Abstract
Dendritic cells are professional antigen-presenting cells that are specialized in antigen uptake and presentation. Allergy to cat has increased substantially in recent years and has been shown to be positively associated with asthma. We have recently shown that the mannose receptor (MR), a C-type lectin expressed by dendritic cells, recognizes various glycoallergens from diverse sources and is involved in promoting allergic responses to a major house dust mite allergen in vitro. Here we investigated the potential role of MR in allergic responses to Fel d 1, a major cat allergen. Fel d 1 binding to MR was confirmed by ELISA. Using blocking, gene silencing (siRNA) experiments, and MR knock-out (MR−/−) cells, we have demonstrated that MR plays a major role in internalization of Fel d 1 by human and mouse antigen-presenting cells. Intriguingly, unlike other glycoallergens, recognition of Fel d 1 by MR is mediated by the cysteine-rich domain, which correlates with the presence of sulfated carbohydrates in natural Fel d 1. WT and MR−/− mice were used to study the role of MR in allergic sensitization to Fel d 1 in vivo. MR−/− mice sensitized with cat dander extract and Fel d 1 produced significantly lower levels of total IgE, Fel d 1-specific-IgE and IgG1, the hallmarks of allergic response, compared with WT mice. Our data show for the first time that Fel d 1 is a novel ligand of the cysteine-rich domain of MR and that MR is likely to play a pivotal role in allergic sensitization to airborne allergens in vivo.
Keywords: Dendritic Cell, Glycoprotein, Immunology, Innate Immunity, Lectin, Allergen, Allergic Sensitization, Allergy, Glycoallergen, Mannose Receptor
Introduction
Allergic sensitization to aeroallergens, particularly those derived from cat, has increased considerably over the past few decades and has been shown to be positively associated with asthma as a strong predisposing factor (1–4). Dander of the domestic cat (Felis domesticus) is the main source of cat allergens, and five allergenic proteins have been identified so far, namely Fel d 1–Fel d 5, with Fel d 1 being the major immunodominant protein accounting for 60–90% of the total allergenicity of cat dander (5–8).
Dendritic cells (DCs)4 are professional antigen-presenting cells and are key regulators of the immune response (9, 10). DCs efficiently sample their microenvironment for foreign antigens through a wide range of receptors; of those receptors, C-type lectin-like receptors appear to be of paramount importance in the recognition and uptake of glycoprotein antigens (11–13). Many of the C-type lectin-like receptors that have been identified on DCs function mainly as antigen uptake receptors, including mannose receptor (MR, CD206) (14–16), DC-SIGN (CD209) (17), and DEC-205 (CD205) (18). MR, a 175-kDa type I integral transmembrane glycoprotein, is a C-type lectin-like receptor abundantly expressed on DCs and known to perform a panoply of functions (19). MR recognizes a wide range of carbohydrates on microbial cell surfaces and mediates endocytic clearance of host derived glycoproteins (20, 21). The extracellular portion of MR contains three regions: an NH2-terminal cysteine rich (CR) domain, a fibronectin type II-like domain (FNII), and eight C-type lectin-like domains (CTLDs) followed by a hydrophobic transmembrane region and a short COOH-terminal hydrophilic cytoplasmic domain (20, 22).
MR is a multifunctional receptor with two lectin activities, involving Ca2+-dependant recognition of carbohydrates through CTLDs, as well as Ca2+-independent binding of acidic glycans sulfated at positions 3 or 4 via the CR domain (23–25), whereas the FNII mediates collagen binding (26, 27). Recently, we have shown that MR expressed on human DCs is a common receptor for several clinically relevant allergens (Der p 1, Der p 2, Ara h 1, Can f 1, and Bla g 2) and that recognition of these allergens is mediated by the CTLD4–7 region of MR (28). Here we show, for the first time, that MR is also an endocytic receptor for the uptake of the major cat allergen Fel d 1 by human and mouse antigen-presenting cells, but intriguingly, unlike Der p 1, Der p 2, Ara h 1, Can f 1, and Bla g 2, the recognition of Fel d 1 by MR is mediated through the CR domain of MR. Furthermore, using WT and MR knock-out (MR−/−) mice, we demonstrate that MR plays a pivotal role in the allergic sensitization to Fel d 1.
EXPERIMENTAL PROCEDURES
Generation of Antigen-presenting Cells
Immature monocyte-derived (Mo)-DCs were generated from the blood of healthy individuals (obtained with informed consent and after ethical committee approval) as previously described (29). Briefly, peripheral blood mononuclear cells were separated by standard density gradient centrifugation on Histopaque-1077 (Sigma). Monocytes were then isolated from peripheral blood mononuclear cells using mouse anti-human CD14 mAb conjugated to magnetic beads (Miltenyi Biotec). CD14+ cells were cultured in RPMI 1640 supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine (all from Sigma), and 10% low endotoxin FBS (Autogen Bioclear). Monocyte differentiation was carried out for 6 days in the presence of IL-4 (250 IU/ml) and GM-CSF (50 ng/ml) (R & D Systems). Bone marrow-derived dendritic cells (BM-DCs) were generated in culture from WT and MR knock-out mice as described elsewhere (30) with minor modifications. Briefly, bone marrow was isolated from bones of the hind legs (femurs and tibias) washed in 70% ethanol and subsequently in RPMI 1640 medium. The bone marrow was then cultured for 6 days in complete medium (RPMI 1640 containing 10% FBS supplemented with 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin; all from Sigma) in the presence of 1000 units/ml murine GM-CSF (R & D Systems). Bone marrow-derived macrophages were generated as previously described (31). Briefly, bone marrow collected from each mouse was resuspended in complete medium (as previously described for BM-DCs), and macrophages were cultured in complete medium containing L-cell conditioned medium for 6 days. Macrophages were collected by treating cells with cold PBS containing 10 mm EDTA.
Lectin ELISA for the Detection of Fel d 1-MR Binding
Binding of the major cat allergen Fel d 1 to various MR constructs was investigated using ELISA. All washes and incubations were carried out in lectin buffer consisting of 10 mm Tris-HCl, pH 7.5, 10 mm Ca2+, 154 mm NaCl, and 0.05% (w/v) Tween 20. Natural Fel d 1 (Indoor Biotechnologies), affinity-purified from cat hair (32) with >95% purity, was used in all experiments. Fel d 1 (5 μg/ml), as well as 2 μg/ml of MR carbohydrate ligands (Mannan (Sigma), sulfated galactose (SO4-3-Gal-PAA), or galactose (Gal-PAA) (Lectinity, Moscow, Russia)), was used to coat the wells of Maxisorp ELISA plates (Nunc) by overnight incubation in PBS at 4 °C. The plates were washed three times and incubated with 2 μg/ml of different domains of mouse MR fused to the Fc portion of human IgG1 (CR-Fc, CTLD4–7-Fc, CR-FNII-CTLD1-Fc, or CR-FNII-CTLD1–3-Fc) for 2 h (33). After three washes, the binding was detected by incubation with anti-human IgG γ-chain-specific alkaline phosphatase conjugate diluted 1/1,000 (Sigma). The plates were then washed three times with lectin buffer and two times with 100 mm Tris-HCl, 100 mm NaCl and 5 mm MgCl2 (pH 9.5) and finally developed with 1 mg/ml of p-nitro-phenylphosphate (Sigma). Absorbance was measured at 405 nm on a plate reader.
To further confirm the binding of Fel d 1 to the CR domain of MR, dose-dependent binding and inhibition assays were performed. Briefly, the plates were coated overnight with various concentrations (0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, or 12.8 μg/ml) of Fel d 1, SO4-3-Gal-PAA, or Gal-PAA and incubated with CR-Fc (2 μg/ml) for 2 h. Alternatively, inhibition assays were performed by preincubation or not of CR-Fc with different concentrations (0.001, 0.01, 0.1, 1, or 10 μg/ml) of mannan or SO4-3-Gal-PAA for 20 min prior to addition to wells of Maxisorp plates that had previously been coated overnight with 5 μg/ml of Fel d 1. All of the assays were carried out in triplicate.
Glycan Analysis of Fel d 1 Using In-gel Protein Digestion and Nanoflow LC-MS/MS Fourier Transform Ion Cyclotron Resonance
These experiments were carried out in the Proteomics Center of the Sahlgrenska Academy (University of Gothenburg, Gothenburg, Sweden). The method for in-gel protein digestion with trypsin described by Shevchenko et al. (35) was applied with some minor modifications (34). Briefly, the gel pieces were destained by washing three times in 25 mm NH4HCO3 in 50% CH3CN and one time in 25 mm NH4HCO3 in 50% CH3OH. Gel pieces were dried in a vacuum centrifuge and incubated with digestion buffer (50 mm NH4HCO3, 10 ng/μl trypsin) at 37 °C overnight. The peptides were extracted in 50% CH3CN, 1% CH3COOH, and the supernatant was evaporated to dryness in a vacuum centrifuge. Prior to MS analysis, the peptides were reconstituted in 0.2% HCOOH.
Two-microliter sample injections were made with an HTC-PAL autosampler (CTC Analytics AG, Zwingen, Switzerland) connected to an Agilent 1100 binary pump (Agilent Technologies, Palo Alto, CA). The peptides were trapped on a precolumn (45 × 0.075-mm inner diameter) and separated on a reversed phase column, 200 × 0.050 mm. The flow through of the analytical column was reduced by a split to an ∼100 nl/min. A 40-min gradient 10–50% CH3CN in 0.2% COOH was used for separation of the peptides.
The nanoflow LC-MS/MS was performed on a hybrid linear ion trap-FT/ICR mass spectrometer equipped with a 7T ICR magnet (LTQ-FT; Thermo Electron, Bremen, Germany). For each scan of FT/ICR, the three most intense, doubly or triply charged ions were sequentially fragmented in the linear trap by collision-induced dissociation. All of the tandem mass spectra were searched by MASCOT (Matrix Science, London). For protein identification, the minimum criteria were one tryptic peptide matched at or above the 99% level of confidence and an additional one peptide match at the 95% level.
Antigen Uptake Assays
Fel d 1 was labeled with Cyanine 5 (Cy5) (Amersham Biosciences) according to the manufacturer's instructions. Uptake assays were carried out in medium consisting of RPMI (Sigma), 30% PBS with Ca2+ and Mg2+ (Invitrogen), and 5% FBS (Autogen Bioclear). Immature Mo-DCs (2 × 105/200 μl) were preincubated for 30 min with different concentrations (50, 100, 200, or 500 μg/ml) of SO4-3-Gal-PAA or Gal-PAA (Lectinity, Moscow, Russia) or mannan (200 μg/ml) and subsequently incubated with 5 μg/ml of Cy5 Fel d 1 for another 30 min. In some experiments, BM-DCs (2 × 105/200 μl) from WT and MR−/− mice were incubated with Cy5 Fel d 1 for 30 min, whereas in other experiments, Mo-DCs (1 × 105/100 μl) that have previously been treated with control (CT) or MR siRNA were incubated with 5 μg/ml of Cy5 Fel d 1 or FITC-Lewisx-PAA (Lectinity) for 30 min. The cells were then washed and fixed in 0.5% formaldehyde, and the quantitative Cy5 Fel d 1 uptake was analyzed by flow cytometry.
RNA Interference
RNA interference was performed by transfecting Mo-DCs with siRNA to specifically knock down MR on DCs as previously described (28). The DNA targeted sequence was 5′-TGGATGGATGATACCTGCGAGAGTA-3′ from 3299 to 3323 bp (Invitrogen), and the nonsilencing CT siRNA used was all star negative control (Qiagen). Gene knockdown was estimated quantitatively at message level by quantitative real time PCR and at protein level by flow cytometry and Western blotting. Cell surface markers were tested before performing functional assays to ascertain MR gene silencing specificity.
Real Time PCR
Real time PCRs were conducted using a MX3005P thermal cycler (Stratagene), and PCR amplifications were performed with the SYBR green method using the following primers: MR (forward), 5′-CGTTTACCAAATGGCTTCGA-3′; MR (reverse), 5′-CCTTGGCTTCGTGATTTCAT-3′); GAPDH (forward), 5′-GAGTCAACGGATTTGGTCGT-3′; and GAPDH (reverse), 5′-GACAAGCTTCCCGTTCTCAG-3′. Each reaction was performed in a final volume of 25 μl comprised of 5 μl of template (cDNA), 12.5 μl of Sybr green master mix (Stratagene), 1 μl of (200 nm) of each primer, and 0.38 μl of ROX dye. The amplifications were performed starting with an initial denaturation step of 95 °C/30 s, followed by 40 cycles of 95 °C/30 s and 56 °C/60 s.
Western Blotting
The samples were run on 12% SDS-PAGE, transferred to nitrocellulose membrane (Amersham Biosciences), blocked with PBS-0.05% Tween 20 containing 5% milk, and subsequently probed with 5 μg/ml of mouse mAb to human CD206 (clone 15.2; AbD Serotec) or mouse anti-human β-actin (diluted 1:2,000; Sigma). The membranes were washed and reprobed with HRP-conjugated rabbit F(ab·)2 anti-mouse IgG diluted 1/5,000 (AbD Serotec). The membranes were washed, and bands were detected with ECL reagent (Amersham Biosciences).
In Vivo Sensitization of Animals
MR−/− mice (36) were provided by M. Nussenzweig (Rockefeller University, New York, NY). These mice were generated originally on a mixed strain of 129SvJ and C57BL/6 background and were backcrossed to the C57BL/6 strain for more than seven generations. Homozygous knock-out mice were bred to provide experimental mice in the Biomedical Services Unit at the University of Nottingham. The mice used in this study were female and 7–8 weeks of age. Control age- and sex-matched C57BL/6 mice were obtained from Charles River Laboratories. WT and MR−/− mice were housed under specific pathogen-free conditions; all of the animals were handled according to institutional guidelines and after obtaining appropriate ethics approval. A total of 18 mice were divided into four groups. Animal sensitization was performed using an established protocol (37) with some modifications. Briefly, at day 0, two groups (WT and MR−/−, seven mice each) were sensitized intraperitoneally (intraperitoneally) with Fel d 1 (15 μg/mouse) emulsified in Al(OH)3 (Imject ALUM; Pierce). Four boosts were given intranasally on days 14, 16, 28, and 30, whereby mice received cat dander extract at 150 μg/50 μl/animal. The mice were challenged intranasally 6 weeks after their first sensitization (day 42) with cat dander extract enriched with 5 μg/ml of purified Fel d 1. Control mice (two WT and two MR−/−) were mock sensitized intraperitoneally with Al(OH)3 and challenged intranasally with PBS. At 24 h after challenge (day 43), the animals were sacrificed, and blood was collected. The sera were stored at −20 °C until use.
Determination of Total and Allergen-specific Antibody Levels
Total IgE responses, as well as allergen-specific IgE, IgG1, and IgG2a responses to Fel d 1, were determined by ELISA. Sera were diluted 1/80, 1/5, 1/20, and 1/12,800 for total IgE, Fel d 1-specific IgE, IgG2a, and IgG1, respectively. Total IgE levels were quantified using an ELISA MAX IgE kit (Biolegend, San Diego, CA) according to the manufacturer's instructions. Briefly, Maxisorp ELISA plates were coated with capture Ab, blocked with assay diluent, incubated with standards and samples for 2 h, and sequentially incubated with biotinylated detection Ab (mouse anti-IgE-biotin), secondary Ab (avidin-HRP), and color was developed using the peroxidase substrate tetramethylbenzidine. Fel d 1-specific IgE (measured using samples that have been depleted of IgG using GammaBind plus-Sepharose (Gamma Bind plus TM SepharoseTM; GE Healthcare Biosciences AB), Fel d 1-specific IgG1, and IgG2a were detected on plates coated with 5 μg/ml Fel d 1 and developed with biotinylated detection Ab (clone R35–118, 2 μg/ml) for IgE or alkaline phosphatase-conjugated Ab (clone X56 for IgG1 and clone R19–15 for IgG2a) (BD Biosciences). The binding of biotinylated antibody was detected with alkaline phosphatase-conjugated streptavidin (Sigma).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software, Inc., San Diego, CA). Student's t test or Mann-Whitney U test was used for pairwise comparisons analyses, whereas multiple comparisons analyses were performed by Kruskal-Wallis with Dunn's post hoc test. Significance was accepted when p values ≤0.05, where * indicates p ≤ 0.05; ** indicates p ≤ 0.01; and *** indicates p ≤ 0.001. The data are expressed as the means ± S.E.
RESULTS
MR Expression Is Required for Uptake of Fel d 1 by Human DCs
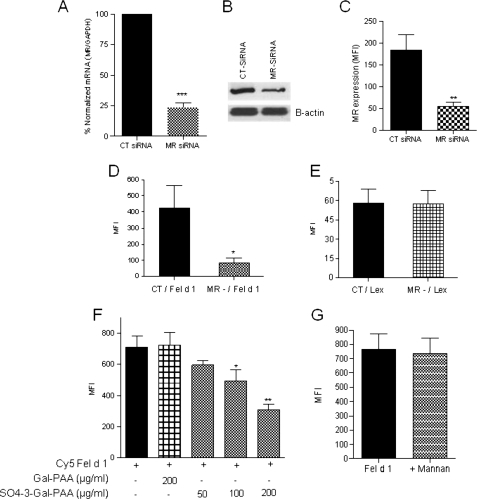
To investigate the contribution of MR to Fel d 1 uptake by human DCs, we used a gene silencing strategy to specifically inhibit the expression of MR on human DCs as described in our previous work (28). MR was successfully down-regulated on DCs by siRNA with percentages of inhibition approaching 70–75% as shown by quantitative real time PCR (Fig. 1A), Western blotting (Fig. 1B), and flow cytometry (Fig. 1C). Immature Mo-DCs that had previously been treated with CT or MR siRNA were incubated with Cy5 Fel d 1, and Fel d 1 uptake was assessed by flow cytometry. We observed a significant inhibition (80%) of Fel d 1 uptake (Fig. 1D) by MR-deficient (mean fluorescence intensity = 83.85 ± 26.78) compared with MR-sufficient (mean fluorescence intensity = 421.9 ± 140.7) DCs. To confirm the specificity of the gene silencing, we used a polyacrylamide (PAA) polymer bearing Lewisx antigen, a specific ligand for DC-SIGN, as a control, and no inhibition of uptake was observed (Fig. 1E). These results indicate that Fel d 1 uptake by human DCs is MR-mediated.
FIGURE 1.
MR silencing and preincubation with a sulfated MR ligand leads to a significant reduction of Fel d 1 uptake by human DCs. A–C, MR expression was determined on Mo-DCs (before and after siRNA treatment) using quantitative real time PCR (A), Western blotting (B) and flow cytometry (C) as described under “Experimental Procedures.” D and E, CT and MR-deficient human Mo-DCs were incubated for 30 min with 5 μg/ml of Cy5 Fel d 1 (D) or FITC-Lewis x (E). Fel d 1 uptake was estimated by flow cytometry, and significant reduction in Fel d 1 uptake was obtained after MR silencing. Mo-DCs were preincubated with increasing concentrations of SO4-3-Gal-PAA or Gal-PAA prior to the addition of Cy5 Fel d 1 and Fel d 1 uptake was quantitatively estimated by flow cytometry (n = 3). F and G, a dose-dependent inhibition of Fel d 1 uptake by SO4-3-Gal-PAA (F) was observed, whereas mannan had no effect (G). *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
Fel d 1 Uptake by Human DCs Is Inhibited by Ligands for the CR Domain of MR
We then investigated the ability of SO4-3-Gal-PAA and mannan, specific ligands for the CR domain and the CTLD4–7 regions of MR, respectively, to inhibit Fel d 1 uptake by human DCs. Immature Mo-DCs were preincubated with different concentrations (50, 100, or 200 μg/ml) of SO4-3-Gal-PAA or mannan prior to the addition of labeled Cy5 Fel d 1. The results shown in Fig. 1F indicate that Fel d 1 uptake (mean fluorescence intensity = 708.7 ± 72.54) was inhibited in a dose-dependent manner by SO4-3-Gal-PAA (p < 0.01, n = 3). The control ligand (Gal-PAA) had no effect on uptake. On the other hand, mannan, the control ligand for the CTLD4–7 region of MR, had no effect on Fel d 1 uptake by Mo-DCs (Fig. 1G), indicating that the CTLD4–7 domains of MR are not involved in Fel d 1 uptake by Mo-DCs.
MR Mediates the Uptake of Fel d 1 by Mouse Macrophages and DCs
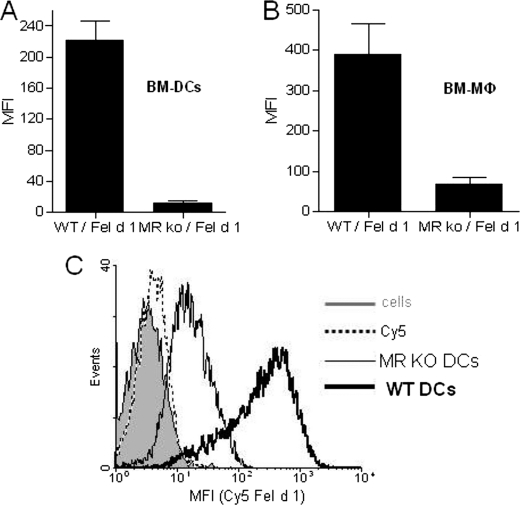
To investigate whether the results obtained with human DCs translate to the mouse system, we investigated Fel d 1 uptake by mouse DCs and macrophages generated from WT and MR−/− animals. The results shown in Fig. 2 demonstrate that both DCs (Fig. 2, A and C) and macrophages (Fig. 2B) from MR-deficient animals display a major reduction in their ability to internalize Fel d 1, demonstrating that MR is a major receptor for Fel d 1 in mouse cells.
FIGURE 2.
MR expression is required for Fel d 1 uptake by mouse antigen-presenting cells. BM-derived DCs (A and C) and macrophages (B) were generated from WT and MR−/− mice. The cells were incubated with 5 μg/ml Cy5 Fel d 1 for 30 min, and Fel d 1 uptake was estimated by flow cytometry (n = 3). The bar diagrams (A and B) show mean fluorescence intensities (MFI) of Fel d 1 uptake BM-DCs and macrophages. C shows a representative histogram profile for Cy5 Fel d 1 uptake by WT and MR−/− BM-DCs.
Fel d 1 Binds to the CR Domain of MR
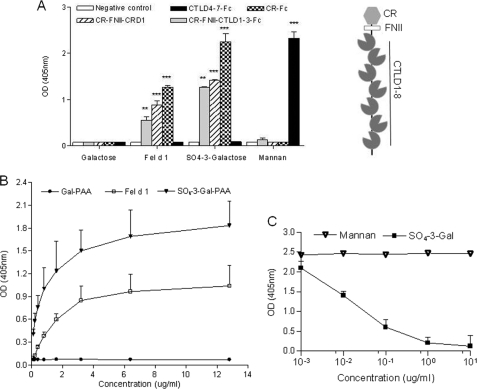
To investigate whether the CR domain of MR could directly bind Fel d 1, we performed ELISA experiments using Fc-derived proteins bearing different domains of mouse MR (Fig. 3A). These assays showed that Fel d 1 binds strongly to CR-Fc, CR-FNII-CTLD1-Fc, and CR-FNII-CTLD1–3-Fc but not to CTLD4–7-Fc. None of the proteins bound to the negative control Gal-PAA (p < 0.0001, n = 5). The control ligand for the CR domain (SO4–3-Gal-PAA) showed strong binding to CR-Fc but not to CTLD4–7-Fc, whereas mannan showed strong binding to CTLD4–7-Fc and no binding to CR-Fc or CR-FNII-CTLD1-Fc and CR-FNII-CTLD1–3-Fc (n = 5). These results demonstrate that Fel d 1 is a ligand for the CR domain of MR. Further assays showed that Fel d 1 binds the CR domain in a dose-dependent manner (Fig. 3B).
FIGURE 3.
Fel d 1 binds to the CR domain of MR. A, binding of Fel d 1 and mannan, SO4-3-Gal-PAA, and Gal-PAA to Fc chimeric proteins containing different MR subfragments (CR-Fc, CR-FNII-CTLD1-Fc, CR-FNII-CTLD1–3-Fc, or CTLD4–7-Fc). Binding was detected by anti-human IgG conjugated to alkaline phosphatase (n ≥ 3). Absorbance was measured at 405 nm, and Kruskal-Wallis with Dunn's post hoc test was used to test for significance. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. The data are expressed as the averages ±S.E. A schematic representation of the structure of the extracellular region of MR indicates the location of the different domains tested. B, dose-dependent binding of Fel d 1 to the CR domain of MR; murine CR-Fc (2 μg/ml) was added to Maxisorp plates that have previously been coated with different concentrations of Fel d 1, SO4–3-Gal-PAA, or Gal-PAA (n = 3). C, binding of CR-Fc to Fel d 1 could be inhibited by SO4–3-Gal-PAA but not by mannan (n = 3). Absorbance was measured at 405 nm.
To further corroborate the binding of Fel d 1 to the CR domain of MR, inhibition ELISA was performed whereby CR-Fc was incubated with different concentrations of SO4-3-Gal-PAA or mannan prior incubation with Fel d 1. The results obtained (Fig. 3C) demonstrate a dose-dependent inhibition of Fel d 1 binding to CR-Fc by SO4-3-Gal-PAA, whereas mannan did not show any effect.
Glycan Composition of Natural Fel d 1
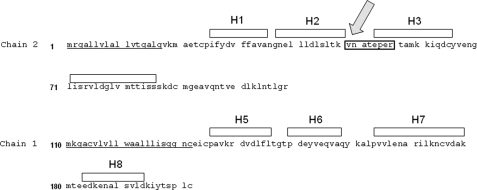
To identify the carbohydrates present in Fel d 1 responsible for binding to the CR domain of MR, we determined the carbohydrate content of Fel d 1 using mass spectroscopic analysis. Fel d 1 is a 35-kDa tetrameric glycoprotein formed by two heterodimers. Each dimer is composed of two chains (chain 1 and 2) derived from two independent genes (38, 39). In agreement with the primary amino acid sequence predicting a single N-glycosylation site at Asn33, we identified the single glycopeptide VNATEPER within Fel d 1 (Fig. 4). This glycopeptide is located in an exposed region of the chain 2 between helix 2 and helix 3 according to Fel d 1 crystal structure (40). The glycan composition of Fel d 1 comprises hybrid and complex carbohydrate structures containing sialic acid and, most importantly, sulfated moieties (Table 1). Supplemental Fig. S1 shows a representative collision-induced dissociation product ion spectrum of the tryptic glycopeptide MH+ = 3776.46. The presence of sulfate groups in the glycans associated with Fel d 1 is consistent with the capacity of Fel d 1 to bind the CR domain of MR.
FIGURE 4.
Localization of the unique N-linked glycosylation site in Fel d 1. Fel d 1 is composed of two heterodimers. Each dimer is composed of two chains (four helices each): chain 2 (H1-H4) and chain 1 (H5-H8) derived from two independent genes. The single glycopeptide VNATEPER (indicated by an arrow) has been identified within Fel d 1 and is located in an exposed region of the chain 2 between helix 2 and helix 3.
TABLE 1.
Composition of the glycoprotein content of Fe l d 1
| Glycoform mass | Δmass | Structure | Type | Peptide mass (M) | Peptide sequence | Theoretical glycopeptide mass |
|---|---|---|---|---|---|---|
| ppm | ||||||
| 2133.772 | 2.086 | (Hex)3 (HexNAc)3 (Deoxyhexose)1 + (Man)3(GlcNAc)2 | hybrid/complex | 914.446 | 49VNATEPER56 | 3049.225 |
| 2424.867 | 3.338 | (Hex)3 (HexNAc)3 (Deoxyhexose)1 (NeuAc)1 + (Man)3(GlcNAc)2 | hybrid/complex | 914.446 | 49VNATEPER56 | 3340.32 |
| 2278.809 | 1.28 | (Hex)3 (HexNAc)3 (NeuAc)1 + (Man)3(GlcNAc)2 | hybrid/complex | 914.446 | 49VNATEPER56 | 3194.262 |
| 2569.905 | 3.575 | (Hex)3 (HexNAc)3 (NeuAc)2 + (Man)3(GlcNAc)2 | hybrid/complex | 914.446 | 49VNATEPER56 | 3485.358 |
| 2504.824 | 2.307 | (Hex)3 (HexNAc)3 (Deoxyhexose)1 (NeuAc)1 (Sulph)1 + (Man)3(GlcNAc)2 | hybrid/complex | 914.446 | 49VNATEPER56 | 3420.277 |
| 2861 | 1.417 | (Hex)3 (HexNAc)3 (NeuAc)3 + (Man)3(GlcNAc)2 | hybrid/complex | 914.446 | 49VNATEPER56 | 3776.453 |
| 2358.766 | 2.822 | (Hex)3 (HexNAc)3 (NeuAc)1 (Sulph)1 + (Man)3(GlcNAc)2 | hybrid/complex | 914.446 | 49VNATEPER56 | 3274.219 |
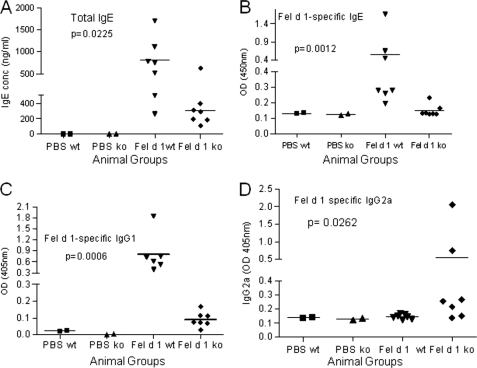
MR Recognition Contributes to Fel d 1 Allergenicity in Vivo
To investigate whether the capacity of Fel d 1 to bind MR could contribute to its allergenicity, we compared the response of WT and MR−/− mice to Fel d 1 sensitization. WT animals produced significantly (p < 0.05) higher total IgE levels (803.7 ± 180.5 ng/ml) compared with MR−/− animals (301.7 ± 64.94 ng/ml), whereas control mice, mock sensitized with PBS, produced undetectable levels of IgE (<0.5 ng/ml) (Fig. 5A). This difference in total IgE levels between WT and MR−/− mice was accompanied by a more significant difference (p < 0.01) in Fel d 1-sepecific IgE (Fig. 5B) and Fel d 1-specific IgG1 (p < 0.001; Fig. 5C) between the two groups of mice. Despite the low levels of Fel d 1-specific IgG2a that could be detected, as expected in a Th2-dominated response, WT mice produced significantly lower levels of IgG2a compared with WT mice (p < 0.03) (Fig. 5D). These results indicate that the allergenic potential of Fel d 1 is enhanced by its capacity to bind MR.
FIGURE 5.
MR contributes to Fel d 1 allergenicity. WT and MR−/− mice were sensitized (intraperitoneal) with Fel d 1 and challenged (intranasal) with cat dander extract and Fel d 1. Antibody responses to Fel d 1 were determined by ELISA as described under “Experimental Procedures.” WT mice produced significantly higher levels of total IgE (A), Fel d 1-specific IgE (B), Fel d 1-specific IgG1 (C), and lower levels of Fel d 1-specific IgG2a (D) compared with MR−/− mice sensitized under similar conditions. Control mice, which received PBS, showed negligible levels of all antibodies.
DISCUSSION
Dendritic cells are sentinels of the immune system with superior ability for antigen recognition and uptake, as well as for priming naïve T cells (41). Cat allergy affects ∼10–15% of the adult population (7), and 20–30% of asthmatic individuals respond to cat allergen exposure (42, 43). Moreover, it has also been shown that up to 40% of asthmatic children are sensitized to cat (1, 44).
In a recent study, we demonstrated that MR is a common receptor on human DCs for the uptake of a number of clinically relevant allergens from diverse sources (28). In this study, we have shown the key role of MR in Fel d 1 uptake by antigen-presenting cells through gene knockdown experiments that specifically target MR and using BM-DC and macrophages obtained from WT and MR−/− mice. These have shown that MR-deficient DCs displayed a significantly reduced capacity to internalize Fel d 1 as compared with MR-sufficient DCs. The substantial reduction/abrogation in Fel d 1 uptake observed with the MR-deficient cells suggests that MR is the main, and most likely the sole, endocytic receptor for the uptake of Fel d 1 by human and mouse antigen-presenting cells. Furthermore, we have identified Fel d 1 as a novel ligand for the CR domain of MR. This was unexpected, because all other tested allergens (Der p 1, Der p 2, Ara h 1, Can f 1, and Bla g 2) have previously been shown to be recognized by the CTLD4–7 domains of MR (28). To date, all other ligands described for the CR domain have been of endogenous origin (45, 46), and it is rather intriguing that the main source of Fel d 1, cat hair/dander, could contain ligands for MR. It is worth noting that Fel d 1 is a member of the secretoglobin family and, together with the hamster Harderian gland proteins and mouse salivary androgen-binding protein, is secreted by exocrine glands and is released to the exterior as a result of licking and grooming (47). It would be of interest to see whether the CR domain could detect other ligands in exocrine organs of other organisms.
Previous studies have identified the major cat allergen, Fel d 1, as a 35-kDa tetrameric glycoprotein with one N-glycosylation site located at residue Asn33 in chain 2 (38, 48). Other studies identified three important IgE epitopes in Fel d 1: two in chain 1 and one in chain 2 (49). The present work shows that Fel d 1 contains a complex glycosylation pattern characterized by the presence of sulfated sugars, as revealed by proteomic (mass spectrometric) analysis (Table 1), and no such glycan structure has previously been reported for glycoallergens. This sulfated sugar moiety may therefore function as a molecular pattern that could be recognized by the CR domain of MR. Moreover, mass spectroscopic analysis of Fel d 1 revealed reduced presence of mannose-type carbohydrate structures. This, therefore, explains our findings regarding the inability of Fel d 1 to bind to the CTLD4–7 domains of MR.
Experimentally induced allergy using either purified Fel d 1 or cat dander extract has previously been reported (37, 50). Following a similar sensitization regime and using WT and MR−/− mice, we investigated whether MR plays a role in deviating the immune response toward a Th2 phenotype after exposure to Fel d 1. Levels of total and allergen-specific IgE were measured as indicators for allergic sensitization (51–54). Measurements of IgG2a (Th1) and IgE and IgG1 (Th2) were used as markers for their respective Th subset responses (55–57). Interestingly, MR−/− mice produced significantly less total IgE, Fel d 1-specific IgE and IgG1 than WT mice sensitized under similar conditions. By contrast, MR−/− mice produced significantly higher amounts of Fel d 1-specific IgG2a, the Th1-dependent isotype, compared with WT mice. These findings clearly indicate for the first time that MR has a major role in mediating Th2 polarization to an airborne allergen in an in vivo setting. This study and our previous work involving human in vitro experiments point strongly to the central role played by MR in allergic sensitization to multiple glycolallergens.
Identifying putative receptors on antigen-presenting cells that play a central role in allergen uptake and downstream events leading to IgE production could certainly lead to better understanding of early events leading to allergic sensitization and pave the way for the rational design of novel therapeutic strategies.
Acknowledgments
We thank the Proteomics Center at Sahlgrenska Academy (funded by a grant from the Knut and Alice Wallenberg Foundation) (University of Gothenburg) for technical assistance with regard to Glycan analysis and Nanoflow LC-MS/MS Fourier Transform Ion Cyclotron Resonance experiments.
This work was supported in part by Asthma UK Research Grant 06/001.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- DC
- dendritic cells
- MR
- mannose receptor
- CTLD
- C-type lectin-like domain
- CT
- control
- CR
- cysteine-rich
- FNII
- fibronectin type II-like domain
- MR−/−
- MR knock-out
- Mo-DC
- monocyte-derived dendritic cells
- BM-DC
- bone marrow-derived dendritic cell
- Cy5
- Cyanine 5
- Ab
- antibody.
REFERENCES
- 1. Gelber L. E., Seltzer L. H., Bouzoukis J. K., Pollart S. M., Chapman M. D., Platts-Mills T. A. (1993) Am. Rev. Respir. Dis. 147, 573–578 [DOI] [PubMed] [Google Scholar]
- 2. Platts-Mills T. A., Vervloet D., Thomas W. R., Aalberse R. C., Chapman M. D. (1997) J. Allergy Clin. Immunol. 100, S2–24 [DOI] [PubMed] [Google Scholar]
- 3. Ichikawa K., Iwasaki E., Baba M., Chapman M. D. (1999) Clin. Exp. Allergy 29, 754–761 [DOI] [PubMed] [Google Scholar]
- 4. Roost H. P., Künzli N., Schindler C., Jarvis D., Chinn S., Perruchoud A. P., Ackermann-Liebrich U., Burney P., Wüthrich B. (1999) J. Allergy Clin. Immunol. 104, 941–947 [DOI] [PubMed] [Google Scholar]
- 5. Løwenstein H., Lind P., Weeke B. (1985) Allergy 40, 430–441 [DOI] [PubMed] [Google Scholar]
- 6. de Groot H., van Swieten P., van Leeuwen J., Lind P., Aalberse R. C. (1988) J. Allergy Clin. Immunol. 82, 778–786 [DOI] [PubMed] [Google Scholar]
- 7. Kleine-Tebbe J., Kleine-Tebbe A., Jeep S., Schou C., Løwenstein H., Kunkel G. (1993) Int. Arch. Allergy Immunol. 100, 256–262 [DOI] [PubMed] [Google Scholar]
- 8. Adédoyin J., Grönlund H., Oman H., Johansson S. G., van Hage M. (2007) J. Allergy Clin. Immunol. 119, 640–645 [DOI] [PubMed] [Google Scholar]
- 9. Banchereau J., Steinman R. M. (1998) Nature 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 10. Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. (2000) Annu. Rev. Immunol. 18, 767–811 [DOI] [PubMed] [Google Scholar]
- 11. McGreal E. P., Miller J. L., Gordon S. (2005) Curr. Opin. Immunol. 17, 18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cambi A., Koopman M., Figdor C. G. (2005) Cell Microbiol. 7, 481–488 [DOI] [PubMed] [Google Scholar]
- 13. Gijzen K., Cambi A., Torensma R., Figdor C. G. (2006) Curr. Protein Pept. Sci. 7, 283–294 [DOI] [PubMed] [Google Scholar]
- 14. Engering A. J., Cella M., Fluitsma D. M., Hoefsmit E. C., Lanzavecchia A., Pieters J. (1997) Adv. Exp. Med. Biol. 417, 183–187 [DOI] [PubMed] [Google Scholar]
- 15. Deslée G., Charbonnier A. S., Hammad H., Angyalosi G., Tillie-Leblond I., Mantovani A., Tonnel A. B., Pestel J. (2002) J. Allergy Clin. Immunol. 110, 763–770 [DOI] [PubMed] [Google Scholar]
- 16. Syme R. M., Spurrell J. C., Amankwah E. K., Green F. H., Mody C. H. (2002) Infect. Immun. 70, 5972–5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cambi A., Figdor C. G. (2003) Curr. Opin. Cell Biol. 15, 539–546 [DOI] [PubMed] [Google Scholar]
- 18. Kato M., Neil T. K., Fearnley D. B., McLellan A. D., Vuckovic S., Hart D. N. (2000) Int. Immunol. 12, 1511–1519 [DOI] [PubMed] [Google Scholar]
- 19. East L., Isacke C. M. (2002) Biochim. Biophys. Acta 1572, 364–386 [DOI] [PubMed] [Google Scholar]
- 20. Pontow S. E., Kery V., Stahl P. D. (1992) Int. Rev. Cytol. 137B, 221–244 [DOI] [PubMed] [Google Scholar]
- 21. Stahl P. D., Ezekowitz R. A. (1998) Curr. Opin. Immunol. 10, 50–55 [DOI] [PubMed] [Google Scholar]
- 22. Harris N., Super M., Rits M., Chang G., Ezekowitz R. A. (1992) Blood 80, 2363–2373 [PubMed] [Google Scholar]
- 23. Taylor M. E., Bezouska K., Drickamer K. (1992) J. Biol. Chem. 267, 1719–1726 [PubMed] [Google Scholar]
- 24. Mullin N. P., Hall K. T., Taylor M. E. (1994) J. Biol. Chem. 269, 28405–28413 [PubMed] [Google Scholar]
- 25. Taylor P. R., Gordon S., Martinez-Pomares L. (2005) Trends Immunol. 26, 104–110 [DOI] [PubMed] [Google Scholar]
- 26. Napper C. E., Drickamer K., Taylor M. E. (2006) Biochem. J. 395, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinez-Pomares L., Wienke D., Stillion R., McKenzie E. J., Arnold J. N., Harris J., McGreal E., Sim R. B., Isacke C. M., Gordon S. (2006) Eur. J. Immunol. 36, 1074–1082 [DOI] [PubMed] [Google Scholar]
- 28. Royer P. J., Emara M., Yang C., Al-Ghouleh A., Tighe P., Jones N., Sewell H. F., Shakib F., Martinez-Pomares L., Ghaemmaghami A. M. (2010) J. Immunol. 185, 1522–1531 [DOI] [PubMed] [Google Scholar]
- 29. Horlock C., Shakib F., Mahdavi J., Jones N. S., Sewell H. F., Ghaemmaghami A. M. (2007) Genome Biol. 8, R30.1–R30.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inaba K., Swiggard W. J., Steinman R. M., Romani N., Schuler G., Brinster C. (2009) Curr. Protoc. Immunol. 86, 3.7.1–3.7.19 [DOI] [PubMed] [Google Scholar]
- 31. Peiser L., Gough P. J., Kodama T., Gordon S. (2000) Infect. Immun. 68, 1953–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chapman M. D., Aalberse R. C., Brown M. J., Platts-Mills T. A. (1988) J. Immunol. 140, 812–818 [PubMed] [Google Scholar]
- 33. Martinez-Pomares L. (2009) Methods Mol. Biol. 531, 103–122 [DOI] [PubMed] [Google Scholar]
- 34. Carlsohn E., Nyström J., Karlsson H., Svennerholm A. M., Nilsson C. L. (2006) J. Proteome Res. 5, 3197–3204 [DOI] [PubMed] [Google Scholar]
- 35. Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 36. Lee S. J., Evers S., Roeder D., Parlow A. F., Risteli J., Risteli L., Lee Y. C., Feizi T., Langen H., Nussenzweig M. C. (2002) Science 295, 1898–1901 [DOI] [PubMed] [Google Scholar]
- 37. Campbell J. D., Buckland K. F., McMillan S. J., Kearley J., Oldfield W. L., Stern L. J., Grönlund H., van Hage M., Reynolds C. J., Boyton R. J., Cobbold S. P., Kay A. B., Altmann D. M., Lloyd C. M., Larché M. (2009) J. Exp. Med. 206, 1535–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kristensen A. K., Schou C., Roepstorff P. (1997) Biol. Chem. 378, 899–908 [DOI] [PubMed] [Google Scholar]
- 39. Griffith I. J., Craig S., Pollock J., Yu X. B., Morgenstern J. P., Rogers B. L. (1992) Gene 113, 263–268 [DOI] [PubMed] [Google Scholar]
- 40. Kaiser L., Grönlund H., Sandalova T., Ljunggren H. G., van Hage-Hamsten M., Achour A., Schneider G. (2003) J. Biol. Chem. 278, 37730–37735 [DOI] [PubMed] [Google Scholar]
- 41. Guermonprez P., Valladeau J., Zitvogel L., Théry C., Amigorena S. (2002) Annu. Rev. Immunol. 20, 621–667 [DOI] [PubMed] [Google Scholar]
- 42. Liam C. K., Loo K. L., Wong C. M., Lim K. H., Lee T. C. (2002) Respirology 7, 345–350 [DOI] [PubMed] [Google Scholar]
- 43. Sarsfield J. K., Boyle A. G., Rowell E. M., Moriarty S. C. (1976) Arch. Dis. Child 51, 186–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Custovic A., Simpson A., Pahdi H., Green R. M., Chapman M. D., Woodcock A. (1998) Thorax 53, 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fiete D. J., Beranek M. C., Baenziger J. U. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2089–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leteux C., Chai W., Loveless R. W., Yuen C. T., Uhlin-Hansen L., Combarnous Y., Jankovic M., Maric S. C., Misulovin Z., Nussenzweig M. C., Feizi T. (2000) J. Exp. Med. 191, 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karn R. C., Laukaitis C. M. (2003) Biochemistry 42, 7162–7170 [DOI] [PubMed] [Google Scholar]
- 48. Duffort O. A., Carreira J., Nitti G., Polo F., Lombardero M. (1991) Mol. Immunol. 28, 301–309 [DOI] [PubMed] [Google Scholar]
- 49. van Milligen F. J., van 't Hof W., van den Berg M., Aalberse R. C. (1994) J. Allergy Clin. Immunol. 93, 34–43 [DOI] [PubMed] [Google Scholar]
- 50. Terada T., Zhang K., Belperio J., Londhe V., Saxon A. (2006) Clin. Immunol. 120, 45–56 [DOI] [PubMed] [Google Scholar]
- 51. Hamilton R. G., MacGlashan D. W., Jr., Saini S. S. (2010) Immunol. Res. 47, 273–284 [DOI] [PubMed] [Google Scholar]
- 52. Wachholz P. A., Dearman R. J., Kimber I. (2005) J. Immunotoxicol. 1, 189–199 [DOI] [PubMed] [Google Scholar]
- 53. Glovsky M. M. (2007) Methods Mol. Biol. 378, 205–219 [DOI] [PubMed] [Google Scholar]
- 54. Niggemann B., Nilsson M., Friedrichs F. (2008) Pediatr. Allergy Immunol. 19, 325–331 [DOI] [PubMed] [Google Scholar]
- 55. Mountford A. P., Fisher A., Wilson R. A. (1994) Parasite Immunol. 16, 521–527 [DOI] [PubMed] [Google Scholar]
- 56. Adel-Patient K., Créminon C., Bernard H., Clément G., Négroni L., Frobert Y., Grassi J., Wal J. M., Chatel J. M. (2000) J. Immunol. Methods 235, 21–32 [DOI] [PubMed] [Google Scholar]
- 57. Ormstad H., Groeng E. C., Duffort O., Løvik M. (2003) Toxicology 188, 309–318 [DOI] [PubMed] [Google Scholar]