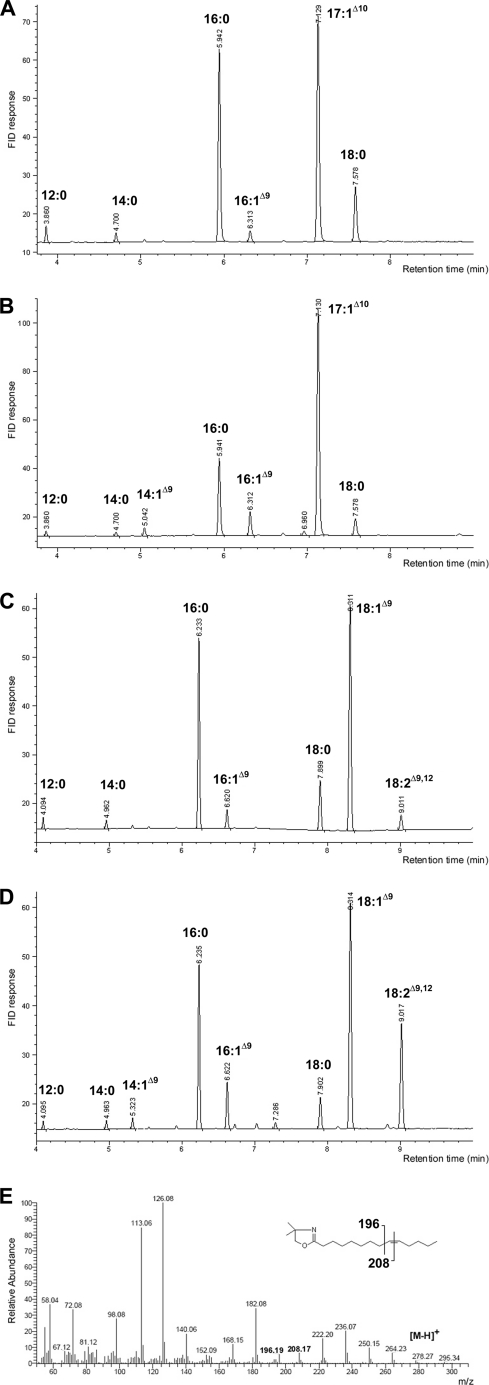
Analysis of fatty acid methyl esters revealed some interesting differences between the activity profiles of the selected desaturase mutants and the wild type enzyme (Figs. 3 and 4). The random mutants 2.1–2.3 all exhibited between 3.9- and 5.9-fold significantly improved Δ9-desaturation activities on 16:0 (p < 0.01), which explains their selection based on the ole1Δ complementation assay. In addition, expression of desaturase mutants 2.2 and 2.3 resulted in significantly increased Δ12-desaturation activities on 18:1Δ9 (p < 0.01). Comparison of peak retention times with those of standard isomers showed that the newly introduced Δ9 and Δ12 bonds in 16:1 and 18:2 were in the cis-conformation (data not shown). In addition, increased levels of 14:1Δ9 were also observed with the regioselectivity of the double bond confirmed by 4,4-dimethyloxazoline derivatization (Fig. 4). Interestingly, only minimal Δ9-desaturation activity was detected on 18:0.
FIGURE 3.
Regioselectivity of wild type (AdD12/9des) and mutant cricket Δ12/Δ9-desaturases. 16:0 and 18:1 conversion efficiencies (%) were calculated from relative substrate and product levels (percentage of total fatty acids) as 100 × product/(substrate + product). Data represent means ± S.D. of three replicate cultures.
FIGURE 4.
A–D, GC analysis of ole1Δ cells expressing the wild type cricket Δ12/Δ9-desaturase (A and C) or desaturase mutant 2.2 (B and D) when supplied with 17:1Δ10 (A and B) or 18:1Δ9 (C and D). FID, flame ionization detector. E, mass spectrum of the 4,4-dimethyloxazoline derivative of the 14:1Δ9 peak in ole1Δ cells expressing the cricket desaturase mutant 2.2. A gap of 12 mass units between the ions m/z = 196 and 208 is diagnostic for a double bond at position Δ9.
Analysis of Individual Desaturase Mutations
To better understand the individual contributions of each of the selected random amino acid substitutions, we constructed a series of single mutants of the cricket Δ12/Δ9-desaturase (Table 1). In addition, homologous mutations were also introduced separately into the cricket Δ9-desaturase (Table 1). Because residues Glu-22 and Glu-37 of the cricket Δ12/Δ9-desaturase are not conserved in the cricket Δ9-desaturase (Ala-22 and Arg-39, respectively), we also exchanged amino acids at these two positions between the two desaturases (Table 1). For reasons of clarity, amino acid substitutions and residues of the cricket Δ9 and Δ12/Δ9 desaturases are referred to below as AdD9 and AdD12/9, respectively.
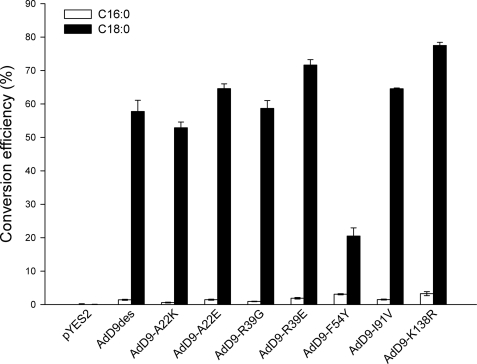
Overall, targeted mutagenesis of the cricket Δ12/Δ9-desaturase resulted in only minor changes in regioselectivity (Fig. 3). Significant increases (p < 0.01) in both Δ9-desaturation and Δ12-desaturation activities were observed upon substituting AdD12/9-K136 for arginine. This mutation is present in mutant 2.2 and explains in part the high activity of this particular variant. The AdD12/9-F52Y substitution caused a significant 23-fold reduction in the Δ12-desaturation activity (p < 0.01), whereas Δ9-desaturation of 16:0 was unaffected. This is in agreement with the change toward Δ9-desaturation as observed for mutant 2.1. The similar substitution AdD9-F54Y introduced into the cricket Δ9-desaturase resulted in a 6-fold reduction in the 18:0/16:0 desaturation ratio (Fig. 5).
FIGURE 5.
Δ9-desaturation activity of wild type (AdD9des) and mutant cricket Δ9-desaturases. 16:0 and 18:0 conversion efficiencies (%) were calculated from relative substrate and product levels (percentage of total fatty acids) as 100 × product/(substrate + product). Data represent means ± S.D. of three replicate cultures.
Results obtained with the cricket Δ12/Δ9- and Δ9-desaturase single mutants point to an important role of AdD12/9-F52 and AdD9-F54 for specific activity on 18:1Δ9 and 18:0 fatty acid substrates, respectively. The functional role of this particular residue in both desaturases was therefore explored further by saturation mutagenesis. Analysis of the individual AdD12/9-F52 saturation mutants revealed that only the original tyrosine substitution and to a lesser extent asparagine resulted in an increased Δ9/Δ12-desaturation ratio (data not shown). Similarly, saturation mutagenesis of AdD9-F54 showed that the shift in substrate specificity toward 16:0 is unique to the AdD9-F54Y substitution (data not shown).
Engineering of a Cricket Δ12/Δ9-desaturase Variant with Increased Δ9 Regioselectivity
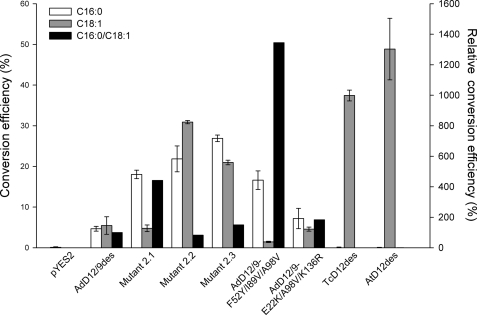
Based on the effect of the single amino acid substitutions, we explored the possibility of further altering the regioselectivity of the cricket Δ12/Δ9-desaturase by recombining some of the selected random mutations. In the first experiment, we redesigned variant 2.2 in an attempt to further improve Δ12-desaturation activity on 18:1 to levels obtained with the T. castaneum and A. thaliana Δ12-desaturases (Fig. 6). The AdD12/9-A98V substitution that was lost during the second selection round was reintroduced, and residue AdD12/9-E37 was restored. Desaturation activity of the triple mutant was reduced and comparable with the wild type enzyme. Secondly, we attempted to improve the Δ9/Δ12-desaturation ratio of variant 2.1 by introducing AdD12/9-I89V and reversing AdD12/9-E37G. The %16:0/%18:1 conversion ratio of the new triple variant was 3.1- and 13.5-fold higher relative to that of the parent mutant 2.1 and the wild type cricket Δ12/Δ9-desaturase, respectively, illustrating a further increase in specificity toward 16:0 (Fig. 6).
FIGURE 6.
Regioselectivity of the T. castaneum Δ12-desaturase (TcD12des), A. thaliana FAD2 Δ12-desaturase (AtD12des), wild type cricket Δ12/Δ9-desaturase (AdD12/9des), and targeted and random mutants of the cricket Δ12/Δ9-desaturase. 16:0 and 18:1 conversion efficiencies (%) were calculated from relative substrate and product levels (percentage of total fatty acids) as 100 × product/(substrate + product). 16:0/18:1 conversion ratios are shown relative to the wild type AdD12/9des desaturase. Data represent means ± S.D. of three replicate cultures.
Acyl Carrier Specificity of Wild Type and Mutant Desaturases
Previous in vitro biochemical studies with isolated insect microsomes have demonstrated that Δ12-desaturation takes place on fatty acid substrates esterified to CoA rather than on phospholipid-linked acyl groups (27, 28). However, such acyl-CoA specificity has not been confirmed directly for the Δ12/Δ9- and Δ12-desaturases recently cloned from the house cricket and the red flour beetle (T. castaneum), respectively (16). To rule out differences in the acyl carrier specificity of the wild type cricket Δ12/Δ9-desaturase and the high activity mutant 2.2, we performed a Δ9-elongase coexpression experiment as described by Sayanova et al. (29). Any acyl-CoA Δ12-desaturase hereby provides the direct substrate for subsequent elongation in the same metabolic pool by the Δ9-elongase from I. galbana, which only accepts 18:2Δ9,12-CoA and 18:3Δ9,12,15-CoA substrates. Elongation should therefore proceed more efficiently than in the case of a phospholipid-specific desaturase such as the A. thaliana FAD2 Δ12-desaturase, which produces 18:2Δ9,12 in a different lipid fraction.
Analysis of total lipids confirmed elongation of the Δ12-desaturation product 18:2Δ9,12 to 20:2Δ11,14 after a 12- and 24-h induction (Table 2). Expression of the A. thaliana FAD2 Δ12-desaturase resulted in the highest 18:2Δ9,12 product levels. Despite the lower desaturation activities of the wild type and mutant cricket desaturases, between 40 and 50% of the available desaturation product was further elongated by the coexpressed Δ9-elongase. Sayanova et al. (29) reported a similar elongation efficiency (57%) when 18:2Δ9,12 was supplied to yeast cultures. Efficient coupling between desaturation and elongation is to be expected when both reactions take place in the acyl-CoA lipid fraction. In the case of the A. thaliana FAD2 Δ12-desaturase, on the other hand, efficiencies of the subsequent elongation step were found to be markedly lower at both time points. Additional acyltransferases that are required to shuttle the desaturation products from the phospholipids to the acyl-CoA lipid pool thereby possibly restrict the flux between acyl-lipid-specific desaturation and acyl-CoA-dependent elongation.
TABLE 2.
Total lipid composition of S. cerevisiae S288C cells expressing the I. galbana Δ9-elongase in combination with the A. thaliana FAD2 Δ12-desaturase (AtD12des), wild type cricket Δ12/Δ9-desaturase (AdD12/9des), or the cricket desaturase mutant 2.2
Desaturation and elongation efficiencies (%) were calculated from relative substrate and product levels (% of total fatty acids) as 100 × product/(substrate + product). Data represent means ± S.D. of three replicate cultures after a 6-, 12-, or 24-h induction.
| Fatty acid | AtD12des |
AdD12/9des |
Mutant 2.2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 6 h | 12 h | 24 h | 6 h | 12 h | 24 h | |
| 16:0 | 9.9 ± 0.3 | 7.3 ± 0.2 | 5.8 ± 0.1 | 10.1 ± 0.6 | 7.0 ± 0.4 | 5.2 ± 0.2 | 10.5 ± 0.2 | 8.2 ± 0.3 | 5.6 ± 0.1 |
| 16:1Δ9 | 25.7 ± 0.5 | 25.7 ± 0.4 | 21.7 ± 0.3 | 26.6 ± 1.4 | 26.5 ± 0.1 | 22.9 ± 0.2 | 25.5 ± 0.1 | 27.5 ± 1.4 | 22.9 ± 0.5 |
| 18:0 | 9.3 ± 0.2 | 8.8 ± 0.3 | 11.1 ± 0.1 | 9.0 ± 0.3 | 8.3 ± 0.1 | 9.8 ± 0.3 | 9.5 ± 0.1 | 8.6 ± 0.4 | 9.7 ± 0.1 |
| 18:1Δ9 | 53.2 ± 0.8 | 50.1 ± 0.6 | 41.9 ± 2.3 | 52.7 ± 1.7 | 55.9 ± 0.3 | 58.2 ± 0.7 | 52.7 ± 0.4 | 51.8 ± 1.7 | 51.5 ± 1.1 |
| 18:2Δ9,12 | 0.4 ± 0.0 | 6.0 ± 0.9 | 14.9 ± 2.1 | 0.1 ± 0.0 | 0.3 ± 0.0 | 1.2 ± 0.0 | 0.2 ± 0.0 | 1.2 ± 0.4 | 4.5 ± 0.1 |
| 20:2Δ11,14 | 0.0 ± 0.0 | 0.4 ± 0.1 | 3.2 ± 0.3 | 0.0 ± 0.0 | 0.2 ± 0.1 | 1.0 ± 0.1 | 0.0 ± 0.0 | 1.1 ± 0.3 | 4.3 ± 0.3 |
| Other | 1.5 | 1.7 | 1.5 | 1.6 | 1.7 | 1.7 | 1.6 | 1.7 | 1.6 |
| % of desaturation | 0.7 | 11.3 | 30.2 | 0.2 | 0.9 | 3.6 | 0.4 | 4.3 | 14.6 |
| % of elongation | 0.0 | 6.3 | 17.7 | 0.0 | 40 | 45.5 | 0.0 | 47.8 | 48.9 |
To obtain further evidence of the acyl-CoA specificity of the wild type cricket Δ12/Δ9-desaturase and its mutant 2.2, NL and different phospholipids of induced yeast cells were separated by TLC. Expression of the A. thaliana FAD2 Δ12-desaturase resulted in an enrichment of 18:2Δ9,12 in PC after a 24-h induction (Table 3). Such enrichment was not observed for either the wild type or the mutant cricket desaturases. Instead similar or even higher levels of 18:2Δ9,12 were detected in the NL. Similar results were obtained after a 12- and 6-h induction (data not shown). These findings are in agreement with previous results obtained with Δ5- and Δ6-acyl-CoA desaturases from microalgae (30, 31). Although we cannot rule out additional acyl-lipid-specific desaturation, the biochemical characterization of the wild type cricket Δ12/Δ9-desaturase and mutant 2.2 provides evidence for the acyl-CoA substrate specificity of both enzymes.
TABLE 3.
Fatty acid composition of PC and NL fractions of S. cerevisiae S288C cells expressing the I. galbana Δ9-elongase in combination with the A. thaliana FAD2 Δ12-desaturase (AtD12des), wild type cricket Δ12/Δ9-desaturase (AdD12/9des), or the cricket desaturase mutant 2.2
Data represent means ± S.D. of three replicate cultures after a 24-h induction.
| Fatty acid | AtD12des |
AdD12/9des |
Mutant 2.2 |
|||
|---|---|---|---|---|---|---|
| NL | PC | NL | PC | NL | PC | |
| 16:0 | 4.3 ± 0.1 | 6.9 ± 0.8 | 4.0 ± 0.3 | 3.5 ± 0.3 | 4.4 ± 0.7 | 3.8 ± 0.1 |
| 16:1Δ9 | 20.4 ± 0.2 | 42.2 ± 1.6 | 20.5 ± 0.6 | 41.5 ± 1.3 | 21.2 ± 1.1 | 41.9 ± 1.4 |
| 18:0 | 12.8 ± 0.8 | 7.7 ± 0.8 | 11.0 ± 0.4 | 4.8 ± 2.0 | 11.0 ± 1.3 | 4.2 ± 0.8 |
| 18:1Δ9 | 45.1 ± 1.5 | 22.0 ± 2.9 | 54.9 ± 2.0 | 47.0 ± 1.8 | 49.0 ± 2.0 | 43.4 ± 0.8 |
| 18:2Δ9,12 | 10.0 ± 1.3 | 17.9 ± 4.3 | 1.1 ± 0.1 | 1.1 ± 0.3 | 4.1 ± 0.2 | 3.0 ± 0.3 |
| 20:2Δ11,14 | 3.9 ± 0.4 | 1.4 ± 0.4 | 1.2 ± 0.1 | 0.4 ± 0.3 | 5.4 ± 0.6 | 1.7 ± 0.0 |
| Other | 3.5 | 1.9 | 7.3 | 1.7 | 4.8 | 1.9 |
DISCUSSION
In nature, the nutritionally important docosahexaenoic acid 22:6Δ4,7,10,13,16,19 is an apt example of the wide range in regioselectivity displayed by fatty acid desaturases. However, many other fatty acid modifications such as hydroxylation that are performed by closely related enzymes are restricted to only a handful of specific positions within the acyl chain of the fatty acid substrate (32). Therefore, a better understanding of the structure-function relationship determining the regioselectivity of fatty acid-modifying enzymes not only opens up further possibilities to manipulate double bond positioning but could also lead to novel and high value designer fatty acids containing various functional groups at tailored positions that are of immediate importance as renewable chemical feedstocks.
The evolution of fatty acid desaturases with novel regioselectivities in insects and other organisms is thought to have occurred via gene duplication or retrotransposition on multiple independent occasions (32–37). Most likely, subsequent accumulation of mutations first resulted in a generalist desaturase displaying a broader substrate acceptance or exhibiting some degree of relaxed catalytic activity (38). Any low level side activity would then serve as a potential starting point for the evolution of a new enzymatic specificity such as altered regioselectivity. As an example, several bifunctional v+3/ω3-desaturases have been cloned from fungi and are believed to be an intermediate form during the change from v+3 toward ω3 regioselectivity (9–12). Unlike v+3- and ω3-fatty acid desaturases, however, Δx-desaturases cluster together in a distinct phylogenetic class (33–35). The bifunctional cricket Δ12/Δ9-desaturase forms a notable exception given its homology to the Δ9-desaturase from the same insect. Furthermore, its broad specificity and low general activity are typical for a generalist enzyme and hint at a relatively recent Δ9-desaturase duplication event. As such, this particular desaturase provides a unique evolutionary and structural link between Δx and v+3 types of regioselectivity.
Despite the high amino acid identity between the cricket Δ9- and Δ12/Δ9-desaturases, both enzymes differ markedly in their specificity. Unlike the Δ9-desaturase, the Δ12/Δ9-desaturase displays low but significant activity on a variety of saturated and mono-unsaturated acyl chains and uniquely combines both Δx and v+3 types of regioselectivity depending on the chain length of the fatty acid substrate. Particularly intriguing is the minimal Δ9-desaturation activity on 18:0. A reduction in size at the bottom of the substrate binding channel has been known to influence acyl chain length specificity of soluble desaturases as fewer carbon atoms can be accommodated between the terminal double bond and the methyl end of the acyl chain (4, 39). Although a different geometry has been suggested for the binding cavity of membrane-bound desaturases (40), it is tempting to speculate that a similar effect occurs in the cricket Δ12/Δ9-desaturase, thereby preventing a correct orientation of the 18:0 substrate with its C9-C10 carbon pair in an eclipsed conformation in close proximity to the diiron active site.
Generalist enzymes such as the cricket Δ12/Δ9-desaturase are ideal starting points for directed evolution experiments aimed at improving promiscuous activities (41, 42). Analysis of the fatty acid products of three desaturase variants that were selected using the ole1Δ yeast complementation assay revealed significant shifts in their chain length specificity and regioselectivity preference (Figs. 3 and 4). Mutants 2.2 and 2.3 can be regarded as improved bifunctional Δ12/Δ9-desaturases yielding significant elevated levels of 16:1Δ9 and 18:2Δ9,12. Mutant 2.1 displayed increased Δ9-desaturation of 16:0, whereas Δ12-desaturation activity on 18:1 was comparable with that of the wild type enzyme. Western blot analysis of ole1Δ yeast cell lysates expressing wild type and mutant desaturases fused to an N-terminal FLAG epitope suggested that changes in the fatty acid product levels as observed for mutants 2.1 and 2.3 were the result of altered specificity and/or increased catalytic activity rather than improved protein stability or expression (data not shown). Such a stability or expression effect could not be completely ruled out for the high activity mutant 2.2, however. Coexpression of the I. galbana Δ9-elongase in yeast allowed us to determine whether a possible change in the acyl carrier specificity of the desaturase mutants in combination with different substrate availabilities in the acyl-CoA and phospholipid pools of induced yeast cultures accounted for some of the observed differences in their fatty acid profiles. Analysis of desaturation and elongation products in NL and PC confirmed no change in acyl carrier specificity of the wild type cricket Δ12/Δ9-desaturase and mutant 2.2 (Tables 2 and 3). This is to our knowledge the first biochemical evidence for the acyl-CoA specificity of a cloned Δ12-desaturase and is of great interest for the production of polyunsaturated fatty acids such as docosahexaenoic acid in oilseed crops via an exclusive acyl-CoA-dependent pathway (31). Although unconfirmed, desaturase mutants 1.1, 2.1, and 2.3 are likely to display a similar specificity for acyl-CoA substrates because they differ in only 3–4 amino acids with mutant 2.2 and all share the AdD12/9-E37G substitution.
Each of the selected desaturase random mutants contained three amino acid substitutions with AdD12/9-E37G and AdD12/9-A98V being shared by more than one desaturase variant (Fig. 2B and Table 1). When we combined the mutations AdD12/9-F52Y, AdD12/9-I89V, and AdD12/9-A98V in the wild type Δ12/Δ9-desaturase, a highly specific 16:0 Δ9-desaturase was obtained that exhibited only minimal Δ12-desaturation activity on 18:1Δ9 (Fig. 6). Our random and targeted mutagenesis results thus demonstrate that a limited number of amino acid changes can have a significant effect on the regioselectivity and chain length specificity of an acyl-CoA fatty acid desaturase. Similarly, the substitution of as little as 1 or 2 amino acids in some cyanobacterial and fungal acyl-lipid desaturases has been shown to change the positioning of the introduced double bond (10, 43).
Although the lack of a crystal structure precludes a detailed structural interpretation of the selected mutations and the majority of fatty acid desaturase mutagenesis studies are based on acyl-lipid desaturases, mapping onto a topological model of the cricket Δ12/Δ9-desaturase and analysis of desaturase single mutants allowed for some interesting observations. Two mutations, AdD12/9-A98V and AdD12/9-K136R, were found to be located within or close to the first two conserved histidine boxes that together with the third histidine box are believed to form the active site (44). Residues in the vicinity of the histidine boxes and particularly in the amino acid stretch following the second histidine box have been reported to influence the catalytic reaction outcome as well as the chain length specificity of FAD2-related enzymes such as the Lesquerella fendleri Δ12-hydroxylase and the Crepis alpina Δ12-acetylenase (45–47). Among the cricket Δ12/Δ9-desaturase single mutants, AdD12/9-K136R displayed the largest increase in desaturase activity. A similar substitution, AdD9-K138R, in the cricket Δ9-desaturase also increased catalytic activity. Therefore, this residue seems to be of importance for desaturation in general rather than influencing regioselectivity or chain length specificity.
Residues AdD9-F54 and AdD12/9-F52 appear to influence the specificity of the desaturation reaction in the cricket Δ9- and Δ12/Δ9-desaturase, respectively. Exchanging AdD9-F54 for tyrosine shifted the substrate specificity of the Δ9-desaturase from 18:0 to 16:0. Such a change toward shorter fatty acid substrates without an immediate effect on regioselectivity is reminiscent of soluble fatty acid desaturase mutants with reduced binding pockets behind the active site (4, 39). In the cricket Δ12/Δ9-desaturase, a single AdD12/9-F52Y mutation reduced Δ12-desaturation significantly, whereas Δ9-desaturation of 16:0 was comparable with the wild type enzyme. The effect of this mutation is more difficult to interpret because steric hindrance at the end of the substrate binding cavity does not explain the reduction in Δ12-desaturation activity. Possibly, direct or long distance effects in the region that is in close contact with the CoA thioester or changes in the upper portion of the substrate binding channel interfere with a correct positioning of the 18:1Δ9 substrate relative to the active site. A similar mechanism was suggested for changes in the regioselectivity of soluble desaturases (3, 4). In a topological model of the cricket Δ12/Δ9-desaturase, AdD12/9-F52 is located in the first predicted transmembrane region close to the membrane surface. When the first two putative transmembrane helices were exchanged between the Δ6 fatty acid desaturase and the Δ8 sphingolipid desaturase from Borago officinalis, the chimeric desaturase displayed a preference for 16:0 and 14:0 fatty acids instead of 18:0 (48). Although the cricket desaturase recognizes a different lipid head group, our results suggest that a similar region is also involved in a substrate binding site in acyl-CoA desaturases.
In summary, our results demonstrate for the first time that directed evolution can provide a valuable alternative to previous targeted mutagenesis and domain-swapping studies when studying the structural basis behind regioselectivity of fatty acid desaturases. Key residues in the cricket Δ12/Δ9-desaturase including AdD12/9-K136 and AdD12/9-F52 provide promising targets for further activity or specificity improvement by means of iterative or combinatorial saturation mutagenesis (39, 49). The results presented in this work are a step forward in our understanding of the structure-function relationship of membrane-bound fatty acid desaturases and will aid in fully unlocking the remarkable enzymatic plasticity displayed by this class of enzymes.
Acknowledgments
We thank J. Petrie for providing the I. galbana Δ9-elongase gene, V. Haritos for critical reading of the manuscript and supplying the T. castaneum Δ12-desaturase gene, Alec Zwart for help with statistical analyses, and X. R. Zhou for providing the cricket and A. thaliana desaturase genes as well as for technical expertise during GC-MS analysis.
- SC
- synthetic complete drop-out
- NL
- neutral lipids
- PC
- phosphatidylcholine.
REFERENCES
- 1. Shanklin J., Somerville C. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 2510–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindqvist Y., Huang W., Schneider G., Shanklin J. (1996) EMBO J. 15, 4081–4092 [PMC free article] [PubMed] [Google Scholar]
- 3. Guy J. E., Whittle E., Kumaran D., Lindqvist Y., Shanklin J. (2007) J. Biol. Chem. 282, 19863–19871 [DOI] [PubMed] [Google Scholar]
- 4. Cahoon E. B., Lindqvist Y., Schneider G., Shanklin J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4872–4877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guy J. E., Abreu I. A., Moche M., Lindqvist Y., Whittle E., Shanklin J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17220–17224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whittle E. J., Tremblay A. E., Buist P. H., Shanklin J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 14738–14743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shanklin J., Cahoon E. B. (1998) Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 611–641 [DOI] [PubMed] [Google Scholar]
- 8. Meesapyodsuk D., Reed D. W., Savile C. K., Buist P. H., Ambrose S. J., Covello P. S. (2000) Biochemistry 39, 11948–11954 [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann M., Hornung E., Busch S., Kassner N., Ternes P., Braus G. H., Feussner I. (2007) J. Biol. Chem. 282, 26666–26674 [DOI] [PubMed] [Google Scholar]
- 10. Meesapyodsuk D., Reed D. W., Covello P. S., Qiu X. (2007) J. Biol. Chem. 282, 20191–20199 [DOI] [PubMed] [Google Scholar]
- 11. Damude H. G., Zhang H., Farrall L., Ripp K. G., Tomb J. F., Hollerbach D., Yadav N. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9446–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang S., Sakuradani E., Ito K., Shimizu S. (2007) FEBS Lett. 581, 315–319 [DOI] [PubMed] [Google Scholar]
- 13. Zhang X., Wei D., Li M., Qi Y., Xing L. (2009) Mol. Biol. Rep. 36, 567–573 [DOI] [PubMed] [Google Scholar]
- 14. Jäckel C., Kast P., Hilvert D. (2008) Annu. Rev. Biophys. 37, 153–173 [DOI] [PubMed] [Google Scholar]
- 15. Turner N. J. (2009) Nat. Chem. Biol. 5, 567–573 [DOI] [PubMed] [Google Scholar]
- 16. Zhou X. R., Horne I., Damcevski K., Haritos V., Green A., Singh S. (2008) Insect Mol. Biol. 17, 667–676 [DOI] [PubMed] [Google Scholar]
- 17. Riddervold M. H., Tittiger C., Blomquist G. J., Borgeson C. E. (2002) Insect Biochem. Mol. Biol. 32, 1731–1740 [DOI] [PubMed] [Google Scholar]
- 18. Winzeler E. A., Shoemaker D. D., Astromoff A., Liang H., Anderson K., Andre B., Bangham R., Benito R., Boeke J. D., Bussey H., Chu A. M., Connelly C., Davis K., Dietrich F., Dow S. W., El Bakkoury M., Foury F., Friend S. H., Gentalen E., Giaever G., Hegemann J. H., Jones T., Laub M., Liao H., Liebundguth N., Lockhart D. J., Lucau-Danila A., Lussier M., M'Rabet N., Menard P., Mittmann M., Pai C., Rebischung C., Revuelta J. L., Riles L., Roberts C. J., Ross-MacDonald P., Scherens B., Snyder M., Sookhai-Mahadeo S., Storms R. K., Véronneau S., Voet M., Volckaert G., Ward T. R., Wysocki R., Yen G. S., Yu K., Zimmermann K., Philippsen P., Johnston M., Davis R. W. (1999) Science 285, 901–906 [DOI] [PubMed] [Google Scholar]
- 19. Vanhercke T., Ampe C., Tirry L., Denolf P. (2005) Anal. Biochem. 339, 9–14 [DOI] [PubMed] [Google Scholar]
- 20. Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 21. Hofmann K., Stoffel W. (1993) Biol. Chem. Hoppe Seyler 374, 166. [DOI] [PubMed] [Google Scholar]
- 22. Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001) J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 23. Kyte J., Doolittle R. F. (1982) J. Mol. Biol. 157, 105–132 [DOI] [PubMed] [Google Scholar]
- 24. Stukey J. E., McDonough V. M., Martin C. E. (1990) J. Biol. Chem. 265, 20144–20149 [PubMed] [Google Scholar]
- 25. Man W. C., Miyazaki M., Chu K., Ntambi J. M. (2006) J. Biol. Chem. 281, 1251–1260 [DOI] [PubMed] [Google Scholar]
- 26. McDonough V. M., Stukey J. E., Martin C. E. (1992) J. Biol. Chem. 267, 5931–5936 [PubMed] [Google Scholar]
- 27. Cripps C., Borgeson C., Blomquist G. J., de Renobales M. (1990) Arch. Biochem. Biophys. 278, 46–51 [DOI] [PubMed] [Google Scholar]
- 28. Borgeson C. E., de Renobales M., Blomquist G. J. (1990) Biochim. Biophys. Acta 1047, 135–140 [DOI] [PubMed] [Google Scholar]
- 29. Sayanova O., Haslam R., Guschina I., Lloyd D., Christie W. W., Harwood J. L., Napier J. A. (2006) J. Biol. Chem. 281, 36533–36541 [DOI] [PubMed] [Google Scholar]
- 30. Domergue F., Abbadi A., Zähringer U., Moreau H., Heinz E. (2005) Biochem. J. 389, 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoffmann M., Wagner M., Abbadi A., Fulda M., Feussner I. (2008) J. Biol. Chem. 283, 22352–22362 [DOI] [PubMed] [Google Scholar]
- 32. Cahoon E. B., Kinney A. J. (2005) Eur. J. Lipid Sci. Technol. 107, 239–243 [Google Scholar]
- 33. Sperling P., Ternes P., Zank T. K., Heinz E. (2003) Prostaglandins Leukot. Essent. Fatty Acids 68, 73–95 [DOI] [PubMed] [Google Scholar]
- 34. Hashimoto K., Yoshizawa A. C., Okuda S., Kuma K., Goto S., Kanehisa M. (2008) J. Lipid Res. 49, 183–191 [DOI] [PubMed] [Google Scholar]
- 35. Gostincar C., Turk M., Gunde-Cimerman N. (2010) J. Membr. Biol. 233, 63–72 [DOI] [PubMed] [Google Scholar]
- 36. Roelofs W. L., Rooney A. P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9179–9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fang S., Ting C. T., Lee C. R., Chu K. H., Wang C. C., Tsaur S. C. (2009) Mol. Biol. Evol. 26, 1447–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shanklin J. (2008) in Advances in Plant Biochemistry and Molecular Biology (Bohnert H. J., Nguyen H., Lewis N. G. eds) Vol. 1, pp. 29–47, Elsevier Science Ltd., Oxford, U.K [Google Scholar]
- 39. Whittle E., Shanklin J. (2001) J. Biol. Chem. 276, 21500–21505 [DOI] [PubMed] [Google Scholar]
- 40. Shanklin J., Guy J. E., Mishra G., Lindqvist Y. (2009) J. Biol. Chem. 284, 18559–18563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tracewell C. A., Arnold F. H. (2009) Curr. Opin. Chem. Biol. 13, 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aharoni A., Gaidukov L., Khersonsky O., McQ Gould S., Roodveldt C., Tawfik D. S. (2005) Nat. Genet. 37, 73–76 [DOI] [PubMed] [Google Scholar]
- 43. Hongsthong A., Subudhi S., Sirijuntarat M., Cheevadhanarak S. (2004) Appl. Microbiol. Biotechnol. 66, 74–84 [DOI] [PubMed] [Google Scholar]
- 44. Shanklin J., Whittle E., Fox B. G. (1994) Biochemistry 33, 12787–12794 [DOI] [PubMed] [Google Scholar]
- 45. Broun P., Shanklin J., Whittle E., Somerville C. (1998) Science 282, 1315–1317 [DOI] [PubMed] [Google Scholar]
- 46. Broadwater J. A., Whittle E., Shanklin J. (2002) J. Biol. Chem. 277, 15613–15620 [DOI] [PubMed] [Google Scholar]
- 47. Gagné S. J., Reed D. W., Gray G. R., Covello P. S. (2009) Biochemistry 48, 12298–12304 [DOI] [PubMed] [Google Scholar]
- 48. Libisch B., Michaelson L. V., Lewis M. J., Shewry P. R., Napier J. A. (2000) Biochem. Biophys. Res. Comm. 279, 779–785 [DOI] [PubMed] [Google Scholar]
- 49. Reetz M. T., Carballeira J. D. (2007) Nat. Protoc. 2, 891–903 [DOI] [PubMed] [Google Scholar]