Abstract
Rice (Oryza sativa) plants carrying the Pi-i resistance gene to blast fungus Magnaporthe oryzae restrict invaded fungus in infected tissue via hypersensitive reaction or response (HR), which is accompanied by rapid ethylene production and formation of small HR lesions. Ethylene biosynthesis has been implicated to be important for blast resistance; however, the individual roles of ethylene and cyanide, which are produced from the precursor 1-aminocyclopropane-1-carboxylic acid, remain unevaluated. In this study, we found that Pi-i-mediated resistance was compromised in transgenic rice lines, in which ethylene biosynthetic enzyme genes were silenced and then ethylene production was inhibited. The compromised resistance in transgenic lines was recovered by exogenously applying cyanide but not ethephon, an ethylene-releasing chemical in plant tissue. In a susceptible rice cultivar, treatment with cyanide or 1-aminocyclopropane-1-carboxylic acid induced the resistance to blast fungus in a dose-dependent manner, while ethephon did not have the effect. Cyanide inhibited the growth of blast fungus in vitro and in planta, and application of flavonoids, secondary metabolites that exist ubiquitously in the plant kingdom, enhanced the cyanide-induced inhibition of fungal growth. These results suggested that cyanide, whose production is triggered by HR in infected tissue, contributes to the resistance in rice plants via restriction of fungal growth.
When infected, a plant carrying a resistance (R) gene to the invading pathogen induces hypersensitive reaction or response (HR), which is a typical resistance response in plants to restrict the pathogen at the initial site of infection. This process often induces or accompanies the rapid formation of small HR lesions (HRLs), which is a hallmark of programmed cell death of infected tissue in the host plants. The triggering of HR starts when the R gene product recognizes the corresponding pathogen avirulence gene product (Staskawicz et al., 1995; Dangl and Jones, 2001), and ethylene production follows it accompanying with HRL formation (de Laat and van Loon, 1983; Hammond-Kosack et al., 1996). The ethylene burst is one of the earliest events in HR, but the role of ethylene itself for disease resistance remains unresolved (Bröekaert et al., 2006). In the ethylene biosynthesis pathway in plants, S-adenosyl Met is converted to 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS), and then ACC is oxidized to ethylene by ACC oxidase (ACO; Bleecker and Kende, 2000). In the latter step, cyanide is produced in stoichiometrically equivalent amounts with ethylene (Peiser et al., 1984). However, the role of cyanide coproduced with ethylene also remains unevaluated.
Cyanide makes the cells of an organism unable to use oxygen, primarily through the inhibition of cytochrome c oxidase in mitochondria, and induces cyanide poisoning in animals (Isom and Way, 1984). However, in plants and fungi, even when cyanide-sensitive respiration is inhibited in the presence of cyanide, cyanide-resistant respiration is rapidly induced in the mitochondria. The alternative respiration involves activation of alternative oxidase, and short cuts the electron transport by transferring electrons directly from reduced ubiquinons to oxygen, helping to avoid incomplete reduction of oxygen to water as a source for reactive oxygen species (Day et al., 1995). Further, plants have the detoxifying enzyme of cyanide, β-cyanoalanine synthase (CAS), whose activity is constitutively found in many plant species at a considerable level (Miller and Conn, 1980). Thus, cyanide may be overlooked as a by-product of ethylene biosynthesis, and the role of cyanide production for disease resistance seems to be unevaluated. Only a limited evidence has been reported on cyanide production via ACC in plants, including cyanide occurrence in HR in Tobacco mosaic virus (TMV)-infected tobacco (Nicotiana tabacum) plants (Grossmann, 1996), and implication of auxin herbicide-induced accumulation of cyanide in the induction of herbicide phytotoxicity (Tittle et al., 1990).
Our previous study proposed the hypothesis that HR-associated ethylene and cyanide biosynthesis from ACC is important for the resistance to blast fungus, Magnaporthe oryzae, in resistant rice (Oryza sativa) plants, and cyanide may contribute to the resistance rather than ethylene (Iwai et al., 2006). As a defense signal in plants, ethylene has been studied extensively, but treatment with ethylene or ethephon was reported to increase either susceptibility or resistance to pathogens in plants depending on the plant-pathogen interaction (Bröekaert et al., 2006). The use of mutants defective in ethylene signaling has indicated a limited role of ethylene in the resistance to some biotrophic pathogens. For example, the resistance to avirulent fungus Cladosporium fulvum in tomato (Solanum lycopersicum) containing the resistance genes Cf-2 and Cf-9 is unaltered in ethylene-insensitive mutants (Brading, 1997; Brading et al., 2000). Arabidopsis (Arabidopsis thaliana) ethylene signaling mutant ein2 shows no alteration in susceptibility to the biotrophic oomycete Peronospora parasitica or biotrophic bacterium Pseudomonas syringae pv tomato (Pieterse et al., 1998). A tobacco line transformed with a dominant negative allele of the Arabidopsis ethylene receptor, etr1, exhibited unaltered resistance to TMV (Knoester et al., 1998). The importance of salicylic acid (SA) in HR-mediated resistance has been shown in many plant-microbe interactions as another defense signal in plants. However, SA is probably not critically important for disease resistance in all cases. For example, in tomato, SA is not required for Cf-2- and Cf-9-dependent resistance to C. fulvum (Brading et al., 2000). In young rice plants, SA treatment induces no clear resistance to blast fungus while SA-dependent fungal resistance is detected in adult rice plants (Iwai et al., 2007). These results indicate the presence of another defense signaling pathway except for ethylene- and SA-signaling pathways in some plant-pathogen interactions.
To study the role of cyanide on disease resistance in plants, we used the same experimental system as reported by Iwai et al. (2006, 2007), utilizing both susceptible and resistant young rice plants to blast fungus, which causes substantial yield losses in the rice crops in the world. The rice R gene Pi-i confers resistance to blast fungus race 003, and the rice cv Nipponbare is susceptible to the race 003. IL7, a near isogenic line of Nipponbare carrying the Pi-i gene, is resistant to the race (Ise and Horisue, 1988). Iwai et al. (2006) showed that blast fungus infection induces rapid HRL formation accompanying transient ethylene burst with enhanced expression of specific ACS and ACO genes in resistant IL7, but not in susceptible Nipponbare. Treatment of IL7 leaves with an ethylene biosynthesis inhibitor compromised the defense response in IL7, but an ethylene signaling inhibitor could not. These results proposed the idea that cyanide is possible defense signal rather than ethylene in the system. To prove the idea, further experiments were conducted in this study. We generated ACO and/or ACS knockdown IL7 rice lines to reveal that HR-associated cyanide/ethylene biosynthesis is required for the resistance to blast fungus. Next, we studied the effect of exogenous cyanide and ethylene on growth of blast fungus in vitro and in planta. These results indicated an important role of HR-associated cyanide occurrence for fungal resistance in resistant rice plants. Furthermore, flavonoids, which are widely distributed in plants, were found to function in resistance to blast fungus in cooperation with cyanide.
RESULTS
Breakdown of the Pi-i-Dependent Resistance in ACS- or ACO-Silenced Resistant Rice Plants
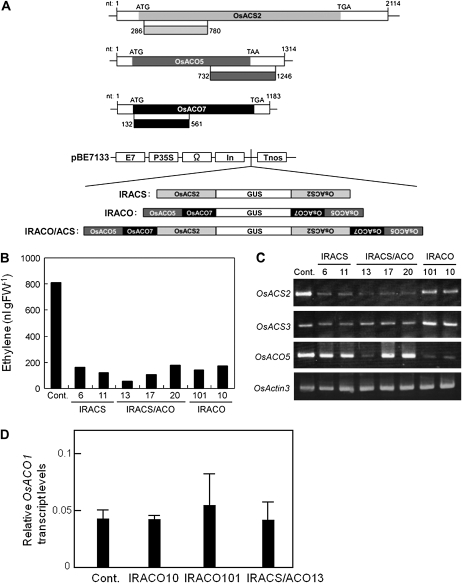
To obtain direct evidence for the involvement of cyanide/ethylene biosynthesis in resistance to blast fungus, ACS- and/or ACO-silenced IL7 plants were generated. Because transcriptional activation of ACS and ACO enhances ethylene biosynthesis (Bleecker and Kende, 2000; Wang et al., 2002), the HR-inducible rice ACS and ACO genes, OsACS2, OsACO5, and OsACO7 (Iwai et al., 2006), were silenced in IL7 line using inverted repeat (IR) constructs that included portions of the corresponding cDNAs (Fig. 1A). Transgenic lines could grow and fertilize in an isolated green house, and wound-induced ethylene production was inhibited in almost all lines. However the growth rate was slightly suppressed and their fertility was lower compared with the vector control. Transgenic lines with relatively high seed yields, such as IRACO10, IRACO101, and IRACS/ACO13, were selected for further analysis. Transgenic plants exhibited reduced ethylene production (Fig. 1B), and reduced expression on OsACS2 and/or OsACO5 (Fig. 1C), while no clear differences from control lines were detected in the expression levels of OsACS3 (Fig. 1C) and OsACO1 (Fig. 1D), which did not respond to HR (Iwai et al., 2006).
Figure 1.
Generation of ACO- and ACS/ACO-silenced plants. A, Structure of the OsACS2, OsACO5, and OsACO7 genes and schematic representation of the constructs used to transform rice plants. Boxes below individual cDNAs indicate the regions used for construction. B, Emission of ethylene from leaves after wounding. Leaves were cut into small pieces and incubated in a sealed vial at 25°C for 24 h under continuous light. A transgenic IL7 line transformed with the vector alone was used as a control (Cont.). C, Reverse transcription-PCR analysis of rice ACS and ACO genes in leaves at 24 h after wounding. OsActin3, which is constitutively expressed in leaves, was used as an internal control. D, Quantitative real-time PCR analysis of the OsACO1 gene in leaves at 48 hpi. Values are means ± se (n = 12).
When blast fungus is inoculated onto young rice seedlings by spraying a suspension of blast conidia, ethylene emissions from the inoculated leaves peak at 36 to 60 h postinoculation (hpi), at which time HR lesions become visible (Iwai et al., 2006). Compared with control plants, which include wild-type IL7 and transgenic IL7 lines expressing only an empty vector, ethylene emissions via blast infection during this period were reduced (Fig. 2A) as well as the transcript levels of OsACO5, OsACO7, and OsACS2 (Fig. 2, B and C) in the IRACO10, IRACO101, and IRACS/ACO13 lines. In blast-inoculated leaves of the susceptible cv Nipponbare wild type, large, whitish, expanding lesions (ELs) formed (Fig. 2D, Nipponbare), while small necrotic HRLs were found in resistant IL7 (Fig. 2D, IL7-WT). Iwai et al. (2006) showed that treatment of blast fungus-inoculated IL7 leaves with an ethylene biosynthesis inhibitor, aminooxyacetic acid, results in induction of formation of ELs, indicating that ethylene biosynthesis is important for the resistance. In the ACO- and ACS/ACO-silenced IL7 lines, about 10% of HRLs expanded and turned whitish brown (Fig. 2D). At 8 d postinoculation (dpi), the mean areas of these ELs (2.2–3.6 mm2) in transgenic lines were considerably larger than those of the HRLs (approximately 0.1 mm2) in control IL7 plants, indicating the breakdown of Pi-i-mediated resistance in ACO- and ACS/ACO-silenced lines. Because the mean lesion areas (2.2–3.6 mm2) in transgenic lines were one order of magnitude smaller than those (32.5 mm2) in susceptible cv Nipponbare (Fig. 2D, Nipponbare versus the IR lines), the knockdown of ACO/ACS partly affected the Pi-i-dependent resistance. Next, transgenic rice lines at four-leaf stage in pots with soil were inoculated with blast fungus, and grown in an isolated green house. When the phenotype of inoculated plants was observed, it was clear that the knockdown affects the resistance to blast fungus. The aerial parts of eight of nine IRACO10 plants and all six IRACO101 plants tested began to wilt within 20 dpi. About half of the wilted plants died by 50 dpi, and the remainder died by 70 dpi (Fig. 2E). The IRACS/ACO line exhibited a similar response to infection as the IRACO lines. In contrast, all six fungus-inoculated control IL7 plants survived without systemic wilting, and produced seeds similar to healthy IL7 plants (Fig. 2E, vector control).
Figure 2.
Breakdown of Pi-i-originated blast resistance in ACO/ACS knockdown rice plants. A, Ethylene emission from leaves. Ethylene concentrations emitted from leaves at 36 to 60 hpi were normalized with the amount of fungal DNA extracted from the inoculated leaves at 60 hpi. B and C, Relative transcript levels of OsACO5 (B), OsACO7 (B), and OsACS2 (C) genes in leaves at 48 hpi, which were normalized with fungal DNA levels. For A to C, the values are means ± se (n = 12). D, Distinct lesion types were apparent in leaves at 8 dpi. Numbers of ELs with an area greater than 0.5 mm2 per leaf and their mean areas were determined using 11 leaves for wild-type (WT) Nipponbare, 30 leaves for wild-type IL7, 12 leaves for IRACO10, 19 leaves for IRACO101, and three leaves for IRACS/ACO13. Values given are means ± se. One leaf per plant was used. White arrowheads indicate typical ELs in whitish brown. E, Phenotypes of ACO-silenced and control IL7 plants grown in a greenhouse. Left, 50 dpi; right, 70 dpi. For A to E, similar results to wild-type IL7 were obtained by transgenic IL7 plants transformed with the vector alone.
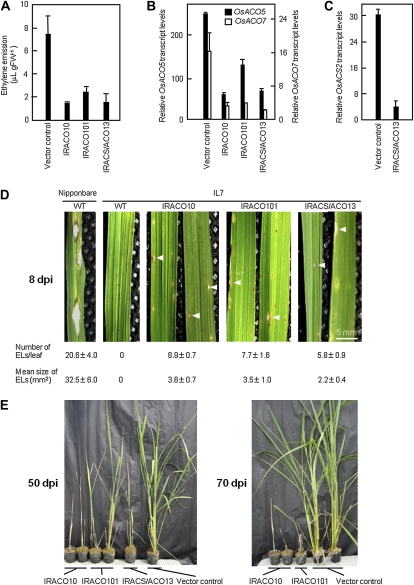
The lowered resistance in transgenic lines was confirmed by a cytological study. The inside of a leaf sheath, which was cut into 4-cm-long sections, was inoculated with a blast conidia suspension in according with Takahashi (1958), and incubated in a moist box. Dark-brown HR lesions (DBLs) were formed in the inoculated area at 2 dpi, and they did not expand, maintaining similar phenotype thereafter in control IL7 plants (Fig. 3A, control, arrowheads). DBLs contained appressoria (Fig. 3B, control, Ap) and necrotized mycelia (Fig. 3B, control, white arrows). In contrast, expanded brownish lesions (BLs) with about 10-fold or more large brown areas than DBLs were found in the leaf sheaths of ACO- and ACS/ACO-silenced lines at 5 dpi (Fig. 3A, black arrows). Transparent mycelia (Fig. 3B, red arrows) and clumps of mycelia (Fig. 3B, red dashed circles) were observed around the BLs, indicating that the fungus escaped from BLs and grew vigorously in the tissue around BLs. These results indicated that knockdown of ACO/ACS in IL7 plants compromised the original resistance to blast fungus.
Figure 3.
Breakdown of Pi-i-originated blast resistance in the leaf sheath of ACO/ACS knockdown rice plants. The inner sides of intact leaf sheaths of each transgenic IL7 line were inoculated with a suspension of blast conidia and incubated at 25°C in a humidified transparent box. After 5 d, the inoculated leaf sheaths were observed using a microscope. A, Left, Inoculated leaf sheaths at 5 dpi. Right, Schematic representation of lesions in the left section. Black arrowheads and black arrows indicate DBLs and BLs, respectively. Bar indicates 5 mm. B, Photograph of leaf sheath around DBLs in control and that around BLs in IRACS/ACO13 lines. Left, A close-up view of a lesion highlighted using red lines in the right section. In the control, necrotized mycelia were detected (white arrows). In the IRACS/ACO13 line, transparent mycelia (red arrows) and clumps of the mycelia, which are highlighted using red dashed circles, were observed. Representative images are shown. Ap, Appressorium. Bar indicates 50 μm.
Cyanide But Not Ethylene Complements Blast Resistance in ACS/ACO Knockdown Rice Plants
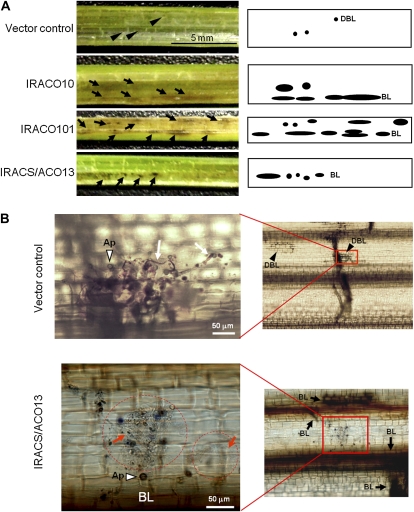
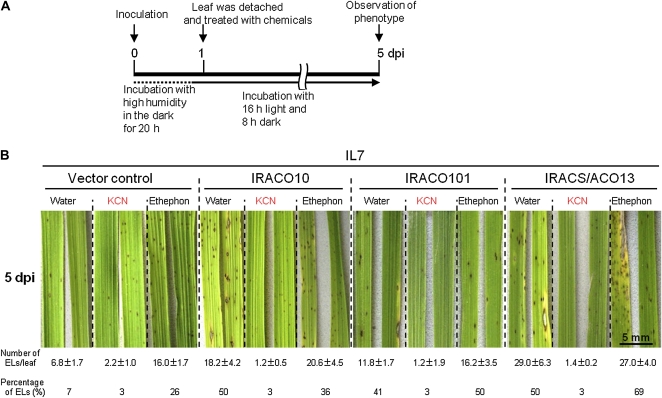
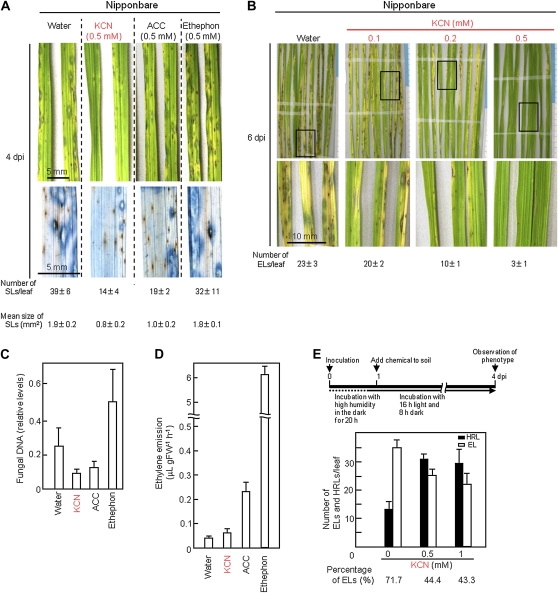
Using detached leaves of ACO- and ACS/ACO-silenced plants, complementation of suppressed resistance by exogenous cyanide and ethylene was analyzed (Fig. 4). In detached leaves, the size of HRLs became slightly larger than those in intact leaves of nonwounded IL7 plants. In detached control IL7 leaves into which water was absorbed from the leaf base in a test tube, some HRLs were enlarged, and about 7% of HRLs grew to more than 0.3 mm2 each at 5 dpi (Fig. 4B, control, water). In water-treated ACO- and ACS/ACO-silenced plants, both the number and percentage of ELs increased. Treatment with 0.5 mm KCN solution reduced the number and percentage of ELs in control plants and also in ACO- and ACS/ACO-silenced plants. In contrast, treatment with ethephon, an agent that releases ethylene in plant tissues, exhibited no inhibitory effect on the formation of ELs. These results indicated that reduced resistance in transgenic lines was complemented by cyanide, but not ethylene. In these conditions, chemical injury was not observed by treatment with KCN at 0.5 and 1 mm, or with ethephon at 0.1 and 0.5 mm. However, KCN treatment at 2 mm induced the injuries such as necrosis and/or chlorosis in the treated leaves.
Figure 4.
Exogenous cyanide but not ethephon complements the reduced blast resistance in transgenic IL7 plants. A, Timeline of the assay with detached leaves. B, Inoculated leaves were detached and treated with water, 0.5 mm KCN, or 0.1 mm ethephon solution in an unsealed tube. The number per leaf of ELs more than 0.3 mm2 and their percentage in total lesions were determined at 5 dpi and are shown at the bottom. Values are means ± se (n = 6). Bar indicates 5 mm.
Exogenous Cyanide and ACC But Not Ethylene Induce Blast Resistance in the Susceptible Rice Plants
Using detached leaves of the susceptible rice cv Nipponbare, the effect of exogenous cyanide on blast resistance was tested in accordance with the experimental procedure shown in Figure 4A. In water-treated leaves, ELs became visible at 3 to 4 dpi (Fig. 5A, top). For the easier observation of the fungal spread, inoculated leaves were cut into 20-mm-long sections and subjected to lactophenol-trypan blue staining. Stained mycelia in blue were found around ELs but not around small brown lesions (Fig. 5A, bottom), which were similar to the HRLs in IL7 (Fig. 4B, control, water) but slightly larger than the HRLs. As the stained region indicates the spread of the mycelia, we designated this as a stained lesion (SL). Exogenous KCN and ACC, the biosynthetic precursor of ethylene and cyanide, but not ethephon reduced the number and size of SLs (Fig. 5A). Figure 5B shows that the inhibitory effect of cyanide for EL formation was dose dependent, and the effect was clearly detected at more than 0.2 mm of cyanide. Consistent with the confinement of SLs and ELs, the amount of M. oryzae was reduced by treatment with KCN or ACC but not ethephon (Fig. 5C). Application of ACC increased ethylene emission (Fig. 5D) and reduced the number and size of SLs (Fig. 5A). Because ethephon, whose treatment was shown to release a large amount of ethylene in this system (Fig. 5D), did not reduce the number and size of SLs (Fig. 5A), ACC-induced resistance in Nipponbare leaves is likely due to ACC-originated cyanide production and not due to ethylene production. In blast fungus-inoculated intact Nipponbare plants grown in soil, exogenous KCN at 0.5 and 1 mm decreased the rate of ELs, indicating enhanced fungal resistance due to cyanide in planta (Fig. 5E), although the effect appeared smaller than in detached leaves (Fig. 5A). This could be because of enhanced consumption of cyanide in the paddy.
Figure 5.
Exogenous cyanide induces blast resistance in intact Nipponbare plant. A, Detached leaves were treated with 0.5 mm KCN, 0.5 mm ACC, and 0.5 mm ethephon at 1 dpi in an unsealed tube. Top, Lesion phenotypes observed in the leaves at 4 dpi. Bottom, SLs observed in the leaves at 4 dpi after the treatment of lactophenol-trypan blue. The number of SLs per leaf and their mean area are shown at the bottom. B, Dose-dependent effect of cyanide on EL formation. Top, Lesion phenotypes at 6 dpi. Bottom, Close-up view of lesion-forming regions bordered by black lines in the top section. The numbers of ELs greater than 0.5 mm2 per leaf are shown at the bottom. C, The amounts of M. oryzae DNA in the treated leaves in A. D, Ethylene emission from leaves shown in A. The leaves harvested at 4 dpi were cut into pieces and incubated in a sealed tube for 3 h, and the level of ethylene released was normalized with the amount of fungal DNA. For A to D, values are means ± se of three independent experiments, each with eight to 14 leaves. E, Exogenous cyanide induces blast resistance in intact Nipponbare leaves. Top, Timeline of the assay with intact leaves. One day after inoculation, 500 mL of a solution containing the indicated concentrations of KCN or water alone was applied to Nipponbare plants grown in a pot containing 500 mL of plant cultivation soil. Bottom, The numbers per leaf of ELs more than 3.2 mm2 and their percentage of total lesions were determined at 5 dpi. The numbers of HRLs per leaf were also counted. Values are means ± se (n = 12).
Ethylene Gas Itself Has No Effect on the Induction of Blast Resistance in Young Rice Plants
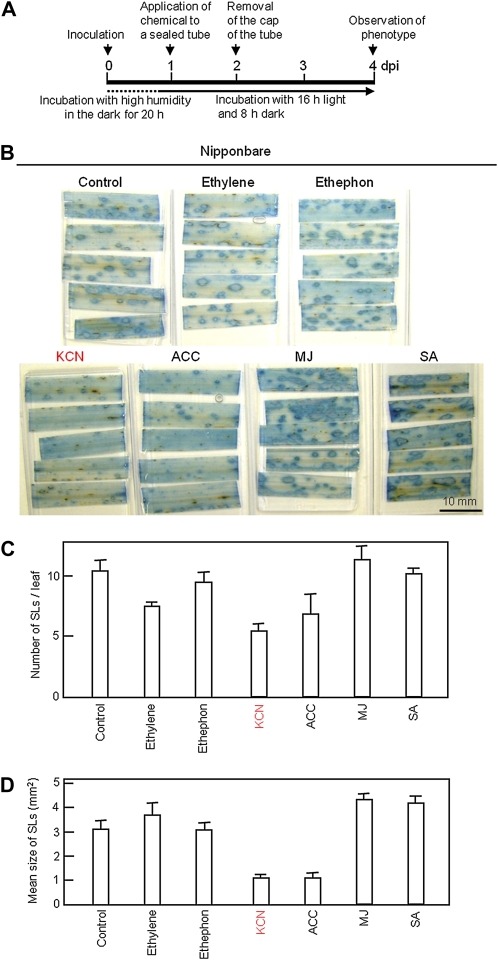
It has been reported that there is different effects of ethephon, ACC, and ethylene on expression of defense genes (Zhang and Wen, 2010). To check whether ethylene itself induces blast resistance, ethylene gas at the final concentration of 5 μmol, which was equivalent to the concentration of ethylene produced from 0.5 mm ethephon in the same experimental system, was directly introduced into a sealed glass tube in which inoculated leaves were placed. The treated leaves are incubated and subjected to observation of lesions stained by lactophenol-trypan blue at 4 dpi (Fig. 6A). The induction effect of ethephon, KCN, and ACC at 0.5 mm was also examined using the same experimental system. There was no major difference in the number and size of SLs among control, ethylene treatment, and ethephon treatment (Fig. 6, control, ethylene, ethephon). The same inhibitory effect of KCN and ACC on reduction in the number and size of SLs was observed as did in the system with unsealed tubes shown in Figures 4 and 5, further supporting that cyanide and ACC, but not ethylene, induce blast resistance in rice.
Figure 6.
Exogenous ethylene has no effect on induction of blast resistance. A, Timeline of the assay with detached leaves. Detached leaves were treated with water (Control), ethylene, or a 0.5 mm solution containing ethephon, KCN, ACC, MJ, or SA in a sealed tube. B, SLs observed in the leaves at 4 dpi after the treatment of lactophenol-trypan blue. Bar indicates 10 mm. The number (C) per leaf of SLs more than 0.5 mm2 and their mean area (D) were determined. Values for C and D are means ± se (n = 5).
Because jasmonic acid and SA play an important role in signal transduction of disease resistance in plants, the effect of methyl jasmonate (MJ), a methyl ester form of jasmonic acid, and SA on induced resistance to blast fungus was also examined in the closed system as described above. Neither MJ nor SA reduced the number and size of SLs. The result on SA’s effect is consistent with our previous result showing that exogenous SA induced resistance to blast fungus in adult, but not young rice plants (Iwai et al., 2007).
Flavonoids and Cyanide Cooperatively Inhibit Fungal Growth in Vitro
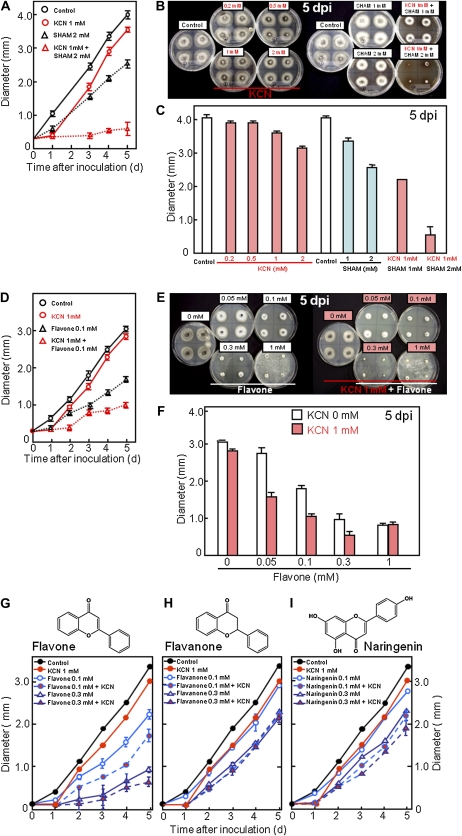
These results indicated that HR-induced production of cyanide but not ethylene is necessary for resistance to blast fungus in rice. Although cyanide inhibits the cytochrome c oxidase respiratory pathway in mitochondria (Isom and Way, 1984), most plants and microorganisms including blast fungus, can rapidly respire using a cyanide-resistant respiratory system (Solomos, 1977; Mizutani et al., 1995). Therefore, we studied on cyanide-sensitive respiration and cyanide-resistant respiration in our experimental system via quantification of fungal growth. Four small mycelium discs were put on potato dextrose agar (PDA) in a petri dish, in which a cyanide solution was supplied. The diameter of the disc, whose increase reflects fungal growth, was increased with time as shown in Figure 6A, but this increase was inhibited by cyanide at 1 mm (Fig. 7A, KCN). Inhibition of fungal growth by cyanide was found at 5 dpi in a concentration-dependent manner (Fig. 7, B and C, KCN). At 1 d, the increase was completely inhibited in the presence of KCN at 1 mm, however it was restored at 2 d and continued with similar rate as control thereafter (Fig. 7A). The phenomenon indicated possible occurrence of cyanide-resistant respiration and/or disappearance of cyanide in the medium, although inhibition by cyanide was very effective within 1 d after the treatment. Therefore, we analyzed the effect of salicylhydroxamic acid (SHAM), an inhibitor for cyanide-resistant respiration. Inhibition of fungal growth by SHAM was clearly enhanced in the presence of cyanide (Fig. 7, A–C, KCN + SHAM). Such synergistic effect of cyanide and SHAM indicated that inhibition of both cyanide-sensitive and cyanide-resistant respiration is fatal for fungus. SHAM is a synthesized molecule and absent in plants. Then, effect of cyanide may be enhanced by natural accessory compounds instead of SHAM to repress cyanide-resistant respiration in plants. As potential accessory compounds, flavonoids, which are possible inhibitors for cyanide-resistant respiration of blast fungus (Mizutani et al., 1996), are found ubiquitously in the plant kingdom. When the effect of flavone was analyzed as a model flavonoid at first, the growth of blast fungus in vitro was inhibited in a concentration-dependent manner, and the inhibition was enhanced in the presence of KCN (Fig. 7, D–F). Next, the effects of flavanone and naringenin, which are central molecules in the biosynthetic pathways for production of specific flavonoid subclasses in plants, were compared with that of flavone. Although flavone was most effective among the three flavonoids used here, clear inhibition of fungal growth was detected by flavanone and naringenin at 0.1 and 0.3 mm (Fig. 7, G–H). In the presence of 1 mm KCN, the inhibition was enhanced, indicating cooperation of flavonoids with cyanide.
Figure 7.
Cyanide inhibits the growth of blast fungus cooperationally with SHAM or flavonoids in vitro. Mycelial layers of M. oryzae grown on PDA at 25°C for 10 d were cut into pieces (about 10 mm2), and four pieces were placed on a petri dish (9 cm in diameter) containing PDA and chemicals. After incubation at 25°C in the dark for the indicated time period, the diameter of mycelial colonies was measured. Methanol was used as the control. A to C, Effect of cyanide or/and SHAM. D to F, Effect of cyanide or/and flavone. Values given are means ± sd of four independent measurements. G to I, Effect of flavonoids on the growth of blast fungus. Flavone (G), flavanone (H), or naringenin (I) was added at 0.1 mm or 0.3 mm in the presence or absence of 1 mm KCN. Values given are means ± sd of four independent measurements. At the top of G to I, structure of each flavonoid is shown.
Exogenous Flavone Induces Blast Resistance in Cooperation with Cyanide in Rice
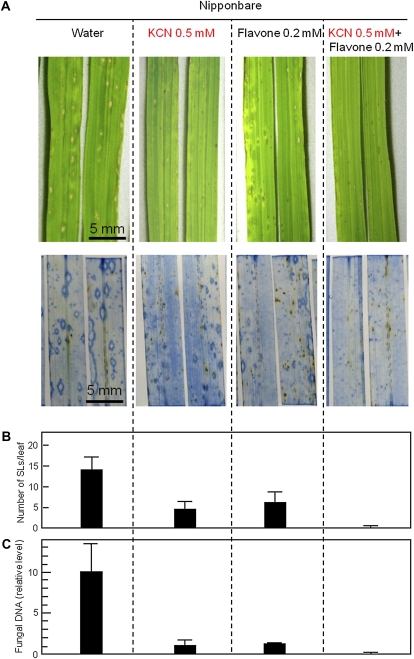
In blast-inoculated Nipponbare leaves, exogenous flavone induced the resistance to blast fungus at 0.2 mm (Fig. 8, flavone). The level of induced resistance was as similar to that by treatment with 0.5 mm KCN. Application of both flavone and cyanide resulted in further resistance, reducing both the numbers of trypan blue-stained lesions (Fig. 8B, KCN + flavone) and fungal mass (Fig. 8C, KCN + flavone). These results suggested that the flavonoid inhibits fungal growth in planta in cooperation with cyanide.
Figure 8.
Exogenous flavone induces blast resistance in Nipponbare cooperatively with KCN. Blast fungus-inoculated Nipponbare leaves detached at 1 dpi were treated with water, 0.5 mm KCN, 0.2 mm flavone, or both 0.5 mm KCN and 0.2 mm flavone in test tubes. A, Top, Phenotypes of the leaves at 4 dpi. Bottom, Phenotypes of the leaves at 4 dpi after staining with lactophenol-trypan blue. B, The number of SLs with an area of more than 0.5 mm2 per leaf at 4 dpi. C, The amounts of M. oryzae DNA extracted from the similar leaves shown in A. Values for B and C are means ± se (n = 6).
DISCUSSION
These results provide direct evidence indicating that HR-associated cyanide production contributes to the resistance in blast-infected rice plants. Cyanide may confer inhibition of cyanide-sensitive respiration in blast fungus, and flavonoids, which are natural constituents in plants, would help the resistance to blast fungus via suppression of cyanide-insensitive respiration of the fungus. Our conclusion is based on the following results that (1) silencing the genes encoding HR-inducible ethylene biosynthetic enzymes broke down the Pi-i-originated resistance to blast fungus, and exogenous cyanide but not ethylene complemented it; (2) exogenous cyanide and the biosynthetic precursor ACC inhibited growth of blast fungus in vitro and in vivo while ethylene did not; and (3) the cyanide-enhanced resistance to blast fungus was increased in the presence of flavonoids in vitro and in vivo.
Cyanide is coproduced with ethylene biosynthesis via ACC, and it is unstable at aerobic conditions and disrupted by CAS in plant tissues (Yip and Yang, 1988). Actually, cyanide in air-exposed agar medium was rapidly disappeared in a clean bench (Supplemental Fig. S1A). Disappearance of cyanide in a solution at room temperature was enhanced in the presence of detached rice leaves. This enhancement may be due to degradation of cyanide by cyanide-destabilizing molecules, such as CAS, released into the solution (Supplemental Fig. S1B). Generally, little attention has been probably paid to the role of cyanide in disease resistance. The results presented here shed the light on the importance of HR-associated cyanide production for blast resistance in rice, and indicated a mechanism how HR contributes to blast resistance in plants.
Exogenous cyanide inhibited fungal growth in vivo (Fig. 5) and in vitro (Fig. 7). The inhibition could be caused by a restriction of cyanide-sensitive respiration in fungus. Recovery of the inhibition in vitro was found after 2 d (Fig. 7D), possibly via the acquisition of cyanide-resistant respiration by fungus or via natural disappearance of cyanide in an aerobic condition. When SHAM, an inhibitor of mitochondrial cyanide-resistant respiration via inhibition of alternative oxidase activity, was added, fungal growth was further restricted. The function of SHAM could be replaced by that of natural plant constituents flavonoids (Fig. 7, D–I). Flavonoids represent one of the largest classes of plant secondary metabolites, conferring a multitude of physiological functions, including UV protection, insect attraction, symbiosis, and variation of flower color. Fofana et al. (2005) reported that disruption of a flavonoid synthesis pathway suppressed the induced resistance against powdery mildew in cucumber (Cucumis sativus), indicating the involvement of flavonoids in fungal disease resistance in plants. Mycelial growth of four fungi, Cladosporium herbarum, Fusarium oxysporum, Trichoderma harzianum, and Alternaria alternata, was inhibited in vitro in the presence of flavone, flavanone, flavonal, and/or 7-hydroxyflavone (Weidenbörner and Jha, 1993). Most conjugated flavonoids in plants, such as anthocyanins and flavonol- and flavon-glycosides, are found primarily in the vacuole (Zhao and Dixon, 2010). As a cellular suicide strategy of plants upon HR, cell death triggered by vacuolar collapse in TMV-infected tobacco plants was proposed (Hatsugai et al., 2006). An increase in a flavanone phytoalexin, sakuranetin, was detected in rice leaves after infection with blast fungus (Kodama et al., 1992). Thus, flavonoids are expected to support the disease resistance in plants, and some of them could be increased after pathogen infection. Further their enhanced release from the vacuole to apoplast and/or cytoplasm by HR may serve for amplification of the resistance. Altogether, these results indicated that naturally occurring flavonoids confer to blast resistance in cooperation with HR-associated cyanide production.
Cyanide production may occur rapidly and locally at initial infection sites in Pi-i-carrying rice leaves associated with HRL formation. The emphasized cyanide increment at HRL could be effective in restricting the invaded fungus within or around HRL at early infection period, even cyanide would be metabolized thereafter. For the host plant, transient increase in cyanide only around infected area would be safe and economical because prolonged accumulation of the toxic cyanide in a large area would be a disadvantage for the host. Now, how much cyanide is actually produced at HRL? Ethylene production has a maximum rate of 8 nL g−1 h−1 fresh weight and continues for at least 3 h at 2 dpi in blast-infected IL7 leaves (Iwai et al., 2006), which indicates that about 0.4 nmol of ethylene is produced per g leaf in 1 h. The area of HRL at 2 dpi was assumed to be lower than about 0.1% of the whole leaf, when 50 to 100 HRL with about 0.005 mm2 each were formed in a leaf of 500 mm2. Therefore, the predicted cyanide production at HRL can be calculated to about 0.4 μmol g−1 h−1 leaf−1. The local concentration of cyanide is enough to induce blast resistance in susceptible Nipponbare (Fig. 5B). Attempts to quantify the predicted cyanide increase in blast fungus-inoculated IL7 leaves at 2 to 3 dpi remain unsuccessful, probably because cyanide is extremely unstable in plant tissues. In addition, the predicted cyanide concentration in the whole rice leaf is about 0.4 nmol g−1 fresh weight. It is roughly calculated as 1/1,000 of that around HRL, and it may be too low to quantify.
A novel alkoxyiminoacetamide, metominostrobin (SSF-126), is a fungicide that has been widely used for protection of rice from fungal pathogens (Mizutani et al., 1995). The agrochemical is similar in mode of action to cyanide, inhibiting the mitochondrial oxidative respiration chain of blast fungus, and functions cooperatively with flavonoids to suppress the growth of the fungus (Mizutani et al., 1996). This chemical is also used to prevent rice sheath blight caused by Rhizoctonia solani (Ichiba et al., 2000) and rice brown spot disease by Cochliobolus miyabeanus. This fungicide was selected from chemically synthesized molecules, and has the similar effect to inhibit fungal mitochondrial respiration as cyanide. On the other hand, resistant rice plants are considered to utilize the cyanide, which is rapidly produced upon programmed cell death in pathogen-infected plants, to inhibit growth of blast fungus. It is interesting that the inhibitory mechanism of the fungicide Oribright happens to similar to cyanide, a predicted natural defense signal of plants proposed in this study.
This study has demonstrated that HR-associated cyanide production plays an important role in rice blast resistance. It would be expected that a similar role of cyanide is found in other plant-pathogen interactions accompanying HR-induced cyanide production, because flavonoids inhibit growth of some pathogenic fungi (Weidenbörner and Jha, 1993) and because Oribright has been used to restrict pathogenic fungi other than blast fungus (Ichiba et al., 2000).
Although our results suggest that cyanide produced during HR contributes to blast resistance through inhibition of fungal growth, it is possible that cyanide induces a defense response that leads to induction of blast resistance. We also cannot exclude the possibility that cyanide is indirectly involved in the induction of resistance to blast fungus through perturbation of plant metabolism. Further studies to investigate the possibilities will be required. Furthermore, analysis using ACS- or ACO-overexpressing Nipponbare rice lines in which a large amount of cyanide would accumulate will be required to clarify whether endogenous cyanide can induce resistance to blast fungus.
MATERIALS AND METHODS
Plant Materials
Rice (Oryza sativa ‘Nipponbare’) susceptible to blast fungus (Magnaporthe oryzae) race 003 (isolate Kyu89-241) and the resistant line IL7 (Ise and Horisue, 1988), which carries the R gene Pi-i against race 003 in Nipponbare background, were grown in a greenhouse at 25°C with 16 h of light and 8 h of dark at 450 μmol m−2 s−1. Unless otherwise stated, the fourth leaf of approximately 2-week-old plants at the four-leaf stage was used for each analysis.
Vector Construction
Silencing constructs were made by introducing duplicate PCR-amplified gene fragments into a binary vector in an inverted tandem orientation separated by a 989-bp region of the GUS gene (corresponding to positions 821–1,809; Jefferson et al., 1986; Fig. 1A). A 495-bp region of the OsACS2 cDNA (corresponding to positions 286–780) was amplified and inserted into pBE7113 (Mitsuhara et al., 1996) to obtain the IRACS construct. Likewise, a 515-bp region of the OsACO5 cDNA (corresponding to positions 732–1,246) and a 430-bp region of the OsACO7 cDNA (corresponding to positions 132–561) were inserted into pBE7113 to obtain the IRACO construct. The amplified OsACS2, OsACO5, and OsACO7 fragments were inserted into pBE7113 to obtain the IRACS/ACO construct.
Rice Transformation and Screening of Transformants
All constructs were used to transform IL7 using Agrobacterium tumefaciens LBA4404 by the method described by Toki et al. (2006). Transformants that grew on the medium containing geneticin (G-418, Wako Pure Chemical) at 50 μg mL−1 were screened for reductions in both wound-induced ethylene production and accumulation of target gene transcripts (Fig. 1, B and C), because OsACS2, OsACO5, and OsACO7 are also wound responsive (Iwai et al., 2006). Transgenic IL7 plants expressing only the original vector pBE2113 were also generated as a control. Geneticin-resistant seedlings from T1 seeds of the transgenic lines were transferred to pots containing a plant cultivation soil (Bonsol no. 1, Sumitomo Chemical) at 7 d after the imbibition of seeds and grown as described previously (Iwai et al., 2006). The phenotypes of three lines (IRACO10, IRACO101, and IRACS/ACO13) and wild-type IL7 and vector control plants were similar. However, the growth and fertility of transgenic lines were inferior compared with wild-type and control plants.
Assay for Fungal Resistance
Conidia of M. oryzae race 003 were prepared as described previously (Iwai et al., 2006). A conidia suspension (1 × 105 mL−1) containing 0.025% (v/v) Tween 20 was sprayed onto rice plants. The inoculated plants were incubated at 25°C with high humidity in the dark for 20 h and then moved to a greenhouse maintained at 25°C with 16 h of light and 8 h of dark. Lactophenol-trypan blue staining of blast mycelia in leaves was performed as described previously (Iwai et al., 2006).
RNA Extraction
Total RNA was extracted from leaves using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions.
Reverse Transcription-PCR
Total RNA was extracted from leaves using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription-PCR was performed using a reverse transcription-PCR High-Plus kit (Toyobo) with the following primers: OsACS2, 5′-GACCTGATCCACGTCGTGTA-3′ and 5′-CATGGTCTCGTCGTCCATGTT-3′; OsACS3, 5′-GCTGCTTCATCAAGAAATGGGAGC-3′ and 5′-GATGGAGCCATAGAGGGAGC-3′; OsACO5, 5′-GCAGATCGACAATGGCTGCG-3′ and 5′-GTCGTCCTGGAACAGCAGGA-3′; and OsActin3, 5′-actaccactgctgaacggga-3′ and 5′-ggacccgactcatcatactc-3′.
Quantitative Real-Time PCR
Total RNA was extracted as described above. First-strand c-DNA was synthesized using iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR was conducted using iQ SYBR Green Supermix (Bio-Rad) and the following primers: 5′-TAGCGTGTGTAACAGGGGTATC-3′ and 5′-CCGATCATTATTCCTTGCCATCTG-3′ for OsACS2, 5′-GTAAGGTTTAGTCGTGTTCGTAGTG-3′ and 5′-GCCACACCAACTGACAATTATCC-3′ for OsACO1, 5′-TACATAACCGCTTTTGCTATTCAAG-3′ and 5′-CCGTGACACACTAAAATACCAAAC-3′ for OsACO5, and 5′-AGCACTTGGGGATTATATTTTGTTG-3′ and 5′-GGGAACCCTGCGTACTACAC-3′ for OsACO7. The data were normalized by the values of a rice actin transcript (AK060893). At least three independent biological samples were used with gene-specific primers.
Chemical Treatments
KCN, ethephon, ACC, and flavone were purchased from Wako Pure Chemical Co., and flavanone and naringenin were purchased from Sigma-Aldrich. For Figures 4, 5, and 8, inoculated leaves were detached at 1 dpi, placed in a glass test tube (40 mm internal diameter, 20 cm long), fed through the cut base with 10 mL of various concentrations of chemicals, and incubated in a chamber maintained at 25°C with 16 h of light and 8 h of dark.
For Figure 6, inoculated leaves detached at 1 dpi were placed in a 70-mL glass test tube containing 10 mL of water (control) or of a 0.5 mm solution of chemicals. At this step, for ethylene treatment, 112 μL ethylene gas was added to the tube containing water, and the cap of tube was completely sealed. For other chemical treatments, the cap was sealed without adding any gas. After incubation for 24 h, the cap was removed, and the open tube was incubated for 2 d. The treated leaves were cut into pieces (2 cm long) and used for staining with lactophenol-trypan blue.
Measurement of Ethylene
Leaves were cut into 1-cm pieces and incubated in a sealed 9-mL glass vial containing 50 μL of water for 3 h (Fig. 5D) or 24 h (Figs. 1B and 2A). After incubation, a 0.1 to 1 mL sample was withdrawn from the headspace and analyzed for ethylene as described previously (Iwai et al., 2006). All data in Figures 2A and 5D were normalized to the amount of M. oryzae DNA in each sample.
Quantification of M. oryzae DNA
Rice and fungal DNA was extracted from inoculated leaves using ISOPLANT (Nippon Gene) and quantified by real-time PCR in accordance with the method of Qi and Yang (2002) using two specific primer pairs, which were designed based on the 3′ noncoding region of a MPG1 gene in M. oryzae (forward, 5′-GGGATGATGGTGGTGGAGGAC-3′; and reverse, 5′-GCCAGGTGCTTAGGACGAAAC-3′). These data were normalized with the DNA amount of a rice actin gene (AK060893), which was quantified using the following primers (forward, 5′-GAGTATGATGAGTCGGGTCCAG-3′; and reverse, 5′-ACACCAACAATCCCAAACAGAG-3′).
Fungal Growth Assay
Blast fungus was cultured on a petri dish containing PDA at 25°C in the dark. Fifty days after incubation, four mycelial layers (2 mm2 in area) were plated onto a petri dish (9 cm in diameter) containing PDA and chemicals and incubated at 25°C in the dark. Fungal growth was estimated by measuring diameters of mycelial colonies formed on agars.
Measurement of Cyanide
Cyanide was colorimetrically measured according to Lambert et al. (1975) with some modifications to increase the sensitivity. A PDA sample (0.5 g) was transferred to a 50-mL polypropylene conical tube with a screw cap. Immediately after adding 6 mL of extraction buffer (5% H2SO4, 70 mm MgSO47H2O, and 30 mm sulfamic acid; Tittle et al., 1990) to the tube, the cap was closed tightly, and contents in the tube were mixed completely. The liberated cyanide was trapped in 300 μL of 0.1 n NaOH solution at the top of the tube at 45°C for 18 h. To 100 μL of the trapped NaOH solution, 10 μL of 5 m acetic acid, 50 μL of 2.5% succinimide/0.25% N-chlorosuccinimide, and 100 μL of 3% barbituric acid in 30% pyridine reagent were added. After incubating for 10 min, the A580 was determined using a spectrophotometer. Using the above condition, 0.01 to 2.0 nmol cyanide can be quantified linearly. The recovery of trapping to NaOH solution was 84%, and the recovery of cyanide from the agar medium was 35%, which was calculated from the values after adding 1 nmol of cyanide to agar medium and to water.
National Center for Biotechnology Information accession numbers for the genes mentioned in this article are as follows: OsACS2 (AK064250), OsACO1 (AK065039), OsACO5 (AK061064), OsACO7 (AK102472), Osactin3 (AK060893), and MPG1 (L20685).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Rapid disappearance of exogenous cyanide in vitro.
Supplementary Material
Acknowledgments
We thank Tokio Imbe and Ikuo Ando for providing IL7 seeds, Nagao Hayashi for providing the M. oryzae strain, Hiroshi Takatsuji for critical comment on the manuscript, and Yoko Goto, Yumi Naito, Masumi Teruse, and Chiaki Kimoto for technical assistance.
References
- Bleecker AB, Kende H. (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Brading PA. (1997) Functional analysis of Cf gene-dependent defense responses in tomato. PhD thesis University of East Anglia, Norwich, UK [Google Scholar]
- Brading PA, Hammond-Kosack KE, Parr A, Jones JD. (2000) Salicylic acid is not required for Cf-2- and Cf-9-dependent resistance of tomato to Cladosporium fulvum. Plant J 23: 305–318 [DOI] [PubMed] [Google Scholar]
- Bröekaert WF, Delauré SL, De Bolle MF, Cammue BP. (2006) The role of ethylene in host-pathogen interactions. Annu Rev Phytopathol 44: 393–416 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JD. (2001) Plant pathogens and integrated defence responses to infection. Nature 411: 826–833 [DOI] [PubMed] [Google Scholar]
- Day DA, Whelan J, Millar AH, Siedow JN, Wiskich JT. (1995) Regulation of the alternative oxidase in plants and fungi. Aust J Plant Physiol 22: 497–509 [Google Scholar]
- de Laat AMM, van Loon LC. (1983) The relationship between stimulated ethylene production and symptom expression in virus-infected tobacco leaves. Physiol Plant Pathol 22: 261–273 [Google Scholar]
- Fofana B, Benhamou N, McNally DJ, Labbé C, Séguin A, Bélanger RR. (2005) Suppression of induced resistance in cucumber through disruption of the flavonoid pathway. Phytopathology 95: 114–123 [DOI] [PubMed] [Google Scholar]
- Grossmann K. (1996) A role for cyanide, derived from ethylene biosynthesis, in the development of stress symptoms. Physiol Plant 97: 772–775 [Google Scholar]
- Hammond-Kosack KE, Silverman P, Raskin I, Jones J. (1996) Race-specific elicitors of Cladosporium fulvum induce changes in cell morphology and the synthesis of ethylene and salicylic acid in tomato plants carrying the corresponding Cf disease resistance gene. Plant Physiol 110: 1381–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Kuroyanagi M, Nishimura M, Hara-Nishimura I. (2006) A cellular suicide strategy of plants: vacuole-mediated cell death. Apoptosis 11: 905–911 [DOI] [PubMed] [Google Scholar]
- Ichiba T, Kinuko K, Kashino H, Nanba K, Mizutani A, Miki N. (2000) Effect of metrominostrobin on respiratory activity of Rhizoctonia solani and its efficacy for controlling rice sheath blight. J Pestic Sci 25: 398–401 [Google Scholar]
- Ise K, Horisue N. (1988) Characteristics of several near-isogenic lines of rice for blast resistance gene. Breed Sci 38: 404–405 [Google Scholar]
- Isom GE, Way JL. (1984) Effect of oxygen on the antagonism of cyanide intoxication: cytochrome oxidase, in vitro. Taxicol Appl Pharm 15: 57–62 [DOI] [PubMed] [Google Scholar]
- Iwai T, Miyasaka A, Seo S, Ohashi Y. (2006) Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol 142: 1202–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai T, Seo S, Mitsuhara I, Ohashi Y. (2007) Probenazole-induced accumulation of salicylic acid confers resistance to Magnaporthe grisea in adult rice plants. Plant Cell Physiol 48: 915–924 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Burgess SM, Hirsh D. (1986) β-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci USA 83: 8447–8451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester M, Hennig J, Bol JF, Linthorst HJ, Bol JF, Linthorst HJ, van Loon LC, van den Heuvel J. (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95: 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama O, Miyakawa J, Akatsuka T, Kiyosawa S. (1992) Sakuranetin, a flavanone phytoalexin from ultraviolet-irradiated rice leaves. Phytopathology 31: 3807–3809 [Google Scholar]
- Lambert JL, Ramasamy J, Paukstelis JV. (1975) Stable reagents for the colorimetric determination of cyanide by modified König reaction. Anal Chem 47: 916–918 [Google Scholar]
- Miller JM, Conn EE. (1980) Metabolism of hydrogen cyanide by higher plants. Plant Physiol 65: 1199–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S, et al. (1996) Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol 37: 49–59 [DOI] [PubMed] [Google Scholar]
- Mizutani A, Miki N, Yukioka H, Tamura H, Masuko M. (1996) A possible mechanism of control of rice blast disease by a novel alkoxyiminoacetamide fungicide, SSF126. Phytopathology 86: 295–300 [Google Scholar]
- Mizutani A, Yukioka H, Tamura H, Miki N, Masuko M, Takeda R. (1995) Respiratory characteristics in Pyricularia oryzae exposed to a novel alkoxyiminoacetamide fungicide. Phytopathology 85: 306–311 [Google Scholar]
- Peiser GD, Wang TT, Hoffman NE, Yang SF, Liu H-W, Walsh CT. (1984) Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carboxylic acid during its conversion to ethylene. Proc Natl Acad Sci USA 81: 3059–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, van Wees SC, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon LC. (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Yang Y. (2002) Quantification of Magnaporthe grisea during infection of rice plants using real-time PCR and northern blot/phosphoimaging analyses. Phytopathology 92: 870–876 [DOI] [PubMed] [Google Scholar]
- Solomos T. (1977) Cyanide-resistant respiration in higher plants. Annu Rev Plant Physiol 28: 279–297 [Google Scholar]
- Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG, Jones JD. (1995) Molecular genetics of plant disease resistance. Science 268: 661–667 [DOI] [PubMed] [Google Scholar]
- Takahashi Y. (1958) A method for forecasting blast disease using the leaf sheath inoculation technique (in Japanese). Plant Protection 12: 339–345 [Google Scholar]
- Tittle FL, Goudey JS, Spencer MS. (1990) Effect of 2,4-dichlorophenoxyacetic acid on endogenous cyanide, β-cyanoalanine synthase activity, and ethylene evolution in seedling of soybean and barley. Plant Physiol 94: 1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H. (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47: 969–976 [DOI] [PubMed] [Google Scholar]
- Wang KL, Li H, Ecker JR. (2002) Ethylene biosynthesis and signaling networks. Plant Cell (Suppl) 14: S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenbörner M, Jha HC. (1993) Antifungal activity of flavonoids and their mixtures against different fungi occurring on grain. Pestic Sci 38: 347–351 [Google Scholar]
- Yip WK, Yang SF. (1988) Cyanide metabolism in relation to ethylene production in plant tissues. Plant Physiol 88: 473–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wen CK. (2010) Preparation of ethylene gas and comparison of ethylene responses induced by ethylene, ACC, and ethephon. Plant Physiol Biochem 48: 45–53 [DOI] [PubMed] [Google Scholar]
- Zhao J, Dixon RA. (2010) The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci 15: 72–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.