Abstract
We report a new series of bis-cyclohexano-fused Mn(III) complexes of bis-hydroxyphenyl-dipyrromethenes (DIPYs) 4a–c as potent and orally active peroxynitrite scavengers. Complexes 4a–c have been shown to reduce peroxynitrite through a 2-electron mechanism thereby forming the corresponding Mn(V)O species, which have been characterized by UV, NMR, and LCMS methods. Mn(III) complex 4b and its strained BODIPY analogue 9b have been analyzed by x-ray crystallography. Finally, complex 4a has been shown to be an orally active and potent analgesic in a model carrageenan-induced hyperalgesia known to be driven by the overproduction of peroxynitrite.
The overproduction of reactive oxygen species (ROS) in vivo is now widely recognized as a key contributor to numerous pathologies.1 One particularly damaging situation results from the diffusion controlled radical coupling of the central ROS, superoxide, with nitric oxide to form peroxynitrite.2 The highly reactive peroxynitrite is a powerful biological oxidant which leaves a trail of dysfunctional oxidized and nitrated proteins, lipids and nucleotides, in its wake.3 From a pharmacological perspective, peroxynitrite is considered a potent proinflammatory and proapoptotic species which plays a critical role in pain of several etiologies as demonstrated initially by our team and then by others.4–6 Accordingly, the discovery of pharmaceutically relevant agents which can effectively decompose peroxynitrite should have significant therapeutic value.2,3
As a result of the early discoveries of Groves7 and Stern,8 Mn(III) and Fe(III) porphyrins have emerged as an important class of peroxynitrite reductase and isomerase catalysts, respectively (Figure 1 A). Elegant mechanistic studies have revealed that the more pharmacologically-suitable Mn(III) porphyrins decompose peroxynitrite primarily in a one-electron fashion and require a biological co-reductant such as ascorbate to complete the reductase catalytic cycle.9 One electron reduction of peroxynitrite produces the potentially damaging nitrogen dioxide radical which is also thought to undergo rapid reduction by endogenous antioxidants.5 Thus, if endogenous antioxidants are plentiful, Mn(III)-pophryins can fully detoxify peroxynitrite in vivo. From this class, the isomeric Mn(III)-tetrakis-(meso-N-alkylpyridinium)porphyrins (e.g. Mn(III)-4-TMPyP5+, 1) are the most well-studied, both as peroxynitrite reductase catalysts and superoxide dismutase (SOD) mimics.9,10
Figure 1.
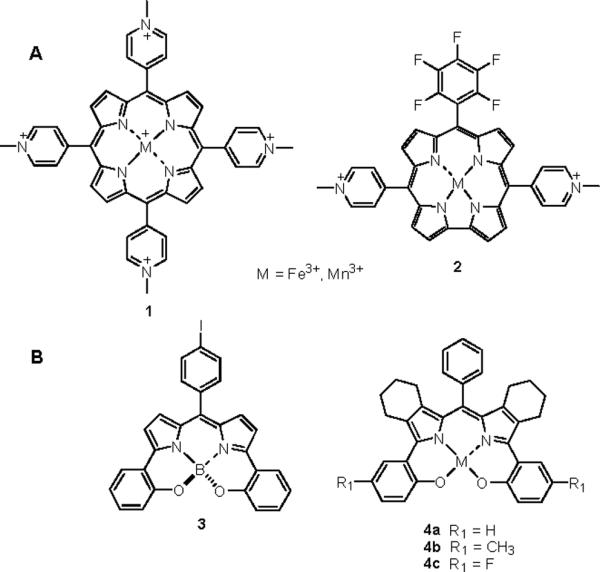
(A) Peroxynitrite decomposition catalysts 1 and 2. (B) Bishydroxyphenyldipyrromethene analogues 3 and 4.
Recently, Gross has reported that Mn(III) and Fe(III) corroles are also excellent peroxynitrite decomposition catalysts.11 Remarkably, the Mn(III) corroles operate through a 2-electron cycle, reducing peroxynitrite to nitrite instead of nitrogen dioxide through a novel disproportionation mechanism. The most important finding from this work was that Mn(III) corroles can decompose peroxynitrite in a catalytic fashion (in contrast to Mn(III) porphyrins) and therefore do not require the assistance of endogenous co-reductants.
Although Mn(III) porphyrins, such as Mn(III)-4-TMPyP5+ 1, and Mn(III) corrole systems, such as compound 2, have proven to be powerful pharmacological tools in animal studies demonstrating the benefits of destroying peroxynitrite in vivo,11–14 they are not optimal as therapeutic candidates. While these types of polycationic complexes have excellent catalytic activities and their high water solubility is a useful property for laboratory measurements, their corresponding high polarity renders them poorly membrane soluble. More amphiphilic metallocorroles have indeed shown great promise as orally active peroxynitrite decomposition catalysts.12c Unfortunately, synthetic methods for accessing polyfunctional corrole systems remain quite challenging and therefore not particularly suited for iterative structure-activity relationship (SAR) studies. To this end we have been keenly interested in the design, synthesis and evaluation of new catalyst systems with enhanced drug-like properties.
In our search for alternative trianionic ligand systems capable of supporting a Mn(V)O intermediate arising from the 2-electron reduction of peroxynitrite (similar to Mn(III) corroles), we were intrigued by the B,O-chelated boron-dipyrromethene (BODIPY®) dye 3 reported originally by Burgess (Figure 1 B).15 These dyes are constrained systems due to the chelate effect and thus have improved fluorescence properties over their non-chelated and well-known BODIPY congeners. The overall “ligand set” in these systems is not only perfect for mimicking the trianionic corrole but these compounds are also amenable to modular synthesis in good to excellent yields.16 Herein we report the synthesis and evaluation of new bis-cyclohexano-fused Mn(III) complexes of bis-hydroxyphenyl-dipyrromethenes (DIPYs) as potent peroxynitrite scavengers with drug-like properties.
The syntheses commenced with readily available tetrahydroisoindole 517 which was converted to the Boc-protected 3-bromo-tetrahydroisoindole 6 in 95% yield for two steps. Next, compound 6 underwent smooth Suzuki couplings with a representative set of protected hydroxyphenylboronic acids, 7a–c in 70–89% yield, followed by a one pot decarboxylation/deprotection procedure to furnish the corresponding 2-benzyloxy- or 2-methoxyphenyl-tetrahydroisoindole derivatives 8a–c. Compounds 8a–c were then treated with benzaldehyde and TFA under Lindsey conditions18 to form the corresponding dipyrromethane derivative, followed by oxidation to the dipyrromethene with p-chloranil19 and subsequent phenol-ether deprotection with boron tribromide.19,20 This sequence was carried out without intervening purifications. The boron tribromide deprotection step afforded the crude BODIPY systems 9a–c which were then directly converted to the Mn(III) complexes 4a–c by reaction with manganese(II) chloride under basic aerobic conditions. The dark emerald green complexes 4a–c behave as simple lipophilic “organic” molecules and were thus amenable to purification by flash chromatography on silica gel providing analytical materials for characterization and activity studies. The overall yield of this sequence ranged from 16–32% (unoptimized) and is therefore quite competitive with porphyrin and corrole syntheses which require the preparation of functionalized pyrrole and/or dipyrromethane units followed by an often low yield cyclization/oxidation step.18
Magnetic susceptibility measurement confirmed that representative complex 4b is high-spin d4 Mn(III). 1H NMR spectra were recorded for 4a in CD2Cl2 which revealed broad and shifted peaks indicative of the paramagnetic Mn(III) complex. Oxidation of 4a with m-CPBA in CD2Cl2 afforded normal sharpened peaks characteristic of the corresponding diamagnetic low-spin d2 Mn(V)O complex (Figures S21 and S22).21
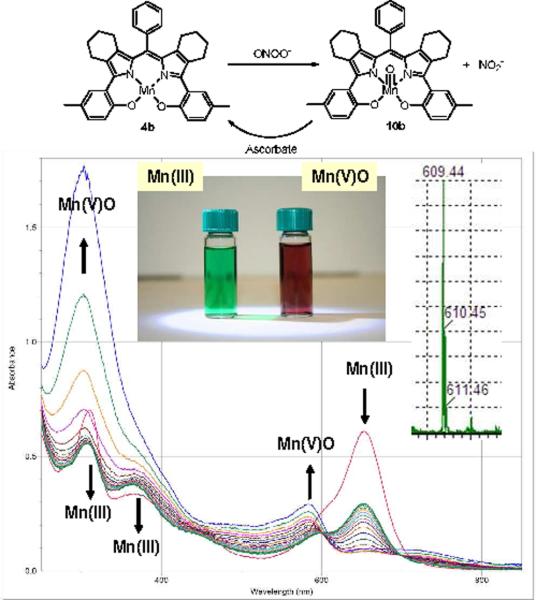
Treatment of a green-colored methanolic solution of each Mn(III) complex 4a–c with excess peroxynitrite (in 0.1N NaOH)22 afforded the corresponding red-colored Mn(V)O intermediate (Figure 2). In methanol solution, the Mn(V)O species persist for 20–30 minutes and are stable enough to have been confirmed by LCMS (Figure 2 inset) and UV spectroscopy. The UV/Vis spectral changes are analogous to those observed during the oxidative generation and subsequent reduction of Mn(V)O-corroles23 and in the elegant studies of highly functionalized Mn(V)O-corrolazines reported by Goldberg.21 In all three complexes (4a–c) the oxo-species are clearly observable using positive ion mode LCMS as [M + H]+ species confirming the formal oxidation state of [Mn(V)O]+3 (accounting for the trianionic ligand set). Treatment of the methanolic solutions of Mn(V)O species with 5 equivalents of ascorbate in phosphate buffer (pH = 7.2) resulted in the apparent instantaneous conversion back to the green Mn(III) form, effecting a possible reductase mode of action.
Figure 2.
UV-Vis spectral changes for 4b (33 ηM, MeOH, 25 °C) after treatment with 0–20 equivalents peroxynitrite (spectra collected every 2 min. See supplementary data) Center inset: samples of 4b and 10b. Right inset: LCMS [M+H]+ for Mn(V)O specie 10b.
The results shown in Figure 2 are of interest only for identifying the putative 2-electron oxidation of 4b with subsequent study of the Mn(V)O species 10b by spectroscopic methods. However, to confirm that complexes can indeed decompose peroxynitrite more rapidly than its spontaneous decomposition at physiological pH, a relevant rapid throughput in vitro assay was sought.
Complexes 4a–c were therefore assayed for their peroxynitrite decomposition activity by determining their ability to inhibit aryl boronate oxidation.24 Oxidation of 4-acetylphenylboronic acid to 4-acetylphenol by peroxynitrite is a clean conversion devoid of any observable intermediates and the second order rate constant for this reaction has been accurately measured at k = 1.6 × 106 M−1s−1 by stopped-flow spectrophotometric methods.24 Inhibition results for complexes 4a–c and Mn(III)-4-TMPyP5+, 1 are presented in Table 1. From the percent inhibition values we have calculated the corresponding apparent second order rate constants for oxidation of the Mn(III) form to the Mn(V)O form for 4a–c and the Mn(IV)O form for 1 at 25 °C in phosphate buffer (pH = 7.2). The numbers match up well for the rate constants reported in the literature for the well known complex 1.25 Thus, this assay provides a reliable method for the rapid in vitro measurement of activity toward decomposing peroxynitrite prior to in vivo studies. Our data reveals that analogues 4a–c have estimated apparent rate constants in the range of those observed for manganese corrole systems.11 If the resulting Mn(V)O forms are more rapidly reduced back to the Mn(III) resting state by endogenous reductants, the k values in Table 1 are thus the catalytic rate constants for reductase-type activity.9 In that manganese corroles have been shown to possess peroxynitrite decomposition activity without the need for endogenous reductants,11 complexes 4a–c may also operate via a similar catalytic cycle as they possess a similar trianionic ligand environment. Future mechanistic studies will address this possible mode of catalysis.
Table 1.
Inhibition of Arylboronic Acid Oxidation
 | |||
|---|---|---|---|
| Catalyst | LogPa | % Inhibition (25 °C) | Second-order rate constant, k, M−1s−1 (25 °C) |
| 4a | +4.18 ± 0.19 | 32.0 ± 2.40 | b7.5 ± 0.6 × 105 |
| 4b | +4.31 ± 0.02 | 25.3 + 2.21 | b5.4 ± 0.5 × 105 |
| 4c | +4.05 ± 0.15 | 31.5 ± 2.50 | b7.3 ± 0.6 × 105 |
| 1 | −4.54 ± 0.16 | 62.4 ± 0.68 |
b2.6 ± 0.2 × 106 c1.8 × 106 |
Measured by the slow-stir method27
apparent second order rate constant for the oxidation of complex (1 equivalent) by peroxynitrite (1 equivalent) estimated from % inhibition in 100 mM phosphate buffer (pH = 7.2); no secondary antioxidants added; determined by LCMS after 1 min reaction time
second order rate constant measured by stopped flow methods.25
Single crystal x-ray analysis of 4b and its BODIPY analogue and synthetic precursor 9b provided very interesting structural information for this new ligand class (Figure 3). The BODIPY system 9b shows a significant distortion of the tetrahedral boron center, similar to that observed for the related non-cyclohexano system.15
Figure 3.
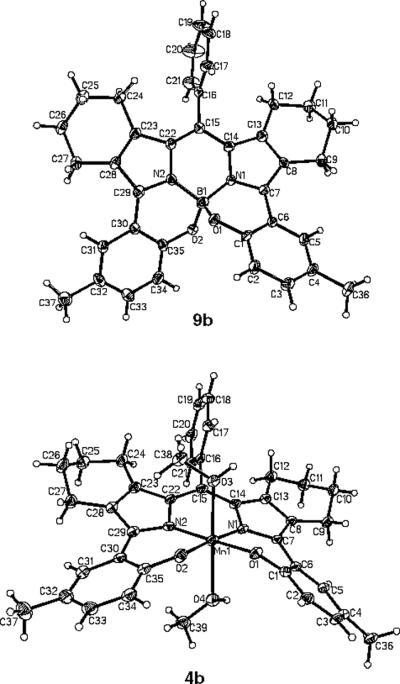
Single crystal structural analysis for BODIPY analogue 9b and manganese(III) complex 4b.
In the case of 9b, the O-B-O angle is 108.4, but the N1-B-O2 and N2-B-O1 angles are 114.4 and 113.1 respectively. The N-B-N angle is pinched in to 106.5 most likely in response to the phenyl bisecting the planar dipyrromethene unit and therefore feeling the close steric interaction of the CH2 groups of the neighboring fused-cyclohexano rings. The Mn(III) complex 4b crystallized with coordinated axial methanol molecules (from CH2Cl2-MeOH solution) to afford a Jahn-Teller distorted octahedral array around the manganese atom. The phenyl-dipyrromethene unit in 4b is displaced upward relative to the equatorial plane with the coordinating phenolate groups displaced slightly downward. The release in strain associated with the distorted tetrahedron of 9b most likely drives its direct conversion to 4b without the need for isolation of the free phenol ligand. Both axial Mn-O(MeOH) bonds are considerably longer than in similar structures observed for Mn(III) corroles (2.19 Å)11c and Mn(III) corrolazines (2.107 Å)21b most likely due to the Jahn-Teller effect.26 The Mn-O3 bond distance is 2.226 Å and the longer Mn-O4 bond distance is 2.342 Å, possibly also influenced by steric interactions with the saddled hydroxyphenyl groups.
In the well known Hargreaves model28 intraplantar injection of carrageenan led to the time-dependent development of thermal hyperalgesia in rats, an inflammatory response known to be driven by high levels of peroxynitrite flux (Figure 3).29 This hyperalgesic effect was potently inhibited by 4a when given by oral gavage (Figure 4). Nearly 100% inhibition is seen for 2 h with a substantial inhibitory effect maintained out to 5 h. At the 2h time point, a similar degree of inhibition was observed with the with the non-selective COX-1/COX-2 inhibitor ibuprofen at 300 mg/kg (99±5% inhibition, n=6). Under the same conditions and at 300 mg/kg, acetaminophen or aspirin attenuated hyperalgesia by 20±4% and 50±6% respectively (n=6, P < 0.05 compared to carrageenan alone at the 2h time point). Complexes 4b and 4c have also been shown to possess potent oral activity in this model. All three new complexes have LogP values in the range of +4, indicating high lipid solubility while Mn(III)-4-TMPyP5+ 1 and related catalysts possess highly negative values (Table 1).30
Figure 4.
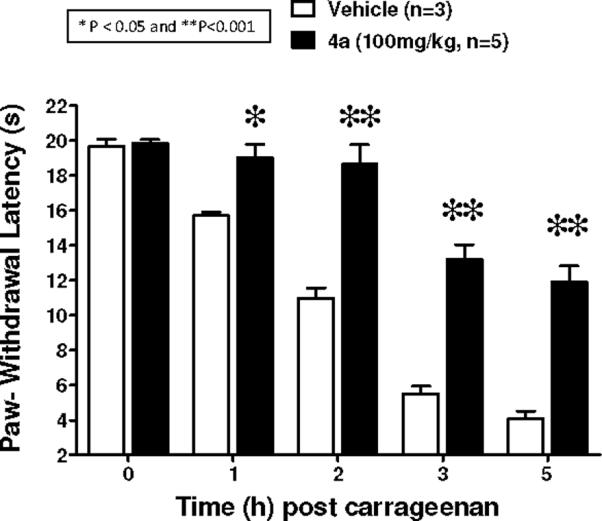
The time-dependent development of carrageenan-induced thermal hyperalgesia (n=3) in rats was blocked by oral administration of 4a (100 mg/kg, n=5). Results are expressed as mean ± SEM and analyzed by two-way ANOVA with Bonferroni post hoc tests where **P<0.05 and **P<0.001 vs. Vehicle (n = number of animals).
Both 4b and 4c were designed to divert or block the potential metabolic hydroxylation para to the chelated phenol through methyl- and fluoro-substitution, respectively. Full SAR studies incorporating further electron donating and withdrawing functionality will be the subject of future reports.
In conclusion, the results presented herein demonstrate the modular synthesis of a new class of orally active Mn(III) complexes which function as peroxynitrite scavengers. These studies suggest that this new complex design may afford improved in vivo performance through the 2-electron reduction of peroxynitrite to nitrite.
Supplementary Material
Scheme 1.
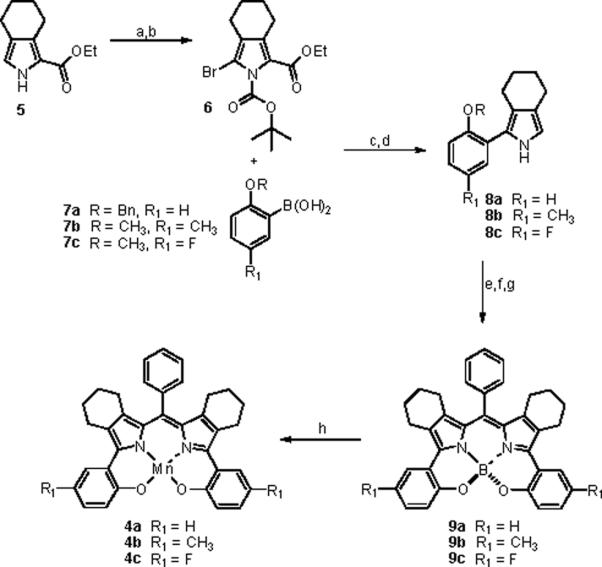
Synthesis of Mn(III) complexes of bishydroxyphenyl-DIPYsa
a(a) NBS, THF, 100%, (b) Boc2O, 4-DMAP, CH3CN, 95% (c) 5% Pd(PPh3)4, Na2CO3-H2O, CH3OH, toluene, 70–89%, (d) (CH2OH)2, KOH, 195 °C, 70–85%, (e) PhCHO, cat. TFA, 52–61%, (f) p-chloranil, CH2Cl2, (g) BBr3, CH2Cl2, (h) MnCl2, CHCl3, CH3OH, 2,6-Lutidine, air, 68–79% for 3 steps (from f).
Acknowledgement
Support of this research by NIH (NIAMS, RC1AR058231) is gratefully acknowledged. The authors also thank Dr. A. Michael Crider of the SIUE School of Pharmacy and Dr. Michael Shaw of the SIUE Department of Chemistry for many helpful discussions. The authors thank the Donald Danforth Plant Science Center Proteomics and Mass Spectrometry group and the Washington University Resource for Biomedical and Biological Mass Spectrometry for HRMS analyses. Funding from the National Science Foundation (MRI, CHE-0420497) for the purchase of the ApexII diffractometer is acknowledged.
Footnotes
Supporting Information Available: Experimental procedures, characterization data for all new compounds, LCMS, NMR, UV/Vis, boronate oxidation assay, oxidation studies, magnetic susceptibility measurement, pharmacological methods, and crystallographic data. This material is available free of charge via the internet at http://pubs.acs.org.
References
- (1).Batinic-Haberle I, Reboucas JS, Spasojevic I. Antioxid. Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ferrer-Sueta G, Radi R. ACS Chem. Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- (3).Szabo C, Ischiropoulos H, Radi R. Nat. Rev. Drug Discov. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- (4).Salvemini D, Neumann W. Life Sci. 2010;86:604–14. doi: 10.1016/j.lfs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- (5).Salvemini D, Neumann WL. Trends Pharmacol. Sci. 2009;30:194–202. doi: 10.1016/j.tips.2008.12.005. [DOI] [PubMed] [Google Scholar]
- (6).Salvemini D. Arch. Biochem. Biophys. 2009;484:238–44. doi: 10.1016/j.abb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Groves JT, Marla SS. J. Am. Chem. Soc. 1995;117:9578–9579. [Google Scholar]
- (8).Stern MK, Jensen MP, Kramer K. J. Am. Chem. Soc. 1996;118:8735–8736. [Google Scholar]
- (9).Lee J, Hunt JA, Groves JT. J. Am. Chem. Soc. 1998;120:6053–6061. [Google Scholar]
- (10).Ferrer-Sueta G, Vitturi D, Batinic-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. J. Biol. Chem. 2003;278:27432–8. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- (11).(a) Mahammed A, Gross Z. Angew. Chem. Int. Ed. 2006;45:6544–7. doi: 10.1002/anie.200601399. [DOI] [PubMed] [Google Scholar]; (b) Kupershmidt L, Okun Z, Amit T, Mandel S, Saltsman I, Mahammed A, Bar-Am O, Gross Z, Youdim MB. J. Neurochem. 2010;113:363–73. doi: 10.1111/j.1471-4159.2010.06619.x. [DOI] [PubMed] [Google Scholar]; (c) Gershman Z, Goldberg I, Gross Z. Angew. Chem. Int. Ed. 2007;46:4320. doi: 10.1002/anie.200700757. [DOI] [PubMed] [Google Scholar]
- (12).(a) Okun Z, Kupershmidt L, Amit T, Mandel S, Bar-Am O, Youdim MBH, Gross Z. ACS Chem. Biol. 2009;4:910. doi: 10.1021/cb900159n. [DOI] [PubMed] [Google Scholar]; (b) Kanamori A, Catrinescu M-M, Mahammed A, Gross Z, Levin LA. J. Neurochem. 2010;114:488–498. doi: 10.1111/j.1471-4159.2010.06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Haber A, Mahammaed A, Fuhrman B, Volkova N, Coleman R, Hayek T, Aviram M, Gross Z. Angew. Chem. Int. Ed. 2008;47:7896. doi: 10.1002/anie.200801149. [DOI] [PubMed] [Google Scholar]
- (13).Drel VR, Pacher P, Vareniuk I, Pavlov I, Ilnytska O, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. Eur. J. Pharmacol. 2007;569:48–58. doi: 10.1016/j.ejphar.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Vareniuk I, Pavlov IA, Drel VR, Lyzogubov VV, Ilnytska O, Bell SR, Tibrewala J, Groves JT, Obrosova IG. Exp. Neurol. 2007;205:425–36. doi: 10.1016/j.expneurol.2007.03.019. [DOI] [PubMed] [Google Scholar]
- (15).Kim H, Burghart A, Welch MB, Reibenspies J, Burgess K. Chem Commun (Camb) 1999:1889–1890. [Google Scholar]
- (16).Burghart A, Kim H, Welch MB, Thoresen LH, Reibenspies J, Burgess K. J. Org. Chem. 1999;64:7813–7819. [Google Scholar]
- (17).May J, D.A., Lash TD. J. Org. Chem. 1992;57:4820–4828. [Google Scholar]
- (18).(a) Lindsey JS, Schreiman IC, Hsu HC, Kearney PC, Marguerettaz AM. J. Org. Chem. 1987;52:827. [Google Scholar]; (b) Littler BJ, Ciringh Y, Lindsey JS. J. Org. Chem. 1999;64:2864–2872. doi: 10.1021/jo982452o. [DOI] [PubMed] [Google Scholar]
- (19).Parhi AK, Kung MP, Ploessl K, Kung HF. Tetrahedron Lett. 2008;49:3395–3399. doi: 10.1016/j.tetlet.2008.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Loudet A, Bandichhor R, Burgess K, Palma A, McDonnell SO, Hall MJ, O'Shea DF. Org. Lett. 2008;10:4771–4774. doi: 10.1021/ol8018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Similar behavior was observed for the manganese(V)-oxo corrolazines: Mandimutsira BS, Ramdhanie B, Todd RC, Wang H, Zareba AA, Czernuszewicz RS, Goldberg DP. J. Am. Chem. Soc. 2002;124:15170. doi: 10.1021/ja028651d.. Lansky DE, Mandimutsira BS, Ramdhanie B, Clausen M, Penner-Hahn J, Zvyagin SA, Telser J, Kryzstek J, Zhan R, Ou Z, Kadish KM, Zakharov L, Rheingold AL, Golberg DP. Inorg. Chem. 2005;44:4485. doi: 10.1021/ic0503636.. Goldberg DP. Acc. Chem. Res. 2007;40:626. doi: 10.1021/ar700039y..
- (22).Uppu RM, Pryor WA. Anal. Biochem. 1996;236:242–9. [PubMed] [Google Scholar]
- (23).Liu HY, Yam F, Xie YT, Li XY, Chang CK. J. Am. Chem. Soc. 2009;131:12890–1. doi: 10.1021/ja905153r. [DOI] [PubMed] [Google Scholar]
- (24).Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Free Radic. Biol. Med. 2009;47:1401–7. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Hunt JA, Lee J, Groves JT. Chem. Biol. 1997;4:845–58. doi: 10.1016/s1074-5521(97)90117-4. [DOI] [PubMed] [Google Scholar]; (b) Differences in pH (7.2 versus 7.4) for the boronate assay versus stopped flow experiments are in part responsible for the minor differences in the kinetic data.
- (26).(a) Lieb D, Zahl A, Shubina TE, Ivanovic-Burmazovic I. J. Am. Chem. Soc. 2010;132:7282. doi: 10.1021/ja1014585. [DOI] [PubMed] [Google Scholar]; (b) Hoogenraad M, Ramkisoensing K, Driessan WL, Kooijman H, Spek AL, Brouwman E, Haasnoot JG, Reedijk J. Inorg. Chim. Acta. 2001;320:117. [Google Scholar]
- (27).Lodge K. J. Chem. Eng. Data. 1999;4:1321–1324. [Google Scholar]
- (28).Measurement of paw-withdrawl latency from a thermal stimulus (pawwithdrawl from a warm surface): Hargreaves K, Dubner R, Brown F, Flores C, Joris J. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7..
- (29).Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Eur. J. Pharmacol. 1996;303:217–20. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- (30).(a) Kos I, Benov L, Spasojevic I, Reboucas JS, Batinic-Haberle I. J. Med. Chem. 2009;52:7868–72. doi: 10.1021/jm900576g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Reboucas JS, Spasojevic I, Salvemini D. Free Radic. Biol. Med. 2009;46:212–9. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.