Abstract
Transplastomic tobacco (Nicotiana tabacum) plants expressing β-glucosidase (Bgl-1) show modified development. They flower 1 month earlier with an increase in biomass (1.9-fold), height (1.5-fold), and leaf area (1.6-fold) than untransformed plants. Trichome density on the upper and lower leaf surfaces of BGL-1 plants increase by 10- and 7-fold, respectively, harboring 5-fold more glandular trichomes (as determined by rhodamine B staining), suggesting that BGL-1 lines produce more sugar esters than control plants. Gibberellin (GA) levels were investigated because it is a known regulator of flowering time, plant height, and trichome development. Both GA1 and GA4 levels are 2-fold higher in BGL-1 leaves than in untransformed plants but do not increase in other organs. In addition, elevated levels of other plant hormones, including zeatin and indole-3-acetic acid, are observed in BGL-1 lines. Protoplasts from BGL-1 lines divide and form calli without exogenous hormones. Cell division in protoplasts is enhanced 7-fold in the presence of exogenously applied zeatin-O-glucoside conjugate, indicating the release of active hormones from their conjugates. Whitefly (Bemisia tabaci) and aphid (Myzus persicae) populations in control plants are 18 and 15 times higher than in transplastomic lines, respectively. Lethal dose to kill 50% of the test population values of 26.3 and 39.2 μg per whitefly and 23.1 and 35.2 μg per aphid for BGL-1 and untransformed control exudates, respectively, confirm the enhanced toxicity of transplastomic exudates. These data indicate that increase in sugar ester levels in BGL-1 lines might function as an effective biopesticide. This study provides a novel strategy for designing plants for enhanced biomass production and insect control by releasing plant hormones or sugar esters from their conjugates stored within their chloroplasts.
In plants, β-glucosidases have been implicated in key developmental processes, such as growth, pathogen defense, and hormone hydrolysis (Esen, 1993; Kleczkowski and Schell, 1995). Recently, considerable progress has been made in elucidating the functions of β-glucosidases for chemical plant defense against pathogens (Morant et al., 2008) and activation of plant hormone groups, including auxins, abscisic acid (ABA), and cytokinins (Wiese and Grambow, 1986; Brzobohatý et al., 1993; Lee et al., 2006). Fungal β-glucosidases efficiently hydrolyze GA-13-O-glucosides. In contrast, enzyme from plants exhibit very low activity (Schliemann and Schneider, 1979; Schliemann, 1984; Sembdner et al., 1994). However, very little is known about the contribution of β-glucosidases to GA homeostasis (Schneider and Schliemann, 1994).
Hormones play an important role in regulating plant growth and development (Davies, 2004). Their regulating properties appear in the course of the biosynthetic and signaling pathways and are followed by catabolic processes. All these metabolic steps are irreversible except for some processes including the formation of glucoside ester or ether conjugates, where the free hormone can be liberated by β-glucosidase enzymatic hydrolysis. For each class of the plant hormones, conjugates have been found (Kleczkowski and Schell, 1995). After characterization of the first GA glucoside, GA8-2-O-β-d-glucoside from Phaseolus coccineus fruits (Schreiber et al., 1970), the term GA conjugate was used for a GA covalently bound to another low-molecular-weight compound. There is now evidence that hormone conjugates act as reversible deactivated storage forms and are important in the regulation of physiologically active hormone levels (Schneider and Schliemann, 1994). The conjugation process is an important aspect of hormone metabolism in plants but has not yet been explored in enhancing growth or productivity.
The most common GA conjugates isolated from plants are connected to Glc. These conjugates are divided into two groups: glucosyl ethers (or O-glucosides) and glucosyl esters. GA Glc conjugates are biologically inactive. The degree of hydrolysis reflects the activity of released parent GA (Sembdner et al., 1980). The loss of biological activity in the course of the conjugation process and the increased polarity of GA glucosyl conjugates favor GA conjugates for their deposition into the plant cell vacuole, but their storage within chloroplasts has not yet been investigated. Because of their preferential formation and accumulation during seed maturation, it has been proposed that GA Glc conjugates may function as storage products (Schneider et al., 1992). The easy formation and hydrolysis of GA glucosyl conjugates results in reversible deactivation/activation and facilitates the regulation of free GA pools.
Plastids play an important role in early biosynthetic steps of plant hormones, including auxins, cytokinins, ABA, and GAs (Davies, 2004). Proplastids of the apical meristem are reported to contain enzymes involved in the early biosynthesis of GAs, but their activities are not detected in mature chloroplasts (Aach et al., 1997; Yamaguchi et al., 2001). Although cytokinins affect a number of processes in chloroplasts, their metabolism has not been fully understood. Chloroplasts from dark-treated tobacco (Nicotiana tabacum) leaves were reported to contain zeatin riboside-O-glucoside and dihydrozeatin riboside-O-glucoside and relatively high cytokinin oxidase activity, suggesting that chloroplasts may contain cytokinins, their conjugates, and the enzymatic activity necessary for their metabolism (Benková et al., 1999). However, a similar role of plastids for subcellular hormone homeostasis is not known for GAs, auxins, and ABA (Nambara and Marion-Poll, 2005; Woodward and Bartel, 2005; Marion-Poll and Leung, 2006).
Sap-sucking insects belonging to the order Homoptera include some of the most devastating insect pests worldwide. Most serious damage caused by these pests is due to their role as vectors of plant viruses. Morphologically specialized structures such as trichomes located on the plant surface may serve as physical barriers to prevent insect feeding. It is well known that trichomes secrete secondary metabolites that are toxic to insects. Among these metabolites, Suc esters are predominant and highly toxic to whiteflies (Bemisia tabaci; Severson et al., 1984; Lin and Wagner, 1994). Cembrenoid diterpene has neurotoxic, cytotoxic, and antimitotic activities (Guo and Wagner, 1995).
Here, we describe transplastomic tobacco plants expressing a fungal β-glucosidase, which is likely to release plant hormones from their conjugates, and its effect on tobacco development. The transplastomic lines show early flowering and increases in biomass, height, internode length, leaf area, and density of leaf globular trichomes that contain more sugar esters that confer protection from whitefly and aphid (Myzus persicae) attacks. Many of the observed effects are typical for plants with altered GA levels (Pimenta Lange and Lange, 2006). In addition trans-zeatin, indole-3-acetic acid (IAA), and ABA levels were evaluated in different plant tissues or organs. These observations open new avenues to modify plants for enhanced biomass, confer novel traits, provide direct evidence for storage of hormone conjugates within chloroplasts, and help to understand basic concepts of the release, transport, metabolism, and signaling of plant hormones in different cellular compartments.
RESULTS
Chloroplast Transformation Vector
The coding sequence of the β-glucosidase gene (bgl1) was amplified from Trichoderma reesei genomic DNA using a PCR-based method (An et al., 2007). To create tobacco plants expressing β-glucosidase, leaf explants from tobacco were transformed with the chloroplast transformation vector (pLD) containing the bgl1 gene (Fig. 1B). Site-specific integration of bgl1 into the trnI/trnA spacer region of the chloroplast genome was achieved through the pLD vector containing the homologous recombination sequences as described previously (Verma and Daniell, 2007; Daniell et al., 2009). This site of integration has several unique advantages (Daniell et al., 2004). The psbA promoter/5′ untranslated region inserted upstream of the bgl-1 gene is anticipated to increase transcription and translation in the light, and the 3′ untranslated region is believed to increase transcript stability. The constitutive 16S rRNA promoter regulates expression of the aminoglycoside 3′ adenylyltransferase (aadA) gene to confer spectinomycin resistance.
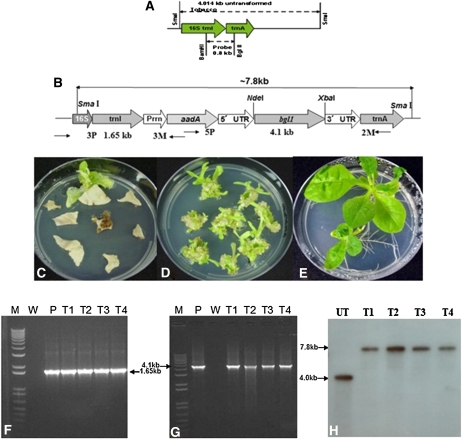
Figure 1.
Chloroplast vectors, plant transformation, and transgene integration. A and B, Schematic representations of the chloroplast flanking sequences used for homologous recombination, probe DNA sequence (0.81 kb; A), and primer annealing sites (3P/3M, 5P/2M; B). C, First round of selection and primary transplastomic shoots. D, Second round of selection. E, Regenerated shoots on rooting medium for the third round of selection; all selection media contained spectinomycin (500 mg L−1). F and G, PCR analysis using primer pairs 3P/3M and 5P/2M for evaluation of site-specific integration of the transgene cassette into the chloroplast genome. M, One-kilobase plus DNA ladder; P, positive control; T1 to T4, transplastomic lines; W, wild-type control. H, Southern blot hybridized with the flanking sequence probe. The UT chloroplast genome shows a 4.0-kb fragment, while BGL-1 lines show a 7.8-kb hybridizing fragment. [See online article for color version of this figure.]
Integration of the Expression Cassette and Homoplasmy
After bombardment of the chloroplast integration and expression vector pLD-utr-bgl1, several spectinomycin-resistant shoots appeared from the bombarded tobacco leaves in the first round of selection (Fig. 1C). Homoplasmic shoots were obtained after the second round of selection (Fig. 1D). The third round of selection on half-strength Murashige and Skoog (MS) medium (Fig. 1E) established transplastomic lines. To confirm the integration of transgene cassettes into the chloroplast genome, the putative transformed plantlets were screened by PCR. Two pairs of primers were used for screening. The 3P and 3M primers were used to check integration of the selectable marker gene (aadA) into the chloroplast genome. The 5P and 2M primers were used to confirm integration of the transgene expression cassette. DNA samples from the BGL-1 shoots showed PCR-positive results with both primers (Fig. 1, F and G). The 3P-3M PCR product size for BGL-1 plants was 1.65 kb, and the 5P-2M PCR product size was 4.1 kb.
Southern-blot analysis was performed to investigate whether BGL-1 transplastomic lines achieved homoplasmy. The probe used was made by digesting the flanking sequences trnI and trnA with BamHI and BglII (Fig. 1A). Flanking sequence probe hybridized with a single 4.0-kb fragment in untransformed (UT) chloroplast genomes. In the BGL-1 lines, one 7.8-kb hybridizing fragment was observed (Fig. 1H). Absence of the 4.0-kb wild-type fragment suggests that all the chloroplast genomes are transformed (to the detection limit of Southern blots) and therefore are homoplasmic.
Transgene Segregation and Phenotype of BGL-1 Transplastomic Lines
T1 transplastomic BGL-1 and UT seeds were germinated on half-strength MS medium containing spectinomycin (500 mg L−1). Transplastomic BGL-1 seeds germinated and grew normally into green seedlings, whereas UT seedlings were bleached soon after germination (Fig. 2A). The lack of Mendelian segregation of transgenes in the BGL-1 line is evident in the progeny.
Figure 2.
Evaluation of transgene segregation and the phenotype of transplastomic (BGL-1) and UT plants. A, UT and transplastomic seeds germinated on half-strength MS medium containing spectinomycin (500 mg L−1) confirm the lack of Mendelian segregation. B, Plants at 3 weeks after seed germination: UT (left) and BGL-1(right). C, Two-month-old transplastomic (left) and UT (right) plants. D, Leaves of transplastomic (bottom row) and UT (top row) plants. E, Mature (3-month-old) transplastomic (right) and UT (left) plants.
In order to investigate the phenotypes (plant height, internode length, flowering time, leaf area, biomass, etc.) of UT and transgenic lines, 12 transgenic plants from three independent transplastomic BGL-1 lines and 12 UT plants were grown in the greenhouse at 25°C, fertilized, and irrigated according to standard procedures. Figure 2 (B and C) shows the same age of BGL-1 and UT seedlings, and significant differences in their size and height are evident. The average flowering time of BGL-1 plants was 1 month earlier than the UT control. The height of the mature BGL-1 line increased 150% when compared with the UT plants, because transplastomic plants have longer internodes (Table I). The leaf area of BGL-1 plants also increased by 160% (Fig. 2D); the average leaf area of BGL-1 was 780 cm2 and that of UT was 490 cm2 (Table I). The average biomass of the mature transplastomic plants was 190% higher than the UT line (Table I).
Table I. Phenotypic assessment of transplastomic BGL-1 and UT plants.
| Plant Type | Internode Length ± sd | Plant Height ± sd | Leaf Area ± sda | Flowering Time ± sd | Biomass ± sdb | Density of Trichomes ± sd |
|
| Upper Side | Lower Side | ||||||
| cm | cm2 per leaf | d | g per plant | mm2 | |||
| UT | 3.2 ± 0.3 | 135.3 ± 5 | 492.2 ± 116 | 152 ± 6 | 584.2 ± 33 | 14.5 ± 3 | 21.6 ± 4 |
| BGL-1 | 5.8 ± 0.4 | 204.3 ± 12 | 779.4 ± 113 | 120 ± 5 | 1,104.3 ± 55 | 149.9 ± 10 | 160.6 ± 5 |
A total of 24 mature leaves (counted from the top, the seventh, and eighth) from 12 BGL-1 and 12 UT plants were measured with the 3,100 leaf area meter.
Five fully grown plants (with 14–16 leaves) from BGL-1 and UT were weighed after removal of soil. The whole plants (including the leaves, stems, and roots) were weighed on a Metter electronic balance.
High Levels of β-Glucosidase Activity in BGL-1 Leaves
Young, mature, and old leaves from transplastomic BGL-1 plants were collected, and β-glucosidase activity was measured using p-nitrophenyl β-d-glucopyranoside (pNPG) as the substrate. One unit of β-glucosidase is defined as the amount of enzyme that released 1 μmol of p-nitrophenol from pNPG substrate under the assay conditions described in “Materials and Methods.” β-Glucosidase activity (44.4 units g−1 mature fresh leaves) was 160-fold more in transplastomic BGL-1 lines than in UT plants (0.27 units g−1 mature fresh leaves). To calculate the yield of β-glucosidase enzyme in whole plants, leaves were collected and grouped into young, mature, and old. The yield of β-glucosidase enzyme was very high in transplastomic plants because the leaf biomass yield was almost 2-fold higher than in UT plants. Because 8,000 tobacco plants can be grown in 1 acre, it is possible to produce 130 million units per cutting, which is 390 million units per year (based on three cuttings; Table II).
Table II. BGL-1 enzyme yield in transplastomic tobacco plants.
One unit of BGL-1 enzyme is defined as the amount of enzyme that released 1 μmol of p-nitrophenol from pNPG substrate under the assay conditions described in “Materials and Methods.” Enzyme yield per acre per year was determined using the following information: 1,5941.4 BGL-1 units per plant × 8,000 plants per acre × three cuttings per year = 382.59 million units per acre per year.
| Enzyme | Leaf Age | No. of Leaves per Plant | Average Weight per Leaf | Units g−1 in Leaf | Units |
Whole Plant Yield | Units (Millions) per Acre per Cutting | Units (Millions) per Acre per Year | |
| Per Leaf | Per Age Group | ||||||||
| g | units | ||||||||
| BGL-1 | Young | 5.7 | 7.1 | 9.24 | 65.6 | 373.92 | 1,5941.4 | 127.53 | 382.59 |
| Mature | 14.2 | 16.5 | 44.41 | 732.77 | 10,405.33 | ||||
| Old | 8.2 | 14.9 | 42.25 | 629.53 | 5,126.15 | ||||
High Density of Trichomes in BGL-1 Leaves
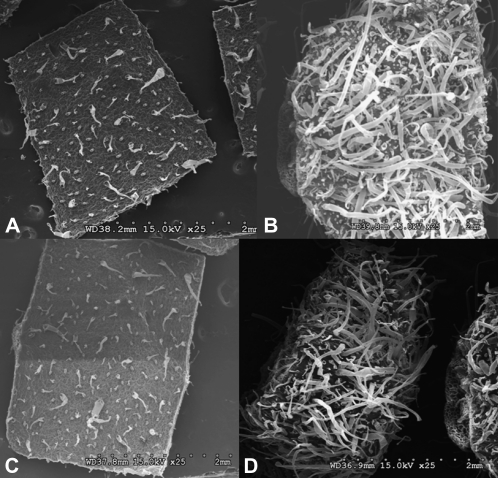
Trichomes are specialized unicellular or multicellular structures derived from the epidermal cell layer and may have various functions depending on the plant species and organs (Wagner et al., 2004). Scanning electron microscopy analysis revealed two kinds of trichomes on both surfaces of tobacco leaves: glandular and nonglandular trichomes. The glandular trichomes differ in morphology and in the spectrum of compounds that are secreted. The glandular trichomes, with large heads, were found on both the lower and upper leaf surfaces. Trichome density was 10 times higher on the upper surface (Fig. 3, A and B) and 7.4 times higher on the lower surface (Fig. 3, C and D) of transplastomic BGL-1 lines when compared with UT controls (Table I).
Figure 3.
Evaluation of leaf surface by scanning electron microscopy. A, Trichomes on leaf upper surface of a UT plant. B, Trichomes on leaf upper surface of a BGL-1 plant. C, Trichomes on leaf lower surface of a UT plant. D, Trichomes on leaf lower surface of a BGL-1 plant.
GA Hormone Levels Are Elevated in BGL-1 Leaves
Endogenous GA levels were investigated because many of the phenotypical changes that occurred in the transplastomic line are known to be regulated by GA. Glucosyl conjugates of GAs are common endogenously occurring metabolites. They are expected to play a significant role in the regulation of the active hormone level (homeostasis) as well as in the processes of transport and storage (Schneider and Schliemann, 1994; Sembdner et al., 1994). The metabolic formation of GA conjugates has been described (Sembdner et al., 1994; Schneider et al., 2000). The reconversion of both GA-O-glycosides and GA glucosyl esters to free GAs has also been observed (Rood et al., 1983, 1986; Schneider and Schliemann, 1994). The metabolism of intact GA-O-glucosides, however, has not yet been detected. GA conjugates may play an important role in the control of growth and development of higher plants. It has been suggested that GA glucosyl esters are “deactivated” GAs that can be enzymatically reconverted to active GAs, thus serving as a reserve form of biologically active GAs. Two parallel GA biosynthetic pathways occur, the “nonhydroxylated” and the 13-hydoxylated pathways (Pimenta Lange and Lange, 2006), the latter one of which has been identified to be the major one in tobacco, and this was confirmed in this study for both BGL-1 and UT plants (Jordan et al., 1995; Table III). Therefore, we investigated GA hormones, their precursors, and metabolites of both pathways in UT control plants and BGL-1 lines (Table III).
Table III. Quantification of endogenous GAs from different parts of UT and BGL-1 transplastomic lines.
Results are means of two determinations.
| GA | Mature Leaf |
Inflorescence |
Shoot Tip |
Internode |
Young Leaf |
|||||
| UT | BGL-1 | UT | BGL-1 | UT | BGL-1 | UT | BGL-1 | UT | BGL-1 | |
| ng g21 dry wt | ||||||||||
| GA53 | 0.3 | 0.2 | 2.8 | 3.1 | 0.9 | 1.0 | 5.0 | 7.0 | 0.2 | 0.4 |
| GA44 | 0.0 | 0.1 | 0.0 | 0.3 | 0.3 | 0.7 | 0.7 | 3.9 | 0.1 | 0.3 |
| GA19 | 5.7 | 10.6 | 12.6 | 11.2 | 14.3 | 20.8 | 18.7 | 23.9 | 13.0 | 23.8 |
| GA20 | 2.4 | 7.6 | 0.5 | 0.6 | 5.9 | 8.1 | 3.9 | 4.8 | 19.4 | 36.8 |
| GA1 | 3.3 | 6.7 | 0.9 | 0.3 | 1.9 | 1.5 | 1.4 | 0.4 | 10.0 | 15.6 |
| GA8 | 1.5 | 2.9 | 1.0 | 0.7 | 2.1 | 0.8 | 0.7 | 0.7 | 0.8 | 0.7 |
| GA4 | 0.4 | 0.6 | 0.3 | 0.7 | 0.4 | 0.6 | 1.0 | 0.2 | 2.3 | 6.6 |
| GA34 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 |
Highest GAs levels were detected in leaves (Table III). There is a 2-fold increase in levels of GA precursor (GA53, GA44, GA19, and GA20), hormone (GA1), and catabolite (GA8) in the BGL-1 line when compared with UT controls (Table III). Therefore, the major GA pathway (13-hydroxylated pathway) is up-regulated in leaves of transplastomic lines. In other plant organs, GA precursor levels were similar (inflorescence) or even lower (shoot tip and internodes) in transplastomic lines when compared with UT plants. When compared with leaves in these organs, only low GA hormone levels were detectable, and they were similar (inflorescence) or even lower (shoot tip and internodes) in transplastomic lines than in UT plants.
In addition, the nonhydroxylated pathway was analyzed: GA12, GA15, GA24, and GA9 precursor levels (data not shown) and GA34 catabolite levels (Table III) were more than 5 times lower compared with the respective GAs of the 13-hydroxylated pathway, confirming the latter one as the major pathway. However, GA4 hormone levels were higher in leaves of transplastomic lines than of the UT plant (Table III). Taken together, an increase in active GA hormone levels is observed only in leaves where chloroplasts are abundant and β-glucosidase is expressed. All GAs analyzed form Glc conjugates, as they contain COOH (ester) and OH (ether) groups. Precursors can form conjugates, as they have COOH and OH side groups within the molecule. Therefore, release of active hormones from conjugates of precursors and mature forms by β-glucosidase is anticipated based on our hypothesis.
IAA and trans-Zeatin, But Not ABA, Levels Are Higher in BGL-1 Lines
Several hormones form Glc conjugates, including ABA, zeatin, and IAA (Sembdner et al., 1994). Also, some of the phenotypes observed in BGL-1 lines (e.g., large leaf size) could be due to the action of other plant hormone groups. Therefore, all hormones or conjugates that we had the ability to evaluate and quantify were investigated using ELISA kits. In BGL-1 samples, the levels of IAA increased in all the tissues or organs. There was 130%, 140%, and 120% more IAA in inflorescence, shoot tip, and internode of BGL-1 than in the UT control. However, the IAA levels increased by 280% in mature leaves of BGL-1 lines (Fig. 4B), where more chloroplasts are present.
Figure 4.
Endogenous ABA, IAA, and trans-zeatin concentration in BGL-1 and UT plants. A, The trans-zeatin concentrations of BGL-1 and UT plants. B, The IAA concentrations of BGL-1 and UT plants. C, The ABA concentrations of BGL-1 and UT plants. ABA, IAA, and trans-zeatin concentrations were calculated as ng per g fresh weight (FW). Each measurement was replicated three to four times using different pooled samples and the Phytodetek competitive ELISA kit.
The trans-zeatin levels in BGL-1 samples also increased significantly. When compared with UT controls, trans-zeatin levels in inflorescence, shoot tip, and internode increased by 170%, 170%, and 160%, respectively. Notably, there were 230% and 210% higher trans-zeatin levels in mature and young leaves of BGL-1 than in the UT control (Fig. 4A), where more chloroplasts are present. In contrast, there was no significant increase of ABA levels in the BGL-1 samples when compared with the UT control, even in the young and mature leaves of the transplastomic BGL-1 lines (Fig. 4C), supporting the idea that ABA conjugates may be irreversible (Sembdner et al., 1994).
Protoplast Culture without Exogenous Hormones or with Hormone Conjugates
The enzyme cocktail with 2% (w/v) cellulase and 0.5% macerozyme could digest the leaf samples completely and release intact protoplasts. The yield of protoplasts from BGL-1 and the UT control was around 4 to 5 × 106 g fresh weight, comparable to a previous report (Rao and Prakash, 1995). To evaluate the effects of hormones or hormone conjugates on protoplast division, six hormones or conjugate combinations (Table IV) were tested in the protoplast culture. Protoplasts from UT leaves did not divide and form cell colonies in the culture medium without exogenous hormone or when supplied with zeatin-O-glucoside only (Fig. 5, A, C, and E; Table IV). In contrast, protoplasts from BGL-1 leaves divided continuously (Fig. 5, B and D) and developed into cell colonies (Fig. 5F) in different types of culture even in the absence of added hormones (Table IV). Compared with the protoplasts from UT, the protoplasts from BGL-1 showed higher division and plating efficiency (Table IV). Most importantly, protoplasts from the BGL-1 line utilized exogenously supplied zeatin-O-glucoside, increasing the efficiency of their cell division by 670% when compared with UT protoplasts (Table IV).
Table IV. Division and plating efficiency of protoplasts derived from BGL-1 and UT.
| Hormone Combination (mg L−1) | No. of Protoplasts Observed | No. of Divisions | Division Efficiencya | No. of Protoplasts Observed | No. of Calli | Plating Efficiencyb |
| % | % | |||||
| Free hormone | ||||||
| UT | 382 | 0 | 0 | 532 | 0 | 0 |
| 428 | 0 | 432 | 0 | |||
| 469 | 0 | 470 | 0 | |||
| BGL-1 | 482 | 17 | 2.8 ± 0.7 | 411 | 9 | 2.0 ± 0.3 |
| 478 | 13 | 485 | 11 | |||
| 467 | 10 | 421 | 7 | |||
| Zeatin (1.0) + naphthylacetic acid (3.0) | ||||||
| UT | 510 | 134 | 28.0 ± 1.7 | 531 | 51 | 9.1 ± 0.6 |
| 514 | 145 | 504 | 47 | |||
| 481 | 142 | 469 | 40 | |||
| BGL-1 | 412 | 141 | 34.0 ± 2.0 | 410 | 62 | 14.1 ± 1.4 |
| 514 | 185 | 519 | 77 | |||
| 489 | 156 | 481 | 60 | |||
| Naphthylacetic acid (3.0) | ||||||
| UT | 397 | 22 | 4.8 ± 0.7 | 389 | 12 | 3.3 ± 0.2 |
| 413 | 20 | 421 | 14 | |||
| 419 | 17 | 456 | 16 | |||
| BGL-1 | 567 | 41 | 9.1 ± 1.8 | 512 | 25 | 4.3 ± 0.5 |
| 418 | 45 | 504 | 20 | |||
| 421 | 39 | 462 | 19 | |||
| Naphthylacetic acid (3.0) + zeatin-O-glucoside (1.0) | ||||||
| UT | 501 | 18 | 4.1+0.5 | 512 | 18 | 3.4+0.4 |
| 402 | 19 | 461 | 17 | |||
| 479 | 20 | 470 | 14 | |||
| BGL-1 | 510 | 132 | 27.5 ± 2.2 | 439 | 52 | 11.4 ± 1.0 |
| 408 | 122 | 481 | 59 | |||
| 412 | 110 | 477 | 49 | |||
| Zeatin (1.0) | ||||||
| UT | 512 | 35 | 6.8 ± 0.9 | 514 | 23 | 4.6 ± 0.3 |
| 427 | 32 | 525 | 26 | |||
| 501 | 30 | 426 | 19 | |||
| BGL-1 | 433 | 55 | 10.6 ± 0.8 | 421 | 28 | 6.2 ± 0.4 |
| 479 | 49 | 513 | 31 | |||
| 534 | 48 | 401 | 24 | |||
| Zeatin-O-glucoside (1.0) | ||||||
| UT | 521 | 0 | 0 | 458 | 0 | 0 |
| 520 | 0 | 421 | 0 | |||
| 524 | 0 | 521 | 0 | |||
| BGL-1 | 551 | 36 | 7.5 ± 1.2 | 471 | 18 | 3.7 ± 0.3 |
| 478 | 42 | 440 | 15 | |||
| 499 | 37 | 502 | 20 | |||
Number of protoplasts dividing per number of total protoplasts in the same visual field of the microscope.
Number of protoplasts dividing and forming cell groups per number of total protoplasts in the same visual field of the microscope.
Figure 5.
Protoplasts and protoplast-derived cells and cell colonies. A, Protoplasts from UT leaf could not divide in the medium without hormones. B, First division of BGL-1 protoplast without hormones. C, Protoplasts from UT leaf could not divide in the medium with zeatin-O-glucoside. D, First cell division of BGL-1 sample in the medium with zeatin-O-glucoside. E, Protoplasts from UT leaf could not form calli in the medium without hormones. F, Protoplast-derived calli of BGL-1 sample in the medium without hormones. Bars = 60 μm. [See online article for color version of this figure.]
High Accumulation of Sugar Ester in BGL-1 Glandular Trichomes
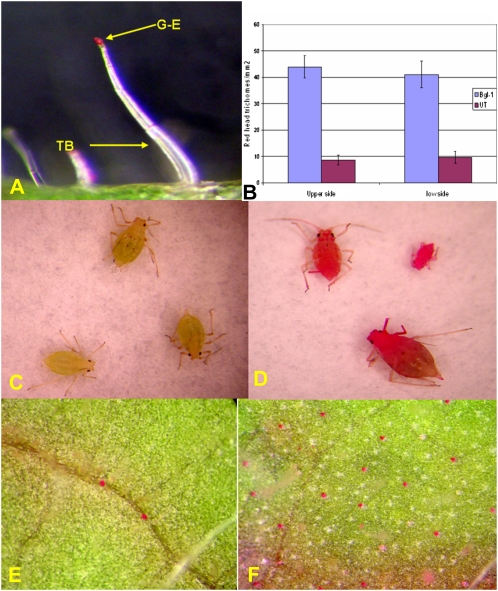
Natural sugar esters have been shown to be effective biopesticides against a range of insect species. Soft-bodied arthropods, including mites, lepidopteran larvae, aphids, whiteflies, and psyllids, are killed rapidly upon contact. In addition, sugar esters have demonstrated ovipositional and feeding deterrence against mites, whiteflies, and leaf miners (McKenzie et al., 2005). It has been well documented that the trichome gland is the only site of exudate sugar ester synthesis in tobacco (Severson et al., 1984), and our light microscope observations confirmed this. Tissues with glandular trichomes were stained by 0.2% rhodamine B, and only the trichome gland was stained (Fig. 6A). Aphids were placed on rhodamine B-stained leaf segments on glass slides and allowed to walk on the surface for 30 min. Slides were then placed in a chloroform atmosphere to anesthetize insects and then mounted for photography. It was observed that aphids walking on BGL-1 leaves were extensively contaminated with sugar ester (Fig. 6D), while the stain of wild-type aphids was very faint (Fig. 6C). It is well known that sugar ester, 4,8,13-duvatriene-1,3-diol, and labdanoids are predominant biopesticides excreted by tobacco glandular trichomes (Severson et al., 1984). This procedure was specific for sugar ester (4,8,13-duvatriene-1,3-diol and labdanoids are not stained) and can be used as a quantitative measure of sugar ester (Severson et al., 1984; Lin and Wagner, 1994). Figure 6 (E and F) revealed that there are 4 to 5 times more glandular trichomes with red secretory heads from both BGL-1 leaf surfaces than on the UT leaves (Fig. 6B), suggesting that transplastomic plants produced more sugar ester than UT plants.
Figure 6.
Histochemical staining of sugar ester. A, Glandular trichomes stained by rhodamine B. B, Density of trichomes with red glandular heads stained by rhodamine B. C, Aphids from a UT plant showing lower staining intensity. D, Red aphids walking on the surface of a BGL-1 leaf. E, Glandular trichomes stained by rhodamine B from a UT plant. F, Higher density of red glandular trichomes from a BGL-1 leaf.
BGL-1 Lines Confer Protection against Whiteflies and Aphids
Both BGL-1 (from two independent lines) and UT samples for aphid and whitefly colonization tests included 10 plants each. Colonization tests of whitefly and aphid were performed in the greenhouse. Transplastomic and UT plants were covered with mesh bags in which 30 aphids or whiteflies were released (Fig. 7, A and B). The population buildup was recorded 25 d later (Fig. 7C). Significant differences in oviposition and the immature and adult populations were observed when whiteflies or aphids were released on control or transplastomic plants (Table V). The total number of whiteflies (eggs/pupae/adults) on the UT plants was 18-fold higher than on the BGL-1 transplastomic lines. Figure 7 (D and E) show a large population of whiteflies on the UT leaves, whereas only a few of whiteflies were found on the transplastomic plants (Fig. 7F). Similarly, heavy colonization of aphids on the control plants was very apparent (Fig. 7, G and H) when compared with transplastomic plants (Fig. 7I). The size of the aphid population on the UT plants was 15 times more than on the BGL-1 transplastomic lines (Table V).
Figure 7.
Aphid and whitefly bioassays on BGL-1 and UT plants. A, The mesh-bag cage placed on each pot (40-d-old plants, six- to seven-leaf stage) on day 0 for insect bioassays. B, Plants 25 d after insect bioassays. C, Release of plants from the cage at 25 d after insect bioassays. D and E, A UT plant heavily colonized with mature and immature whiteflies. E shows an enlarged view of the circled area in D. F, BGL-1 transplastomic plants with negligible colonization of whiteflies. G and H, A UT plant heavily colonized with mature and immature aphids. H shows an enlarged view of the circled area in G. I, BGL-1 transplastomic plants with negligible aphids.
Table V. Aphid and whitefly colonization tests on BGL-1 and UT plants.
Whole plants (40 d old, six- to seven-leaf stage) were confined to insect-proof nylon mesh bags and maintained at 25°C for 25 d as shown in Figure 7. For the aphid bioassay, 30 neonatal nymphs were introduced with a hair brush to each plant. Thirty newly emerged adult whiteflies were released in each mesh bag .Twenty-five days after release, the mesh bags were removed, and the total number of adults and immature-stage insects that emerged was recorded on the whole plant. Both BGL-1 and UT lines for aphid or whitefly colonization tests had 10 plants each. Values shown are numbers per plant ± sd.
| Plant Type | Whitefly Population |
Aphid Population |
||||
| Eggs/Pupae | Adults | Total | Nymphs | Adults | Total | |
| UT | 1,257.8 ± 171 | 580.6 ± 71 | 1,838.4 ± 222 | 568.9 ± 101 | 388.9 ± 61 | 957.8 ± 156 |
| BGL-1 | 75.0 ± 15 | 25.6 ± 5 | 100.6 ± 17 | 42.0 ± 13 | 22.3 ± 5 | 64.3 ± 15 |
In addition to colonization tests, the toxicity of exudates was also evaluated using the method reported by Jackson and Danehower (1996) and Wang et al. (2001). Purified exudates from tobacco leaf surface were applied as droplets of 0.2 μL size in a solvent of Suc acetate isobutyrate:acetone (2:1, v/v) to dissolve exudates. This solvent alone caused no mortality over the 66-h time period of toxicity tests (Wang et al., 2001). According to Spearman-Karber method dose-response analysis of whitefly to total exudates, LD50 values (lethal dose to kill 50% of the test population) of 26.3 and 39.2 μg per whitefly for BGL-1 and UT were obtained. Similarly, the LD50 values for aphid were 23.1 and 35.2 μg per aphid for BGL-1 and UT exudates (Fig. 8). These results are very similar to the results reported by Wang et al. (2001), in which genetically modified plants showed a LD50 value of 20.8 when compared with 32.2 in UT plants.
Figure 8.
Toxicity LD50 values for whiteflies and aphids from trichome exudates of UT and BGL-1 plants. Analysis is of composite data from four independent experiments with separate exudate isolates. Mortality was assessed after 66 h. LD50 values were estimated according to the Karber method.
DISCUSSION
The protoplast culture system offers an ideal platform to investigate cell wall regeneration, cell division, and plant regeneration. The success of a protoplast culture system primarily relies on consistent yields of a large population of uniform and viable protoplasts and culture medium with optimal hormone combinations. Cytokinins and auxins are two kinds of important hormones for protoplast culture, and nearly all protoplast culture systems use these two hormones. Hormone glucoside conjugates are the metabolic products of free hormones and are inactive unless hydrolyzed and released from their conjugates by enzymes. In the protoplast culture system shown here, protoplasts from transplastomic leaves could divide and form calli in the medium without exogenous hormone or utilizing hormone conjugates. Therefore, protoplasts could survive and divide utilizing the endogenous free hormones released by β-glucosidase from their conjugates or exogenously supplied zeatin-O-glucosides. In contrast, protoplasts from UT plants could not utilize the exogenously supplied hormone conjugate or divide in the absence of hormones. Also, these investigations suggest the transport of free hormones between chloroplasts and the cytoplasm. While thousands of proteins are imported into chloroplasts, not a single protein has been conclusively shown to be exported from chloroplasts. Most recently, we showed that antimicrobial peptides expressed within chloroplasts conferred high levels of antiviral (Tobacco mosaic virus) and antibacterial (Erwinia) protection. In this study, antimicrobial peptides were fused with GFP to see whether they are exported, but confocal studies showed GFP expression exclusively within chloroplasts (Lee et al., 2010). Therefore, it is very likely that β-glucosidase is compartmentalized within chloroplasts unless they are lysed by osmotic stress.
Furthermore, there was a significant increase in endogenous levels of all hormones tested except ABA. In mature leaves, there was more catabolic product GA8 present than in young leaves. There were about 2 times more GA precursors (GA53, GA44, GA19, and GA20), plant hormone GA1, and catabolite GA8 in BGL-1 lines than in UT controls. Therefore, the major GA pathway (13-hydroxylated pathway) was up-regulated in leaves of transplastomic lines. Such increases in active GA precursors, hormones, and metabolic products were observed only in leaves where β-glucosidase was expressed and not in other parts where chloroplasts are not abundant (e.g. inflorescence). Previously, GA conjugates have been shown to be present mainly in vacuoles of the cell (Garcia-Martinez et al., 1981). However, our results suggest the chloroplast as another important cell compartment harboring GA conjugates (precursors, hormones, and/or catabolites).
In BGL-1 lines, internode length, plant height, biomass, leaf area, and trichome density increase, and they flower 1 month earlier than in UT plants (Table I). All these developmental steps are known to be regulated by GAs. Although the internodes of the BGL-1 lines are clearly longer when compared with those of UT plants (Table I; Fig. 1H), only the GA precursor levels, but not the GA hormone levels, are elevated in internodes of the BGL-1 line. This observation suggests a rapid turnover due to feed-forward up-regulation of the catabolic steps of the pathway in the BGL-1 lines (Pimenta Lange and Lange, 2006).
Besides quantification of GA metabolism, IAA, trans-zeatin, and ABA concentrations were also analyzed by ELISA. The IAA level increased more (approximately 280%) in mature leaves of BGL-1 lines, where more chloroplasts are present. The trans-zeatin level in BGL-1 samples also increased significantly (more than 200%), especially in mature and young leaves, where more chloroplasts are present. In contrast, there was no significant increase of ABA level in the BGL-1 samples, even in leaves, confirming the suggestion that ABA conjugates may be irreversible (Sembdner et al., 1994).
GLABROUS1 (GL1), encoding a MYB transcription factor, is one of the genes necessary for trichome initiation (Schwab et al., 2000). GL1 expression can be induced by treatment with GAs. The Arabidopsis (Arabidopsis thaliana) ga1 mutation results in less abundant GL1 and fewer trichomes. Application of GA reversed both effects (Perazza et al., 1998). More recently, Curaba et al. (2004) observed that Arabidopsis lec2 and fus3 mutants with higher levels of bioactive GAs and GL1 transcript (50- to 60-fold) resulted in higher trichome density. β-Glucosidases hydrolyze β1-4 bonds between two Glc molecules or Glc-substituted molecules (not only from hormone conjugates). This should result in increases of free hormones and, to a much larger extent, in an increase of Glc within the plastids. The free hormone GA increases the formation of trichomes. The additional Glc might be utilized in the formation of additional sugar esters that were identified in the glandular trichomes of BGL-1 lines. Our results indicate that β-glucosidase releases active hormones, including GAs, from their conjugates stored within plastids and facilitate a 740% to 1,033% increase in trichome density in transplastomic lines. Moreover, rhodamine B staining confirmed an increase in sugar esters located within the globular trichomes of the BGL-1 lines. Insect bioassays showed that the whitefly and aphid (eggs/pupae/adults) populations in the UT plants were 18- and 15-fold higher than in the BGL-1 transplastomic lines. Again, the fact that sugar esters were not hydrolyzed by β-glucosidase within the globular trichomes supports the view that this enzyme is compartmentalized within chloroplasts. Taken together, our data suggest that plastid-specific expression of β-glucosidase releases endogenous plant hormones, including GAs, with dramatic impact on growth, flowering, biomass, and pathogen defense. These observations open new avenues to engineer plants for enhanced biomass and to confer novel traits, in addition to the production of an important enzyme for the biofuel industry.
A recent study sponsored by the U.S. Departments of Energy and Agriculture concluded that up to 1.3 billion dry tons of biomass could be produced annually in the United States by 2030 for bioenergy production, in addition to present agricultural and forestry production (Perlack et al., 2005). Based on a theoretical yield of 113 gallons of ethanol per ton of dry corn (Zea mays) stover, a 5% increase in biomass would deliver an additional 621 million gallons of ethanol annually, which is equal to 10% of 2007 corn ethanol production (Carroll and Somerville, 2009; Salas Fernandez et al., 2009). Therefore, doubling the biomass should increase ethanol production 20-fold and have a profound effect in the biofuel industry. Even though several approaches have been considered for increasing hormone biosynthesis to enhance biomass (Salas Fernandez et al., 2009) or understand regulation/signaling (Achard and Genschik, 2009), release of active hormones from stored conjugates or utilization of stored reserves within plant cells has not yet been investigated. Therefore, this paper provides a novel option for releasing active hormones to understand basic concepts or enhance biomass.
One of the major limitations in the production of ethanol from plant biomass is the cost and availability of the enzymes required for hydrolysis (Sticklen, 2008). Therefore, both the nuclear and chloroplast genomes have been modified to produce glycosyl hydrolases in different cellular compartments (Leelavathi et al., 2003; Wei et al., 2004; Yu et al. 2007; Taylor et al., 2008; Ziegelhoffer et al., 2009). Recently, the chloroplast genome has been engineered to produce low-cost enzyme cocktails that are efficient in biomass hydrolysis (Verma et al., 2010a). Based on observed expression levels in this study, up to 382.59 million units of β-glucosidases can be produced annually, per acre of tobacco, significantly reducing their production cost. Recent advances in our ability to transform different crop species via the chloroplast genome (Verma and Daniell, 2007; Ruhlman et al., 2010) and produce vaccines in leaves (Arlen et al., 2008; Davoodi-Semiromi et al., 2010) for oral delivery make these findings broadly applicable.
It is known that functionally inactive hormone conjugates (e.g. β-glucosides) are stored in vacuoles in both monocotyledons and eudicotyledons in plant cells (Morant et al., 2008). However, in this study, we have utilized for the first time hormone conjugates stored in chloroplasts. The bioactivating β-glucosidases in eudicotyledons are stored in the apoplast as an enzyme or as protein bodies. In monocotyledons, β-glucosidases are localized in plastids or other compartments. While thousands of proteins are imported into chloroplasts, no protein is known to be exported. Therefore, active hormones released from conjugates within chloroplasts must be transported to other cellular compartments or organs, resulting in enhanced trichome density, internode length, and biomass or early flowering. This provides unique opportunities to study hormone transport and signaling from leaves (chloroplasts) to other organs. Therefore, in order to understand the basic concepts of release, transport, metabolism, and signaling of plant hormones in different cellular compartments, β-glucosidase could be targeted to the vacuole or expressed in chloroplasts to release active hormones from their conjugates. Thus, this study opens the door for understanding basic concepts in addition to various biotechnology applications.
MATERIALS AND METHODS
Isolation of the bgl1 Gene and Construction of the Chloroplast Vector
Full-length cDNA of bgl1 (U09580) was amplified using overlapping primers for three exons and genomic DNA of Trichoderma reesei (American Type Culture Collection) as the template by a PCR-based method (An et al., 2007). The forward primers for three exons are as follows: 5′-GAATTCCATATGCGTTACCGAACAGCAGCTGCGCTGGCACTTGCCACTGGGCCCTTTGCTAGGGCAGACAGTCACTCAACATCGGGGGCC-3′ (exon 1); 5′-CTAGGGCAGACAGTCACTCAACATCGGGGGCCTCGGCTG-3′ (exon 2); and 5′-CACGCCGCGGTACGAGTTCGGCTATGGACTGTCTTACACCAAGTTCAACTACTCACGCC-3′ (exon 3). The reverse primers for exons 2 and 3 are as follows: 5′-ACAGTCCATAGCCGAACTCGTACCG-3′ (exon 2); and 5′-CTCTCTAGACTACGCTACCGACAGAGTGCTCGTC-3′ (exon 3). Sequences for restriction enzymes NdeI and XbaI were added in forward and reverse primers to facilitate cloning into the pLD vector. Full-length amplified bgl1 was ligated into the pCR Blunt II Topo vector (Invitrogen) and sequenced (Genewiz) to detect any PCR errors. The bgl1 gene was released from the Topo vector by digestion with NdeI and XbaI and cloned into the pLD vector (Daniell et al., 2001; Verma et al., 2010b) to make the tobacco (Nicotiana tabacum) chloroplast expression vector. All cloning steps were carried out in Escherichia coli according to Sambrook and Russell (2001).
Bombardment and Selection of Transplastomic Lines
Tobacco leaves were bombarded using the Bio-Rad PDS 1000/He biolistic device as described previously (Daniell et al., 2004). Bombarded leaves were then subjected to three rounds of selection. First and second rounds of selection were performed on the regeneration medium of plants, and the third round of selection was on hormone-free half-strength MS medium. All growth media were supplemented with 500 mg L−1 spectinomycin as described previously (Verma et al., 2008). After selection, confirmed transplastomic lines were transferred to the pots in the greenhouse for further growth.
PCR Evaluation of Transplastomic Lines
Total plant DNA was isolated from UT and transplastomic tobacco leaves using the DNeasy Plant Mini Kit (Qiagen). PCR was set up with two pairs of primers, 3P-3M and 5P-2M (Verma et al., 2008), to investigate the integration of transgene expression cassettes into the tobacco chloroplast genome. The 3P primer (5′-AAAACCCGTCCTCAGTTCGGATTGC-3′) anneals with the native chloroplast genome in the 16S rRNA gene, while 3M primer (5′-CCGCGTTGTTTCATCAAGCCTTACG-3′) anneals with the aadA gene. This pair of primers was used to investigate site-specific integration of selectable marker genes into the chloroplast genome. The 5P primer (5′-CTGTAGAAGTCACCATTGTTGTGC-3′) anneals with the aadA gene, while the 2M primer (5′-TGACTGCCCACCTGAGAGCGGACA-3′) anneals with the trnA gene, which was used to confirm the integration of the transgene expression cassette.
Confirmation of Homoplasmy and Transgene Segregation
Southern-blot analysis was performed to evaluate homoplasmy according to laboratory protocols (Kumar and Daniell, 2004). In brief, total tobacco genomic DNA (2–4 μg) isolated from UT or transformed leaves after the third round of selection was digested with SmaI and separated on a 0.8% agarose gel and then transferred to a nylon membrane (Nytranspc; Whatman). The chloroplast flanking sequence probe was prepared by digesting pUC-Ct vector (Verma et al., 2008) DNA with BamHI and BglII, which generated a 0.81-kb probe (Fig. 1A). After labeling the probe with [α-32P]dCTP, the membrane was hybridized with the probe using the Stratagene Quick-Hyb hybridization solution and protocol.
T1 seeds from transplastomic line BGL-1 and UT tobacco seeds were geminated on half-strength MS medium containing 500 mg L−1 spectinomycin for the evaluation of segregation of transgenes.
Phenotypic Evaluation of Transplastomic Lines
In order to investigate the phenotypes (plant height, internode length, flowering date, leaf area, biomass, etc.) of UT and transgenic lines, 12 transgenic plants from three independent transplastomic BGL-1 lines and 12 UT plants were transferred to jiffy pellets, kept initially for 2 weeks under high humidity, and then moved to the greenhouse for further growth at 25°C, fertilized, and irrigated according to standard procedures.
Scanning Electron Microscopic Evaluation of Leaf Surface and Histochemical Staining of Sugar Ester
Leaves were washed with distilled water to remove any dirt and dead bodies of insects. A drop of fixative (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 m phosphate buffer) was added in a petri dish or on a glass plate. Small pieces were dissected (3–4 mm) from mature leaves of transplastomic and UT plants in the presence of the fixative. At room pressure, the specimens sunk to the bottom. Tissues were fixed for 3 to 4 h at room temperature. Tissues were washed with 0.1 m phosphate buffer four times for 15 min each and then rinsed with distilled water three times for 5 min each. Tissues were dehydrated with a graded series of ethanol: 30% ethanol for 10 min; 50% to 70% to 80% to 90% to 95% ethanol for 20 min each; and finally, 100% ethanol for 20 min three times. Leaf cross-sections were loaded into a gasket and placed into the critical point drier (Bomb; Electron Microscopy Sciences). After drying, samples were placed on carbon strips facing up. Gold-Palladium was coated with the Emitech K 550 Sputter Coater for 2 min to reach 200 Å. Pieces were excised and examined with a Hitachi S-3500N scanning electron microscope. The densities of trichomes were determined on both the upper and lower sides of the leaves.
For sugar ester staining, tissue pieces were submerged in 0.2% rhodamine B in water for 60 min, then submerged in four separate vessels containing distilled water (5 s in each) to remove unbound stain. Samples were photographed using a Stemi V6 stereomicroscope.
Evaluation of Aphid and Whitefly Toxicity
Exudates were washed from five to seven developmentally matched leaves of healthy BGL-1 and UT plants by acetonitrile using a protocol essentially as described by Wang et al. (2001). Washes were evaporated, and exudates were dried and weighed. Dilutions were prepared in 2:1 (v/v) Suc acetate isobutyrate:acetone. In three of the four experiments, doses were 5, 12.5, 25, and 50 μg. In the fourth experiment, doses of 6, 10, 20, 38, and 50 μg were used. Mature aphids (Myzus persicae) and whiteflies (Bemisia tabaci) were collected from greenhouse-grown UT tobacco plants and distributed among seven leaf discs (1.5 cm diameter) on 2% agar on petri plates (20 aphids per plate, one plate per dose). The discs were cut from leaves washed with 20% (v/v) Tween 20 to remove exudate and then with water. One drop of exudate-containing solution (0.2 μL) was applied to dorsa of each aphid (20 aphids or whiteflies per dose per experiment). After 66 h at 22°C and a 16-h-light/8-h-dark cycle, mortality was assessed. The LD50 values for 66 h were estimated according to the Karber method (Zhang et al., 2004).
Evaluation of Aphid and Whitefly Colonization
BGL1-1 and UT plants were challenged by aphids using the Yao et al. (2003) protocol and by whiteflies using the Jindal and Dhaliwal (2009) protocol. Plants were grown inside the greenhouse, fertilized, and irrigated according to standard procedures. During the bioassay, the whole plant (40 d old, six- to seven-leaf stage) was confined to an insect-proof nylon mesh bag and maintained at 25°C for 25 d. This system allowed aphids access to the entire plant but confined them to a single plant. Thirty neonatal nymphs were introduced with a hair brush to each tobacco plant on day 0. For the whitefly bioassay, 30 newly emerged adults were released in mesh bags as described above. The mesh bags were removed after 25 d, and the total number of adult and immature insects that emerged was recorded on the whole plant. Both BGL-1 (from two independent lines) and UT samples for aphid and whitefly colonization tests included 10 plants each.
β-Glucosidase Enzyme Assay
Total soluble protein was extracted from 1 g of fresh leaf tissue ground in liquid nitrogen using 2 mL of ice-cold 100 mm citrate buffer (pH 5.2) with the protease inhibitor cocktail (Roche). Protein concentration was measured according to the Bradford method using the Bio-Rad protein assay kit. β-Glucosidase activity was estimated by the Berghem and Pettersson (1974) method with the following modifications: 100 μg of total soluble protein extracted from leaf sample was incubated with 4 mm pNPG (Sigma) in 1 mL of 100 mm citrate buffer, pH 5.2, at 50°C for 10 min. The reaction was terminated by adding 2 mL of 1 m Na2CO3. Enzymatic release of nitrophenol was spectrophometrically determined immediately (after adding Na2CO3) at 405 nm. The standard curve of p-nitrophenol was prepared under alkaline conditions using 1 m Na2CO3. The concentration of nitrophenol present in the reaction was analyzed by measuring the absorbance and extrapolating the concentration from the nitrophenol standard curve. One pNPG unit is defined as 1 μmol of p-nitrophenol formed per min at 50°C under these assay conditions.
Evaluation of GAs, Precursor, and Metabolites by Gas Chromatography-Mass Spectrometry
Five fully grown plants (with 14–16 leaves) from BGL-1 and UT were sampled for GA analysis. Five-gram fresh samples from mature leaves (seventh or eighth), inflorescence, shoot tip, internodes, and young leaves (top first or second) were ground into fine powder in liquid nitrogen and freeze dried in a lyophilizer. All the samples were stored at –20°C until analysis. Freeze-dried plant material (200 mg dry weight) was spiked with 17,17-d2-GA standards (2 ng each; from Prof. L. Mander). Samples were extracted, purified, derivatized, and analyzed by combined gas chromatography-mass spectrometry using selected ion monitoring as described by Lange et al. (2005). Six successive GAs of the nonhydroxylated pathway (GA12, GA15, GA24, GA9, GA4, and GA34) and six of the 13-hydroxylated pathway (GA53, GA44, GA19, GA20, GA1, and GA8) were further analyzed.
ABA, IAA, and Zeatin Detection by ELISA
The ABA, IAA, and zeatin concentrations in shoot tip, inflorescence, internode, and mature and young leaves from BGL-1 and UT control plants were measured using the Phytodetek competitive ELISA kits (Agdia). The hormone extraction was done based on the Oliver et al. (2007) protocol. BGL-1 and control plants were grown in the greenhouse in the same conditions, and then shoot tip, inflorescence, internode, and mature and young leaves were collected at a fixed time of day, frozen, and ground in liquid nitrogen. The powder was extracted overnight at 4°C in cold 80% methanol. The mixture was then centrifuged at 5,000 rpm for 5 min, and the supernatant was collected. The pellet was washed three times in cold 80% methanol. Then, the supernatant of all the samples was pooled and dried in a Speed-Vac until approximately 50 μL of liquid remained. Tris-buffered saline (25 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1 mm MgCl2, and 3 mm NaN3) was added to a final volume of 200 μL. These extracts were diluted 10-fold in Tris-buffered saline and used in the ELISA according to the Phytodetek kit protocol. A standard curve of different ABA, IAA, and zeatin dilutions was constructed to calculate the sample ABA, IAA, and trans-zeatin concentrations. ABA, IAA, and trans-zeatin concentrations were calculated as ng per g fresh weight. Each measurement was replicated three to four times using pooled samples.
Protoplast Isolation and Culture
The protocol of the protoplast isolation was modified from the method of Rao and Prakash (1995). Leaves from BGL-1 and UT were cut into small pieces and placed on the surface of the enzyme solution with the lower surface down. Incubation was carried out in the dark at a temperature of 25°C ± 1°C for 8 h to release the protoplasts. The enzymatic cocktail contained 2% (w/v) cellulase Onozuka R-10, 0.5% (w/v) macerozyme R-10, 0.2% (w/v) dextran potassium sulfate, 5 mm CaCl2·2H2O, and 0.7 m mannitol, pH 5.8. After removing undigested materials and digested debris by filtration through a 400-m steel mesh, the enzymatic mixture was centrifuged at 1,000 rpm for 10 min. The protoplasts were resuspended in 5 mL of washing medium, and the process was repeated three times. Protoplasts were cultured at a density of 2 × 105 mL−1 in 5-cm petri dishes in 3 mL of culture medium (Rao and Prakash, 1995) supplemented with different hormone or conjugate hormone regimes (Table IV). The cultures were kept at 25°C in the dark. After 7 d, fresh medium was added to each petri dish, and the addition of medium was repeated on the 11th d of culture. Star-shaped microcalli developed within 21 d of culture. After the development of microcalli visible to the naked eye, the cultures were transferred to light.
Acknowledgments
We thank Anja Liebrandt, Rosalía García-Torres, Katherine Gruenberg, and N. Dolendro Singh for technical assistance, Dr. Richard Beeson for the leaf area meter, and Dr. Pappachan E. Kolattukudy for discussions on hormone conjugates.
References
- Aach H, Bode H, Robinson DG, Graebe JE. (1997) ent-Kaurene synthase is located in proplastids of meristematic shoot tissues. Planta 202: 211–219 [Google Scholar]
- Achard P, Genschik P. (2009) Releasing the brakes of plant growth: how GAs shutdown DELLA proteins. J Exp Bot 60: 1085–1092 [DOI] [PubMed] [Google Scholar]
- An X, Lu J, Huang JD, Zhang B, Liu D, Zhang X, Chen J, Zhou Y, Tong Y. (2007) Rapid assembly of multiple-exon cDNA directly from genomic DNA. PLoS ONE 2: e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. (2008) Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect Immun 76: 3640–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Witters E, Van Dongen W, Kolár J, Motyka V, Brzobohatý B, Van Onckelen HA, Machácková I. (1999) Cytokinins in tobacco and wheat chloroplasts: occurrence and changes due to light/dark treatment. Plant Physiol 121: 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghem LE, Pettersson LG. (1974) The mechanism of enzymatic cellulose degradation: isolation and some properties of a beta-glucosidase from Trichoderma viride. Eur J Biochem 46: 295–305 [DOI] [PubMed] [Google Scholar]
- Brzobohatý B, Moore I, Kristoffersen P, Bako L, Campos N, Schell J, Palme K. (1993) Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science 262: 1051–1054 [DOI] [PubMed] [Google Scholar]
- Carroll A, Somerville C. (2009) Cellulosic biofuels. Annu Rev Plant Biol 60: 165–182 [DOI] [PubMed] [Google Scholar]
- Curaba J, Moritz T, Blervaque R, Parcy F, Raz V, Herzog M, Vachon G. (2004) AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by LEAFY COTYLEDON2 and FUSCA3 in Arabidopsis. Plant Physiol 136: 3660–3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Lee SB, Panchal T, Wiebe PO. (2001) Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol 311: 1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Ruiz ON, Dhingra A. (2004) Chloroplast genetic engineering to improve agronomic traits. Methods Mol Biol 286: 111–137 [DOI] [PubMed] [Google Scholar]
- Daniell H, Singh ND, Mason H, Streatfield SJ. (2009) Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci 14: 669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ. (2004) Plant Hormones: Biosynthesis, Signal Transduction, Action! Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- Davoodi-Semiromi A, Schreiber M, Nalapalli S, Verma D, Singh ND, Banks RK, Chakrabarti D, Daniell H. (2010) Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J 8: 223–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esen A. (1993) β-Glucosidases. Esen A, , β-Glucosidases: Biochemistry and Molecular Biology. ACS Symposium Series. American Chemical Society, Washington, DC, pp 1–14 [Google Scholar]
- Garcia-Martinez JL, Ohlrogge JB, Rappaport L. (1981) Differential compartmentation of gibberellin a(1) and its metabolites in vacuoles of cowpea and barley leaves. Plant Physiol 68: 865–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Wagner GJ. (1995) Biosynthesis of cembratrienols in cell-free extracts from trichomes of Nicotiana tabacum. Plant Sci 110: 1–10 [Google Scholar]
- Jackson DM, Danehower DA. (1996) Integrated case study: Nicotiana leaf-surface components and their effects on insect pests and diseases. Kerstiens G, , Plant Cuticles: An Integrated Functional Approach. BIOS Scientific Publishers, Oxford, pp 231–254 [Google Scholar]
- Jindal V, Dhaliwal GS. (2009) Elucidating resistance in cotton genotypes to whitefly, Bemisia tabaci, by population buildup studies. Phytoparasitica 37: 137–145 [Google Scholar]
- Jordan ET, Hatfield PM, Hondred D, Talón M, Zeevaart JAD, Vierstra RD. (1995) Phytochrome A overexpression in transgenic tobacco. Correlation of dwarf phenotype with high concentrations of phytochrome in vascular tissue and attenuated gibberellin levels. Plant Physiol 107: 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski K, Schell J. (1995) Phytohormone conjugates: nature and function. Crit Rev Plant Sci 14: 283–298 [Google Scholar]
- Kumar S, Daniell H. (2004) Engineering the chloroplast genome for hyperexpression of human therapeutic proteins and vaccine antigens. Methods Mol Biol 267: 365–383 [DOI] [PubMed] [Google Scholar]
- Lange T, Kappler J, Fischer A, Frisse A, Padeffke T, Schmidtke S, Lange MJ. (2005) Gibberellin biosynthesis in developing pumpkin seedlings. Plant Physiol 139: 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I. (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126: 1109–1120 [DOI] [PubMed] [Google Scholar]
- Lee SB, Li BC, Jin SX, Daniell H. (2010) Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotech J (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelavathi S, Gupta N, Maiti S, Ghosh A, Reddy VS. (2003) Overproduction of an alkali- and thermo-stable xylanase in tobacco chloroplasts and efficient recovery of the enzyme. Mol Breed 11: 59–67 [Google Scholar]
- Lin Y, Wagner GJ. (1994) Surface disposition and stability of pest-interactive, trichome-exuded diterpenes and sucrose esters of tobacco. J Chem Ecol 20: 1907–1921 [DOI] [PubMed] [Google Scholar]
- Marion-Poll A, Leung J. (2006) Abscisic acid synthesis, metabolism and signal transduction. In Hedden P, Thomas SG, eds, Plant Hormone Signaling, Annual Plant Reviews, Vol 24 Blackwell Publishing, Oxford, pp 1–36 [Google Scholar]
- McKenzie CL, Weathersbee AA, III, Puterka GJ. (2005) Toxicity of sucrose octanoate to egg, nymphal, and adult Bemisia tabaci (Hemiptera: Aleyrodidae) using a novel plant-based bioassay. J Econ Entomol 98: 1242–1247 [DOI] [PubMed] [Google Scholar]
- Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, Bak S. (2008) β-Glucosidases as detonators of plant chemical defense. Phytochemistry 69: 1795–1813 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Oliver SN, Dennis ES, Dolferus R. (2007) ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol 48: 1319–1330 [DOI] [PubMed] [Google Scholar]
- Perazza D, Vachon G, Herzog M. (1998) Gibberellins promote trichome formation by up-regulating GLABROUS1 in Arabidopsis. Plant Physiol 117: 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlack RD, Wright LL, Turhollow AF, Graham RL, Stokes BJ, Erbach DC. (2005) Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply. Study Report DOE/GO-102005-2135 (ORNL/TM, -2005/66) Oak Ridge National Laboratory, Oak Ridge, TN [Google Scholar]
- Pimenta Lange MJ, Lange T. (2006) Gibberellin biosynthesis and the regulation of plant development. Plant Biol (Stuttg) 8: 281–290 [DOI] [PubMed] [Google Scholar]
- Rao SK, Prakash AH. (1995) A simple method for the isolation of plant protoplasts. J Biosci 20: 645–655 [Google Scholar]
- Rood SB, Beall FD, Pharis RP. (1986) Photocontrol of gibberellin metabolism in situ in maize. Plant Physiol 80: 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood SB, Pharis RP, Koshioka M. (1983) Reversible conjugation of gibberellins in situ in maize. Plant Physiol 73: 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Verma D, Samson N, Daniell H. (2010) The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol 152: 2088–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas Fernandez MG, Becraft PW, Yin Y, Lübberstedt T. (2009) From dwarves to giants? Plant height manipulation for biomass yield. Trends Plant Sci 14: 454–461 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. (2001). Molecular Cloning: A Laboratory Manual, Ed 3 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Schliemann W. (1984) Hydrolysis of conjugated gibberellins by β-glucosidases of dwarf rice (Oryza sativa L. cv. ‘Tan ginbozu’). J Plant Physiol 116: 123–132 [DOI] [PubMed] [Google Scholar]
- Schliemann W, Schneider G. (1979) Untersuchungen zur enzymatischen: Hydrolyse von Gibberellin-O-glucosiden. I. Hydrolysegeschwindigkeiten von Gibberellin-13-Oglucosiden. Biochem Physiol Pflanzen 174: 738–745 [Google Scholar]
- Schneider G, Jensen E, Spray CR, Phinney BO. (1992) Hydrolysis and reconjugation of gibberellin A20 glucosyl ester by seedlings of Zea mays L. Proc Natl Acad Sci USA 89: 8045–8048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G, Koch M, Fuchs P, Schmidt J. (2000) Identification of metabolically formed glucosyl conjugates of [17-D2] GA34. Phytochem Anal 11: 232–235 [Google Scholar]
- Schneider G, Schliemann W. (1994) Gibberellin conjugates: an overview. Plant Growth Regul 15: 247–260 [Google Scholar]
- Schreiber K, Weiland J, Sembdner G. (1970) Isolierung von Gibberellin-A8-O(3)-β-d-glucopyranosid aus Frtüchten von Phaseolus coccineus. Phytochemistry 9: 189–198 [Google Scholar]
- Schwab B, Folkers U, Ilgenfritz H, Hülskamp M. (2000) Trichome morphogenesis in Arabidopsis. Philos Trans R Soc Lond B Biol Sci 355: 879–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sembdner G, Atzorn R, Schneider G. (1994) Plant hormone conjugation. Plant Mol Biol 26: 1459–1481 [DOI] [PubMed] [Google Scholar]
- Sembdner G, Grofl D, Liebisch HW, Schneider G. (1980) Biosynthesis and metabolism of plant hormones. MacMillan J, , Encyclopedia of Plant Physiology. Springer-Verlag, Berlin, pp 281–444 [Google Scholar]
- Severson RF, Arrendale RF, Chortyk OT, Johnson AW, Jackson DM, Gwynn GR, Chaplin JF, Stephenson MG. (1984) Quantitation of the major cuticular components from green leaf of different tobacco types. J Agric Food Chem 32: 566–570 [Google Scholar]
- Sticklen MB. (2008) Plant genetic engineering for biofuel production: towards affordable cellulosic ethanol. Nat Rev Genet 9: 433–443 [DOI] [PubMed] [Google Scholar]
- Taylor LE, II, Dai Z, Decker SR, Brunecky R, Adney WS, Ding SY, Himmel ME. (2008) Heterologous expression of glycosyl hydrolases in planta: a new departure for biofuels. Trends Biotechnol 26: 413–424 [DOI] [PubMed] [Google Scholar]
- Verma D, Daniell H. (2007) Chloroplast vector systems for biotechnology applications. Plant Physiol 145: 1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Kanagaraj A, Jin SX, Singh ND, Kolattukudy PE, Daniell H. (2010a) Chloroplast-derived enzyme cocktails hydrolyse lignocellulosic biomass and release fermentable sugars. Plant Biotechnol J 8: 332–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H. (2010b) Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA 107: 7101–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Samson NP, Koya V, Daniell H. (2008) A protocol for expression of foreign genes in chloroplasts. Nat Protoc 3: 739–758 [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Wang E, Shepherd RW. (2004) New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot (Lond) 93: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Wang R, DeParasis J, Loughrin JH, Gan S, Wagner GJ. (2001) Suppression of a P450 hydroxylase gene in plant trichome glands enhances natural-product-based aphid resistance. Nat Biotechnol 19: 371–374 [DOI] [PubMed] [Google Scholar]
- Wei S, Marton I, Dekel M, Shalitin D, Lewinsohn E, Bravdo BA, Shoseyov O. (2004) Manipulating volatile emission in tobacco leaves by expressing Aspergillus nigerbeta-glucosidase in different subcellular compartments. Plant Biotechnol J 2: 341–350 [DOI] [PubMed] [Google Scholar]
- Wiese G, Grambow HJ. (1986) Indole-3-methanol-β-d-glucoside and indole-3-carboxylic acid-β-glucoside are products of indole-3-acetic acid degradation in wheat leaf segments. Phytochemistry 25: 2451–2455 [Google Scholar]
- Woodward AW, Bartel B. (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kamiya Y, Sun TP. (2001) Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J 28: 443–453 [DOI] [PubMed] [Google Scholar]
- Yao J, Pang Y, Qi H, Wan B, Zhao X, Kong W, Sun X, Tang K. (2003) Transgenic tobacco expressing Pinellia ternata agglutinin confers enhanced resistance to aphids. Transgenic Res 12: 715–722 [DOI] [PubMed] [Google Scholar]
- Yu LX, Gray BN, Rutzke CJ, Walker LP, Wilson DB, Hanson MR. (2007) Expression of thermostable microbial cellulases in the chloroplasts of nicotine-free tobacco. J Biotechnol 131: 362–369 [DOI] [PubMed] [Google Scholar]
- Zhang XF, Zhang SF, Shi XY, Li J, Yang RL. (2004) Acute LD50 determination of toosendanin in mice by ip injection. J Traditional Chinese Veterinary Med 3: 12–13 [Google Scholar]
- Ziegelhoffer T, Raasch JA, Austin-Phillips S. (2009) Expression of Acidothermus cellulolyticus E1 endo-beta-1,4-glucanase catalytic domain in transplastomic tobacco. Plant Biotechnol J 7: 527–536 [DOI] [PubMed] [Google Scholar]