Abstract
CD38, a multifunctional enzyme that catalyzes the synthesis of intracellular Ca2+ messengers, cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP), is known to be expressed on platelets. However, the role of CD38 in platelets remains unclear. Our present results show that treatment of platelets with thrombin results in a rapid and sustained Ca2+ signal, resulting from a coordinated interplay of Ca2+-mobilizing messengers, inositol 1,4,5-trisphosphate, cADPR, and NAADP. By dissecting the signaling pathway using various agents, we delineated that cADPR and NAADP are sequentially produced through CD38 internalization by protein kinase C via myosin heavy chain IIA following phospholipase C activation in thrombin-induced platelets. An inositol 1,4,5-trisphosphate receptor antagonist blocked the thrombin-induced formation of cADPR and NAADP as well as Ca2+ signals. An indispensable response of platelets relying on cytosolic calcium is the surface exposure of phosphatidylserine (PS), which implicates platelet procoagulant activity. Scrutinizing this parameter reveals that CD38+/+ platelets fully express PS on the surface when stimulated with thrombin, whereas this response was decreased on CD38−/− platelets. Similarly, PS exposure and Ca2+ signals were attenuated when platelets were incubated with 8-bromo-cADPR, bafilomycin A1, and a PKC inhibitor. Furthermore, in vivo, CD38-deficient mice exhibited longer bleeding times and unstable formation of thrombus than wild type mice. These results demonstrate that CD38 plays an essential role in thrombin-induced procoagulant activity of platelets and hemostasis via Ca2+ signaling mediated by its products, cADPR and NAADP.
Keywords: Calcium Intracellular Release, Endocytosis, Hemostasis, NAD, Platelet, Protein Kinase C (PKC), Thrombin, CD38, NAADP, Cyclic ADP-ribose
Introduction
An increase in intracellular calcium ion concentrations ([Ca2+]i) is a major event for the activation of platelets (1). Ca2+ is mobilized from intracellular Ca2+ stores by various second messengers, including inositol 1,4,5-trisphosphate (IP3),3 cyclic ADP-ribose (cADPR), and nicotinic acid adenine dinucleotide phosphate (NAADP). IP3 is produced by activated phospholipase C (PLC), whereas cADPR and NAADP are produced by the type II transmembrane glycoprotein CD38 (2). These Ca2+ messengers act on their specific receptors/channels on intracellular stores: IP3 receptors (IP3R), ryanodine receptors (RyRs), and two-pore channels, respectively (2–4).
Since the presence of CD38 on the surface of platelets was first reported, a number of attempts have been made to illustrate the role of cADPR and NAADP in platelets (5). Although no direct evidence has been presented yet, cADPR formation in platelets has been demonstrated (5, 6), and the association of CD38 with the cytoskeleton of platelets upon thrombin stimulation has been shown (7, 8). Platelet cytoskeletal reorganization, upon thrombin stimulation, is a highly dynamic process involving protein phosphorylation (9). Particularly, protein kinase C (PKC) phosphorylates non-muscle myosin heavy chain IIA (MHCIIA) at Ser-1917 of the C-terminal end in mast cells (10), and in platelets (11–16). In platelets, at least four PKC isoforms (α, β, δ, θ) are expressed (17, 18), and it has become clear that each isoform plays a different role in platelet function, even including a negative role (19, 20). CD38 has been shown to be internalized by associating with MHCIIA for the synthesis of cADPR in lymphokine-activated killer cells by forming a complex of phospho-MHCIIA, Lck, and CD38 (21). Therefore, it is important to clarify the possible role of PKC in the activation of CD38 in platelets.
The initial response of platelets upon stimulation, depending on the stimuli, is a rapid and sustained [Ca2+]i rise followed by functional responses of platelets. In quiescent platelets, PS is maintained in the inner leaflet of the plasma membrane. Stimulation of platelets subsequently switches on the activity of a Ca2+-dependent phospholipid scramblase (22, 23), resulting in surface exposure of negatively charged PS, required for the binding of coagulation factor complexes (24). PKC isoforms variably regulate platelet Ca2+ signals and have influences on PS exposure (19, 25, 26). Furthermore, different stimulants of platelets play variable roles in PS exposure; for instance, collagen results in increased PS exposure, whereas thrombin as a co-agonist additionally contributes to PS exposure (27–30). Collagen as a stimulant depends on extracellular Ca2+ (31, 32), whereas thrombin very efficiently mobilizes intracellular Ca2+ from internal stores (33–35), and therefore, PS exposure is additionally enhanced with thrombin.
Human platelets possess two intracellular Ca2+ stores (1, 36). These stores respond differentially to physiological stimuli; thrombin evokes Ca2+ release from these stores, which requires IP3 and NAADP, whereas ADP and vasopressin exert an IP3-dependent release of Ca2+ in human platelets (37). The function of the IP3-dependent, dense tubular system can be inhibited by a low dose of thapsigargin, whereas the function of NAADP-dependent stores is impaired by vacuolar H+-ATPase inhibitors, such as nigericin and bafilomycin A1 (38), suggesting the acidic nature of the NAADP-sensitive Ca2+ store (39).
The question whether CD38 is involved in the mobilization of Ca2+ from intracellular stores upon activation of platelets has been an important issue. Therefore, to decipher the role of CD38 in platelets, we employed platelets from CD38−/− and CD38+/+ mice and compared the various events related to Ca2+ signaling induced by thrombin. Our study reveals that platelets from CD38−/− mice showed low amplitude of sustained Ca2+ signal and reduced PS exposure in response to thrombin, indicating that CD38 plays an essential role in Ca2+ signaling in thrombin-induced platelets.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Thrombin, 8-bromo-cADPR (8-Br-cADPR), RO318220 (R136), U73122, ATP assay kit, nicotinamide guanine dinucleotide, and FITC-annexin V were purchased from Sigma-Aldrich. Xestospongin C (XeC) and BAPTA-AM were purchased from Calbiochem (Darmstadt, Germany). Bafilomycin A1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Fura-2 AM and Fluo-3 were purchased from Invitrogen. Ned-19 was purchased from Aurora Fine Chemicals (San Diego, CA). Antibodies (Abs) were obtained from the following sources. Anti-mouse CD38, LAMP-1, and FITC-CD38 monoclonal antibodies (mAbs) were from BD Biosciences; anti-MHCIIA polyclonal antibody (pAb) was from Covance (Emeryville, CA); and Ab against RyR and V-ATPase, GRP78, horseradish peroxidase-conjugated anti-mouse IgG anti-goat IgG, and anti-rabbit IgG were from Santa Cruz Biotechnology. Anti-Lck pAb was from Upstate Biotech Millipore (Lake Placid, NY). Anti-NaK-ATPase mAb was from Novus Biologicals (Littleton, CO). Anti-goat TRITC-conjugated Abs were purchased from Sigma-Aldrich.
Platelet Preparation
CD38−/− mice were bought from The Jackson Laboratory. All animal studies were performed according to a protocol approved by the Institutional Animal Care and Use Committee of Chonbuk National University Medical School. Blood was drawn by cardiac puncture into an acid citrate dextrose solution (20 mm citric acid, 110 mm sodium citrate, and 5 mm glucose) in a 1:10 ratio v/v. Platelets were prepared as described previously by centrifugation. In brief, acid citrate dextrose solution and blood were centrifuged at 2,000 rpm for 6 min at room temperature. Platelet-rich plasma was removed from the top in a separate tube containing 200 μl of modified Tyrode's buffer (TB) (134 mm NaCl, 5 mm HEPES, 5 mm d-glucose, 2.9 mm KCl, 0.34 mm Na2HPO4, 1 mm MgCl2, and 12 mm NaHCO3; pH 7.3). Platelet-rich plasma collected in one tube was spun again at 5,000 rpm for 40 s for the removal of red blood cells and white blood cells that are usually present. Platelet-rich plasma was then transferred to another tube and centrifuged at 6,000 rpm for 6 min. Pelleted platelets were resuspended to the required density in TB, and platelet count was adjusted to 0.5 × 109/ml with an optical method as described previously (40). Platelets in TB along with 0.1% bovine serum albumin (BSA) were then rested for 60 min at 37 °C prior to their use for the experiment. For the calcium study, 0.4 units/ml apyrase was added during the resting period to minimize the effect of ADP and ATP so that only the thrombin effect was evident.
In Vitro Thrombus Formation
In vitro thrombus aggregation and formation were assessed in a clear flat-bottomed, 96-well plate as described previously (41, 42). However, at the end of each experiment, images were captured from the respective wells. The Thermomax microplate reader was turned on at least 30 min before the experiment and prewarmed to 37 °C. Appropriate volumes of TB-suspended platelets were added and stimulated with 0.5 units/ml thrombin or the same volume of vehicle in the control by using a multipipette. The plate was stirred for 5 s in a microplate reader at 650 nm, and then after every 15 s, it was agitated for 3 s. After 10 min, the extra platelets were removed from one corner by tilting the plate. Reactions were stopped by adding 200 μl of 3% ice-cold formalin diluted in PBS, which was removed after 15 min and washed twice with ice-cold PBS. Phase contrast images from five random microscopic fields were acquired by using a Nikon Eclipse TS100 at a total magnification of 100. Images acquired were captured using AVT-ActiveCam Viewer version 1.1.0. Aggregated clusters of platelets were visually counted.
Platelet Procoagulant Index and CD38 Internalization
The procoagulant index assessment was performed in 24-well plates. Washed platelets were added in respective wells and stimulated with 0.5 units/ml thrombin. Extra platelets were removed from one corner by tilting the plate. Respective wells were washed twice with PBS. The aggregated platelets were post-labeled with FITC-annexin A5 (0.5 μg/ml) and CD38 antibody. After 60 min of incubation in the dark, respective wells were washed with PBS, and TRITC-conjugated secondary antibody was added for CD38 Ab. After 30 min of incubation with secondary antibody, the wells were washed again. Fluorescence images were recorded under respective wavelengths from at least 5–7 randomly chosen microscopic fields with a Zeiss confocal microscope at a total magnification of 400. Images were analyzed with Carl Zeiss LSM Image examiner software for mean intensity of fluorescence, total area, and pixel density. Of these, the latter two essentially remain the same for all images. The procoagulant index of thrombi was determined as the ratio of the mean intensity of the surface coverage of PS-exposing platelets to the pixel density.
ATP Assay
TB-suspended platelets were stimulated with 0.5 units/ml thrombin for the desired time at 37 °C in micro tubes. After the desired time period, tubes were then spun at 13,000 rpm for 1 min at 4 °C. Appropriate volumes of the supernatant were transferred into other tubes containing ice-cold perchloric acid and left on ice after vortex. After centrifugation at 13,000 rpm for 10 min, the supernatant was transferred to another tube, and perchloric acid was removed with KHCO3 in a ratio of 3:1. After centrifugation, ATP in the supernatant was measured with a luciferase luciferin mixture by adding the appropriate volume in the sample with Lumat LB 9507. Values obtained were plotted against the standard value of ATP.
Determination of ADPR Cyclase Activity
ADPR cyclase activity was determined by fluorometrically using nicotinamide guanine dinucleotide as substrate. TB-suspended platelets were incubated with or without 0.5 units/ml thrombin at 37 °C for 5 min. After 5 min, 200 μm nicotinamide guanine dinucleotide was added and incubated for another 5 min. The reaction was stopped with the appropriate amount of 10% TCA. The samples were centrifuged at 13,000 rpm for 10 min at 4 °C. Supernatant (80 μl) was diluted with 720 μl of sodium phosphate buffer, pH. 7.2. Fluorescence of cyclic GDP-ribose produced was determined at an excitation/emission wavelength of 297/410 nm.
Measurement of [Ca2+]i
Washed platelets were incubated with 5 μm Fura 2-AM in TB containing 1% BSA for 40 min. In some experiments, an inhibitor was also added and incubated. After the required time of incubation and washing once, 1 ml of Fura 2-AM loaded platelets was subjected to Photon Technology International in a magnetically stirred cuvette at 37 °C with excitation wavelengths of 340 and 380 nm and emission at 500 nm. Platelets were then stimulated with 0.5 units/ml thrombin. Changes in [Ca2+]i were monitored using a 340/380 nm fluorescence ratio.
cADPR and NAADP Assay
TB-suspended platelets were stimulated with thrombin, 0.5 units/ml, for a specific time period with or without an inhibitor. The rest of the protocol for their measurement was essentially the same as described previously (43).
Immunoprecipitation and Western Blotting
Washed platelets were stimulated with 0.5 units/ml thrombin after incubation with or without an inhibitor for 5 min. The reaction was stopped with ice-cold cell lysis buffer containing 20 mm HEPES (pH 7.2), 1% (v/v) Triton X-100, 10% glycerol, 100 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 50 mm NaF, 1 mm Na3VO4, 10 g/ml leupeptin, 10 μg/ml pepstatin, and 10 μg/ml aprotinin. Samples were left on ice for 30 min, and supernatants were obtained after centrifugation at 13,000 rpm for 10 min. Protein concentration of the supernatant was determined using a Bio-Rad protein assay kit against known concentrations of BSA as the standard. For immunoprecipitation, cell lysates were precleared with protein G-agarose for 120 min, and the supernatants were incubated with anti-CD38 mAb overnight at 4 °C and then further incubated with protein G-agarose at 4 °C for 120 min. The immunoprecipitates were washed three times with cell lysis buffer and boiled for 10 min. The immunoprecipitated proteins were subjected to SDS-PAGE on 10% gel. After transferring to nitrocellulose membranes, the blots were incubated in blocking buffer (10 mm Tris-HCl (pH 7.6), 150 mm NaCl, and 0.05% Tween 20) containing 3% BSA for 120 min at room temperature and then incubated with primary Ab, MHCIIA (1:2,000 dilution) in 1% BSA blocking buffer overnight at 4 °C. The blots were rinsed four times with blocking buffer and incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000 dilution) in blocking buffer at room temperature for 60 min. The immunoreactive proteins with the respective secondary Ab were determined using an enhanced chemiluminescence kit (Amersham Biosciences AB) and exposed to a LAS-1000 ImageReader Lite (Fujifilm).
Flow Cytometry
Washed platelets were stimulated with 0.5 units/ml thrombin for 5 min at 37 °C after incubating with or without an inhibitor. After the appropriate time, the reaction was stopped by adding 1 ml of 3% ice-cold formalin diluted in PBS. After 15 min of fixation on ice, the tubes were spun at 6,000 rpm for 6 min at 4 °C and washed once with ice-cold PBS. After washing, 500 μl of PBS containing 3% BSA was added and left on a rotator in a cold room for 60 min. The tubes were centrifuged again at 6,000 rpm for 6 min at 4 °C and washed once with ice-cold PBS. After washing, pellets were resuspended in 500 μl of PBS containing 1% BSA and were incubated with CD38 Abs conjugated with FITC for 120 min in dark cold room. After incubation, platelets were washed once and resuspended in the appropriate volume, and the sample was subjected to FACS along with the control, which did not have the FITC antibody. A total of 10,000 events per sample were collected with a BD Biosciences FACSCalibur using the CellQuest program. A color- and light-sensitive substance was run with the control for calibration purposes.
Analysis of Bleeding Time
The procedure was conducted on 20–25-g male mice. Mice were anesthetized using ketamine. An equal length of the tail was cut using a sharp blade. The tail was immersed in a BD Biosciences Falcon tube containing normal saline, which was prewarmed to 37 °C. The appearance of a blood streak was considered as the start time, and disappearance or cessation of bleeding was recorded as the end time for bleeding.
Assessment of in Vivo Thrombus Formation after Injury Induced by FeCl3
Mice weighing 20–25 g were anesthetized using ketamine by intraperitoneal injection. Mice were placed on a prewarmed surface. Bilateral peritoneal incisions were made, and the abdominal contents were exposed. Exposed intestines were carefully examined, and the superior mesenteric artery was located (44). A feces-free area that was directly supplied by the superior mesenteric artery was located. Saline-soaked filter paper was placed, and the segment of intestine was placed on top of it and was studied for perfusion. The intestine was sprayed with prewarmed saline after every 2 min. A 1 × 2-mm patch of No. 1 Whatman filter paper, soaked in 10% FeCl3, was applied to the exposed artery for 1 min (45). Blood perfusion was recorded using a small animal laser Doppler flow meter (model BLF 21, Transonic Systems Inc.) for up to 30 min. Thrombus formation, as defined by lack of arterial flow, was recorded for data analysis. Occlusive thrombi were considered stable if total occlusion lasted for greater than 10 min.
Statistics
Statistical analysis was carried out on raw data using Sigma Plot 9 by using the unpaired Student's t test; p < 0.05 was considered statistically significant. Values are expressed as mean, and n indicates number of experiment.
Supplemental Methods
Methods for cellular fractionation preparation of T cells, RNA isolation, and RT-PCR are described in the supplemental file.
RESULTS AND DISCUSSION
Thrombin-induced Platelet Activation Is Compromised by CD38 Deficit
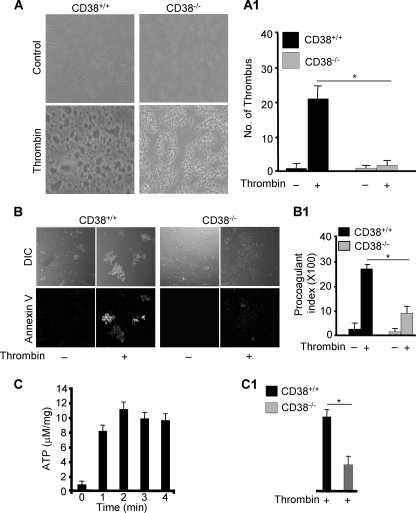
We noticed a significant difference in thrombus formation of platelets from CD38−/− mice in response to thrombin when compared with those from CD38+/+ mice (Fig. 1A), suggesting that CD38 plays an important role in thrombin-induced platelet activation. To confirm this notion, we compared thrombin-induced PS exposure in platelets in CD38−/− and wild type mice. Platelets from CD38−/− mice showed a significant decrease in the thrombin-induced PS exposure when compared with those from CD38+/+ mice (Fig. 1B). In addition, degranulation of dense granules was greatly impaired in platelets from CD38−/− mice in response to thrombin when compared with those from CD38+/+ mice (Fig. 1, C and C1). Because CD38 is a mammalian prototype enzyme for producing Ca2+ signal messengers (2) and cytoplasmic Ca2+ is known to be necessary for thrombin-induced platelet activation (33–35), we measured intracellular Ca2+ concentration ([Ca2+]i) in platelets from CD38−/− and wild type mice before and after treatment of thrombin. Platelets from CD38−/− mice showed significantly reduced amplitude of thrombin-induced Ca2+ signals when compared with those from wild type mice (Fig. 2A). These findings indicate that CD38 plays an essential role in thrombin-induced platelet activation through producing Ca2+ signal messenger(s).
FIGURE 1.
Deficiency of CD38 attenuates thrombus formation, PS exposure, and degranulation. Washed platelets of CD38+/+ and CD38−/− mice were simultaneously processed and stimulated with 0.5 units/ml thrombin for in vitro thrombus formation (A) and PS exposure (B). The respective bar graphs (A1 and B1) represent the mean value ± S.D. from 3–4 independent platelet preparations; *, p < 0.01. DIC, differential interference contrast. C, ATP secretion. Platelets were stimulated with 0.5 units/ml thrombin for the indicated times, and secreted ATP was assessed from the supernatant. C1, platelets of CD38+/+ and CD38−/− mice were stimulated with 0.5 units/ml thrombin for 2 min, and secreted ATP was measured. The bar graphs represent the mean value ± S.D. from 2–4 independent platelet preparations; *, p < 0.01.
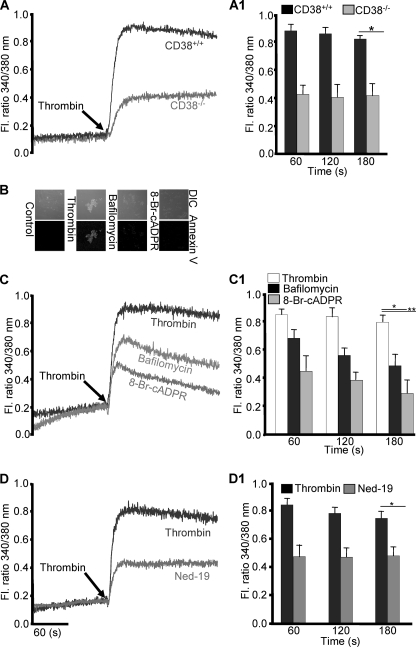
FIGURE 2.
CD38 products, cADPR and NAADP, are required for thrombin-induced Ca2+ signals and PS exposure. A, CD38+/+ and CD38−/− washed platelets were stimulated with 0.5 units/ml thrombin for [Ca2+]i measurement. Washed platelets from CD38+/+ mice were suspended in an appropriate volume of TB with or without incubation with 300 nm bafilomycin A1 or 100 μm 8-Br-cADPR for 30 min and treated with 0.5 units/ml thrombin for PS exposure (B) and [Ca2+]i (C). D, platelets incubated with or without 1 mm Ned-19 for 25 min and then treated with 0.5 units/ml thrombin for [Ca2+]i measurement. The respective bar graphs (A1, C1, and D1) represent the mean value ± S.D. of the fluorescence (FL) ratio of [Ca2+]i from 4–5 independent platelet preparations. *, p < 0.03 and **, p < 0.02.
Role of cADPR and NAADP in Ca2+ Signals of Platelets
Next, we further characterized thrombin-induced Ca2+ signals in platelets using various agents. Pretreatment with an antagonistic analog of cADPR, 8-Br-cADPR, significantly abolished thrombin-induced PS exposure and Ca2+ signals (Fig. 2, B and C), indicating that one of the CD38 products, cADPR, is involved in thrombin-induced PS exposure. Similarly, the role of NAADP, another product of CD38, on PS exposure was assessed by using bafilomycin A1, a specific inhibitor of vacuolar H+-ATPase. This is required for maintaining the acidity of NAADP-sensitive Ca2+ stores, as reported earlier (38), as lysosomal-like organelles in human platelets. Pretreatment of platelets with the agent also decreased thrombin-induced PS exposure as well as Ca2+ signals (Fig. 2, B and C). To confirm the involvement of NAADP in thrombin-induced Ca2+ signals, we used a specific inhibitor of the NAADP receptor, Ned-19, and we found that the agent also significantly inhibited thrombin-induced Ca2+ signals (Fig. 2D). The difference between the inhibitory effect of bafilomycin A1 and 8-Br-cADPR on thrombin-induced Ca2+ signals may be because 8-Br-cADPR blocks NAADP formation (Fig. 3B1) as well as the action of cADPR, whereas bafilomycin A1 interferes with the function of NAADP but not of cADPR. Consistently, a putative target for cADPR, RyR, and the enzyme localized in a putative Ca2+ store for NAADP, vacuolar H+-ATPase, were proved by immunoblot analysis of cellular fractions (supplemental Fig. S1A), which was further proved by the fact that the treatment of respective fractions with cADPR and NAADP resulted in responses of Ca2+ signals (supplemental Fig. S1B). Furthermore, reverse transcription-PCR (RT-PCR) of RyRs showed the presence of RyR-2, but not RyR-1 and RyR-3 (supplemental Fig. S4). Moreover, pretreatment of platelets with IP3R antagonist XeC abrogated all the Ca2+ signals induced by thrombin (supplemental Fig. S2A1). Taken together, these results suggest that cADPR- and NAADP-mediated Ca2+ signals play important roles in thrombin-induced PS exposure and that IP3-mediated Ca2+ signaling is upstream of CD38 activation.
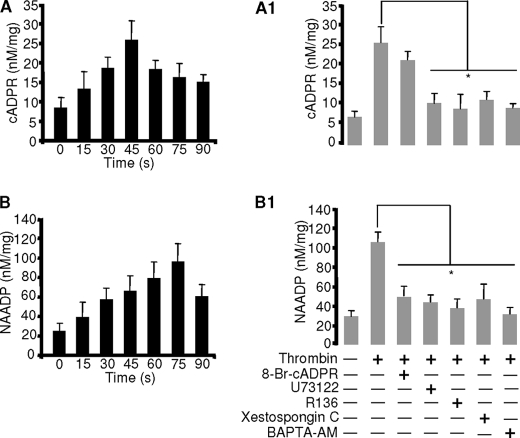
FIGURE 3.
Thrombin induces formation of cADPR and NAADP via PLC/IP3/PKC. Cyclic methods were employed as described under “Experimental Procedures.” Washed platelets were stimulated with 0.5 units/ml thrombin for cADPR (A) and NAADP (B). Washed platelets were preincubated with 100 μm 8-Br-cADPR, 2 μm U73122, 5 μg/ml PKC inhibitor, 2 μm XeC, or 10 μm BAPTA-AM stimulated with 0.5 units/ml thrombin for cADPR (A1) and NAADP (B1). The respective bar graphs represent the mean value ± S.D. from 3–4 independent experiments. *, p < 0.03.
PLC/IP3/PKC Is Involved in Thrombin-induced Formation of cADPR and NAADP
To confirm the above finding that thrombin induces activation of CD38 in platelets, we directly measured intracellular concentrations of cADPR and NAADP after stimulation with thrombin. Thrombin induced production of cADPR and NAADP with peaks at 45 and 75 s, respectively (Fig. 3, A and B). Because the above finding revealed that XeC completely blocked the thrombin-induced Ca2+ signal, this suggests that PLC/IP3/PKC may be involved upstream of CD38 activation. To confirm this notion, we examined various inhibitors on thrombin-induced CD38 activation. Pretreatment of platelets with a PLC inhibitor U73122, an IP3R antagonist XeC, a PKC inhibitor R136, or the intracellular Ca2+ chelator BAPTA-AM abolished thrombin-induced formation of both messengers (Fig. 3, A1 and B1). However, pretreatment of platelets with 8-Br-cADPR abolished the production of NAADP, suggesting that cADPR is required for NAADP production. Together, these results indicate that PLC/IP3/PKC are upstream effectors for the activation of CD38 and that cADPR formation precedes NAADP.
PKC Is Required for CD38-mediated Calcium Signaling
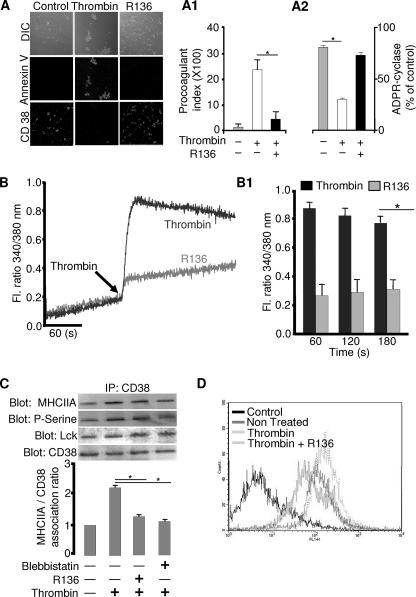
Because the above finding suggests that PKC is an upstream effector for the activation of CD38, we examined whether PKC is directly involved in thrombin-induced PS exposure and Ca2+ signals. A PKC inhibitor, R136, significantly reduced thrombin-induced PS exposure, CD38 internalization, and Ca2+ signals (Fig. 4, A and B), suggesting that PKC is involved. Furthermore, ectocellular CD38 enzyme activity was abolished with thrombin and the thrombin-induced down-regulation of cyclase activity was blocked by pretreatment with R136 (Fig. 4A2). PKC is known to be activated and phosphorylate MHCIIA in stimulated platelets. Therefore, we reasoned that PKC may be involved in CD38 activation by its phosphorylation of MHCIIA, which associates with CD38 via Lck. To prove this hypothesis, we examined the effects of the PKC inhibitor on the association of CD38 via Lck and phospho-MHCIIA in platelets before and after treatment of thrombin. We first identified Lck in mouse platelets by immunoblotting (supplemental Fig. S3), which is in accordance with an earlier observation by Pestina et al. (46). Interestingly, association of CD38 and Lck was not affected by thrombin alone or with R136 (Fig. 4C), suggesting that Lck is constitutively associated with CD38. However, R136 significantly reduced the thrombin-induced association of CD38 and MHCIIA along with inhibiting serine phosphorylation of MHCIIA (Fig. 4C). Furthermore, blebbistatin, a MHCIIA inhibitor, also decreased the thrombin-induced association of the molecules (Fig. 4C). FACS data also showed that R136 abolished the thrombin-induced CD38 internalization (Fig. 4D). These results suggest that PKC is involved in thrombin-induced CD38 internalization through association of CD38 with MHCIIA via Lck in platelets, through serine phosphorylation of MHCIIA.
FIGURE 4.
PKC induces the internalization of CD38 through the association of CD38 via Lck with MHCIIA for thrombin-induced Ca2+ signals and PS exposure. A and B, effects of PKC inhibitor on thrombin-induced PS exposure, CD38 internalization (A), and [Ca2+]i increase (B) in platelets. The bar graphs represent the mean value ± S.D. of the procoagulant index (A1), ectocellular ADPR cyclase activity (A2), and fluorescence ratio of [Ca2+]i (B1) from 3–5 independent platelet preparations. *, p < 0.01. DIC, differential interference contrast. C, washed platelets (n = 3) were incubated with 5 μg/ml R136 for 25 min or 50 μm blebbistatin for 30 min and then stimulated with 0.5 units/ml thrombin. The immunoprecipitated (IP) proteins were analyzed by immunoblotting with anti-CD38 mAb, anti-Lck pAb, anti-phosphoserine (P-Serine), or anti-MHCIIA pAb. The respective bar graph represents the association ratio of CD38 with MHCIIA. The first column is assigned a value of 1 for relative comparison. *, p < 0.03. D, washed platelets (n = 4) were preincubated with or without R136 and then stimulated with 0.5 units/ml thrombin. CD38 fluorescence intensity measured in flow cytometry.
Ablation of CD38 Markedly Impedes Thrombus Formation in Vivo
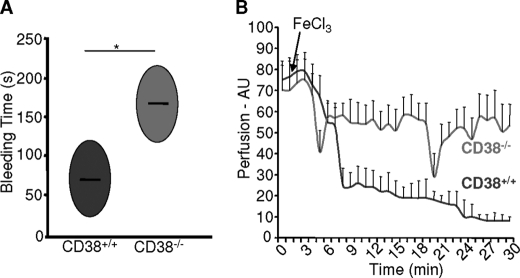
Having demonstrated in our in vitro data the importance of CD38 for the formation of cADPR and NAADP in thrombin-induced PS exposure and Ca2+ signals, it was imperative to demonstrate its direct role in hemostasis and thrombosis. Therefore, we directly compared the bleeding time of two genotypes, CD38+/+ and CD38−/− mice, in an in vivo tail bleeding assay. Bleeding times for CD38−/− mice were significantly longer than those for CD38+/+ mice (163 ± 29 s versus 66 ± 16 s; Fig. 5A). Details of this finding were captured on video (supplemental video data). We further examined a role of CD38 in platelet function by comparing the stable clot formation following chemical-induced injury of the superior mesenteric artery in CD38+/+ and CD38−/− mice. CD38+/+ mice rapidly formed occlusive thrombi that were stable over 30 min in the length of the experiment, whereas stable arterial occlusion was not detected in CD38−/− mice, whereas a 20% increase in perfusion was evident (Fig. 5B).
FIGURE 5.
Deficiency of CD38 impedes thrombus formation in vivo. A, in vivo hemostasis was assessed between the two genotypes. The areas of the ovals represent the average bleeding time of two individual experiments with four mice per group. *, p < 0.05. B, arterial thrombus formation was assessed in CD38+/+ and CD38−/− mice. The perfusion of intestine and time are shown on the y axis and x axis, respectively. Data represent two individual experiments with 3–4 mice per group. AU, arbitrary units.
CD38-mediated Ca2+ Signaling in Thrombin-induced Activated Platelets
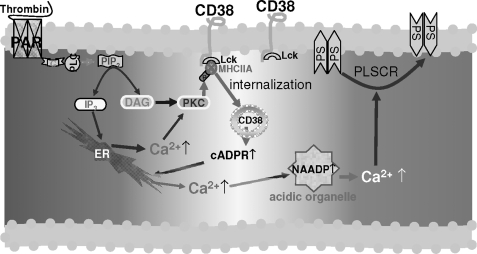
In the present study, we provide evidence for a key role of CD38 in platelet function and thrombosis in mice. CD38−/− platelets displayed significantly diminished PS exposure and Ca2+ signaling in response to thrombin. Consistently, our data showed that the thrombin-induced PS exposure and Ca2+ signals were blocked by antagonistic analogs or receptor inhibitors of cADPR and NAADP, indicating that thrombin-induced platelet activation is dependent on cADPR/NAADP-mediated Ca2+ signaling. Activation of CD38 requires its internalization from the cell surface to the cytosol. The finding that CD38 internalization was blocked by an IP3R blocker and a PKC inhibitor indicates that IP3-mediated Ca2+ signals and PKC are essential for CD38 activation. This suggests that PKC isozyme(s), which is responsible for CD38 internalization, is a Ca2+-dependent conventional isoform of PKC. Our present and previous data (21) showed that CD38 internalization is mediated through PKC-mediated phosphorylation of MHCIIA, resulting in cADPR formation. NAADP production seems to require cADPR-mediated Ca2+ signals. These findings enabled us to draw a final picture of the pathway that leads to CD38 internalization/activation, and thereby, cADPR/NAADP-mediated Ca2+ signaling (Fig. 6).
FIGURE 6.
Proposed model of CD38 activation in platelets. Thrombin receptor, protease-activated receptor (PAR), couples with G protein and stimulates PLC, producing two second messengers, IP3 and diacylglycerol (DAG). IP3-mediated Ca2+ release along with diacylglycerol activates PKC, which induces the phosphorylation of MHCIIA. The Phospho-MHCIIA makes complexes with CD38 via Lck, which results in internalization of CD38 and subsequently allows the formation of cADPR and NAADP in platelets. Phospholipid scramblase (PLSCR), a Ca2+-dependent enzyme, flips the PS from inner membrane to the outer membrane.
These in vitro data with an in vivo study showing unstable thrombosis with prolonged bleeding times in CD38−/− mice demonstrate that CD38 is critical for complete Ca2+ signaling in platelet function. To establish that targeting of CD38 may be effective to prevent atherosclerosis and other hyperthrombosis diseases, such as deep venous thrombosis, further studies with human platelets are required.
This work was supported by the Korea Science and Engineering Foundation (National Research Laboratory Grant R0A-2007-000-20121-0) (to U.-H. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental methods, Figs. S1–S4, and supplemental movies.
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- IP3 receptor
- cADPR
- cyclic ADP-ribose
- 8-Br-cADPR
- 8-bromo-cADPR
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- PLC
- phospholipase C
- RyR
- ryanodine receptor
- PS
- phosphatidylserine
- TB
- Tyrode's buffer
- TRITC
- tetramethylrhodamine isothiocyanate
- XeC
- xestospongin C
- BAPTA-AM
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- Ab
- antibody.
REFERENCES
- 1. Jardín I., López J. J., Pariente J. A., Salido G. M., Rosado J. A. (2008) Trends Cardiovasc. Med. 18, 57–61 [DOI] [PubMed] [Google Scholar]
- 2. De Flora A., Guida L., Franco L., Zocchi E., Bruzzone S., Benatti U., Damonte G., Lee H. C. (1997) J. Biol. Chem. 272, 12945–12951 [DOI] [PubMed] [Google Scholar]
- 3. Lee H. C. (2000) J. Membr. Biol. 173, 1–8 [DOI] [PubMed] [Google Scholar]
- 4. Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramaschi G., Torti M., Festetics E. T., Sinigaglia F., Malavasi F., Balduini C. (1996) Blood 87, 2308–2313 [PubMed] [Google Scholar]
- 6. Ohlmann P., Leray C., Ravanat C., Hallia A., Cassel D., Cazenave J. P., Gachet C. (1998) Biochem. J. 331, 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torti M., Festetics E. T., Bertoni A., Sinigaglia F., Balduini C. (1998) FEBS. Lett. 428, 200–204 [DOI] [PubMed] [Google Scholar]
- 8. Torti M., Tolnai Festetics E., Bertoni A., Sinigaglia F., Balduini C. (1998) FEBS. Lett. 431, 19–22 [DOI] [PubMed] [Google Scholar]
- 9. Lyons R. M., Stanford N., Majerus P. W. (1975) J. Clin. Invest. 56, 924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ludowyke R. I., Elgundi Z., Kranenburg T., Stehn J. R., Schmitz-Peiffer C., Hughes W. E., Biden T. J. (2006) J. Immunol. 177, 1492–1499 [DOI] [PubMed] [Google Scholar]
- 11. Moussavi R. S., Kelley C. A., Adelstein R. S. (1993) Mol. Cell. Biochem. 127, 219–227 [DOI] [PubMed] [Google Scholar]
- 12. Kriajevska M., Tarabykina S., Bronstein I., Maitland N., Lomonosov M., Hansen K., Georgiev G., Lukanidin E. (1998) J. Biol. Chem. 273, 9852–9856 [DOI] [PubMed] [Google Scholar]
- 13. Watson S. P., McNally J., Shipman L. J., Godfrey P. P. (1988) Biochem. J. 249, 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikebe M., Reardon S. (1990) Biochemistry 29, 2713–2720 [DOI] [PubMed] [Google Scholar]
- 15. Kawamoto S., Bengur A. R., Sellers J. R., Adelstein R. S. (1989) J. Biol. Chem. 264, 2258–2265 [PubMed] [Google Scholar]
- 16. Straussman R., Even L., Ravid S. (2001) J. Cell. Sci. 114, 3047–3057 [DOI] [PubMed] [Google Scholar]
- 17. Crosby D., Poole A. W. (2002) J. Biol. Chem. 277, 9958–9965 [DOI] [PubMed] [Google Scholar]
- 18. Khan W. A., Blobe G., Halpern A., Taylor W., Wetsel W. C., Burns D., Loomis C., Hannun Y. A. (1993) J. Biol. Chem. 268, 5063–5068 [PubMed] [Google Scholar]
- 19. Harper M. T., Poole A. W. (2010) J. Biol. Chem. 285, 19865–19873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crosby D., Poole A. W. (2003) J. Biol. Chem. 278, 24533–24541 [DOI] [PubMed] [Google Scholar]
- 21. Rah S. Y., Park K. H., Nam T. S., Kim S. J., Kim H., Im M. J., Kim U. H. (2007) J. Biol. Chem. 282, 5653–5660 [DOI] [PubMed] [Google Scholar]
- 22. Zwaal R. F., Comfurius P., Bevers E. M. (2005) Cell. Mol. Life Sci. 62, 971–988 [DOI] [PubMed] [Google Scholar]
- 23. Williamson P., Christie A., Kohlin T., Schlegel R. A., Comfurius P., Harmsma M., Zwaal R. F., Bevers E. M. (2001) Biochemistry 40, 8065–8072 [DOI] [PubMed] [Google Scholar]
- 24. Wolfs J. L., Wielders S. J., Comfurius P., Lindhout T., Giddings J. C., Zwaal R. F., Bevers E. M. (2006) Blood 108, 2223–2228 [DOI] [PubMed] [Google Scholar]
- 25. Gilio K., Harper M. T., Cosemans J. M., Konopatskaya O., Munnix I. C., Prinzen L., Leitges M., Liu Q., Molkentin J. D., Heemskerk J. W., Poole A. W. (2010) J. Biol. Chem. 285, 23410–23419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siffert W., Akkerman J. W. (1988) J. Biol. Chem. 263, 4223–4227 [PubMed] [Google Scholar]
- 27. Gilio K., van Kruchten R., Braun A., Berna-Erro A., Feijge M. A., Stegner D., van der Meijden P. E., Kuijpers M. J., Varga-Szabo D., Heemskerk J. W., Nieswandt B. (2010) J. Biol. Chem. 285, 23629–23638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heemskerk J. W., Vuist W. M., Feijge M. A., Reutelingsperger C. P., Lindhout T. (1997) Blood 90, 2615–2625 [PubMed] [Google Scholar]
- 29. Keuren J. F., Wielders S. J., Ulrichts H., Hackeng T., Heemskerk J. W., Deckmyn H., Bevers E. M., Lindhout T. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1499–1505 [DOI] [PubMed] [Google Scholar]
- 30. Heemskerk J. W., Kuijpers M. J., Munnix I. C., Siljander P. R. (2005) Trends Cardiovasc. Med. 15, 86–92 [DOI] [PubMed] [Google Scholar]
- 31. Ardlie N. G., Garrett J. J., Bell L. K. (1986) Thrombosis Res. 42, 115–124 [DOI] [PubMed] [Google Scholar]
- 32. Roberts D. E., McNicol A., Bose R. (2004) J. Biol. Chem. 279, 19421–19430 [DOI] [PubMed] [Google Scholar]
- 33. Giambelluca M. S., Gende O. A. (2007) Blood Coagul. Fibrinolysis 18, 303–308 [DOI] [PubMed] [Google Scholar]
- 34. Paul B. Z., Daniel J. L., Kunapuli S. P. (1999) J. Biol. Chem. 274, 28293–28300 [DOI] [PubMed] [Google Scholar]
- 35. Davies T. A., Drotts D. L., Weil G. J., Simons E. R. (1989) J. Biol. Chem. 264, 19600–19606 [PubMed] [Google Scholar]
- 36. Rosado J. A., Nuñez A. M., Lopez J. J., Pariente J. A., Salido G. M. (2006) Arch. Biochem. Biophys. 452, 9–16 [DOI] [PubMed] [Google Scholar]
- 37. López J. J., Redondo P. C., Salido G. M., Pariente J. A., Rosado J. A. (2006) Cell. Signal. 18, 373–381 [DOI] [PubMed] [Google Scholar]
- 38. López J. J., Camello-Almaraz C., Pariente J. A., Salido G. M., Rosado J. A. (2005) Biochem. J. 390, 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Michelangeli F., Ogunbayo O. A., Wootton L. L. (2005) Curr. Opin. Cell. Biol. 17, 135–140 [DOI] [PubMed] [Google Scholar]
- 40. Lee V. S., Tarassenko L. (1992) J. Biochem. Biophys. Methods 24, 215–223 [DOI] [PubMed] [Google Scholar]
- 41. Bednar B., Condra C., Gould R. J., Connolly T. M. (1995) Thromb. Res. 77, 453–463 [DOI] [PubMed] [Google Scholar]
- 42. Ribeiro J. M., Francischetti I. M. (2001) J. Exp. Biol. 204, 3887–3894 [DOI] [PubMed] [Google Scholar]
- 43. Rah S. Y., Park K. H., Han M. K., Im M. J., Kim U. H. (2005) J. Biol. Chem. 280, 2888–2895 [DOI] [PubMed] [Google Scholar]
- 44. Renné T., Pozgajová M., Grüner S., Schuh K., Pauer H. U., Burfeind P., Gailani D., Nieswandt B. (2005) J. Exp. Med. 202, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang X., Xu L. (2005) Thromb. Res. 115, 95–100 [DOI] [PubMed] [Google Scholar]
- 46. Pestina T. I., Stenberg P. E., Druker B. J., Steward S. A., Hutson N. K., Barrie R. J., Jackson C. W. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 3278–3285 [DOI] [PubMed] [Google Scholar]