Abstract
Human umbilical cord blood-derived stromal cells (hUCBDSCs), a novel population isolated from CD34+ cells by our laboratory, exerted an immunosuppressive effect on xenogenic T cells. This study aimed to investigate whether hUCBDSCs play a critical role in the suppression of acute graft-versus-host disease (aGVHD). The hUCBDSCs were co-cultured with splenocytes (SPCs) of donor C57BL/6 mice. The aGVHD in the recipient (B6×BALB/c) F1 mice was induced by the infusion of bone marrow cells and SPCs from donor mice following sublethal irradiation. The shift in vivo for hUCBDSCs was detected. The proliferation and cell cycle of SPCs were tested by cell counting kit-8 and flow cytometry, respectively. The expression of CD49b natural killer (NK) cells and CD3 T cells was detected by flow cytometry in co-culture and post-transplantation. IL-4, and IFN-γ were detected by ELISA in the serum of co-culture and post-transplantation. The survival time, body weight, clinical score, and histopathological score were recorded for mice post-transplantation. The hUCBDSCs promoted the proliferation of SPCs and significantly increased the ratio of the S and G2/M phase (p < 0.05). The hUCBDSCs significantly increased the expression of CD49b NK cells and IL-4 protein and decreased the expression of CD3 T cells and IFN-γ protein both in vitro and in vivo. The survival time of mice with co-transplantation of hUCBDSCs was significantly prolonged, and decreased clinical and histopathological scores were also observed. The hUCBDSCs were continually detected in the target organs of GVHD. These results suggest that hUCBDSCs possess the capability of suppressing aGVHD, possibly via their influence on CD3 T cells, NK cells, and cytokines.
Keywords: Bone Marrow, Cell-Cell Interaction, Cellular Regulation, Immunology, Stromal Cell, Graft-versus-host Disease, Haploidentical Stem Cell Transplantation
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT)3 constitutes an effective therapy for hematopoietic disorders (1–3). The haploidentical/mismatch-related HSCT has been limited by the high risk of severe graft-versus-host disease (GVHD), graft rejection, and life-threatening infections (4). Alloreactive donor T cells, which recognize MHC-mismatched host cells and primarily target the skin, gut, and liver, play a crucial role in the induction of GVHD (5). Although conventional immunosuppressive drugs and/or T cell depletion of the graft represent cardinal therapeutic approaches to prevent and treat GVHD, 30–70% of recipients suffer from GVHD after HSCT despite the application of immunosuppressive drugs (6). Despite the decreased incidence of GVHD, T cell depletion has been associated with an increased incidence of graft failure, tumor relapse, and opportunistic infections (7). Natural killer (NK) cells, a major effector cell of innate immunity, are known to display strong cytolytic activity against tumor or virally infected cells (8). Alloreactive NK could eliminate leukemia relapse and graft rejection and protect recipients against GVHD, providing a powerful tool for enhancing the efficacy and safety of allogeneic HSCT (9–11). The pretransplant infusion of alloreactive NK cells obviated the need for high intensity conditioning and reduced GVHD.
Cellular therapy for GVHD and graft-versus-leukemia has attracted much attention. Mesenchymal stromal cells (MSCs), which exist in bone marrow, exhibit the greatest potential for such therapy. It has been demonstrated that human, baboon, and murine MSCs not only fail to elicit allogeneic or xenogeneic T cell response, but are also able to exert an immunosuppressive effect on T cell activation and proliferation stimulated by alloantigen, mitogen, and CD3/CD28 antibody in vitro (12). In accordance with these in vitro studies, the administration of MSCs has been found to be efficacious in protecting against GVHD in several animal models and clinical trials (13–16).
We previously isolated a novel population of adherent fibroblast-like cells from human umbilical cord blood CD34+ cells (hUCBDSCs) and confirmed that hUCBDSCs possess immunomodulatory effects on T cells in vitro across the MHC species barrier that are identical to MSCs (17, 18). The aim of the present study was to clarify whether the administration of hUCBDSCs decreases the incidence and severity of aGVHD in mice haploidentical stem cell transplantation and the possible mechanism of this effect.
EXPERIMENTAL PROCEDURES
Mice
Male C57BL/6 (H-2b, donor) mice and female (B6×BALB/c) F1 (H-2b/d, recipient) mice were purchased from the Experimental Animals Center at the Third Military Medical University (qualified certification number: CQA 0101015# and 0103017#, Chongqing, China). All mice were used at 8–10 weeks of age and housed under standard conditions. The experimental protocol used in this study was approved by the Animal Care Committee of the University and was in agreement with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Isolation and Culture of hUCBDSCs
Full-term hUCB samples were harvested with the informed consent of mothers. This study was approved by the local ethical committee. Cell culture was performed according to our previous report (19). Briefly, the mononuclear cell fractions were separated on Percoll density-gradient fractionation columns (density = 1.077 g/liter; Pharmacia Biotech) after depleting red blood cells through 6% gelatin sedimentation. The CD34+ cells, separated from mononuclear cells using the magnetic cell-sorting system (Miltenyi Biotec, Bergisch Gladbach) were cultured using standard Dexter complete medium (i.e. DMEM (Invitrogen) supplemented with 12.5% fetal bovine serum (Hyclone), 12.5% horse serum (Invitrogen), 10–6 m/liter hydrocortisone, 10 ng/ml SCF (Sigma), and 1 ng/ml basic fibroblast growth factor (Sigma)). The culture medium was replaced 48 h later, and thereafter, half of the amount was changed weekly. The cells were passaged at a ratio of 1:2 after growing to 80% confluence.
Isolation and Culture of Human Bone Marrow Stromal Cells (hBMSCs)
The bone marrow was taken from healthy volunteers, and then the mononuclear cell fractions were separated as above and cultured using DMEM. The culture medium was replaced 48 h later and, thereafter, half of the amount was changed weekly. The cells were passaged at a ratio of 1:2 after growing to 80% confluence.
Preparation of Mice Bone Marrow Cells and Splenocytes
The mice bone marrow cells (BMCs) were flushed from both the femora and tibiae with RPMI 1640 medium. The suspension was passed through a 40-μm filter to remove clumps and washed twice by centrifugation at 1000 rpm for 5 min in PBS. The BMCs were then resuspended in RPMI 1640 medium.
The spleens were aseptically obtained from a C57BL/6 (H-2b) donor mouse. The spleen single-cell suspension was prepared by gently grinding and filtering the spleens through nylon mesh (40-μm-diameter pores). Following the lysis of red blood cells with Tris-NH4Cl solution and two washes with PBS, the SPCs were resuspended in RPMI 1640 medium and cultured for 24 h. The nonadherent cells were then obtained for the next experiment.
Proliferative Assays
1 × 105 nonadherent SPCs from the C57BL/6 mouse were seeded in triplicate onto 96-well plates in 200 μl of complete RPMI 1640 medium. The following five groups were performed: simple culture with SPCs alone (control group), the last concentration 2 μg/liter PHA with SPCs (PHA group), 1 × 106 hUCBDSCs/well with SPCs, 1 × 106 hUCBDSCs with SPCs in the presence of rhIL-2 (1000 units/ml) and 1 × 106 hUCBDSCs with SPCs in the presence of the supper serum of cultured hUCBDSCs. Seven days later, cell proliferation was determined using cell counting kit-8 (CCK-8; Dojindo, Japan). Briefly, the cultured SPCs were collected and seeded onto 96-well plates in 200 μl of complete RPMI 1640 medium. Subsequently, 20 μl of CCK-8 solution was added to each well, which was then incubated for another 4 h. A values were tested at a wavelength of 450 nm using a microplate reader. For each group, three plates were made, and three experiments were conducted.
Cell Cycle
The SPC were cultured with or without hUCBDSCs for 7 days. The SPCs were then collected and stained with propidium iodide (Sigma). Nuclei were analyzed with flow cytometry (FACScalibur; BD Biosciences). The percentage of cells at each phase of the cell cycle was estimated from their DNA content histograms.
Cell Co-culture
Nonadherent SPCs from the male C57BL/6 mice (1 × 105/well) were plated on 12-well plates in the absence or presence of hUCBDSCs and hBMSCs (1 × 106/well). Seven days later, nonadherent cells were collected for the determination of CD3 T cells and CD49b NK cells using flow cytometry. The supper serum was collected for the determination of IFN-γ and IL-4 using an enzyme-linked immunosorbent assay (ELISA).
Mouse Haploidentical HSCT and Evaluation of aGVHD
Recipient (B6×BALB/c) F1 mice were irradiated with a single dose of 7.0 Gy using a 60Co γ-ray source at a dose rate of ∼0.5 Gy/min. Subsequently, 1 × 106 BMCs and 1 × 106 SPCs from donor C57BL/6 mice with or without 1 × 106 hUCBDSCs were injected intravenously into each recipient mouse within 4 h after conditioning. Nongrafted irradiated mice served as controls. Each group included 20 mice. Survival was monitored daily, and the body weights and appearance of the mice were measured weekly. The degrees of clinical aGVHD were assessed using a scoring system (20). A clinical index was obtained by adding the scores of five parameters: weight loss, posture, activity, fur texture, and skin integrity. Each parameter was graded from 0 to 2, and the maximum index was 10.
For histological examination, the mice were sacrificed on day 30. Tissue samples from the skin, liver, and small intestine were harvested, fixed in 4% formaldehyde solution, embedded in paraffin, and stained with H&E. The intensity of aGVHD was blindly assessed. The aGVHD histopathological scoring was conducted according to previous reports (21). The severity of aGVHD for the liver and small intestine was graded from 0 to 4. Skin was scored on a scale of 0 to 3. The final aGVHD histopathological score was the sum of these three parameters.
Flow Cytometry
The monoclonal antibodies phycoerythrin anti-mouse CD3 and PE anti-mouse CD49b were purchased from eBioscience. Mice were either sacrificed on day 7, 14, and 21 after transplantation.
The expression of CD3 and CD49b in the spleen of recipients was tested by flow cytometry. Briefly, the splenocytes were generated according to the above-mentioned method. The splenocytes were then incubated at 4 °C for 30 min with antibodies against CD3 and CD49b. Equal aliquots of cells were labeled with isotype monoclonal antibodies to determine nonspecific reaction. Finally, cells were washed and assayed using flow cytometry.
ELISA Assay
Mice were sacrificed on day 7, 14, or 21 after transplantation, and the serum was collected. IFN-γ and IL-4 were detected by ELISA according to the manufacturer's protocol. For each group, this detection was performed three times.
Tracking of hUCBDSCs
The cultured hUCBDSCs were washed with PBS. Subsequently, 1 × 106 hUCBDSCs were incubated for 5 min at 37 °C and then for an additional 15 min at 4 °C with 1 μl CM-DiI dye. The cells were washed with PBS and suspended in fresh medium with 1 × 107/ml.
As described above, 1 × 107 labeling hUCBDSCs were infused into the haploidentical transplantation mice. Cryostat slides were prepared from the skin, liver, intestine, kidney, spleen, and lung of the recipients on day 3, 7, 14, and 21 after transplantation. The fluorescence photos were taken immediately.
Analysis of Chimerism
Four weeks after transplantation, the peripheral blood cells were analyzed to determine the number of recipient- or donor-type cells by flow cytometry. Briefly, the peripheral blood was incubated with phycoerythrin-labeled anti-mouse H-2d and fluoresceine isothiocyanate-conjugated anti-2b mAb, followed by hemolysis using BD PharM Lyse (BD Biosciences). The stained cells were analyzed using FACScan (BD Biosciences). The percent donor chimerism is defined as donor/(donor + host) × 100%.
Statistical Analysis
The results are expressed as mean ± S.E. Statistical evaluation was performed using Student's t-tests with SPSS software (version 10.0). The survival curve was derived using a Kaplan-Meier analysis, and the survival rate and mortality were analyzed using a log-rank test. A significance level of p < 0.05 was used.
RESULTS
Growth Characteristics of hUCBDSCs
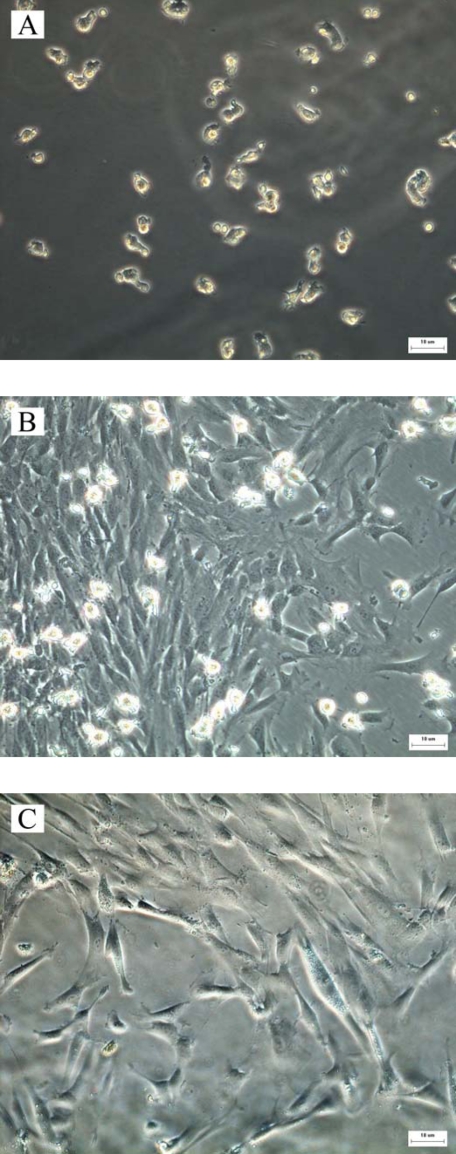
The cultured cells appeared as small fusiform, triangular, or spherical cells in the early phase. As the culture time increased, these cells gradually became larger. At 8–12 days, the stromal cell colonies began to emerge, and the maximum of colonies was achieved at 14–17 days (Fig. 1A). At 20–28 days, the stromal cells increased significantly and filled the base of the Petri dish. Using an inverted microscope, it was observed that the hUCBDSCs were differentiated into three types: fibroblast-like cells (56–60%), macrophage-like cells (34–36%), and small, sphere-like cells (3–5%; Fig. 1B). After subculture, the cell morphology tended to be uniform. Fibroblast-like cells were the most frequent type, whereas the number of macrophage-like cells and small-sphere-like cells was greatly decreased (Fig. 1C).
FIGURE 1.
Growth characteristics of hUCBDSCs. A, cultured hUCBDSCs after 3 days. The cells appeared as small fusiform, triangular, or spherical. B, primary cultured hUCBDSCs after 28 days. The cell appeared as fibroblast-like, macrophage-like, and small sphere-like. C, subcultured hUCBDSCs with the type of fibroblast-like cells. Each experiment was performed three times. Scale bars, 10 μm.
Proliferation Assay of SPCs
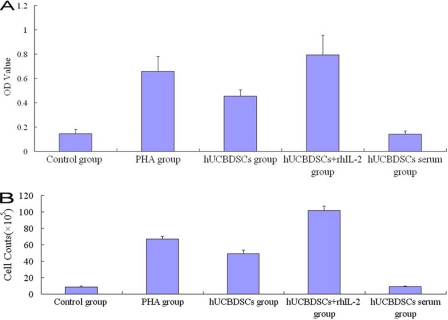
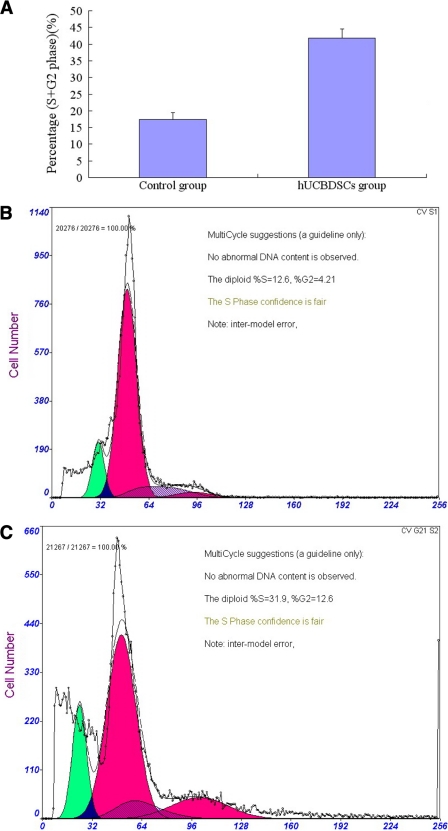
The hUCBDSCs promoted the proliferation of SPCs; there are differences between the PHA-positive group and hUCBDSCs group (p < 0.05), and their role for hUCBDSCs was much stronger. When they were combined with recombinant human IL-2 (p < 0.05); however, the role of proliferation was not detected by the culture supernatants of hUCBDSCs (Fig. 2A). The trypan blue staining indicated that the cell viability was 97% ± 2.1%. From the calibration curve, we calculated that the cell numbers were about 8-, 6-, and 12-fold for the PHA group, hUCBDSCs group, and hUCBDSCs + rhIL-2 group, respectively (Fig. 2B). We further examined the cell cycle of SPCs by flow cytometry. The hUCBDSCs significantly increased the ratio of the S phase and G2/M phase of the SPCs co-cultured for 7 days (p < 0.05; Fig. 3).
FIGURE 2.
Proliferation assays. The hUCBDSCs were co-cultured with mice splenocytes for 7 days; the nonadherent splenocytes were collected for the proliferation assays with CCK-8. The hUCBDSCs promoted the proliferation of SPCs; there are differences between the PHA-positive group and hUCBDSC group (p < 0.05), and their role for hUCBDSCs was much stronger if they were combined with recombinant human IL-2 (p < 0.05). However, proliferation was not detected in the culture supernatants of hUCBDSCs. A, A values for different groups. B, cell numbers after culture for different groups. Each experiment was performed three times.
FIGURE 3.
Cell cycle assays. After co-culturing hUCBDSCs with SPCs for 7 days, the cell cycles of hUCBDSCs were detected. The hUCBDSCs significantly increased the ratio of the S phase and G2/M phase of SPCs relative to the simple cultured SPCs (p < 0.05). A, percentage of cells in the S phase and G2/M phase. B, flow cytometry of the cell cycle of SPCs in the control group. C, flow cytometry of the cell cycle of SPCs in the hUCBDSCs group. Each experiment was performed three times.
Expression of CD3 T Cells and CD49b NK Cells and Cytokines in in Vitro Co-culture
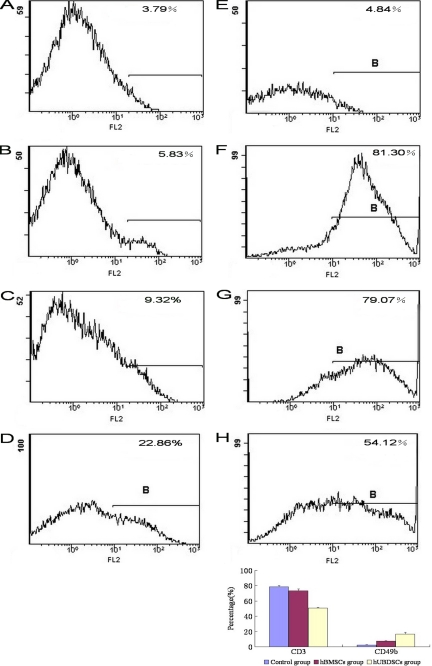
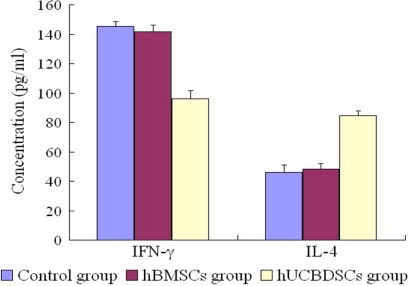
The hUCBDSCs and hBMSCs increased the expression of CD49b NK cells and decreased the expression of CD3 T cells. The hUCBDSCs were much stronger than the hBMSCs in CD49b NK-cell and CD3 T-cell expression (p < 0.05; Fig. 4). It is much stronger in promoting the expression of IL-4 and decreasing the expression of IFN-γ (p < 0.05; Fig. 5).
FIGURE 4.
Regulation of CD49b NK cell and CD3 T cell expression by hUCBDSCs. After co-culturing hUCBDSCs and hBMSCs with SPCs for 7 days, the SPCs were collected. The expression of CD49b NK cells and CD3 T cells was then examined by flow cytometry. The hUCBDSCs and hBMSCs increased the expression of CD49b NK cells and decreased the expression of CD3 T cells; however, hUCBDSCs were much stronger than hBMSCs in CD49b NK cell and CD3 T cell expression (p < 0.05). A, isotype control of CD49 NK cells. B, expression of CD49 NK cells in control group. C, expression of CD49 NK cells in hBMSC group. D, expression of CD49 NK cells in hUCBDSC group. E, isotype control of CD3 T cells. F, expression of CD3 T cells in control group. G, expression of CD3 T cells in hBMSC group. H, expression of CD3 T cells in hUCBDSC group. Each experiment was performed three times.
FIGURE 5.
Regulation of hUCBDSCs on the expression of the cytokines of IL-4 and IFN-γ in vitro. After co-culturing hUCBDSCs and hBMSCs with SPCs for 7 days, the co-cultured serum was collected; the expression of IL-4 and IFN-γ was then examined by ELISA. It is much stronger in promoting the expression of IL-4 and decreasing the expression of IFN-γ in hUCBDSCs than in hBMSCs (p < 0.05). Each experiment was performed three times.
hUCBDSCs Suppressed aGVHD in Mice HSCT
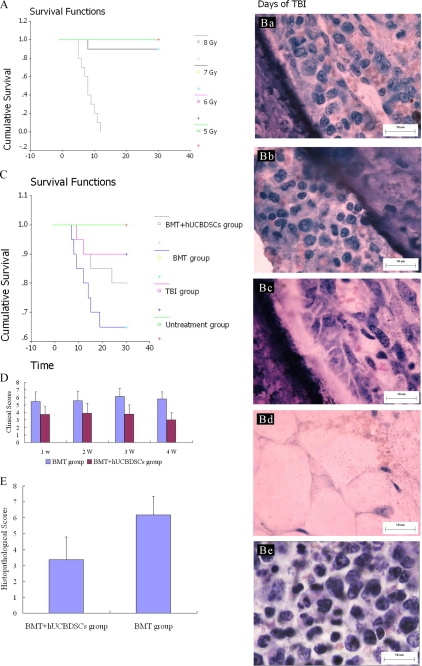
First, we irradiated recipient (B6×BALB/c) F1 mice with a 5.0, 6.0, 7.0, and 8.0 Gy 60Co γ-ray source, respectively. All of the mice that received 8.0 Gy died within 14 days; all of the mice that received 5.0 Gy, 6.0 Gy 60Co γ-ray lived more than 30 days, and 1 of the 10 mice that experienced 7.0 Gy 60Co γ-ray died. The living other mice that experienced 7.0 Gy 60Co γ-ray lived more than 30 days (Fig. 6A). Furthermore, we observed a change of bone marrow after 7 days of irradiation. The hematopoietic tissues were extremely rare in the mice that received 8.0 Gy 60Co γ-ray. Although the hematopoietic tissues decreased in other mice, this decrease was not obvious, and all of the mice experienced hematopoietic recovery after 14 days. However, the hematopoietic tissues in the mice that received 7.0 Gy were the smallest relative to the mice that received 5.0, 6.0, and 7.0 Gy 60Co γ-ray (Fig. 6B). Therefore, we selected the 7.0 Gy 60Co γ-ray as the sublethal irradiation dose.
FIGURE 6.
Survival rate of mice and the change of bone marrow with varying 60Co γ-ray irradiation and the survival rate of mice and the aGVHD clinical and histopathological scores after transplantation. A, Kaplan-Meier curve for mice that received varying doses of 60Co γ-ray irradiation. All of the mice that received 8.0 Gy died within 14 days; all of the mice that experienced 5.0 Gy and 6.0 Gy 60Co γ-ray irradiation lived more than 30 days, and 1 of 10 mice died after receiving 7.0 Gy 60Co γ-ray. The other living mice receiving 7.0 Gy 60Co γ-ray lived more than 30 days. B, bone marrow biopsies of mice that experienced varying doses of 60Co γ-ray irradiation (a, 5.0 Gy; b, 6.0 Gy; c, 7.0 Gy; d, 8.0 Gy; e, normal mice). The hematopoietic tissues were extremely rare in the mice that received 8.0 Gy 60Co γ-ray irradiation. Although the hematopoietic tissues decreased in other mice, this decrease was not obvious. All of the mice experienced hematopoietic recovery after 14 days. However, the hematopoietic tissues in the mice that received 7.0 Gy were the least relative to the mice that received 5.0, 6.0, and 7.0 Gy 60Co γ-ray irradiation. Scale bars, 10 μm C, Kaplan-Meier curve for mice after transplantation. All of the mice from untreated group survived more than 30 days. 2 of 20 mice died within 30 days for total body irradiation group. 7 of the 20 mice in the BMC group died within 30 days, and 4 mice died in BMT + hUCBDSCs group. All the mice from the BMT group developed moderate to severe GVHD clinical signs such as weight loss, hunched posture, and ruffled fur, and 80% of the mice survived for 30 days. However, the percentage of survival in the BMT + hUCBDSCs group was significantly increased (23%) compared with BMT group (p < 0.05). D, clinical scores after transplantation. The GVHD clinical scores of BMT + hUCBDSCs group were significantly lower than those of BMT group on days 7, 14, 21, and 28 after transplantation (p < 0.05). E, histopathological score after transplantation. The histopathological score in BMT + hUCBDSCs group was significantly lower than that in BMT group (p < 0.05). 20 mice were used in each group, and 3 mice were used at each time point.
In this study, there were four groups: group A, the normal mice; group B, irradiated without treatment; group C, irradiated and treated with BMCs and SPCs; and group D, irradiated and treated with BMCs, SPCs, and hUCBDSCs. All of the mice in group A survived more than 30 days. 2 of the 20 mice in group B died within 30 days. 7 of the 20 mice in group C died within 30 days, and 4 mice died in group D. All the mice in group C developed moderate to severe aGVHD clinical signs such as weight loss, hunched posture, and ruffled fur, and 80% of the mice survived for 30 days. However, the percentage of survival in group D was significantly higher (23%) compared with group C (p < 0.05; Fig. 6C). The aGVHD clinical scores of group D were also significantly lower than those of group C on days 7, 14, 21 and 28 after transplantation (p < 0.05; Fig. 6D). On day 28 after transplantation, three mice in each group were humanely sacrificed to observe lymphocytic infiltration and cell damage in aGVHD target organs such as the skin, liver, and small intestine. Meanwhile, the aGVHD histopathological score was evaluated as previously described. The aGVHD histopathological score in group D was significantly lower than that in group C (p < 0.05), indicating that the infusion of hUCBDSCS may decrease the severity of aGVHD in mice subjected to haploidentical stem cell transplantation (Fig. 6E).
Donor Chimerism
The proportion of chimerism was 74.95 ± 3.20% in the BMT + hUCBDSCs group, which was higher than that of the BMT group (64.27 ± 3.68%) at 28 days post-transplantation (p < 0.05).
Effect of hUCBDSCs in Vivo
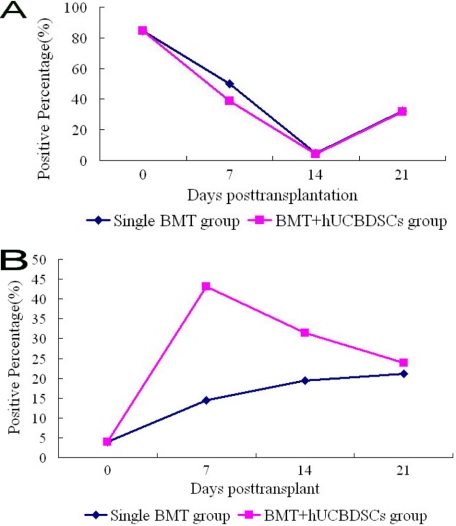
We tested the expression of CD49b NK and CD3 T cells from the spleen by flow cytometry and the IL-4 and IFN-γ in the serum of mice by ELISA at the indicated time points after haploidentical stem cell transplantation. The CD3 T cells were decreased in both groups and reached the lowest point on day 14, after which they gradually increased; however, the level of decrease was much lower in the BMT + hUCBDSCs group than in the simple BMT group (Fig. 7A). The NK cells were increased in both groups and reached their highest point on day 7, after which their expression gradually decreased in the BMT + hUCBDSCs group but continually increased in the BMT group. However, the level of NK cells was higher in the BMT + hUCBDSCs group than in the simple BMT group throughout the experiment (Fig. 7B).
FIGURE 7.
Expression of CD49b NK and CD3 T cells in the spleen after haploidentical stem cell transplantation. The results showed that the hUCBDSCs group significantly increased the expression of CD49b NK cells and decreased the expression of CD3 T cells (p < 0.05). A, expression of CD3 T cells after transplantation. B, expression of CD49b NK cells after transplantation. Three mice were used at each time point.
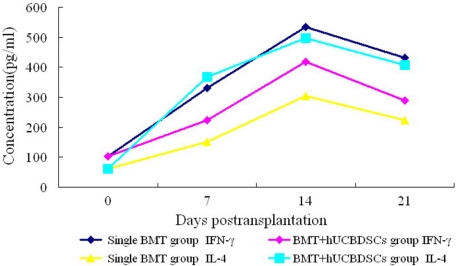
The expression of IL-4 and IFN-γ continually increased in both groups and reached its highest point on day 14, after which their expression decreased. However, the increase of IL-4 was much higher in the BMT + hUCBDSCs group than in the simple BMT group, and the increase of IFN-γ was much lower in the BMT + hUCBDSCs group than in the simple BMT group (p < 0.05; Fig. 8).
FIGURE 8.
Expression of IL-4 and IFN-γ in the haploidentical stem cell transplantation mice. The results showed that the hUCBDSCs group significantly promoted the expression of IL-4 and inhibited IFN-γ in blood serum (p < 0.05). Three mice were used at each time point.
Tracking of hUCBDSCs
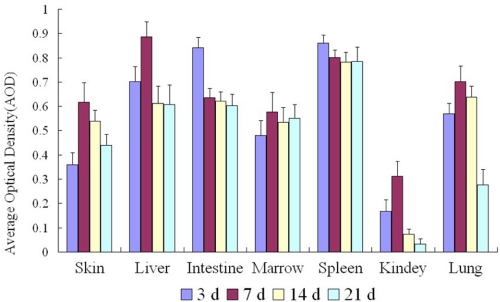
The hUCBDSCs marked with CM-DiI shifted to all tissues/organs in the early phase in mouse haploidentical hematopoietic stem cell transplantation and reached the highest point in the spleen and intestine on day 3 and in the bone marrow, skin, liver, lung, and kidney at day 7. A slight decrease was then observed in the bone marrow, skin, liver, spleen, and intestine, but a high level of hUCBDSCs was continually observed. However, a significant decrease was observed in the kidney and lung (Fig. 9).
FIGURE 9.
Movement of hUCBDSCs marked with CM-DiI in the haploidentical stem cell transplantation mice. The hUCBDSCs shifted to all tissues/organs in the early phase in mouse haploidentical HSCT. Continued expression was detected in the bone marrow, spleen, and target organ of GVHD, but little expression was observed in other organs. Three mice were used at each time point.
DISCUSSION
GVHD is the immune response of donor T lymphocytes responding to the recipient's alloantigens. Cellular and cytokine mechanisms primarily drive GVHD. Selective T cell depletion was used to prevent GVHD without causing immune deficiency. Other cells in the graft, such as NK cells, regulatory T cells, and MSCs, can also retain immune capability without causing GVHD (22). During the elimination or suppression of GVHD, it is very important to preserve GVL, which contributes to the cure of hematological malignancies in allogeneic HSCT.
The hBMSCs are well studied with regard to their immunomodulatory function in vitro and in vivo. Previous studies have shown that hBMSCs possess the capability of suppressing human T cell proliferation in vitro that is induced by alloantigens, mitogens, and CD3 and CD28 antibodies (23, 24). To exploit the immunomodulatory function of hBMSCs, culture-expanded hBMSCs have been experimentally infused into patients to prevent GVHD. The patient was completely cured after repeated administration of MSCs derived from his mother (13). Subsequently, a multicenter clinical trial demonstrated the effectiveness of hBMSCs infusion in the treatment of GVHD (25).
However, bone marrow collection is an invasive procedure, and the number and expansion capacity of bone marrow MSCs decline with age. Furthermore, the underlying mechanisms of hBMSCs immunosuppression in vivo remain unclear. Because hUCB has many advantages, such as its simple and safe procurement and its high frequency of immature stem and progenitor populations, it is the most attractive source of MSCs. A novel population of adherent spindle-shaped fibroblastoid cells from CD34+ cells in the hUCB, called hUCBDSCs, has been successfully isolated and cultured in vitro by our laboratory (17). A previous study confirmed the hematopoiesis-supporting properties of hUCBDSCs in vitro (17). We also found that hUCBDSCs exerted similar immunomodulatory effects on xenogeneic T cells and allogeneic T cells in vitro (18).
In the present study, we found that hUCBDSCs promoted the proliferation of SPCs. However, another study showed that MSCs suppressed the proliferation of lymphocytes (26). The reason for this discrepancy may be that the other study was performed using purified T lymphocytes, whereas we utilized whole spleen lymphocytes. Many studies have shown that the role of T suppression of MSCs may play through the secreted cytokines. In the current study, we did not observe this mechanism for hUCBDSCs. We also detected the cell cycle of co-cultured SPCs. The results showed that the S+G2/M phase was much higher in hUCBDSCs than in the simple cultured group, which further supported our proliferation assays. Many studies have shown that MSCs suppress the proliferation of T and B lymphocytes and enhance the expression of NK (27, 28). The change of the co-cultured SPC was explored in the current study. Our study showed that the hUCBDSCs significantly decreased the expression of T lymphocytes and promoted the expression of NK cells. This effect was more significant than that of hBMSCs, indicating that hUCBDSCs may be more effective in immunoregulation. Cytokines, which are currently being explored as a method of enhancing GVL while minimizing the risk of GVHD, have the ability to enhance certain cellular subsets, such as regulatory T cells, after transplantation (29). IL-4 decreased GVHD and IFN-γ increased GVHD. We also examined the expression of cytokines in vitro. Our study showed that hUCBDSCs significantly decreased the expression of IFN-γ and promoted the expression of IL-4.
Co-infusion of MSCs reduced the incidence of graft and enhanced the numbers and activity of NK cells (30, 31). Interestingly, a reduced incidence of aGVHD has also been observed in HLA-identical HSCT when NK recovery was rapid after HSCT (32). Our results in vitro showed that hUCBDSCs may suppress GVHD and preserve GVL. We further explored the function of hUCBDSCs in haploidentical stem cell transplantation in sublethally irradiated mice. Our study showed that hUCBDSCs significantly decreased the mortality and prolonged the survival time of mice. The degree of aGVHD was much lower in the hUCBDSC group than in the groups without hUCBDSCs. The hUCBDSCs significantly deleted the expression of T lymphocytes and promoted the expression of NK cells. The hUCBDSCs inhibited the expression of IFN-γ and promoted the expression of IL-4.
After being infused into experimental animals, MSCs improve the outcome of renal, neural, and lung injury and prolong survival of skin allografts (33). MSCs may be immunosuppressive in vivo as they reverse severe steroid-resistant acute GVHD of the gut and liver (13). Our study in vitro and in vivo showed that hUCBDSCs suppress GVHD and preserve GVL. The movement of hUCBDSCs is unclear in haploidentical transplantation. We further tracked the movement of hUCBDSCs by labeling them with CM-DiI. The continued expression of hUCBDSCs was detected in the bone marrow, spleen, and target organ of GVHD, which further testifies to the role of hUCBDSCs in the regulation of GVHD.
In conclusion, the results of previous studies have been controversial with regard to the prophylactic and treatment effect of MSCs on aGVHD. The cell type, cell dose, animal model, administration timing, and evaluation system might account for these differences. Our results showed that hUCBDSCs decrease the incidence and severity of aGVHD in mice haploidentical stem cell transplantation. This effect may be due to the promotion of the proliferation of mice SPCs via the suppression of T lymphocytes and the consequent enhancement of NK cells and IFN-γ and IL-4 expression. The function of enhanced NK in vitro and in vivo requires in-depth research.
This work was supported by National Natural Science Foundation Grant 30971109, Natural Science Foundation Project of Chong Qing Chongqing Science and Technology Commission 2009BA5011, and the Innovation Foundation for Young Scientists of Third Military Medical University Grant 2009D226.
- HSCT
- hematopoietic stem cell transplantation
- aGVDH
- acute GVDH
- BMC
- bone marrow cell
- BMT
- bone marrow transplant
- GVDH
- graft-versus-host disease
- Gy
- gray
- hBMSC
- human bone marrow stromal cell
- hUCBDSC
- human umbilical cord-derived stromal cell
- MSC
- mesenchymal stromal cell
- NK
- natural killer
- PHA
- phytohemagglutinin
- SPC
- splenocyte.
REFERENCES
- 1. Chen X. H., Gao L., Zhang X., Gao L., Zhang C., Kong P. Y., Liu H., Peng X. G., Sun A. H., Qi D. G., Gong Y., Wang Q. Y. (2009) Blood Cells Mol. Dis. 43, 98–104 [DOI] [PubMed] [Google Scholar]
- 2. Koh L. P., Chao N. J. (2008) Blood Cells Mol. Dis. 40, 20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang C., Chen X. H., Zhang X., Gao L., Gao L., Kong P. Y., Peng X. G., Sun A. H., Gong Y., Zeng D. F., Wang Q. Y. (2010) Transfus. Med. 20, 169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X. H., Zhang C., Zhang X., Gao L., Gao L., Kong P. Y., Peng X. G., Qi D. G., Sun A. H., Zeng D. F., Liu H., Gong Y., Wang Q. Y. (2009) Biol. Blood Marrow Transplant. 15, 266–273 [DOI] [PubMed] [Google Scholar]
- 5. Toubai T., Sun Y., Reddy P. (2008) Best Pract. Res. Clin. Haematol. 21, 101–117 [DOI] [PubMed] [Google Scholar]
- 6. Nash R. A., Antin J. H., Karanes C., Fay J. W., Avalos B. R., Yeager A. M., Przepiorka D., Davies S., Petersen F. B., Bartels P., Buell D., Fitzsimmons W., Anasetti C., Storb R., Ratanatharathorn V. (2000) Blood 96, 2062–2068 [PubMed] [Google Scholar]
- 7. Zeiser R., Marks R., Bertz H., Finke J. (2004) Ann. Hematol. 83, 551–565 [DOI] [PubMed] [Google Scholar]
- 8. Ruggeri L., Mancusi A., Burchielli E., Capanni M., Carotti A., Aloisi T., Aversa F., Martelli M. F., Velardi A. (2008) Blood Cells Mol. Dis. 40, 84–90 [DOI] [PubMed] [Google Scholar]
- 9. Ruggeri L., Mancusi A., Burchielli E., Perruccio K., Aversa F., Martelli M. F., Velardi A. (2006) Semin. Cancer Biol. 16, 404–411 [DOI] [PubMed] [Google Scholar]
- 10. Schulze A., Schirutschke H., Oelschlägel U., Schmitz M., Füssel M., Wassmuth R., Ehninger G., Bornhäuser M., Platzbecker U. (2008) Exp. Hematol. 36, 378–389 [DOI] [PubMed] [Google Scholar]
- 11. Tanaka J., Sugita J., Asanuma S., Arita K., Shono Y., Kikutchi M., Shiratori S., Wakasa K., Yasumoto A., Shigematu A., Kondo T., Kobayashi T., Asaka M., Imamura M. (2009) Hum. Immunol. 70, 701–705 [DOI] [PubMed] [Google Scholar]
- 12. Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A., Santarlasci V., Mazzinghi B., Pizzolo G., Vinante F., Romagnani P., Maggi E., Romagnani S., Annunziato F. (2006) Stem Cells 24, 386–398 [DOI] [PubMed] [Google Scholar]
- 13. Ringdén O., Uzunel M., Rasmusson I., Remberger M., Sundberg B., Lönnies H., Marschall H. U., Dlugosz A., Szakos A., Hassan Z., Omazic B., Aschan J., Barkholt L., Le Blanc K. (2006) Transplantation 81, 1390–1397 [DOI] [PubMed] [Google Scholar]
- 14. Itakura S., Asari S., Rawson J., Ito T., Todorov I., Liu C. P., Sasaki N., Kandeel F., Mullen Y. (2007) Am. J. Transplant. 7, 336–346 [DOI] [PubMed] [Google Scholar]
- 15. Tisato V., Naresh K., Girdlestone J., Navarrete C., Dazzi F. (2007) Leukemia 21, 1992–1999 [DOI] [PubMed] [Google Scholar]
- 16. Min C. K., Kim B. G., Park G., Cho B., Oh I. H. (2007) Bone Marrow Transplant. 39, 637–645 [DOI] [PubMed] [Google Scholar]
- 17. Gao L., Chen X., Zhang X., Liu Y., Kong P., Peng X., Liu L., Liu H., Zeng D. (2006) Blood Cells Mol. Dis. 36, 322–328 [DOI] [PubMed] [Google Scholar]
- 18. Hao L., Zhang C., Chen X. H., Zou Z. M., Zhang X., Kong P. Y., Liang X., Gao L., Peng X. G., Sun A. H., Wang Q. Y. (2009) Croat. Med. J. 50, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang C., Chen X. H., Zhang X., Gao L., Kong P. Y., Liu H., Liang X., Peng X. G., Wang Q. Y. (2008) Zhongguo Shi Yan Xue Ye Xue Za Zhi 16, 1437–1441 [PubMed] [Google Scholar]
- 20. Cooke K. R., Kobzik L., Martin T. R., Brewer J., Delmonte J., Jr., Crawford J. M., Ferrara J. L. (1996) Blood 88, 3230–3239 [PubMed] [Google Scholar]
- 21. Grass J. A., Wafa T., Reames A., Wages D., Corash L., Ferrara J. L., Lin L. (1999) Blood 93, 3140–3147 [PubMed] [Google Scholar]
- 22. Cao D., Hu L., Wang Y., Wang L., Zheng W., Ma W. (2009) Transpl. Immunol. 20, 243–248 [DOI] [PubMed] [Google Scholar]
- 23. Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P. D., Matteucci P., Grisanti S., Gianni A. M. (2002) Blood 99, 3838–3843 [DOI] [PubMed] [Google Scholar]
- 24. Tse W. T., Pendleton J. D., Beyer W. M., Egalka M. C., Guinan E. C. (2003) Transplantation 75, 389–397 [DOI] [PubMed] [Google Scholar]
- 25. Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M. E., Remberger M., Dini G., Egeler R. M., Bacigalupo A., Fibbe W., Ringdén O. (2008) Lancet 371, 1579–1586 [DOI] [PubMed] [Google Scholar]
- 26. Rasmusson I., Ringdén O., Sundberg B., Le Blanc K. (2003) Transplantation 76, 1208–1213 [DOI] [PubMed] [Google Scholar]
- 27. Spaggiari G. M., Capobianco A., Becchetti S., Mingari M. C., Moretta L. (2006) Blood 107, 1484–1490 [DOI] [PubMed] [Google Scholar]
- 28. Boissel L., Tuncer H. H., Betancur M., Wolfberg A., Klingemann H. (2008) Biol. Blood Marrow Transplant. 14, 1031–1038 [DOI] [PubMed] [Google Scholar]
- 29. Alyea E. P. (2008) Best Pract. Res. Clin. Haematol. 21, 239–250 [DOI] [PubMed] [Google Scholar]
- 30. Ball L. M., Bernardo M. E., Roelofs H., Lankester A., Cometa A., Egeler R. M., Locatelli F., Fibbe W. E. (2007) Blood 110, 2764–2767 [DOI] [PubMed] [Google Scholar]
- 31. Ho V. T., Cutler C. (2008) Best Pract. Res. Clin. Haematol. 21, 223–237 [DOI] [PubMed] [Google Scholar]
- 32. Savani B. N., Mielke S., Adams S., Uribe M., Rezvani K., Yong A. S., Zeilah J., Kurlander R., Srinivasan R., Childs R., Hensel N., Barrett A. J. (2007) Leukemia 21, 2145–2152 [DOI] [PubMed] [Google Scholar]
- 33. Barrett A. J., Le Blanc K. (2008) Best Pract. Res. Clin. Haematol. 21, 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]