Abstract
Single-channel conductance in Cys-loop channels is controlled by the nature of the amino acids in the narrowest parts of the ion conduction pathway, namely the second transmembrane domain (M2) and the intracellular helix. In cationic channels, such as Torpedo ACh nicotinic receptors, conductance is increased by negatively charged residues exposed to the extracellular vestibule. We now show that positively charged residues at the same loop 5 position boost also the conductance of anionic Cys-loop channels, such as glycine (α1 and α1β) and GABAA (α1β2γ2) receptors. Charge reversal mutations here produce a greater decrease on outward conductance, but their effect strongly depends on which subunit carries the mutation. In the glycine α1β receptor, replacing Lys with Glu in α1 reduces single-channel conductance by 41%, but has no effect in the β subunit. By expressing concatameric receptors with constrained stoichiometry, we show that this asymmetry is not explained by the subunit copy number. A similar pattern is observed in the α1β2γ2 GABAA receptor, where only mutations in α1 or β2 decreased conductance (to different extents). In both glycine and GABA receptors, the effect of mutations in different subunits does not sum linearly: mutations that had no detectable effect in isolation did enhance the effect of mutations carried by other subunits. As in the nicotinic receptor, charged residues in the extracellular vestibule of anionic Cys-loop channels influence elementary conductance. The size of this effect strongly depends on the direction of the ion flow and, unexpectedly, on the nature of the subunit that carries the residue.
Keywords: GABA Receptors, Ion Channels, Membrane Biophysics, Neurotransmitter Receptors, Single Molecule Biophysics, Glycine Receptors, Single Channel Conductance
Introduction
Channels in the nicotinic superfamily are formed by five subunits arranged quasi-symmetrically around the central pore. Structural information from the Torpedo acetylcholine nicotinic receptor and related prokaryotic channels, such as GLIC and ELIC (all cation-permeable), shows that the conduction path for ions starts with a wide vestibule formed by the extracellular domains (1–3). This tapers to a narrower transmembrane region, which is lined mainly by the second transmembrane domains (M2) of each of the five subunits. In eukaryotic channels, this is connected to the cytoplasm by openings in the subunits intracellular domains, which are formed by the M3-M4 loop. The rate of ion flow along this pathway (and therefore the single-channel conductance) is strongly affected by the nature of the residues that line it, especially those that line the narrowest parts. In particular, there are rings of charged amino acids at the extracellular and intracellular ends of the transmembrane part of the pore (positions −4′ and 20′ in the M2 domain (4) and around the fenestrations of the cytoplasmic domain (5). It was recently found that single-channel conductance can also be influenced by residues in the extracellular domain (ECD). The first clue came from the structure of a member of the family of ACh-binding proteins (AChBP), molluscan homopentameric soluble proteins, which are homologous to the ECD part of nicotinic channels. In the structure of the Aplysia AChBP crystallized in the presence of sulfate or phosphate ions, a positively charged Arg residue in position 97 in loop 5 coordinates the negatively charged sulfate or phosphate ion (6, 7). AChBPs do not function as ion channels, but cation-permeable nicotinic channels, such as the Torpedo ACh receptor and the 5-HT3A receptor, have a negatively charged Asp in the equivalent position (97 for the α1 Torpedo nicotinic subunit). Reversing or neutralizing this charge markedly decreases the single-channel conductance of these receptors (6, 8).
In the anion-permeable channels in this superfamily, such as glycine and γ-aminobutyric acid (GABA)2 channels, the amino acid in this position is a positively charged Lys, suggesting that this residue is exposed to the vestibule and is important as a conductance determinant in the whole nicotinic superfamily. In the present study, we investigated the effect of reversing from positive to negative the charge in this position in homomeric and heteromeric glycine channels and in GABAA channels. We show that this decreases the single channel conductance especially for outward currents (i.e. when chloride ions enter the vestibule and the channel from the extracellular medium). An unexpected feature of our findings is that the magnitude of the conductance change depends very strongly on which subunit carries the mutation. No effect is seen if the β subunit (in glycine receptors) or the γ subunit (in GABAA receptors) is mutated, unless other subunits in the receptors are also mutated. In glycine receptors, the unequal contribution of the α and β subunits was not due to the different number of copies of each subunit in the pentamer.
EXPERIMENTAL PROCEDURES
Heterologous Expression of Glycine and GABAA Receptors
Human embryonic kidney 293 cells (HEK-293) (American Type Culture Collection-CRL-1573; LGC Promochem) were maintained at 37 °C in a 95% air/5% CO2 incubator in DMEM supplemented with 0.11 g/liter sodium pyruvate, 10% v/v heat-inactivated fetal bovine serum, 100 unit/ml penicillin G, 100 μg/ml streptomycin sulfate, and 2 mm l-glutamine (all from Invitrogen). Cells (passaged every 2–3 days, up to 30 times) were plated 2 h before transfection by calcium phosphate-DNA co-precipitation (details in Ref. 9). Rat α1 and β GlyR subunits (GenBankTM accession numbers AJ310834 and AJ310839) were transfected at a ratio of 1:40 to minimize contamination by homomeric α1 receptors (10) with a total amount of 3 μg of cDNA per dish. Rat α1, β2, and γ2L (long isoform) GABAA receptor subunits (GenBankTM accession numbers AY574250, AY574251, AY574252) were transfected at a ratio of 1:1:4. All recordings were carried out 24–72 h after transfection. All subunits were subcloned into the pCI vector (Promega). Site-directed mutagenesis was performed using the QuikChange mutagenesis kit (Stratagene). All constructs were full-length sequenced. As shown in alignment in Fig. 1E, the position of the extracellular conductance determinant is: for GlyR subunits Lys-104 in α1 or Lys-127 in β; for GABAA receptor subunits Lys-105 and Lys-106 in α1, Lys-101 and Lys-102 in β2, Lys-117 and Lys-118 in γ2 (numbering as in the mature peptide).
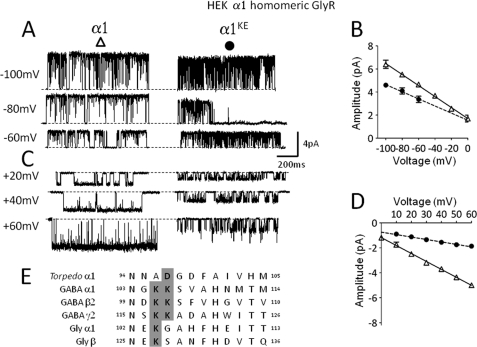
FIGURE 1.
Effects on the single channel slope conductance of α1 homomeric GlyR of a charge reversal mutation of the extracellular domain conductance determinant in loop 5. A and C are examples of inward (A) and outward (C) currents (low-pass filtered at 1 kHz) recorded from HEK cells in the cell-attached configuration in the presence of 0.2 mm Gly. Imposed holding potentials are as indicated. For inward currents only (recorded in a Na+-based external solution) the true transmembrane potential will depend also on the cell resting membrane potential. Outward currents were recorded in K+-based external solution, which effectively abolishes the cell resting potential. The baseline is indicated by a dashed line for each trace. B and D are current-voltage relationships for wild type (continuous line, Δ) and mutant receptors (dashed line, ●), for inward and outward currents, respectively. E, alignment of loop 5 of Torpedo α1, GABAA, and Gly subunits. The conserved charged residues are bold and highlighted in gray. Charge reversal mutations at a position homologous to Asp-97 of the α1 Torpedo (6) replace Lys with Glu and are indicated throughout by the superscript KE.
Preparation of α1_β and β_α1 Tandem Constructs
Gly α1 and β subunits were linked by an 18-amino acid linker (six repeats of Ala-Gly-Ser) connecting the C-terminal of the α1 subunit to the N-terminal of the β subunit. 2 runs of PCR were performed on each subunit. The first run eliminated the stop codon from the α1 subunit and the signal peptide from the β subunit. The second run of PCR added EcoRI at the 5′-end of the α1 subunit and an (AGS)3 repeat followed by XhoI at the 3′-end. At the 5′-end of the β subunit XhoI was added followed by an (AGS)3 repeat, whereas at the 3′-end NotI was inserted for subsequent cloning into a plasmid. The α1 and β subunits were then subcloned one at a time into a new pCI vector between EcoRI/XhoI and XhoI/NotI. The whole construct was then fully sequenced. The resulting construct is very similar to that previously used successfully to express a 2α:3β stoichiometry in oocytes (11), with the slight difference that in that study the α1 C terminus was shortened by 4 amino acids and 7 AGS repeats were used.
An identical approach was used to create β_α1 dimers. To maintain the effective length of the linker between subunits despite the much shorter C-terminal of the leading β subunit (1 amino acid only), β_α1 dimers were joined with AGS repeats of different length (6, 8, and 10) and we tested all of these constructs.
For expression in oocytes, all cDNAs were linearized immediately downstream of the 3′-UTR with BamHI, and capped cRNAs were transcribed using the T7 mMessage mMachine kit (Ambion, UK). The quality of the cRNA molecules was checked by RNA gel-electrophoresis.
Xenopus Oocyte Preparation and cRNA Injection
Female Xenopus laevis frogs, anesthetized by immersion in neutralized ethyl m-aminobenzoate solution (tricaine, methanesulfonate salt; 0.2% solution w/v, Sigma), were killed by concussion immediately followed by decapitation and destruction of brain and spinal cord before removal of the ovarian lobes. This procedure is in accordance with United Kingdom Home Office regulations. The study protocol does not fall under the remit of the UCL Research Ethics Committee.
Clumps of stage V-VI oocytes were dissected in a sterile modified Barth's solution consisting of (in mm): 88 NaCl, 1 KCl, 0.82 MgCl2, 0.77 CaCl2, 2.4 NaHCO3, 15 Tris-HCl, with 10 units/ml of penicillin and 10 μg/ml of streptomycin (Invitrogen, Breda, The Netherlands) in HPLC water; pH 7.4, adjusted with NaOH. The dissected oocytes were placed in OR2 solution (82.5 mm NaCl, 2.5 mm KCl, 1 mm MgCl2, 5 mm Hepes, pH adjusted to 7.6 with NaOH) and defolliculated manually.
In the same manner as for HEK cells, wild-type or mutant RNAs coding for the α1 and β subunits were injected at a 1:40 molar ratio to minimize contamination by α1 homomeric channels. Wild-type or mutant α1_β tandem and β monomer constructs were injected at a 1:2 molar ratio. When attempting to express receptors with 3α:2β stoichiometry, α1_β tandem and α1monomer constructs were injected at a 40:1 ratio. Each oocyte was injected with a total of 20 ng of cRNA (in 36 nl of RNase-free water), using a Nanoject Automatic Oocyte Injector (Drummond Scientific, Broomall, PA), and then incubated for ∼72 h at 18 °C in fresh Barth's solution containing 5% heat-inactivated horse serum. Experiments were carried out 3–4 days from injection.
Single-channel Recordings
All recordings from HEK293 cells or Xenopus oocytes were performed in the cell-attached configuration. Electrodes were pulled from thick-walled Clark borosilicate glass GC150F (Harvard Instruments), coated near the tip with Sylgard 184® (Dow Corning) and fire-polished prior to use to a final resistance of 6–12 MΩ. The oocyte vitelline membrane was removed by hand after incubating the oocytes for 10 min in stripping solution (Table 1).
TABLE 1.
Solutions used throughout the recordings
Glycine receptor conductances were measured using S1 and S2 solutions for negative and positive voltages respectively. Due to the low conductance of GABAA receptors we bathed the cells with S2 solution in combination with S3 in the recording pipette to obtain the maximal achievable conductance (12). Gly 200 μm and GABA 10 mm were the concentration of agonists added to the pipette solution. Concentrations are expressed in mm.
| Compound | Stripping solution | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| Na-gluconate | 20 | 5.4 | |||
| K-gluconate | 200 | ||||
| NaCl | 102.7 | 5.4 | 5.4 | ||
| KCl | 20 | 2 | 142 | 170 | 273.6 |
| CaCl2 | 2 | 2 | 2 | 2 | |
| MgCl2 | 1 | 1 | 1 | 1 | 1 |
| HEPES | 10 | 10 | 10 | 10 | |
| EGTA | 10 | ||||
| MES | 10 | ||||
| TEA-Cl | 20 | 20 | 20 | ||
| Sucrose | 15 | 15 | |||
| pH | 7.6 | 7.4 | 7.4 | 6.0 | 7.4 |
Single-channel currents were recorded with an Axopatch 200B amplifier, pre-filtered at 10 kHz (using the 4-pole Bessel filter of the amplifier). The signal was then passed through an 8-pole Bessel filter (fc = 5 kHz) and digitized to the computer with a sampling rate of 50 kHz, using a Digidata 1322A and Clampex 8.1.
All recording solutions are listed in Table 1. Glycine and GABA were used to elicit channel opening and were freshly added to the pipette solutions from a 1 m frozen stock (final concentrations, 0.2 and 10 mm, respectively).
Single-channel current-voltage relationships were obtained from different patches for inward and outward currents, using different recording solutions. This was done to make the signal of interest as big as possible. Thus, inward currents from either glycine or GABAA receptors were recorded in the sodium-based S1 external solution (Table 1) both in the bath and in the pipette. In this case the true transmembrane potential is the pipette holding potential minus the cell resting potential (this is not important as we measure slope conductances throughout). For glycine channels, outward currents (i.e. at positive holding potentials) were recorded with high- K+ S2 solution (both in the bath and in the pipette). In this solution, the resting potential of the cell is depolarized to 0 mV. Consequently, the pipette holding potential values, as shown in the figures and in “Results” are the true transmembrane potentials.
Because of the low conductance of GABAA receptors, recording of outward currents were performed with the high K+ S2 solution in the bath and with a low-pH, high-chloride (200 mm) solution (S3) in the pipette to increase the channel conductance. The effects of both pH and chloride concentration on the GABAA conductance are well-known (12, 13).
At the agonist concentrations we used, openings occurred in clusters. Before analysis the traces were digitally filtered at 1 KHz using Clampfit 9.2 (Molecular Devices). Openings were detected by threshold-crossing measurement using Clampfit 9.2. Slope conductance values at positive and negative holding potentials were obtained by plotting current-voltage curves, and by fitting a straight line to the data points (each point was the average of 50 to 500 openings) in Equation 1,
where γ is the slope of the line, I is the single current measured, V is the voltage and C is the reversal potential (Prism 5, Graphpad). Only openings longer than 0.8 ms (more than twice the value of rise-time of the filter) were included in the analysis. Statistical analysis was performed by one-way ANOVA followed by Tukey's multiple comparison test (Prism 5, Graphpad). For clarity, the tables show only the significance of comparisons of each mutant to the appropriate wild-type, and other comparisons are reported in the text.
RESULTS
A Residue in the Extracellular Domain Affects the Conductance of GlyR Channels
The alignment of loop 5 sequences of Cys-loop receptors in Fig. 1E shows that the position of the extracellular conductance determinant (6) is occupied by a negatively charged Asp (bold) in the α1 subunit of the Torpedo ACh nicotinic receptor, which is cation-permeable, and by positively charged Lys residues in subunits from the glycine (Gly) and GABAA receptors, which are anion-permeable.
The traces in Fig. 1, A and C show the changes in single-channel currents of α1 Gly homomeric receptors produced by reversing the charge at this position (104) by mutating Lys to Glu. We measured the channel slope conductance from cell-attached recordings such as those shown, both at negative transmembrane potentials (where the current is inward because chloride exits the cell, Fig. 1, A and B) and at positive transmembrane potentials (where chloride enters the cell, Fig. 1, C and D). These experiments were done on different patches to allow us to use different recording solutions, chosen to maximize the signal in each of the two protocols. The traces and the plot show that charge reversal in the extracellular vestibule of α1 (i.e. five copies of Lys mutated to Glu) produced only a modest decrease in the slope conductance of inward currents (about 22.0%, Table 2). The same mutation had a much more marked effect on the outward channel conductance, which decreased by 72.4%. It is not particularly surprising that a mutation in the extracellular vestibule has a greater effect on the flow of chloride ions from the external medium to the inside of the cell. Previous work on the single-channel conductance of Gly receptors reported that mutations in the intracellular membrane-associated helix (in the cytoplasmic M3-M4 loop) affected more markedly the flow of chloride in the opposite direction, from the cytoplasm to the extracellular medium (14). In nicotinic channels, Konno et al. (15) reported that mutations in the extracellular ring of charges in M2 have a greater effect on single channel inward currents (i.e. the cation inflow) than on outward currents, with the opposite pattern for mutations in the intracellular ring (but see Ref. 16). While our report was in preparation, it was reported that, in homomeric α1 GlyR expressed in HEK cells, charge neutralization at the same position (by mutation to Ala) reduced slope conductance to a smaller extent (−40%, Ref. 7).
TABLE 2.
Inward and outward slope conductance values for α1 and α1β Gly receptors expressed in HEK cells
Average values ± S.E. are in pS.
| Receptor type | Inward γ | na | Outward γ | na |
|---|---|---|---|---|
| α1 | 47.3 ± 2.1 | 3 | 65.3 ± 6.6 | 5 |
| α1K104E | 36.9 ± 0.6b | 5 | 19.8 ± 0.7c | 5 |
| α1β | 24.2 ± 1.4 | 5 | 33.2 ± 0.5 | 6 |
| α1K104Eβ | 22.5 ± 1.0 | 3 | 19.6 ± 1.5d | 4 |
| α1βK127E | 23.0 ± 3.3 | 4 | 30.5 ± 0.6 | 7 |
| α1K104EβK127E | 24.7 ± 1.5 | 6 | 10.7 ± 0.8d | 6 |
a n is the number of patches.
b p < 0.05 compared to wild-type conductance (one-way ANOVA followed by Dunnett's post-hoc test).
c p < 0.001 compared to wild-type conductance (one-way ANOVA followed by Dunnett's post-hoc test).
d p < 0.0001 compared to wild-type conductance (one-way ANOVA followed by Dunnett's post-hoc test).
Asymmetrical Contribution of α and β Subunits to the Conductance of Heteromeric GlyR
We next investigated the effect of the same charge reversal mutation on the conductance of heteromeric α1β GlyR, where residues in the α1 and β subunits can be mutated independently. The traces in Fig. 2A (rightmost column) show that the extracellular charge reversal mutations had virtually no effect on the conductance of inward currents through heteromeric glycine receptors, even if all the subunits were mutated and five negative charges are inserted (double mutants, α1KEβKE, 24.7 ± 1.5 pS, Fig. 2B and Table 2). This is in contrast with the modest, but consistent decrease in inward conductance observed with five copies of the homologous mutation in homomeric channels.
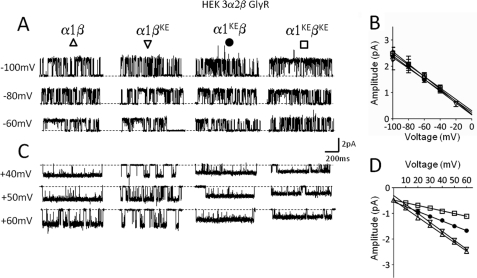
FIGURE 2.
Effects on the single channel slope conductance of the α1β heteromeric GlyR of charge reversal mutations in the loop 5 extracellular conductance determinant. Representative recordings of inward (A) and outward (C) currents recorded from HEK cells in cell-attached mode at different voltages (display fc = 1 kHz). B and D are inward and outward current-voltage relationships for wild type and mutant receptors: α1β (Δ), α1KEβ (●), α1βKE (▿), α1KEβKE (□). Note that inward currents are insensitive to charge reversal mutations in this position, even when all five subunits carry the mutation. On the contrary, the conductance of outward currents is markedly decreased by this mutation, but only when the mutation is carried either by the α1 or by both the α1 and the β subunits. Mutating the β subunit on its own does not affect outward slope conductance. Slope conductance values for each subunit combination are reported in Table 2.
Next, we measured the outward slope conductance for the heteromeric α1β GlyR mutated in either the α or the β subunit or in both. As in homomeric receptors, the outward conductance was much more sensitive to charge reversal in the extracellular domain than the inward conductance. Unexpectedly, the contribution of α and β subunits was very different. In particular, replacing Lys with Glu in the β subunit alone (α1βKE) had very little effect (−8%, 30.5 ± 0.6 pS), whereas the homologous mutation on the α1 subunit (α1KEβ) decreased outward conductance by as much as 41% (19.6 ± 1.5 pS, p < 0.001, Table 2). With the double mutant channel (α1KEβKE), which contains five copies of the charge reversal mutation, we observed a pronounced decrease in conductance (−67.8%, 10.7 ± 0.8 pS), greater than that produced by mutating either the α or the β subunit alone (p < 0.001) (Fig. 2C, rightmost column, and square symbols in the current-voltage plots in Fig. 2D). The size of this effect was similar to that seen when all subunits are mutated in the homomeric channel (α1KE, a 72.4% reduction from wild-type conductance).
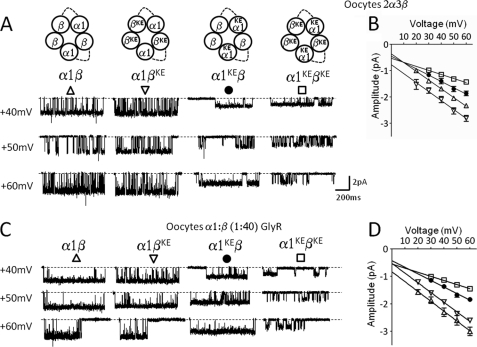
In the Torpedo nicotinic receptor, a minimum of three subunits had to be mutated in the homologous position for the change in conductance to be large enough to be detectable (6). Similarly, conductance mutations in the intracellular domain have no effect if present only in two of the five subunits (4, 5). Therefore, it is possible that mutations in the β subunit appear to have no effect on α1β GlyR conductance because this channel contains only two copies of β (as proposed by (10, 17, 18), but see (11). To test this hypothesis, we constrained the heteromeric receptor to have three copies of the β subunit and a 2α:3β stoichiometry by means of a tandem construct (α1_β), as previously described, i.e. with a linker made of 7 AGS repeats (11).
Because expression in HEK cells of α1_β together with a monomeric β subunit failed to produce any whole-cell current (data not shown), we performed the experiments in Xenopus oocytes. In this expression system, the same combination expressed robust currents that were dependent on co-injection of monomer β subunit constructs (i.e. α1_β tandem construct alone did not express functional GlyR, see supplemental Fig. S1). The cell-attached traces in Fig. 3A show the effect on outward GlyR currents of mutating the extracellular conductance determinant Lys to Glu either in the α or the β subunits in these constrained stoichiometry GlyRs. Surprisingly, even when all the three β subunits carried the reverse charge mutation (α1βKE), no effect was seen on the outward slope conductance (Fig. 3A, 34.9 ± 0.9 pS, second column and Table 3). On the other hand, mutating the two α subunits (α1KEβ, Fig. 3A, third column) still produced a clear decrease in conductance (−27.3%, 24.0 ± 1.0 pS, p < 0.05). Combining the (ineffective) mutations in β with the mutations in α to express a double mutant receptor (α1KEβKE) produced a greater decrease in conductance (−54.4%, 16.4 ± 1.6 pS) than mutating the α subunit alone (p < 0.05).
FIGURE 3.
Effects of charge reversal mutations on the outward conductance of α1β heteromeric Gly receptors expressed by coinjection of the α1 and β subunit constructs, or constrained to contain two α and three β subunits by means of concatenated constructs. (α1_β) tandem subunit constructs were co-injected with a monomeric β subunit into Xenopus oocytes and the outward currents were recorded at different voltages. A shows examples of traces recorded at positive voltages for different combinations of wild-type and mutated subunits (display fc = 1 kHz). Slope conductance values for each combination of mutant and wild-type subunits are reported in Table 3. B shows current-voltage plots for wild type and mutant receptors. α1β (Δ), α1KEβ (●), α1βKE (▿),α1KEβKE (□). Mutations carried by the β subunit had no effect on conductance, even though the stoichiometry of this receptor is 2α:3β. C shows examples of traces for the same wild type and mutant combinations expressed by co-injecting un-tethered α1 and β subunits. D shows current-voltage plots for wild-type and mutant un-tethered receptors.
TABLE 3.
Outward slope conductance values for α1β Gly receptors expressed in Xenopus oocytes as tandem subunits together with a free β subunit (α1_β+β) or as untethered subunits (α1:β, 1:40 injection ratio)
Average values ± S.E. are in pS.
| Receptor type (tethered) | Outward γ | na | Receptor type (un-tethered) | Outward γ | na |
|---|---|---|---|---|---|
| α1β | 33.0 ± 2.0 | 5 | α1β | 35.3 ± 1.3 | 3 |
| α1K104Eβ | 24.0 ± 1.0b | 3 | α1K104Eβ | 23.3 ± 0.6b | 4 |
| α1βK127E | 34.9 ± 0.9 | 7 | α1βK127E | 34.7 ± 1.0 | 3 |
| α1K104EβK127E | 16.4 ± 1.6c | 6 | α1K104EβK127E | 15.9 ± 0.6b | 3 |
a n is the number of patches.
b p < 0.001 compared to wild-type conductance (one-way ANOVA followed by Dunnett's post-hoc test).
c p < 0.0001 compared to wild-type conductance (one-way ANOVA followed by Dunnett's post-hoc test).
Thus, charge reversal in the extracellular domain of the β subunit does not change conductance in the heteromeric α1β receptor, even when β subunits are present in higher number than α subunits. Mutations in the β subunit only have a detectable effect when the conductance has already been reduced by mutating the α subunit.
As artifacts with tandem subunits have been described for other members of the nicotinic superfamily (19–21), we measured, for comparison, the conductance of heteromeric receptors expressed in oocytes from un-tethered α1 and β subunits, both wild type and mutant. We found that the effects of mutations were identical to those observed in receptors constrained to have a 2α:3β stoichiometry in oocytes (Fig. 3, C and D, Table 3).
To better explore whether the subunit copy number influences the effects of the conductance mutation in the same heterologous system, we tried to express a pure 3α:2β receptor population by injecting in oocytes the α1_β tandem together with an α1 subunit at 40:1 ratio. Robust whole-cell currents were recorded (supplemental Fig. S1), but patches from these oocytes contained exclusively α1 homomeric channels, as indicated by their conductance (6 patches from 2 different batches of oocytes).
Because of that, we tried an alternative strategy and constructed β_α1 tandems, linked by (AGS)6/8/10 repeats. Unfortunately, and in contrast with the α1_β constructs described above and in Ref. 11, all these β_α1 tandems displayed robust whole-cell current when injected alone in oocytes, making them useless for our purposes (see supplemental Fig. S1).
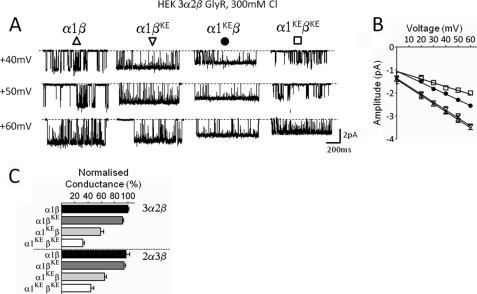
In Gly and GABAA channels, single-channel conductance increases with increasing chloride concentration above the physiological 150 mm chloride concentration used in our experiments so far, reaching a maximum above 300 mm (12). We next tested whether the effect of charge reversals in the different subunits is affected if single-channel conductance is increased by using a chloride concentration that is close to saturating (300 mm).
We therefore recorded outward currents from all mutant and wild-type combinations of α1β GlyR in the presence of 300 mm chloride in the extracellular (pipette) solution (solution S4 in Table 1). However, as shown in Fig. 4, the results were very similar to those obtained in the experiments with lower chloride concentrations (150 mm), both in the magnitude of the effect and in the pattern seen with mutations in different subunits. Again, introducing the charge reversal mutation in the β subunit had no effect on the conductance of α1β GlyR heteromers, unless the α subunit was also mutated (Table 4).
FIGURE 4.
In the α1β Gly channels the pattern of effect of mutations in the different subunits is not altered by increasing permeant ion concentration. Representative traces of outward currents recorded in cell-attached patches at different voltages (display fc = 1 kHz) in 300 mm chloride (solution S4 in Table 1). A shows example of traces recorded at positive voltages for different combinations of wild-type and mutated subunits for the heteromeric α1β Gly channels. B, outward current-voltage relationships for the wild-type and mutant heteromeric α1β receptor: α1β (Δ), α1βKE (▿), α1KEβ (●), α1KEβKE (□). C, summary of outward slope conductance values for GlyRs with 3α:2β (HEK, upper panel) and 2α:3β (expressed as concatenated subunits in oocytes, lower panel) stoichiometries. Values are normalized to the conductance value of the wild type. Note that when both subunits are mutated the conductance decreases more markedly in the 3α form (32.2% of the wild type) rather than in the 2α form of the receptor (45.6% of the wild type).
TABLE 4.
Outward slope conductance values for α1 and α1β Gly receptors expressed in HEK cells in presence of 300 mm of Cl in the pipette solution
Average values ± S.E. are in pS.
| Receptor type | Outward γ | na |
|---|---|---|
| α1 | 83.7 ± 1.2 | 6 |
| α1K104E | 36.3 ± 1.8b | 3 |
| α1β | 36.5 ± 1.7 | 5 |
| α1K104Eβ | 22.7 ± 2.4b | 4 |
| α1βK127E | 36.4 ± 1.7 | 3 |
| α1K104EβK127E | 17.7 ± 1.2b | 4 |
a n is the number of patches.
b Significance after one-way ANOVA and Dunnett's post-hoc test or an unpaired t-test.
Asymmetrical Contribution of γ versus α or β Subunits on the Single-channel Conductance of GABAA Receptors
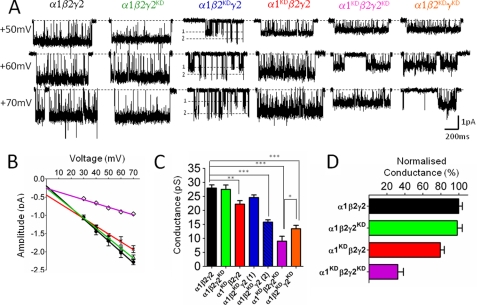
The α1β2γ2 GABAA receptor pentamer contains two copies each of the α and β subunit and one of the γ subunit (Ref. 22 and references therein): the 2:2:1 stoichiometry of this receptor makes it an ideal testing ground to examine whether each subunit type has an equivalent effect on channel conductance and how their contribution sums. As shown in the sequence alignment in Fig. 1E, in GABAA receptors, α, β, or γ subunits bear two positive charges at this site, as they contain two consecutive Lys residues at the position equivalent to Ala-96 and Asp-97 of α1 Torpedo (6). Because we do not know whether both of these residues are exposed to the open channel, we reversed charges at both of these positions, by mutating the two Lys residues to Asp in the α1, β2, and γ2 subunits. The traces and the I/V plot in Fig. 5, A and C show that charge reversal mutations only in the γ2 subunit (α1β2γ2KD) had no effect on the outward conductance of the GABAA channel (27.5 ± 1.5 pS, see Table 5). By contrast, charge reversal in either α1 (α1KDβ2γ2) or β2 (α1β2KDγ2) subunit caused detectable decreases in conductance. When the α1 subunit carried the mutation (α1KDβ2γ2), outward conductance decreased from its control value of 28.0 ± 1.1 pS in the wild-type channel, to 22.2 ± 1.2 pS for the mutant receptor (−20.8%, p < 0.01). When the β2 subunit was mutated, the resulting receptor, α1β2KDγ2 showed two distinct conductance levels (24.6 ± 0.9 and 15.8 ± 0.8 pS). This suggested that the influence of the α and β subunits on the single channel conductance of GABAA receptors is comparable in magnitude, but not identical.
FIGURE 5.
Charge reversal mutations in the extracellular domain affect outward conductance of the α1β2γ2 GABAA receptor. α1, β2, and γ2 subunits were expressed in HEK cells at a 1:1:4 ratio. A shows representative outward currents recorded in the cell-attached configuration at several voltages from wild-type and mutant receptors (display fc = 1 kHz). Each mutant subunit carries two charge reversal mutations (KK to DD) at homologous position to Asp-97 of the α1 Torpedo (see text). Conductance values are reported in Table 5. B, current-voltage plots for representative mutant and wild-type receptors. α1β2γ2 (black line, ●), α1KDβ2γ2 (red line, x), α1 β2γ2KD (green line, Δ), α1KDβ2γ2KD (violet line and ♢). C, conductance values for all subunit combination shown as bar graph. Introduction of charge reversal mutations at homologous positions in the α1, β2, or γ2 subunit decreases conductance in a subunit-specific manner. Asterisks denote the significance after a one-way ANOVA test followed by Dunnett's test. D, bar graph of outward conductance values normalized for their wild type. The γ2 subunit does not affect conductance when it is the only subunit carrying the charge reversal mutation. However, note the γ2 mutation decreases synergistically conductance in combination with mutations in either the α1 or the β2 subunit.
TABLE 5.
Outward slope conductance values for GABA α1β2γ2 receptor
Average values ± S.E. are in pS.
| Receptor type | Outward γ | n |
|---|---|---|
| α1β2γ2 | 28.0 ± 1.1 | 4 |
| α1K105/6Dβ2γ2 | 22.2 ± 1.2a | 5 |
| α1 β2 γ2K117/8D | 27.5 ± 1.5 | 4 |
| α1 β2K101/2Dγ2 | 24.6 ± 0.9 (cond 1) | 6 |
| 15.8 ± 0.8b(cond 2) | ||
| α1K105/6Dβ2 γ2K117/8D | 9.0 ± 1.7b | 3 |
| α1 β2K101/2D γ2K117/8D | 13.4 ± 1.2b | 3 |
a Significance after one-way ANOVA and a Dunnett's post test. p < 0.001.
b Significance after one-way ANOVA and a Dunnett's post test. p < 0.0001.
Next, we wanted to investigate whether, in the α1β2γ2 GABAA receptor, combining mutations in multiple subunits adds their effects on the channel conductance. We could not record single-channel currents from receptors bearing mutations in both the α1 and β2 subunits (α1KDβ2KDγ2), possibly because the conductance becomes very small. Despite the lack of effect of mutations in the γ2 subunit alone, combining α1 and γ2 mutated subunits (α1KDβ2γ2KD) produced a receptor with a conductance of 9.0 ± 1.7 pS, much smaller than that of the α1KDβ2γ2 combination (see Table 5) p < 0.001. Combining mutations in the β2 and γ2 subunits (α1β2KD γ2KD) yielded a receptor with a single conductance level of 13.4 ± 1.2 pS. It is worth noting that despite the fact that both α1KDβ2γ2KD and α1β2KD γ2KD contain the same number of charge reversal mutations, a slightly greater reduction in conductance is achieved with the former combination (p < 0.05). Thus, mutating γ2 clearly reduces conductance if other subunits are mutated, and this also confirms that in our expression system the γ subunit is reliably incorporated into the pentamer.
DISCUSSION
Here we show that charged residues in the extracellular domain of Gly and GABAA receptors have a strong effect on single-channel conductance, and that the effects depend on the direction of chloride flow and on the subunit carrying the mutation.
Several details of the mechanisms of ion permeation and selectivity in the nicotinic superfamily remain unclear, in part because of the lack of high-resolution structural data for the pore domain. Channel conductance is increased by charges strategically placed at the cytoplasmic portals formed by M3-M4 loops (5), in M2 (4) and in the extracellular vestibule (6). In nicotinic receptors, the negatively charged residues at positions −4′, 0′, and 20′ of M2 have been extensively investigated. At the intracellular and extracellular end of M2, the effect of each charge is roughly equivalent across subunits. The overall change in conductance produced when more than one subunit is mutated is proportional to the net change in the total charge in the pentamer at each position (4). The appealing regularity in this pattern would seem to suggest that the channel is roughly symmetrical in these positions and that the negative charges act simply by adding to the electrostatic potential in a relatively wide, water-filled part of the pore (∼20 Å in the Torpedo structure at 4 Å resolution (1). This would increase conductance by concentrating permeant cations, funnelling them to the narrowest point of the channel, which may correspond to the charges at position 0′ of M2. Nevertheless, a simple electrostatic model fails to account for other results, especially the fact that the effects of extracellular ring mutations are unaffected by changes in ionic strength (16). The extracellular conductance determinant is also thought to act by adding to the electrostatic surface potential of the vestibule. In computations of the surface potential of the Torpedo pore, this negatively charged residue corresponds to a negative peak as high as that produced by the M2 extracellular ring of charges (23). Brownian dynamics simulations suggest that the electrostatic effect to which these charges contribute increases the concentration of sodium ions in the extracellular vestibule to be about 4-fold that of the counterion chloride (24).
We do not have any structural information for anionic Cys-loop channels and the usefulness of homology models derived from the pore of cationic channels is limited by major sequence differences. The α subunits of anionic channels have a Pro residue at the intracellular end of M2 (−2′), which is absent in most of the non-α subunits and in all the cationic channels. This Pro may make the narrowest part of the pore narrower in anionic channels and force permeating ions to lose more of their solvation shells (25). In Gly channels, the M2 domain is the main conductance determinant, and it is the difference between the sequence of the α and the β subunit M2 domain that makes conductance lower in heteromers than in homomers (26). Other domains, such as the intracellular M3-M4 loop, are less important than in cationic channels (14).
We now show that, in line with the Torpedo results (6), the conductance of anionic channels is strongly affected by extracellular domain mutations. The most surprising finding of our study is that, in the α1β Gly receptor, mutation of the α1 subunit markedly affected conductance, whereas β subunit mutations, in isolation, had no effect. In muscle nicotinic receptors (αβαγδ) asymmetries in conductance effects are not uncommon when mutating residues in the narrowest portion of the pore, such as 2′ in M2 (27–29), and this may reflect asymmetry in the channel structure. However, in this early work, none of the mutated muscle nicotinic subunits completely failed to alter conductance, as we observed here for the β subunit in the α1βKE Gly receptor and for the γ2 subunit of GABAA receptors.
Examples of asymmetric effects of the different Gly and GABAA receptor subunits have been reported for phenomena other than conductance. For example, in the α1β Gly receptor activation by agonists is markedly affected by mutations in the α1 subunit, but not in the homologous β mutant subunits (30). In the GABAA receptor, asymmetric contribution of subunits has been reported for permeability (31), effect of gain-of-function mutations (L9′ and F6′) (32–34) and mutations that ablate pH modulation (35).
In addition to that, there are reports of threshold effects for conductance mutations. For instance, in muscle nicotinic receptors, no effect is observed if only the (two copies of the) α subunits are mutated in the intracellular domain (4, 5) or at the extracellular residue equivalent to the one we investigated here (6). The conductance of α1β Gly and α1β2γ2 GABAA receptors is more sensitive to charge reversal here than that of nicotinic receptors, and a decrease is detectable with two copies of the mutation. A threshold effect could explain why mutating the single copy of the γ subunit in GABAA receptors had no effect, but cannot account for the lack of effect of β mutations in glycine channels. There is disagreement in the literature on whether wild-type recombinant glycine channels incorporate two or three copies of β in the pentamer (10, 11, 18), but we have used tandem constructs to force channels to contain three copies of β. These tandem-expressed receptors do seem to contain two α (and therefore three β) subunits because they are less sensitive to mutating α alone (27.7% conductance decrease versus a 41% decrease) than unconstrained receptors, where we probably have three copies of α1. Even though three β subunits are present in tandem-expressed receptors, mutating them still has no effect on conductance. This suggests a marked asymmetry in the extracellular domain of the glycine receptor, where it would appear that this residue in loop 5 is exposed to the vestibule only for the α subunits. Given that this position is at the end of the loop A segment of the principal agonist binding domain, it does not seem so surprising that the mutation may be reporting a difference between the principal binding subunit and the complementary one (regardless of whether this is α or β) (11). Why this should be the case for glycine receptors, but not for GABAA receptors we cannot say. Probing the accessibility of these residues from the pore may yield useful clues for the conformation of the different subunits in these receptors.
Structural asymmetry in the vestibule across different subunit types cannot explain why mutating the “ineffective” subunits becomes detectable when the other subunits have also been mutated. Non-linear summation of effects can easily arise even in symmetrical channels because of the topology of subunit arrangement. For instance, the order of mutated subunits modulates the effect of mutations in the CNG tetramer (36): mutations in subunits on opposite sides of the pore have bigger effects on conductance than mutations of adjacent subunits, probably because having big residues on opposite sides narrows the pore to a greater extent. Another way in which non-linear summation of effects could occur is if a subunit interacts with its neighbors. Again you would expect a greater effect when mutating non-adjacent pairs of subunits than adjacent ones (as seen when mutating consecutive or non-consecutive binding sites (37)). We have reported non-linear summation for gain of function 9′ mutations in M2 of α3β4α5 neuronal nicotinic receptors (38), where mutating the α5 reduces the EC50 only if the other subunits are mutated, possibly because the mutation has more than one effect (e.g. on activation and desensitization), which result in opposite actions on the macroscopic experimental measurement.
It is hard to see why non-linear summation should occur on conductance here. It could be that mutating all the subunits around the pore causes the mutations to start interacting and no longer have a localized effect. Expecting summation would then be unrealistic. However, the channel vestibule is 30 Å wide at this level (in the Torpedo structure) and it is hard to see how the side chains could interact at this distance. Even if we revise this diameter (6) on the basis of the α1 muscle nicotinic structure (39) the diameter here is at least 20 Å, almost identical to that at the level of the extracellular ring of charges, where a relatively simple pattern of summation is generally seen.
Our work shows that the extracellular domain contributes to determine the rate of ion flow through anionic Cys-loop receptors. Single-channel conductance measurements have often been used as a tool to address the question of receptor stoichiometry and topology (36, 40–42). For this tool to be valid, it must be assumed that the contribution of each subunit to conductance changes sums in a regular, predictable pattern. Our results show that this is not always the case. The lack of equivalence across subunits for these mutations may report on the conformation of the nicotinic channels in the extracellular vestibule, at the back of the principal side of the binding site and as such warrants further investigation.
Acknowledgment
We thank Dr. Christopher Miller (Brandeis University, Waltham) for commenting on a draft of the manuscript.
This work was supported by the MRC (Programme Grant G0400869, to L. G. S.) and by a Nuffield Science Bursary (to C. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- GABA
- γ-aminobutyric acid
- GlyR
- glycine receptor.
REFERENCES
- 1. Unwin N. (2005) J. Mol. Biol. 346, 967–989 [DOI] [PubMed] [Google Scholar]
- 2. Hilf R. J., Dutzler R. (2009) Nature 457, 115–118 [DOI] [PubMed] [Google Scholar]
- 3. Bocquet N., Nury H., Baaden M., Le, Poupon C., Changeux J. P., Delarue M., Corringer P. J. (2009) Nature 457, 111–114 [DOI] [PubMed] [Google Scholar]
- 4. Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. (1988) Nature 335, 645–648 [DOI] [PubMed] [Google Scholar]
- 5. Kelley S. P., Dunlop J. I., Kirkness E. F., Lambert J. J., Peters J. A. (2003) Nature 424, 321–324 [DOI] [PubMed] [Google Scholar]
- 6. Hansen S. B., Wang H. L., Taylor P., Sine S. M. (2008) J. Biol. Chem. 283, 36066–36070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brams M., Gay E. A., Sáez J. C., Guskov A., van, Elk R., van der Schors R. C., Peigneur S., Tytgat J., Strelkov S. V., Smit A. B., Yakel J. L., Ulens C. (2011) J. Biol. Chem. 286, 4420–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Livesey M., Cooper M., Lambert J., Peters J. (2010) Proc. Br. Pharmacol. Soc. 7, 37P [Google Scholar]
- 9. Groot-Kormelink P. J., Beato M., Finotti C., Harvey R. J., Sivilotti L. G. (2002) J. Neurosci. Methods 113, 207–214 [DOI] [PubMed] [Google Scholar]
- 10. Burzomato V., Groot-Kormelink P. J., Sivilotti L. G., Beato M. (2003) Recept. Channels 9, 353–361 [DOI] [PubMed] [Google Scholar]
- 11. Grudzinska J., Schemm R., Haeger S., Nicke A., Schmalzing G., Betz H., Laube B. (2005) Neuron 45, 727–739 [DOI] [PubMed] [Google Scholar]
- 12. Bormann J., Hamill O. P., Sakmann B. (1987) J. Physiol. 385, 243–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mortensen M., Ebert B., Wafford K., Smart T. G. (2010) J. Physiol 588, 1251–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carland J. E., Cooper M. A., Sugiharto S., Jeong H. J., Lewis T. M., Barry P. H., Peters J. A., Lambert J. J., Moorhouse A. J. (2009) J. Biol. Chem. 284, 2023–2030 [DOI] [PubMed] [Google Scholar]
- 15. Konno T., Busch C., von Kitzing E., Imoto K., Wang F., Nakai J., Mishina M., Numa S., Sakmann B. (1991) Proc. Roy. Soc. Lond. 244, 69–79 [DOI] [PubMed] [Google Scholar]
- 16. Kienker P., Tomaselli G., Jurman M., Yellen G. (1994) Biophys. J. 66, 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langosch D., Thomas L., Betz H. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 7394–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhse J., Laube B., Magalei D., Betz H. (1993) Neuron 11, 1049–1056 [DOI] [PubMed] [Google Scholar]
- 19. Groot-Kormelink P. J., Broadbent S. D., Boorman J. P., Sivilotti L. G. (2004) J. Gen. Physiol. 123, 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou Y., Nelson M. E., Kuryatov A., Choi C., Cooper J., Lindstrom J. (2003) J. Neurosci. 23, 9004–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minier F., Sigel E. (2004) Trends Pharmacol. Sci. 25, 499–503 [DOI] [PubMed] [Google Scholar]
- 22. Sieghart W., Sperk G. (2002) Curr. Top. Med. Chem. 2, 795–816 [DOI] [PubMed] [Google Scholar]
- 23. Meltzer R. H., Lurtz M. M., Wensel T. G., Pedersen S. E. (2006) Biophys. J. 91, 1315–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song C., Corry B. (2009) Biochim. Biophys. Acta 1788, 1466–1473 [DOI] [PubMed] [Google Scholar]
- 25. Keramidas A., Moorhouse A. J., Schofield P. R., Barry P. H. (2004) Prog. Biophys. Mol. Biol. 86, 161–204 [DOI] [PubMed] [Google Scholar]
- 26. Bormann J., Rundström N., Betz H., Langosch D. (1993) EMBO J. 12, 3729–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imoto K., Konno T., Nakai J., Wang F., Mishina M., Numa S. (1991) FEBS Lett. 289, 193–200 [DOI] [PubMed] [Google Scholar]
- 28. Villarroel A., Herlitze S., Koenen M., Sakmann B. (1991) Proc. Roy. Soc. Lond. B 243, 69–74 [DOI] [PubMed] [Google Scholar]
- 29. Villarroel A., Herlitze S., Koenen M., Sakmann B. (1992) Proc. Roy. Soc. Lond. B 249, 317–324 [DOI] [PubMed] [Google Scholar]
- 30. Shan Q., Nevin S. T., Haddrill J. L., Lynch J. W. (2003) J. Neurochem. 86, 498–507 [DOI] [PubMed] [Google Scholar]
- 31. Jensen M. L., Timmermann D. B., Johansen T. H., Schousboe A., Varming T., Ahring P. K. (2002) J. Biol. Chem. 277, 41438–41447 [DOI] [PubMed] [Google Scholar]
- 32. Chang Y., Wang R., Barot S., Weiss D. S. (1996) J. Neurosci. 16, 5415–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalziel J. E., Cox G. B., Gage P. W., Birnir B. (2000) Mol. Pharmacol. 57, 875–882 [PubMed] [Google Scholar]
- 34. Gonzales E. B., Bell-Horner C. L., Dibas M. I., Huang R. Q., Dillon G. H. (2008) Neurosci. Lett. 431, 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilkins M. E., Hosie A. M., Smart T. G. (2002) J. Neurosci. 22, 5328–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu D. T., Tibbs G. R., Siegelbaum S. A. (1996) Neuron 16, 983–990 [DOI] [PubMed] [Google Scholar]
- 37. Rayes D., De Rosa M. J., Sine S. M., Bouzat C. (2009) J. Neurosci. 29, 6022–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Groot-Kormelink P. J., Boorman J. P., Sivilotti L. G. (2001) Br. J. Pharmacol. 134, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dellisanti C. D., Yao Y., Stroud J. C., Wang Z. Z., Chen L. (2007) Nat. Neurosci. 10, 953–962 [DOI] [PubMed] [Google Scholar]
- 40. Cooper E., Couturier S., Ballivet M. (1991) Nature 350, 235–238 [DOI] [PubMed] [Google Scholar]
- 41. Béhé P., Stern P., Wyllie D. J. A., Nassar M., Schoepfer R., Colquhoun D. (1995) Proc. Roy. Soc. Lond. B 262, 205–213 [DOI] [PubMed] [Google Scholar]
- 42. Premkumar L. S., Auerbach A. (1997) J. Gen. Physiol. 110, 485–502 [DOI] [PMC free article] [PubMed] [Google Scholar]