Abstract
Corneal cross-linking using riboflavin and ultraviolet-A (RFUVA) is a clinical treatment targeting the stroma in progressive keratoconus. The stroma contains keratocan, lumican, mimecan, and decorin, core proteins of major proteoglycans (PGs) that bind collagen fibrils, playing important roles in stromal transparency. Here, a model reaction system using purified, non-glycosylated PG core proteins in solution in vitro has been compared with reactions inside an intact cornea, ex vivo, revealing effects of RFUVA on interactions between PGs and collagen cross-linking. Irradiation with UVA and riboflavin cross-links collagen α and β chains into larger polymers. In addition, RFUVA cross-links PG core proteins, forming higher molecular weight polymers. When collagen type I is mixed with individual purified, non-glycosylated PG core proteins in solution in vitro and subjected to RFUVA, both keratocan and lumican strongly inhibit collagen cross-linking. However, mimecan and decorin do not inhibit but instead form cross-links with collagen, forming new high molecular weight polymers. In contrast, corneal glycosaminoglycans, keratan sulfate and chondroitin sulfate, in isolation from their core proteins, are not cross-linked by RFUVA and do not form cross-links with collagen. Significantly, when RFUVA is conducted on intact corneas ex vivo, both keratocan and lumican, in their natively glycosylated form, do form cross-links with collagen. Thus, RFUVA causes cross-linking of collagen molecules among themselves and PG core proteins among themselves, together with limited linkages between collagen and keratocan, lumican, mimecan, and decorin. RFUVA as a diagnostic tool reveals that keratocan and lumican core proteins interact with collagen very differently than do mimecan and decorin.
Keywords: Collagen, Eye, Glycosaminoglycan, Protein Cross-linking, Protein-Protein Interactions, Collagen, Cornea, Cross-linking, Proteoglycans, Riboflavin Ultraviolet-A
Introduction
The cornea is the transparent, dome-shaped tissue covering the front of the eye. It is a powerful refracting surface, providing 65–75% of the eye's focusing power, and is made up of three distinct layers: epithelium, stroma, and endothelium. The stroma comprises about 90% of cornea thickness in humans (1). Although it is very highly innervated, it does not contain any blood vessels (2–4). Collagen gives the cornea its strength, elasticity, and form (5). The unique molecular shape, paracrystalline arrangement, and very regular fine diameter of the evenly spaced collagen fibrils are essential in producing a transparent cornea (6, 7).
In the corneal stromal extracellular matrix, glycosaminoglycan (GAG)2 polysaccharides are the most abundant negatively charged macromolecules and are classified on the basis of their repeating disaccharide structures into four main groups: keratan sulfate (KS), chondroitin sulfate/dermatan sulfate (CS/DS), heparan sulfate and heparin, and hyaluronan. Glycosaminoglycans, normally covalently attached to proteoglycan (PG) core proteins, play important roles in corneal transparency, nerve growth cone guidance, and cell adhesion, largely dependent on their patterns of sulfation or lack of it (8, 9). The corneal stroma is composed primarily of collagen fibrils, each of which consists of a core of type V collagen coated with type I collagen (10, 11), coated in turn by two classes of PGs (12): keratan sulfate proteoglycan and chondroitin sulfate/dermatan sulfate proteoglycan. Through N-linked oligosaccharides, KS chains are attached to three core proteins, lumican, keratocan, and mimecan, to form keratan sulfate proteoglycans (13–15). These three core proteins belong to a class of proteins known as small leucine-rich repeat proteins (16–18). Through O-linked oligosaccharide, CS/DS chains are attached to core protein decorin (19, 20). In the case of decorin, a single CS/DS linkage site is present near the amino terminus of the core protein (21, 22), whereas lumican and keratocan possess four or five potential KS attachment sites in the central leucine-rich repeat region of each core protein molecule (13, 23, 24), and mimecan has two potential KS attachment sites (25, 26). Current molecular models of the corneal stroma suggest that these proteoglycan core proteins wrap themselves laterally around the collagen fibrils in a manner that folds their hydrophobic domains inside, against the collagen fibrils (27). In contrast, the highly sulfated GAG chains (together with their associated water molecules of hydration) are thought to stick out laterally away from the sides of the collagen fibrils, forming an exterior hydrophilic shell. The thickness of that shell matches the thickness of the shell surrounding adjoining fibrils, producing a very precise center-to-center spacing between the collagen fibrils characteristic of the corneal stroma and necessary for its transparency (28). Through this interaction with collagen (mostly with type I), PGs play important biological roles in collagen fibrillogenesis and matrix assembly (29). They also may serve as binders of other proteins, some of which may be neurorepellants and neuroattractants (30).
Keratoconus is a vision disorder of unknown molecular etiology but with some genetic basis that occurs when the normally round cornea begins to bulge outward. This abnormal shape, arising as the central region becomes thinner, prevents the light entering the eye from being focused correctly on the retina and causes distortion of vision (31). Keratoconus may progress for 10–20 years and then slow in its progression. Each eye may be affected differently. Keratoconus affects 1 in 2000 people (32). Treatment options include conservative approaches, aimed at maintaining visual acuity, such as rigid contact lenses placed to straighten corneal aberrations (33). Alternatively, more invasive methods are applied using intrastromal corneal implants (34) or performing anterior lamellar keratoplasty or penetrating keratoplasty in extreme cases (35, 36). However, all of these techniques only correct the refractive errors of keratoconus and do not treat the cause underlying the corneal ectasia and therefore do not stop the progression of keratoconus.
Recently, a new technique of corneal cross-linking was devised that directly improves the biomechanical rigidity of the corneal stroma (37). This approach consists of irradiation with ultraviolet-A (UVA) in the presence of the photosensitizer, riboflavin (RF), as a chromophore, to stop progression of the keratoconus syndrome. The basic principle of corneal cross-linking is thought to be that in the presence of UVA at 370 nm, riboflavin is excited into its triplet state, generating singlet oxygen, which can react further with various molecules, inducing chemical covalent bonds bridging amino groups of collagen fibrils (38). In brief, the central 7 mm of the corneal epithelium is removed to allow better diffusion of riboflavin into the stroma. Before irradiation with UVA, a 0.1% riboflavin solution (10 mg of riboflavin 5-phosphate in 10 ml of dextran 20% solution) is applied onto the debrided central cornea every 5 min for 30 min. Then, as the application of riboflavin continues, the irradiation is performed from a 1-cm distance for 30 min with UVA at 370 nm and irradiance of 3 milliwatt/cm2 (5.4 J/cm2) (39). Clinical studies have shown that the progression of keratoconus is effectively stopped (40), and the biomechanical strength of a keratoconus cornea is significantly increased by 70–300% (41, 42). In the anterior stroma, average collagen fibril diameter in the treated cornea is significantly increased by 12.2%, and in the posterior stroma, it is increased by 4.6% (43). Currently, laboratory studies are mainly focused on documenting biomechanical effects (44), thermomechanical effects (45), morphological changes (46), effects on keratocytes (47), localization of cross-linking (48), and effects on collagenase resistance (49, 50). However, the chemical mechanisms of collagen and PG interactions catalyzed by riboflavin + UVA treatment during corneal cross-linking have not been elucidated except in recent work demonstrating that singlet oxygen and carbonyl groups are required (51). Some additional mechanisms are reported here.
EXPERIMENTAL PROCEDURES
Materials
Bovine eyeballs were freshly collected from Alta Vista Locker (Alta Vista, KS). Collagen type I from bovine skin was purchased from INAMED (Fremont, CA). Collagen type III and type IV from human placenta and RF 5′-monophosphate sodium salt were purchased from Sigma (St. Louis, MO). KS from bovine cornea was purchased from Seikagaku America (East Falmouth, MA) and was purified further by chromatography and chondroitinase ABC treatment (sodium salt), as described in detail below. The average molecular mass of KS was ∼15 kDa. CS from bovine trachea was purchased from Sigma. CS was purified by chemical treatment, as described in detail below. Recombinant human keratocan and human lumican proteins were purchased from Abnova Corp. (Taipei, Taiwan). Recombinant mouse mimecan and recombinant human decorin proteins were purchased from R&D Systems (Minneapolis, MN). Anti-human keratocan polyclonal antibody was purchased from Abnova Corp. Anti-human lumican antibody, anti-mouse mimecan antibody, and anti-human decorin antibody were purchased from R&D Systems (Minneapolis, MN). Rabbit polyclonal antibody to collagen type I (ab34710) was purchased from Abcam Inc. (Cambridge, MA). NuPAGE® Novex® BisTris 4–12% precast gels (8 cm × 8 cm × 1.5 mm), NuPAGE® Novex® Tris acetate 3–8% precast gels (8 cm × 8 cm × 1.5 mm), and chromogenic Western blot kits were purchased from Invitrogen (Carlsbad, CA).
Preparation of Free KS Polysaccharide Chains
Based on the manufacturer's protocol for isolation of KS, there is residual core peptide at the reducing terminus. N-Glycanase was employed to release N-linked KS chains from the short peptides. Following the procedure suggested by Prozyme, the mixture of KS and N-Glycanase was incubated overnight at 37 °C. Enzymatic digestions were terminated by heating for 10 min in boiling water and then cooled to room temperature. Subsequently, 1 volume of 100% ice-cold trichloroacetic acid (TCA) was added to 4 volumes of the digestion sample, mixed, and kept at 4 °C overnight. The digestion sample was centrifuged at 14,000 rpm and at 4 °C for 30 min to remove precipitated N-glycanase. The supernatant, containing KS, was transferred to Ultra free-MC centrifugal filter units (5,000 NMWL; Millipore) and washed extensively with water to remove salt, and then the retentate was lyophilized.
Preparation of Free CS Polysaccharide Chains
To obtain CS chains free of residual core protein peptides for cross-linking analysis, an alkaline β-elimination reaction was carried out in the presence of NaBH4. The CS sample was adjusted to 1.0 m NaBH4, 0.05 m NaOH, incubated at 45 °C for 24 h (52), neutralized with 1 m acetic acid, washed extensively with water using Ultra free-MC centrifugal filter units (5,000 NMWL; Millipore) to remove salt, and then the retentate, containing CS, was lyophilized.
Cross-linking Model System
Purified collagen type I was used to assess the mechanisms of cross-linking induced by the photosensitizer RF and UVA under conditions that resemble those used for clinical treatment of progressive keratoconus. The mixture of purified collagen type I (1 μg/μl in PBS) and RF (0.1% in PBS), in solution, was irradiated with UVA of 370 nm for 30 min at a distance of 5 cm from the light source (UV-X Radiation System for Corneal Cross-linking CXL; IROC Medical, Zurich, Switzerland). The volume of reaction solution was 20 μl in 0.5-ml plastic centrifugal tubes. The sample solutions were irradiated with the UVA shining down directly onto the surface of the solutions, mixed two times by vortexing at 10-min intervals while it was being irradiated.
Cross-linking Treatment on Intact Whole Corneas ex Vivo
The cross-linking procedure was performed as described previously (51). In brief, the bovine corneal epithelium was mechanically removed using a blunt knife, and riboflavin 0.1% (w/v) solution (RF + PBS) was applied beginning 30 min before irradiation and continuing every 5 min during irradiation. The corneas were irradiated with UVA of 370 nm for 30 min at a distance of 5 cm from the light source.
Guanidine HCl Extraction of Corneal Tissue
The de-epithelialized bovine corneas (0.6 g wet weight), treated or untreated with RFUVA, were frozen by liquid nitrogen, pulverized, homogenized further in 4 m guanidine HCl (GHCl) containing protease inhibitors (53), and then incubated for 24 h at 0–4 °C with gentle agitation. The tissue residue was removed by centrifugation at 10,000 × g for 30 min, and the supernatant was retained as the extract. The tissue residue pellet was re-extracted for a second 24 h with fresh 4 m GHCl solution. The two extracts were combined together and then applied to an Amicon Ultra centrifugal filter (regenerated cellulose, 3,000 Mr cutoff; Millipore), centrifuged to desalt, and concentrated to one-fifth of the original volume. The retentates that did not pass through the filter were used for collagen cross-linking evaluation without any further processing.
Pepsin Extraction of Corneal Tissue
For pepsin extraction, the frozen, pulverized, and homogenized corneal tissue (0.6 g wet weight) was extracted for 48 h in 20 volumes of 0.5 m acetic acid with 10 mg/ml pepsin (800–2,500 units/mg, EC 3.4.23.1; Sigma) with gentle stirring movement at 0–4 °C (54). The tissue residue was removed by centrifugation at 10,000 × g for 30 min. The supernatant was collected and neutralized by the addition of NaOH and then desalted and concentrated as described above. These retentates were used for collagen cross-linking evaluation.
SDS-PAGE
For analysis of collagen and PG cross-linking, 5 μl of NuPAGE LDS sample buffer (Invitrogen) and 2 μl of NUPAGE reducing agent (Invitrogen) were added into each sample solution, heated at 70 °C for 10 min, and then loaded onto NuPAGE® Novex® BisTris 4–12% gels (8 cm × 8 cm × 1.5 mm precast gel; Invitrogen) and subjected to electrophoresis (100 mA/gel for 60 min) under reducing conditions. After electrophoresis, the gels were stained with 0.1% (w/v) Coomassie Brilliant Blue R-250.
Western Blot Analysis of Collagen Interaction with Proteoglycan Core Proteins
For detection of possible cross-linking between collagen and proteoglycan core proteins, samples were loaded onto NuPAGE® Novex® Tris acetate 3–8% polyacrylamide gels (8 cm × 8 cm × 1.5 mm precast gel; Invitrogen) and subjected to electrophoresis (40 mA/gel for 60 min) under reducing conditions. Following electrophoresis, proteins were transferred to nitrocellulose (Fisher) by electroblotting (BioTrans (Ann Arbor, MI) semidry electrophoretic transfer unit). Protein transfer buffer was prepared following instructions of BioTrans: pH 8.4, 48 mm Tris base, 39 mm glycine hydrochloride, and 1.3 mm SDS in 20% methanol. Voltage was set at 150 V. Transfer time was 45 min. Subsequently, chromogenic immunodetection was performed following the kit protocol for small membranes (Invitrogen). Collagen type I and core proteins were identified using anti-collagen type I antibody (ab34710), anti-keratocan antibody (Abnova), anti-lumican antibody (AF2846), anti-mimecan antibody (AF2949), and anti-decorin antibody (AF143), respectively. The specificity of each of these four PG core protein antibodies was confirmed by Western blotting of electrophoretic gels of all four core proteins (supplemental Fig. S1).
Analysis of the Interaction of GAGs and Collagen Type I with Mass Spectrometry
When gel electrophoresis was finished, the areas containing cross-linked collagen (the area from 150 kDa up to the base of the gel sample well) and KS/CS (the area from 100 kDa down to the bottom of the gel) were each cut off and eluted from unstained SDS gels with electrophoretic elution (model 422 Electro-Eluter, Bio-Rad). Eluted molecules were centrifuged through an Ultra free-MC centrifugal filter unit (5,000 NMWL; Millipore) to remove buffer salts. For analysis of KS-sulfated disaccharides, the retentates were recovered in 100 μl of 0.1 m ammonium acetate buffer (pH 6.0) containing 0.1 milliunits/μl keratanase II and digested for 24 h at 37 °C. For analysis of CS-sulfated disaccharides, the retentates instead were recovered in 100 μl of 50 mm ammonium acetate buffer (pH 8.0) containing 1 milliunit/μl chondroitinase ABC and digested for 24 h at 37 °C. The enzymes were then inactivated at 100 °C for 5 min. Digest solutions (10 μl) of each set were diluted by adding 70 μl of MeOH, 5 μl of 5 mm ammonium acetate buffer (pH 7.5), 5 μl of 2 mm (NH4)2SO4, and 10 μl of water. The mixtures were centrifuged at 3,800 rcf for 30 min at 4 °C (Ultra free-MC centrifugal filter unit, 5,000 NMWL; Millipore) with subsequent analysis of the retentates by electrospray ionization-MS/MS (55, 56). Mass spectra were obtained using an electrospray ionization source on a quadrupole ion trap instrument (Bruker Daltonics Esquire 3000). Parameters used for analysis of all internal standards and corneal stromal tissue digests were as follows: spray voltage, 3.5 kV; dry gas (nitrogen), flow, 5.0 liters/min; drying temperature, 180 °C. The mass range scanned was m/z 50–1000. Data acquisition software used was Bruker Daltonics Data Analysis version 3.0.
Evaluation of Collagen Cross-linking in Whole Corneas
Antibodies to collagen type I and to each of the PG core proteins were used to assess the cross-linking of collagen and PG core proteins. Cleavage of collagen chains and non-collagen proteins at methionine residues with cyanogen bromide (CNBr) was performed in 70% formic acid under argon covered at room temperature as described previously (57). Samples were treated with N-glycanase or O-glycanase according to the manufacturer's protocols (ProZyme), to remove intact chains of KS or CS/DS, respectively. Briefly, 2 μl of N-glycanase or O-glycanase was added to the reaction mixture (50 μl) and incubated for 24 h at 37 °C. Other samples were digested with keratanase II (100 milliunits/ml in 0.1 m ammonium acetate buffer, pH 6.0) or with chondroitinase ABC (1000 milliunits/ml in 50 mm ammonium acetate buffer, pH 8.0) for 24 h at 37 °C, respectively (56) to depolymerize chains of KS or CS/DS while leaving their linkage region sugars still attached to the core protein. Enzymatic digestions were terminated by heating for 10 min in boiling water and then lyophilized. The same proportions of each digested sample were subjected to electrophoresis on 4–12% SDS gels and then to Western blot analysis, as described above.
RESULTS
Establishment of Cross-linking Model System
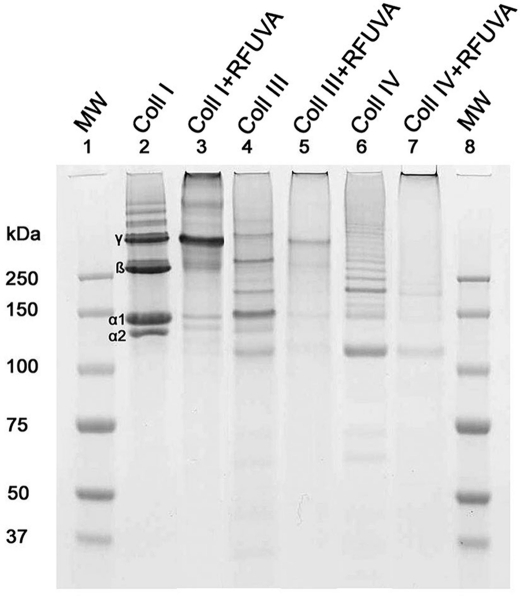
Three purified collagens, type I, type III, and type IV, were chosen to assay the reactivity of collagen cross-linking under the clinical conditions of treatment of keratoconus patients with UVA and riboflavin. The reaction was monitored by SDS-PAGE. The results showed that collagen types I, III, and IV each display a banding pattern distinct from the other two. For collagen type I, RFUVA causes (a) α1, α2, and β chains to almost disappear; (b) γ chains to increase slightly in intensity; and (c) protein staining at the base of the well to increase (Fig. 1, lane 3). For collagen types III and IV, similar results were observed, as shown for the RFUVA treatment in Fig. 1, lane 5 (Type III) and lane 7 (Type IV). These results suggest that collagen cross-linking definitely happens when irradiating with UVA in the presence of riboflavin. The anterior corneal stroma is the location of the major effects of riboflavin + UVA treatment, and the stromal fibrils are primarily composed of collagen type I. This collagen type therefore was chosen for the following experiments. Based on the initial experiment shown in Fig. 1, we used the following standard reaction conditions in the rest of this study (unless stated otherwise): collagen type I (1 μg/μl) in 0.1 m PBS (pH 7.2), 0.1% riboflavin in 0.1 m PBS (pH 7.2), and UVA at 370 nm for 30 min at a distance of 5 cm from the light source. This reaction model system was in a 0.5-ml plastic centrifuge tube and mixed two times by vortexing at 10-min intervals while it was being irradiated from directly above the sample solution.
FIGURE 1.
SDS-PAGE patterns of cross-linking collagens. Collagen types I (lane 2), III (lane 4), and IV (lane 6) each displayed a banding pattern distinct from the other two. RFUVA causes collagen type I (lane 3) α and β chains to almost disappear, γ chains to increase slightly in intensity, and protein staining at the base of the gel well to increase. A similar pattern of band disappearance is seen also with type III collagen (lane 5) and type IV collagen (lane 7), with increased staining at the top of the gel (base of the sample well). Lane 1, molecular mass standards. Gels were stained with Coomassie Blue.
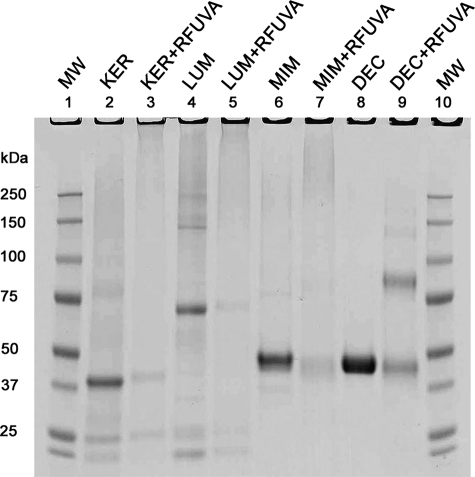
Core Protein Cross-linking
Under experimental conditions described above for Fig. 1, core proteins keratocan, lumican, mimecan, and decorin (all at a concentration of 0.2 μg/μl) each migrate as a monomer band. However, after exposure to RFUVA, each such monomer band virtually disappears (Fig. 2). For keratocan, lumican, and mimecan, RFUVA does not cause a distinct higher molecular mass band to form but rather causes formation of polymers of a very wide range of molecular mass values from 100/150 kDa up to the bottom of the gel sample well (Fig. 2, lanes 3, 5, and 7). In contrast, in the case of decorin, RFUVA causes an entirely new band to form of approximately twice the size of the monomer band (Fig. 2, lane 9).
FIGURE 2.
SDS-PAGE patterns of cross-linking core proteins. RFUVA caused the monomer band of each PG to virtually disappear. For keratocan, lumican, and mimecan, RFUVA + PG did not cause a higher Mr band to form, instead forming new higher Mr polymers of a wide range of sizes from 100/150 kDa to the base of the gel well (lanes 3, 5, and 7). For decorin, RFUVA caused an entirely new band to form of approximately twice the size of the monomer band (lane 9). Gels were stained with Coomassie Blue.
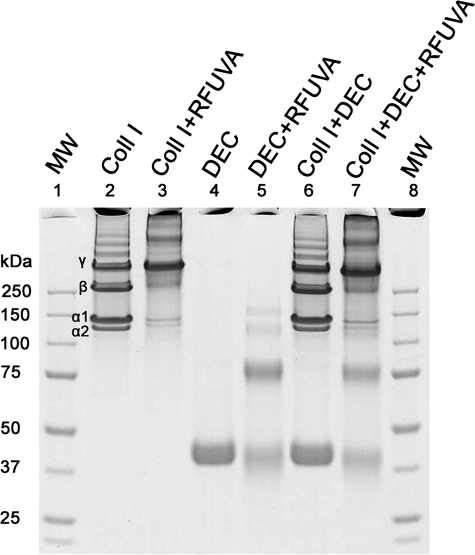
Interaction of Collagen and Core Proteins
To detect possible collagen interactions with core proteins during RFUVA, collagen type I in solution, in the presence or absence of PG core proteins, was irradiated by UVA in the presence of RF. The relative intensity profiles of individual bands of SDS gels (Figs. 3 and 4) are summarized in Table 1 as a percentage of the total protein in each scanned sample. When collagen type I in solution (Fig. 3, lane 2) was exposed to RFUVA (Fig. 3, lane 3), the relative intensity of collagen α chains and β chains significantly decreased, and the band at the very top of the gel increased. However, when the same reaction was performed in the presence of keratocan (Fig. 3, lane 5) or lumican (Fig. 3, lane 7), the relative intensity of collagen α chains and β chains did not decrease as significantly as in their absence, suggesting that keratocan and lumican strongly inhibit the effect of RFUVA. In other words, keratocan and lumican both appear to inhibit collagen cross-linking with other collagen molecules. However, in contrast to keratocan and lumican, in the presence of mimecan, the intensity of collagen α and β chains significantly decreased after RFUVA, as if mimecan were not present (Fig. 3, compare lane 3 versus lane 9), indicating that mimecan does not interfere with collagen cross-linking (Fig. 3, compare lane 3 with lane 9), resulting in ∼40% decrease in the relative intensities of α chains and β chains, whereas the relative intensity of γ chains (Fig. 3, lane 3 versus lane 9) was increased ∼20%. Similarly to mimecan, decorin does not interfere with collagen cross-linking in the presence of RFUVA (Fig. 4, lane 3 versus lane 7), suggesting that neither mimecan nor decorin interferes with RFUVA collagen cross-linking.
FIGURE 3.
Interaction of core proteins keratocan, lumican, and mimecan with type I collagen. Presence of keratocan or lumican prevented RFUVA from causing diminished staining of α chains and β chains of collagen (lanes 5 and 7). In contrast, mimecan did not interfere with collagen cross-linking, thus allowing α chains and β chains to be cross-linked to form high Mr polymers near γ chains in size and toward the top range of the gel, and staining intensity increased sharply at the base of the sample well (lane 9). Gels were stained with Coomassie Blue.
FIGURE 4.
Interaction of core protein decorin with type I collagen. In the presence of RFUVA (lane 7), decorin did not interfere with collagen cross-linking, as evidenced by the virtual disappearance of α and β chains, the increased density of γ chains, and the same increased density of staining in the upper region of the gel as in the absence of decorin (lane 3). Gels were stained with Coomassie Blue.
TABLE 1.
Scanning density analysis of SDS-PAGE bands using Image Quantity Software TL (band intensity, percentage of total detected protein stain in each sample, n = 3)
The density of each band is compared with the integrated total of all the bands (and diffuse staining) in that individual sample. For any individual sample, the sum of staining from the α1, α2, β, and γ bands will be less than 100%; the remainder represents the diffuse staining in other Mr regions of that sample.
| α2 | α1 | β | γ | |
|---|---|---|---|---|
| Collagen I | 10.75 ± 0.39 | 24.37 ± 0.43 | 27.36 ± 0.60 | 23.06 ± 0.16 |
| Collagen I + RFUVA | 5.89 ± 0.41 | 10.99 ± 0.39 | 15.34 ± 0.61 | 44.47 ± 1.07 |
| Collagen I + KER + RFUVA | 7.71 ± 0.16 | 17.63 ± 0.29 | 32.56 ± 0.76 | 42.34 ± 0.79 |
| Collagen I + LUM + RFUVA | 7.58 ± 0.19 | 19.13 ± 0.41 | 28.21 ± 0.23 | 34.83 ± 0.78 |
| Collagen I + MIM + RFUVA | 3.22 ± 0.22 | 7.28 ± 0.20 | 11.76 ± 0.58 | 54.07 ± 0.61 |
| Collagen I + DEC + RFUVA | 2.25 ± 0.28 | 5.11 ± 0.22 | 15.11 ± 0.35 | 59.86 ± 1.69 |
Western Blot Analysis of Collagen Interaction with Core Proteins
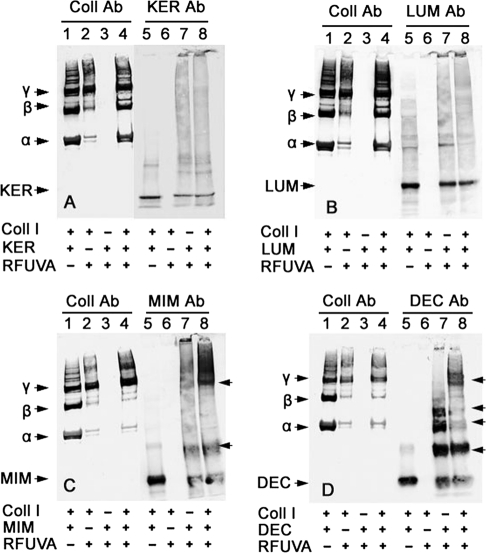
In the SDS-PAGE experiments, the core protein-to-collagen ratio was 1:5. These same ratios were used in all Western blot experiments. In case of keratocan (Fig. 5A), keratocan partly inhibits RFUVA cross-linking of collagen I (lane 2 versus lane 4), yet keratocan does not appear to become bound to collagen I by RFUVA (lane 5 versus lane 7 versus lane 8). Interestingly, if RFUVA is applied to lumican in the presence of collagen I (Fig. 5B, lanes 4 and 8), lumican displays a pattern of interaction with collagen similar to that of keratocan in that RFUVA cross-linking of collagen I is partly inhibited (Fig. 5B, lane 2 versus lane 4). Thus, keratocan and lumican interact with collagen I and respond to RFUVA in ways that correspond closely with one another. In sharp contrast with that pattern, mimecan does not prevent RFUVA from causing collagen I to undergo cross-linking (Fig. 5C, lane 2 versus lane 4). Conversely, however, the presence of collagen does affect the cross-linking response of mimecan to RFUVA (Fig. 5C, lane 7 versus lane 8). Thus, in the absence of collagen (Fig. 5C, lane 7), mimecan in the presence of RFUVA forms higher molecular weight regions, with an especially strong one at the bottom of the sample well. However, in the presence of collagen I (Fig. 5C, lane 8), mimecan is found in two higher molecular weight regions, especially in the upper region of the gel, including in bands in the same position as γ chains of collagen (arrowhead). In comparison with mimecan, the responses of decorin to RFUVA are even more dramatic in the new banding patterns created when collagen is also present, especially in the appearance not only of the apparent dimer form seen in Fig. 4 (lane 4 versus lanes 5 and 7) but also of three even higher molecular weight forms (Fig. 5D, lane 7). The presence of collagen and RFUVA causes decorin to migrate even less as its single monomer band, leaves the dimer band approximately the same, but instead, causes two of its four higher molecular weight polymers to display greatly diminished staining intensity, and causes a new group of bands to form near the top of the gel co-migrating with the γ chains of collagen (arrowhead) (Fig. 5D, lane 8). Thus, although mimecan and decorin do not inhibit RFUVA-induced cross-linking of collagen I with itself as do keratocan and lumican, both mimecan and decorin do respond significantly to RFUVA and to the simultaneous presence of collagen I in ways that correspond closely with one another and contrast greatly with the pattern shown by keratocan and lumican. Thus, the pattern of interaction of keratocan and lumican with collagen appears to be very different from the pattern of interaction of mimecan and decorin with collagen.
FIGURE 5.
Western blot of proteoglycan core protein interaction with type I collagen induced by RFUVA. Western blot is shown. A, the presence of keratocan prevented RFUVA from causing collagen type I to undergo cross-linking (lane 4 versus lane 2). The intensities of α and β chains (lane 4) were almost the same as the corresponding bands in lane 1. B, lumican inhibited collagen cross-linking (lane 4 versus lane 2). C, mimecan did not inhibit RFUVA-induced collagen cross-linking (lane 2 versus lane 4), whereas mimecan formed a new band near γ chains and in the top range of the gel (lane 8 versus lane 7). D, decorin did not inhibit RFUVA-induced collagen cross-linking (lane 2 versus lane 4), whereas decorin formed a new band near γ chains (lane 8 versus lane 7). Decorin dimers and oligomers induced by RFUVA were observed (lane 7).
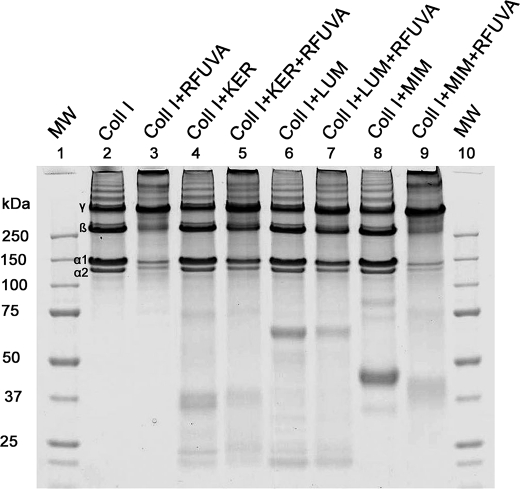
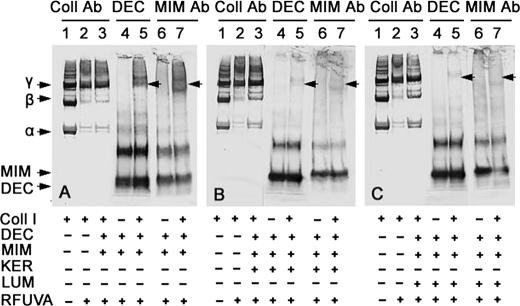
Fig. 6 shows that keratocan and lumican not only interfere with RFUVA cross-linking of collagen but also interfere with RFUVA cross-linking of both mimecan and decorin. In the absence of keratocan and lumican, but even in the simultaneous presence of both mimecan and decorin, collagen undergoes cross-linking (α and β chains almost disappear) (Fig. 6A, lane 1 versus lanes 2 and 3). Decorin undergoes cross-linking in the presence (Fig. 6, lane 5) or absence (Fig. 6, lane 4) of collagen I, but in its presence, decorin also occurs in a band in the same position as collagen I γ chains (arrowhead), suggesting that some decorin is bound to the γ chain, three-chain polymer form of collagen I. Similar to decorin, mimecan also occurs in a band in the same position as collagen I γ chains (arrowheads), suggesting that some mimecan is bound to the γ polymer form of collagen I (Fig. 6A, lane 7). In the presence of keratocan, as well as mimecan and decorin, collagen I cross-linking by RFUVA is partly inhibited (Fig. 6B, lane 3 versus lane 2). Moreover, for both decorin and mimecan, RFUVA-induced cross-linking in the presence or absence of collagen I is inhibited by the presence of keratocan (Fig. 6B, lane 4 versus lane 5 and lane 6 versus lane 7 in comparison with those same lanes in Fig. 6A). Thus, the presence of mimecan or decorin in the same position as collagen I γ chains is not seen in Fig. 6B, lanes 5 and 7 (arrowheads), in comparison with those same lanes in Fig. 6A (arrowheads), suggesting that keratocan can prevent both decorin and mimecan from cross-linking with themselves and also can prevent their interaction with collagen. Importantly, lumican displays the same inhibitory ability as keratocan (Fig. 6C, lane 4 versus lane 5 and lane 6 versus lane 7, arrowheads).
FIGURE 6.
Effect of keratocan or lumican on RFUVA cross-linking of collagen in the simultaneous presence of both mimecan and decorin. Western blot is shown. A, in the simultaneous presence of both mimecan and decorin, collagen cross-linking in response to RFUVA is not inhibited, and both mimecan and decorin each undergo their respective patterns of cross-linking. B, in contrast, in the presence of keratocan, as well as mimecan and decorin, collagen I cross-linking by RFUVA was partly inhibited (lane 3 versus lane 2). In addition, RFUVA-induced cross-linking of both decorin and mimecan in the presence or absence of collagen I are inhibited (lane 4 versus lane 5 and lane 6 versus lane 7). C, similarly, in the presence of lumican, collagen I cross-linking by RFUVA was partly inhibited (lane 3 versus lane 2). In addition, RFUVA-induced cross-linking of both decorin and mimecan in the presence or absence of collagen I is inhibited (lane 4 versus lane 5 and lane 6 versus lane 7).
Interaction of Collagen with KS and CS
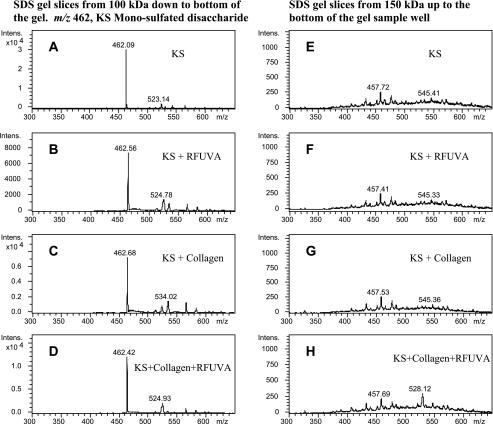
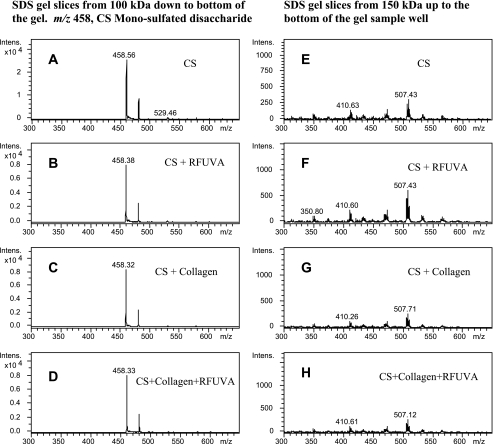
To determine if KS or CS might be involved in RFUVA-induced collagen cross-linking, mass spectrometry was used to analyze the SDS gel samples. As can be seen from Fig. 7, A–D, a strong signal intensity detected at m/z 462.0 corresponds to KS-monosulfated disaccharide released by keratanase II from gel slices (area from 100 kDa down to the bottom of the gel), whereas no signal at m/z 462.0 (Fig. 7, E–H) was observed with the corresponding gel slice area from 150 kDa up to the base of the gel sample well, suggesting an absence of higher molecular polymer forms involving KS. As can be seen from Fig. 8, A–D, in the case of CS, a strong signal intensity was detected at m/z 458.0 for CS-monosulfated disaccharide (4S/6S) from gel slices (area from 100 kDa down to the bottom of the gel), whereas no signal at m/z 458.0 (Fig. 8, E–H) was observed with the corresponding gel slice area from 150 kDa up to the base of the gel sample well, suggesting an absence of higher molecular polymer forms involving CS. These results indicate that neither KS nor CS is involved in RFUVA-induced corneal cross-linking.
FIGURE 7.
Analysis to detect possible interaction of KS and collagen type I, using mass spectrometry. Peaks at m/z 462.0 (A–D) correspond to the characteristic KS-monosulfated disaccharide released by keratanase II from gel slices (area from 100 kDa down to the bottom of the SDS gel). There are no peaks at m/z 462.0 (E–H) observed with gel slices in the area from 150 kDa up to the base of the gel sample well.
FIGURE 8.
Analysis to detect possible interaction of CS and collagen type I, using mass spectrometry. Peaks at m/z 458.0 (A–D) correspond to the characteristic CS monosulfated disaccharide (4S/6S) released by chondroitinase ABC from gel slices (area from 100 kDa down to the bottom of the SDS gel). There are no peaks at m/z 458.0 (E–H) observed with gel slices in the area from 150 kDa to the base of the gel sample well.
Collagen and Core Proteins Cross-linking in Whole Cornea ex Vivo
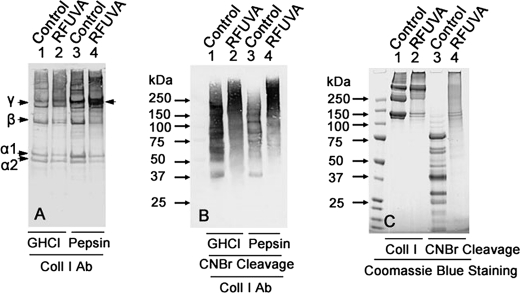
Antibodies to collagen type I and to each of the PG core proteins were used to assay for possible cross-linking between collagen and PG core proteins in native cornea with RFUVA. Fig. 9A shows the electrophoretic migration pattern of collagen type I from whole cornea treated by RFUVA and then extracted with either GHCl or with pepsin; the latter, by cleaving only their telopeptide domains, leaves the triple-chain helical domains of collagen molecules intact. Compared with control samples (Fig. 9A, lanes 1 and 3), the staining intensities of α1 chains, α2 chains, and β chains in the presence of RFUVA were decreased, whereas the staining intensities of γ chains were significantly increased (Fig. 9A, lanes 2 and 4), thus indicating that collagen molecules were undergoing cross-linking even in the presence of the normal mixture of all four natively GAG-glycosylated PGs ex vivo.
FIGURE 9.
Effect of RFUVA on collagen type I cross-linking in whole cornea ex vivo versus collagen type I in solution. A, patterns of collagen type I from whole cornea treated by RFUVA. Lanes 1 and 2, GHCl extraction; lanes 3 and 4, pepsin extraction. B, patterns of collagen type I from whole cornea treated by RFUVA and then digested with CNBr. Lanes 1 and 2, GHCl extraction; lanes 3 and 4, pepsin extraction. C, patterns of electrophoretic migration of purified collagen type I in solution, treated by RFUVA, and digested with CNBr. All peptide domains of collagen type I participate in cross-linking to form a range of high Mr molecules. A and B, Western blot; C, Coomassie Blue staining.
Fig. 9C indicates that, in the absence of RFUVA treatment, incubation of samples of purified collagen with CNBr causes collagen type I to be cleaved at methionine residues, generating the collagen type I-specific pattern of lower molecular size peptides expected (Fig. 9C, lane 3). However, CNBr incubation of collagen type I that was first treated with RFUVA demonstrates that all domains of the collagen type I molecule are involved in cross-linking into higher Mr molecules, as evidenced by the absence of low Mr collagen peptides and, instead, the appearance of a broad range of higher Mr molecules (Fig. 9C, lane 4). Fig. 9B indicates that regardless of whether whole corneal samples were extracted from corneas using the GHCl protocol standard for extracting PGs (53) or the pepsin protocol standard for extracting the triple-helical domains of collagens (54), when such samples are derived from corneas treated with RFUVA, chemical cleavage with CNBr reveals a range of collagen peptides that migrate in a high MW range (Fig. 9B, lanes 2 and 4), just as do the CNBr peptides from collagen type I (Fig. 9C, lane 4).
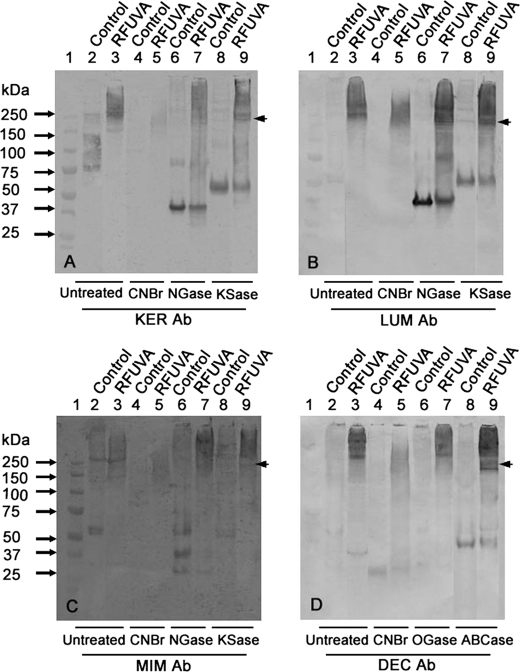
Fig. 10, A–D, displays the profiles of each individual PG core protein extracted with GHCl from whole corneas with or without exposure to RFUVA and with or without treatments to remove GAG chains from their core proteins. Exposure of whole corneas to RFUVA causes some proportions of all proteoglycan core proteins to migrate in the same band positions as collagen type I γ chains (Fig. 9), shown here as arrowheads, regardless of whether glycosaminoglycan chains of KS were intact (untreated lanes) or were removed with N-glycanase or with keratanase II, consistent with PGs in solutions containing type I collagen also co-migrating with collagen γ chains (Figs. 5 and 6). Moreover, in corneas exposed to RFUVA, even after cleavage of core proteins to small peptides with CNBr, a range of reactivity was detected in the high Mr region (arrowheads), whereas none of the core proteins were detected in that high Mr region in samples from control corneas treated with CNBr. Thus, these results suggest that RFUVA causes some cross-linking to occur between PG core proteins and collagen I in whole cornea, ex vivo.
FIGURE 10.
Patterns of cross-linking collagens and PG core proteins in whole cornea ex vivo. Western blot is shown. A, interaction of keratocan and collagen type I from corneas (with or without RFUVA treatment) treated by CNBr (lanes 4 and 5), N-glycanase (NGase) (lanes 6 and 7), and keratanase II (KSase) (lanes 8 and 9). B, interaction of lumican and collagen type I from corneas (with or without RFUVA treatment) treated by CNBr (lanes 4 and 5), N-glycanase (lanes 6 and 7), and keratanase II (lanes 8 and 9). C, interaction of mimecan and collagen type I from corneas (with or without RFUVA treatment) treated by CNBr (lanes 4 and 5), N-glycanase (lanes 6 and 7), and keratanase II (lanes 8 and 9). D, interaction of decorin and collagen type I from corneas (with or without RFUVA treatment) treated by CNBr (lanes 4 and 5), O-glycanase (OGase) (lanes 6 and 7), and chondroitinase ABC (ABCase) (lanes 8 and 9). All four PG core proteins in the cornea show some molecules that co-migrate in the same region as collagen γ chains (arrowheads).
DISCUSSION
Corneal cross-linking by RFUVA represents a new method for treatment of progressive keratoconus in Europe (40) and currently is under clinical study in the United States. Here, we have used both an in vitro and an ex vivo model reaction system to investigate the effects of RFUVA specifically on the interaction of collagen and PGs, an interaction very likely to occur during corneal cross-linking. Our data demonstrate that irradiation with UVA in the presence of riboflavin cross-links collagen α and β chains into high molecular weight polymers. In addition, RFUVA can induce purified, non-glycosylated PG core proteins themselves to cross-link to form higher Mr polymers. When both collagen and PG core proteins are present together in solution, both of the corneal PG core proteins, keratocan and lumican, inhibit collagen cross-linking. However, in sharp contrast, two other corneal PG core proteins, mimican and decorin, not only do not interfere with collagen cross-linking, they in fact form cross-links with collagen to form new bands in high molecular weight regions of electrophoretic gels. When studied in isolation from their normal PG core proteins, the corneal GAGs, KS and CS, are not induced by RFUVA to cross-link among themselves or to form cross-links with collagen. Thus, based on interactions between these purified proteins in solution in vitro, RFUVA mainly appears to involve cross-linking of collagen molecules with themselves, PG core proteins with themselves, and collagen molecules with the two specific PG core proteins, mimecan and decorin. These results suggest that in the absence of RFUVA, collagen interacts non-covalently with keratocan and lumican very differently from the way interacts with mimecan and decorin. Data from ex vivo exposure of whole corneas to RFUVA lead to these same conclusions. This reveals two sharply distinct styles of molecular interactions between collagen and PGs.
Type I collagen, the most abundant collagen in cornea (11), is composed of three polypeptide chains and two types of single chains, α1 and α2, both of which are accessible to polymerization through intra- and intermolecular bonds (58). Dimers of α chains are called β-components (composed of α1 α2 or α1 α1 chains). Trimers of three α chains are called γ-components. In this study, RFUVA mainly causes α1, α2, β11, and β12 chains to cross-link into macromolecules at least as large as γ-components, and larger (Fig. 1, lane 3) and thus to disappear from their normal α and β chain positions on electrophoretic gels. Intermicrofibrillar cross-links significantly affect the mechanical properties of the corneal collagen tissue (59). The strength of the collagen fibers depends on the formation of covalent cross-links between the telopeptide terminal domains and adjacent helical domains of collagen molecules (60). Riboflavin-sensitized photodynamic modification of collagen causes formation of cross-linked molecules, in which an energy transfer between the excited riboflavin and molecular oxygen produces singlet oxygen that then interacts with the collagen fibrils in an oxidation reaction to form physical, covalent cross-links (61). Results presented here provide new and direct evidence that covalent cross-links between collagen molecules, especially involving α1, α2, β11, and β12 chains, are initiated by RFUVA and are consistent with earlier data indicating that singlet oxygen plays a primary role in corneal cross-linking (51).
Data presented here indicate that collagen types I, III, and IV, in isolation from one another and from PGs, undergo cross-linking reactions that all share some characteristics (Fig. 1). Both α and β chains virtually disappear, presumably because of their covalent cross-linking into higher molecular weight polymers that either migrate as γ chains (∼300 kDa) to some extent or as even larger aggregates, some of which barely enter the gel at the bottom of the sample well (increased density at top of lane 3 in Fig. 1). In response to RFUVA, normal corneal PG core proteins, keratocan, lumican, mimecan, and decorin (but non-glycosylated (i.e. lacking their normal GAG chains)), similarly appear to undergo cross-linking reactions with themselves that cause their virtual disappearance as monomer molecules (Fig. 2). One PG core protein, decorin, appears to form distinct dimer molecules (∼80 kDa) (Fig. 2, lane 9). When collagen type I is subjected to RFUVA in the simultaneous presence of these PG core proteins (Fig. 3), collagen cross-linking is drastically inhibited if either keratocan or lumican is present (Fig. 3, lanes 5 and 7). In sharp contrast, however, the presence of mimecan does not inhibit collagen cross-linking (Fig. 3, lane 9). In those same solutions containing collagen type I, PG core proteins keratocan and lumican appear to undergo almost normal cross-linking with themselves (as indicated by a diminution of their monomer bands) as a result of RFUVA (Fig. 3, lanes 5 and 7), as does mimecan (Fig. 3, lane 9). These results suggest that keratocan and lumican can block those sites on collagen type I that normally allow collagen to cross-link with itself. However, even when any one of those three PG core proteins is present, RFUVA treatment still produces large molecular aggregates that are present at the bottom of the sample wells (Fig. 3, lanes 5, 7, and 9), as it does in their absence (Fig. 3, lane 3), perhaps representing some of the cross-linked collagen seen in Fig. 1. Thus, none of the core proteins appears to produce molecular aggregates with collagen type I, at least in the region in which molecules can enter the gel (with the possible exception of mimecan (Fig. 3, lane 9)). Decorin (Fig. 4), like mimecan, does not inhibit the cross-linking of collagen type I (Fig. 4, lane 7) in response to RFUVA; likewise, DEC undergoes its normal cross-linking with itself in response to RFUVA, forming a prominent dimer molecule in the absence (lane 5) and presence (Fig. 4, lane 7) of collagen type I. Also similar to mimecan, decorin appears to form a new macromolecule (>300 kDa) when exposed to RFUVA in the presence of collagen type I (Fig. 4, lane 7). Altogether, the results in Figs. 1–4 indicate that keratocan and lumican interact with collagen type I in solution very differently than do mimecan and decorin.
In cornea and in other tissues, any of these core proteins may exist either in forms lacking glycosylation or with glycosylation consisting of GAG chains. Such glycosaminoglycan polysaccharides (which do not appear to participate in RFUVA cross-linking, as shown here in Figs. 7–9) may sterically hold PG core proteins at sufficient distances from collagen molecules in adjacent fibrils as to prevent formation of cross-links between them in response to RFUVA in vivo. However, such PG core proteins, even when heavily glycosylated with GAG chains, are modeled as binding with their leucine-rich domains directly to the side of collagen fibrils (62, 63), physical proximity that apparently does not facilitate RFUVA cross-linking, at least between collagen and purified, non-glycosylated keratocan or lumican in solution in vitro. The mechanism by which keratocan and lumican so drastically inhibit collagen cross-linking with itself by RFUVA therefore suggests a mechanism of steric blockage that does not put collagen molecules in reactive juxtaposition with either non-glycosylated PG core protein. In contrast, although both mimecan and decorin fail to inhibit cross-linking of collagen with itself, they each produce a new band at >300 kDa (Fig. 5, C (lane 8) and D (lane 8)) that appears to represent co-polymers of collagen-PG core protein that have not been described previously.
Binding antibodies to collagen type I and to each of the PG core proteins (in both glycosylated and non-glycosylated forms) allowed an independent way to assess degrees of cross-linking between collagen and PG core proteins. In the absence of core proteins, collagen type I in solution in vitro forms higher molecular weight polymers, especially leading both to the disappearance of most collagen α chains and β chains and to some enhancement of triple-chain polymers (γ polymers; 300 kDa) (Fig. 5, lane 2 in each gel). In contrast, the simultaneous presence of purified non-glycosylated keratocan or lumican in solution in vitro virtually eliminated such collagen cross-linking with itself (Fig. 5, A and B, lanes 4). In those same solutions, keratocan and lumican do not undergo cross-linking in the absence of RFUVA (lanes 5), but they do appear in a range of higher molecular weight polymers in response to RFUVA, in the absence (lanes 7) or presence of collagen type I (lanes 8). Thus, no purified, non-glycosylated keratocan or lumican appears to be bound to collagen during RFUVA in solution in vitro, although those core proteins form cross-links with themselves. In sharp contrast, although neither mimecan nor decorin inhibit the ability of collagen molecules to cross-link with themselves in response to RFUVA, both of those purified, non-glycosylated core proteins in the simultaneous presence of collagen form higher molecular weight polymer forms with themselves and even larger ones in the presence of collagen (Fig. 5, C and D, lanes 8) than in its absence (lanes 7), suggesting that they cross-link to collagen type I.
When collagen type I was exposed to RFUVA in solution in vitro in the presence of both mimecan and decorin, together with either keratocan or mimecan, (all as purified, non-glycosylated proteins), collagen cross-linking with itself was inhibited (as expected, because of the presence of keratocan and lumican) (Fig. 6, A versus B or A versus C, lanes 3) but not as much as if mimecan and decorin had been absent. In the absence of keratocan or mimecan, both mimecan and decorin epitopes are detected in macromolecules (equal to and >300 kDa) in the presence of collagen (Fig. 6A, lanes 5 and 7). However, if either keratocan (gel B) or lumican (gel C) is present in that mixture in solution in vitro, the amount of mimecan and decorin detected in the very high molecular size molecules is reduced although not eliminated. Thus, in a complete cornea in vivo with abundant collagen type I and all four PG core proteins in glycosylated form, some collagen molecules would undergo cross-linking with mimecan and decorin and might contribute substantively to tissue strength, even if such collagen-PG core protein cross-links did not represent a large proportion of the molar reaction yield of product.
Evidence presented in Fig. 8 suggests that the GAG components of native PG molecules do not themselves participate in RFUVA cross-linking. The smallest molecules were categorized as those in a size range arbitrarily below 100 kDa (Figs. 8 (A–D) and 9 (A–D)), whereas the largest were in a size range of 150 kDa and above (to the bottom of the sample well). These analyses probed for evidence of cross-linking to keratan sulfate (Fig. 8, A–H), chondroitin sulfate (Fig. 9, A–H), or any protein, but no such new indicator molecules were detected in response to exposure to RFUVA, consistent with an apparent absence of RFUVA causing visible cross-linking of corneal GAGs with themselves or with collagen.
Data presented in Fig. 9 indicate that collagen type I, present in the corneal stroma and examined here ex vivo, displays RFUVA cross-linking in the presence of the normal mixture of all four native PGs (i.e. PG core proteins with their GAG chains attached). Although the presence of the purified, non-glycosylated core proteins, keratocan and lumican, suppresses collagen cross-linking when both molecules are in solution in vitro (Figs. 1, 3, and 4), when those same molecules are present in the polymerized gel of the corneal stroma and in their native, glycosylated form, cross-linking of these PG core proteins to collagen is detected (Fig. 10). Future work will be needed to determine if this difference in the ability of both keratocan and lumican to form RFUVA cross-links with collagen type I arises because the presence of KS chains affects the conformation of these two core proteins as they bind to collagen (enhancing their RFUVA cross-linking to collagen) or because the higher densities of molecules in the native stroma bring collagen and these two core proteins close enough together to undergo RFUVA cross-linking. In contrast to the behavior of both keratocan and lumican (in solution in vitro versus in native glycosylated form ex vivo), both mimecan and decorin appear able to form RFUVA cross-links with collagen in solution in vitro (Fig. 5, C (lane 8) and D (lane 8)) and also in their native glycosylated form ex vivo (Fig. 10, C and D, lane 9). These results strikingly demonstrate that, as revealed here by using RFUVA as a type of molecular diagnostic probe, both keratocan and lumican interact with collagen type I very differently than do both mimecan and decorin. Elucidating the exact nature of those two styles of molecular interaction with collagen type I will be the subject of future experiments.
Protocols involving treatment of corneas with RFUVA are being used for an ever wider set of clinical conditions (40, 64, 65). It therefore is essential to elucidate as completely as possible the precise molecular effects of that treatment, not only on the cellular populations exposed (66) but also on the component molecules of the extracellular matrix. That has been the purpose of the experiments performed here. Many other interactions catalyzed by RFUVA in the cornea remain to be characterized in this same manner (e.g. possible interactions between collagens type I and type V within individual collagen fibrils and interactions between the collagen type IV of the epithelial basal lamina and laminin and the stromal proteoglycans). Using RFUVA reactivity as a molecular diagnostic probe may therefore prove useful in formulating new hypotheses about the native conformations of molecules in the extracellular matrix in general.
This work was supported, in whole or in part, by National Institutes of Health Grant R01EY000952 (to G. W. C.). This work was also supported by the Research Career Development Core (Brychta) in the Division of Biology at Kansas State University GOBO000657 (to G. W. C.), by National Science Foundation Major Research Instrumentation Program Grant 0521587 (to Kansas State University), and by the Terry C. Johnson Center for Basal Cancer Research at Kansas State University.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- GAG
- glycosaminoglycan
- RF
- riboflavin
- UVA
- ultraviolet-A
- PG
- proteoglycan
- KER
- keratocan
- LUM
- lumican
- MIM
- mimecan
- DEC
- decorin
- Ab
- antibody
- Coll
- collagen
- KS
- keratan sulfate
- CS
- chondroitin sulfate
- GHCl
- guanidine HCl
- DS
- dermatan sulfate
- NMWL
- normal molecular weight limit
- RFUVA
- riboflavin and ultraviolet-A
- rcf
- relative centrifuge force.
REFERENCES
- 1. Beuerman R. W., Pedroza L. (1996) Microsc. Res. Tech. 33, 320–335 [DOI] [PubMed] [Google Scholar]
- 2. Marfurt C. F., Cox J., Deek S., Dvorscak L. (2010) Exp. Eye Res. 90, 478–492 [DOI] [PubMed] [Google Scholar]
- 3. Al-Aqaba M. A., Fares U., Suleman H., Lowe J., Dua H. S. (2010) Br. J. Ophthalmol. 94, 784–789 [DOI] [PubMed] [Google Scholar]
- 4. Müller L. J., Pels L., Vrensen G. F. (1996) Invest. Ophthalmol. Vis. Sci. 37, 476–488 [PubMed] [Google Scholar]
- 5. Worthington C. R. (1984) Q. Rev. Biophys. 17, 423–451 [DOI] [PubMed] [Google Scholar]
- 6. Robert L., Legeais J. M., Robert A. M., Renard G. (2001) Pathol. Biol. 49, 353–363 [DOI] [PubMed] [Google Scholar]
- 7. Hay E. D. (1985) J. Cell. Biochem. 27, 143–156 [DOI] [PubMed] [Google Scholar]
- 8. Cardin A. D., Weintraub H. J. (1989) Arteriosclerosis 9, 21–32 [DOI] [PubMed] [Google Scholar]
- 9. Ruoslahti E., Engvall E. (1980) Biochim. Biophys. Acta 631, 350–358 [DOI] [PubMed] [Google Scholar]
- 10. Hassell J. R., Birk D. E. (2010) Exp. Eye Res. 91, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newsome D. A., Gross J., Hassell J. R. (1982) Invest. Ophthalmol. Vis. Sci. 22, 376–781 [PubMed] [Google Scholar]
- 12. Scott J. E. (1991) Biochem. Soc. Trans. 19, 877–881 [DOI] [PubMed] [Google Scholar]
- 13. Funderburgh J. L., Funderburgh M. L., Brown S. J., Vergnes J. P., Hassell J. R., Mann M. M., Conrad G. W. (1993) J. Biol. Chem. 268, 11874–11880 [PubMed] [Google Scholar]
- 14. Funderburgh J. L., Corpuz L. M., Roth M. R., Funderburgh M. L., Tasheva E. S., Conrad G. W. (1997) J. Biol. Chem. 272, 28089–28095 [DOI] [PubMed] [Google Scholar]
- 15. Corpuz L. M., Funderburgh J. L., Funderburgh M. L., Bottomley G. S., Prakash S., Conrad G. W. (1996) J. Biol. Chem. 271, 9759–9763 [DOI] [PubMed] [Google Scholar]
- 16. Funderburgh J. L., Funderburgh M. L., Mann M. M., Conrad G. W. (1991) Biochem. Soc. Trans. 19, 871–876 [DOI] [PubMed] [Google Scholar]
- 17. Kao W. W., Liu C. Y. (2002) Glycoconj. J. 19, 275–285 [DOI] [PubMed] [Google Scholar]
- 18. Carlson E. C., Liu C. Y., Chikama T., Hayashi Y., Kao C. W., Birk D. E., Funderburgh J. L., Jester J. V., Kao W. W. (2005) J. Biol. Chem. 280, 25541–25547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li W., Vergnes J. P., Cornuet P. K., Hassell J. R. (1992) Arch. Biochem. Biophys. 296, 190–197 [DOI] [PubMed] [Google Scholar]
- 20. Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. (1990) J. Histochem. Cytochem. 38, 1549–1563 [DOI] [PubMed] [Google Scholar]
- 21. Kjellén L., Lindahl U. (1991) Annu. Rev. Biochem. 60, 443–475 [DOI] [PubMed] [Google Scholar]
- 22. Spiro R. C., Freeze H. H., Sampath D., Garcia J. A. (1991) J. Cell Biol. 115, 1463–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corpuz L. M., Dunlevy J. R., Hassell J. R., Conrad A. H., Conrad G. W. (2000) Matrix Biol. 19, 699–704 [DOI] [PubMed] [Google Scholar]
- 24. Dunlevy J. R., Neame P. J., Vergnes J. P., Hassell J. R. (1998) J. Biol. Chem. 273, 9615–9621 [DOI] [PubMed] [Google Scholar]
- 25. Corpuz L. M., Dunlevy J. R., Hassell J. R., Conrad A. H., Conrad G. W. (2000) Matrix Biol. 19, 693–698 [DOI] [PubMed] [Google Scholar]
- 26. Chen R., Jiang X., Sun D., Han G., Wang F., Ye M., Wang L., Zou H. (2009) J. Proteome Res. 8, 651–661 [DOI] [PubMed] [Google Scholar]
- 27. Iozzo R. V. (1999) J. Biol. Chem. 274, 18843–18846 [DOI] [PubMed] [Google Scholar]
- 28. Meek K. M., Quantock A. J., Boote C., Liu C. Y., Kao W. W. (2003) Matrix Biol. 22, 467–475 [DOI] [PubMed] [Google Scholar]
- 29. Christiansen D. L., Huang E. K., Silver F. H. (2000) Matrix Biol. 19, 409–420 [DOI] [PubMed] [Google Scholar]
- 30. Conrad A. H., Zhang Y., Tasheva E. S., Conrad G. W. (2010) Invest. Ophthalmol. Vis. Sci. 51, 4500–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edrington T. B., Zadnik K., Barr J. T. (1995) Optom. Clin. 4, 65–73 [PubMed] [Google Scholar]
- 32. Romero-Jiménez M., Santodomingo-Rubido J., Wolffsohn J. S. (2010) Cont. Lens Anterior Eye 33, 157–166 [DOI] [PubMed] [Google Scholar]
- 33. Garcia-Lledo M., Feinbaum C., Alio J. L. (2006) Compr. Ophthalmol. Update 7, 47–52 [PubMed] [Google Scholar]
- 34. Ertan A., Colin J. (2007) J. Cataract Refract. Surg. 33, 1303–1314 [DOI] [PubMed] [Google Scholar]
- 35. Pramanik S., Musch D. C., Sutphin J. E., Farjo A. A. (2006) Ophthalmology 113, 1633–1638 [DOI] [PubMed] [Google Scholar]
- 36. Tan D. T., Por Y. M. (2007) Curr. Opin. Ophthalmol. 18, 284–289 [DOI] [PubMed] [Google Scholar]
- 37. Wollensak G., Spörl E., Seiler T. (2003) Ophthalmologe 100, 44–49 [DOI] [PubMed] [Google Scholar]
- 38. Krishna C. M., Uppuluri S., Riesz P., Zigler J. S., Jr., Balasubramanian D. (1991) Photochem. Photobiol. 54, 51–58 [DOI] [PubMed] [Google Scholar]
- 39. Seiler T., Hafezi F. (2006) Cornea 25, 1057–1059 [DOI] [PubMed] [Google Scholar]
- 40. Wollensak G., Spoerl E., Seiler T. (2003) Am. J. Ophthalmol. 135, 620–627 [DOI] [PubMed] [Google Scholar]
- 41. Wollensak G., Spoerl E. (2004) J. Cataract Refract. Surg. 30, 689–695 [DOI] [PubMed] [Google Scholar]
- 42. Wollensak G., Spoerl E., Seiler T. (2003) J. Cataract Refract. Surg. 29, 1780–1785 [DOI] [PubMed] [Google Scholar]
- 43. Wollensak G., Wilsch M., Spoerl E., Seiler T. (2004) Cornea 23, 503–507 [DOI] [PubMed] [Google Scholar]
- 44. Wollensak G., Iomdina E. (2009) J. Cataract Refract. Surg. 35, 540–546 [DOI] [PubMed] [Google Scholar]
- 45. Spoerl E., Wollensak G., Dittert D. D., Seiler T. (2004) Ophthalmologica 218, 136–140 [DOI] [PubMed] [Google Scholar]
- 46. Dhaliwal J. S., Kaufman S. C. (2009) Cornea 28, 62–67 [DOI] [PubMed] [Google Scholar]
- 47. Mencucci R., Marini M., Paladini I., Sarchielli E., Sgambati E., Menchini U., Vannelli G. B. (2010) Clin. Exp. Ophthalmol. 38, 49–56 [DOI] [PubMed] [Google Scholar]
- 48. Kohlhaas M., Spoerl E., Schilde T., Unger G., Wittig C., Pillunat L. E. (2004) J. Cataract Refract. Surg. 32, 279–283 [DOI] [PubMed] [Google Scholar]
- 49. Spoerl E., Wollensak G., Seiler T. (2004) Curr. Eye Res. 29, 35–40 [DOI] [PubMed] [Google Scholar]
- 50. Schilde T., Kohlhaas M., Spoerl E., Pillunat L. E. (2008) Ophthalmologe 105, 165–169 [DOI] [PubMed] [Google Scholar]
- 51. McCall A. S., Kraft S., Edelhauser H. F., Kidder G. W., Lundquist R. R., Bradshaw H. E., Dedeic Z., Dionne M. J., Clement E. M., Conrad G. W. (2010) Invest. Ophthalmol. Vis. Sci. 51, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Isemura M., Ikenaka T. (1975) Biochim. Biophys. Acta 411, 11–21 [DOI] [PubMed] [Google Scholar]
- 53. Conrad G. W., Ager-Johnson P., Woo M. L. (1982) J. Biol. Chem. 257, 464–471 [PubMed] [Google Scholar]
- 54. Newsome D. A., Gross J., Hassell J. R. (1982) Invest. Ophthalmol. Vis. Sci. 22, 376–381 [PubMed] [Google Scholar]
- 55. Zhang Y., Kariya Y., Conrad A. H., Tasheva E. S., Conrad G. W. (2005) Anal. Chem. 77, 902–910 [DOI] [PubMed] [Google Scholar]
- 56. Zhang Y., Conrad A. H., Tasheva E. S., An K., Corpuz L. M., Kariya Y., Suzuki K., Conrad G. W. (2005) Invest. Ophthalmol. Vis. Sci. 46, 1604–1614 [DOI] [PubMed] [Google Scholar]
- 57. Epstein E. H., Jr., Scott R. D., Miller E. J., Piez K. A. (1971) J. Biol. Chem. 246, 1718–1724 [PubMed] [Google Scholar]
- 58. Makareeva E., Mertz E. L., Kuznetsova N. V., Sutter M. B., DeRidder A. M., Cabral W. A., Barnes A. M., McBride D. J., Marini J. C., Leikin S. (2008) J. Biol. Chem. 283, 4787–4798 [DOI] [PubMed] [Google Scholar]
- 59. Parry D. A. (1988) Biophys. Chem. 29, 195–209 [DOI] [PubMed] [Google Scholar]
- 60. Sung H. W., Chang W. H., Ma C. Y., Lee M. H. (2003) J. Biomed. Mater. Res. A 64, 427–438 [DOI] [PubMed] [Google Scholar]
- 61. de La Rochette A., Birlouez-Aragon I., Silva E., Morlière P. (2003) Biochim. Biophys. Acta 1621, 235–241 [DOI] [PubMed] [Google Scholar]
- 62. Funderburgh J. L. (2000) Glycobiology 10, 951–958 [DOI] [PubMed] [Google Scholar]
- 63. Kao W. W., Funderburgh J. L., Xia Y., Liu C. Y., Conrad G. W. (2006) Exp. Eye Res. 82, 3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kampik D., Ralla B., Keller S., Hirschberg M., Friedl P., Geerling G. (2010) Invest. Ophthalmol. Vis. Sci. 51, 3929–3934 [DOI] [PubMed] [Google Scholar]
- 65. Snibson G. R. (2010) Clin. Exp. Ophthalmol. 38, 141–153 [DOI] [PubMed] [Google Scholar]
- 66. Wollensak G., Spörl E., Reber F., Pillunat L., Funk R. (2003) Ophthalmic Res. 35, 324–328 [DOI] [PubMed] [Google Scholar]