Abstract
The low density lipoprotein receptor-related protein-1 (LRP1) is known to serve as a chylomicron remnant receptor in the liver responsible for the binding and plasma clearance of apolipoprotein E-containing lipoproteins. Previous in vitro studies have provided evidence to suggest that LRP1 expression may also influence high density lipoprotein (HDL) metabolism. The current study showed that liver-specific LRP1 knock-out (hLrp1−/−) mice displayed lower fasting plasma HDL cholesterol levels when compared with hLrp1+/+ mice. Lecithin:cholesterol acyl transferase and hepatic lipase activities in plasma of hLrp1−/− mice were comparable with those observed in hLrp1+/+ mice, indicating that hepatic LRP1 inactivation does not influence plasma HDL remodeling. Plasma clearance of HDL particles and HDL-associated cholesteryl esters was also similar between hLrp1+/+ and hLrp1−/− mice. In contrast, HDL secretion from primary hepatocytes isolated from hLrp1−/− mice was significantly reduced when compared with that observed with hLrp1+/+ hepatocytes. Biotinylation of cell surface proteins revealed decreased surface localization of the ATP-binding cassette, subfamily A, member 1 (ABCA1) protein, but total cellular ABCA1 level was not changed in hLrp1−/− hepatocytes. Finally, hLrp1−/− hepatocytes displayed reduced binding capacity for extracellular cathepsin D, resulting in lower intracellular cathepsin D content and impairment of prosaposin activation, a process that is required for membrane translocation of ABCA1 to facilitate cholesterol efflux and HDL secretion. Taken together, these results documented that hepatic LRP1 participates in cellular activation of lysosomal enzymes and through this mechanism, indirectly modulates the production and plasma levels of HDL.
Keywords: ABC Transporter, HDL, Hepatocyte, Lipoprotein-like Receptor (LRP), Lipoprotein Secretion, Lysosomal Glycoproteins
Introduction
Low density lipoprotein receptor-related protein-1 (LRP1),2 the largest member of the low density lipoprotein (LDL) receptor gene family, is composed of an extracellular 515-kDa polypeptide covalently linked to an 85-kDa polypeptide that spans the plasma membrane and extrudes into the cytosol (1). The extracellular domain of LRP1 includes 31 cysteine-rich complement-like repeats responsible for ligand binding and 22 cysteine-rich repeats that are homologous to the β-propeller domains in the epidermal growth factor precursor protein (1). Structural similarities between these cysteine-rich repeats of LRP1 and the ligand-binding domain of the LDL receptor have led to its identification as the chylomicron remnant receptor responsible for hepatic clearance of apolipoprotein E (apoE)-containing lipoproteins (2–5). Subsequent studies revealed that LRP1 is also capable of binding and mediating cellular uptake of other ligands, including activated α2-macroglobulin, protease-protease inhibitor complexes, and lysosomal hydrolases such as the saposin precursor protein prosaposin (6, 7). Additionally, recent evidence also established LRP1 as an integrator of signal transduction events in cell regulation, including the modulation of PDGF receptor and TGFβ-1 receptor activation (8–10), Wnt signaling (11), and participation in neurotransmitter-dependent postsynaptic responses (12).
The function of LRP1 as a cargo transporting endocytic receptor or a cell signaling receptor appears to be tissue- and cell type-specific (13). For example, LRP1 serves as a signaling receptor during adipocyte differentiation via activation of Wnt5a/β-catenin pathway and inhibition of acetyl-CoA carboxylase phosphorylation (11). In contrast, LRP1 is a cargo transporting receptor in mature adipocytes responsible for the uptake of lipid nutrients from triglyceride-rich lipoproteins for storage and utilization (14). The function of LRP1 in smooth muscle cells is also unrelated to cargo transport, but its modulation of PDGF receptor and TGFβ-1 receptor activities is essential for maintenance of vascular reactivity and limiting hypercholesterolemia-induced atherosclerosis and injury-induced neointimal hyperplasia (8–10). In the neurons of dendritic synapses, LRP1 interacts with the N-methyl-d-aspartate receptor to modulate neuronal calcium signaling (15). Neuronal expression of LRP1 also activates protein kinase B/Akt signaling in protection against stress-induced apoptosis (16). In addition, LRP1 has been shown to participate in focal adhesion and cell migration via direct association with integrins and/or modulation of calreticulin/thrombospondin signaling events (17, 18).
In the liver, LRP1 serves as a cargo transport endocytic receptor with a well documented role in chylomicron remnant uptake (13, 19). Plasma levels of coagulation factor VIII, von Willebrand factor, and tissue-type plasminogen activator are also elevated in mice with defective hepatic LRP1 expression (20), indicating that LRP1 also participates in plasma clearance of these macromolecules. Interestingly, the inactivation of LRP1 expression in livers of mice that also lack both LDL receptor and apoE resulted in a 2-fold increase of atherosclerotic lesion area when compared with that observed in Ldlr−/−apoE−/− mice with normal hepatic LRP1 expression (20). Surprisingly, plasma cholesterol and triglyceride levels were lower in mice with hepatic LRP1 inactivation, primarily due to decreased levels of the atherogenic lipoproteins very low density lipoprotein (VLDL) and LDL (20). Based on these observations, the authors proposed that hepatic LRP1 deficiency promotes atherosclerosis due to defective coagulation factor and protease-protease inhibitor complex clearance but is independent of plasma cholesterol and lipoprotein metabolism (20). However, it is important to note that these studies on the influence of hepatic LRP1 on lipoprotein levels and atherosclerosis were performed with hypercholesterolemic mice that also lacked LDL receptor and apoE. Therefore, the influence of hepatic LRP1 expression on plasma lipoprotein levels in mice with full complements of LDL receptor and apoE expression and under normal dietary conditions remains unknown. In view of the well documented role of hepatic LRP1 as a chylomicron remnant receptor (3, 5), as well as its potential role in high density lipoprotein (HDL) metabolism (21–23), this study was undertaken to examine the impact of hepatic LRP1 inactivation on lipoprotein metabolism under normal dietary conditions.
EXPERIMENTAL PROCEDURES
Animals and Diets
Animals were maintained in a pathogen-free facility, and all experimental procedures were performed with protocols approved by the University of Cincinnati Institutional Animal Use and Care Committee. All mice were maintained on alternating 12-h light and dark cycles under controlled environmental conditions with free access to food and water. The Lrp1flox/flox mice were originally obtained from Dr. Joachim Herz at the University of Texas Southwestern Medical Center and were backcrossed in our facility with C57BL/6 mice for 10 generations to achieve congenic Lrp1flox/flox mice (10, 14). Liver-specific LRP1-null (hLrp1−/−) mice were generated by cross-breeding Lrp1flox/flox mice with albumin promoter-driven cre recombinase transgenic mice in the C57BL/6 background (The Jackson Laboratory, Bar Harbor, ME). Male littermates of hLrp1−/− mice and Lrp1flox/flox mice without the cre transgene, heretofore referred to as hLrp1+/+ mice, at the age of 8–16 weeks were used for all experiments.
Plasma Chemistry and Enzyme Analyses
Animals were maintained on normal rodent diet (LM485 from Teklad) and fasted for 10 h prior to blood collection from the tail vein into EDTA-containing tubes. Triglycerides and total cholesterol levels were measured enzymatically with commercially available kits (Infinity kits, Thermo Scientific). Non-esterified fatty acids were measured using non-esterified fatty acid reagents from Wako Chemicals. Plasma glucose levels were determined using blood glucose strips and glucometer (Accu-Check, Roche Diagnostics). Plasma insulin concentrations were determined by ELISA (Crystal Chem Inc., Downers Grove, IL). Plasma lecithin:cholesterol acyltransferase (LCAT) activity was measured using fluorescent assay reagents (EMD Biosciences, San Diego, CA). Hepatic lipase activity in 2 μl of plasma was assessed based on the hydrolysis of the 4-methylumbelliferyl heptanoate substrate as described (24). For lipoprotein analysis, plasma from 10 mice were pooled, and 220 μl was separated via fast performance liquid chromatography (FPLC) on a Superose 6 HR 10/30 column (Amersham Biosciences). The columns were equilibrated with FPLC buffer containing 0.05 m Tris-HCl, pH 7.5, 0.15 m NaCl, 1 mm EDTA, and 0.05% NaN3. Fractions (0.5 ml) were collected for cholesterol determinations.
Liver Lipid Measurements
Livers from euthanized mice were perfused with ice-cold phosphate-buffered saline (PBS) through the hepatic portal vein and then excised, weighed, and flash-frozen in liquid nitrogen. Lipids were extracted in chloroform:methanol:water (2:1:3) from homogenized livers. An aliquot of each sample was dried under nitrogen stream and solubilized in cholesterol or triglyceride detection reagents (Thermo Scientific). Lipid extraction efficiency differences between samples were normalized based on the recovery of trace amounts of [3H]cholesterol added to the samples before extraction.
Preparation of Radiolabeled HDL
Human HDL (d = 1.063–1.21 g/ml) was prepared from normolipemic plasma by sequential density ultracentrifugation and labeled with [1,2-3H]cholesteryl oleoyl ether (American Radiolabeled Chemicals, Inc.) by exchange from donor liposomal particles using partially purified human plasma cholesterol ester transfer protein (25). After exchange, donor particles were removed by centrifugal flotation at d 1.08 g/ml density, and the radiolabeled HDL was reisolated by flotation at d 1.21 g/ml. The [3H]cholesteryl ether-containing HDL was subsequently labeled with 125I-N-methyl-tyramine-cellobiose by incubating 0.8 mol of 125I-N-methyl-tyramine-cellobiose/mol of HDL proteins in 0.1 m sodium phosphate buffer, pH 8.0, for 1 h at 37 °C as described (26). The resulting doubly labeled HDL was exhaustively dialyzed against PBS, pH 7.4, containing 0.1% EDTA. The final specific activities of doubly labeled HDL were 20 cpm/ng of protein for 125I and 33 dpm/ng of protein for 3H.
Plasma HDL Clearance
Male mice (n = 5/group) were fasted for 10 h prior to intravenous injection through the orbital plexus with 250 μl of PBS containing the doubly labeled HDL (1 × 106 dpm of 3H). Blood was collected from the tail vein, plasma was immediately separated by centrifugation, and 5 μl of plasma was used for 125I radioactivity determination. A tracer amount of [14C]cholesterol along with 0.1 mg/ml carrier cholesterol was added to each 15-μl plasma sample, and total lipids were then extracted twice with hexane:isopropyl alcohol (3:2, v/v). The pooled organic phases were back-extracted with distilled water, dried under nitrogen, and resolubilized in ethanol. The amount of [3H]cholesteryl ether present in 15 μl of plasma samples was determined by liquid scintillation and corrected for extraction efficiency based on the recovery of the [14C]cholesterol. Plasma radioactivity at 0.5 min after injection time point was used as 100% of the injected dose to represent the initial plasma HDL pool. The logarithm of the amount of lipoprotein remaining in the plasma was plotted against time, and the decay curves were fitted using a second-order polynomial equation.
Primary Hepatocyte Culture
Primary hepatocytes were isolated from anesthetized mice (ketamine, 80 mg/kg of body weight, and xylazine, 16 mg/kg of body weight in 0.9% NaCl, Midwest Veterinary Supply) by perfusion with a collagenase solution (4). Briefly, the livers were first flushed at 37 °C with calcium-free Krebs-Henseleit buffer containing 0.5 mm EGTA and then infused with a digestion solution containing Krebs-Henseleit buffer plus 125 units/ml collagenase (Sigma) and 2% (w/v) bovine serum albumin at a rate of 6 ml/min/g of liver. Cells were dissociated in William's E medium supplemented with 5% fetal bovine serum, 100 nm insulin, 100 nm dexamethasone, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin and passed through a 100-μm cell strainer. Following two washes in William's E medium, viable hepatocytes were sedimented in 50% Percoll (GE Biosciences) and cultured in supplemented William's E medium. Adherent cells were washed after 4 h and then cultured in HepatoZYME-SFM containing 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen).
Hepatic Secretion of HDL
Primary hepatocytes in 6-well dishes (400,000 cells/well) were incubated with 10 μCi/ml [3H]mevalonate for 16 h in HepatoZYME-SFM medium to radiolabel endogenous cholesterol. The cells were washed after the incubation period and then equilibrated with medium in the absence of radiolabel. The cells were then incubated for 6 h in 1.5 ml medium with or without 10 μg/ml purified human apoA-I. The secretion of [3H]cholesterol as nascent HDL or efflux to human apoA-I was determined after immunoprecipitation of the conditioned medium with antibodies against mouse or human apoA-I antibodies (Abcam) and captured on protein G-Sepharose beads (GE Healthcare). Control immunoprecipitation was performed using equal masses of species- and isotype-specific IgG. The immunoprecipitates were collected by centrifugation for determination of radioactivity.
Cell Surface Labeling and Immunoblotting
Primary hepatocytes plated in 100-mm tissue culture dishes (2 × 106 cells/dish) were washed five times in ice-cold phosphate-buffered saline and then incubated twice for 20 min at 4 °C with PBS containing 0.5 mg/ml sulfo-NHS-SS-biotin (Pierce) (6). Unreacted biotin was quenched using two 10-min washes with saline containing 50 mm Tris-HCl, pH 7.6, on ice. Cells were lysed in buffer containing 5 μm Tris-HCl, pH 8.0, 0.15 m NaCl, 0.1% SDS, 0.5% sodium deoxycholate, and 1% Triton X-100 with protease inhibitor and phosphatase inhibitor cocktails (Roche Diagnostics). Equal protein concentrations of post-mitochondrial supernatants were mixed with immobilized streptavidin (Pierce) overnight at 4 °C. Following three washes in PBS, cell surface protein-biotin complexes were eluted from the streptavidin beads with 4× Tris-glycine gel loading buffer containing 10% (v/v) β-mercaptoethanol at 37 °C for 30 min. Biotinylated proteins eluted from the streptavidin beads, total hepatocyte cell lysates, and proteins in conditioned medium were resolved by SDS-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Bio-Rad). The membranes were blocked in PBS containing 0.1% Tween 20 and 5% nonfat dry milk and then incubated with primary antibodies overnight at 4 °C. Primary antibodies used in this study include mouse apoA-I, LDL receptor, human apoA-I, and prosaposin antibodies from Abcam (Cambridge, MA); anti-mouse apoE and anti-VLDL receptor from Santa Cruz Biotechnology (Santa Cruz, CA); anti-ABCA1 from Trend Pharma & Tech (Surrey, British Columbia, Canada); anti-α-tubulin from Sigma; and cathepsin D and scavenger receptor-BI (SR-BI) antibodies from Novus Biologicals (Littleton, CO). Antibodies against the 85- and 515-kDa subunits of LRP1 were made in rabbits using peptides corresponding to sequences in the carboxyl and amino regions, respectively. Primary antibodies were detected using horseradish peroxidase (HRP)-conjugated secondary antibodies and visualized using enhanced chemiluminescence reagents (Amersham Biosciences).
Hepatocyte Glycosphingolipid Quantification
Primary hepatocytes from hLrp1+/+ and hLrp1−/− mice cultured at a density of 2.0 × 106 cells/well in 10-cm cluster dishes were washed three times in PBS and scraped in lysis buffer consisting of 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, and 0.25 m sucrose. Samples were submitted for mass spectral data acquisition at the Medical University of South Carolina Lipidomics Facility where 1 mg was used for mass spectrometry analysis of glycosphingolipids. Lipids were extracted and analyzed using established protocols in the facility (27). An aliquot was used for the quantification of total protein in cell lysates using BCA analysis for normalization.
Cathepsin D Binding to Hepatocytes
A plasmid containing the mouse cathepsin D cDNA under the control of T7 promoter (clone ID 5253319) was obtained from Invitrogen. The cDNA was transcribed and translated in vitro in the presence of [35S]methionine using the TnT-coupled reticulocyte lysate system (Promega). A nonspecific antisense control was generated using the SP6 promoter in the same plasmid. Primary hepatocytes isolated from hLrp1+/+ and hLrp1−/− littermates were seeded in a 24-well dish at 5 × 104 cells/well and then cultured overnight in HepatoZYME-SFM before experiments. The hepatocytes were preincubated for 30 min at 37 °C with or without 40 μg/ml of the LRP1 ligand binding inhibitor GST-RAP (28) prior to the addition of [35S]cathepsin D. The incubation was continued at 4 °C for 4 h. Cell monolayers were washed extensively with cold PBS, and extracts were prepared as described above. The amount of [35S]cathepsin D bound to the cells was determined by liquid scintillation counting and normalized to total cellular protein content in each well. Receptor-ligand binding curves were generated using mean ± S.D. of quadruplicate experiments.
Statistical Analysis
Statistical analysis was performed with SigmaStat Version 3.5. Values were expressed as mean ± S.D. Multiple comparisons were first tested by Student's t test or analysis of variance. When analysis of variance demonstrated significant differences, individual mean differences were analyzed with the Student-Newman-Keuls test. A value of p < 0.05 was considered to be statistically significant.
RESULTS
Hepatic LRP1 Deficiency Decreases Plasma HDL Level in Mice
Hepatocyte-specific Lrp1 knock-out (hLrp1−/−) mice generated by crossing Lrp1flox/flox mice (29) with albumin promoter-driven cre recombinase transgenic mice showed the absence of LRP1 in liver extracts when compared with control hLrp1+/+ littermates (Fig. 1). When these animals were maintained on a normal diet with mouse chow containing 4% fat with no cholesterol or cholate supplementation, no difference in fasting plasma levels of triglyceride, non-esterified fatty acid, glucose, and insulin was observed between hLrp1+/+ and hLrp1−/− mice (Table 1). Triglyceride and cholesterol levels in tissues related to lipid metabolism were also similar between the two groups of animals. In contrast, plasma cholesterol levels were significantly lower, by ∼33%, in hLrp1−/− mice when compared with hLrp1+/+ mice (Table 1). Fractionation of plasma lipoproteins by FPLC revealed that the reduction of plasma cholesterol levels in hLrp1−/− mice was due entirely to decreased levels of HDL (Fig. 1).
FIGURE 1.
Analysis of plasma lipoproteins in hLrp1+/+ and hLrp1−/− mice. A total of 0.25 ml of pooled plasma samples from fasting hLrp1+/+ (●) and hLrp1−/− (○) littermates was applied to FPLC. Total cholesterol in each 0.5-ml fraction was determined colorimetrically. The FPLC elution profile of standard VLDL, LDL, and HDL is indicated for comparison. The inset shows immunoblot analysis of liver protein extracts from hLrp1+/+ (+) and hLrp1−/− (−) mice with antibodies against the 85-kDa subunit of LRP1.
TABLE 1.
Plasma and tissue lipid levels in hLrp1+/+ and hLrp1−/− mice
NEFA, non-esterified fatty acids.
| hLrp1+/+ (n = 18) | hLrp1−/− (n = 14) | |
|---|---|---|
| Plasma | ||
| Triglycerides (mg/dl) | 59 ± 14 | 58 ± 15 |
| Cholesterol (mg/dl) | 112 ± 38 | 74 ± 27a |
| NEFA (mmol/l) | 0.48 ± 0.27 | 0.36 ± 0.16 |
| Glucose (mg/dl) | 165 ± 16 | 156 ± 11 |
| Insulin (ng/ml) | 1.37 ± 0.48 | 1.42 ± 0.72 |
| Liver | ||
| Triglycerides (μg/mg) | 16.95 ± 7.63 | 21.78 ± 6.18 |
| Cholesterol (μg/mg) | 1.51 ± 0.23 | 1.62 ± 0.17 |
| Heart | ||
| Triglycerides (μg/mg) | 4.49 ± 1.30 | 4.08 ± 1.13 |
| Cholesterol (μg/mg) | 0.73 ± 0.17 | 0.63 ± 0.08 |
| Skeletal muscle | ||
| Triglycerides (μg/mg) | 12.30 ± 5.31 | 11.65 ± 4.83 |
| Cholesterol (μg/mg) | 0.39 ± 0.14 | 0.32 ± 0.06 |
| White adipose | ||
| Triglycerides (μg/mg) | 103.62 ± 24.02 | 110.76 ± 10.91 |
| Cholesterol (μg/mg) | 0.11 ± 0.07 | 0.17 ± 0.16 |
a Denotes significant differences from hLrp1+/+ at p < 0.01.
Hepatic LRP1 Inactivation Does Not Influence HDL Remodeling and Catabolism
The mechanism by which hepatic LRP1 deficiency decreases plasma HDL level was explored initially with a focus on the activities of HDL remodeling enzymes in plasma of hLrp1+/+ and hLrp1−/− mice. These studies were prompted by in vitro evidence of interaction between the LRP1 ligand α2-macroglobulin and LCAT and of LRP1-mediated clearance of the LCAT-α2-macroglobulin complex that may decrease LCAT bioavailability for HDL remodeling (23). However, plasma LCAT activity was found to be similar between hLrp1+/+ and hLrp1−/− mice (Fig. 2A). Another LRP1 ligand in mouse plasma that can potentially influence HDL remodeling is hepatic lipase (30). Therefore, we also compared hepatic lipase activity in plasma of hLrp1+/+ and hLrp1−/− mice. Surprisingly, plasma hepatic lipase activity was lower in hLrp1−/− mice when compared with that observed in hLrp1+/+ mouse plasma (Fig. 2B). These results indicate that hepatic LRP1 plays a minor role in hepatic lipase clearance in vivo. Moreover, because hepatic lipase knock-out mice have a slight elevation of plasma HDL level when compared with wild type mice (31), the decrease in hepatic lipase activity observed in hLrp1−/− mice likely cannot account for the decreased HDL level observed in these animals. Additional experiments showed that HDL catabolism was also similar between hLrp1+/+ and hLrp1−/− mice as intravenously injected HDL-associated [3H]cholesteryl esters and 125I-labeled HDL were cleared from circulation at similar rates in the two groups of mice (Fig. 2, C and D).
FIGURE 2.
Plasma enzyme activities and HDL clearance in hLrp1+/+ and hLrp1−/− mice. A, plasma activity of the HDL remodeling enzyme LCAT in hLrp1+/+ and hLrp1−/− mice was determined using the assay kit from EMD Biosciences, based on conversion of the fluorescent LCAT substrate measured at 470 nm to product measured at 390 nm. OD, optical density. B, plasma activity of hepatic lipase was assessed using 2 μl of plasma based on the hydrolysis of the fluorescent substrate 4-methylumbelliferyl heptanoate. The error bars represent mean ± S.E. of data obtained from 10 fasting animals per group. * indicates statistical significance at p < 0.05. The clearance rates of [3H]cholesteryl ether-labeled HDL (C) and 125I-HDL (D) were determined by quantifying the amount of isotope remaining (percentage of original HDL cholesterol) in plasma at the indicated times after injection. Radioactivity present in the plasma 30 s after injection was used as 100% of the injected dose. The data represent the mean ± S.E. obtained with four animals per group.
Hepatic LRP1 Deficiency Decreases HDL Production
The lower plasma HDL level but similar HDL catabolic rates in hLrp1−/− mice when compared with hLrp1+/+ mice suggested that hepatic LRP1 deficiency alters HDL production by the liver. This possibility was addressed directly with primary hepatocytes isolated from hLrp1+/+ and hLrp1−/− mice. Cellular cholesterol was prelabeled by incubating freshly isolated hepatocytes with [3H]mevalonate for 6 h in serum-free medium. The cells were incubated for an additional 6 h in the absence or presence of exogenous apoA-I added to the culture medium, and the amount of [3H]cholesterol transferred to the culture medium as apoA-I-immunoprecipitable HDL was measured to assess HDL produced by lipidation of intracellularly produced apoA-I as well as that produced by lipidation of exogenous apoA-I, respectively (32, 33). Results showed significant decrease of HDL [3H]cholesterol secretion in hLrp1−/− hepatocytes when compared with hLrp1+/+ hepatocytes when incubated without exogenous apoA-I (Fig. 3A). Additionally, [3H]cholesterol efflux to exogenous apoA-I was also reduced by ∼55% (Fig. 3B).
FIGURE 3.
Nascent HDL synthesis and cholesterol efflux to exogenous apoA-I by hLrp1+/+ and hLrp1−/− hepatocytes. Endogenous cholesterol in primary hepatocytes (400,000 cells/well) from hLrp1+/+ and hLrp1−/− mice were prelabeled by incubation for 16 h with 10 μCi/ml [3H]mevalonate. Nascent HDL synthesis (A) and exogenous apoA-I-mediated cholesterol efflux (B) were determined by measuring anti-apoA-I-immunoprecipitable [3H]cholesterol secreted into the culture medium after incubation for 6 h in the absence or presence of exogenously added apoA-I, respectively. The data represent mean ± S.E. from six mice per group in two separate experiments. * indicates statistical significant difference at p < 0.05.
LRP1 Deficiency Alters Apolipoprotein Secretion and Decreases Cell Surface ABCA1 in Hepatocytes
Analysis of conditioned medium from hLrp1+/+ and hLrp1−/− hepatocytes revealed 4.6-fold more apoE and 50% less apoA-I secretion by LRP1-deficient hepatocytes (Fig. 4A). The elevated level of apoE in the culture medium of hLrp1−/− hepatocytes is consistent with the well documented role of LRP1 in uptake and clearance of apoE-containing lipoproteins as well as its role in recapturing and sequestration of apoE on the cell surface (34). Importantly, the decreased level of apoA-I in the medium confirms the decreased nascent HDL secretion by hLrp1−/− hepatocytes. An important regulator of hepatic HDL production and plasma HDL level is ABCA1 (35, 36). Therefore, cell lysates were prepared from hLrp1+/+ and hLrp1−/− hepatocytes for immunoblot analysis of ABCA1 expression. Results showed that LRP1 deficiency did not influence the total amount of ABCA1 present in hepatocytes (Fig. 4B). However, when cell surface proteins of hLrp1+/+ and hLrp1−/− hepatocytes were biotinylated with sulfo-NHS-biotin and then precipitated with immobilized streptavidin prior to immunoblotting, significantly less ABCA1 (∼50%) was found to be present on the cell surface of hLrp1−/− hepatocytes when compared with hLrp1+/+ hepatocytes (Fig. 4C). In contrast, cell surface expression of SR-BI, LDL receptor, and VLDL receptor was similar between hLrp1+/+ and hLrp1−/− hepatocytes (Fig. 4C). Similar cell surface expression level of SR-BI is consistent with results showing similar HDL cholesteryl ester clearance rates between hLrp1+/+ and hLrp1−/− mice. Moreover, the similar levels of three different cell surface lipoprotein receptors between hLrp1+/+ and hLrp1−/− hepatocytes also indicate that the decrease of cell surface ABCA1 is specific and not a generalized phenomenon of defective cell surface protein transport in the absence of LRP1.
FIGURE 4.
Apolipoprotein secretion and lipoprotein receptor expression in hLrp1+/+ and hLrp1−/− hepatocytes. Primary hepatocytes from hLrp1−/− and hLrp1+/+ mice were cultured in serum-free medium for 20 h. A, equal volumes of conditioned media were resolved by SDS-polyacrylamide gels and immunoblotted using antibodies against apoA-I and apoE. B, equal protein concentrations of whole cell lysates were immunoblotted with antibodies against ABCA1. C, cell surface proteins were labeled with sulfo-NHS-SS-biotin and then precipitated using immobilized streptavidin. The precipitates were electrophoretically resolved and immunoblotted using antibodies against the 515-kDa subunit of LRP1, ABCA1, SR-BI, LDL receptor (LDLR), and VLDL receptor (VLDLR). Each lane in the immunoblots represents a sample from an individual well. The immunoblots were scanned, and the intensity of the bands was quantified by image analysis. The average pixel density of the bands from hLrp1+/+ hepatocyte cultures was arbitrarily set at 1.0 in each case. The error bars indicate S.D. from the mean values with * indicating significant differences from the hLrp1+/+ groups at p < 0.01.
Hepatic LRP1 Deficiency Alters Cathepsin D and Prosaposin Processing
Membrane localization of ABCA1 is regulated by glycosphingolipid metabolism, with defective lysosomal conversion of hexosylceramides, including galactosyl-, glucosyl-, and lactosylceramide, to ceramide, resulting in reduced trafficking of ABCA1 to the cell surface (37). Comparison of total glycosphingolipid contents in hLrp1+/+ and hLrp1−/− hepatocytes revealed elevated levels of long-chain hexosylceramides in hLrp1−/− hepatocytes (Fig. 5), which is consistent with the interpretation that LRP1 deficiency reduces glycosphingolipid degradation and thereby decreases cell surface ABCA1. One potential mechanism underlying defective glycosphingolipid metabolism in hLrp1−/− hepatocytes may be related to the impairment of trafficking and/or processing of the LRP1 ligand prosaposin, the precursor of the glycosphingolipid-hydrolyzing saposins. To test this possibility, protein extracts were prepared from primary hepatocytes from hLrp1+/+ and hLrp1−/− mice for immunoblot analysis. Surprisingly, although LRP1 has been shown to bind and internalize prosaposin in fibroblasts (6), prosaposin was not detected in the culture medium, but its intracellular level was increased by almost 3-fold in hLrp1−/− hepatocytes (Fig. 6A). The accumulation of prosaposin in hLrp1−/− hepatocytes suggested defective conversion of prosaposin to activated saposins, a process that is mediated by cathepsin D (38). Indeed, intracellular levels of cathepsin D were comparatively lower in hLrp1−/− hepatocytes than hLrp1+/+ hepatocytes (Fig. 6). Interestingly, significantly higher amounts of cathepsin D were found in the hLrp1−/− hepatocyte culture medium when compared with the medium of hLrp1+/+ hepatocyte cultures (Fig. 6B). Similar elevation of prosaposin and decreased cathepsin D levels were also observed in freshly isolated liver of hLrp1−/− mice (Fig. 6C). Taken together, these results suggest that decreased intracellular levels of the lysosomal enzyme cathepsin D along with the accompanying defect in prosaposin activation are responsible for reduced cell surface ABCA1 and decreased HDL production in hLrp1−/− hepatocytes. The direct relationship between reduced cathepsin D levels and decreased HDL production was substantiated by additional experiments showing that inactivation of cathepsin D with pepstatin A also reduced HDL production in normal hLrp1+/+ hepatocytes (Fig. 7). The latter observation is consistent with previous data showing reduced cell surface ABCA1 expression and cholesterol efflux from macrophages after cathepsin D inhibition with pepstatin or siRNA (35).
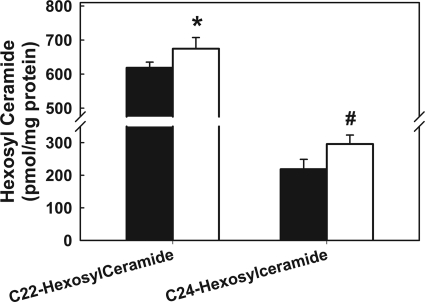
FIGURE 5.
Long-chain hexosylceramide levels in hLrp1+/+ and hLrp1−/− hepatocytes. Primary hepatocytes from hLrp1+/+ and hLrp1−/− mice cultured at a density of 2.0 × 106 cells/well in 10-cm cluster dishes were lysed in buffer consisting of 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, and 0.25 m sucrose. One mg of each sample was used for mass spectrometry analysis of glycosphingolipids. Sample recovery was normalized to protein content in each sample. Results represent mean ± S.D. of triplicate determinations from two separate experiments. * indicates difference from hLrp1+/+ hepatocytes at p = 0.05; # indicates difference from hLrp1+/+ hepatocytes at p = 0.03.
FIGURE 6.
Prosaposin and cathepsin D levels in hLrp1+/+ and hLrp1−/− mouse livers. Cell lysates (A) and conditioned media (B) from primary hepatocytes of hLrp1+/+ and hLrp1−/− mice were resolved on SDS-polyacrylamide gels and immunoblotted with antibodies against prosaposin (ProSAP) or cathepsin D (CTSD). Immunoblots with tubulin antibodies were used as loading control for primary hepatocyte experiments. Each lane represents a sample from an individual well. The immunoblots were scanned, and the intensity of the bands was quantified by image analysis. The average pixel density of the bands from hLrp1+/+ hepatocyte cultures was arbitrarily set at 1.0 in each case. The error bars indicate S.D. from the mean values with * indicating significant differences from the hLrp1+/+ groups at p < 0.01. C, immunoblots of proteins isolated from fresh liver homogenates of hLrp1+/+ and hLrp1−/− mice.
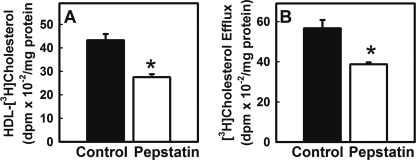
FIGURE 7.
Effect of cathepsin D inhibition on nascent HDL synthesis and cholesterol efflux to exogenous apoA-I. Endogenous cholesterol in primary hepatocytes from hLrp1+/+ mice was prelabeled by incubation for 16 h with 10 μCi/ml [3H]mevalonate and then incubated for 3 h with pepstatin (25 μg/ml) or vehicle control (Control) prior to determination of HDL synthesis and apoA-I lipidation. Nascent HDL synthesis (A) and exogenous apoA-I-mediated cholesterol efflux (B) were determined by measuring anti-apoA-I-immunoprecipitable [3H]cholesterol secreted into the culture medium over a subsequent 6-h period in the absence or presence of exogenously added apoA-I, respectively, with continued presence of pepstatin or vehicle. The data represent mean ± S.E. from three mice per group in two separate experiments. * indicates statistical significant difference at p < 0.05.
Cathepsin D Binding to Hepatic LRP1
The decreased intracellular cathepsin D levels along with higher amounts of the enzyme in conditioned medium of hLrp1−/− hepatocytes suggested that LRP1 may participate in the cathepsin D trafficking and reuptake process. To test this possibility, cathepsin D was radiolabeled with [35S]methionine by in vitro translation of its cDNA and then added to the culture medium of hLrp1+/+ and hLrp1−/− hepatocytes. Results showed that [35S]cathepsin D bound to the hepatocytes via a concentration-dependent saturable process (Fig. 8). The total amount of [35S]cathepsin D bound to the hLrp1−/− hepatocytes was ∼45% less than that bound to hLrp1+/+ hepatocytes (Fig. 8). The binding of [35S]cathepsin D to hLrp1+/+ hepatocytes was reduced to similar levels as observed with hLrp1−/− hepatocytes in the presence of GST-conjugated receptor-associated protein (RAP) that inhibits LRP1 ligand binding (Fig. 8). However, the addition of GST-RAP had no effect on [35S]cathepsin D binding to hLrp1−/− hepatocytes (Fig. 8).
FIGURE 8.
Cathepsin D binding to hLrp1+/+ and hLrp1−/− hepatocytes. Primary hepatocytes from hLrp1+/+ (filled symbols) and hLrp1−/− (open symbols) mice were preincubated at 37 °C for 30 min in the absence (circles) or presence (triangles) of 40 μg/ml GST-RAP prior to the addition of [35S]cathepsin D produced from in vitro translation of cathepsin D cDNA. The incubation was continued at 4 °C for 4 h, and cell lysates were prepared for liquid scintillation counting to determine [35S]cathepsin D associated with the hepatocytes. The data represent mean ± S.E. from quadruplicate experiments. Note that S.E. was too small to visualize in some cases. # indicates significant difference from the other three curves at p < 0.01. The inset shows SDS-PAGE analysis documenting the purity of the in vitro translated product from the sense (A) and antisense (B) direction of the cathepsin D cDNA.
DISCUSSION
Several genetic association studies have identified LRP1 gene polymorphism as a risk factor for premature coronary artery disease in humans (39, 40). Inactivation of the Lrp1 gene in liver and smooth muscle cells has also been shown to increase vascular occlusive diseases in mice (8, 10, 20). The increased atherosclerosis and neointimal formation in mice with smooth muscle-specific LRP1 inactivation are due to aberrant cell signal regulation with constitutive PDGF receptor and TGFβ receptor activation (9, 10). In contrast, increased atherosclerosis observed with liver-specific LRP1 inactivation has been attributed to defective clearance of coagulation factors and protease-protease inhibitor complexes including the atherogenic protein plasminogen activator inhibitor-1 (20). Results of the current study showed that liver-specific LRP1 deficiency reduces plasma HDL levels by decreasing HDL synthesis, thereby identifying an additional mechanism by which LRP1 activity reduces atherosclerosis risk. Our observations of decreased plasma HDL levels in hLrp1−/− mice are different from previous reports showing similar plasma and HDL cholesterol levels between chow-fed hLrp1+/+ and hLrp1−/− mice (4). The apparent discrepancy between our results with previous studies may be due to the method of generating liver-specific Lrp1−/− mice. The Rohlmann study (4) achieved hepatic LRP1 inactivation by breeding Lrp1flox/flox mice with MX1-driven cre transgenic mice and then induction of cre expression by polyinosinic acid-polycytidylic acid (poly(I-C)) injection. The earlier study also examined hLrp1−/− mice in a mixed genetic background, whereas the hLrp1−/− mice used in the current study were in the C57BL/6 background. In this regard, reduced plasma HDL level due to hepatic LRP1 deficiency was also reported in two separate studies comparing hLrp1−/−Ldlr−/− with hLrp1+/+Ldlr−/− mice (4, 20).
The potential importance of LRP1 in regulating plasma HDL level has been implicated previously by several indirect experiments. For example, one study showed that the LRP1 ligand α2-macroglobulin binds to the HDL cholesterol esterification enzyme LCAT to inactivate its enzyme activity (23). Therefore, defects in α2-macroglobulin clearance due to hepatic LRP1 deficiency may conceivably increase plasma α2-macroglobulin concentration, leading to overall decrease in LCAT activity and reduction of plasma HDL levels. However, our results showing comparable LCAT activity in plasma of hLrp1+/+ and hLrp1−/− mice indicate that hepatic LRP1 plays at best a minor role in regulating LCAT activity under chow-fed dietary conditions in vivo, and α2-macroglobulin-LCAT interaction cannot account for the difference in plasma HDL levels between hLrp1+/+ and hLrp1−/− mice. Another study showed that animals lacking the LRP1 ligand hepatic lipase displayed elevated plasma HDL levels when compared with wild type mice (31), suggesting the possibility that hepatic LRP1 deficiency may elevate hepatic lipase level, resulting in decreased plasma HDL. Results of the current study showing that hepatic lipase activity was actually lower in hLrp1−/− mice ruled out this possibility.
Another enzyme that participates in regulation of plasma HDL level and remodeling is endothelial lipase (41). Although the current study did not compare endothelial lipase activity in hLrp1+/+ and hLrp1−/− mice directly, it is unlikely that differences in HDL levels between these animals are due to differences in HDL remodeling by endothelial lipase because SR-BI-mediated selective uptake is required for the latter process (42) and no difference in SR-BI level and selective uptake of HDL cholesteryl esters were noted between hLrp1+/+ and hLrp1−/− mice. Moreover, reduction of endothelial lipase activity is also expected to increase plasma HDL cholesterol level (43, 44) instead of the decreased HDL cholesterol level observed in the hLrp1−/− mice. Finally, LRP1 has been shown to participate in selective cellular uptake of HDL-associated cholesteryl esters in adipocytes and liposarcoma cells via a novel efflux-recapture mechanism (21, 45). Our results showing no difference in fractional catabolic rates for HDL-associated proteins and cholesteryl esters despite the lower plasma HDL levels in hLrp1−/− when compared with the hLrp1+/+ mice indicated that hepatic LRP1 does not participate in HDL cholesteryl ester selective uptake in vivo.
In the current study, we found that the lower plasma HDL level in hLrp1−/− mice reduces cell surface expression of ABCA1, thereby decreasing hepatic nascent HDL synthesis and cholesterol efflux to exogenous apoA-I. Our results also showed that LRP1 deficiency reduces intracellular cathepsin D levels, with more enzyme secreted into extracellular medium and less being recaptured and endocytosed into intracellular compartments. Previous studies have shown that cathepsin D inhibition decreases prosaposin conversion to the saposin peptides, resulting in glycosphingolipid accumulation in late endosomes/lysosomes (37) and decreased availability of ceramide to promote ABCA1-mediated cholesterol efflux (46). Therefore, the alteration in cathepsin D trafficking is most likely the direct cause of reduced hepatic HDL secretion and lower plasma HDL levels in hLrp1−/− mice. The relationship between LRP1, cathepsin D activation of prosaposin, and ABCA1-mediated HDL secretion is consistent with previous studies observed in Chinese hamster ovary cells showing that up-regulation of the LRP1 engulfment adaptor protein (GULP) impairs LRP1 ligand uptake, leading to glycosphingolipid retention in late endosome/lysosome compartments and decreased ABCA1-mediated cholesterol efflux, whereas the knockdown of GULP has the opposite effect (22). The results reported herein provided direct evidence showing the participation of LRP1 in this pathway in the liver under physiological setting and showing that reduced cathepsin D activity is directly responsible for decreased production and plasma HDL levels in hLrp1−/− mice. However, it is important to note that although our results implicate cathepsin D effects on ABCA1 trafficking, we cannot rule out the possibility that other LRP1 effects on lysosome function may also affect HDL formation.
A recent study identified another mechanism by which LRP1 modulates ABCA1 expression and cholesterol transport. In smooth muscle cells, LRP1 serves as a co-receptor with the PDGF receptor to participate in cell signal transduction (9, 10). In the absence of LRP1, PDGF receptor signaling events including mitogen-activated protein kinase, protein kinase B/AKT, and cytosolic phospholipase A2 phosphorylation are constitutively activated (47). The increase in cytosolic phospholipase A2 activity leads to overproduction of arachidonic acid from plasma membrane phospholipid hydrolysis, and the excessive arachidonic acid suppresses ABCA1 gene expression by inactivating liver X receptor/retinoid X receptor (47). However, this mechanism is unlikely to occur in the liver because PDGF receptor is minimally expressed in hepatocytes under normal physiological conditions. Results showing similar levels of total ABCA1 protein in hepatocytes of hLrp1+/+ and hLrp1−/− are supportive of this conclusion. Our study showed that the LRP1 defect in the liver attenuates cell surface ABCA1 activity in hepatocytes due to reduced intracellular level of cathepsin D and the accompanying defect in prosaposin activation. Taken together, these results document that depending on the cell type, LRP1 may regulate ABCA1 expression and activity via either its cell signal regulatory properties or its function as a cargo transporting endocytic receptor. More importantly, these results revealed the importance of LRP1 function in regulating plasma HDL level and reverse cholesterol transport in addition to its well documented role in the metabolism and clearance of atherogenic triglyceride-rich lipoproteins.
Results of the current study showing that LRP1 participates in the cathepsin D binding and reuptake process in hepatocytes also add to the long list of ligands for LRP1 and expand our understanding of the numerous mechanisms by which LRP1 affects cell functions. Classically, cathepsin D synthesized in the rough endoplasmic reticulum is transported to the lysosomes by clathrin-coated vesicles via its interaction with mannose 6-phosphate receptors (48). However, the mannose 6-phosphate receptors are not sufficient for proper lysosomal enzyme targeting, and alternative mechanisms for enzyme transport to the lysosomes exist in a cell type-specific manner (49, 50). In fact, the capturing of lysosomal enzymes and their targeting to the lysosomes is remarkably effective in hepatocytes with defective expression of both the 300-kDa and the 46-kDa mannose 6-phosphate receptors (50). In vitro interaction of cathepsin D with LRP1 on the fibroblast cell surface has been reported recently (51). The current study documents that LRP1 is likely to be the additional endocytic receptor in hepatocytes responsible for uptake of lysosomal enzymes independent of the mannose 6-phosphate receptors. Whether cathepsin D trafficking defect due to LRP1 inactivation also causes other functional abnormalities in the liver in addition to reduced HDL secretion remains to be determined.
This work was supported, in whole or in part, by National Institutes of Health Grants DK74932 (to D. Y. H.) and HL62542 (to W. S. D.).
- LRP1
- LDL receptor-related protein-1
- apoE
- apolipoprotein E
- LCAT
- lecithin:cholesterol acyltransferase
- ABCA1
- ATP-binding cassette, subfamily A, member 1
- SR-BI
- scavenger receptor class B type 1
- RAP
- receptor associated protein.
REFERENCES
- 1. Herz J., Kowal R. C., Goldstein J. L., Brown M. S. (1990) EMBO J. 9, 1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kowal R. C., Herz J., Goldstein J. L., Esser V., Brown M. S. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 5810–5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ishibashi S., Herz J., Maeda N., Goldstein J. L., Brown M. S. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 4431–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rohlmann A., Gotthardt M., Hammer R. E., Herz J. (1998) J. Clin. Invest. 101, 689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu K. C., Chen W., Cooper A. D. (2001) J. Clin. Invest. 107, 1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hiesberger T., Hüttler S., Rohlmann A., Schneider W., Sandhoff K., Herz J. (1998) EMBO J. 17, 4617–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strickland D. K., Gonias S. L., Argraves W. S. (2002) Trends Endocrinol. Metab. 13, 66–74 [DOI] [PubMed] [Google Scholar]
- 8. Boucher P., Gotthardt M., Li W. P., Anderson R. G., Herz J. (2003) Science 300, 329–332 [DOI] [PubMed] [Google Scholar]
- 9. Boucher P., Li W. P., Matz R. L., Takayama Y., Auwerx J., Anderson R. G., Herz J. (2007) PLoS ONE 2, e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basford J. E., Moore Z. W., Zhou L., Herz J., Hui D. Y. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1772–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terrand J., Bruban V., Zhou L., Gong W., El Asmar Z., May P., Zurhove K., Haffner P., Philippe C., Woldt E., Matz R. L., Gracia C., Metzger D., Auwerx J., Herz J., Boucher P. (2009) J. Biol. Chem. 284, 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. May P., Rohlmann A., Bock H. H., Zurhove K., Marth J. D., Schomburg E. D., Noebels J. L., Beffert U., Sweatt J. D., Weeber E. J., Herz J. (2004) Mol. Cell. Biol. 24, 8872–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lillis A. P., Van Duyn L. B., Murphy-Ullrich J. E., Strickland D. K. (2008) Physiol. Rev. 88, 887–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hofmann S. M., Zhou L., Perez-Tilve D., Greer T., Grant E., Wancata L., Thomas A., Pfluger P. T., Basford J. E., Gilham D., Herz J., Tschöp M. H., Hui D. Y. (2007) J. Clin. Invest. 117, 3271–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bacskai B. J., Xia M. Q., Strickland D. K., Rebeck G. W., Hyman B. T. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11551–11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuentealba R. A., Liu Q., Kanekiyo T., Zhang J., Bu G. (2009) J. Biol. Chem. 284, 34045–34053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orr A. W., Elzie C. A., Kucik D. F., Murphy-Ullrich J. E. (2003) J. Cell Sci. 116, 2917–2927 [DOI] [PubMed] [Google Scholar]
- 18. Orr A. W., Pedraza C. E., Pallero M. A., Elzie C. A., Goicoechea S., Strickland D. K., Murphy-Ullrich J. E. (2003) J. Cell Biol. 161, 1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herz J., Chen Y., Masiulis I., Zhou L. (2009) J. Lipid Res. 50, S287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Espirito Santo S. M., Pires N. M., Boesten L. S., Gerritsen G., Bovenschen N., van Dijk K. W., Jukema J. W., Princen H. M., Bensadoun A., Li W. P., Herz J., Havekes L. M., van Vlijmen B. J. (2004) Blood 103, 3777–3782 [DOI] [PubMed] [Google Scholar]
- 21. Vassiliou G., McPherson R. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1669–1675 [DOI] [PubMed] [Google Scholar]
- 22. Kiss R. S., Ma Z., Nakada-Tsukui K., Brugnera E., Vassiliou G., McBride H. M., Ravichandran K. S., Marcel Y. L. (2006) J. Biol. Chem. 281, 12081–12092 [DOI] [PubMed] [Google Scholar]
- 23. Krimbou L., Marcil M., Davignon J., Genest J., Jr. (2001) J. Biol. Chem. 276, 33241–33248 [DOI] [PubMed] [Google Scholar]
- 24. Gilham D., Lehner R. (2005) Methods 36, 139–147 [DOI] [PubMed] [Google Scholar]
- 25. Pittman R. C., Knecht T. P., Rosenbaum M. S., Taylor C. A., Jr. (1987) J. Biol. Chem. 262, 2443–2450 [PubMed] [Google Scholar]
- 26. Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr., Attie A. D. (1983) Biochem. J. 212, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Methods 39, 82–91 [DOI] [PubMed] [Google Scholar]
- 28. Williams S. E., Ashcom J. D., Argraves W. S., Strickland D. K. (1992) J. Biol. Chem. 267, 9035–9040 [PubMed] [Google Scholar]
- 29. Rohlmann A., Gotthardt M., Willnow T. E., Hammer R. E., Herz J. (1996) Nat. Biotechnol. 14, 1562–1565 [DOI] [PubMed] [Google Scholar]
- 30. Vergés M., Bensadoun A., Herz J., Belcher J. D., Havel R. J. (2004) J. Biol. Chem. 279, 9030–9036 [DOI] [PubMed] [Google Scholar]
- 31. Homanics G. E., de Silva H. V., Osada J., Zhang S. H., Wong H., Borensztajn J., Maeda N. (1995) J. Biol. Chem. 270, 2974–2980 [DOI] [PubMed] [Google Scholar]
- 32. Wang M. D., Franklin V., Sundaram M., Kiss R. S., Ho K., Gallant M., Marcel Y. L. (2007) J. Biol. Chem. 282, 22525–22533 [DOI] [PubMed] [Google Scholar]
- 33. Chisholm J. W., Burleson E. R., Shelness G. S., Parks J. S. (2002) J. Lipid Res. 43, 36–44 [PubMed] [Google Scholar]
- 34. Fazio S., Linton M. F., Swift L. L. (2000) Trends Cardiovasc. Med. 10, 23–30 [DOI] [PubMed] [Google Scholar]
- 35. Basso F., Freeman L., Knapper C. L., Remaley A., Stonik J., Neufeld E. B., Tansey T., Amar M. J., Fruchart-Najib J., Duverger N., Santamarina-Fojo S., Brewer H. B., Jr. (2003) J. Lipid Res. 44, 296–302 [DOI] [PubMed] [Google Scholar]
- 36. Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., Hayden M. R., Maeda N., Parks J. S. (2005) J. Clin. Invest. 115, 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haidar B., Kiss R. S., Sarov-Blat L., Brunet R., Harder C., McPherson R., Marcel Y. L. (2006) J. Biol. Chem. 281, 39971–39981 [DOI] [PubMed] [Google Scholar]
- 38. Gopalakrishnan M. M., Grosch H. W., Locatelli-Hoops S., Werth N., Smolenová E., Nettersheim M., Sandhoff K., Hasilik A. (2004) Biochem. J. 383, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCarthy J. J., Parker A., Salem R., Moliterno D. J., Wang Q., Plow E. F., Rao S., Shen G., Rogers W. J., Newby L. K., Cannata R., Glatt K., Topol E. J. (2004) J. Med. Genet. 41, 334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pocathikorn A., Granath B., Thiry E., Van Leuven F., Taylor R., Mamotte C. (2003) Atherosclerosis 168, 115–121 [DOI] [PubMed] [Google Scholar]
- 41. Badellino K. O., Rader D. J. (2004) Curr. Opin. Cardiol. 19, 392–395 [DOI] [PubMed] [Google Scholar]
- 42. Nijstad N., Wiersma H., Gautier T., van der Giet M., Maugeais C., Tietge U. J. (2009) J. Biol. Chem. 284, 6093–6100 [DOI] [PubMed] [Google Scholar]
- 43. Jin W., Millar J. S., Broedl U., Glick J. M., Rader D. J. (2003) J. Clin. Invest. 111, 357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edmondson A. C., Brown R. J., Kathiresan S., Cupples L. A., Demissie S., Manning A. K., Jensen M. K., Rimm E. B., Wang J., Rodrigues A., Bamba V., Khetarpal S. A., Wolfe M. L., Derohannessian S., Li M., Reilly M. P., Aberle J., Evans D., Hegele R. A., Rader D. J. (2009) J. Clin. Invest. 119, 1042–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vassiliou G., Benoist F., Lau P., Kavaslar G. N., McPherson R. (2001) J. Biol. Chem. 276, 48823–48830 [DOI] [PubMed] [Google Scholar]
- 46. Witting S. R., Maiorano J. N., Davidson W. S. (2003) J. Biol. Chem. 278, 40121–40127 [DOI] [PubMed] [Google Scholar]
- 47. Zhou L., Choi H. Y., Li W. P., Xu F., Herz J. (2009) PLoS One 4, e6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ludwig T., Le Borgne R., Hoflack B. (1995) Trends Cell Biol. 5, 202–206 [DOI] [PubMed] [Google Scholar]
- 49. Kasper D., Dittmer F., von Figura K., Pohlmann R. (1996) J. Cell Biol. 134, 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dittmer F., Ulbrich E. J., Hafner A., Schmahl W., Meister T., Pohlmann R., von Figura K. (1999) J. Cell Sci. 112, 1591–1597 [DOI] [PubMed] [Google Scholar]
- 51. Beaujouin M., Prébois C., Derocq D., Laurent-Matha V., Masson O., Pattingre S., Coopman P., Bettache N., Grossfield J., Hollingsworth R. E., Zhang H., Yao Z., Hyman B. T., van der Geer P., Smith G. K., Liaudet-Coopman E. (2010) J. Cell Sci. 123, 3336–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]