Abstract
One isoform of callose synthase, Glucan Synthase-Like7 (GSL7), is tightly coexpressed with two isoforms of sucrose synthase (SUS5 and SUS6) known to be confined to phloem sieve elements in Arabidopsis (Arabidopsis thaliana). Investigation of the phenotype of gsl7 mutants of Arabidopsis revealed that the sieve plate pores of stems and roots lack the callose lining seen in wild-type plants. Callose synthesis in other tissues of the plant appears to be unaffected. Although gsl7 plants show only minor phenotypic alterations during vegetative growth, flowering stems are reduced in height and all floral parts are smaller than those of wild-type plants. Several lines of evidence suggest that the reduced growth of the inflorescence is a result of carbohydrate starvation. Levels of sucrose, hexoses, and starch are lower in the terminal bud clusters of gsl7 than in those of wild-type plants. Transcript levels of “starvation” genes expressed in response to low sugars are elevated in the terminal bud clusters of gsl7 plants, at the end of the night, and during an extended night. Pulse-chase experiments with 14CO2 show that transport of assimilate in the flowering stem is much slower in gsl7 mutants than in wild-type plants. We suggest that the callose lining of sieve plate pores is essential for normal phloem transport because it confers favorable flow characteristics on the pores.
The pores in the sieve plates of phloem elements are key determinants of the velocity of transport of Suc and other phloem contents over long distances in the plant. From theoretical models, phloem conductance is predicted to be directly related to the density of pores on the sieve plate and to the length and diameter of the pores (Thompson and Holbrook, 2003; Thompson, 2006). During phloem development, pore formation is preceded by deposition of the β1,3-glucan callose between the plasma membrane and the cell wall, both as “platelets” on the faces of the end wall and around the plasmodesmatal channel. The pore is formed by widening of the plasmodesmatal channel, a process that involves removal of some of the callose. The precise sequence of events, and the importance of callose in these events, remain unclear. Some authors propose that callose largely replaces cellulose in the region of the wall in which the sieve plate pore will form. Pore formation is then due primarily to callose degradation (Esau and Thorsch, 1985; Thorsch and Esau, 1988). Others reject the idea that callose replaces original cell wall material and propose that pore formation involves degradation of the original material as well as some callose. According to this view, the main function of callose deposition is to restrict wall thickening in the region in which the pore will form (Evert et al., 1966; Deshpande, 1974, 1975).
However it is derived, the mature pore usually has a callose lining (Bouck and Cronshaw, 1965; Deshpande, 1974, 1975; Thorsch and Esau 1988; Eleftheriou, 1990; for summary, see Sjölund, 1997). If mature phloem elements are damaged, massive callose synthesis occurs on the sieve plate, resulting in occlusion of the pores and retention of phloem contents behind the callose plugs (Evert and Derr, 1964; Hao et al., 2008; Mullendore et al., 2010). Indeed, because callose is formed very rapidly upon damage to the phloem, its existence in sieve plate pores in undamaged plants has been disputed in the past: some authors have argued that its presence is an artifact of sectioning and preparation techniques (Evert and Derr, 1964; Walsh and Melaragno, 1976; Spanner, 1978; Sjölund, 1997; for summary, see van Bel, 2003). However, callose linings have been observed in sieve plate pores from a wide range of plant species and organs and with a range of techniques designed to minimize the possibility of callose synthesis during tissue preparation (Ehlers et al., 2000; van Bel et al., 2002). They are almost certainly a feature of mature sieve plates in many species.
Callose synthesis occurs during normal growth in many locations in plants. In addition to sieve plate pores, it is deposited in the cell plate during cytokinesis (Samuels et al., 1995), in cell walls at the neck region of plasmodesmata (Turner et al., 1994), and during pollen formation (microgametogenesis) and pollen tube growth (Rae et al., 1985; McCormick, 1993). It is also deposited on the inner face of cell walls in response to pathogen attack, forming a barrier against fungal penetration (Jacobs et al., 2003; Nishimura et al., 2003). Callose is synthesized from the sugar nucleotide UDPglucose via callose synthases that span the plasma membrane. In both vascular and nonvascular plants, callose synthases are encoded by multigene families, and different isoforms have different locations and functions in the plant (Voigt et al., 2006; Dong et al., 2008; Schober et al., 2009; Schuette et al., 2009). Of the 12 Glucan Synthase-Like (GSL; also called CalS) genes in Arabidopsis (Arabidopsis thaliana), five are suggested or proven from mutational and correlative analyses to encode isoforms with important functions in pollen development (GSL1, GSL2, GSL5, GSL8, and GSL10; Enns et al., 2005; Nishikawa et al., 2005; Töller et al., 2008; Huang et al., 2009). Two genes have been implicated in cytokinesis, cell plate formation, and cell patterning (GSL6 and GSL8; Hong et al., 2001; Chen et al., 2009; Thiele et al., 2009) and one in response to attack by fungal pathogens (GSL5; Jacobs et al., 2003; Nishimura et al., 2003; Dong et al., 2008). Expression of the GSL6 gene is elevated and callose is deposited locally in response to phloem feeding by silverleaf whitefly nymphs (Bemisia sp.; Kempema et al., 2007) and the aphid Brevicoryne brassicae (Kuśnierczyk et al., 2008). The isoform(s) responsible for the synthesis of callose in sieve plate pores has yet to be identified.
Interest in the control of phloem transport has increased with the demonstration of its importance in the transmission of developmental and environmentally triggered signaling molecules involved in the induction of flowering and of systemic resistance to pathogens (Turgeon and Wolf, 2009). There has been recent progress in modeling phloem transport and in the precise measurement of anatomical features and transport velocity (Van As, 2007; Mullendore et al., 2010). These approaches reveal that callose deposition in sieve plate pores after wounding can drastically reduce phloem conductivity within minutes. They also suggest that there is a much more complex relationship between phloem conductivity (estimated from anatomical measurements) and the velocity of phloem transport (measured by MRI) across species than is predicted from simple microfluidic principles. Further progress in this area is likely to be slow without new tools to manipulate anatomical components of the phloem in a precise, specific, and noninvasive manner within a single species.
We discovered recently that the synthesis of the callose lining of pores in the phloem sieve plates of the flowering stem of Arabidopsis is partially dependent upon the presence of two isoforms of Suc synthase, SUS5 and SUS6. In a mutant lacking both isoforms (the sus5sus6 mutant), the thickness of the callose lining is markedly reduced but callose formation is normal in other parts of the plant (Barratt et al., 2009). SUS5 and SUS6 appear to be present exclusively in the phloem. In roots, both transcripts are reported to be phloem located (Birnbaum et al., 2003; http://www.arexdb.org/root-images/deconRoot.html), and in situ hybridizations detected SUS6 transcript only in phloem initial cells (Barratt et al., 2009). Immunoblots of tissue prints revealed SUS5 and SUS6 proteins only in phloem tissue in stems and mature hypocotyls. Taken together, these data indicate that SUS5 and SUS6 may have a specific role in the provision of the UDPglucose substrate for the synthesis of callose in the wall of sieve plate pores.
To discover further components of the pathway of callose synthesis in the sieve plate, and thus provide tools for exploring the role of this polymer in the development and function of the phloem, we looked for genes that are coexpressed with SUS5 and/or SUS6. We found that SUS5, SUS6, and one callose synthase, GSL7, are tightly coexpressed. Here, we show that GSL7 is expressed in the phloem and is necessary for callose synthesis in the sieve plate. In gsl7 mutants, growth of the floral stem and all floral parts is reduced. We provide evidence that this is due to carbon starvation caused by reduced phloem conductivity.
RESULTS
Identification of GSL7 as a Gene Coexpressed with SUS5 and SUS6
Two different coexpression databases (ATTED-II, the Arabidopsis trans-factor and cis-element prediction database [Obayashi et al., 2007], http://atted.jp/; AthCoR@CSB.DB, the Arabidopsis coresponse database, http://csbdb.mpimp-golm.mpg.de/csbdb/dbcor/ath/ath_tsgq.html, “developmental series”) revealed a strong correlation between the abundance of transcripts for SUS5, SUS6, and a gene encoding a putative callose synthase, GSL7 (CalS7; At1g06490). This correlation is particularly striking because it was identified in both databases. The lists of genes coexpressed with SUS5 otherwise showed very little similarity between the two databases (Supplemental Tables S1 and S2).
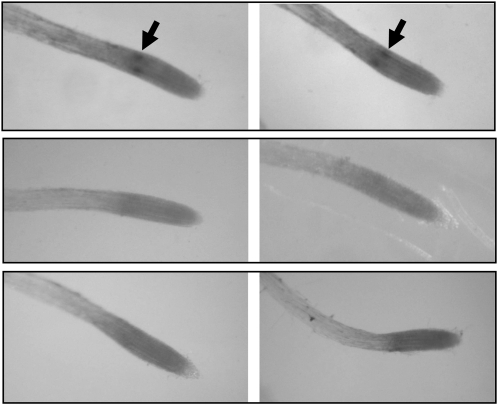
In whole-mount in situ hybridization experiments on roots, GSL7 transcript was detected only in two discrete regions in the differentiating stele. No transcript was detected using a sense probe as a control (Fig. 1). This distribution is very similar to that of SUS6 transcript (Barratt et al., 2009) and is consistent with the idea that GSL7 transcript may be located in the phloem initial cells. Further evidence of a phloem location is provided by global analyses of patterns of gene expression in the Arabidopsis root (Birnbaum et al., 2003; Brady et al., 2007; Cartwright et al., 2009; Arex, the Arabidopsis Gene Expression database, http://www.arexdb.org/). Transcript levels for SUS5, SUS6, and GSL7 are strongly enhanced in the phloem relative to all other cell types in the root (Supplemental Fig. S1).
Figure 1.
Location of transcription of the GSL7 gene in roots. Whole-mount preparations of roots were treated with antisense and sense probes from the GSL7 gene. A, Two examples of roots from wild-type plants probed with a 350-bp antisense probe. The arrows indicate two regions of hybridization within the root. B, Two examples of wild-type roots probed with a 350-bp sense probe. C, Two examples of roots from gsl7 mutant plants probed with a 350-bp antisense probe.
Loss of GSL7 Eliminates Callose from Phloem Sieve Plates
To discover the role and importance of GSL7, we obtained insertion mutants from the SALK and Syngenta Arabidopsis Insertion Library (SAIL) collections. Homozygous lines were named gsl7-1 (SALK_048921) and gsl7-2 (SAIL_114_A01). GSL7 transcript was not detectable in stems of either of these lines (Supplemental Fig. S2).
To visualize callose, sections of flowering stem were stained with aniline blue, a dye that fluoresces when it binds callose. Callose was present in the phloem region in hand-cut sections of stems of wild-type plants but not gsl7 mutant plants (Fig. 2A, arrows). Prolonged (18-h) incubation of cut stems in water prior to fixation and sectioning failed to induce callose formation in the phloem in mutant plants (Fig. 2B). However, callose formation was induced in cells adjacent to the xylem in both wild-type and mutant plants (Fig. 2B, stars).
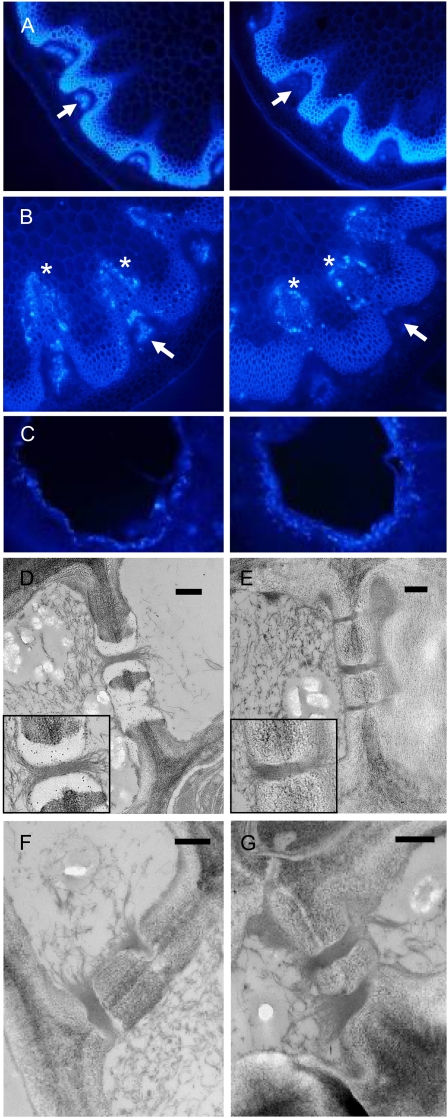
Figure 2.
Loss of callose from the phloem in flowering stems of the gsl7 mutant. All images are representative of results obtained from several different wild-type and gsl7 mutant plants. The cell walls of the xylem and fibers are autofluorescent. A, The basal 1 to 2 cm of flowering stems from 45-d-old plants was excised, and hand-cut sections were stained with aniline blue and viewed with a UV epifluorescence microscope. Left, wild-type plant; right, gsl7 mutant plant. Arrows indicate the position of the phloem. Fluorescence indicating the presence of callose is associated with the phloem in the wild type but not in the gsl7 mutant. B, As in A, but excised stems were incubated in water for 18 h prior to staining. Left, wild-type plant; right, gsl7 mutant plant. Fluorescence indicating the presence of callose is associated with cells adjacent to the xylem in both wild-type and gsl7 mutant stems (asterisks) but is associated with the phloem only in wild-type stems (arrows). C, Fully expanded leaves from 42-d-old plants were punctured with a plastic tip and then harvested the next day, stained with aniline blue, and viewed with a UV epifluorescence microscope. Left, wild-type plant; right, gsl7 mutant plant. D to G, Electron micrographs of phloem elements in sections of the basal 1 to 2 cm of flowering stems from wild-type (D) and gsl7 mutant (E–G) 45-d-old plants excised directly into glutaraldehyde fixative. Sections in D and E are immunogold labeled with an anti-callose antiserum. Note the presence of gold particles in the electron-transparent sieve plate lining of wild-type plants and their absence from an equivalent position in mutant plants (compare enlarged insets in D and E). Bars = 500 nm.
Aniline blue staining of whole roots also indicated a specific role for GSL7 in callose production in the phloem. In wild-type roots, callose was abundant in single files of cells in the stele of older parts of the root. No callose was visible in this location in gsl7 mutant roots (Fig. 3).
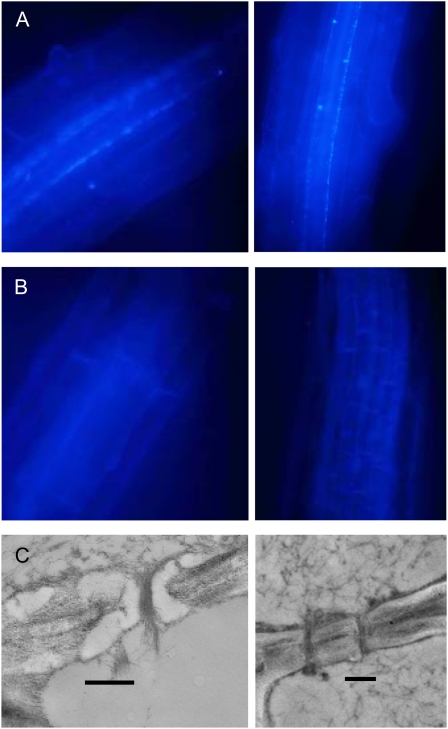
Figure 3.
Loss of callose from the phloem in roots of the gsl7 mutant. All images are representative of results obtained from several different wild-type and gsl7 mutant plants. Photographs are of roots from 4-d-old seedlings. A and B, Regions behind the expansion zones of roots from two different seedlings stained with aniline blue. In wild-type seedlings (A), fluorescence is associated with two cell files in the stele. In gsl7 seedlings (B), no fluorescence is visible in these cell files. C, Electron micrographs of phloem elements in sections of the proximal region of roots of wild-type (left) and gsl7 mutant (right) seedlings. An electron-transparent lining is present in the sieve plate pores of wild-type but not gsl7 plants. Bars = 500 nm.
These observations suggested that GSL7 is responsible for the production of callose in the phloem but not for the production of callose in other cell types, either when intact or in response to wounding. To test this idea further, we examined callose production in response to mechanical wounding of mature leaves. The deposition of callose around mesophyll cells bordering the wound site was indistinguishable in wild-type and gsl7 plants (Fig. 2C).
To discover the location of GSL7-dependent callose in the phloem and to establish whether GSL7 is responsible for callose in the phloem of intact as well as wounded plants, we examined transmission electron micrographs of longitudinal sections of flowering stems and older regions of seedling roots. To prevent the production of wound callose, tissues were excised into fixative and immediately vacuum infiltrated. In wild-type plants, sieve plate pores in both flowering stems and roots were lined with callose (Figs. 2 and 3; Barratt et al., 2009). By contrast, no callose lining was visible in sieve plate pores in either stems or roots of the gsl7 mutant. Immunogold labeling with a callose-specific antiserum confirmed the presence of callose in the lining of the sieve plate pores in the wild type but failed to detect callose in the pore walls of the mutant (Fig. 2; data not shown).
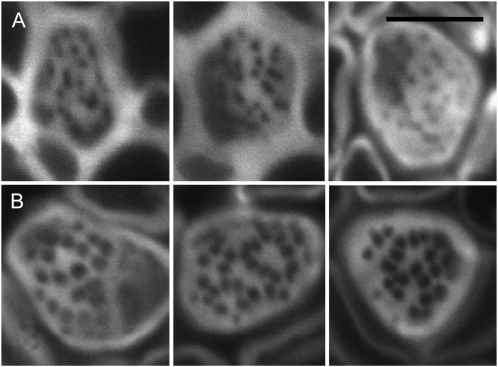
To obtain more information about the impact of the loss of GSL7 on sieve plate anatomy, we used high-resolution confocal laser scanning microscopy on thick sections of flowering stems after rapid fixation and pseudo-Schiff propidium iodide staining. As reported previously (Truernit et al., 2008), this allowed transverse and longitudinal resolution of sieve plates (Fig. 4). In wild-type plants, sieve plate pores were large and unobstructed (Fig. 4A). Measurements of 20 sieve plates (from at least two stems in each of two separate preparations) gave pore diameters between 0.30 and 0.55 μm. These data accord well with previous estimates for Arabidopsis stems of approximately 0.5 μm (1-week-old stems; Truernit et al., 2008) and 0.52 μm (stems of unspecified age; Thompson and Wolniak, 2008). By contrast, although pore numbers per sieve plate were approximately the same as in wild-type plants, sieve plate pores in stems of mutant plants appeared much smaller in diameter than those in wild-type plants. In two out of three independent preparations, pores were barely visible (Fig. 4B). In the third preparation, pores were of greater diameter (Fig. 5). Pore diameter was 0.22 ± 0.01 μm (mean ± se of measurements on 13 sieve plates), whereas in equivalent tissue from wild-type plants grown at the same time it was 0.35 ± 0.01 μm (mean ± se of measurements on 10 sieve plates). The sieve plates themselves were similar in diameter in mutant and wild-type plants. In the preparation shown in Figure 5, sieve plate diameter was 5.40 ± 0.12 μm (10 sieve plates) in wild-type plants and 5.19 ± 0.20 μm (13 sieve plates) in mutant plants. This is comparable with previous estimates for Arabidopsis stems of between 5 and 6.2 μm (Truernit et al., 2008).
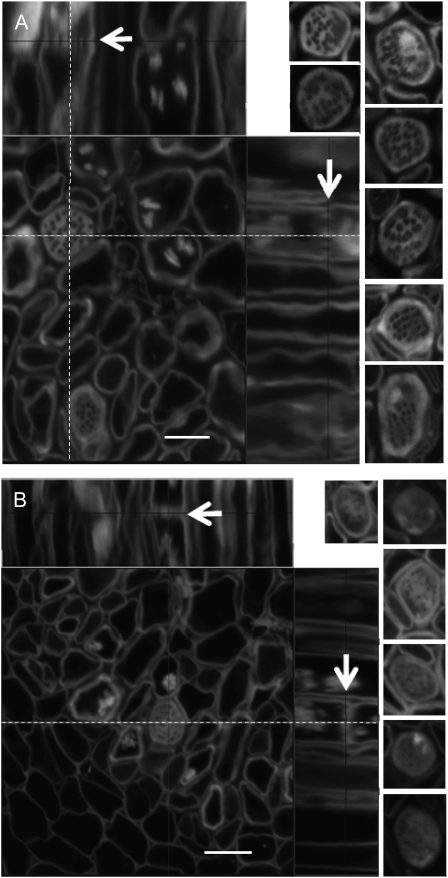
Figure 4.
Sieve plate pores in the base of the stem are small or partially occluded in the gsl7 mutant. Main images are confocal laser scanning micrographs of thick sections from the basal 4 cm of the flowering stems of 6-week-old plants. At this stage, wild-type stems were approximately 13 cm high and gsl7 mutant stems were 10 to 11 cm high (Supplemental Fig. S3). Images were processed using Zeiss LSM Image Browser software. Sieve elements are seen in transverse section and as longitudinal projections (top and right). The dotted lines indicate the positions of the longitudinal sections with respect to the transverse sections. The arrows in the longitudinal sections are positioned at the plane of the transverse section and indicate the same sieve plate. The small images to the left of the main images are typical individual sieve plates seen in transverse sections, taken from independent preparations of stems from three different batches of plants in the wild type and two batches in the case of gsl7 mutant plants. All images are at the same scale. A, The wild type. B, The gsl7 mutant. Note that sieve plate pores are smaller in diameter in all mutant examples than in wild-type examples. Bars = 5 μm.
Figure 5.
Phloem sieve plates in stems of gsl7 mutant and wild-type plants. Images are taken from the batch of gsl7 mutant plants with the largest sieve plate pores. Pore sizes were smaller in two further independently grown batches of mutant plants (Fig. 4). Images were obtained as described for Figure 4. All images are at the same magnification. A, The gsl7 mutant. B, The wild-type, grown under the same conditions and at the same time as the gsl7 mutant plants shown in A. Bar = 5 μm.
Stems and Floral Parts Are Reduced in Size in the gsl7 Mutant
The gsl7 mutants displayed distinct but mild phenotypes during vegetative growth (Supplemental Fig. S3). The growth rate and general morphology of gsl7 and wild-type rosettes were very similar, as were the root and aboveground biomass at the point of emergence of the flowering stem (Fig. 6A; Supplemental Table S3). The degree of purple pigmentation of gsl7 rosettes was greater than in wild-type plants of the same age, particularly in older rosettes (Supplemental Fig. S3).
Figure 6.
Vegetative and reproductive growth of the gsl7 mutant. A, Top, plants after 45 d of growth in 8 h of light and 16 h of dark; bottom, plants after about 35 d of growth in 12 h of light and 12 h of dark. Plants to the left of the dashed line are the wild type, and those to the right are gsl7 mutants grown in the same tray. B, Plants of the wild type (left), gsl7-1 (middle), and gsl7-2 (right) after 49 d of growth in 12 h of light and 12 h of dark. C, Apex of flowering stems after 42 d of growth of wild-type (left) and gsl7 mutant (right) plants photographed from above. Photographs of plants of this age from the side are shown in Supplemental Figure S4. D, Typical flowers from the apex of the flowering stem after 42 d of growth from a gsl7 mutant (top) and a wild-type (bottom) plant. The two images are at the same magnification. E, Fully expanded siliques from flowering stems after 42 d of growth from a gsl7 mutant (top) and a wild-type (bottom) plant. The two images are at the same magnification.
Marked differences between gsl7 and wild-type plants became apparent as soon as the flowering stem started to elongate. The stem was thinner in gsl7 than in wild-type plants (Supplemental Table S3), its growth rate was slower, and the main stem and side branches of gsl7 plants typically achieved only about half the height of those of wild-type plants. Side branches were longer in relation to the main stem in gsl7 than in wild-type plants (Fig. 6B; Supplemental Fig. S3). The number and size of flowers and the length of peduncles and siliques were also reduced in gsl7 mutants. Whereas sepals were of near-normal size, the lengths of petals, stamens, and carpels were reduced by one-third to one-half in mutant relative to wild-type plants (Fig. 6, C–E).
Phloem Transport Rates and Carbohydrate Availability Are Reduced in the Inflorescences of the gsl7 Mutant
We reasoned that the reduced size of stems and floral parts in the gsl7 mutants could be due to a limited supply of Suc from the leaves brought about by malfunction of phloem sieve elements in the stem.
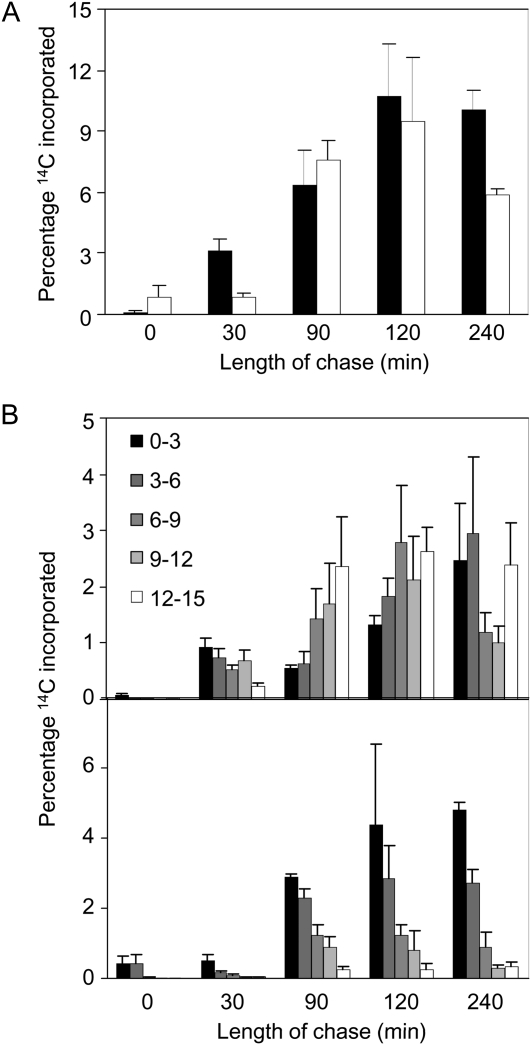
To investigate the efficiency of the transport of Suc through the flowering stem, single leaves of intact plants with 15-cm-high flowering stems were exposed to 14CO2 in the light for 5 min. Progression of assimilated 14C up the main flowering stem was measured at intervals during a 240-min chase period in the light in air (Fig. 7). Although different individual plants incorporated different amounts of 14C, there was no difference between wild-type and mutant plants and the pattern of distribution of 14C in the plant was not related to the total amount incorporated. Over the first 120 min of the chase period, the percentage of total incorporated 14C found in the stem rose to values of around 9% in both wild-type and mutant plants (Fig. 7A). However, the distribution of 14C within the stem differed markedly between the genotypes. In wild-type plants, a large fraction of the 14C rapidly reached the top 3 cm of the flowering stem (12–15 cm; Fig. 7B). This section contained 26% or more of the total label in the stem from 90 min onward. In gsl7 plants, the biggest proportion of label remained in the lowest 3 cm of the stem throughout the chase (0–3 cm; Fig. 7B). This lowest section contained at least 40% of the total 14C in the stem throughout the chase period. The percentage of 14C in the top section rose very slowly during the chase period to a maximum value of only 3.8% of the total 14C in the stem.
Figure 7.
Rates of 14C movement in the flowering stem are very different in wild-type and gsl7 mutant plants. A 5-min pulse of 14CO2 was supplied to a mature leaf of plants with flowering stems 15 cm high, and then plants were incubated in normal air for up to 240 min. Plants were harvested immediately after the pulse and at intervals thereafter. 14C was measured in the supplied leaf and in 3-cm sections of the stem, and contents of stem sections are expressed as percentages of the total 14C content (sum of supplied leaf and stem contents). Values are means of measurements on five plants for each time point, and se is indicated. A, Percentage of 14C present in the stem. Black bars, the wild-type; white bars, the gsl7 mutant. B, Percentage of 14C present in successive 3-cm sections of the stem. Section 0 to 3 is the basal section, and section 12 to 15 is the top section. Top graph, wild-type plants; bottom graph, gsl7 mutant plants.
The difference in inflorescence architecture between wild-type and gsl7 plants is unlikely to account for the radical differences in the rate of movement of 14C in the stem. First, we compared mutant and wild-type plants with stems of the same height (rather than plants of the same age, which would have stems of different heights). Second, although gsl7 inflorescences have different side-branch growth rates from wild-type plants, the lowest 3 cm of the stem had no or only single branches in all the plants we used. A more likely explanation of the difference in 14C movement is that the rate of phloem transport in stems of gsl7 mutants is much lower than that of wild-type plants.
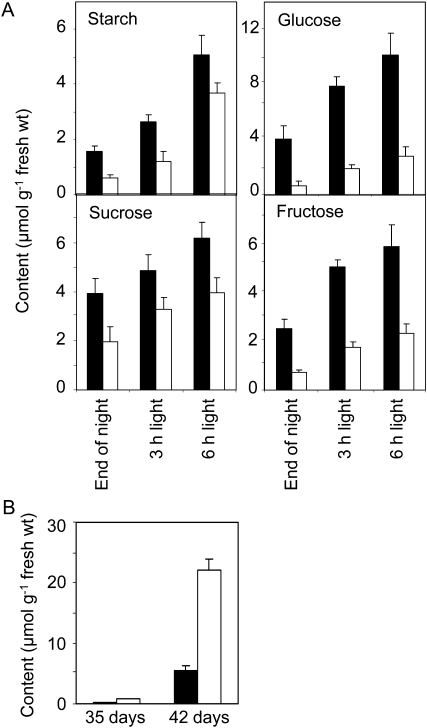
Restricted phloem transport in the flowering stem of gsl7 mutants might be expected to lead to reduced carbohydrate availability at the top of the inflorescence. Accordingly, we measured carbohydrates in the terminal cluster of flowers and buds in mutant and wild-type plants. At the end of the night, gsl7 mutant inflorescences had half of the Suc content of wild-type inflorescences, 40% of the starch content, 30% of the Fru content, and less than 20% of the Glc content. These differences decreased through the day as contents of all carbohydrates increased (Fig. 8).
Figure 8.
Carbohydrate contents of the apices of flowering stems and of leaves of mutant and wild-type plants. Values are means of measurements on five samples per time point, and se is indicated. Black bars, the wild type; white bars, the gsl7 mutant. A, Starch and sugars in apices of flowering stems. Plants were grown for 46 d in 12 h of light and 12 h of dark. Samples were harvested at the end of the night and 3 and 6 h into the light period. Each sample consisted of two flower heads (approximately the apical 1 cm of the main stem, consisting of the terminal cluster of flowers and buds). B, Starch in leaves. Plants were grown for either 35 or 42 d. Each sample was harvested at the end of the night and consisted of three fully expanded leaves from a single plant.
Iodine staining revealed that the difference in starch content between mutant and wild-type inflorescences was particularly marked in the parenchymatous pith at the top of the stem. At the end of the night, this tissue stained darkly in wild-type plants but not in mutant plants (Supplemental Fig. S4).
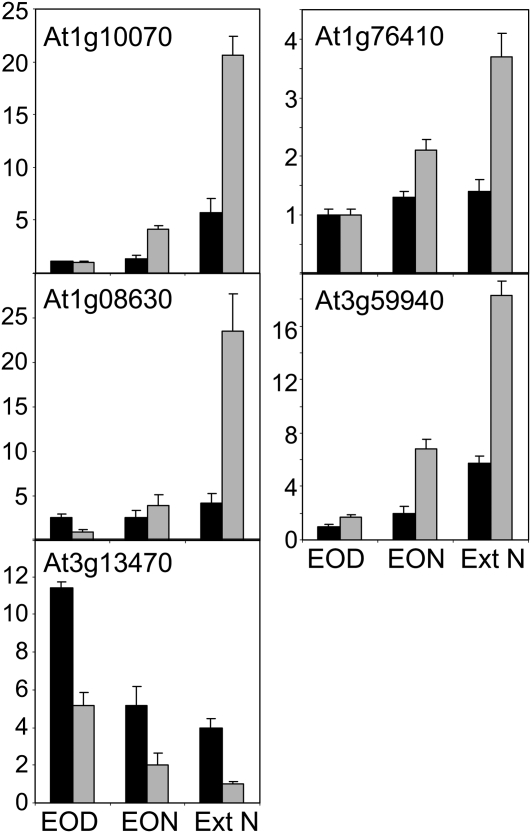
In leaves, abnormally low carbohydrate contents lead to widespread changes in gene expression. This “starvation response” is seen, for example, when the night is extended beyond the normal dawn and in mutants defective in the storage or utilization of starch reserves during the night (Smith and Stitt, 2007). The starvation response can be monitored by measuring transcript levels of genes for which expression is known to be specifically responsive to carbohydrate levels (Gibon et al., 2004; Bläsing et al., 2005; Osuna et al., 2007; Usadel et al., 2008; Graf et al., 2010). We investigated whether the reduced carbohydrate levels in the terminal cluster of flowers and buds of gsl7 inflorescences triggered a starvation response by determining the expression of four starvation-induced genes (At1g76410, At3g59940, At1g08630, and At1g10070) and one sugar-induced gene (At3g13470; Supplemental Table S4). Transcript levels of the four starvation-induced genes were very low in both wild-type and mutant inflorescences at the end of the day. In the wild type, transcript levels remained low throughout the normal night. In the gsl7 mutant, transcript levels of all four genes increased significantly during the night. Levels for three of the genes were significantly higher in mutant than in wild-type inflorescences at the end of the night (Fig. 9). An extension of the night by 4 h markedly increased this difference: levels were 2.7 to more than five times higher in mutant than in wild-type inflorescences. For the sugar-induced gene, transcript levels in wild-type inflorescences were more than twice as high as those in mutant inflorescences at the end of the day. This difference was maintained as transcript levels fell through the night and during the extended night (Fig. 9).
Figure 9.
Expression of starvation marker genes in the apices of flowering stems of mutant and wild-type plants. Plants were grown for 42 d in 12 h of light and 12 h of dark. Samples were harvested at the end of the night (EON), end of the day (EOD), and after a 4-h extension of the night (Ext N). Each sample consisted of four flower heads (approximately the apical 1 cm of the main stem, consisting of the terminal cluster of flowers and buds). Total RNA was extracted, cDNA was synthesized, and transcript levels were measured by quantitative real-time PCR and normalized against a UBIQUITIN10 control. Details of genes are given in Supplemental Table S4. Values are means of measurements on five samples per time point, and sd is indicated. Black bars, the wild type; gray bars, the gsl7 mutant.
Reduced capacity for the transport of sugars to the inflorescence might be expected to lead to an accumulation of carbohydrate in leaves. Although the pattern of carbohydrate turnover in leaves of flowering Arabidopsis plants varies somewhat between batches of plants (B. Kular, M. Pike, and A.M. Smith, unpublished data), we consistently observed elevated levels of starch in leaves of the gsl7 mutant from the onset of flowering. Leaves of 7-week-old mutant plants stained more darkly with iodine at the end of the night than leaves of wild-type plants of the same age (Supplemental Fig. S4). Mutant leaves had three times more starch than wild-type leaves at the end of the night at 5 weeks old and four times more at 6 weeks old (Fig. 8B).
Suc Feeding Increases Flower Size in the gsl7 Mutant
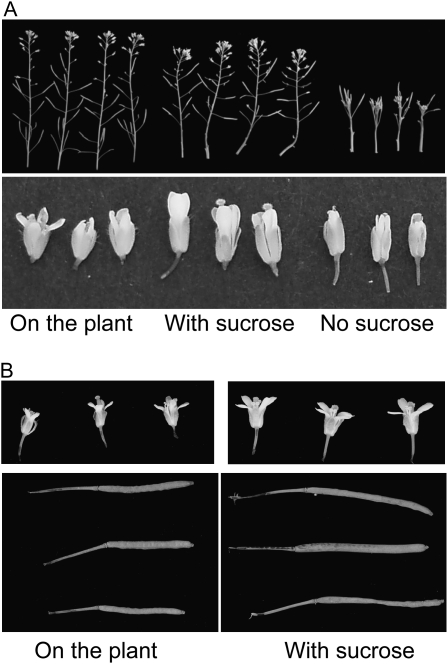
If the reduced growth of floral parts of the gsl7 mutant is indeed due to limited carbohydrate availability, an increased supply of Suc might be expected to increase flower size. Accordingly, we identified plants of 6 to 7 weeks old with inflorescences of the same height (total stem length of approximately 10 cm), removed the top 2 cm of the flowering stem from some of them, and placed the bases of the cut stems in a nutrient solution with or without 167 mm (3%, w/v) Suc. After 7 d of further growth under the same conditions of light, temperature, and humidity, cut inflorescences were compared with those developed for the same length of time on the intact control plants. Although the size of flowers and siliques on the cut inflorescences varied somewhat between experiments, those incubated with Suc were in every case (three independent experiments) markedly larger than those developed on the plant (Fig. 10). This effect was entirely dependent on the presence of Suc in the nutrient solution. Almost no growth occurred in cut inflorescences in the nutrient solution without Suc (Fig. 10) or in the nutrient solution in which Suc was replaced by the same concentration of mannitol (data not shown).
Figure 10.
Effects of Suc feeding on the inflorescence of the gsl7 mutant. The apical 2 cm of flowering stems of half of a batch of gsl7 plants was harvested when flowering stems were approximately 10 cm in height, and the cut base was incubated in a solution with or without 167 mm Suc. Cultured stems and the remaining intact plants were held in the same environmental conditions for 7 d. A, Inflorescences (top) and flowers (bottom) of intact plants (left), stems cultured with Suc (middle), and stems cultured without Suc (right). B, Flowers (top) and siliques (bottom) from an experiment on a separately grown batch of plants. Left, Organs from intact plants; right, organs from stems cultured with Suc. The two images of flowers are the same magnification, as are the two images of siliques.
DISCUSSION
GSL7 Is Responsible for Callose Deposition in Sieve Plate Pores
We provide evidence that the callose synthase GSL7 is exclusively responsible for the synthesis of callose associated with the pores of sieve plates. In the root, transcription of GSL7 is confined to the phloem. Loss of expression of this gene results in complete loss of the callose lining of sieve plate pores between phloem sieve elements in both roots and flowering stems. Thus, it appears that none of the other 11 GSL isoforms in the plant is able to compensate for loss of GSL7 with respect to the synthesis of callose in stem or root phloem. Callose in several other tissues of the plant seems to be unaffected. Plants lacking GSL7 do not display the loss of fertility seen in plants in which callose is not deposited during male gametogenesis (gsl8 and gsl10 mutants; Töller et al., 2008) or the extreme growth phenotypes seen when callose deposition on cell plates during cytokinesis is compromised (gsl8 mutants; Töller et al., 2008; Chen et al., 2009). Loss of GSL7 does not affect the formation of wound callose in leaf mesophyll cells and in parts of the stem other than the phloem. Thus, the synthesis of callose via GSL7 seems to be confined to the phloem sieve elements.
Our data indicate that GSL7 is responsible for the synthesis of callose in the sieve plate pore both as part of the normal process of phloem maturation in intact plants and also in response to wounding. First, callose is present in phloem sieve plates of wild-type plants but absent from those of gsl7 mutants when precautions are taken to avoid a wound response by fixing excised tissue as rapidly as possible. Second, the striking reduction in growth of the stem in the gsl7 mutant is consistent with a role for GSL7 in the intact, nonwounded plant. Third, callose is not deposited in the phloem of gsl7 mutant plants after wounding, implying that GSL7 is required for the rapid occlusion of the sieve plate by callose in response to wounding. The pattern of expression of GSL7 is consistent with roles in both the developing and the mature phloem. We detected GSL7 transcript in phloem initial cells in the root by in situ hybridization. GSL7 protein synthesized in these cells is likely to be responsible for callose deposition during sieve plate formation. GSL7 transcript is also present in the mature phloem (detected by microarray analysis of marked, sorted cell types in the root; Arex database, http://www.arexdb.org/). We suggest that GSL7 protein synthesized in the companion cells of the mature phloem is responsible for the production of callose on the sieve plates of the sieve elements in response to wounding. It is also possible that GSL7 is involved in callose turnover in mature sieve plate pores in intact plants. Turnover of callose deposits in the neck regions of plasmodesmata is proposed to alter the diameter of the plasmodesmatal pore and thus control plasmodesmatal fluxes (Levy et al., 2007). Similarly, turnover of callose in the sieve plate pore in response to developmental and/or environmental factors may alter the diameter of the pore and thus modulate phloem conductivity.
Callose Synthesis in the Phloem May Differ from That in Other Parts of the Plant
It has been suggested that UDPglucose for the synthesis of cell wall components is supplied via a specific association between SUS and cell wall biosynthetic enzymes (Amor et al., 1995; Verma and Hong, 2001; Salnikov et al., 2003; Persia et al., 2008). SUS is frequently associated with plasma membranes, consistent with the idea that it supplies UDPglucose to either cellulose synthase or callose synthase or both. However, complete loss of SUS (in a sus1sus2sus3sus4 mutant) from all cell types except the phloem in Arabidopsis has negligible effects on growth in standard conditions (Barratt et al., 2009). This suggests that SUS is not required for either cellulose or callose synthesis in most organs of the plant. Thus, UDPglucose generated in the cytosol by an alternative route (e.g. UDPglucose pyrophosphorylase) must be sufficient.
The situation in the phloem appears to be different. We showed previously that the presence of callose in the sieve plate pores is partially dependent on two isoforms of SUS located in the phloem sieve element, SUS5 and SUS6. A mutant lacking both of these isoforms had much thinner layers of callose in the sieve plate pores of the stem than wild-type plants, although inflorescence growth was normal (Barratt et al., 2009). Thus, it seems likely that these SUS isoforms synthesize the UDPglucose substrate for GSL7 from Suc in phloem. Callose synthases are membrane-spanning proteins, taking substrate from the cytosol and generating callose outside the cell. The fact that SUS5 and SUS6 are exclusively and tightly associated with a cell wall fraction in extracts of Arabidopsis tissues (requiring high salt and detergent treatments for their release; Barratt et al., 2009) suggests that they may form part of a complex involved in callose synthesis on the sieve plate. However, it seems highly unlikely that SUS5 and SUS6 are outside the cell. Both have kinetic properties, including a neutral pH optimum, similar to other, intracellular SUS isoforms (Bieniawska et al., 2007). We suggest that GSL7, SUS5, and SUS6 may form part of a tight complex that spans the plasma membrane of the sieve element and links into the wall. In this context, it is interesting that both SUS isoforms have C-terminal extensions not found in other isoforms (Baud et al., 2004); these may be involved in complex formation.
Although the callose lining of sieve plate pores is reduced in thickness in the sus5sus6 mutant, it is not abolished. This implies that GSL7 can obtain substrate from another source, at least in this mutant. It is possible that the SUS1 isoform fulfills this role. Unlike SUS2, SUS3, and SUS4, which are entirely soluble, a minor fraction of the SUS1 protein is consistently present in the cell wall fraction of Arabidopsis extracts (Barratt et al., 2009).
The specific requirement for SUS for normal callose synthesis via GSL7 in the sieve element may reflect the highly unusual metabolic status of the sieve element. The concentration of Suc is extremely high, and concentrations of many other primary metabolites are likely to be low in relation to other cell types. Direct utilization of Suc to provide UDPglucose for callose synthesis, via SUS, may thus be favored over the synthesis of UDPglucose via UDPglucose pryophosphorylase from Glc-1-P. A direct association of SUS with the callose synthase complex may be essential to ensure an adequate concentration of UDPglucose at the active site. The source of UDP for UDPglucose synthesis in the sieve element remains an open and interesting question.
The Callose Lining of Sieve Plate Pores Is Necessary for Normal Phloem Transport in the Stem
Our results provide direct and unambiguous evidence that the callose lining of the sieve plate pore is essential for normal phloem transport in the flowering stem. In gsl7 mutants, the growth of the stem, flowers, and siliques is strongly reduced. Labeling experiments showed that transport of Suc in the flowering stem was very strongly reduced in the gsl7 mutant. Although the rate of entry of 14C into the stem after supply of 14CO2 to a leaf was approximately the same in wild-type and gsl7 mutant plants, its rate of movement to the top of stem of the same height was greatly retarded in the mutant. The most likely explanation of this difference is that loss of callose from the sieve plate pores drastically reduces phloem conductivity.
Several different features of our results suggest that restricted transport of Suc in gsl7 phloem resulted in metabolic and transcriptional changes in the inflorescences, which in turn account for their reduced size. Starch and sugar contents were lower in gsl7 than in wild-type inflorescences, especially at the end of the night. Photosynthesis in the inflorescence of the mutant during the light period may compensate to some extent for the reduced supply of Suc via the phloem. The reduction in carbohydrate availability in the mutant inflorescence enhanced the expression of starvation genes and repressed the expression of a sugar-activated gene. Induction of expression of the starvation genes in leaves is associated with a strong reduction or cessation of growth in both leaves and roots (Smith and Stitt, 2007; Graf et al., 2010, and refs. therein).
Further evidence for a link between reduced growth of the inflorescence of gsl7 plants and Suc supply is provided by experiments in which Suc was supplied directly to gsl7 inflorescences via the cut stem. Suc supplied in this way probably moves to the flowers via the xylem rather than the phloem. The result was an increase in size of all floral parts relative to those on intact mutant plants. These data are consistent with the idea that a lack of Suc alone could account for the reduced growth. None of our experiments preclude the possibility that reduced growth of the inflorescence is at least in part due to reduced transport of a hormone or other signaling molecule necessary for normal growth. However, the simplest interpretation is that reduced growth results from low carbohydrate availability brought about by reduced phloem conductivity in the stem.
The effect of the loss of GSL7 is much more marked in the inflorescence than in the rosette. The inflorescence is a major sink for carbon from the rosette, and the marked phenotype likely reflects its dependence on, and distance from, source organs. It is interesting that there is no marked impact of the gsl7 mutation on root mass in mature rosettes even though root sieve plate pores lack callose. This difference in impact of the mutation between stems and roots may reflect a higher demand for carbon by the developing inflorescence than by the root system. We have not examined the phloem of developing (sink) leaves and petioles. It remains possible that another enzyme in part compensates for the loss of GSL7 in these organs.
The Callose Lining May Determine the Flow Characteristics of the Sieve Plate Pore
It is not immediately apparent why loss of the callose lining of the sieve plate pore should cause a dramatic reduction in phloem transport in the stem. One possibility is that callose is required for normal sieve plate pore development. Some authors have proposed that during this process existing wall components around plasmodesmata are replaced by callose, which is then degraded to form a pore (see introduction). If this is the case, loss of callose is expected to prevent or very seriously restrict pore formation. Our data do not support this idea. It is clear that degradation of noncallose wall material in the gsl7 mutant allows the formation of pores at approximately the same frequency as in wild-type plants. From transmission electron microscopy (TEM) images, the pores in gsl7 plants appear to be of approximately the same dimensions as those in wild-type plants. Thus, we suggest that callose deposition is not required for pore formation per se. However, callose formation during pore development may nonetheless be important in determining the flow characteristics of the pore. Some authors have noted that deposition of callose on the face of the end wall where the pore will form is associated with the cessation of wall thickening at that point, so that in the mature sieve plate, the pore openings are in slight depressions in the face of the wall (Evert et al., 1966; Deshpande, 1974, 1975). It is proposed that callose actually restricts wall growth. The length of the pore is important in determining phloem conductivity (Thompson and Holbrook, 2003), so this proposed role for callose may be of significance in the function of the mature phloem. Our TEM images are consistent with the suggestion that callose deposition may reduce effective pore length, although further work would be required to confirm this point. Whereas pore openings in the gsl7 mutant tend to be flush with the surface of the wall, those in wild-type plants are surrounded by callose-lined depressions (Fig. 2).
An alternative explanation for the importance of the callose in the sieve plate pore is that it provides necessary mechanical strength to the pore walls. Phloem conductivity and transport velocity are highly dependent on pore diameter (Thompson and Holbrook, 2003). In an intact plant, the pores must remain open against the mechanical stress imposed by the mass flow of viscous material in the sieve element. It seems likely that the callose lining renders the sieve plate pore a rigid, noncompressible tube with good flow characteristics. In the absence of a rigid lining, the diameter of the pore may vary with flow velocity, the walls tending to deform under pressure. There may also be variation within the cross-section of the wall in the tendency to deform under pressure, to the further detriment of the flow characteristics. Although the mechanical properties of callose are not well understood, it is clear that it provides resistance to both tension and compression stress in the walls of pollen tubes. Enzymatic removal of callose from pollen tubes halved the stiffness (n m−1) of the side wall of the tube (Parre and Geitmann, 2005). We suggest that in the gsl7 mutant, the walls of the sieve plate pore tend to collapse under mass flow, reducing phloem conductivity and hence the supply of Suc to the inflorescence. It is not yet clear why our confocal laser scanning microscopy views of sieve plates showed a large reduction in the apparent diameter of sieve plate pores in mutant stems relative to wild-type stems when this was not seen in TEM images. This difference could reflect plasticity of the wall of the pore of the mutant. In the absence of a rigid callose lining, the pore walls may swell or shrink differentially in the two different fixation and preparation techniques used for TEM and confocal laser scanning microscopy.
In summary, we suggest that callose deposition is not required for pore formation per se but is a very important determinant of the flow characteristics of the pore. Callose may influence both the effective length of the pore, by preventing wall thickening around the developing pore, and its flow characteristics, by providing a rigid lining and hence an invariant diameter under mass flow conditions.
After acceptance of this paper, we became aware of another paper published online that also reports the importance of GSL7 for callose synthesis in the phloem (Xie et al., 2010). The authors propose that the loss of callose in gsl7 mutants hampers sieve plate pore formation, whereas our work indicates that pore function rather than pore formation is hampered. Further research will be required to resolve this issue.
MATERIALS AND METHODS
Plant Material
Unless otherwise stated, Arabidopsis (Arabidopsis thaliana) plants were grown in compost at 20°C with a 12-h-light/12-h-dark cycle, 150 to 200 μmol quanta photosynthetically active radiation m−2 s−1, and 75% relative humidity. For seedlings and seedling roots, seeds were surface sterilized, sown on plates of solid medium, and grown vertically at 22°C with 6 h of dark and 18 h of light according to Barratt et al. (2009). Homozygous mutants were identified by screening T-DNA insertion lines from the SALK collection and SAIL lines with gene- and T-DNA-specific primers. Studies were carried out on the gsl7-1 mutant unless otherwise stated. Primer sequences are given in Supplemental Table S5. Genomic DNA was extracted from leaves according to Krysan et al. (1996). The PCR products obtained using T-DNA left border primers and gene-specific primers were sequenced to confirm the locations of the inserts. A wild-type line was selected as a control for each mutant line from the segregating F2 population from which the mutant was selected.
Inflorescence Culture
Flowering stems of 6- to 7-week-old plants were cut 2 cm below the apex and then immediately recut under sterile water. Cut ends were sterilized in 0.025% (v/v) sodium hypochlorite for 5 min. The cut ends were then inserted through holes in the lid of a petri dish containing filter-sterilized medium (one-quarter-strength Arabidopsis nutrient solution [Arteca and Arteca, 2000]) with 3% (w/v) Suc, 20 mm l-Gln, and 10 mm l-Asn to a depth of about 0.75 cm. The stems were cultured for 7 d, and the medium was changed daily. Culture was in the same controlled-environment conditions used for the growth of intact plants.
In Situ Hybridization
Primer sequences are given in Supplemental Table S5. A DNA template for a partial cDNA for GSL7 was generated from a stem cDNA library by PCR, and T3 (sense) and T7 (antisense) promoter sequences were incorporated by PCR to give 350-bp probes. Whole-mount in situ hybridization on 4-d-old seedlings using digoxigenin-labeled probes was carried out according to Carol et al. (2005) and Barratt et al. (2009).
Microscopy
For confocal laser scanning microscopy on thick sections, the basal 4 cm of primary flowering stems of 6-week-old plants was excised and immediately fixed in ice-cold 50% (v/v) methanol and 10% (v/v) acetic acid for at least 24 h. The stem was recut, and sections of the basal 2 cm were sectioned under absolute ethanol on a Vibratome. Sections (200 μm) were transferred to fixative and then subjected to pseudo-Schiff propidium iodide staining (Truernit et al., 2008). Samples were viewed with a confocal microscope at an excitation wavelength of 488 nm.
TEM and immunogold labeling of phloem in longitudinal sections of the basal 4 cm of primary flowering stems of 6-week-old plants and of the basal 2 cm of the main root of 11-d-old seedlings, grown on solid medium, were carried out as described by Barratt et al. (2009). To ensure rapid cessation of metabolism, excised tissue was immediately placed into fixative (2.5% [v/v] glutaraldehyde in 0.05 m sodium cacodylate, pH 7.3), rapidly sliced into 1-mm pieces, and then vacuum infiltrated with fixative.
Aniline Blue and Iodine Staining
Hand-cut sections of primary flowering stems from 6- to 7-week-old plants were stained with 0.1% (w/v) aniline blue (water soluble; Sigma) in 0.1 m sodium phosphate (pH 9.0) and then viewed with a UV epifluorescence microscope.
For leaf wounding, fully expanded leaves from 6-week-old plants were punctured with a plastic tip and then harvested the next day. After removal of chlorophyll by washing in methanol, leaves were rinsed in water and stained overnight with aniline blue as above.
For roots, whole 4-d-old seedlings were vacuum infiltrated with 0.1% (w/v) aniline blue in 0.1 m sodium phosphate (pH 9.0) and incubated in the dark for 1 to 2 h.
Starch in the apical region of primary flowering stems was visualized by staining with Lugol’s iodine solution after removal of chlorophyll at 80°C in 80% (v/v) ethanol.
Metabolite Assays
For starch and sugar measurements, tissue was frozen in liquid nitrogen immediately after harvest. Tissue was extracted with dilute perchloric acid. Sugars were assayed enzymatically on the neutralized soluble fraction using spectrophotometric assays coupled to NAD reduction via Glc-6-P dehydrogenase (Chia et al., 2004). Starch was assayed as Glc in the insoluble fraction after washing, gelatinization, and enzymatic digestion with α-amylase and α-amyloglucosidase (Smith and Zeeman, 2006).
Quantitative Real-Time PCR Analysis
Each sample consisted of the terminal cluster of flowers and buds from the primary flowering stems of four 6-week-old plants. Total RNA was isolated using the RNeasy kit (Qiagen) and digested with RQ1 RNase-Free DNase 1 (Promega). cDNA was synthesized using the SuperScript 111 kit (Invitrogen). Quantitative real-time PCR was performed on a 96-well plate with a DNA Engine Opticon 2 Real-Time PCR Detection System (Bio-Rad) in a reaction mix containing 5 μL of SYBR Green JumpStart Taq ReadyMix (Sigma). Thermal cycling was 95°C for 2 min and 40 cycles of 95°C for 10 s, 60°C for 1 min. Primer sequences are given in Supplemental Table S5. Values were normalized against a UBIQUITIN10 control, and relative expression was calculated by setting the wild-type value to 1.
14CO2 Pulse-Chase Labeling
Labeling experiments were performed 2 h into the photoperiod on intact flowering plants in principal growth stage 6 (Boyes et al., 2001) with a primary flowering stem height of 15 cm. In a sealed reservoir chamber, 14CO2 with a specific activity of 59 mCi mmol−1 was released by the acidification of sodium 14C-bicarbonate. A small plexiglass chamber connected to the reservoir was clamped to a mature adult leaf of a plant illuminated with 150 μmol quanta photosynthetically active radiation m−2 s−1. 14CO2 was circulated for 5 min, after which the plexiglass chamber was removed and the plants were kept in normal air for a chase period of 0 to 240 min.
Three-centimeter sections of the flowering stem and the labeled leaf were harvested separately into tubes containing 5 mL of 80% (v/v) boiling ethanol. Samples were homogenized using a glass homogenizer and successively extracted for 5 min in 3 mL of 80% (v/v) ethanol, 50% (v/v) ethanol, 20% (v/v) ethanol, water, and finally 80% (v/v) ethanol. Between each extraction, insoluble material was removed by centrifugation. For each sample, the combined supernatants were dried down under an air stream. This fraction was redissolved in 2 mL of water. Any water-insoluble residue was dissolved in 1 mL of 98% (v/v) ethanol. The insoluble material was resuspended in tissue solubilizer (NCS; GE Healthcare) and dissolved overnight at 23°C. Liquid scintillation counting was used to determine the 14C in each fraction.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Location in roots of transcripts for SUS5, SUS6, and GSL7.
Supplemental Figure S2. Absence of GSL7 transcript in two lines carrying T-DNA insertions in the GSL7 gene.
Supplemental Figure S3. Growth of wild-type and gsl7 mutant plants.
Supplemental Figure S4. Loss of starch from the inflorescence and altered starch turnover in the leaves of the gsl7 mutant.
Supplemental Table S1. Top 20 genes coexpressed with SUS5 (At5g37180), from ATTED-II.
Supplemental Table S2. Top 40 genes coexpressed with SUS5 (At5g37180), from CSB.DB.
Supplemental Table S3. Weights of rosettes, leaves, and stem bases of wild-type and mutant plants.
Supplemental Table S4. Starvation marker genes used in this study.
Supplemental Table S5. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Susan Bunnewell and Paul Derbyshire (both John Innes Centre) for help with TEM and advice on in situ hybridization, respectively.
References
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP. (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92: 9353–9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteca RN, Arteca JM. (2000) A novel method for growing Arabidopsis thaliana plants hydroponically. Physiol Plant 108: 188–193 [Google Scholar]
- Barratt DHP, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM. (2009) Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc Natl Acad Sci USA 106: 13124–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Vaultier MN, Rochat C. (2004) Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J Exp Bot 55: 397–409 [DOI] [PubMed] [Google Scholar]
- Bieniawska Z, Barratt DHP, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM. (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49: 810–828 [DOI] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN. (2003) A gene expression map of the Arabidopsis root. Science 302: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M. (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck GB, Cronshaw J. (1965) The fine structure of differentiating sieve tube elements. J Cell Biol 25: 79–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J. (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. (2007) A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806 [DOI] [PubMed] [Google Scholar]
- Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. (2005) A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438: 1013–1016 [DOI] [PubMed] [Google Scholar]
- Cartwright DA, Brady SM, Orlando DA, Sturmfels B, Benfey PN. (2009) Reconstructing spatiotemporal gene expression data from partial observations. Bioinformatics 25: 2581–2587 [DOI] [PubMed] [Google Scholar]
- Chen XY, Liu L, Lee E, Han X, Rim Y, Chu H, Kim SW, Sack F, Kim JY. (2009) The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiol 150: 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM. (2004) A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J 37: 853–863 [DOI] [PubMed] [Google Scholar]
- Deshpande BP. (1974) Development of the sieve plate in Saxifraga sarmentosa L. Ann Bot (Lond) 38: 151–158 [Google Scholar]
- Deshpande BP. (1975) Differentiation of the sieve plate of Cucurbita: a further view. Ann Bot (Lond) 39: 1015–1022 [Google Scholar]
- Dong X, Hong Z, Chatterjee J, Kim S, Verma DPS. (2008) Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 229: 87–98 [DOI] [PubMed] [Google Scholar]
- Ehlers K, Knoblauch M, van Bel AJE. (2000) Ultrastructural features of well-preserved and injured sieve elements: minute clamps keep the phloem transport conduits free for mass flow. Protoplasma 214: 80–92 [Google Scholar]
- Eleftheriou EP. (1990) Microtubules and sieve plate development in differentiating protophloem sieve elements of Triticum aestivum L. J Exp Bot 41: 1507–1515 [Google Scholar]
- Enns LC, Kanaoka MM, Torii KU, Comai L, Okada K, Cleland RE. (2005) Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol Biol 58: 333–349 [DOI] [PubMed] [Google Scholar]
- Esau K, Thorsch J. (1985) Sieve plate pores and plasmodesmata, the communication channels of the symplast: ultrastructural aspects and developmental relations. Am J Bot 72: 1641–1653 [Google Scholar]
- Evert RF, Derr WF. (1964) Callose substance in sieve elements. Am J Bot 51: 552–559 [Google Scholar]
- Evert RF, Murmanis L, Sachs IB. (1966) Another view of the ultrastructure of Cucurbita phloem. Ann Bot (Lond) 30: 563–585 [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M. (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39: 847–862 [DOI] [PubMed] [Google Scholar]
- Graf A, Schlereth A, Stitt M, Smith AM. (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci USA 107: 9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao P, Liu C, Wang Y, Chen R, Tang M, Du B, Zhu L, He G. (2008) Herbivore-induced callose deposition on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol 146: 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Zhang Z, Olson JM, Verma DPS. (2001) A novel UDP-glucose transferase is part of the callose synthase complex and interacts with phragmoplastin at the forming cell plate. Plant Cell 13: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Chen XY, Rim Y, Han X, Cho WK, Kim SW, Kim JY. (2009) Arabidopsis glucan synthase-like 10 functions in male gametogenesis. J Plant Physiol 166: 344–352 [DOI] [PubMed] [Google Scholar]
- Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GB. (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15: 2503–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempema LA, Cui X, Holzer FM, Walling LL. (2007) Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs: similarities and distinctions in responses to aphids. Plant Physiol 143: 849–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Tax F, Sussman MR. (1996) Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc Natl Acad Sci USA 93: 8145–8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuśnierczyk A, Winge P, Jørstad TS, Troczyńska J, Rossiter JT, Bones AM. (2008) Towards global understanding of plant defence against aphids: timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ 31: 1097–1115 [DOI] [PubMed] [Google Scholar]
- Levy A, Erlanger M, Rosenthal M, Epel BL. (2007) A plasmodesmata-associated β-1,3-glucanase in Arabidopsis. Plant J 49: 669–682 [DOI] [PubMed] [Google Scholar]
- McCormick S. (1993) Male gametophyte development. Plant Cell 5: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullendore DL, Windt CW, Van As H, Knoblauch M. (2010) Sieve tube geometry in relation to phloem flow. Plant Cell 22: 579–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Preuss D. (2005) Callose (β-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301: 969–972 [DOI] [PubMed] [Google Scholar]
- Obayashi T, Kinoshita K, Nakai K, Shibaoka M, Hayashi S, Saeki M, Shibata D, Saito K, Ohta H. (2007) ATTED-II: a database of co-expressed genes and cis elements for identifying co-regulated gene groups in Arabidopsis. Nucleic Acids Res 35: D863–D869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Gibon Y, Bläsing OE, Höhne M, Günter M, Kamlage B, Trethewey R, Scheible WR, et al. (2007) Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J 49: 463–491 [DOI] [PubMed] [Google Scholar]
- Parre E, Geitmann A. (2005) More than a leak sealant: the mechanical properties of callose in pollen tubes. Plant Physiol 137: 274–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persia D, Cai G, Del Casino C, Faleri C, Willemse MTM, Cresti M. (2008) Sucrose synthase is associated with the cell wall of tobacco pollen tubes. Plant Physiol 147: 1603–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae AL, Harris PJ, Bacic A, Clarke AE. (1985) Composition of the cell walls of Nicotiana alata Link et Otto pollen tubes. Planta 166: 128–133 [DOI] [PubMed] [Google Scholar]
- Salnikov VV, Grimson MJ, Seagull RW, Haigler CH. (2003) Localization of sucrose synthase and callose in freeze-substituted secondary-wall-stage cotton fibers. Protoplasma 221: 175–184 [DOI] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH, Jr, Staehelin LA. (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober MS, Burton RA, Shirley NJ, Jacobs AK, Fincher GB. (2009) Analysis of the (1,3)-β-D-glucan synthase gene family of barley. Phytochemistry 70: 713–720 [DOI] [PubMed] [Google Scholar]
- Schuette S, Wood AJ, Geisler M, Geisler-Lee J, Ligrone R, Renzaglia KS. (2009) Novel localization of callose in the spores of Physcomitrella patens and phylogenomics of the callose synthase gene family. Ann Bot (Lond) 103: 749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund RD. (1997) The phloem sieve element: a river runs through it. Plant Cell 9: 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Stitt M. (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC. (2006) Quantification of starch in plant tissues. Nat Protoc 1: 1342–1345 [DOI] [PubMed] [Google Scholar]
- Spanner DC. (1978) Sieve-plate pores, open or occluded? A critical review. Plant Cell Environ 1: 7–20 [Google Scholar]
- Thiele K, Wanner G, Kindzierski V, Jürgens G, Mayer U, Pachl F, Assaad FF. (2009) The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J 58: 13–26 [DOI] [PubMed] [Google Scholar]
- Thompson MV. (2006) Phloem: the long and the short of it. Trends Plant Sci 11: 26–32 [DOI] [PubMed] [Google Scholar]
- Thompson MV, Holbrook NM. (2003) Application of a single-solute non-steady-state phloem model to the study of long-distance assimilate transport. J Theor Biol 220: 419–455 [DOI] [PubMed] [Google Scholar]
- Thompson MV, Wolniak SM. (2008) A plasma membrane-anchored fluorescent protein fusion illuminates sieve element plasma membranes in Arabidopsis and tobacco. Plant Physiol 146: 1599–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsch J, Esau K. (1988) Ultrastructural aspects of primary phloem: sieve elements in poinsettia (Euphorbia pulcherrima, Euphorbiaceae). IAWA Bull 9: 363–373 [Google Scholar]
- Töller A, Brownfield L, Neu C, Twell D, Schulze-Lefert P. (2008) Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J 54: 911–923 [DOI] [PubMed] [Google Scholar]
- Truernit E, Bauby H, Dubreucq B, Grandjean O, Runions J, Barthélémy J, Palauqui JC. (2008) High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 20: 1494–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. (2009) Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Turner A, Wells B, Roberts K. (1994) Plasmodesmata of maize root tips: structure and composition. J Cell Sci 107: 3351–3361 [DOI] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M. (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van As H. (2007) Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance water transport. J Exp Bot 58: 743–756 [DOI] [PubMed] [Google Scholar]
- van Bel AJE. (2003) The phloem, a miracle of ingenuity. Plant Cell Environ 26: 125–149 [Google Scholar]
- van Bel AJE, Ehlers K, Knoblauch M. (2002) Sieve elements caught in the act. Trends Plant Sci 7: 126–132 [DOI] [PubMed] [Google Scholar]
- Verma DPS, Hong Z. (2001) Plant callose synthase complexes. Plant Mol Biol 47: 693–701 [DOI] [PubMed] [Google Scholar]
- Voigt CA, Schäfer W, Salomon S. (2006) A comprehensive view on organ-specific callose synthesis in wheat (Triticum aestivum L.): glucan synthase-like gene expression, callose synthase activity, callose quantification and deposition. Plant Physiol Biochem 44: 242–247 [DOI] [PubMed] [Google Scholar]
- Walsh MA, Melaragno JE. (1976) Ultrastructural features of developing sieve elements in Lemna minor L.: sieve plate and lateral sieve areas. Am J Bot 63: 1174–1183 [Google Scholar]
- Xie B, Wang X, Zhu M, Zhang Z, Hong Z. (November 9, 2010) CalS7 encodes a callose synthase responsible for callose deposition in the phloem. Plant J http://dx.doi.org/10.1111/j.1365-313X.2010.04399.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.