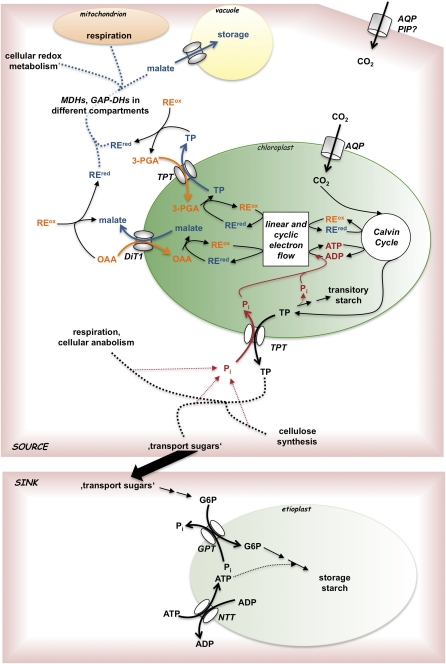
Metabolite transport proteins occupy key positions in the metabolic networks of highly compartmentalized eukaryotic cells. In such cells, at least one and frequently more than one membrane delineate organelles from the cytosol. Thereby compartments with different reactions milieus are created that are characterized by low pH or high-energy status, or by highly reductive or oxidative conditions, as compared to the cytosol. The membranes bounding these compartments act as effective diffusion barriers to most organic metabolites and inorganic ions. However, cellular metabolic networks frequently extend over several compartments. Hence, substrates, intermediates, and products of pathways need to pass the bounding membranes in a controlled manner that retains the compartment-specific conditions while allowing the passage of defined metabolites. Transport proteins that are embedded in the membrane enable the passage of metabolites and thereby connect metabolic networks beyond organellar boundaries (Linka and Weber, 2010).
Transport proteins can be broadly classified into three groups: channels or pores, primary active transporters, and secondary active transporters, respectively (Heldt, 1999). Channels or pores permit the diffusion of molecules along a concentration gradient or electrochemical potential. Since diffusion of solutes through pores and channels does not involve binding of the substrate to the channel protein but its passage through the hydrophilic channel pore, diffusion occurs very fast, up to 106 molecules per second (Heldt, 1999). In contrast, carrier proteins, similar to enzymes, bind their substrates and undergo a conformational change upon binding and transport. Hence, transport processes mediated by carrier proteins are several orders of magnitude slower than those mediated by channels, ranging between 10 and several thousand molecules per second (Heldt, 1999). Primary active transporters split energy-rich bonds such as those in ATP or inorganic pyrophosphate to transport metabolites or ions against a concentration gradient. Secondary active transport proteins act as either symporters or antiporters, respectively. That is, they transport one molecule against its concentration gradient, whereas another is either transported in the same (symport) or the opposite direction (antiport) along its concentration gradient. This cotransport mode is mandatory, meaning that under physiological conditions, transport of one molecule cannot occur without the other. The larger, favorable change in free energy of one substrate drives the flux of the second molecule against its electrochemical potential difference. Especially for secondary transporters, it is important to consider the net transport activity, which can be calculated from the symport by addition and from the antiport by subtraction. The majority of transport proteins involved in transporting metabolites resulting from photosynthesis are of the secondary active transporter type. Since secondary transporters, as outlined above, have low turnover numbers, relatively large amounts of such proteins are required if large fluxes have to be accommodated.
The core reactions of photosynthesis occur exclusively in the chloroplast: (1) the light-driven photosynthetic electron transport chain, which generates reducing equivalents in the form of NADPH and energy equivalents in the form of ATP, and (2) the Calvin-Benson cycle, which uses reducing and energy equivalents to assimilate CO2 into triosephosphates (TPs). Organic carbon in the form of TPs represents the principle output of the Calvin-Benson cycle. TPs can either be exported from the chloroplast to the remainder of the cell or they can be metabolized within the chloroplast, for example during transitory starch biosynthesis (Heldt, 1999; Fig. 1, center). Both energy and reducing power generated by the photosynthetic light reactions are also used in the chloroplast for a number of additional anabolic reactions, such as nitrogen and sulfur assimilation, amino acid and lipid biosynthesis, and production of precursors for secondary metabolism. However, the chloroplast is not autonomous—it depends on the remainder of the cell for photosynthesis to function: TPs exported to the cytosol are predominantly converted to transport sugars, such as Suc, and to structural carbohydrates, such as cellulose. Inorganic phosphate (Pi) released from TPs during these biosyntheses is returned to the chloroplast, which is essential for continuous operation of photosynthesis. Indeed, the one-to-one stoichiometry for TP/Pi exchange by the TP/phosphate translocator (TPT) provides a regulatory link between photosynthetic rates and cytosolic carbon metabolism. For example, if Suc synthesis in the cytoplasm slows down, Pi availability drops and the absence of Pi returning to the chloroplast slows photosynthesis. In addition, a toxic by-product of the Rubisco reaction, phosphoglycolate, must be detoxified, excess reducing power needs to be diffused, and cofactors for the photosynthetic reactions need to be imported from other parts of the cell. Hence, efficient operation of photosynthesis critically depends on the presence of transport proteins that connect the chloroplast with its surroundings.
Figure 1.
Schematic representation of pathways and transport proteins with impact on photosynthetic capacity. REred, Reduced reducing equivalent; REox, oxidized reducing equivalent; G6P, Glc-6-P; MDH, malate dehydrogenase; GAP-DH, glyceraldehyde phosphate dehydrogenase; for transport protein abbreviations, see legend to Table I. The complex sink source interface is only schematically indicated by an arrow connecting source with sink.
Chloroplasts, the site of photosynthesis, are surrounded by two membranes, the inner envelope membrane and the outer envelope membrane. The inner envelope is traditionally considered to represent the specificity barrier with a set of highly specific transport proteins, while the outer envelope is considered to be less selective with a set of broad specificity pores.
THE CORE TRANSPORT PROCESSES INVOLVED IN PHOTOSYNTHESIS
The single most abundant protein in the inner envelope is the TPT, which represents the major pathway for carbon export during the day (Flügge and Heldt, 1984). It functions as an antiporter. That is, it transports TPs in a 1:1 counter exchange with Pi (Flügge and Heldt, 1984). The net result of each transport step is equivalent to three reduced carbon atoms, with no net transport of phosphate (Table I; Fig. 1). Dissecting the physiological role of this transport protein is complex since TPs can either be exported to the cytosol, or stored inside the chloroplast in the form of transitory starch. If synthesis of Suc that is synthesized from TPs in the cytosol (and/or Suc export to sinks) becomes limited, as indicated by falling cytosolic phosphate concentrations, phosphate becomes unavailable as a counter substrate for TP at the transporter. TP can thus no longer be exported and is rerouted into transitory starch biosynthesis (Flügge and Heldt, 1984). This rerouting provides sufficient metabolic flexibility to allow the plant to survive under laboratory conditions, even if the activity of TPT is compromised by knockdown or knockout (Häusler et al., 2000b, 2000c; Schneider et al., 2002). Transitory starch is broken down during the night predominantly to maltose and to a minor degree to Glc, which are both exported to the cytosol for conversion to Suc that is loaded into the phloem. The amylolytic mobilization of transitory starch does not conserve all of the energy contained in the glycosidic bonds of the starch polymer (Weise et al., 2004) and mobilization and phloem loading are fueled by respiration during the night (Häusler et al., 2000a).
Table I. List of transport proteins with impact on photosynthetic capacity.
| Name | Shortcut | Arabidopsis Gene | Substrates | Mode of Transport | Net Transport |
| Aquaporin | AQP | Analyzed in tobacco (Nicotiana tabacum) | CO2 | Channel | CO2 |
| Triosephosphate/phosphate translocator | TPT | At5g46110 | TP, phosphate | Antiport | Three carbon moieties |
| TP, 3-PGA | Antiport | One reducing equivalent, reduced | |||
| Dicarboxylate translocator 1 | DiT1 | At5g12860 | OAA, malate | Antiport | One reducing equivalent, reduced |
| Suc symporter 1 | SUT1 | Characterized in sugar beet (Beta vulgaris) | Suc, protons | Symport | 12 carbon moieties |
| Glc-6-P/phosphate translocator | GPT | At5g54800, At1g61800 | Glc-6-P, phosphate | Antiport | Six carbon moieties |
| Adenine nucleotide transporter | NTT | At1g80300, At1g15500 | ATP, ADP | Antiport | Energy, phosphate |
| Dicarboxylate translocator 1 | DiT1 | At5g12860 | 2-Oxoglutarate, malate | Antiport | One amino group (together with DiT2) |
| Dicarboxylate translocator 2 | DiT2 | At5g64290, At5g64280 | Glu, malate | Antiport | One amino group (together with DiT1) |
| Phosphoenolpyruvate/phosphate translocator | PPT | At5g33320, At3g01550 | Phosphoenolpyruvate, phosphate | Antiport | Three carbon moieties |
| S-adenosyl-Met transporter | SAMT | At4g39460 | S-adenosyl-Met, S-adenosyl homo-Cys | Antiport | Activate one carbon group |
In contrast to photosynthesis in ambient CO2 concentrations, TPT strongly limits photosynthetic carbon fixation under elevated CO2 conditions (Häusler et al., 2000c). Under these conditions, both the maximum rate of transitory starch biosynthesis and TP export from the chloroplast colimit the rate of CO2 assimilation. This indicates that if CO2 concentrations continue to rise or if photosynthesis is engineered to increase the flux through the pathway, the TPT will become limiting for the rate of CO2 assimilation.
In contrast to organic carbon, which can be stored if in excess, excess reducing power cannot be stored in the chloroplast and thus must be dissipated. In addition to the chloroplast-intrinsic pathways for dissipation, there exist at least two potential shuttles for the export of reducing power: (1) the TP/3-phosphoglyceric acid (3-PGA) shuttle and (2) the malate/oxaloacetate (OAA) shuttle. The TPT of higher plants is not only capable of exporting reduced carbon but also of reducing power (Flügge and Heldt, 1984). That is, the TPT can exchange TP for 3-PGA, which equals the net movement of one reducing equivalent: TP is exported to the cytosol, oxidized to 3-PGA by cytosolic glyceraldehyde phosphate dehydrogenase, and the resulting 3-PGA is returned to the chloroplast. Analyzing the physiological role of TPT as redox shuttle in knockdown or knockout plants is difficult since the effects of carbon export limitation may confound those of redox export limitation. Introducing a TPT that does not accept 3-PGA and that is therefore unable to act as a reducing equivalent shuttle into a TPT knockout mutant would allow to separately address the two roles of TPT and its possible role as an in vivo reducing equivalent shuttle (Linka et al., 2008).
A second possible reducing equivalent shuttle is the dicarboxylate translocator DiT1 (Taniguchi et al., 2002; Renné et al., 2003). It can exchange OAA for malate; hence, it catalyzes the net movement of one reducing equivalent without net C4 acid transport (Taniguchi et al., 2002; Renné et al., 2003): Malate is exported to the cytosol, where it is oxidized to OAA, which is returned to the chloroplast. Since DiT1, together with DiT2 in a shuttle that involves two transport proteins, also plays a major role in nitrogen assimilation (Weber and Flügge, 2002), similar to TPT these dual roles cannot easily be dissected. Relatively mild repression of DiT1 already limits photosynthesis. This limitation is likely based in part or completely on its role in nitrogen metabolism and not as a redox shuttle (Schneidereit et al., 2006). The reducing equivalents exported by either the TP/3-PGA and/or the OAA/malate shuttle can be recovered and stored in any organelles that have glyceraldehyde phosphate dehydrogenase or malate dehydrogenase activity (Scheibe, 2004), oxidized by the mitochondria (Raghavendra and Padmasree, 2003), or used for redox reactions. Recently, it was also shown that C4 acids such as malate play an important role as carbon store fueling nocturnal cellular metabolism (Fahnenstich et al., 2007), which requires the vacuole as a storage compartment for malate. Of course, the movement of redox pairs across other organellar membranes and the storage of malate require transport proteins, such as those located in the tonoplast membrane (Emmerlich et al., 2003).
In contrast to both organic carbon and reducing power, ATP transport across the leaf chloroplast envelope likely does not play a major role during the day. The capacity for ATP transport in the chloroplast envelope is 100-fold lower than that for TPs and the major effect of a knockout of the plastidial ATP transporter NTT is on nocturnal, not on photosynthetic metabolism (Reiser et al., 2004).
Transitory starch stored during the day is broken down by the amylolytic pathway during the night and the resulting products maltose and Glc are exported to the cytosol (Weise et al., 2004). The export of maltose is mediated by the maltose exporter MEX1 (Niittylä et al., 2004) and Glc is exported by the plastidic Glc transporter pGlcT (Weber et al., 2000). The nightly mobilization rate of transitory starch is adjusted by the plant so that the starch reservoir lasts until the night ends (Usadel et al., 2008). Inhibition of nightly starch degradation severely inhibits plant growth (Niittylä et al., 2004; Lu and Sharkey, 2006), indicating that sufficient breakdown capacity is critical to optimal use of carbon stored during the day.
In addition to transport proteins that export the products of photosynthesis, the import of substrates also warrants consideration. Until recently, it was assumed that CO2 entry into the chloroplast occurs by diffusion through the membrane. However, dedicated CO2 pores belonging to the aquaporin protein family were recently discovered in the plasma membrane and in the chloroplast envelope (Uehlein et al., 2003, 2008). A knockdown of the CO2 transporting aquaporin resulted in a 15% decrease in the maximal photosynthetic rate, which was most likely due to higher resistance to CO2 conductance at the chloroplast envelope (Uehlein et al., 2008). The consequence of CO2 aquaporin overexpression has not yet been reported. However, it is likely that the CO2 pores are evolutionarily adapted to present day CO2 concentrations and present day photosynthetic rates in the plants’ native environment. Growth in elevated CO2 concentrations, which we will be facing due to global change, or in lowered CO2 conditions such as closed stomates due to drought, may alter flux in a way that requires altered CO2 conductance.
TRANSPORT PROCESSES PERIPHERALLY ASSOCIATED WITH PHOTOSYNTHESIS
Sink strength is a major determinant of photosynthetic capacity. Photoassimilates are exported from source cells via the phloem to the sink tissues. At least one transport protein at this interface, the Suc proton symporter SUT1 of the phloem companion cells, controls the photoassimilate transport rate to the sinks (Vaughn et al., 2002). Its abundance and therefore its maximal transport capacity are mediated by Suc in the phloem that in turn depends on sink strength (Vaughn et al., 2002). In sink tissues storage starch is produced from Glc-6-P imported into the amyloplasts. Since amyloplasts are not capable of producing ATP by photophosphorylation, it must be imported from the remainder of the cell. Simultaneous overexpression of the plastidial Glc-6-P/phosphate translocator (GPT) and the ATP transporter NTT in potato (Solanum tuberosum) tubers leads to increased sink strength and 19% higher tuber yield. The starch content was increased overall (44%) and on per tuber basis (28%; Zhang et al., 2008). This case clearly demonstrates flux control at the transport protein level, rather than at the level of enzyme activity.
Several other transport proteins apparently limit photosynthesis, although it is not exactly known whether the effect is directly on photosynthetic metabolism or due to pleiotropic effects on cellular metabolism. For example, a mutation in the plastidic phosphoenolpyruvate/phosphate translocator (PPT) causes a reticulate leaf phenotype and affects the electron transfer rates but not overall carbon assimilation rates (Streatfield et al., 1999). PPT imports one of the substrates of the shikimic acid pathway, phosphoenolpyruvate, from the cytosol. The shikimate pathway provides one of the substrates for plastoquinone biosynthesis, which might explain the observed effects on electron transfer rates (Voll et al., 2003). A mutation in the plastidic S-adenosyl-Met transporter SAMT, affects prenyllipid synthesis and therefore the synthesis of a range of cofactors essential to photosynthesis, among them chlorophyll and plastoquinone. Consequentially, knockdowns in SAMT appear pale and have retarded growth, indicating less net carbon gain (Bouvier et al., 2006). These examples show that it is not always straightforward to dissect direct effects on photosynthetic metabolism from pleiotropic metabolic effects that indirectly feed back onto photosynthesis.
Although the core reactions of photosynthesis occur exclusively in the chloroplast, photorespiration, the recycling of carbon lost through the oxygenation reaction of Rubisco, requires peroxisomes and mitochondria (Bauwe et al., 2010). Since up to one-third of inorganic carbon fixed during the day is lost again through photorespiration in C3 plants, the flux through this pathway is very high, in mature leaves probably second only to photosynthesis itself (Bauwe et al., 2010). The enzymatic reactions of photorespiration have been mostly resolved at the molecular level, however, all transport proteins, except for those involved in nitrogen reassimilation, are unknown (Bauwe et al., 2010). It is thus difficult to estimate whether transport proteins exert control over the flux through this pathway or whether transport proteins are present in excess, such as the enzymes involved in the pathway (Zhu et al., 2007). Although metabolic channeling through organellar extensions, such as stromules and matrixules has been suggested, it is more likely that controlled metabolite exchange by transport proteins ensures flux through the pathway (Foyer and Noctor, 2007). Physical proximity of peroxisomes and mitochondria to chloroplasts, as it is known from electron micrographs, is apparently crucial for efficient photorespiration. A mutation in the peroxisomal PEX10 protein causes loss of physical association and a mild photorespiratory phenotype, indicating that short pathways for diffusion between organelles are important for maintaining high flux through the pathway (Schumann et al., 2007). The role of peroxisomes in photosynthetic metabolism beyond photorespiration has not yet been fully resolved.
Photorespiration aside, mitochondrial metabolism is vital for chloroplast function and photosynthetic capacity. Mitochondrial inhibitors like oligomycin, sodium azide, or antimycin A and transgenic modifications of the mitochondrial electron transport chain have marked influence on photosynthetic capacity (Raghavendra and Padmasree, 2003; Noctor et al., 2007). At least one transport protein is known to be involved in this intricate balance, the uncoupling protein UCP1. A UCP1 mutant in Arabidopsis (Arabidopsis thaliana) is characterized by lower CO2 assimilation rates, although its stomatal conductance was not altered. It was suggested that lack of uncoupling protein function adversely affects mitochondrial redox poise, which in turn impacts photosynthesis (Sweetlove et al., 2006). Thus, photosynthesis, although confined to the chloroplast, is part of an intricate cross-compartment network that is well connected throughout the cell and dependent on its connections. Attempts to increase flux through photosynthesis thus likely require adjustments to the transport capacity of the chloroplast membrane and of other organellar membranes as well.
C4 PHOTOSYNTHESIS: NATURE’S SUCCESSFUL SOLUTION TO SUPERCHARGING PHOTOSYNTHESIS
C4 photosynthesis is highly efficient due to the reduction of carbon loss by photorespiration. This is achieved, at the expense of additional ATPs per CO2 fixed, through increasing the CO2 concentration in the vicinity of Rubisco, thereby suppressing the oxygenation reaction of Rubisco to less than 1% of that observed in C3 plants (von Caemmerer and Furbank, 2003). The C4 photosynthetic reactions occur in different cell types, mesophyll and bundle sheath cells, and involve at least two distinct chloroplast types, one in each cell type (Hatch, 1987). The flux through the C4 pathway is higher than the apparent rate of CO2 assimilation and likely represents one of the highest metabolite fluxes known in plants (Weber and von Caemmerer, 2010). Comparative quantitative proteomics as well as transcriptomics clearly demonstrated that the high metabolite flux needed to sustain C4 photosynthesis is achieved by strongly increased transport protein abundance (Bräutigam et al., 2008, 2011; Friso et al., 2010). Hence, during evolution of the C4 photosynthetic pathway, transport capacity became limiting and the amounts of transport proteins were increased to cope with the increased demand on flux. C4 photosynthesis thus serves as a prime example demonstrating the importance of transport proteins for achieving high rates of photosynthetic carbon assimilation.
CONCLUDING REMARKS
We set out to address the question—do metabolite transport processes limit photosynthesis? Unfortunately, the question cannot be answered with a simple yes or no. Plants have evolved somewhat elastic transport capacities to cope with environmental variations, such as shading or high light intensities. However, this innate flexibility is rather limited, as has been demonstrated by knockdown and knockout plant lines showing reduced capacity of TP transport by TPT. Global change, leading to increasing CO2 concentrations in the atmosphere, as well as attempts at increasing photosynthetic rates by engineering of the photosynthetic pathway may easily overburden the extant capacity of the transport systems both in and outside of the chloroplast. While altering transport capacity alone is unlikely to change photosynthetic capacity, altering photosynthetic capacity by other means may quickly render transport capacity as a limiting factor, as demonstrated by the strong control exerted by TPT over the maximal rate of photosynthesis at elevated CO2 concentrations. A further case in point is C4 photosynthesis, in which increased amounts and activities of enzymes of the C4 pathway are accompanied by strongly increased amounts of the required transporter proteins.
Acknowledgments
We regret omission of many relevant citations due to space constraints.
References
- Bauwe H, Hagemann M, Fernie AR. (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15: 330–336 [DOI] [PubMed] [Google Scholar]
- Bouvier F, Linka N, Isner JC, Mutterer J, Weber APM, Camara B. (2006) Arabidopsis SAMT1 defines a plastid transporter regulating plastid biogenesis and plant development. Plant Cell 18: 3088–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Hoffmann-Benning S, Weber AP. (2008) Comparative proteomics of chloroplast envelopes from C3 and C4 plants reveals specific adaptations of the plastid envelope to C4 photosynthesis and candidate proteins required for maintaining C4 metabolite fluxes. Plant Physiol 148: 568–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Kajala K, Wullenweber J, Sommer M, Gagneul D, Weber KL, Carr KM, Gowik U, Maß J, Lercher MJ, et al. (2011) An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiol 155: 142–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerlich V, Linka N, Reinhold T, Hurth MA, Traub M, Martinoia E, Neuhaus HE. (2003) The plant homolog to the human sodium/dicarboxylic cotransporter is the vacuolar malate carrier. Proc Natl Acad Sci USA 100: 11122–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnenstich H, Saigo M, Niessen M, Zanor MI, Andreo CS, Fernie AR, Drincovich MF, Flügge UI, Maurino VG. (2007) Alteration of organic acid metabolism in Arabidopsis overexpressing the maize C4 NADP-malic enzyme causes accelerated senescence during extended darkness. Plant Physiol 145: 640–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge UI, Heldt HW. (1984) The phosphate-triose phosphate-phosphoglycerate translocator of the chloroplast. Trends Biochem Sci 9: 530–533 [Google Scholar]
- Foyer CH, Noctor G. (2007) Shape-shifters building bridges? Stromules, matrixules and metabolite channelling in photorespiration. Trends Plant Sci 12: 381–383 [Google Scholar]
- Friso G, Majeran W, Huang M, Sun Q, van Wijk KJ. (2010) Reconstruction of metabolic pathways, protein expression, and homeostasis machineries across maize bundle sheath and mesophyll chloroplasts: large-scale quantitative proteomics using the first maize genome assembly. Plant Physiol 152: 1219–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. (1987) C-4 photosynthesis—a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106 [Google Scholar]
- Häusler RE, Baur B, Scharte J, Teichmann T, Eicks M, Fischer KL, Flügge UI, Schubert S, Weber A, Fischer K. (2000a) Plastidic metabolite transporters and their physiological functions in the inducible crassulacean acid metabolism plant Mesembryanthemum crystallinum. Plant J 24: 285–296 [DOI] [PubMed] [Google Scholar]
- Häusler RE, Schlieben NH, Flügge UI. (2000b) Control of carbon partitioning and photosynthesis by the triose phosphate/phosphate translocator in transgenic tobacco plants (Nicotiana tabacum). II. Assessment of control coefficients of the triose phosphate/phosphate translocator. Planta 210: 383–390 [DOI] [PubMed] [Google Scholar]
- Häusler RE, Schlieben NH, Nicolay P, Fischer K, Fischer KL, Flügge UI. (2000c) Control of carbon partitioning and photosynthesis by the triose phosphate/phosphate translocator in transgenic tobacco plants (Nicotiana tabacum L.). I. Comparative physiological analysis of tobacco plants with antisense repression and overexpression of the triose phosphate/phosphate translocator. Planta 210: 371–382 [DOI] [PubMed] [Google Scholar]
- Heldt HW. (1999) Plant Biochemistry, Ed 3 Elsevier Academic Press, San Diego [Google Scholar]
- Linka M, Jamai A, Weber APM. (2008) Functional characterization of the plastidic phosphate translocator gene family from the thermo-acidophilic red alga Galdieria sulphuraria reveals specific adaptations of primary carbon partitioning in green plants and red algae. Plant Physiol 148: 1487–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linka N, Weber APM. (2010) Intracellular metabolite transporters in plants. Mol Plant 3: 21–53 [DOI] [PubMed] [Google Scholar]
- Lu Y, Sharkey TD. (2006) The importance of maltose in transitory starch breakdown. Plant Cell Environ 29: 353–366 [DOI] [PubMed] [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303: 87–89 [DOI] [PubMed] [Google Scholar]
- Noctor G, De Paepe R, Foyer CH. (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12: 125–134 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K. (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 8: 546–553 [DOI] [PubMed] [Google Scholar]
- Reiser J, Linka N, Lemke L, Jeblick W, Neuhaus HE. (2004) Molecular physiological analysis of the two plastidic ATP/ADP transporters from Arabidopsis. Plant Physiol 136: 3524–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renné P, Dressen U, Hebbeker U, Hille D, Flügge UI, Westhoff P, Weber APM. (2003) The Arabidopsis mutant dct is deficient in the plastidic glutamate/malate translocator DiT2. Plant J 35: 316–331 [DOI] [PubMed] [Google Scholar]
- Scheibe R. (2004) Malate valves to balance cellular energy supply. Physiol Plant 120: 21–26 [DOI] [PubMed] [Google Scholar]
- Schneider A, Häusler RE, Kolukisaoglu U, Kunze R, van der Graaff E, Schwacke R, Catoni E, Desimone M, Flügge UI. (2002) An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J 32: 685–699 [DOI] [PubMed] [Google Scholar]
- Schneidereit J, Häusler RE, Fiene G, Kaiser WM, Weber APM. (2006) Antisense repression reveals a crucial role of the plastidic 2-oxoglutarate/malate translocator DiT1 at the interface between carbon and nitrogen metabolism. Plant J 45: 206–224 [DOI] [PubMed] [Google Scholar]
- Schumann U, Prestele J, O’Geen H, Brueggeman R, Wanner G, Gietl C. (2007) Requirement of the C3HC4 zinc RING finger of the Arabidopsis PEX10 for photorespiration and leaf peroxisome contact with chloroplasts. Proc Natl Acad Sci USA 104: 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streatfield SJ, Weber A, Kinsman EA, Häusler RE, Li JM, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flügge UI, Chory J. (1999) The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell 11: 1609–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Lytovchenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, Eickmeier I, Fernie AR. (2006) Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci USA 103: 19587–19592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Taniguchi Y, Kawasaki M, Takeda S, Kato T, Sato S, Tabata S, Miyake H, Sugiyama T. (2002) Identifying and characterizing plastidic 2-oxoglutarate/malate and dicarboxylate transporters in Arabidopsis thaliana. Plant Cell Physiol 43: 706–717 [DOI] [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425: 734–737 [DOI] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. (2008) Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20: 648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M. (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn MW, Harrington GN, Bush DR. (2002) Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci USA 99: 10876–10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll L, Häusler RE, Hecker R, Weber A, Weissenböck G, Fiene G, Waffenschmidt S, Flügge UI. (2003) The phenotype of the Arabidopsis cue1 mutant is not simply caused by a general restriction of the shikimate pathway. Plant J 36: 301–317 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank RT. (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77: 191–207 [DOI] [PubMed] [Google Scholar]
- Weber A, Flügge UI. (2002) Interaction of cytosolic and plastidic nitrogen metabolism in plants. J Exp Bot 53: 865–874 [DOI] [PubMed] [Google Scholar]
- Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gröner F, Hebbeker U, Flügge UI. (2000) Identification, purification, and molecular cloning of a putative plastidic glucose translocator. Plant Cell 12: 787–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber APM, von Caemmerer S. (2010) Plastid transport and metabolism of C3 and C4 plants—comparative analysis and possible biotechnological exploitation. Curr Opin Plant Biol 13: 257–265 [DOI] [PubMed] [Google Scholar]
- Weise SE, Weber APM, Sharkey TD. (2004) Maltose is the major form of carbon exported from the chloroplast at night. Planta 218: 474–482 [DOI] [PubMed] [Google Scholar]
- Zhang L, Häusler RE, Greiten C, Hajirezaei MR, Haferkamp I, Neuhaus HE, Flügge UI, Ludewig F. (2008) Overriding the co-limiting import of carbon and energy into tuber amyloplasts increases the starch content and yield of transgenic potato plants. Plant Biotechnol J 6: 453–464 [DOI] [PubMed] [Google Scholar]
- Zhu XG, de Sturler E, Long SP. (2007) Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol 145: 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]