Abstract
Tyrosine O-sulfation is a post-translational modification catalyzed by two tyrosylprotein sulfotransferases (TPST-1 and TPST-2) in the trans-Golgi network. Tpst2-deficient mice have male infertility, sperm motility defects, and possible abnormalities in sperm-egg membrane interactions. Studies here show that compared with wild-type sperm, fewer Tpst2-null sperm bind to the egg membrane, but more of these bound sperm progress to membrane fusion. Similar outcomes were observed with wild-type sperm treated with the anti-sulfotyrosine antibody PSG2. The increased extent of sperm-egg fusion is not due to a failure of Tpst2-null sperm to trigger establishment of the egg membrane block to polyspermy. Anti-sulfotyrosine staining of sperm showed localization similar to that of IZUMO1, a sperm protein that is essential for gamete fusion, but we detected little to no tyrosine sulfation of IZUMO1 and found that IZUMO1 expression and localization were normal in Tpst2-null sperm. Turning to a discovery-driven approach, we used mass spectrometry to characterize sperm proteins that associated with PSG2. This identified ADAM6, a member of the A disintegrin and A metalloprotease (ADAM) family; members of this protein family are associated with multiple sperm functions. Subsequent studies revealed that Tpst2-null sperm lack ADAM6 and ADAM3. Loss of ADAM3 is strongly associated with male infertility and is observed in knockouts of male germ line-specific endoplasmic reticulum-resident chaperones, raising the possibility that TPST-2 may function in quality control in the secretory pathway. These data suggest that TPST-2-mediated tyrosine O-sulfation participates in regulating the sperm surface proteome or membrane order, ultimately affecting male fertility.
Keywords: ADAM ADAMTS, Cell-Cell Interaction, Membrane Fusion, Oocyte, Reproduction, Sperm, Spermatozoa, Tyrosine Sulfation, Fertilization
Introduction
Tyrosine O-sulfation is a post-translational modification catalyzed by tyrosylprotein sulfotransferases (TPSTs)3 (1). Although tyrosine O-sulfation was first described more than 50 years ago (2), the TPSTs were identified just a decade ago (3–5). Tyrosine-sulfated proteins and/or TPST activity have been observed in animals and plants but not in prokaryotes or fungi (1, 6). Most animals' genomes appear to have two genes encoding TPSTs, although only one Tpst gene has been identified in Drosophila (1, 6); a TPST also has recently been identified in Arabidopsis (7). The mammalian enzymes are known as TPST-1 and TPST-2; these two enzymes are broadly expressed in human and murine tissues and are co-expressed in the majority of cell types (1). Tyrosine O-sulfation occurs in the trans-Golgi network, with the luminally oriented catalytic domains of TPSTs mediating the transfer of sulfate from the universal sulfate donor 3′-phosphoadenosine 5′-phosphosulfate to tyrosine residues in polypeptides (3–5, 8–10).
TPST substrates include a variety of secreted and membrane-anchored proteins, including adhesion molecules, G-protein-coupled receptors, and extracellular matrix proteins. Tyrosine O-sulfation is implicated in protein-protein interactions and in optimization of protein function (1, 11). In CCR5 (chemokine (C-C motif) receptor 5), the sulfation of one or more tyrosine residues in the N-terminal extracellular domain enhances the ability of CCR5 to bind the CC chemokines CCL3, CCL4, and CCL5, and mutation of the four tyrosine residues in the CCR5 N-terminal extracellular domain to phenylalanines resulted in ∼100-fold reduction in affinity for CCL3 and CCL5 (12). Tyrosine O-sulfation affects other protein-protein interactions, including ligand binding to several chemokine receptors and G-protein-coupled receptors (CXCR4, CCR2B, CX3CR1, CCR8, CXCR3, C5a, C3a, SIP1, and the FSH, LH, and TSH receptors), P-selectin glycoprotein ligand-1 (PSGL-1, CD162) binding to P-selectin and L-selectin, and the thrombin inhibitor hirudin binding to thrombin (1, 13–20). Tyrosine O-sulfation plays a role in the formation of a complex of two Xenopus Wnt family members; disruption of this Wnt complex formation by depletion of Tpst1 causes deficient Wnt signaling and abnormal dorsal axis formation in embryos that developed from Tpst1-depleted Xenopus oocytes (21). Tyrosine O-sulfation also is required for optimal proteolysis of several proteins, such as the processing of progastrin to gastrin, proteolysis of the complement C4α chain by C1s, and proteolytic activation of coagulation factors V and VIII by thrombin (22–24).
Tpst1−/−, Tpst2−/−, and Tpst1/Tpst2 double knock-out mice have been characterized (25–27). Most pups deficient in both Tpst1 and Tpst2 die within 1–2 days of birth; Tpst1/Tpst2 double knock-out pups delivered at embryonic day 19.5 by Cesarean section show signs of cardiopulmonary dysfunction (25). Tpst1−/− and Tpst2−/− mice show modest growth delays; the Tpst2−/− mice attain normal body weight at 10 weeks of age, although they have primary hypothyroidism (25–27). Tpst1−/− males have normal fertility, but Tpst1−/− females have smaller litters due to fetal death around midgestation (27). Tpst2−/− mice have normal female fertility, but Tpst2−/− males are infertile (26). Several components of male reproductive function of the Tpst2−/− mice appear normal, including vaginal plugging frequency; testicular weight and histology; and serum levels of follicle-stimulating hormone, luteinizing hormone, and testosterone (26). Epididymal sperm from Tpst2−/− males are normal in number, morphology, and motility; appear to capacitate in vitro (as assessed by cholera toxin β-subunit staining); and undergo acrosome exocytosis spontaneously and in response to calcium ionophore (26). However, Tpst2-null sperm are defective in motility in viscous medium and in their ability to fertilize cumulus-enclosed eggs (26). Additionally, in vitro fertilization (IVF) experiments with zona pellucida (ZP)-free eggs revealed that sperm from Tpst2−/− males adhered poorly to the egg plasma membrane and, surprisingly and somewhat paradoxically, appeared to show an increased extent of sperm-egg fusion (26).
In this work, we sought to gain insights into these abnormalities in sperm membrane function in Tpst2-null sperm. We first tested two hypotheses (not mutually exclusive) for the apparent increase in sperm-egg fusion with Tpst2-null sperm: (a) the Tpst2-null sperm have a diminished ability to trigger the establishment of the membrane block to polyspermy, and/or (b) they have an increased ability to fuse with eggs as compared with wild type. We find that Tpst2-null sperm are capable of inducing egg activation and membrane block establishment. On the other hand, the Tpst2-null sperm appear to have an increased propensity to fuse with eggs as compared with wild-type sperm. The decreased sperm-egg binding and increased sperm-egg fusion appears to be linked at least in part with tyrosine-sulfated proteins on the sperm surface because sperm treated with an anti-sulfotyrosine monoclonal antibody also show decreased binding and increased fusion with ZP-free eggs. We also considered the molecular basis of these differences in sperm function. We first examined IZUMO1, a sperm protein that is essential for sperm-egg fusion in mice (28) that we speculated could be modified in Tpst2-null sperm. The localization of sulfated tyrosines on sperm is similar to the localization of IZUMO1, but we find (a) that very little if any IZUMO1 is sulfated, and (b) that IZUMO1 expression and localization are normal in Tpst2-null sperm. With this finding that IZUMO1 was not significantly altered in Tpst2-null sperm, we turned to an unbiased, discovery-driven approach. Sperm tyrosine-sulfated proteins (and proteins associated with these proteins) were isolated on an anti-sulfotyrosine affinity column and then analyzed by mass spectrometry. This identified ADAM6, a member of the A disintegrin and A metalloprotease (ADAM) family of proteins that participate in sperm-egg interactions. ADAM6 forms complexes with ADAM3, a sperm-specific protein that is essential for male fertility based on knock-out mouse studies (29, 30). Interestingly, we found that Tpst2-null sperm lack ADAM6 as well as ADAM3, providing a key insight into the molecular basis of the infertility of Tpst2-null males.
EXPERIMENTAL PROCEDURES
Animals
Tpst2−/− (Tpst2tm1Klm, MGI:3512111) mice on the 129S6/SvEvTac background were generated, housed, and fed as described previously (26, 31). Tpst2−/− mice were generated by crossing Tpst2+/− males and females. Mice were bred at the Oklahoma Medical Research Foundation, and most experimental studies were performed at Johns Hopkins University. All animal procedures were approved by the Institutional Animal Care and Use Committees of the Oklahoma Medical Research Foundation and Johns Hopkins University.
Gamete Collection and in Vitro Fertilization
Metaphase II eggs were collected from 6–8-week-old female CF-1 mice (Harlan, Indianapolis, IN) that were injected with 5 IU of pregnant mare's serum gonadotropin (Sigma-Aldrich) and then 5 IU of human chorionic gonadotropin (Sigma-Aldrich) 46–48 h later; collection of ovulated eggs was performed at 12–13 h post-human chorionic gonadotropin. Cumulus cells were removed by treating the eggs briefly (<5 min) with 0.025% Type IV-S hyaluronidase (Sigma-Aldrich) in Whitten's medium (109.5 mm NaCl, 4.7 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 5.5 mm glucose, 0.23, mm pyruvic acid, 4.8 mm lactic acid hemicalcium salt (32) with 8 mm NaHCO3, 15 mm HEPES (hereafter referred to as Whitten's-HEPES) and 30 mg/ml bovine serum albumin (BSA; Albumax I; Invitrogen). Eggs were then washed through Whitten's medium with 22 mm NaHCO3 (hereafter referred to as Whitten's-bicarbonate). For IVF assays using ZP-free eggs, the ZPs were removed by brief incubation (∼10 s) in acidic medium-compatible buffer (116.4 mm NaCl, 5.4 mm KCl, 1 mm NaH2PO4, 0.8 mm MgSO4, 10 mm HEPES, pH 1.5); the ZP-free eggs were then cultured for 60 min at 37 °C in Whitten's-bicarbonate containing 15 mg/ml BSA prior to insemination.
Sperm were collected from Tpst2+/+ or Tpst2−/− males or from CD-1 retired breeders (Harlan) by mincing two caudae epididymides and vasa deferentia in 125 μl of Whitten's-bicarbonate containing 15 mg/ml BSA. Sperm were allowed to swim out of the tissue for 10–15 min, and the tissue was removed from the medium. The 125 μl of sperm-containing medium was then carefully pipetted into the bottom of a 12 × 75-mm tube containing 750 μl Whitten's-bicarbonate with 15 mg/ml BSA. After 45 min, the 220 μl from the top of the tube was recovered; this swim-up sperm suspension was cultured for an additional 1.5–2 h (total time, 2.5–3 h) to allow for capacitation and spontaneous acrosome exocytosis.
ZP-free eggs were inseminated at 100,000 sperm/ml (10-μl drops, 10 eggs/drop) for 60 min. To assess sperm-egg membrane binding, eggs were washed three times with a ∼100–120-μm diameter pipette. Inseminated eggs were fixed in 3.7–4.0% paraformaldehyde (Sigma-Aldrich) in PBS 15–60 min and then stained with 4′,6′-diamidino-2-phenylindole (DAPI) by mounting eggs in Vectashield (Vector Laboratories, Burlingame, CA) containing 1.5 μg/ml DAPI. Eggs were scored for the number of sperm bound, sperm fused, and exit from metaphase II arrest.
Reinsemination to Assess Membrane Block Establishment
The reinsemination assay to examine establishment of the membrane block to polyspermy was adapted from our past work (33, 34). For the first insemination (IVF1), eggs were inseminated with 100,000 sperm/ml for 20 min, washed three times in Whitten's-bicarbonate containing 15 mg/ml BSA to detach any loosely attached sperm, and then cultured for 100 min to allow time for membrane block establishment (total time, 120 min). After this incubation, eggs were inseminated with a second batch of sperm. Sperm for this second insemination were freshly collected from a CD-1 retired breeder, capacitated, and then, 15 min before the start of the second insemination, labeled with the mitochondrial marker MitoTracker Green (Molecular Probes, Inc.; diluted from a 1 mm stock in DMSO to a final concentration of 100 nm; mixed with 106 sperm/ml for 10 min in the dark). Eggs were inseminated with 250,000 MitoTracker-labeled sperm/ml for 1 h. These experiments included control unfertilized eggs inseminated in parallel to assess the base-line level of sperm fused per egg from the second insemination (“IVF2 only” control eggs). DAPI staining was performed as described above, and eggs were examined for the presence of decondensing sperm heads with no detectable green fluorescence in the midpiece (from the first insemination), and decondensing sperm heads with an associated MitoTracker Green-labeled midpiece (from the second insemination).
In Vitro Fertilization with Sperm Pretreated with the Anti-sulfotyrosine Antibody PSG2
Sperm from CD-1 males were collected as described above. For inseminations of ZP-free eggs, the sperm were capacitated for 2 h, after which the sperm were diluted to a concentration of 100,000 sperm/ml in Whitten's-bicarbonate containing 15 mg/ml BSA and 1, 5, or 10 μg/ml of the anti-sulfotyrosine antibody PSG2 (31) or nonimmune isotype control human IgG4-λ (Sigma-Aldrich). These sperm suspensions were used to prepare 10-μl IVF culture droplets. These drops were covered with oil and incubated for 60 min, after which ZP-free eggs were added to the droplet (10 eggs/10 μl; final sperm/egg ratio, 100:1). Eggs were inseminated for 60 min and then assessed for sperm-egg binding and fusion as described above. We observed no labeling of the egg plasma membrane with 10 or 100 μg/ml PSG2 and only very modest staining with 250 μg/ml PSG2 (data not shown). PSG2-treated and control sperm suspensions were also used to prepare sperm lysates for analysis by anti-ADAM6, anti-ADAM3, and anti-ADAM2 immunoblotting (for details, see below).
Immunofluorescence of Sperm
Caudal epididymal sperm from Tpst2−/− mice or Tpst2+/+ control mice were collected by mincing two epididymides in 900 μl of Whitten's-bicarbonate containing 4 mg/ml BSA. The sperm were cultured at 37 °C for 2.5–3 h and then washed three times with PBS. Sperm were pipetted onto Fisherbrand SuperFrost slides (Fisher; 25,000–50,000 sperm/slide) and allowed to dry at room temperature for 1.5 h. PBS containing 0.1% Tween 20 was used for anti-IZUMO1 staining (rabbit polyclonal antibody (28); gift of Masaru Okabe, Osaka University), and TBS containing 0.1% Tween 20 was used for PSG2 staining; these will be referred to generically as “buffer” throughout this section. Sperm were fixed for 10 min at room temperature with 3.7% formaldehyde in buffer and then permeabilized with 0.5% Triton X-100 in buffer for 5 min at room temperature. Slides were blocked overnight at 4 °C (0.5% BSA in buffer for IZUMO1; 5% milk in buffer for PSG2). Primary antibody incubations were performed for 2 h at room temperature (10 μg/ml in blocking buffer; anti-IZUMO1 rabbit polyclonal antibody, PSG2 monoclonal antibody, or nonimmune rabbit IgG or human IgG4-λ). Washes were performed in Coplan jars. Texas Red-conjugated secondary antibodies (15 μg/ml anti-rabbit IgG or 10 μg/ml anti-human IgG, Fcγ; Jackson Immunoresearch) and fluorescein-conjugated peanut agglutinin (100 μg/ml; Vector Laboratories) were incubated for 2 h at room temperature. Slides were mounted with VectaShield containing 1.5 μg/ml DAPI.
Immunoprecipitation
Caudal epididymal sperm lysates for immunoprecipitation were prepared in lysis buffer (150 mm NaCl, 25 mm Tris-HCl, pH 8.0, 1% Triton X-100 supplemented with 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Sigma), 1 μg/ml of leupeptin (Sigma), and 1 μg/ml of pepstatin (Sigma)). Samples were incubated for 30 min on ice and then subjected to two successive centrifugations (4 °C, 10,000 × g, 10 min each). Sperm lysates (∼4.6 × 107 sperm each) were precleared for 1 h at 4 °C with 40 μl of packed Protein G-agarose beads (Upstate/Millipore, Billerica, MA). Anti-IZUMO1 monoclonal antibody (monoclonal antibody 125 (35); gift of Masaru Okabe, Osaka University) or nonimmune rat IgG was coupled to Protein G-agarose beads by tumbling for 2 h at 4 °C. The precleared lysates were then incubated for 4 h at 4 °C with antibody-coupled Protein G-agarose beads. The beads were then washed three times with ice-cold lysis buffer and once with 20 mm Tris-HCl, pH 7.5, and then were resuspended in 2× SDS-PAGE sample buffer (2% SDS, 5.5% sucrose, 0.0006% bromphenol blue, 80 mm Tris-HCl, pH 6.8) and heated at 95 °C for 5 min. Immunoprecipitated samples were resolved by SDS-PAGE and transferred to an Immobilon membrane (Millipore) for IZUMO1 immunoblotting or NitroPlus transfer membrane (MSI, Westboro, MA) for PSG2 immunoblotting. Approximately 9.1 × 106 and 3.6 × 107 sperm equivalents were loaded onto the gels used for IZUMO1 and PSG2 immunblotting, respectively.
Immunoblotting
Caudal epididymal sperm lysates for immunoblotting were prepared in SDS-PAGE sample buffer, boiled for 5 min at 100 °C, and then centrifuged for 5 min. The supernatant was recovered, and 1% β-mercaptoethanol was added; the sample was then heated for 5 min at 100 °C. For testis lysates, two testes were decapsulated and then lysed in 1 ml of lysis buffer (150 mm NaCl, 50 mm Tris-HCl, pH 7.5, 1% Triton X-100, 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Sigma), 1 μg/ml leupeptin (Sigma), and 1 μg/ml pepstatin (Sigma)) with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY). The testis lysates were incubated on ice 30 min and then subjected to two successive centrifugations (4 °C, 10,000 × g, 10 min each). Protein lysates (250,000 sperm/lane, or 2 μg of testis lysate/lane) were resolved by SDS-PAGE and transferred to an Immobilon membrane for immunoblotting with all antibodies except PSG2, for which the NitroPlus transfer membrane was used.
Blocking was performed using 10% cold water fish gelatin (Sigma) in PBS containing 0.1% Tween 20 (IZUMO1, ADAM2, and ADAM3 blots) or 5% nonfat dry milk in TBS containing 0.1% Tween 20 (PSG2 and ADAM6 blots). Primary antibodies were anti-IZUMO1 (rat monoclonal antibody 125 (35)), anti-sulfotyrosine antibody PSG2 (0.05 μg/ml (31)), anti-ADAM2 (1 μg/ml; clone 9D2.2; Chemicon International/Millipore), anti-ADAM3 antibody (1 μg/ml; clone 7C1.2; Chemicon International/Millipore), and anti-ADAM6 (0.2 μg/ml; M-145; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). HRP-conjugated secondary antibodies were from Jackson Immunoresearch or Sigma-Aldrich and used at 0.16–0.7 μg/ml. For PSG2 immunoblots, HRP-conjugated Protein A was used (0.2 μg/ml; Pierce). Primary and secondary antibodies were incubated for 1 h at room temperature. Blots were rinsed after blocking and after each antibody incubation, prior to detection with Supersignal chemiluminescent substrate (Pierce).
Blots were probed with anti-lactate dehydrogenase C (LDHC; also known as LDH-C4) as a loading control (36). For reprobing with anti-LDHC, the membrane was stripped with Restore Western blot stripping buffer (Pierce) for 15 min at room temperature with shaking, washed three times for 10 min each, and then probed with Supersignal chemiluminescent substrate (Pierce) to confirm the success of the stripping. The membrane was then blocked with TBS containing 0.1% Tween 20, 5% milk, followed by anti-LDHC antibody (gift of Erwin Goldberg (Northwestern University, Chicago, IL); diluted 1:10,000 (37)) in TBS containing 0.1% Tween 20, 5% milk for 30 min and then 0.04 μg/ml HRP-conjugated goat anti-rabbit IgG in TBS containing 0.1% Tween 20, 5% milk for 1 h. Quantification of band intensities was performed with ImageJ software (available on the National Institutes of Health Web site), with the rectangular selection tool used to outline each lane and the peak intensity determined. The density of each ADAM band was divided by the density of the sample-matched LDHC band; these values were then averaged for each cell type (sperm or testis) for each genotype.
For detection of immunoprecipitated IZUMO1, an anti-IZUMO1 rabbit polyclonal antibody was used (1 μg/ml (35)) followed by HRP-conjugated Protein A (0.2 μg/ml; Pierce); primary antibody and Protein A-HRP were diluted in PBS containing 0.1% Tween-20 and 3% BSA. For anti-sulfotyrosine PSG2 detection of immunoprecipitated proteins, the membrane was blocked for 1 h in 5% nonfat dry milk, followed by 0.05 μg/ml anti-sulfotyrosine PSG2 antibody, and then HRP-conjugated Protein A was used (0.2 μg/ml; Pierce); PSG2 and HRP-protein were diluted in TBS containing 0.1% Tween 20.
Affinity Chromatography on the PSG2 Anti-sulfotyrosine Antibody
Sperm were collected from 15 wild-type (129S6/SvEvTac) males by placing each pair of caudae epididymides and vasa deferentia in 900 μl of PBS; the tissue was removed after 15 min. Sperm were then washed three times with 800 μl of PBS and then lysed by sonication (550 Sonic Dismembranator; Fisher) at a concentration of 50,000 sperm/μl in 0.1 m NaCl, 20 mm MOPS, pH 7.5, with a mixture of protease inhibitors (Complete Mini, Roche Applied Science). The lysate was centrifuged (60 min, 100,000 × g, 4 °C), and the MOPS-soluble supernatant was saved on ice. The remaining pellet was extracted for 60 min at 4 °C with 20 mm TAPS, 100 mm NaCl, and 1% Triton X-100 and then centrifuged (20 min, 25,000 × g, 4 °C). This supernatant was then combined with the MOPS-soluble supernatant.
The sperm lysate was applied at a flow rate of 0.1 ml/min to a PSG2-UltraLink column (0.5 × 16 cm, 4 mg of antibody per ml of resin). The column was washed with 2 bed volumes of 0.1 m NaCl, 20 mm MOPS, pH 7.5, 0.05% Triton X-100, 0.02% NaN3, and then bound proteins were eluted with 2.67 mm sulfated peptide LDsYDF (where sY represents sulfotyrosine) in 0.1 m NaCl, 20 mm MOPS, pH 7.5, 0.05% Triton X-100, 0.02% NaN3 at a flow rate of 0.1 ml/min. Flow-through and elution fractions were separated by SDS-PAGE and then silver-stained or transferred to Whatman Protan BA85 nitrocellulose (Fisher) for PSG2 immunoblotting. Membranes were processed as above, except that 0.2 μg/ml peroxidase-conjugated anti-human IgG, Fcγ (Jackson Immunoresearch) and Amersham Biosciences ECL (GE Healthcare) were used.
The elutions from the PSG2 column were in-gel-digested and identified by tandem mass spectrometry following the protocol as reported previously (31) with minor modifications. An LTQ-ORBITRAPXL mass spectrometer (ThermoFisher Scientific, San Jose, CA) equipped with a nano-HPLC(C18) system was used to collect the spectra. All data were searched against the IPI_Mouse data base (version 3.49) through MASCOT. All identified peptides had scores greater than the significance threshold value at p < 0.05.
Statistical Analysis
Statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria). Error bars in figures represent S.E.
RESULTS
Sperm from Tpst2−/− Mice Trigger Membrane Block Establishment in Eggs
One hypothesis to explain the apparent increased extent of sperm-egg fusion in eggs inseminated with Tpst2-null sperm (26) is that these sperm have a reduced ability to trigger the egg's membrane block to polyspermy. To assess membrane block establishment, reinsemination assays were performed (38, 39), in which zygotes created with sperm from either Tpst2+/+ or Tpst2−/− mice (IVF1 in Fig. 1A) were challenged with a second batch of wild-type (CD-1) MitoTracker-labeled sperm (IVF2 in Fig. 1A); the zygotes were then assessed for whether the MitoTracker-labeled sperm from this second insemination were able to fuse with the zygote membrane (Fig. 1A). If the sperm from the first insemination (IVF1) triggers membrane block establishment, then few or no MitoTracker-labeled sperm would fuse with the zygote membrane. On the other hand, if the sperm from IVF1 do not trigger membrane block establishment, then the membrane of the zygote would remain receptive to sperm and thus labeled sperm from IVF2 would be able to fuse.
FIGURE 1.
Assessment of membrane block establishment in eggs fertilized by Tpst2−/− mice. A, schematic diagram of the reinsemination assay used to assess membrane block establishment. ZP-free eggs were inseminated with sperm from Tpst2+/+ or Tpst2−/− mice to generate zygotes (IVF1). These zygotes were cultured for a total of 120 min and then were challenged with a second insemination (IVF2) using MitoTracker-labeled sperm. As a control, unfertilized naive eggs were inseminated with MitoTracker-labeled sperm in parallel (unfertilized control). B, graph shows the average number of MitoTracker-labeled IVF2 sperm fused per egg ± S.E. (error bars) from three replicate experiments. There was no difference in the ability of Tpst2+/+ and Tpst2−/− sperm to trigger the membrane block to polyspermy. Eggs inseminated with sperm from Tpst2+/+ or Tpst2−/− mice had a similar percentage of zygotes that allowed the fusion of MitoTracker-labeled sperm (Tpst2+/+, 6.5%; Tpst2−/−, 15.5%; p value = 0.13, χ2) as well as a similar number of MitoTracker-labeled sperm fused per egg (Tpst2+/+, 0.09 ± 0.04; Tpst2−/−, 0.21 ± 0.07; p value = 0.12, t test).
87 of 93 zygotes (94%) created with sperm from Tpst2+/+ mice did not have any fused MitoTracker-labeled sperm. These zygotes created with wild-type sperm had an average of 0.09 ± 0.04 MitoTracker-labeled sperm/zygote (Fig. 1B). Similarly, 49 of 58 zygotes (84%) created with sperm from Tpst2−/− mice did not have any fused MitoTracker-labeled sperm, resulting in 0.21 ± 0.07 labeled sperm/zygote (Fig. 1B). Eggs inseminated with sperm from Tpst2+/+ or Tpst2−/− mice had a similar percentage of zygotes that allowed the fusion of MitoTracker-labeled sperm (p value = 0.13; χ2 analysis) as well as a similar number of labeled sperm fused per egg (p value = 0.12; t test). Based on this, we conclude that both wild-type and Tpst2-null sperm are able to trigger the membrane block to polyspermy. The control insemination of “naive” unfertilized eggs (Fig. 1A) showed that the MitoTracker-labeled sperm were able to fertilize ZP-free eggs that had not established the membrane block (1.69 ± 0.16 sperm fused/egg) (Fig. 1B).
Increased Ratio of Fused Sperm to Total Egg-associated Sperm and Increased Extent of Polyspermy from Inseminations Using Tpst2-null Sperm
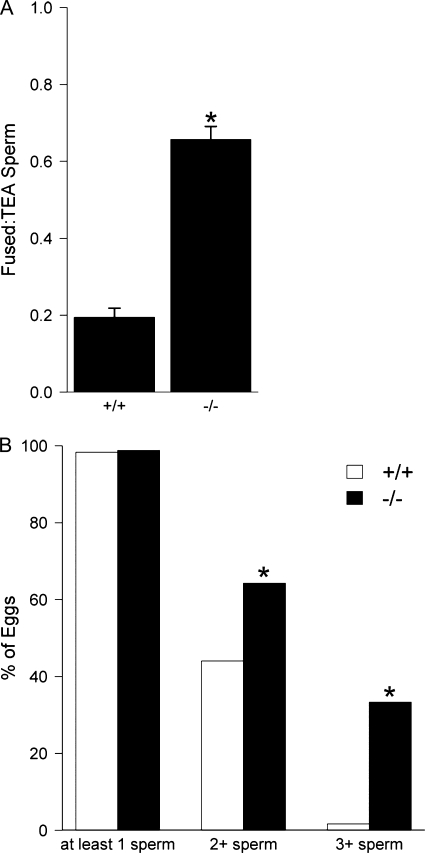
Previous data suggested that ZP-free eggs inseminated with sperm from Tpst2−/− mice have a decreased number of sperm bound per egg and an increased number of sperm fused per egg when compared with eggs inseminated with sperm from Tpst2+/+ mice (26). Here we assessed the extent of sperm-egg fusion while controlling for the reduced binding of Tpst2-null sperm. This involved calculating the ratio of fused sperm to total egg-associated (TEA) sperm, namely dividing the number of sperm fused with an egg by the number of sperm bound plus the number of sperm fused with an egg (TEA sperm = bound sperm + fused sperm). This will be referred to as the fused/TEA ratio. The fused/TEA ratio for eggs inseminated with sperm from Tpst2+/+ mice was 0.19 ± 0.02 and was 0.66 ± 0.03 for eggs inseminated with sperm from Tpst2−/− mice (p value = 0.01; nested ANOVA; Fig. 2A).
FIGURE 2.
Ratio of fused sperm to total egg-associated sperm and extent of polyspermy in ZP-free eggs inseminated with Tpst2-null sperm. ZP-free eggs were inseminated for 60 min with 100,000 sperm/ml (sperm/egg ratio 100:1) with sperm from either Tpst2+/+ or Tpst2−/− mice. The eggs were then washed, fixed, and assessed for sperm-egg binding and fusion. A, the ratio of the number of fused to sperm to the number of TEA sperm was calculated by dividing the number of sperm fused with an egg by the number of sperm bound plus the number of sperm fused. The fused/TEA ratio is expressed as the mean ± S.E. (error bars). B, assessment of the extent of fertilization and polyspermy, showing the percentage of eggs fertilized (i.e. fused with at least one sperm), the percentage of eggs fused with at least two sperm (2+ sperm), and the percentage of eggs fused with three or more sperm (3+ sperm). Open bars show data from eggs inseminated with sperm from Tpst2+/+ males (+/+), and solid bars show data from eggs inseminated with sperm from Tpst2−/− males (−/−). Three wild-type males (59 eggs total) and four Tpst2−/− males (84 eggs total) were tested. *, p < 0.05 in comparisons of −/− with +/+.
We also examined if inseminations with sperm from Tpst2−/− mice resulted in more highly polyspermic eggs when compared with inseminations with control sperm. There were similar percentages of fertilized eggs (i.e. eggs with at least one sperm fused) resulting from inseminations with wild-type or Tpst2-null sperm (+/+, 98%; −/−, 99%; p value = 0.64; χ2). However, inseminations with Tpst2-null sperm resulted in 64% of eggs with 2 or more sperm fused/egg, compared with 44% for inseminations with control sperm (p value = 0.03; χ2). Furthermore, 33% of eggs inseminated with Tpst2-null sperm were trispermic or greater, whereas only 2% of eggs inseminated with control sperm were trispermic or greater (p value = 1.01 × 10−5; χ2; Fig. 2B).
Localization of Sulfated Tyrosine Residues on Sperm and Effects of the Anti-sulfotyrosine Antibody PSG2 on IVF of ZP-free Eggs
Because tyrosine O-sulfation mediated by TPST-2 appears to affect sperm function (26) (Figs. 1 and 2), it was of interest to determine the localization of sulfated tyrosine residues in sperm. Immunofluorescence with the anti-sulfotyrosine antibody PSG2 revealed that acrosome-intact sperm from Tpst2+/+ mice exhibited staining in the acrosomal and equatorial regions as well as faint labeling of the tail (Fig. 3A). Spontaneously acrosome-reacted sperm from Tpst2+/+ mice lost staining in the acrosomal region but exhibited staining in the equatorial region and tail as well as faint staining in the post-acrosomal region (Fig. 3C). The staining pattern of Tpst2-null was largely similar to wild-type sperm, although stronger staining of the post-acrosomal region in both acrosome-intact and acrosome-reacted sperm was observed in the Tpst2-null sperm (Fig. 3, M and O, solid arrowheads) as compared with wild-type sperm (Fig. 3, A and C, open arrowheads). The nonimmune isotype control antibody showed some modest cross-reactivity in the acrosomal region (Fig. 3, B and N), but the staining with the PSG2 antibody resulted in a more intense signal, suggesting that the acrosome contains sulfotyrosine residues (Fig. 3, A and M). It should be noted that Tpst2-null sperm would not necessarily be expected to be devoid of sulfotyrosine because Tpst1 mRNA is detected in Type A and B spermatogonia, spermatocytes, spermatids, Sertoli cells, and the epididymis (see the Washington University Mammalian Reproductive Genetics Web site), and Tpst1 mRNA is expressed at normal wild-type levels in Tpst2-null testes (26).
FIGURE 3.
Localization of sulfotyrosine residues in sperm. Indirect immunofluorescence analysis of sulfotyrosine residues (detected by PSG2) in sperm from Tpst2+/+ mice (A–L) and Tpst2−/− mice (M–X). Images in the left column show sperm labeled with the PSG2 antibody; images in the right column show sperm labeled with the nonimmune isotype control antibody (Nonimmune). Sperm shown here were triple-labeled (i.e. A, E, and I show the same sperm cell, photographed with three different fluorescence filters). Images show the sperm head and a portion of the sperm tail and staining with the PSG2 or nonimmune isotype control antibody (A–D and M–P), peanut agglutinin (PNA) to label the acrosome (B–H and Q–T), and DAPI to label DNA (I–L and U–X). Acrosome-intact sperm (AI) and acrosome-reacted sperm (AR) are shown. The arrowheads indicate the postacrosomal region of the sperm head; anti-sulfotyrosine staining of the post-acrosomal region was observed in Tpst2-null acrosome-intact and acrosome-reacted sperm (M and O; solid arrowheads), whereas little staining of this region was observed in wild-type sperm (A and C; open arrowheads).
To determine the effects of the anti-sulfotyrosine antibody on IVF and if PSG2 treatment would mimic the end result of Tpst2−/− knock-out, wild-type sperm were treated with 1, 5, or 10 μg/ml of the PSG2 anti-sulfotyrosine antibody and then used to inseminate ZP-free eggs for 60 min. (Note that we observed no labeling of the egg plasma membrane with 10 or 100 μg/ml PSG2 and only modest staining with 250 μg/ml PSG2; data not shown.) IVF performed in the presence of PSG2 resulted in decreased numbers of sperm bound per egg and increased numbers of sperm fused per egg (Fig. 4A). This effect of PSG2 on sperm function was dependent on the concentration of PSG2 antibody and was statistically significant when compared with results from sperm treated with the nonimmune isotype control IgG. For example, the fused/TEA ratio for the control IgG-treated group was 0.31 ± 0.03, whereas the fused/TEA ratio for the 10 μg/ml PSG2 treatment group was 0.73 ± 0.04 (p value = 4 × 10−6; Tukey's honestly significant differences; Fig. 4B). The percentage of fertilized eggs in inseminations using control IgG-treated sperm was 72%, and the percentage was 86% in inseminations using sperm treated with 10 μg/ml PSG2 (p value = 0.04; χ2; Fig. 4C). The percentage of eggs that were trispermic or greater was 8% for the control IgG control and was 24% for the 10 μg/ml PSG2 treatment group (p value = 0.02; χ2; Fig. 4C).
FIGURE 4.
Effects of an anti-sulfotyrosine antibody (PSG2) on fertilization of ZP-free eggs. Wild-type sperm were preincubated with 1, 5, or 10 μg/ml anti-sulfotyrosine antibody (PSG2) or 10 μg/ml nonimmune isotype control IgG (nonimmune) or left untreated (no treatment) for 60 min and then used to inseminate ZP-free eggs for 60 min. The eggs were then washed, fixed, and assessed for sperm-egg binding and fusion. A, the average number of sperm bound (open bars) and fused (solid bars) per egg. Values are the mean ± S.E. (error bars). B, the ratio of fused/TEA sperm was calculated as described in the legend to Fig. 2 for the eggs inseminated with untreated sperm, sperm treated with the nonimmune isotype control IgG, and sperm treated with 1, 5, and 10 μg/ml PSG2. Values are the mean ± S.E. C, assessment of the extent of fertilization and polyspermy was done as described in the legend to Fig. 2, showing the percentage of eggs fertilized (i.e. fused with at least one sperm), the percentage of eggs fused with at least two sperm (2+ sperm), and the percentage of eggs fused with three or more sperm (3+ sperm). Bars (from left to right), data from inseminations done in the absence of antibody (open bars), in the presence of the nonimmune isotype control antibody (solid bars), in the presence of 1 μg/ml PSG2 (right hatched bars), in the presence of 5 μg/ml PSG2 (vertically hatched bars), and in the presence of 10 μg/ml PSG2 (left hatched bars). *, p < 0.05 for comparisons with nonimmune isotype control IgG. This experiment was repeated four times with 78–127 eggs/group.
IZUMO1 in Sperm from Tpst2+/+ and Tpst2−/− Mice
The sperm protein IZUMO1 (also known as Izumo) has been characterized through knock-out mouse studies as being essential for sperm-egg fusion (28), and PSG2 staining of sperm (Fig. 3) shows localization reminiscent of the localization of IZUMO1. We hypothesized that an alteration in IZUMO1 expression or localization in Tpst2-null sperm could underlie the increase in sperm-egg fusion observed in eggs inseminated with Tpst2-null sperm. We therefore used immunofluorescence and immunoblotting to examine IZUMO1 in sperm from Tpst2−/− mice. In acrosome-intact sperm from Tpst2−/− and Tpst2+/+ mice, IZUMO1 was localized to the acrosomal region (Fig. 5A, i and vii); after spontaneous acrosome reaction, IZUMO1 relocalized to the equatorial region (Fig. 5A, iv and x), indicating that sperm from Tpst2−/− mice do not have major differences in IZUMO1 localization as compared with wild-type sperm. In addition, IZUMO1 was detected by immunoblotting at comparable levels in sperm from Tpst2−/− and Tpst2+/+ mice (Fig. 5B). We also examined if IZUMO1 cross-reacted with the anti-sulfotyrosine antibody PSG2, with the hypothesis that IZUMO1 was sulfated on a tyrosine residue(s) and thus might not be appropriately modified in the Tpst2-null sperm. We immunoprecipitated IZUMO1 from wild-type sperm using an anti-IZUMO1 antibody and then immunoblotted with the anti-sulfotyrosine antibody PSG2, or, as a control, an anti-IZUMO1 antibody. However, we did not detect a band on the anti-sulfotyrosine blot corresponding to the expected Mr for IZUMO1 (Fig. 5C, ii, lane 2).
FIGURE 5.
IZUMO1 in sperm from Tpst2+/+ and Tpst2−/− mice. A, immunofluorescence analysis of IZUMO1 localization in sperm from Tpst2+/− mice (i–vi) and Tpst2−/− mice (vii–xii). Images show the sperm head, with staining with an anti-IZUMO1 antibody (i, iv, vii, and x), peanut agglutinin (PNA) to label the acrosome (ii, v, viii, and xi), and DAPI to label DNA (iii, vi, ix, and xii). Acrosome-intact sperm (AI; i–iii and vii–ix) and acrosome-reacted sperm (AR, iv–vi and x–xii) are shown. IZUMO1 protein is appropriately localized in sperm from heterozygote and knock-out animals. B, immunoblot analysis of lysates of cauda epididymal sperm from Tpst2+/+ and Tpst2−/− mice using an anti-IZUMO1 antibodies. Anti-LDHC (also known as LDH-C4) was used as a loading control. C, immunoprecipitation of IZUMO1 from cauda epididymal sperm lysates, followed by immunoblotting with anti-IZUMO1 (i) or anti-sulfotyrosine (Anti-SulfoTyr) (ii). Control immunoprecipitations were also performed with a nonimmune IgG. Starting sperm lysate (Input) was run on the gel as a control. The arrowhead to the right of the blot panels indicates the Mr for IZUMO1. D, anti-IZUMO1 immunoblot blot of PSG2 affinity column fractions. Samples were as follows. Input, PSG2 column input; Flow-through, flow-through; Eluate, elution fraction after the addition with sulfated pentapeptide. Mr is indicated to the left of the blots in C and D.
ADAM6 and ADAM3 Are Not Detected in Sperm from Tpst2−/− Mice
To take an unbiased approach to gain insights into the functions of tyrosine-sulfated proteins and the effects of Tpst2 deletion on sperm, PSG2 affinity chromatography was used to isolate epididymal sperm proteins that may be tyrosine-sulfated or that may be associated with tyrosine-sulfated proteins. Eluted proteins were separated by SDS-PAGE, the bands of interest were excised, and peptides were identified by nanoflow reverse phase chromatography tandem mass spectrometry. Additionally, to complement our examination of IZUMO1 sulfation by immunoprecipitation followed by immunoblotting with PSG2 (Fig. 5C), we examined the flow-through and elution fractions of this PSG2 column by immunoblotting with an anti-IZUMO1 antibody (Fig. 5D). This demonstrated that the vast majority of IZUMO1 was in the flow-through fractions, and only very small amounts of IZUMO1 were detected in the elution fractions (Fig. 5D). Furthermore, IZUMO1 was not identified by mass spectrometry in the elution fractions. Mass spectrometry analysis of the elution fractions did identify other proteins; of these, ADAM6 was the most likely candidate to affect sperm-egg membrane interactions. ADAM6 forms complexes with two proteins that can participate in sperm-egg interactions, ADAM2 and ADAM3. Additionally, ADAM6 is greatly reduced in sperm lysates from Adam2 and Adam3 knock-out mice (40), and the Adam2 and the Adam3 knock-out mice have deficiencies in sperm migration into the oviduct, sperm-ZP interactions, and sperm-egg membrane interactions (29, 30, 41). We therefore investigated ADAM6 in more detail.
Anti-ADAM6 immunoblotting detected ADAM6 in the flow-through fractions and in the elutions of the PSG2 column (data not shown). Assessment of ADAM6 tyrosine sulfation by PSG2 immunoblotting was inconclusive (PSG2 and nonimmune IgG immunoblots of anti-ADAM6 immunoprecipitates showed similar patterns of bands; data not shown); therefore, the sulfation of tyrosine residues in ADAM6 (or lack thereof) awaits further analysis. It should be noted that the localization of sulfotyrosine residues (Fig. 3) has some overlaps but is not identical to the localization of ADAM6 (40). Examination of how Tpst2 deletion affected ADAM6 and associated ADAMs ADAM3 and ADAM2 provided significant insights. Immunoblotting of epididymal sperm lysates with an anti-ADAM6 antibody revealed that there is no detectable ADAM6 in Tpst2-null sperm (Fig. 6A, lanes 5–7), but ADAM6 was detected in testis lysates from Tpst2−/− males (Fig. 6B, lanes 5–7). Because ADAM6 associates with ADAM2 and ADAM3 (40), we also examined these ADAMs in epididymal sperm lysates from Tpst2−/− mice. ADAM2 levels were comparable in sperm lysates from Tpst2+/+ and Tpst2−/− mice (Fig. 6E), but ADAM3, like ADAM6, was absent from sperm lysates from Tpst2−/− mice (Fig. 6B, lanes 4–6). Also like ADAM6, ADAM3 was detected in testis lysates (Fig. 6C, lanes 5–7). Thus, these data show that ADAM3 and ADAM6 are translated but then are lost post-translationally at some point as cells progress from the testis to the cauda epididymis. PSG2 treatment of sperm (as used in the IVF experiments shown in Fig. 4) had no impact on ADAM6, ADAM3, and ADAM2 expression (supplemental Fig. 1). This shows that the loss of ADAM6 and ADAM3 from Tpst2-null sperm is due to the genetic deletion of Tpst2 and loss of TPST2 protein and that the presence/stability of ADAM6, ADAM3, and ADAM2 is not affected by PSG2 binding to sulfotyrosine residues on sperm.
FIGURE 6.
ADAM6, ADAM3, and ADAM2 in sperm and testis lysates from Tpst2−/− mice. Cauda epididymal sperm lysates (A, C, and E) and testicular lysates (B and D) from Tpst2+/+ (+/+) and Tpst2−/− (−/−) mice were analyzed by immunoblotting with anti-ADAM6 (A and B), anti-ADAM3 (C and D), and anti-ADAM2 (E). Each lane contains a protein sample from an individual Tpst2+/+ or Tpst2−/− mouse. Blots were stripped and reprobed with an anti-LDHC antibody as a control (lower rows of bands in all panels). Mr is indicated to the right of the blots. The ratios of the ADAM band intensities to LDHC band intensity from these blots also were determined (see supplemental Table 1; the ratio values for ADAM6 in testis, for ADAM3 in testis, and for ADAM2 in sperm were not statistically significantly different between the Tpst2+/+ and Tpst2−/− samples).
DISCUSSION
The work here shows that ZP-free eggs inseminated with Tpst2-null sperm have increased extents of sperm-egg fusion and polyspermy as compared with eggs inseminated with wild-type sperm. This is all the more striking considering that the Tpst2-null sperm bind less well to the egg membrane as compared with wild-type sperm. Tpst2-null sperm and wild-type sperm have similar abilities to induce the establishment of the membrane block to polyspermy; thus, the increased extents of sperm-egg fusion and polyspermy with Tpst2-null sperm are not due to a deficiency in prevention of polyspermy at the level of the egg membrane. The studies here instead indicate that the increased fusion and polyspermy are most likely due to enhanced ability of Tpst2-null sperm to fuse with the egg, which appears to be linked with tyrosine-sulfated proteins on the sperm surface based on the findings that sperm pretreated with the anti-sulfotyrosine antibody PSG2 also show reduced binding and increased extents of sperm-egg fusion and polyspermy. To our knowledge, only one other knock-out model, the Adam24−/− mouse (also known as Testase 1), has shown a phenotype of increased sperm-egg fusion, but the phenotype of the Adam24−/− mouse differs from that of the Tpst2−/− mice in multiple ways. Adam24−/− males are subfertile (42), whereas the Tpst2−/− males are completely infertile. Sperm-egg binding appears to be normal with Adam24-null sperm (42), whereas sperm-egg binding is reduced in Tpst2-null sperm. The increased fusion observed with Adam24-null sperm was speculated to be attributable to an impaired ability of these sperm to trigger membrane block establishment, but this was not tested. Our experimental studies show that the increased fusion with Tpst2-null sperm is not due to a reduced ability of Tpst2-null sperm to trigger the membrane block in the egg.
The sperm protein most strongly implicated in gamete fusion, IZUMO1 (28), appears to be unaffected in Tpst2-null sperm (Fig. 5). Additionally, IZUMO1 does not appear to be a major TPST substrate, nor does it appear to be associated with a tyrosine-sulfated protein (Fig. 5). Taken together, this argues against abnormalities in IZUMO1 being linked with the differences in sperm function in Tpst2−/− mice (although it remains a formal possibility that there is a subtle abnormality in IZUMO1 in Tpst2-null sperm). Moving from this candidate approach with IZUMO1, we next sought to characterize aspects of the “molecular phenotype” of Tpst2-null sperm. A major discovery coming from this work is that Tpst2-null sperm lack detectable ADAM3 and ADAM6. The absence of ADAM3 in Tpst2-null sperm provides key insights into the male infertile phenotype of the Tpst2−/− mice because male infertility is observed in Adam3−/− mice (29, 30, 41). Moreover, other knock-out models have been discovered to lack ADAM3 on sperm, and male infertility is also observed in these as well: Adam1a, Adam2, Clgn (calmegin), and Calr3 (calsperin) (43–46). An additional male infertile knock-out, the Ace−/− (angiotensin-converting enzyme) mouse, has detectable ADAM3 on their sperm, but there are abnormalities in Triton X-114 fractionation of ADAM3 (45, 47). Thus, the appropriate expression of ADAM3 is very tightly correlated with male fertility from seven knock-out models, now including Tpst2−/−. ADAM3 is implicated in multiple aspects of sperm function. Sperm that lack ADAM3 are unable to migrate through the uterotubal junction to the oviduct (41). Sperm from the Adam1a−/−, Adam2−/−, Clgn−/−, Calr3−/−, and Ace−/− animals, which have abnormal ADAM3 or loss of ADAM3, also have defects in transit into the oviduct (43, 44, 46–48). ADAM3 also has been proposed to be involved in ZP interaction, based on the reduced ability of Adam3-null sperm to bind to the ZP (29, 30) and on the detection of an in vitro interaction of solubilized ZP proteins with ADAM3 (49). Tpst2-null sperm have a greatly reduced ability to fertilize cumulus-intact eggs and ZP-intact eggs, defects that are likely to be impacted by the reduced motility in viscous medium of Tpst2-null sperm (26). One candidate proposed to participate in sperm-ZP interaction, milk fat globule protein-EGF factor 8 protein (MFGE8, also known as SED1) (50), is a substrate for TPST-2 (31). Taken together, based on the absence of ADAM3 from Tpst2-null sperm, impaired oviductal transit, ZP binding, and potentially additional factors probably contribute to the male infertility of the Tpst2−/− mice.
The discovery that Tpst2-null sperm lack ADAM3 and ADAM6 also is significant because it suggests that tyrosine O-sulfation by TPST-2 plays a role in regulating the sperm surface proteome, with potential parallels to what has been observed in several other knockouts, particularly the Clgn−/− and Calr3−/− mice (44–46). Based on studies of these knock-out models and on the data here, we propose a model for TPST-2 action in ADAM expression as part of a protein quality control pathway (Fig. 7A). Protein folding in the endoplasmic reticulum (ER) plays a critical role in protein quality control because a significant portion of misfolded proteins are targeted to ER-associated degradation (51). In the ER of male germ cells, the membrane-associated chaperone calmegin (functionally analogous to calnexin in other cell types) and the luminal chaperone calsperin (functionally analogous to calreticulin) are important for appropriate protein expression in sperm, and the Clgn and Calr3 knock-out mice have abnormalities in the sperm surface proteome and sperm function (44–46). Misfolded proteins that progress to more distal parts of the secretory pathway can be retrotranslocated to the ER and subjected to ER-associated degradation. An additional layer of the quality control pathway also occurring past the ER is formation of higher order oligomers of certain proteins in the ER-Golgi intermediate compartment and in the Golgi (51). TPST-2 and its substrates are illustrated in the diagram here to function at this latter stage, based on data showing that sulfation of proteins occurs in the trans-Golgi (8, 9, 52). It is also possible that TPSTs are localized in an earlier portion of the secretory pathway in male germ cells (hybridoma, COS-7, and PC12 cells were used in these other studies (8, 9, 52)), and thus TPSTs may be functioning in an earlier part of the protein quality control pathway as well. There is other evidence that tyrosine O-sulfation plays a role in protein complex formation (21). In support of oligomeric complexes being important for ADAM stability, multiple studies demonstrate that ADAMs are found in several different complexes (nicely summarized in Ref. 40) and that deficiency of one Adam (i.e. by gene knock-out) can lead to the loss of other ADAMs. Adam2-null sperm lack several ADAMs (40, 53), with much of the ADAM3 loss occurring during protein transit from the trans-Golgi to the cell surface, such that little or no ADAM3 was detected on the surfaces of Adam2-null cells (54, 55). Adam3-null sperm also have reduced amounts of several ADAMs (30, 40, 53). Protein folding also is important for ADAM stability because Clgn-null sperm lack detectable ADAM1B, ADAM2, and ADAM3; these proteins are lost post-translationally, probably due to aberrant protein folding due to the Clgn deficiency (44, 45). Calr3-null sperm lack detectable ADAM3 but interestingly do have ADAM1B and ADAM2, suggestive of specificity of calsperin action on ADAM3 (46).
FIGURE 7.
Models for the role of TPST-2 and tyrosine O-sulfation in ADAM expression on sperm. A, diagram showing mechanisms of protein quality control in the secretory pathway and a hypothesized role for TPST-2 and its substrates. As addressed under “Discussion,” protein folding in the ER and oligomerization in latter parts of the secretory pathway play key roles in protein quality control (51). Misfolded proteins, in the ER or retrotranslocated to the ER, are subjected to ER-associated degradation. In the ER of male germ cells, the membrane-associated chaperone calmegin and the luminal chaperone calsperin are important for appropriate protein expression in sperm (46, 56). Based on data in other cell types indicating that TPSTs mediate tyrosine sulfation in the Golgi, this diagram illustrates TPST-2 having actions in the Golgi, although it is also possible that TPST-2 acts earlier in the secretory pathway in male germ cells. Based on the loss of ADAM3 and ADAM6 from Tpst2-null sperm, we speculate that Tpst2 deficiency would lead to aberrant protein oligomerization or other defects in protein quality, and thus these proteins would be subject to degradation. This could occur through targeting of these proteins to a degradation pathway from the Golgi and/or retrotranslocation of these proteins and subsequent ER-associated degradation. B and C, illustration of two models of possible TPST-2 substrates and how these could affect ADAM3 and ADAM6. The model shown in B predicts that ADAM6 is sulfated by TPST-2 (symbolized by the S in the diamond) and that wild-type cells have a complex including ADAM6, ADAM3, and ADAM2 (40). In the Tpst2-null cells, the sulfation of ADAM6 is lost or greatly reduced, and this sulfation is critical for the stability of ADAM6 and ADAM3 but not ADAM2 (i.e. ADAM2 is present in Tpst2-null sperm). The model shown in C predicts that a different protein, Protein X, is sulfated by TPST-2. The loss of sulfation of Protein X, which would occur in Tpst2-null cells, affects the stability of ADAM3 and ADAM6, possibly by affecting formation of the ADAM-containing complex.
The hypothesized effects of Tpst2 deficiency on ADAM expression are illustrated in Fig. 7, B and C, based on two possibilities for the presence of ADAM6 in the elutions from the PSG2 column. ADAM6 could be a TPST-2 substrate, and sulfation of ADAM6 could be critical for stability and/or complex formation involving ADAM6 and ADAM3, and thus ADAM6 and ADAM3 are lost from Tpst2-null sperm (Fig. 7B). TPST-2 also could mediate the sulfation of an unidentified “Protein X” that is responsible for formation of certain ADAM-containing complexes; the loss of the sulfation of this unknown protein may lead to loss of ADAM6 and ADAM3 from Tpst2-null sperm (Fig. 7C). Interestingly, ADAM2 is still present on Tpst2-null sperm, suggesting that tyrosine O-sulfation by TPST-2 is not required for ADAM2 stability on sperm (40). Nevertheless, the loss of ADAM3 and ADAM6 is suggestive that the sperm surface proteome and sperm membrane functionality could be altered, leading to the abnormalities observed in the Tpst2-null sperm. Furthermore, the difference in anti-sulfotyrosine PSG2 localization in Tpst2-null sperm as compared with wild-type sperm raises the possibility that TPST-2 activity affects the localization of sulfated proteins and/or proteins associated with sulfated proteins, in turn affecting sperm membrane order.
In summary, this work provides several insights. First, we have identified additional aspects of the Tpst2-null phenotype: increased propensity of Tpst2-null sperm to undergo sperm-egg fusion and the loss of ADAM6 and ADAM3 from Tpst2-null sperm. Put in context with other knockouts with related phenotypes, this provides new clues into physiological roles of TPSTs. The data here raise the possibility that tyrosine O-sulfation by TPST-2 in the secretory pathway of male germ cells may have a function that is analogous to that of calmegin (56), ultimately affecting sperm functions by altering the sperm surface proteome and membrane order as an end result of changes in protein complex stability and/or composition. This potential role of TPST-2 in a protein quality control capacity provides an additional angle to consider on how tyrosine O-sulfation can impact protein-protein interactions and protein function (1, 11). Furthermore, with regard to understanding sperm function and male infertility, work over the last 10 years on mouse knock-outs clearly shows that abnormalities in a particular sperm function can be due to the loss of specific gene product combined with additional downstream effects of the gene deletion. Examples of these include Clgn, Calr3, and several Adam knockouts (40, 43, 44, 46, 48) as well as knock-out of the testis-specific serine kinase Tssk6 (which affects IZUMO1 localization and gamete fusion (57)), the equatorial segment protein Spesp1 (which affects biochemical and localization characteristics of IZUMO1 and equatorin, sperm membrane morphology, and gamete fusion (58)), and, as shown here, Tpst2. Taken together, an emerging model is that the sperm membrane requires both the correct complement of proteins and the proper organization of these proteins for normal sperm function and normal male fertility.
Acknowledgments
We thank Dr. Masaru Okabe (Osaka University) for the anti-IZUMO1 antibodies, Dr. Erwin Goldberg (Northwestern University) for the anti-LDHC antibody, and Dr. Rafael Irizarry (Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health) and Dr. Karl Broman (Department of Biostatistics and Medical Informatics, School of Medicine and Public Health, University of Wisconsin) for help and advice on statistical analyses.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HD037696 and R01 HD045671 (to J. P. E.) and HD056022 (to K. L. M. and J. A. L.). For a portion of this work, M. R. M. was supported by a training grant from the National Institute of Child Health and Human Development (T32 HD007276).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1 and Table 1.
- TPST
- tyrosylprotein sulfotransferase
- ADAM
- A disintegrin and A metalloprotease
- ZP
- zona pellucida
- IVF
- in vitro fertilization
- LDHC
- lactate dehydrogenase C
- TAPS
- N-tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid
- TEA
- total egg-associated
- ER
- endoplasmic reticulum.
REFERENCES
- 1. Moore K. L. (2003) J. Biol. Chem. 278, 24243–24246 [DOI] [PubMed] [Google Scholar]
- 2. Bettelheim F. R. (1954) J. Am. Chem. Soc. 76, 2838–2839 [Google Scholar]
- 3. Beisswanger R., Corbeil D., Vannier C., Thiele C., Dohrmann U., Kellner R., Ashman K., Niehrs C., Huttner W. B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11134–11139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ouyang Y., Lane W. S., Moore K. L. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2896–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ouyang Y. B., Moore K. L. (1998) J. Biol. Chem. 273, 24770–24774 [DOI] [PubMed] [Google Scholar]
- 6. Moore K. L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14741–14742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Komori R., Amano Y., Ogawa-Ohnishi M., Matsubayashi Y. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 15067–15072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baeuerle P. A., Huttner W. B. (1987) J. Cell Biol. 105, 2655–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosa P., Mantovani S., Rosboch R., Huttner W. B. (1992) J. Biol. Chem. 267, 12227–12232 [PubMed] [Google Scholar]
- 10. Lee R. W., Huttner W. B. (1983) J. Biol. Chem. 258, 11326–11334 [PubMed] [Google Scholar]
- 11. Huttner W. B. (1982) Nature 299, 273–276 [DOI] [PubMed] [Google Scholar]
- 12. Farzan M., Mirzabekov T., Kolchinsky P., Wyatt R., Cayabyab M., Gerard N. P., Gerard C., Sodroski J., Choe H. (1999) Cell 96, 667–676 [DOI] [PubMed] [Google Scholar]
- 13. Choe H., Farzan M. (2009) Methods Enzymol. 461, 147–170 [DOI] [PubMed] [Google Scholar]
- 14. Gao J., Choe H., Bota D., Wright P. L., Gerard C., Gerard N. P. (2003) J. Biol. Chem. 278, 37902–37908 [DOI] [PubMed] [Google Scholar]
- 15. Colvin R. A., Campanella G. S., Manice L. A., Luster A. D. (2006) Mol. Cell. Biol. 26, 5838–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutiérrez J., Kremer L., Zaballos A., Goya I., Martínez-A C., Márquez G. (2004) J. Biol. Chem. 279, 14726–14733 [DOI] [PubMed] [Google Scholar]
- 17. Wilkins P. P., Moore K. L., McEver R. P., Cummings R. D. (1995) J. Biol. Chem. 270, 22677–22680 [DOI] [PubMed] [Google Scholar]
- 18. Pouyani T., Seed B. (1995) Cell 83, 333–343 [DOI] [PubMed] [Google Scholar]
- 19. Sako D., Comess K. M., Barone K. M., Camphausen R. T., Cumming D. A., Shaw G. D. (1995) Cell 83, 323–331 [DOI] [PubMed] [Google Scholar]
- 20. Stone S. R., Hofsteenge J. (1986) Biochemistry 25, 4622–4628 [DOI] [PubMed] [Google Scholar]
- 21. Cha S. W., Tadjuidje E., White J., Wells J., Mayhew C., Wylie C., Heasman J. (2009) Curr. Biol. 19, 1573–1580 [DOI] [PubMed] [Google Scholar]
- 22. Hortin G. L., Farries T. C., Graham J. P., Atkinson J. P. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 1338–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pittman D. D., Tomkinson K. N., Michnick D., Selighsohn U., Kaufman R. J. (1994) Biochemistry 33, 6952–6959 [DOI] [PubMed] [Google Scholar]
- 24. Pittman D. D., Wang J. H., Kaufman R. J. (1992) Biochemistry 31, 3315–3325 [DOI] [PubMed] [Google Scholar]
- 25. Westmuckett A. D., Hoffhines A. J., Borghei A., Moore K. L. (2008) Gen. Comp. Endocrinol. 156, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borghei A., Ouyang Y. B., Westmuckett A. D., Marcello M. R., Landel C. P., Evans J. P., Moore K. L. (2006) J. Biol. Chem. 281, 9423–9431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouyang Y. B., Crawley J. T., Aston C. E., Moore K. L. (2002) J. Biol. Chem. 277, 23781–23787 [DOI] [PubMed] [Google Scholar]
- 28. Inoue N., Ikawa M., Isotani A., Okabe M. (2005) Nature 434, 234–238 [DOI] [PubMed] [Google Scholar]
- 29. Shamsadin R., Adham I. M., Nayernia K., Heinlein U. A., Oberwinkler H., Engel W. (1999) Biol. Reprod. 61, 1445–1451 [DOI] [PubMed] [Google Scholar]
- 30. Nishimura H., Cho C., Branciforte D. R., Myles D. G., Primakoff P. (2001) Dev. Biol. 233, 204–213 [DOI] [PubMed] [Google Scholar]
- 31. Hoffhines A. J., Jen C. H., Leary J. A., Moore K. L. (2009) J. Biol. Chem. 284, 3096–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whitten W. K. (1971) Adv. Biosci. 6, 129–139 [Google Scholar]
- 33. Gardner A. J., Williams C. J., Evans J. P. (2007) Reproduction 133, 383–393 [DOI] [PubMed] [Google Scholar]
- 34. Gardner A. J., Knott J. G., Jones K. T., Evans J. P. (2007) J. Cell. Physiol. 212, 275–280 [DOI] [PubMed] [Google Scholar]
- 35. Inoue N., Ikawa M., Okabe M. (2008) Biochem. Biophys. Res. Commun. 377, 910–914 [DOI] [PubMed] [Google Scholar]
- 36. Goldberg E., Eddy E. M., Duan C., Odet F. (2010) J. Androl. 31, 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Odet F., Duan C., Willis W. D., Goulding E. H., Kung A., Eddy E. M., Goldberg E. (2008) Biol. Reprod. 79, 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wortzman G. B., Evans J. P. (2005) Mol. Hum. Reprod. 11, 1–9 [DOI] [PubMed] [Google Scholar]
- 39. Gardner A. J., Evans J. P. (2006) Reprod. Fertil. Dev. 18, 53–61 [DOI] [PubMed] [Google Scholar]
- 40. Han C., Choi E., Park I., Lee B., Jin S., Kim do H., Nishimura H., Cho C. (2009) Biol. Reprod. 80, 1001–1008 [DOI] [PubMed] [Google Scholar]
- 41. Yamaguchi R., Muro Y., Isotani A., Tokuhiro K., Takumi K., Adham I., Ikawa M., Okabe M. (2009) Biol. Reprod. 81, 142–146 [DOI] [PubMed] [Google Scholar]
- 42. Zhu G. Z., Gupta S., Myles D. G., Primakoff P. (2009) Mol. Reprod. Dev. 76, 1106–1114 [DOI] [PubMed] [Google Scholar]
- 43. Nishimura H., Kim E., Nakanishi T., Baba T. (2004) J. Biol. Chem. 279, 34957–34962 [DOI] [PubMed] [Google Scholar]
- 44. Ikawa M., Nakanishi T., Yamada S., Wada I., Kominami K., Tanaka H., Nozaki M., Nishimune Y., Okabe M. (2001) Dev. Biol. 240, 254–261 [DOI] [PubMed] [Google Scholar]
- 45. Yamaguchi R., Yamagata K., Ikawa M., Moss S. B., Okabe M. (2006) Biol. Reprod. 75, 760–766 [DOI] [PubMed] [Google Scholar]
- 46. Ikawa M., Tokuhiro K., Yamaguchi R., Benham A. M., Tamura T., Wada I., Satouh Y., Inoue N., Okabe M. (2011) J. Biol. Chem. 286, 5639–5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hagaman J. R., Moyer J. S., Bachman E. S., Sibony M., Magyar P. L., Welch J. E., Smithies O., Krege J. H., O'Brien D. A. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2552–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cho C., Bunch D. O., Faure J. E., Goulding E. H., Eddy E. M., Primakoff P., Myles D. G. (1998) Science 281, 1857–1859 [DOI] [PubMed] [Google Scholar]
- 49. Kim E., Baba D., Kimura M., Yamashita M., Kashiwabara S., Baba T. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18028–18033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ensslin M. A., Shur B. D. (2003) Cell 114, 405–417 [DOI] [PubMed] [Google Scholar]
- 51. Anelli T., Sitia R. (2008) EMBO J. 27, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goettsch S., Badea R. A., Mueller J. W., Wotzlaw C., Schoelermann B., Schulz L., Rabiller M., Bayer P., Hartmann-Fatu C. (2006) J. Mol. Biol. 361, 436–449 [DOI] [PubMed] [Google Scholar]
- 53. Kim T., Oh J., Woo J. M., Choi E., Im S. H., Yoo Y. J., Kim D. H., Nishimura H., Cho C. (2006) Biol. Reprod. 74, 744–750 [DOI] [PubMed] [Google Scholar]
- 54. Stein K. K., Go J. C., Primakoff P., Myles D. G. (2005) Biol. Reprod. 73, 1032–1038 [DOI] [PubMed] [Google Scholar]
- 55. Nishimura H., Myles D. G., Primakoff P. (2007) J. Biol. Chem. 282, 17900–17907 [DOI] [PubMed] [Google Scholar]
- 56. Ikawa M., Wada I., Kominami K., Watanabe D., Toshimori K., Nishimune Y., Okabe M. (1997) Nature 387, 607–611 [DOI] [PubMed] [Google Scholar]
- 57. Sosnik J., Miranda P. V., Spiridonov N. A., Yoon S. Y., Fissore R. A., Johnson G. R., Visconti P. E. (2009) J. Cell Sci. 122, 2741–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fujihara Y., Murakami M., Inoue N., Satouh Y., Kaseda K., Ikawa M., Okabe M. (2010) J. Cell Sci. 123, 1531–1536 [DOI] [PubMed] [Google Scholar]