Abstract
This study examined the role of the Gαq signal constituted by Gαq and Gα11 (encoded by Gnαq and Gnα11, respectively), a major intracellular pathway of parathyroid hormone (PTH), in the PTH osteoanabolic action by the gain- and loss-of-function analyses. Transgenic mice with osteoblast-specific overexpression of the constitutively active Gnαq gene under the control of 2.3-kb type I collagen α1 chain (Col1a1) promoter exhibited osteopenia with decreased bone formation parameters and did not respond to the daily PTH treatment. We then established osteoblast-specific Gnαq and Gnα11 double-knock-out (cDKO) mice by crossing the 2.3-kb Col1a1 promoter-Cre recombinase transgenic mice and those with Gnαq gene flanked with loxP and global ablation of Gnα11 (Col1a1-Cre+/−;Gnaqfl/fl;Gna11−/−) and found that the cDKO and single knock-out littermates of Gnαq or Gnα11 exhibited normal bone volume and turnover under physiological conditions. With a daily injection of PTH, however, the cDKO mice, but not the single knock-out mice, showed higher bone volume and turnover than the wild-type littermates. Cultures of primary osteoblasts derived from cDKO and wild-type littermates confirmed enhancement of the PTH osteoanabolic action by the Gαq signal deficiency in a cell-autonomous mechanism, in association with the membrane translocation of protein kinase Cδ. This enhancement was reproduced by overexpression of regulator of G protein signaling-2, a Gαq signal inhibitor, in osteoblastic MC3T3-E1 cells. Hence, the Gαq signal plays an inhibitory role in the PTH osteoanabolic action, suggesting that its suppression may lead to a novel treatment in combination with PTH against osteoporosis.
Keywords: Bone, G Protein-coupled Receptors (GPCRs), G Proteins, Mouse, Protein Kinase C (PKC), Osteoblast, Osteoporosis, Parathyroid Hormone, Regulator of G Protein Signaling-2
Introduction
Bone anabolic action of parathyroid hormone (PTH)2 has attracted considerable clinical attention, and led to the approval of the recombinant human PTH (1–34) for osteoporosis treatment worldwide (1, 2). PTH is known to bind to the type 1 PTH/PTH-related peptide (PTHrP) receptor (PTH1R), a class II G protein-coupled receptor superfamily member (3, 4), in osteoblasts and causes activation of the two major signal pathways via the G protein α subunits Gαs and Gαq (encoded by Gnαs and Gnαq, respectively) (5). Although Gαs leads to activation of adenylyl cyclase and an increase of intracellular levels of cAMP, Gαq activates phospholipase C which results in activation of protein kinase C (PKC) and an increase of intracellular Ca2+ ([Ca2+]i) concentration (6). Accumulated evidence of the Gnαs gene mutations in human bone disorders like fibrous dysplasia, McCune-Albright syndrome, and Albright hereditary osteodystrophy indicates the osteoanabolic role of the Gαs signal (7, 8). This was supported by a study on mice with osteoblast-specific Gnαs deficiency showing an impairment of bone formation (9, 10). Transgenic mice overexpressing constitutively active Pth1r via the Gαs signal in osteoblasts exhibit increased trabecular bone formation (11), and several studies also support the crucial role of the Gαs signal in the PTH osteoanabolic action thorough stimulation of osteoblast differentiation (12). Contrarily, little has been known about the role of the Gαq signal in bone metabolism. Hence, the present study sought to clarify the involvement of the Gαq signal in the PTH osteoanabolic action by examining the effects of gain- and loss-of-functions of the signal in osteoblasts.
Recently, we created transgenic mice with osteoblast-specific overexpression of the constitutively active Gnαq gene under the control of the 2.3-kb type I collagen α1 chain (Col1a1) promoter (Col1a1-Gnaq-tg mice) and found that the mice exhibited osteopenia in trabecular and cortical bones, indicating an inhibitory function of the Gαq signal in bone formation under physiological conditions without PTH stimulation (13). However, mice with global deletion of Gnαq showed normal bone phenotype while exhibiting cerebellar ataxia and platelet dysfunction (14). This may be due to redundant functionality of Gαq and Gα11 (encoded by Gnα11), a Gαq subfamily member that also activates phospholipase C and may compensate for the Gαq insufficiency (15). Although global Gna11 deficient mice showed no abnormality in any organs (16), the global Gnaq and Gna11 double-knock-out mice were embryonically lethal due to cardiomyocyte hypoplasia (17). Hence, to avoid compensation by Gα11 solely in bone, we established Gnαq and Gnα11 double-knock-out mice with osteoblast-specific ablation of Gnαq and global ablation of Gnα11 for the loss-of-function analysis of the Gαq signal.
Upon activation of G protein-coupled receptor, there are several proteins that modulate the G protein-mediated signals including regulator of G protein signaling (RGS) (18–20). Among >20 RGS family members, RGS2 is known to be a selective inhibitor of the Gαq signal via Gαq and Gα11 (21, 22). Hence, we also examined the effects of gain- and loss-of-functions of RGS2 on the PTH osteoanabolic action.
EXPERIMENTAL PROCEDURES
Mice
The transgenic mice overexpressing the constitutively active Gnaq gene or Cre gene under the control of 2.3-kb Col1a1 promoter (Col1a1-Gnaq-tg and Col1a1-Cre+/−, respectively) were generated as previously described (13, 23). The osteoblast-specific Gnαq and Gnα11 double-knock-out (cDKO) mice were generated by crossing the 2.3-kb Col1a1 promoter-Cre mice above (Col1a1-Cre+/−) and those with Gnαq gene flanked with loxP (Gnaqfl/fl) and global ablation of Gnα11 (Gnα11−/−) (24). Intercrosses between Col1a1-Cre+/−;Gnaq+/fl;Gna11+/− mice resulted in the generation of osteoblast-specific cDKO (Col1a1-Cre+/−;Gnaqfl/fl;Gna11−/−) and wild-type (Col1a1-Cre−/−;Gnaqfl/fl;Gna11+/+) as well as single-knock-out mice of either Gnαq (Col1a1-Cre+/−;Gnαqfl/fl;Gnα11+/−) or Gnα11 (Col1a1-Cre+/−;Gnαq+/fl;Gnα11−/−). Genotyping for Gnaq and Gna11 alleles was performed as described previously (17). Mice were on a mixed genetic background with a predominant contribution of the C57BL6/N strain, and male littermates were compared in each experiment. For the PTH treatment, mice received either 80 μg of recombinant rat PTH (1–34) (Sigma-Aldrich) per kg of body weight or the vehicle (PBS; Sigma-Aldrich) by subcutaneous injection five times/week for 4 weeks beginning at 8 weeks of age. All experiments were performed according to the protocol approved by the Animal Care and Use Committee of the University of Tokyo.
Radiological Analyses
Plain radiographs were taken using a soft x-ray apparatus (CMB-2; SOFTEX), and the bone mineral density (BMD) was measured by dual energy x-ray absorptiometry using a bone mineral analyzer (PIXImus Densitometer; GE Medical Systems). Computed tomographic scanning of the femurs was performed using a composite x-ray analyzer (NX-CP-C80H-IL; Nittetsu ELEX Co.) and reconstructed into a three-dimensional feature by the volume-rending method (VIP-Station; Teijin System Technology). Trabecular density was measured using a peripheral quantitative computed tomography (pQCT) analyzer (XCT Research SA+; Stratec Medizintecnik GmbH) at the metaphysis 1.4 mm above the distal growth plate of femurs.
Histological Analyses
For toluidine blue staining, samples were fixed with 70% ethanol, embedded in methyl methacrylate, and sectioned in 5-μm slices. Histomorphometric analyses were performed as described in an area 1.2 mm long from 0.5 mm below the growth plate of the proximal tibias (25), according to the ASBMR nomenclature report (26). For double labeling of the mineralization front, mice were injected subcutaneously with 16 mg/kg body weight of calcein at 5 days and 1 day before sacrifice.
Cell Cultures
For primary osteoblasts, calvariae of neonatal WT and cDKO littermates were digested by 0.1% collagenase and 0.2% dispase five times, and cells were isolated by the last three digestions were combined as an osteoblast population. Mouse osteoblastic MC3T3-E1 cells were purchased from RIKEN Cell Bank (Tsukuba, Japan). Cells were cultured in α-minimal essential medium (α-MEM; Invitrogen) containing 10% FBS (HyClone Laboratories, Inc., Logan, UT). In osteoblast differentiation experiments, medium was supplemented with 50 μg/ml ascorbic acid and 10 mm β-glycerophosphate (Sigma-Aldrich). For the intermittent treatment with PTH or phorbol 12-myristate 13-acetate (PMA), the cells were exposed to the agent for the first 6 h in each incubation cycle of 48 h and then cultured in its absence during the remainder of the cycle (12).
Gene Transfection
To construct the adenovirus expressing green fluorescent protein (GFP) (Ax-GFP) and Rgs2 (Ax-Rgs2), cDNA encoding GFP and Rgs2 was excised and subcloned into an adenoviral vector using an AdenoX Expression System (Takara Bio, Shiga, Japan), according to the manufacturer's instructions. Adenoviruses were amplified in HEK293 cells and purified with an AdenoX Virus Purification Kit (Takara Bio). Ax-GFP and AX-Rgs2 were transfected at 100 multiplicities of infection for 24 h to MC3T3-E1 cells.
Alkaline Phosphatase (ALP) Activity and Staining
For ALP activity measurement, primary osteoblasts or MC3T3-E1 cells adenovirally transfected with GFP or Rgs2 were cultured in the medium above with or without 10 nm recombinant rat PTH (1–34) and 100 nm PMA (Cell Signaling Technology, Beverly, MA). After six cycles (12 days) of intermittent treatment with PTH or PMA as described above, the cells were sonicated in 10 mm Tris-HCl buffer (pH 8.0) containing 1 mm MgCl2 and 0.5% Triton X-100, and ALP activity in the lysate was measured using an ALP kit (Wako Pure Chemical Industry, Ltd., Osaka, Japan). The protein content was determined using BCA protein assay reagent (Pierce). For ALP staining, cells were fixed in 70% ethanol and stained for 10 min with a solution containing 0.01% naphthol AS-MX phosphate, 1% N,N-dimethyl formamide, and 0.06% fast blue BB (Sigma-Aldrich).
Real-time Quantitative RT-PCR
For measurement of Col1a1 and osteocalcin (encoded by Bglap) mRNA, primary osteoblasts from WT and cDKO mice or MC3T3-E1 cells adenovirally transfected with GFP or Rgs2 underwent six cycles (12 days) of intermittent treatment with 10 nm PTH or the vehicle as described above. For measuring Rgs2 mRNA, MC3T3-E1 cells were precultured in α-MEM/10% FBS and further cultured in the presence of 10 nm PTH for the indicated times. Total RNA was extracted with ISOGEN (Wako Pure Chemical), and an aliquot (1 μg) was reverse-transcribed using a PrimeScript RT reagent kit (Takara Bio). For RT-PCR, real-time PCR was performed on an ABI Prism 7000 Sequence Detection System (ABI) using QuantiTect SYBR Green PCR Master Mix (Qiagen, Tokyo), according to the manufacturer's instructions. All reactions were run in triplicate. A set of primers was designed using sequences obtained from the GenBank as follows: 5′-ACGTCCTGGTGAAGTTGGTC-3′ and 5′-CAGGGAAGCCTCTTTCTCCT-3′ for Col1a1 mRNA (U08020.1), 5′-AAGCAGGAGGGCAATAAGGT-3′ and 5′-TTTGTAGGCGGTCTTCAAGC-3′ for Bglap mRNA (NM007541.1), and 5′-CCGAGTTCTGTGAAGAAAACATTG-3′ and 5′-GGGACTCCTGGTCTCATGTAGCAT-3′ for Rgs2 mRNA (NP002914.1).
Immunoblotting and Immunoprecipitation
For the protein expressions of Gαq and Gα11 in tissues, liver, heart, kidney, whole brain, femurs, and tibias from neonatal mice were homogenized in T-PER (Pierce Chemical) on ice using a 200-μl microtissue grinder (Wheaton, Millville, NJ). For long bones, as much surrounding soft tissue was removed as possible, and bones were immediately frozen in liquid nitrogen. Homogenates (25 μg of protein) were then subjected to SDS-PAGE. For the protein expression of PKCs, primary osteoblasts were treated with 10 nm PTH or vehicle for 30 min. The lysates of the whole cells and the cell membrane were prepared using an M-PER kit and a Mem-PER kit (Pierce Chemical) and were subjected to SDS-PAGE. Immunoblotting was performed using antibodies to common epitopes of Gαq and Gα11, Gαs, RGS2, PKCβI, PKCδ, PKCϵ, PKCη (a dilution of 1:1000; Cell Signaling Technology), and actin (1:1,000; Sigma-Aldrich), followed with horseradish peroxidase-conjugated goat anti-mouse IgG and goat anti-rabbit IgG (a dilution of 1:10,000; Amersham Biosciences). For immunoprecipitation, the whole cell lysates (500 μg of total protein) prepared using the M-PER kit were incubated with 2 μg of antibody to Gαs or the Gαq/Gα11 above using a Catch and Release kit (Upstate Biotechnology).
Measurements of Intracellular cAMP and [Ca2+]i
For the measurement of intracellular cAMP, the transfected cells were precultured in serum-free α-MEM containing 0.1% BSA and 1 mm 3-isobutyl-1-methylxanthine (Sigma-Aldrich) for 10 min and further cultured in the presence of 10 nm PTH at 37 °C for 15 min. The amount of cAMP in the cell extracts was determined by cAMP Biotrak EIA System (Amersham Biosciences). For the measurement of [Ca2+]i, transfected MC3T3-E1 cells were loaded with 4 μm fura-2 AM (Invitrogen) in physiological salt solution containing 150 mm NaCl, 4 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 5.6 mm glucose, and 10 mm HEPES and were cultured in the presence of 10 nm PTH for 5 min. The excitation wavelengths of 345 nm and 380 nm were acquired using an inverted microscope (IX70; Olympus, Tokyo) equipped with a cooled charge-coupled device camera (Rolera XR, Qimaging, Canada). Image analysis was carried out using IPLab software (BD Biosciences Bioimaging, Rockville, MD).
Statistical Analyses
Means of groups were compared by ANOVA. Significance of differences was determined by post hoc testing with Bonferroni's method.
RESULTS
Effect of Osteoblast-specific Gnαq Overexpression on Osteoanabolic Action of PTH
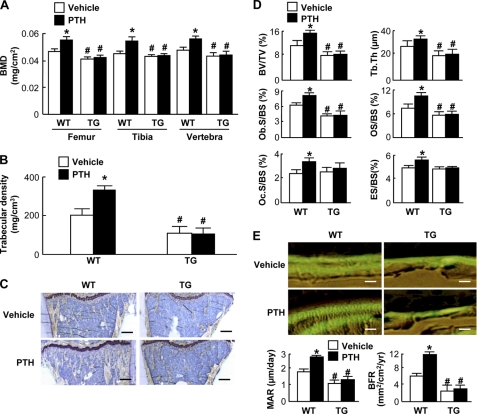
To know the effect of the gain-of-function of Gαq signal on the osteoanabolic action of PTH, we initially used transgenic mice with overexpression of the constitutively active Gnαq gene under the control of 2.3-kb type I collagen α1 chain promoter (Col1a1-Gnaq-tg). As we reported previously (13), the Col1a1-Gnaq-tg mice under the vehicle treatment exhibited radiographic and histologic osteopenia with decreases in static and dynamic histomorphometric parameters for bone formation compared with the wild-type littermates (Fig. 1). In wild-type mice, the daily injection of PTH for 4 weeks caused an increase in bone volume with enhancement of bone formation and resorption parameters compared with the vehicle treatment, consistent with previous reports by others and us (27–29). However, in transgenic mice, there were no significant differences between the vehicle and PTH treatment groups in bone volume or histomorphometric parameters, indicating that the gain-of-function of the Gαq signal in osteoblasts cancels the PTH osteoanabolic action.
FIGURE 1.
Effects of daily PTH injection (80 μg/kg × 5 times × 4 weeks) on bones of 8-week-old Col1a1-Gnaq-tg (TG) and their wild-type (WT) littermates. A, BMD determined by dual energy x-ray absorptiometry of the entire femurs, tibias, and L2–L5 vertebrae. B, trabecular density determined by pQCT of the distal metaphysis of the femurs. C, toluidine blue staining of proximal tibias (scale bars, 200 μm). D, static histomorphometric parameters in the proximal tibias. BV/TV, trabecular bone volume as a percentage of total volume; Tb.Th, trabecular thickness; Ob.S/BS, percentage of the bone surface covered by cuboidal osteoblasts; OS/BS, percentage of the bone surface covered with osteoid; Oc.S/BS, percentage of bone surface covered by mature osteoclasts; ES/BS, percentage of eroded surface. E, top, double labelings with calcein of trabecular bones in the proximal tibias imaged by fluorescent microscopy (scale bars, 20 μm). E, bottom, dynamic histomorphometric parameters based on the double labelings above. MAR, mineral apposition rate; BFR, bone formation rate. Data in all graphs (A, B, D, and E) are expressed as mean (bars) ± S.E. (error bars) for 4 mice/group. *, p < 0.01 versus vehicle; #, p < 0.01 versus WT.
Effect of Osteoblast-specific Gnαq and Gnα11 Double-knock-out on Osteoanabolic Action of PTH
To explore further the role of endogenous Gαq signal in bone, we established conditional Gnαq and Gnα11 double-knock-out (cDKO: Col1a1-Cre+/−;Gnaqfl/fl;Gna11−/−) mice with osteoblast-specific ablation of Gnαq and global ablation of Gnα11 to avoid the compensation by the redundant functionality of Gαq and Gα11. Initially, we looked at expression patterns of Gαq and Gα11 proteins in various tissues of mice of four genotypes depending on deficiency of Gnαq and Gnα11 genes: Col1a1-Cre−/−;Gnαqfl/fl;Gnα11+/+ (wild-type), Col1a1-Cre+/−;Gnαqfl/fl;Gnα11+/−, Col1a1-Cre+/−;Gnαq+/fl;Gnα11−/−, and Col1a1-Cre+/−;Gnαqfl/fl;Gnα11−/− (cDKO). The cDKO mice were confirmed to show a specific defect of both Gαq and Gα11 protein expressions in bone (Fig. 2A). These mice developed and grew normally without abnormality in major organs (Fig. 2B). In addition, there was no significant difference of bone shape or bone volume of the cDKO mice compared with the wild-type littermates or single-knock-out littermates of either Gnαq (Col1a1-Cre+/−;Gnαqfl/fl;Gnα11+/−) or Gnα11 (Col1a1-Cre+/−;Gnαq+/fl;Gnα11−/−) under physiological conditions (Fig. 2C and supplemental Fig. 1).
FIGURE 2.
Phenotypes of Col1a1-Cre+/−;Gnaqfl/fl;Gna11−/− (cDKO) mice under physiological conditions. A, expression patterns of Gαq and Gα11 proteins by Western blotting using an antibody to common epitopes of Gαq and Gα11 (α-Gαq/Gα11) in several tissues of neonatal mice of four genotypes depending on deficiency of Gnαq and Gnα11 genes: Col1a1-Cre−/−;Gnαqfl/fl;Gnα11+/+ (WT), Col1a1-Cre+/−;Gnαqfl/fl;Gnα11+/−, Col1a1-Cre+/−;Gnαq+/fl;Gnα11−/−, and Col1a1-Cre+/−;Gnαqfl/fl;Gnα11−/− (cDKO). B, body weight (left) and tibial length (right) of the WT and cDKO littermates at the indicated ages. Data are expressed as means (symbols) ± S.E. (error bars) for 4 or 5 mice/group per time. There were no significant differences between genotypes at any time points. C, BMD determined by dual energy x-ray absorptiometry of the entire tibias of mice of four genotypes above at 4 and 8 weeks of age. Data are expressed as mean (bars) ± S.E. (error bars) for 3–5 mice/group per time. There were no significant differences among genotypes.
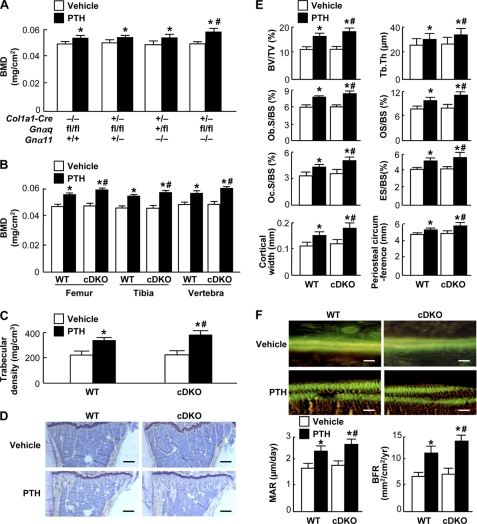
To know the effect of loss-of-function of the Gαq signal on the PTH osteoanabolic action, we administered daily PTH injections to mice of the four genotypes and compared the bone volume and turnover. Under the vehicle treatment, there was no significant difference among genotypes in BMD (Fig. 3, A and B), trabecular density (Fig. 3C), or histomorphometric parameters for bone formation and resorption in either trabecular or cortical bones (Fig. 3, D–F). Here again, the daily PTH treatment was confirmed to cause increases in bone volume, bone formation, and resorption parameters in the wild-type mice. These effects of PTH on bone volume and turnover were more greatly increased in the cDKO mice than in the wild-type littermates, although not in single-knock-out mice of either Gnαq or Gnα11. These indicate that the loss-of-function of the Gαq signal in osteoblasts enhances the osteoanabolic actions of PTH.
FIGURE 3.
Effects of daily PTH injection (80 μg/kg × 5 times × 4 weeks) on bones of 8-week-old Col1a1-Cre+/−;Gnαqfl/fl;Gnα11−/− (cDKO) and the wild-type (WT: Col1a1-Cre−/−;Gnαqfl/fl;Gnα11+/+) littermates. A, BMD determined by dual energy x-ray absorptiometry of the entire tibias of mice of four genotypes: WT, two single-knock-out mice (Col1a1-Cre+/−;Gnαqfl/fl;Gnα11+/− and Col1a1-Cre+/−;Gnαq+/fl;Gnα11−/−), and cDKO. B, BMD of the entire femurs, tibias, and L2–L5 vertebrae. C, trabecular density determined by pQCT of the distal metaphysis of the femurs. D, toluidine blue staining of proximal tibias (scale bars, 200 μm). E, static histomorphometric parameters in the proximal tibias as shown in Fig. 1D, and cortical bone parameters (two bottom graphs). F, top, double labelings with calcein of trabecular bones in the proximal tibias imaged by fluorescent microscopy (scale bars, 20 μm). F, bottom, dynamic histomorphometric parameters based on the double labelings above as shown in Fig. 1E. Data in all graphs (A, B, C, E, and F) are expressed as mean (bars) ± S.E. (error bars) for 4 or 5 mice/group. *, p < 0.01 versus vehicle; #, p < 0.01 versus WT with PTH treatment.
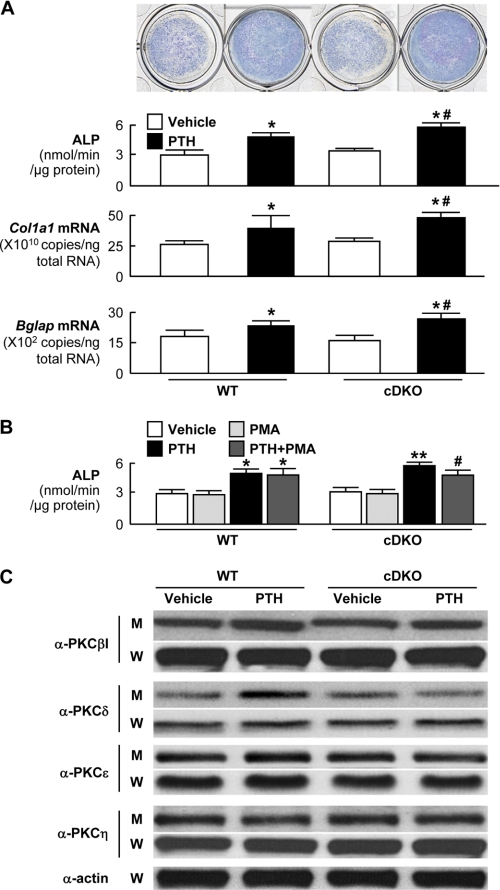
We then compared cultures of primary calvarial osteoblasts derived from cDKO mice with those from wild-type littermates. Intermittent treatment of PTH stimulated differentiation and matrix synthesis of cultured osteoblasts determined by ALP activity and mRNA levels of osteogenesis markers such as Col1a1 and Bglap, and the PTH effects were enhanced in the cultures of cDKO osteoblasts compared with the wild-type cultures, indicating that loss-of-function effect of the Gαq signal on the PTH osteoanabolic action was cell-autonomous in osteoblasts (Fig. 4A). The enhancement of the PTH effect in the cDKO cells was inhibited by addition of PMA, a PKC activator, suggesting that PKC normally retards the PTH effects (Fig. 4B).
FIGURE 4.
Primary cultures of osteoblasts from neonatal calvariae of Col1a1-Cre+/−;Gnαqfl/fl;Gnα11−/− (cDKO) and the wild-type (WT: Col1a1-Cre−/−;Gnαqfl/fl;Gnα11+/+) littermates. A, ALP staining and activity (upper two rows) and mRNA levels of Col1a1 and osteocalcin (encoded by Bglap) (lower two rows) after six cycles (12 days) of intermittent treatment with and without 10 nm PTH. *, p < 0.01 versus vehicle; #, p < 0.01 versus WT with PTH treatment. B, ALP activity after the intermittent treatment above with and without PTH or PMA. *, p < 0.01 versus WT with vehicle; **, p < 0.01 versus WT with PTH; #, p < 0.05 versus cDKO with PTH alone. C, protein levels of PKC isoforms determined by immunoblotting using respective antibodies (α-PKCs) in the membrane extracts (M) and whole cell lysates (W) of primary osteoblasts from two genotypes cultured with and without PTH for 30 min. Data in all graphs (A and B) are expressed as mean (bars) ± S.E. (error bars) for 5 cultures/group.
Hence, we looked at protein levels of PKC isoforms that are known to be expressed in osteoblasts: PKCβI, PKCδ, PKCϵ, and PKCη (30), in primary osteoblasts from the two genotypes cultured with and without PTH. Neither PTH treatment nor the Gαq signal deficiency altered any PKC isoform levels in the whole cell lysates, suggesting that there was no regulation of the PKC isozyme expressions. In the membrane extracts of wild-type osteoblasts, however, only PKCδ level was increased by PTH treatment, and this stimulation was suppressed in the cDKO osteoblasts, implicating that membrane translocation of PKCδ by PTH may be associated with the Gαq signal (Fig. 4C). Taken together, these in vivo and ex vivo results demonstrate the inhibitory role of the Gαq signal in osteoblasts, possibly in association with the PKCδ membrane translocation, in the osteoanabolic action of PTH.
Involvement of RGS2 in the PTH Osteoanabolic Action
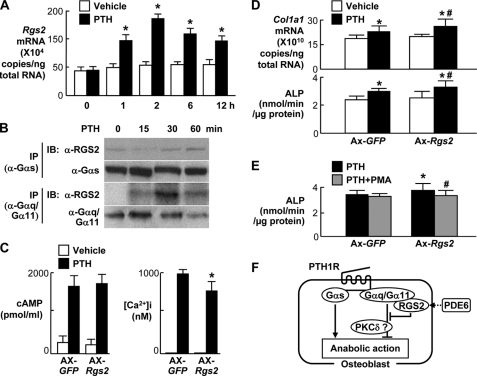
We next examined the involvement of RGS2, a putative inhibitor of the Gαq signal (21, 22), in the PTH osteoanabolic action. The Rgs2 expression was increased in response to PTH treatment in cultured osteoblastic MC3T3-E1 cells (Fig. 5A). Immunoprecipitation/immunoblotting analyses confirmed the physical interaction of RGS2 with Gαq/Gα11 protein, but not with Gαs protein, after the PTH treatment (Fig. 5B). Adenoviral overexpression of Rgs2 caused partial but significant suppression of intracellular Ca2+ elevation, but not cAMP accumulation, by the PTH treatment in MC3T3-E1 cells (Fig. 5C). These confirm that RGS2 binds directly to Gαq/Gα11 protein and partially inhibits the Gαq signal without affecting the Gαs signal. The Rgs2 overexpression enhanced the stimulation of Col1a1 expression and ALP activity by intermittent treatment with PTH in MC3T3-E1 cells (Fig. 5D), just as the genetic deficiency of the Gαq signal did in primary osteoblasts. Again, the enhancement of the PTH effect by the Rgs2 overexpression was inhibited by addition of PMA, suggesting that PKC retards the PTH effects (Fig. 5E).
FIGURE 5.
Involvement of RGS2 in the PTH (10 nm) action on osteoblastic MC3T3-E1 cells. A, time course of the Rgs2 mRNA level by real-time RT-PCR in MC3T3-E1 cells cultured with and without PTH. *, p < 0.01 versus vehicle. B, time course of protein bindings of RGS2 with Gαs and Gαq/Gα11 after PTH treatment by immunoprecipitation (IP)/immunoblotting (IB) analyses using respective antibodies to RGS2 and Gαs (α-RGS2 and α-Gαs) or an antibody to common epitopes of Gαq and Gα11 (α-Gαq/Gα11). C, intracellular cAMP accumulation and Ca2+ ([Ca2+]i) concentration in MC3T3-E1 cells with adenoviral overexpression of Rgs2 (Ax-Rgs2) or the control vector (Ax-GFP) after 15 min and 5 min treatment, respectively, with and without PTH. *, p < 0.01 versus Ax-GFP. D, Col1a1 mRNA level and ALP activity in MC3T3-E1 cells with adenoviral overexpression of Rgs2 (Ax-Rgs2) or the control vector (Ax-GFP) after six cycles (12 days) of intermittent treatment with and without PTH. *, p < 0.01 versus vehicle; #, p < 0.01 versus Ax-GFP with PTH treatment. E, ALP activity after the intermittent PTH treatment above with and without PMA. *, p < 0.05 versus Ax-GFP without PMA; #, p < 0.05 versus Ax-Rgs2 without PMA. Data in all graphs (A, C, D, and E) are expressed as mean (bars) ± S.E. (error bars) for five cultures/group. F, schematic of effects of Gαs and Gαq signals in osteoblasts on the PTH osteoanabolic action.
DISCUSSION
The present gain- and loss-of-function analyses in Col1a1-Gnaq-tg mice and Col1a1-Cre+/−;Gnaqfl/fl;Gna11−/− mice, respectively, demonstrated the inhibitory role of the Gαq signal in the PTH osteoanabolic action (Fig. 5F). Although this suggests that suppression of the signal may lead to a novel treatment in combination with PTH against osteoporosis, there is no agent or drug that directly suppresses the Gαq signal. Hence, we focused on RGS2, a physiological inhibitor of the Gαq signal, and found that the Rgs2 overexpression enhanced the PTH osteoanabolic action in cultured osteoblasts. The RGS2 expression is known to be stimulated by phosphodiesterase (PDE) inhibitors which are now clinically used for treatment of erectile dysfunction, asthma, and pulmonary arterial hypertension (31). In fact, our preliminary examination confirmed that PDE6, a PDE family member, was expressed in MC3T3-E1 cells and primary mouse calvarial osteoblasts (supplemental Fig. 2A). When MC3T3-E1 cells were treated with zaprinast, a PDE6 inhibitor that is now clinically used as a drug for pulmonary arterial hypertension, the Rgs2 expression was significantly up-regulated (supplemental Fig. 2B). Hence, we speculate that PDE inhibitors might possibly be useful for clinical application to osteoporosis treatment as an enhancer of the PTH osteoanabolic action via the RGS2 induction and the Gαq suppression.
To know the in vivo function of RGS2 on bone, our preliminary study compared BMD between mice with global deletion of Rgs2 (Rgs2−/−) and the wild-type littermates. Unlike Col1a1-Gnaq-tg mice that exhibited osteopenia with and without PTH treatment (Fig. 1), there was no significant difference in BMD between Rgs2−/− and wild-type littermates either with or without the treatment (supplemental Fig. 3A). This may possibly be due to insufficient activation of the Gαq signal by the Rgs2 deficiency, as suggested by only a partial inhibition of the intracellular Ca2+ elevation by the Rgs2 overexpression (Fig. 5C). In fact, primary osteoblasts derived from Rgs2−/− mice showed Ca2+ elevation under the PTH treatment similar to that from WT mice, whereas Col1a1-Gnaq-tg osteoblasts exhibited enhanced Ca2+ elevation (supplemental Fig. 3B). Alternatively, because RGS2 is generally deficient in the global Rgs2−/− mice, possible involvement of systemic regulation by the Gαq signal activation in other organs cannot be ruled out. For example, cardiac hypertrophy caused by the myocardial Gαq activation in the Rgs2−/− mice (32) may enhance the general blood flow, causing an increase of circulation in bone tissue. Hence, examination of PTH osteoanabolic action using conditional knock-out mice of Rgs2 will be the next task for us to elucidate the function of RGS2 in bone.
The PTH osteoanabolic action was enhanced only in the cDKO mice, but not in single-knock-out mice of either Gnαq or Gnα11, confirming a redundant functionality of Gαq and Gα11 in osteoblasts (Fig. 3). To date, no human diseases have been attributed to mutations in the Gnαq gene. The lack of syndromes caused by loss-of-function mutations is readily understood because of the redundant functionality, making it necessary to have simultaneous mutations in both genes (17). The absence of a syndrome because of a dominant-acting mutation is less apparent. Mice with global Gnaq and Gna11 double-knock-out were embryonically lethal due to cardiomyocyte hypoplasia (17), and those with parathyroid-specific double-knock-out exhibited a phenotype resembling germ line knock-out of the extracellular Ca2+-sensing receptor: severe hypercalcemia, hyperparathyroidism, hypocalciuria, growth retardation, and early postnatal death (33). Hence, Gαq and Gα11 may compensate each other's function in various tissues.
Although Gnαq transgenic mice in this study showed severe osteopenia (Fig. 1), no significant phenotype was observed in the cDKO mice under physiological conditions (Fig. 2). Similarly, in cultures without PTH treatment, osteoblasts from the transgenic mice showed an increase of PKC activity and decreases in differentiation markers in our previous study (13), whereas osteoblasts from the cDKO mice showed normal PKC activity and differentiation markers (Fig. 4). This is consistent with findings of cardiomyocyte-specific Gnaq and Gna11 double-knock-out mice that showed no cardiac abnormality under physiological conditions but showed a lack of ventricular hypertrophy in response to pressure overload (24). These indicate the existence of other compensatory mechanisms for the Gαq signal deficiency under physiological conditions, which became insufficient under pathological conditions like PTH stimulation or pressure overload.
There was no significant difference between WT and Col1a1-Gnaq-tg in resorption parameters (Oc.S/BS and ES/BS) under the PTH stimulation (p > 0.05) (Fig. 1D). This is consistent with our previous results on ex vivo analyses of cultured osteoblasts derived from the WT and Col1a1-Gnaq-tg mice, which show no difference in mRNA level of RANKL or osteoprotegerin, nor in the RANKL/osteoprotegerin ratio between WT and Col1a1-Gnaq-tg osteoblasts under the PTH treatment (13), implicating involvement of the Gαq signal in the PTH anabolic action rather than in the catabolic action. However, these resorption parameters under the PTH treatment were enhanced in the cDKO mice (Fig. 3E). Although we assume that this catabolic effect may be secondary to the anabolic effect via the coupling between bone formation and resorption, we cannot deny the possibility of direct involvement of the Gαq signal in the PTH catabolic action. Further studies using a model of continuous infusion of PTH by implanting a minipump, instead of the daily and intermittent injection used in the present study, will be needed to answer the question.
The present study used 8-week-old mice, which may seem somewhat young for the experiment of PTH that is indicated for osteoporosis treatment in humans. However, little is known about the age-related osteoanabolic action of PTH in mice. A previous study showed that it is variable depending on the site: in the tibia and femur it is nearly twice as great in young mice as it is in aged mice, whereas in the vertebra it is greater in aged mice (34). Because the present study performed bone analyses mainly in the tibia and femur, we used 8-week-old mice to increase the sensitivity of the assay. Similar analyses in the vertebra of 4–6-month-old mice may add further information for the application to the treatment of osteoporosis in humans.
Previous studies by others and us have shown that insulin-like growth factor (IGF)-I and its signaling molecule insulin-receptor substrate (IRS)-1 at least partly mediate the PTH osteoanabolic action (12, 27, 35, 36). However, PTH is reported to induce IGF-I expression via the cAMP accumulation through the Gαs signal (37, 38). In fact, our analyses revealed that IGF-I concentrations in the serum of Col1a1-Gnaq-tg and cDKO mice were comparable with those of respective wild-type littermates and that the concentration in the culture medium of cDKO osteoblasts was similar to that of the wild-type cultures (data not shown), denying involvement of the Gαq signal in the IGF-I production. Hence, the Gαq signal might possibly affect the intracellular signal lying downstream of the IGF-I receptor like IRS-1/Akt pathway as reported in the process of cardiac hypertrophy and keratinocyte migration (39, 40). Another candidate for the mediator of the PTH osteoanabolic action is sclerostin, an endogenous inhibitor of the Wnt signal (41). However, this signal is also dependent on the Gαs/cAMP pathway (42). So far, there are many other molecules like IL-18, β-arrestin2, connexin43, TGF-β, ATF-4, and CREM, which have been suggested to be involved in the PTH osteoanabolic action using their knock-out mice (43, 44). Moreover, in vitro screenings identified c-fos, RUNX2, and cAMP response element binding-protein as downstream molecules of PTH in osteoblasts (45). However, none of these molecules has been shown to be linked to the Gαq signal in the process of the PTH osteoanabolic action thus far. Elucidation of the molecular network related to the Gαq signal is anticipated to lead to a novel treatment in combination with PTH against osteoporosis.
Acknowledgments
We thank R. Yamaguchi and H. Kawahara for technical assistance.
This work was supported by Grants-in-aid for Scientific Research 21390416, 22659267, and 22390286 from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- PTH
- parathyroid hormone
- ALP
- alkaline phosphatase
- BMD
- bone mineral density
- cDKO
- conditional double-knock-out
- Col1a1
- type I collagen α1 chain
- IGF-I
- insulin-like growth factor-I
- IRS-1
- insulin receptor substrate-1
- PDE
- phosphodiesterase
- PMA
- phorbol 12-myristate 13-acetate
- pQCT
- peripheral quantitative computed tomography
- RANKL
- receptor activator of NF-κB ligand
- RGS
- regulator of G protein signaling
- tg
- transgenic.
REFERENCES
- 1. Neer R. M., Arnaud C. D., Zanchetta J. R., Prince R., Gaich G. A., Reginster J. Y., Hodsman A. B., Eriksen E. F., Ish-Shalom S., Genant H. K., Wang O., Mitlak B. H. (2001) N. Engl. J. Med. 344, 1434–1441 [DOI] [PubMed] [Google Scholar]
- 2. Hodsman A. B., Bauer D. C., Dempster D. W., Dian L., Hanley D. A., Harris S. T., Kendler D. L., McClung M. R., Miller P. D., Olszynski W. P., Orwoll E., Yuen C. K. (2005) Endocr. Rev. 26, 688–703 [DOI] [PubMed] [Google Scholar]
- 3. Jüppner H., Abou-Samra A. B., Freeman M., Kong X. F., Schipani E., Richards J., Kolakowski L. F., Jr., Hock J., Potts J. T., Jr., Kronenberg H. M., et al. (1991) Science 254, 1024–1026 [DOI] [PubMed] [Google Scholar]
- 4. Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 2732–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Datta N. S., Abou-Samra A. B. (2009) Cell. Signal. 21, 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCudden C. R., Hains M. D., Kimple R. J., Siderovski D. P., Willard F. S. (2005) Cell. Mol. Life Sci. 62, 551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spiegel A. M. (1997) Horm. Res. 47, 89–96 [DOI] [PubMed] [Google Scholar]
- 8. Weinstein L. S., Yu S., Warner D. R., Liu J. (2001) Endocr. Rev. 22, 675–705 [DOI] [PubMed] [Google Scholar]
- 9. Sakamoto A., Chen M., Nakamura T., Xie T., Karsenty G., Weinstein L. S. (2005) J. Biol. Chem. 280, 21369–21375 [DOI] [PubMed] [Google Scholar]
- 10. Kronenberg H. M. (2010) Ann. N.Y. Acad. Sci. 1192, 327–329 [DOI] [PubMed] [Google Scholar]
- 11. Calvi L. M., Sims N. A., Hunzelman J. L., Knight M. C., Giovannetti A., Saxton J. M., Kronenberg H. M., Baron R., Schipani E. (2001) J. Clin. Invest. 107, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishizuya T., Yokose S., Hori M., Noda T., Suda T., Yoshiki S., Yamaguchi A. (1997) J. Clin. Invest. 99, 2961–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogata N., Kawaguchi H., Chung U. I., Roth S. I., Segre G. V. (2007) J. Biol. Chem. 282, 35757–35764 [DOI] [PubMed] [Google Scholar]
- 14. Offermanns S., Toombs C. F., Hu Y. H., Simon M. I. (1997) Nature 389, 183–186 [DOI] [PubMed] [Google Scholar]
- 15. Gudermann T., Kalkbrenner F., Schultz G. (1996) Annu. Rev. Pharmacol. Toxicol. 36, 429–459 [DOI] [PubMed] [Google Scholar]
- 16. Wettschureck N., Moers A., Offermanns S. (2004) Pharmacol. Ther. 101, 75–89 [DOI] [PubMed] [Google Scholar]
- 17. Offermanns S., Zhao L. P., Gohla A., Sarosi I., Simon M. I., Wilkie T. M. (1998) EMBO J. 17, 4304–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Vries L., Zheng B., Fischer T., Elenko E., Farquhar M. G. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 235–271 [DOI] [PubMed] [Google Scholar]
- 19. Ross E. M., Wilkie T. M. (2000) Annu. Rev. Biochem. 69, 795–827 [DOI] [PubMed] [Google Scholar]
- 20. Dromey J. R., Pfleger K. D. (2008) Endocr. Metab. Immune Disord. Drug Targets 8, 51–61 [DOI] [PubMed] [Google Scholar]
- 21. Zou M. X., Roy A. A., Zhao Q., Kirshenbaum L. A., Karmazyn M., Chidiac P. (2006) Cell. Signal. 18, 1655–1663 [DOI] [PubMed] [Google Scholar]
- 22. Hao J., Michalek C., Zhang W., Zhu M., Xu X., Mende U. (2006) J. Mol. Cell. Cardiol. 41, 51–61 [DOI] [PubMed] [Google Scholar]
- 23. Dacquin R., Starbuck M., Schinke T., Karsenty G. (2002) Dev. Dyn. 224, 245–251 [DOI] [PubMed] [Google Scholar]
- 24. Wettschureck N., Rütten H., Zywietz A., Gehring D., Wilkie T. M., Chen J., Chien K. R., Offermanns S. (2001) Nat. Med. 7, 1236–1240 [DOI] [PubMed] [Google Scholar]
- 25. Yamada T., Kawano H., Koshizuka Y., Fukuda T., Yoshimura K., Kamekura S., Saito T., Ikeda T., Kawasaki Y., Azuma Y., Ikegawa S., Hoshi K., Chung U. I., Nakamura K., Kato S., Kawaguchi H. (2006) Nat. Med. 12, 665–670 [DOI] [PubMed] [Google Scholar]
- 26. Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. (1987) J. Bone Miner. Res. 2, 595–610 [DOI] [PubMed] [Google Scholar]
- 27. Bikle D. D., Sakata T., Leary C., Elalieh H., Ginzinger D., Rosen C. J., Beamer W., Majumdar S., Halloran B. P. (2002) J. Bone Miner. Res. 17, 1570–1578 [DOI] [PubMed] [Google Scholar]
- 28. Yamaguchi M., Ogata N., Shinoda Y., Akune T., Kamekura S., Terauchi Y., Kadowaki T., Hoshi K., Chung U. I., Nakamura K., Kawaguchi H. (2005) Endocrinology 146, 2620–2628 [DOI] [PubMed] [Google Scholar]
- 29. Kawano T., Troiano N., Adams D. J., Wu J. J., Sun B. H., Insogna K. (2008) Endocrinology 149, 4009–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lampasso J. D., Chen W., Marzec N. (2006) Int. J. Mol. Med. 17, 1125–1131 [PubMed] [Google Scholar]
- 31. Bender A. T., Beavo J. A. (2006) Pharmacol. Rev. 58, 488–520 [DOI] [PubMed] [Google Scholar]
- 32. Takimoto E., Koitabashi N., Hsu S., Ketner E. A., Zhang M., Nagayama T., Bedja D., Gabrielson K. L., Blanton R., Siderovski D. P., Mendelsohn M. E., Kass D. A. (2009) J. Clin. Invest. 119, 408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wettschureck N., Lee E., Libutti S. K., Offermanns S., Robey P. G., Spiegel A. M. (2007) Mol. Endocrinol. 21, 274–280 [DOI] [PubMed] [Google Scholar]
- 34. Knopp E., Troiano N., Bouxsein M., Sun B. H., Lostritto K., Gundberg C., Dziura J., Insogna K. (2005) Endocrinology 146, 1983–1990 [DOI] [PubMed] [Google Scholar]
- 35. Canalis E., Centrella M., Burch W., McCarthy T. L. (1989) J. Clin. Invest. 83, 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shinoda Y., Kawaguchi H., Higashikawa A., Hirata M., Miura T., Saito T., Nakamura K., Chung U. I., Ogata N. (2010) J. Cell. Biochem. 109, 755–763 [DOI] [PubMed] [Google Scholar]
- 37. Umayahara Y., Billiard J., Ji C., Centrella M., McCarthy T. L., Rotwein P. (1999) J. Biol. Chem. 274, 10609–10617 [DOI] [PubMed] [Google Scholar]
- 38. Chang W., Rewari A., Centrella M., McCarthy T. L. (2004) J. Biol. Chem. 279, 42438–42444 [DOI] [PubMed] [Google Scholar]
- 39. Dorn G. W., 2nd, Force T. (2005) J. Clin. Invest. 115, 527–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taboubi S., Garrouste F., Parat F., Pommier G., Faure E., Monferran S., Kovacic H., Lehmann M. (2010) Mol. Biol. Cell 21, 946–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Keller H., Kneissel M. (2005) Bone 37, 148–158 [DOI] [PubMed] [Google Scholar]
- 42. Bellido T. (2006) J. Musculoskelet. Neuronal Interact. 6, 358–359 [PubMed] [Google Scholar]
- 43. Partridge N. C., Li X., Qin L. (2006) Ann. N.Y. Acad. Sci. 1068, 187–193 [DOI] [PubMed] [Google Scholar]
- 44. Qiu T., Wu X., Zhang F., Clemens T. L., Wan M., Cao X. (2010) Nat. Cell Biol. 12, 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swarthout J. T., D'Alonzo R. C., Selvamurugan N., Partridge N. C. (2002) Gene 282, 1–17 [DOI] [PubMed] [Google Scholar]