Abstract
The organ-specific accumulation, spatial distribution, and chemical speciation of selenium (Se) were previously unknown for any species of cactus. We investigated Se in Opuntia ficus-indica using inductively coupled plasma mass spectrometry, microfocused x-ray fluorescence elemental and chemical mapping (μXRF), Se K-edge x-ray absorption near-edge structure (XANES) spectroscopy, and liquid chromatography-mass spectrometry (LC-MS). μXRF showed Se concentrated inside small conic, vestigial leaves (cladode tips), the cladode vasculature, and the seed embryos. Se K-edge XANES demonstrated that approximately 96% of total Se in cladode, fruit juice, fruit pulp, and seed is carbon-Se-carbon (C-Se-C). Micro and bulk XANES analysis showed that cladode tips contained both selenate and C-Se-C forms. Inductively coupled plasma mass spectrometry quantification of Se in high-performance liquid chromatography fractions followed by LC-MS structural identification showed selenocystathionine-to-selenomethionine (SeMet) ratios of 75:25, 71:29, and 32:68, respectively in cladode, fruit, and seed. Enzymatic digestions and subsequent analysis confirmed that Se was mainly present in a “free” nonproteinaceous form inside cladode and fruit, while in the seed, Se was incorporated into proteins associated with lipids. μXRF chemical mapping illuminated the specific location of Se reduction and assimilation from selenate accumulated in the cladode tips into the two LC-MS-identified C-Se-C forms before they were transported into the cladode mesophyll. We conclude that Opuntia is a secondary Se-accumulating plant whose fruit and cladode contain mostly free selenocystathionine and SeMet, while seeds contain mainly SeMet in protein. When eaten, the organic Se forms in Opuntia fruit, cladode, and seed may improve health, increase Se mineral nutrition, and help prevent multiple human cancers.
Selenium (Se) is a naturally occurring semimetallic trace element in alkaline seleniferous soils derived from marine sediments, including Cretaceous shale deposits found in western North America (Emmons et al., 1896). Water-soluble inorganic selenate (SeO42−) is leachable from such soils and can be evapoconcentrated to high levels in reservoirs. SeO42− is then biomagnified in the food chain by aquatic organisms, resulting in the deformation or death of fish and birds. The environmental Se pollution disaster observed at the Kesterson Reservoir National Wildlife Refuge was the first published report of these detrimental effects, which have also occurred in other Se-rich areas (Ohlendorf et al., 1986; Ohlendorf and Santolo, 1994).
The increased accumulation of naturally occurring salts, boron (B), and Se has worsened in some agricultural areas due to limited freshwater supplies, low winter rainfall, and drought conditions, which reduce leaching of salts away from the root zone. Across the globe, lands such as those located on the western side of the San Joaquin Valley (WSJV) in central California have been strongly affected by growing urban populations, changing climatic conditions, and increased governmental restrictions on water supplies used for the environmental rehabilitation of endangered fish species. Consequently, the identification of tolerant crops is critical for agriculture to survive and be sustained in this and other regions with chronic drought, accumulated salts, B, and Se. Growing Se-biofortified crops is an emerging method for the utilization of these agricultural areas, because the neutriceutical benefits of enhancing organic Se, an essential micronutrient in crops, are concomitant with the phytomanagement of inorganic Se pollutants.
Spineless prickly pear cactus (Opuntia ficus-indica USDA No. 248) is an attractive crop in this application because its dark purple fruit have medicinal benefits, and this accession tolerates drought, salt, and B while accumulating and volatilizing Se (Bañuelos and Lin, 2010). Without Se enrichment, Opuntia fruit and cladode already contain chemotherapeutic qualities that, when eaten, have been shown to help protect the human immune and nervous systems, prevent oxidative stress, help treat gastritis, hyperglycemia, arteriosclerosis, diabetes, and prostatic hypertrophy, and help prevent multiple human cancers (Frati et al., 1990; Hegwood, 1990; Palevitch et al., 1993; Dok-Go et al., 2003; Kuti, 2004; Zou et al., 2005; Feugang et al., 2006). Opuntia fruit is also considered an excellent source of mineral nutrition, being enriched in calcium, potassium, and magnesium (Feugang et al., 2006). Under optimum conditions growing in a rangeland for animal feed, Opuntia can produce up to 40 tons dry weight per ha (Guevara et al., 2009). Recently, in a 3-year field trial, Bañuelos and Lin (2010) showed that only certain varieties of spineless Opuntia can tolerate high salt and B concentrations in agricultural sediments, and these varieties exhibited only slight decreases of 20% in plant height and 35% of total fruit weight. Remarkably, in two ideally suited varieties, including USDA No. 248, the mean fruit weight was actually greater in agricultural sediment-grown varieties than when grown in good-quality control soils (Bañuelos and Lin, 2010). These novel observations indicated that these salt- and B-tolerant, Se-accumulating Opuntia varieties could be considered as an alternative crop for drainage-affected, low-water-use agricultural regions such as those of WSJV and that the growth or production of Se-enriched Opuntia may hold additional value as a Se-biofortified food (Bañuelos and Lin, 2010).
Se is an essential micronutrient for animals and humans. Food normally contains low amounts of Se, mainly in the form of selenomethionine (SeMet), while selenocysteine (SeCys) is the second most abundant. The importance of Se to human health is of global interest because Se deficiency occurs to such an extent that many inhabitants of Europe, Australia, New Zealand, India, Bangladesh, and China ingest insufficient amounts of Se (less than 10 μg d−1) in their daily diet (Moreno-Reyes et al., 1998; Tan et al., 2002). This is largely due to inadequate soil and crop Se concentrations in these regions, where deficiency has a large negative health impact on approximately 15% of the world’s human populations (Tan et al., 2002). Se deficiency leads to a weakened immune system, heart disease, and hypothyroidism (Combs, 2000). In contrast, sufficient Se in a human diet has long-term health benefits besides meeting basic nutritional requirements (Finley, 2006). SeCys is present in the active site of key antioxidant enzymes (Stadtman, 1980), including those that play a role in free radical scavenging and thus have anticarcinogenic activities (Ellis and Salt, 2003). Humans need Se in their diet for at least 25 different proteins, among which are the powerful antioxidant enzyme family of selenoglutathione peroxidases (for review, see Whanger, 2002; Ellis and Salt, 2003). Anticarcinogenic activity is described for various organic Se forms against multiple types of cancer (Clark et al., 1996; Reid et al., 2002; Whanger, 2002), most notably for a reduction of lung, colorectal, and prostate cancers in humans (Ip and Ganther, 1992). There is a great deal of variation in the efficacy of inorganic and organic Se compounds in cancer prevention (Ip and Ganther, 1992; Whanger, 2002). The U.S. National Academy of Sciences currently recommends ingestion of 55 μg Se d−1, and the World Health Organization recommends 40 μg Se d−1. However, supplementation at 200 μg Se d−1 is nontoxic and improves immune responses, reduces bacterial and viral infections, reduces the onset of many heart diseases, reduces most cancer rates, especially breast, prostate, lung, and liver cancers, and possibly reduces Alzheimer’s and associated dementias (Spallholz et al., 2001; Ellis and Salt, 2003). Se fights cancer using the antioxidant mechanisms of enzymes like selenoglutathione peroxidases (Ellis and Salt, 2003), when present in various nonenzymatic proteins, and through the formation of highly reactive Se-containing metabolites (Spallholz et al., 2001). In human cells, SeMet may be metabolized into dimethylselenide, a reactive intracellular metabolite that is associated with selective apoptosis of human cancer cells (Spallholz et al., 2001).
While there is no evidence that Se is essential for higher plants, sulfur (S)-loving plants often contain trace levels of Se (Birringer et al., 2002; Ellis and Salt, 2003; Sors et al., 2005). In plant roots and leaves, Se and S most often follow the same molecular pathways for their uptake, enzymatic reduction, metabolism, incorporation into protein, and volatilization (Sors et al., 2005). In roots of Arabidopsis (Arabidopsis thaliana), SeO42− is taken up through a sulfate (SO42−) transporter such as Sultr1;2, which is localized in the root tip, root cortex, and lateral roots (Shibagaki et al., 2002). After uptake into the roots, SeO42− (similar to SO42−) is thought to be transported primarily to the shoot chloroplasts and often reduced and incorporated into multiple organic Se compounds (Leustek, 2002; Ellis and Salt, 2003). Se analogs of S metabolites are indistinguishable to most plant enzymes from their S versions and hence are assimilated and metabolized by S pathways. Thus, most S molecules found in plants also have Se analogs (Leustek, 2002). Most plant species contain less than 25 μg Se g−1 dry weight in their natural environment and cannot tolerate increased Se concentrations (White et al., 2004). When Se is integrated into SeCys and SeMet, these amino acids can be misincorporated and nonspecifically replace Cys and Met in proteins, which often results in Se toxicity to plants (Brown and Shrift, 1981, 1982). In native western Se-hyperaccumulator plants, which can accumulate 100-fold more Se than normal plants, Se misincorporation is prevented by the methylation of SeCys into Se-methyl-SeCys, which may be further converted to γ-glutamyl-Se-methyl-SeCys (Dunnill and Fowden, 1967; Brown and Shrift, 1981; for review, see Sors et al., 2005). Additionally, selenocystathionine (SeCyst) was found to be accumulated at low concentrations in hyperaccumulator plants, in secondary accumulator plants, and in trace amounts in nonaccumulator plants (Shrift and Virupaksha, 1965; Peterson and Butler, 1971; Kotrebai et al., 2000; Montes-Bayon et al., 2002; Dernovics et al., 2007).
There appears to be a clear potential for producing value-added Se-enriched Opuntia as a low-maintenance drought-tolerant crop with nutritional and health benefits. These features are consistent with production when grown in the drought-, salt-, and B-impacted, Se-rich agricultural areas such as those located on the WSJV. However, the biochemistry and speciation of Se in Opuntia, or in any species of cactus, were previously unknown. Based on field measurements, we hypothesized that the spineless prickly pear cactus USDA No. 248 may have a Se metabolism comparable to that of secondary Se-accumulator plant species. Hence, the objectives of this study were to quantify the accumulation, spatial distribution, and chemical speciation of Se in the organs of spineless, salt-tolerant, antioxidant-rich, dark purple fruit-bearing Opuntia USDA No. 248. We achieved this by using a combination of synchrotron x-ray absorption spectroscopy and mass spectrometry techniques to identify the Se compounds within Se-enriched Opuntia tissues. The investigation of the biochemistry and molecular biology of Se in Opuntia organs has yielded insights into the physiology of Se metabolism in this cactus species and has suggested that Opuntia may have global agronomic applications that improve human health and nutrition while remediating Se-polluted environments. Although the health benefits need to be systematically confirmed, the organic Se forms present in Opuntia are strongly suggestive of nutraceutical and dietary applications, with the potential for improving human mineral nutrition while preventing cancer, heart disease, and viral and bacterial infections.
RESULTS
Quantification of Se Accumulation in Opuntia Organs after Acid Digestion
As determined by inductively coupled plasma mass spectrometry (ICPMS), the Se concentrations (μg Se g−1 dry weight; average ± sd) in Opuntia grown in 2008 in the agricultural sediment soil were as follows: 9.2 ± 4.4 in cladode, 2.2 ± 0.4 in fruit flesh, 1.6 ± 0.2 in fruit skin, and 4.8 ± 0.9 in seed. The Se levels in Opuntia collected in 2009 grown in sediment field soil were as follows: 17.2 ± 3.1 in cladode, 2.6 ± 0.9 in fruit flesh, 2.0 ± 0.3 in fruit skin, and 7.3 ± 1.0 in seed. In addition, Bañuelos and Lin (2010) measured that Opuntia volatilized 39.4 ± 4.2 μg Se m−2 d−1 in the agricultural sediment. To test overall Se accumulation abilities and to facilitate chemical analysis, we applied biweekly treatments using 500 mL of a 50 μm sodium selenate (Na2SeO4) solution to 1-year-old Opuntia plants and grew them under controlled conditions in sandy loam soil. Sixty days later, samples were collected and the total Se concentrations (μg Se g−1 dry weight) were determined to be 110.7 ± 15.6 in cladode, 46.7 ± 7.8 in fruit, and 17.0 ± 0.8 in seed.
Microfocused X-Ray Fluorescence and X-Ray Absorption Near-Edge Structure Analyses of Se in Opuntia Organ Tissues
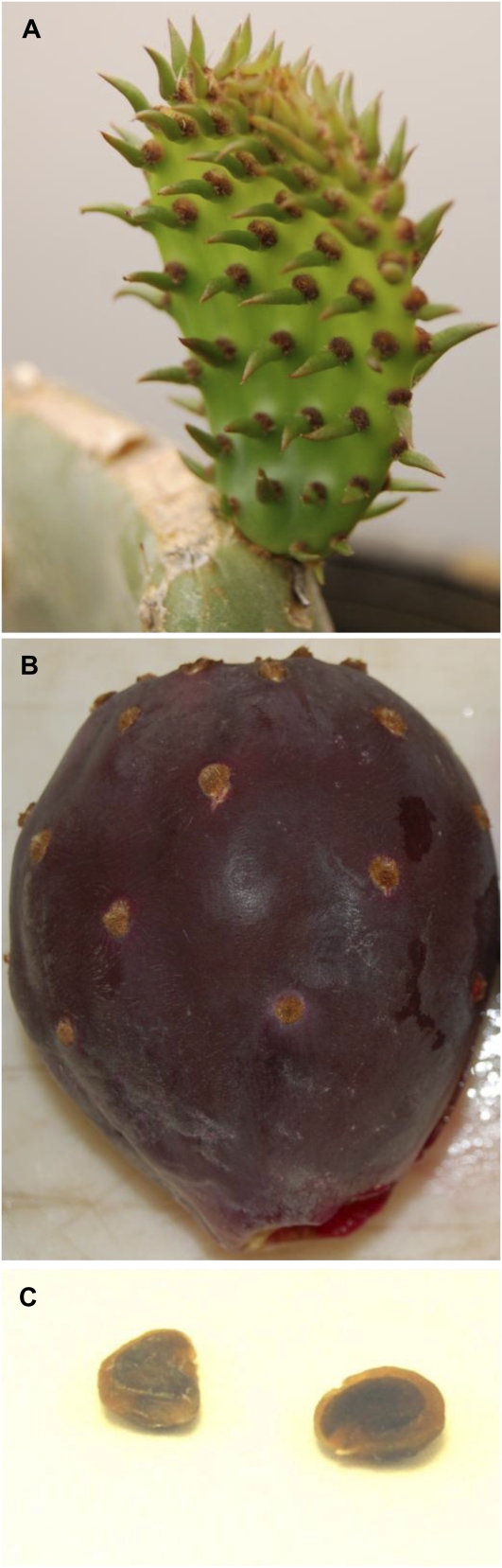
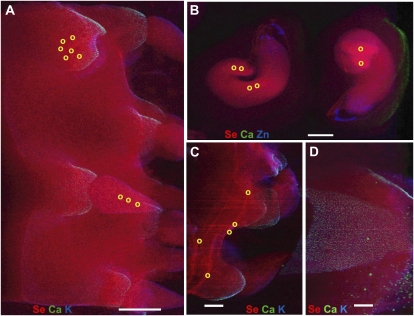
After growth in agricultural sediment, the spatial distribution of Se in the flash-frozen Opuntia organs pictured in Figure 1 (young cladode with tips [A], fruit [B], and seeds [C]) were investigated using microfocused x-ray fluorescence (μXRF). Se was distributed throughout the cladodes, with a slightly higher Se concentration in cladode tips and cladode vasculature (Fig. 2, A and C). Seeds accumulated higher Se in the embryo and particularly in cotyledons, while very little Se was located in the seed coat or endosperm (Fig. 2B). Fruit had a homogeneous Se distribution with no distinct pattern of Se localization (Fig. 2D).
Figure 1.
Photographs showing the organs and tissues of spineless, salt- and B-tolerant, dark purple fruit-bearing Opuntia (USDA No. 248) subjected to biochemical analysis. Young cladode with tips (A), fruit (B), and seeds (C) are shown.
Figure 2.
Distribution and speciation of Se in Opuntia. μXRF map showing spatial distribution of Se (coded in red) and other selected elements (calcium [Ca] in green, potassium [K] or zinc [Zn] in blue) in cladodes (A and C), in seeds (B), and in fruit (D). Bars = 600 μm (A), 1 mm (B), and 900 μm (C and D). Yellow circles in A to C show locations of μXANES scans whose fitting results are reported in Table I.
The chemical forms of Se in Opuntia organs were examined using x-ray absorption near-edge structure (XANES) spectroscopy. Micro-XANES (μXANES) distinguishes the different organic and inorganic forms of Se with high resolution in localized areas of plants. Least squares linear combination fitting (LCF) of Se K-edge μXANES spectra were performed over 12,630 to 12,850 eV using a library of standard selenocompounds. The percentage error associated with LCF is estimated to be ±10%. Of the total Se percentage, small cladode tips had an average of approximately 21% SeO42−79% was detected in organic carbon-Se-carbon (C-Se-C) forms similar to SeMet or SeCyst (Table I). The rest of the cladode base had 91% C-Se-C forms, while a small portion averaging approximately 9% was inorganic selenite (SeO32−). In seeds, approximately 96% of total Se was detected in organic C-Se-C forms, while the remaining approximately 4% of Se was inorganic SeO32− (Table I). The Se concentrations in whole flash-frozen fruit from the field were below the detection limit of μXANES analyses.
Table I. Se speciation in Opuntia tissue by μXANES spectroscopy.
Results of the least squares LCF of Se K-edge XANES spectra are shown. The best LCF was obtained by minimizing the normalized sum-squares residuals [NSS = 100 × Σ(μexp – μfit)2/Σ(μexp)2], where μ is the normalized absorbance. Error on percentages is estimated to ±10%. Selenodiglutathione, SeCys, SeCystine, and elemental Se0 were not detected (ND). Se0 is both red and gray elemental forms.
| Frozen Opuntia Tissue, Location | NSS | SeO42− | SeO32− | SeMet |
| Cladode tip | 8.12E-04 | 21 | ND | 79 |
| Cladode base | 6.67E-04 | ND | 9 | 91 |
| Seed 2B left | 4.81E-04 | ND | 4 | 96 |
| Seed 2B right | 4.24E-04 | ND | 3 | 97 |
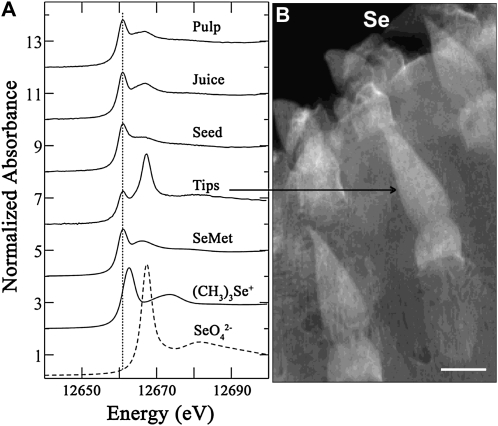
The chemical forms of Se in frozen ground Opuntia organs were then investigated using bulk XANES spectroscopy, and the resulting spectra are shown in Figure 3A. Bulk XANES allows for the mean Se speciation to be averaged over the whole ground tissue, because it has a much larger beam size and provides a higher sensitivity to lower Se concentrations. Bulk XANES-LCF analysis of cladode tips, which had slightly higher relative Se concentrations (μXRF; Figs. 2A and 3B), showed that 58% ± 1% of the Se was present in organic C-Se-C forms such as SeMet or SeCyst, while the remaining total 42% ± 1% of Se was again identified as the unreduced inorganic SeO42− (Table II). Bulk XANES-LCF analysis of Se demonstrated that organic C-Se-C forms constituted 100% ± 1% and 98% ± 1% of the total Se in fruit pulp and clarified fruit juice, respectively, and demonstrated that all of the inorganic Se had been reduced, assimilated, and metabolized. Bulk XANES analyses of seed identified 93% ± 1% of Se also as C-Se-C forms, while the remaining balance was modeled as the trimethylselenonium ion (Table II). However, as a minor component, this is difficult to distinguish from the selenoxide species C2-Se=O. Thus, the bulk XANES analyses of Opuntia sample spectra indicate that organic C-Se-C forms predominate in all Opuntia samples, except in the small tips of immature cladodes. SeMet is frequently observed as a common Se chemical species in plants; however, both XANES techniques (microfocused and bulk) cannot readily distinguish SeMet from other C-Se-C compounds, because they are only sensitive to geometries and electronics peripheral to Se. However, since x-rays penetrate and directly probe the flash-frozen samples, XANES analysis ensures that Se speciation results are not prone to artifacts of extraction or other preprocessing.
Figure 3.
A, Normalized Se K-edge XANES spectra of different Opuntia samples. Reference spectra of SeMet, trimethylselenonium ion [(CH3)3Se+], and SeO42− are shown for comparison. The SeO42− spectrum is shown as a dashed line. The dotted line indicates the peak position of SeMet standard. B, Distribution of Se in Opuntia by μXRF map showing the spatial distribution of Se (coded in white) in young cladode. Bar = 1 mm.
Table II. Se speciation in Opuntia tissue by bulk XANES spectroscopy.
Results of the least squares LCF of Se K-edge XANES spectra are shown. Values are percentage contributions ± 3 times the estimated sd determined in the fit. Fractions lower than 3 times the estimated sd were eliminated, and the fit was repeated. Fit errors (residuals) are also provided. SeO32−SeCys, SeCystine, and elemental Se0 were not detected (ND).
| Frozen Ground Opuntia Tissue | SeO42− | Trimethylselenonium | SeMet | Fit Errors (×10−2) |
| Cladode tips | 42 (1) | ND | 58 (1) | 0.11 |
| Pulp | ND | ND | 100 (1) | 0.02 |
| Juice | 2 (1) | ND | 98 (1) | 0.03 |
| Seed | ND | 7 (1) | 93 (1) | 0.02 |
ICPMS Quantification of Se in Aqueous Extracts following Enzymatic Digestion
Total Se concentrations (μg Se g−1 dry weight) in ICPMS-analyzed Opuntia tissues were as follows: 8.88 ± 0.6 in cladode, 3.07 ± 1.1 in clarified fruit juice, 1.64 ± 4.0 in fruit pulp, and 5.71 ± 1.9 in seeds. Recovery percentages of Se in aqueous extracts following proteinase K digestion were 59 ± 3 for ground cladode, 88 ± 6 for fruit juice, 79 ± 3 for fruit pulp, and 48 ± 3 for ground seed and were enhanced relative to nondigested aqueous extracts by factors of 1.3 ± 0.08 for ground cladode, 1.1 ± 0.03 for clarified fruit juice, 1.0 ± 0.23 for fruit pulp, and 3.0 ± 0.41 for ground seeds. Pretreatment of ground seeds using lipase and proteinase K together increased the Se recovery percentage into the aqueous phase by a factor of 5.3 ± 0.4.
ICPMS Quantification of Se in HPLC Fractions
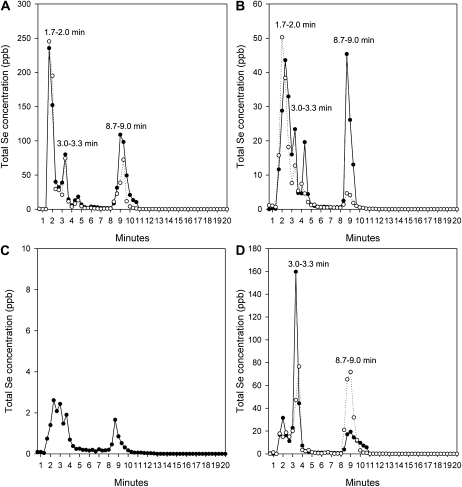
Separation of Se compounds in aqueous extracts was achieved with reverse-phase HPLC fractionation followed by quantification with ICPMS and resulted in the resolution of Se-containing peaks in proteinase-digested and undigested samples (Fig. 4, black and white circles, respectively). Aqueous extracts of the ground cladode, fruit juice, fruit pulp, and ground seed contained multiple Se peaks (Fig. 4). Fruit pulp had the lowest Se concentrations relative to the other tissue samples. All of the total Se injected was recovered off of the column and accounted for in Se peaks (99.2% ± 0.3%).
Figure 4.
Se-containing fractions of Opuntia cladode (A), clarified fruit juice (B), fruit pulp (C), and seed (D). Samples were predigested with proteinase K (black circles) or not digested (white circles). Values were quantified in nL L−1 (ppb) and obtained by reverse-phase C18 HPLC followed by ICPMS.
Liquid Chromatography-Mass Spectrometry Chemical Verification of Se Compounds in Opuntia Supplemented with SeO42−
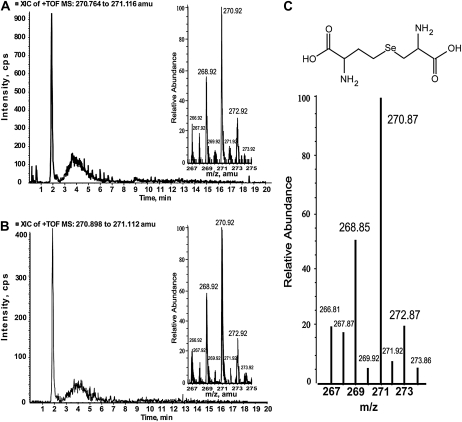
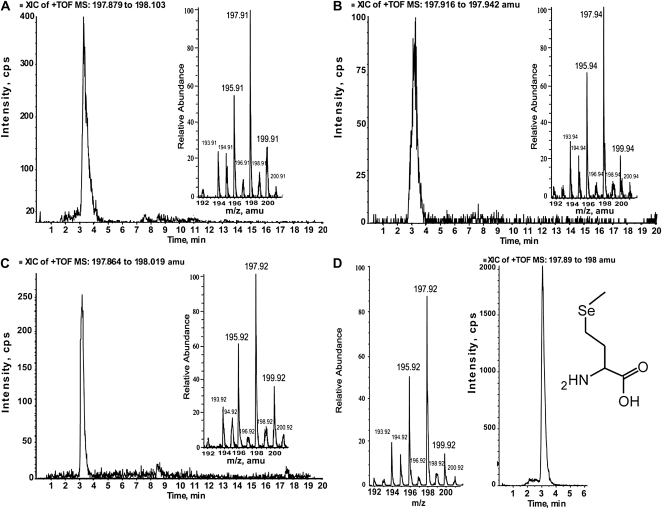
ICPMS quantification of Se in agricultural sediment-grown Opuntia tissues demonstrated that concentrations in each Se-containing HPLC fraction were below the liquid chromatography-mass spectrometry (LC-MS) limit of detection threshold (approximately 500 ng) for SeMet, a likely candidate for the 3.0- to 3.3-min peak (Fig. 4). Accordingly, it was necessary to grow Opuntia plants in a sandy loam soil supplemented biweekly with 500 mL of 50 μm SeO42− (as described in “Materials and Methods”). The addition of Se for 60 d resulted in a multifold Se increase of 6.5 for ground cladode, 17.9 for clarified fruit juice, and 2.4 for ground seed. Fractions were then further concentrated in vacuo approximately 5-fold to facilitate detection and subsequent speciation using an LC-MS system that used identical chromatographic conditions to those used for ICPMS quantification. The fraction collected over 1.7 to 2.0 min from cladode and fruit contained a peak that eluted at 1.87 min (Fig. 5, A and B, respectively) and matches spectrometrically with SeCyst (Fig. 5C). The 3.0- to 3.3-min fraction collected from cladode, fruit, and seed contained a 3.12-min peak, in spectrometric and chromatographic agreement with SeMet (Fig. 6). SeCyst and SeMet are both C-Se-C forms and support our XANES speciation results. In fruit, a minor unknown Se compound was present in the 4.0- to 4.3-min fraction, while the 8.7- to 9.0-min fraction contained a mixture of large molecules, most with a mass spectrum greater than 1,000 atomic mass units. Acid hydrolysis of the 8.7- to 9.0-min fraction in 6 n HCl at 150°C for 90 min resulted in the production of SeCyst and SeMet in cladode and fruit juice, while hydrolysis of the same fraction from the seed produced mainly the 3.0- to 3.3-min peak. These two observations suggested that the 8.7- to 9.0-min fractions contain some selenoproteins. Intriguingly, two organic Se compounds found in some plants, SeCys or selenocystine (SeCystine), were not detected in Opuntia tissues by either LC-MS or XANES analysis. SeO42−SeO32− were also analyzed by LC-MS in negative ion mode and were not detected in any Opuntia tissues. Thirteen other organic Se compounds, none of which were detected, were screened for using an algorithm to extract diagnostic Se isotopic signatures from total ion scans over the range (+) mass-to-charge ratio (m/z) 70 to 1,300 (Table III).
Figure 5.
LC-MS of 1.7- to 2.0-min Se-containing fractions with a mass spectrum matching SeCyst in cladode (A) and fruit juice (B). The published mass spectrum for SeCyst is shown in C (Dernovics et al., 2007; Freeman et al., 2010).
Figure 6.
LC-MS of 3.0- to 3.3-min Se-containing fraction with a mass spectrum and retention time matching SeMet in cladode (A), fruit juice (B), and seed (C). The mass spectrum and retention time for a SeMet standard are shown in D.
Table III. MS detection and screening method for five major Se isotopes in Se compounds (0.1000-atomic mass unit range).
| Se Isotopes | 76 | 77 | 78 | 80 | 82 | Retention Time |
| min | ||||||
| Natural isotopic abundance | 9.37 | 7.63 | 23.77 | 49.61 | 8.73 | |
| [M+H] + m/z positive ion mode | ||||||
| Selenocysteine | 164.9163–165.0163 | 165.9171–166.0171 | 166.9145–167.0145 | 168.9137–169.0137 | 170.9139–171.0139 | 1.93 |
| Selenocystine | 332.8924–332.9924 | 333.8937–333.9937 | 334.8934–334.9934 | 336.883–336.9883 | 338.8904–338.9904 | 1.54 |
| Selenocystathionine | 266.9018–267.0018 | 267.9027–268.0027 | 268.9001–269.0001 | 270.9092–271.0092 | 272.9096–273.0096 | 1.87 |
| Selenohomocysteine | 179.9398–180.0398 | 180.9406–181.0406 | 181.938–182.038 | 183.9371–184.0371 | 185.9374–186.0374 | |
| Selenomethionine | 193.9055–194.0055 | 194.9063–195.0063 | 195.8837–195.9837 | 197.8828–197.9828 | 199.8731–199.9731 | 3.12 |
| Selenomethionine − NH3+ | 176.8468–176.9468 | 177.8402–177.9402 | 178.8958–178.9958 | 180.8356–180.9356 | 182.8401–182.9401 | 3.12 |
| Methylselenomethionine | 208.9789–209.0789 | 209.9798–210.0798 | 210.9772–211.0772 | 212.9763–213.0763 | 214.9766–215.0766 | |
| S-Adenosyl-l-homoselenocysteine | 429.0270–429.1270 | 430.0270–430.1270 | 431.0244–431.1244 | 433.0234–433.1234 | 435.0241–435.1241 | |
| Adenosylselenomethionine | 444.0505–444.1505 | 445.0505–445.1505 | 446.0479–446.1479 | 448.0469–448.1469 | 450.0476–450.1476 | |
| Methylselenocysteine | 179.9398–180.0398 | 180.9406–181.0406 | 181.938–182.038 | 183.9371–184.0371 | 185.9374–186.0374 | 2.14 |
| Methylselenocysteine − NH3+ | 162.9133–163.0133 | 163.9141–164.0141 | 164.9115–165.0115 | 166.9106–167.0106 | 168.9109–169.0109 | 2.14 |
| γGlutamylmethylselenocysteine | 308.9824–309.0824 | 309.9884–310.0884 | 310.9807–311.0807 | 312.9798–313.0798 | 314.9803–315.0803 | 2.42 |
| Selenobetaine | 165.9367–166.0367 | 166.9376–167.0376 | 167.9349–168.0349 | 169.9341–170.0341 | 171.9344–172.0344 | |
| Selenolanthionine | 252.9562–253.0562 | 253.9571–254.0571 | 254.9544–255.0544 | 256.9535–257.0535 | 258.9538–259.0539 | |
| Selenodiglutathione | 705.0009–705.1009 | 706.022–706.1022 | 706.9996–707.0996 | 708.9986–709.0986 | 710.991–711.0991 | |
| Selenocystamine | 244.8927–244.9927 | 245.8939–245.9939 | 246.8914–246.9914 | 248.8905–248.9905 | 250.8906–250.9906 | |
| Selenourea | 119.9061–120.0061 | 120.9068–121.0068 | 121.9042–122.0042 | 123.9034–124.0034 | 125.9036–126.0036 | |
| [M+H] − m/z negative ion mode | ||||||
| Selenite (HSeO31−) | 124.8534–124.9534 | 125.8541–125.9541 | 126.8515–126.9515 | 128.8507–128.9507 | 130.8510–130.9510 | 1.83 |
| Selenate (HSeO41−) | 141.1407–141.2407 | 142.1490–142.2490 | 143.0901–143.1901 | 145.0901–145.1901 | 147.0960–147.1960 | 1.85 |
μXRF Chemical Map Showing the Distribution of Se Compounds in Cladode
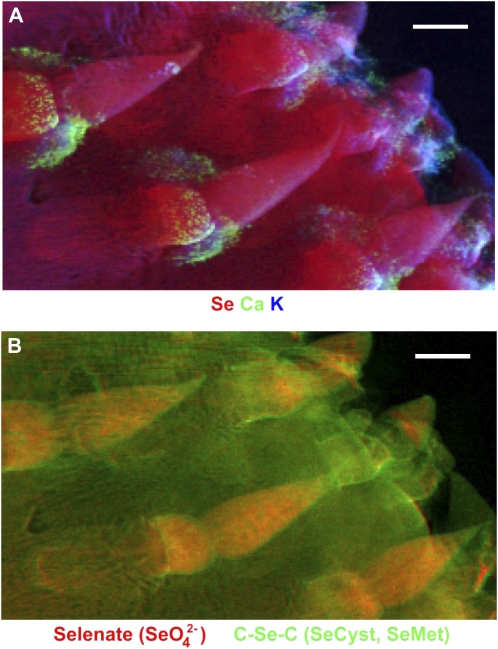
To investigate the spatial distribution and speciation of Se in a young Opuntia cladode, we used μXRF chemical mapping (Pickering et al., 2000; Freeman et al., 2006; Marcus, 2010), which simultaneously detects and maps the multiple forms of Se. The young cladode was first analyzed by μXRF elemental mapping, which confirmed an increased concentration of Se in cladode tips and is shown along with calcium and potassium (Fig. 7A). The Se chemical maps were obtained at incident energies of 12,640, 12,660.5, 12,668, and 13,000 eV to obtain the background, SeMet, SeO42−and total Se signals, respectively. Energy calibration was performed using Red Se (white line maximum set at 13,074.73 eV). They allowed us to pinpoint the distribution of SeO42−the C-Se-C compounds (Fig. 7B; i.e. SeMet and SeCyst first detected by LC-MS). Consistent with our bulk XANES analysis, the μXRF chemical mapping revealed a high concentration of SeO42− located inside cladode tips, while the mesophyll of the cladode pad had a high concentration of the C-Se-C forms.
Figure 7.
A, μXRF map showing spatial distribution of Se (coded in red) and other selected elements (calcium [Ca] in green, potassium [K] in blue) in a young cladode from a plant supplied with 50 μm Na2SeO4. B, Bicolor-coded chemical map of a young cladode showing SeO42− in red and C-Se-C (SeMet or SeCyst) in green, using 35- × 35-μm2 pixel size. Bars = 1 mm.
DISCUSSION
The levels of Se in Opuntia organs (2–17 μg Se g−1 dry weight) are comparable to those found in secondary accumulator Brassicaceae species grown under the same field conditions (Bañuelos et al., 2005). These Se levels in plant tissues used for food are considered ideal and safe for Se-enriched human food and animal feed products in Se-deficient regions. The Se accumulation results are also consistent with secondary Se-accumulating Brassicaceae species already in use for phytomanaging soluble Se in agriculture (Bañuelos et al., 2003). Opuntia showed no visible signs of Se toxicity or reduced growth after the high-Se treatment. These results suggest that Opuntia is a secondary accumulator of Se and may be useful in utilizing soluble Se on a large agricultural scale.
The μXRF map of Se showed that the total Se distribution pattern in the cladode was homogeneous throughout the mesophyll, with higher concentrations in cladode tips. Se concentrations were also higher in the cladode vasculature and in seed embryos, particularly inside cotyledons. XANES was able to differentiate organic and inorganic forms of Se in Opuntia tissues without having to extract or pretreat the samples. μXANES analysis demonstrated that inside the small immature cladode tips, a substantial 21% of Se was identified as the unreduced inorganic SeO42−79% was C-Se-C compounds. Approximately 91% and 96% of Se in the rest of the cladode at its base and in seeds matched C-Se-C compounds. Bulk XANES of Se in fruit juice and in fruit pulp showed that about 98% of Se also matched C-Se-C compounds. The cladode tips, vestigial long shoot leaves, were unique in that their XANES spectra matched approximately 42% SeO42−approximately 58% C-Se-C compounds. Both of the data sets generated using μXANES and bulk XANES suggest that in a young cladode, inorganic SeO42− is first transported into cladode tips, which are the likely ends of transpiration streams. These results established that in small cladode tips, which shrivel up and senesce during early cladode development, SeO42− is accumulated and then reduced and metabolized into C-Se-C compounds. XANES results showed the predominant form of Se in the seed to be 93% C-Se-C. Previous studies have demonstrated that Opuntia has substantial amounts of S-containing amino acids such as Met and cystine inside its seeds (Sawaya et al., 1983). However, neither SeCys nor SeCystine was detected by XANES in Opuntia tissues, including its seeds.
LC-MS measurements were required to structurally discriminate the C-Se-C molecules present in Opuntia that were identified with XANES. The percentage of organic C-Se-C forms was calculated as approximately 75% SeCyst and approximately 25% SeMet in cladodes, approximately 71% SeCyst and approximately 29% SeMet in fruit, and approximately 32% SeCyst and approximately 68% SeMet in seeds. Se chemical speciation results obtained from XANES and LC-MS were fully consistent across all tested Opuntia organ tissues.
Because proteinase K treatment enhanced Se recovery percentages by a factor of only 1.3 ± 0.08 in cladode, 1.1 ± 0.03 in fruit juice, and 1.0 ± 0.23 in fruit pulp, these results support our finding that in these succulent tissues of Opuntia, Se is mostly in the “free” form SeCyst and is nonproteinaceous. In Opuntia seed, however, proteinase K treatment increased Se recoveries by 3.0- ± 0.41-fold, demonstrating that Se is mainly proteinaceous. Digestions of seed samples using proteinase K together with lipase further increased the aqueous Se recoveries 5.3- ± 0.4-fold, and this finding supports the conclusion that in seeds, SeMet-rich proteins are associated with lipids.
When we explored the biochemistry of Se in Opuntia cladodes using μXRF chemical mapping, we illuminated both the inorganic and organic forms of Se in Opuntia simultaneously, which allowed us to distinguish the distribution of SeO42− found mainly in cladode tips and C-Se-C compounds (i.e. SeCyst and SeMet) found mainly in the cladode mesophyll. Considering that the young cladode tips are actually vestigial, conic long shoot leaves, it was interesting that these tips showed a high concentration of not only SeO42− but also substantial amounts of C-Se-C. This result confirmed that, as is known in other leaves (Leustek, 2002), the young cladode tips are highly active sites for not only the initial storage of SeO42− but also the reduction and assimilation of SeO42− into at least one, if not both, of the C-Se-C compounds verified by LC-MS (SeCyst and SeMet) before these forms are transported to other areas of the cladode, fruit, and eventually seed.
SeCyst is a freely pooled selenoamino acid in many secondary Se accumulator plants and has been well documented in both secondary Se accumulator and nonaccumulator plant species, including but not limited to Brassica juncea, Lecythis minor (monkey pot nut), Morinda reticulate, Stanleya albescens, and Arabidopsis (Peterson and Butler, 1971; Montes-Bayon et al., 2002; Dernovics et al., 2007; Freeman et al., 2010). SeCyst is a metabolic intermediate between SeCys and SeMet, and both amino acids can be toxic to plants when incorporated into protein (for review, see Pickering et al., 2003). Additionally, some hyperaccumulator species such as Stanleya pinnata, Neptunia amplexicaulis, and Astragalus pectinatus contain a mixture of 20% to 30% SeCyst and 70% to 80% Se-methyl-SeCys (Horn and Jones, 1941; Shrift and Virupaksha, 1965; Peterson and Butler, 1967; Freeman et al., 2006). These hyperaccumulator plants have reduced Se accumulation abilities when compared with other hyperaccumulator species, and this may be due to accumulating SeCyst, a nonmethylated organic form and a direct precursor of SeMet. An increased accumulation of the precursor SeCyst was observed after watering B. juncea with SeMet (Montes-Bayon et al., 2002). This result first suggested that SeMet may be metabolized in reverse or degraded to yield SeCyst in leaves. In plants, this reverse Se flux may help prevent some SeMet from entering into proteins. It is quite possible that the pooled SeCyst found in Opuntia offers the ability to accumulate Se and may protect from SeCys misincorporation into protein.
SeCys misincorporation into plant proteins is hypothesized to be more toxic than SeMet misincorporation, potentially due to the important function of Cys in disulfide bond formation, which is crucial for protein structure and function (Brown and Shrift, 1981, 1982; Burnell, 1981a, 1981b). It may be of importance that neither SeCys nor SeCystine was detected in Opuntia tissues. This finding suggests that the misincorporation of SeCys into proteins may be prevented as a result of another unique characteristic of Opuntia. This might be achieved through substrate discrimination at the amino acid activation stage of protein synthesis, such as observed for SeCys and its exclusion by the cysteinyl-tRNA synthetase of the Se accumulator Astragalus bisulcatus (Burnell and Shrift, 1979). This may also be achieved via a rapid metabolic flux of SeCys directly into the free pool of SeCyst. Synthesis of SeMet may also be inhibited in cladode and fruit through substrate discrimination by Met synthase, or SeMet could be actively excluded from proteins by a Met-selective methionyl-tRNA synthetase (Burnell, 1981b). Finally, the Se content of proteins might be reduced through enzymic removal of SeCys or SeMet from preformed proteins, comparable to the posttranslational deamination of Gln and Asn residues, which liberates Glu and Asp (Uy and Wold, 1977). All of these molecular mechanisms could result in the prevention of SeCys formation, the selective pooling of SeCyst, and the lack of SeMet in Opuntia proteins, particularly in the cladode and fruit.
Even without considering its accumulation of organic Se and associated health benefits, Opuntia is now known as a medicinal food crop with elevated nutrition and disease prevention properties (for review, see Feugang et al., 2006). Opuntia is commonly eaten raw or cooked in various dishes by people in the western United States, Mexico, and various countries worldwide. Consumption of whole fresh cladode, fruit, or food products such as juices, jams, jellies, wines, and breakfast cereals made from either fruit or cladode may help treat or prevent multiple human ailments, including diabetes, cancer, and heart disease (Frati et al., 1990; Hegwood, 1990; Palevitch et al., 1993; Zou et al., 2005; Feugang et al., 2006). Moreover, studies have shown that the great abundance of flavonoids found in Opuntia fruit can enhance the function of the human immune system and protect the nervous systems through reducing oxidative stress and actively scavenging free radicals (Dok-Go et al., 2003; Kuti, 2004; Feugang et al., 2006). Hence, the additional health benefits of producing Se-enriched Opuntia, which may enhance these existing medicinal chemotherapeutic properties, are very exciting and completely unexplored.
As an example of this potential, we have conducted preliminary in vitro colon cancer cell inhibition assays (C. Stushnoff, T. Zuber, J.L. Freeman, and G.S. Bañuelos, unpublished data). These colon cancer cell inhibition assays used aqueous extracts from the antioxidant-rich purple Opuntia USDA No. 248 fruit that were naturally enriched in Se after growth in agricultural sediment. The preliminary results suggest that the addition of these organic Se compounds may indeed further inhibit colon cancer cell growth when compared with controls with no detectable Se after 24 h of growth. This preliminary result is exciting considering the published finding of a high-Se-treated broccoli (Brassica oleracea) diet that results in a decrease of colon cancer-induced crypt and lesion formation in trials using the colon cancer rat model (Finley et al., 2000).
In conclusion, Opuntia USDA No. 248 can tolerate adverse saline- and B-impacted soil conditions, and our analytical results show that it can also accumulate and metabolize inorganic Se into the two major C-Se-C forms SeCyst and SeMet. These two forms may enhance the health potential of this new organic Se-biofortified nutraceutical food. Because Opuntia thrives under arid conditions with a minimum input of water, successfully growing this crop under rain-fed-only conditions or with poor-quality water may provide water-sensitive Se-laden regions with an important cash-value crop. This is of extreme importance for many agricultural areas, including those in the recently deemed “semiretired lands” within the WSJV. Moreover, the development of unique valued-added Se-enriched nutraceutical food products from Opuntia can improve profitability in these affected areas and thereby increase the realistic potential of successfully producing a truly specialized arid land crop. Developing new Opuntia fruit products and identifying the organic Se chemical forms, associated antioxidants, and anticarcinogenic characteristics contained within these foods could validate and confirm the high marketability of these unique Se-enriched products produced from the WSJV. When grown under adverse soil conditions like those present in the Se-enriched WSJV, Opuntia cladodes, fruit, and seeds could provide organic Se-biofortified nutraceutical foods naturally enriched in health-promoting, potentially cancer-preventing organic Se compounds.
MATERIALS AND METHODS
Propagation and Plantation of Opuntia
Cladodes of spineless 3- to 4-year-old prickly pear cactus (Opuntia ficus-indica clone 248) were initially collected from Mexico and are at present growing at the U.S. Department of Agriculture-Agricultural Research Service Plant Introduction Research Facility in Parlier, California. Prickly pear cactus plantlets were clonally propagated by placing an uprooted cladode (a stem segment) in soil. The cladodes were then transplanted in sandy loam soil without irrigation until new sprouts appeared for several weeks. The plants were lightly watered weekly with one-fifth-strength Hoagland solution for 2 months in a greenhouse and then hardened under outside environmental conditions for 1 month. After 3 months of initial root establishment, the different clones were transplanted into the drainage sediment with a 1.2- × 1.2-m field spacing in each plot. Each clone plot was randomly replicated 10 times on the drainage sediment beds.
Field Growth
A saline-rich drainage sediment with high Se and B concentrations was collected from the top 25-cm layer of residual sediment in the San Luis Drain near Mendota, California, and spread to a depth of 40 cm in an excavated field plot at the U.S. Department of Agriculture Research Facility in Parlier in 1999 (Bañuelos et al., 2005). The drainage sediment was covered with a 4-cm layer of clean sandy loam soil (containing less than 0.1 mg Se kg−1) to enhance biological activity, plant growth, and survival. The major chemical properties of the soil sediment and the control soil (a Hanford sandy loam, coarse-loamy, mixed superactive, nonacid, thermic Typic Xerothents) with soluble 1.2 ± 0.3 μg Se g−1 and 5.1 ± 0.9 μg B g−1 were fully described by Bañuelos and Lin (2010). At the field site, beds were created (33 m long and 1 m wide) on the drainage sediment and control soil plots (Bañuelos et al., 2005). Normal agronomic management practices were applied on the test plots throughout each growing season, including applying a non-S-containing ammonium nitrate fertilizer at an application rate of 50 kg ha−1 year−1. A surface-drip irrigation system was installed consisting of one in-line turbulent flow emitter per bed with an emitter spacing of 0.45 m and a flow rate of 4 L h−1. All cactus plots were drip irrigated with good-quality water (electrical conductivity less than 0.8 dS m−1) based on the rate of evapotranspiration loss recorded at the California Irrigation Management Information System weather station located 2 km away at the University of California Kearney Research Station in Parlier.
Controlled Opuntia Growth and Se Amelioration
Two-year-old Opuntia USDA No. 248 clones were growing in a sandy loam soil that was supplemented biweekly with 500 mL of 50 μm SeO42− solution for 60 d while incubating in a Conviron plant growth chamber on 12 h of full-spectrum light, 30°C day, 27°C night.
Sampling of Cladodes and Fruit for ICPMS
Cladodes and fruits were sampled in both 2007 (year 2) and 2008 (year 3) by removing the two uppermost cladodes (i.e. the most recent growth) of each plant from each replicate (consisting of two plants). Mature fruits were harvested from within each replicate of each clone when the fruit became completely red, purple, or yellow at year 3. Harvested fruits were counted, weighed, and then stored at 10°C overnight before processing. Thereafter, the skin was removed and seeds were separated from the fruit flesh, frozen at −80°C, and processed later for analysis of Se.
ICPMS Analysis of Se
Frozen seed and flesh samples were removed from a freezer, cut, ground into smaller pieces, and oven dried at 50°C. Plant samples for Se analysis were harvested (as described above) and digested with HNO3, H2O2, and HCl (Bañuelos and Akohoue, 1994). Total Se was analyzed by an inductively coupled plasma mass spectrometer (Agilent 7500cx). The National Institute of Standards and Technology Wheat Flour (SRM 1567; Se content of 1.1 ± 0.2 μg g−1 dry mass) and two internal soil standards (sediment collected from Kesterson Reservoir, California, with a total Se content of 7.5 ± 0.25 and 25 ± 0.87 mg kg−1) were used as the quality-control standards for plants and soils, respectively. The Se recovery rates of the standard materials were over 94%.
μXRF/μXANES and Chemical Mapping
Se distribution and local speciation were determined using μXRF mapping and μXANES spectroscopy, respectively. Samples were washed to remove any external Se, flash frozen in liquid nitrogen, and shipped on dry ice to the Advanced Light Source at the Lawrence Berkeley Laboratory for microprobe analyses on Beamline 10.3.2. Frozen samples were transferred onto a Peltier stage kept at −33°C to minimize radiation damage. μXRF elemental maps were recorded at 13 keV, using a 15-μm (horizontal) × 6-μm (vertical) beam, a 15-μm × 15-μm pixel size, and 50-ms dwell time per pixel. The chemical form of Se in particular areas of interest in Opuntia samples was further investigated using Se K-edge μXANES. Maps and XANES spectra were recorded with a seven-element GE solid-state detector. Se XANES spectra were deadtime corrected, preedge background subtracted, and postedge normalized using standard procedures. Red Se (white line maximum set at 13,074.73 eV) was used to calibrate the spectra. Least squares LCF of Se XANES spectra was performed in the 12,630- to 12,850-eV range using a library of nine standard selenocompounds. The error on the percentages of species present is estimated to be ±10%. Aqueous solutions of the various selenocompounds were used as standard materials: Na2SeO4 and Na2SeO3, SeMet, and SeCystine, all purchased from Sigma-Aldrich. Methyl-SeCys, γ-glutamyl-methyl-SeCys, and selenodiglutathione were purchased from PharmaSe, while red and gray elemental Se were a gift from Dan Strawn at the Lawrence Berkeley National Laboratory Advanced Light Source. SeCys was obtained by reducing SeCystine at 25°C overnight in 100 mm sodium borohydride at a 1:1 molar ratio. All data processing and analyses were performed with a suite of custom LabVIEW programs (http://xraysweb.lbl.gov/uxas/Beamline/Software/Software.htm). Chemical mapping of the different Se forms was achieved using the same conditions and methods described previously by Pickering et al. (2000), Freeman et al. (2006), and Marcus (2010).
Bulk XANES
Seeds were removed from whole fresh Se-enriched Opuntia fruit, which were placed in a juicer. The fruit juice was then clarified through multiple centrifugations and decantation. Both the juice and residual pulp were then freeze dried separately. However, these samples were never close to dehydration, and water was clearly present when samples were thawed after analysis. All other frozen tissues were ground in liquid nitrogen, packed into 2-mm pathlength sample cells, and kept frozen in liquid nitrogen for bulk XANES analysis. XANES data were collected at the Stanford Synchrotron Radiation Light Source with the storage ring operating at 3 GeV and with ring currents of 150 to 200 mA. Se K-edge spectra were recorded on Beamline 9-3 with an upstream rhodium-coated collimating mirror, a Si(220) double-crystal monochromator, and a downstream rhodium-coated focusing mirror. The incident x-ray intensity was monitored using a N2-filled ionization chamber. XANES spectra were recorded by monitoring the Se Kα fluorescence using a 30-element germanium detector equipped with Soller slits and an arsenic filter. During data collection, samples were maintained approximately at 10 K in a liquid helium-flow cryostat as described by Andrahennadi et al. (2007). Spectra were also collected in fluorescence on dilute aqueous solutions of standard Se species buffered at pH 7. These included the inorganic Se forms as SeO42−SeO32−the organic forms of SeMet, trimethylselenonium, SeCystine, and SeCys, as well as solid red elemental Se measured in transmittance. The spectrum of each sample was calibrated with respect to the spectrum of hexagonal Se, placed downstream of the sample, and measured simultaneously in transmission, the first energy inflection of which was assumed to be 12,658.0 eV. Background subtraction, normalization, and data analyses were carried out according to standard procedures using the EXAFSPAK program suite available at http://ssrl.slac.stanford.edu/exafspak.html. Similar to the μXANES spectra, bulk XANES spectra were analyzed by least squares LCF (Pickering et al., 1999). When multiple Se species are present, the Se spectrum of the sample is a weighted sum of the spectra from the different species. Since the percentage contribution of a standard spectrum to the sum is equal to the percentage of total Se present in that chemical form, the spectrum may be analyzed by least squares LCF to extract the percentage contribution from each species, assuming that all species present are represented in the set of standards.
Sample Preparation for HPLC, ICPMS, and LC-MS
Freeze-dried samples (1.0–1.5 g) were ground to a powder in liquid nitrogen with a mortar and pestle and placed in a 40-mL glass vial with a Teflon cap. Proteinase K (200 mg) was added to three out of six sample vials (Montes-Bayon et al., 2002). Next, 10 mL of water (18 mΩ) at room temperature was added, the samples were vortexed, and all samples were incubated in a shaker for 20 h at 37°C. After digestion, 17 mL of methanol (ultrapure) was added, and each tube was vortexed, allowed to stand for an additional 2 h, vortexed again, and refrigerated overnight at 4°C. Following this, 8.5 mL of chloroform was added and the tubes were capped, shaken vigorously, and refrigerated at 4°C overnight until tissue was fully extracted and the upper aqueous (methanol-water) phase was clarified. Vials were tapped to break emulsions before the upper phase (approximately 27 mL) was carefully transferred to a 50-mL conical centrifuge tube. One-quarter of the aqueous (methanol-water) phase was then pipetted into 50-mL ICP digestion tubes for drying, acid digestion, and analysis of total aqueous Se by ICPMS. The fully extracted tissue in 8.5 mL of chloroform was then dried, acid digested, and analyzed for total nonaqueous Se phase by ICPMS. Percentage of Se recovery into the aqueous phase was calculated from these ICPMS results as {(total Se in methanol-water phase)/[(total Se in methanol-water phase) + (total Se in chloroform)] × 100}. The remaining aqueous (methanol-water) phase was concentrated to approximately 2.5 mL in vacuo and stored in a freezer. Final cleanup of the concentrate was with Waters Sep-Pak Classic C18 cartridges (360 mg, 55–105 μm). Each cartridge was cleaned by flushing 10 mL of methanol and 5 mL of water (18 mΩ) in succession. The concentrates were thawed and vortexed, and 11 μL of 88% formic acid was added prior to being transferred by disposable Pasteur pipette to the SepPak. The column was loaded with the sample, and elution was with 3 mL of methanol into a 50-mL conical tube. Eluent was then concentrated in vacuo down to approximately 1- to 1.5-mL final volume, which was recorded before samples were transferred into vials in preparation for HPLC fractionation and mass spectrometry (ICPMS and LC-MS).
HPLC Fractionation
A 30-μL injection of each sample extract was repeated 11 times. Fractions resulting from each injection of a sample were collected using an automated collector with 20-s resolution and pooled. Based on ICPMS analysis, 99.2% ± 0.3% of the Se quantified in a sample prior to chromatography was accounted for in collected fractions; no Se was detected in the eluent after 12 min.
ICPMS Analysis of Se-Containing Fractions
Once fractions were collected, 1.5 mL was divided into aliquots in ICPMS tubes and then speed vacuumed to remove acetonitrile. Water (18 MΩ) was used to bring the volume to 1.5 mL before analysis with an ICPMS device (Agilent 7500cx). ICPMS Se data were used to quantify the relative amounts of Se in each fraction over the course of chromatography. Se levels in a sample were quantified with and without fractionation, as well as with and without proteinase K digestion.
LC-MS
Dual Shimadzu LC-10AD pumps, a Shimadzu SC-10A photodiode array detector (PDA), a Phenomenex Hyperclone 5-μm ODS (C18) 120A 250- × 2.00-mm column at 20°C, and an Applied Biosystems QSTAR XL high-resolution quadrupole time-of-flight mass spectrometer were used. A column flow of 0.5 mL min−1 was composed of two eluents: A, 10 mm ammonium formate in deionized water; B, 10 mm ammonium formate in a 10%:90% deionized water:acetonitrile mixture. The mobile phase composition (in terms of percentage ratios) was isocratic at 100 A:0 B for 5 min, ramped to 60 A:40 B over 2 min, ramped to 25 A:75 B over 3 min, held for 5 min, ramped down to 100 A:0 B over 3 min, and held for 2 min. Column effluent was routed through the PDA and to the mass spectrometer. After the PDA, however, a splitter directed approximately 80% of the flow to the fraction collector and approximately 20% (100 μL min−1) to the mass spectrometer. Spectra over (+) m/z 70 to 1,300 were obtained for all organic Se compounds, while spectra over (−) m/z 40 to 700 were obtained for two inorganic Se compounds using an Ion Spray source with a 4.5-mm i.d. capillary sample tube and a spray voltage of ±4,750 V. The declustering potential was ±70 V, the focusing potential was ±230 V, and the ion release delay and width were 6 and 5 (arbitrary units), respectively. Parameters for nitrogen source and curtain gas were 15 p.s.i. and 1.31 L min−1, respectively. An initial detection or screening program method was then created using the five major isotopes and a 0.1-atomic mass unit window of appropriate masses for either positive or negative mode. The 17 Se-containing molecules along with mass ranges and retention times are listed in Table III. Retention times in minutes for Se standards were as follows: SeO32− = 1.83, SeO42− = 1.85, SeCystine = 1.54, SeCys = 1.93, methyl-SeCys = 2.14, γ-glutamyl methyl-SeCys = 2.42, and SeMet = 3.12 (2–3 s).
Acknowledgments
Opuntia USDA No. 248 cladodes were originally provided by the National Arid Land Plant Genetic Resources Unit and the Western Regional Plant Introduction Station located at the U.S. Department of Agriculture-Agricultural Research Service in Parlier, California.
References
- Andrahennadi RM, Wayland M, Pickering IJ. (2007) Speciation of selenium in stream insects using x-ray absorption spectroscopy. Environ Sci Technol 41: 7683–7687 [DOI] [PubMed] [Google Scholar]
- Bañuelos GS, Akohoue S. (1994) Comparison of microwave digestion with block digestion for selenium and boron analysis in plant tissues. Commun Soil Sci Plant Anal 25: 1655–1670 [Google Scholar]
- Bañuelos GS, Lin ZQ. (2010) Cultivation of the Indian fig Opuntia in selenium-rich drainage sediments under field conditions. Soil Use Manage 26: 167–175 [Google Scholar]
- Bañuelos GS, Lin ZQ, Arroyo I, Terry N. (2005) Selenium volatilization in vegetated agricultural sediment from the San Luis Drain, Central California. Chemosphere 60: 1203–1213 [DOI] [PubMed] [Google Scholar]
- Bañuelos GS, Lin ZQ, Wu L, Terry N. (2003) Phytoremediation of selenium contaminated soil and waters: fundamentals and future prospects. Rev Environ Health 17: 291–306 [DOI] [PubMed] [Google Scholar]
- Birringer M, Pilawa S, Flohé L. (2002) Trends in selenium biochemistry. Nat Prod Rep 19: 693–718 [DOI] [PubMed] [Google Scholar]
- Brown TA, Shrift A. (1981) Exclusion of selenium from proteins of selenium-tolerant Astragalus species. Plant Physiol 67: 1051–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Shrift A. (1982) Selenium-toxicity and tolerance in higher plants. Biol Rev Camb Philos Soc 57: 59–84 [Google Scholar]
- Burnell JN. (1981a) Selenium metabolism in Neptunia amplexicaulis. Plant Physiol 67: 316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell JN. (1981b) Methionyl-tRNA synthetase from Phaseolus aureus: purification and properties. Plant Physiol 67: 325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell JN, Shrift A. (1979) Cysteinyl-tRNA synthetase from Astragalus species. Plant Physiol 63: 1095–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, et al. (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. J Am Med Assoc 276: 1957–1963 [PubMed] [Google Scholar]
- Combs GF., Jr (2000) Food system-based approaches to improving micronutrient nutrition: the case for selenium. Biofactors 12: 39–43 [DOI] [PubMed] [Google Scholar]
- Dernovics M, García-Barrera T, Bierla K, Preud’homme H, Lobinski R. (2007) Standardless identification of selenocystathionine and its g-glutamyl derivatives in monkeypot nuts by 3D liquid chromatography with ICP-MS detection followed by nano HPLC-Q-TOF-MS/MS. Analyst (Lond) 132: 439–449 [DOI] [PubMed] [Google Scholar]
- Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, Lee YH, Jin C, Lee YS, Cho J. (2003) Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. Saboten. Brain Res 965: 130–136 [DOI] [PubMed] [Google Scholar]
- Dunnill PM, Fowden L. (1967) The amino acids of the genus Astragalus. Phytochemistry 6: 1659–1663 [Google Scholar]
- Ellis DR, Salt DE. (2003) Plants, selenium and human health. Curr Opin Plant Biol 6: 273–279 [DOI] [PubMed] [Google Scholar]
- Emmons SF, Cross W, Eldridge GH. (1896) Geology of the Denver basin in Colorado. US Geol Surv Monogr 27: 527 [Google Scholar]
- Feugang JM, Konarski P, Zou D, Stintzing FC, Zou C. (2006) Nutritional and medicinal use of cactus pear (Opuntia spp.) cladodes and fruits. Front Biosci 11: 2574–2589 [DOI] [PubMed] [Google Scholar]
- Finley JW. (2006) Bioavailability of selenium from foods. Nutr Rev 64: 146–151 [DOI] [PubMed] [Google Scholar]
- Finley JW, Davis CD, Feng Y. (2000) Selenium from high selenium broccoli protects rats from colon cancer. J Nutr 130: 2384–2389 [DOI] [PubMed] [Google Scholar]
- Frati AC, Jimenez E, Ariza CR. (1990) Hypoglycemic effect of Opuntia ficus indica in non insulin-dependent diabetes mellitus patients. Phytother Res 4: 195–197 [Google Scholar]
- Freeman JL, Tamaoki M, Stushnoff C, Quinn CF, Cappa JJ, Devonshire J, Fakra SC, Marcus MA, McGrath SP, Van Hoewyk D, et al. (2010) Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol 153: 1630–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JL, Zhang LH, Marcus MA, Fakra S, McGrath SP, Pilon-Smits EA. (2006) Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol 142: 124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara JC, Suassuna P, Felker P. (2009) Opuntia forage production systems: status and prospects for rangeland application. Rangeland Ecol Manag 62: 428–434 [Google Scholar]
- Hegwood DA. (1990) Human health discoveries with Opuntia sp. (prickly pear). Hortic Sci 25: 1515–1516 [Google Scholar]
- Horn MJ, Jones DB. (1941) Isolation from Astragalus pectinatus of a crystalline amino acid complex containing selenium and sulphur. J Biol Chem 139: 649–660 [Google Scholar]
- Ip C, Ganther HE. (1992) Relationship between the chemical form of selenium and anticarcinogenic activity. Wattenberg I, Lipkin M, Boon CW, Kellott GJ, , Cancer Chemoprevention. CRC Press, Boca Raton, FL, pp 479–488 [Google Scholar]
- Kotrebai M, Birringer M, Tyson JF, Block E, Uden PC. (2000) Selenium speciation in enriched and natural samples by HPLC-ICP-MS and HPLC-ESI-MS with perfluorinated carboxylic acid ion-pairing agents. Analyst (Lond) 125: 71–78 [DOI] [PubMed] [Google Scholar]
- Kuti JO. (2004) Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem 85: 527–533 [Google Scholar]
- Leustek T. (2002) Sulfate metabolism. Somerville CR, Meyerowitz EM, , The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0017, http://www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus MA. (2010) X-ray photon-in/photon-out methods for chemical imaging. Trends Analyt Chem 29: 508–517 [Google Scholar]
- Montes-Bayon M, Grant T, Meija J, Caruso JA. (2002) Selenium in plants by mass spectrometric techniques: developments in bio-analytical methods. J Anal At Spectrom 17: 1015–1023 [Google Scholar]
- Moreno-Reyes R, Suetens C, Mathieu F, Begaux F, Zhu D, Rivera MT, Boelaert M, Nève J, Perlmutter N, Vanderpas J. (1998) Kashin-Beck osteoarthropathy in rural Tibet in relation to selenium and iodine status. N Engl J Med 339: 1112–1120 [DOI] [PubMed] [Google Scholar]
- Ohlendorf HM, Hoffman DJ, Slaki MJ, Aldrich TW. (1986) Embryonic mortality and abnormalities of aquatic birds: apparent impacts of selenium from irrigation drain water. Sci Total Environ 52: 49–63 [Google Scholar]
- Ohlendorf HM, Santolo GM. (1994) Kesterson reservoir—past, present and future: an ecological risk assessment. Frankenberger WT, Jr, Benson S, , Selenium in the Environment. Marcel Dekker, New York, pp 69–117 [Google Scholar]
- Palevitch D, Earon G, Hevir I. (1993) Treatment of benign prostate hypertrophy with Opuntia ficus indica (L.) Miller. J Herbs Spices Med Plants 2: 45–49 [Google Scholar]
- Peterson PJ, Butler GW. (1967) Significance of selenocystathionine in an Australian selenium-accumulating plant, Neptunia amplexicaulis. Nature 213: 599–600 [Google Scholar]
- Peterson PJ, Butler GW. (1971) The occurrence of selenocystathionine in Morinda reticulata Benth., a toxic seleniferous plant. Aust J Biol Sci 24: 175–177 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, George GN, Van Fleet-Stalder V, Chasteen TG, Prince RC. (1999) X-ray absorption spectroscopy of selenium-containing amino acids. J Biol Inorg Chem 4: 791–794 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, Salt DE, George GN. (2000) Quantitative, chemically specific imaging of selenium transformation in plants. Proc Natl Acad Sci USA 97: 10717–10722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering IJ, Wright C, Bubner B, Ellis D, Persans MW, Yu EY, George GN, Prince RC, Salt DE. (2003) Chemical form and distribution of selenium and sulfur in the selenium hyperaccumulator Astragalus bisulcatus. Plant Physiol 131: 1460–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid ME, Duffield-Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR. (2002) Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev 11: 1285–1291 [PubMed] [Google Scholar]
- Sawaya WN, Khalil JK, Al-Mohammad MM. (1983) Nutritive value of prickly pear seeds, Opuntia ficus-indica. Plant Foods Hum Nutr 33: 91–97 [Google Scholar]
- Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP. (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29: 475–486 [DOI] [PubMed] [Google Scholar]
- Shrift A, Virupaksha TK. (1965) Seleno-amino acids in selenium-accumulating plants. Biochim Biophys Acta 100: 65–75 [DOI] [PubMed] [Google Scholar]
- Sors TG, Ellis DR, Salt DE. (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86: 373–389 [DOI] [PubMed] [Google Scholar]
- Spallholz JE, Shriver BJ, Reid TW. (2001) Dimethyldiselenide and methylseleninic acid generate superoxide in an in vitro chemiluminescence assay in the presence of glutathione: implications for the anticarcinogenic activity of l-selenomethionine and l-Se-methylselenocysteine. Nutr Cancer 40: 34–41 [DOI] [PubMed] [Google Scholar]
- Stadtman TC. (1980) Selenium-dependent enzymes. Annu Rev Biochem 49: 93–110 [DOI] [PubMed] [Google Scholar]
- Tan J, Zhu W, Wang W, Li R, Hou S, Wang D, Yang L. (2002) Selenium in soil and endemic diseases in China. Sci Total Environ 284: 227–235 [DOI] [PubMed] [Google Scholar]
- Uy R, Wold F. (1977) Posttranslational covalent modification of proteins. Science 198: 890–896 [DOI] [PubMed] [Google Scholar]
- Whanger PD. (2002) Selenocompounds in plants and animals and their biological significance. J Am Coll Nutr 21: 223–232 [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, et al. (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55: 1927–1937 [DOI] [PubMed] [Google Scholar]
- Zou DM, Brewer M, Garcia F, Feugang JM, Wang J, Zang R, Liu H, Zou CP. (2005) Cactus pear: a natural product in cancer chemoprevention. Nutr J 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]