Abstract
About 15% of flowering plants accumulate fructans. Inulin-type fructans with β(2,1) fructosyl linkages typically accumulate in the core eudicot families (e.g. Asteraceae), while levan-type fructans with β(2,6) linkages and branched, graminan-type fructans with mixed linkages predominate in monocot families. Here, we describe the unexpected finding that graminan- and levan-type fructans, as typically occurring in wheat (Triticum aestivum) and barley (Hordeum vulgare), also accumulate in Pachysandra terminalis, an evergreen, frost-hardy basal eudicot species. Part of the complex graminan- and levan-type fructans as accumulating in vivo can be produced in vitro by a sucrose:fructan 6-fructosyltransferase (6-SFT) enzyme with inherent sucrose:sucrose 1-fructosyltransferase (1-SST) and fructan 6-exohydrolase side activities. This enzyme produces a series of cereal-like graminan- and levan-type fructans from sucrose as a single substrate. The 6-SST/6-SFT enzyme was fully purified by classic column chromatography. In-gel trypsin digestion led to reverse transcription-polymerase chain reaction-based cDNA cloning. The functionality of the 6-SST/6-SFT cDNA was demonstrated after heterologous expression in Pichia pastoris. Both the recombinant and native enzymes showed rather similar substrate specificity characteristics, including peculiar temperature-dependent inherent 1-SST and fructan 6-exohydrolase side activities. The finding that cereal-type fructans accumulate in a basal eudicot species further confirms the polyphyletic origin of fructan biosynthesis in nature. Our data suggest that the fructan syndrome in P. terminalis can be considered as a recent evolutionary event. Putative connections between abiotic stress and fructans are discussed.
About 45,000 species of angiosperms, approximately 15% of the flowering plants, store fructans, Fru-based oligo- and polysaccharides derived from Suc. Fructans are known to occur in the highly evolved orders of the Poales (Poaceae), Liliales (Liliaceae), Asparagales, Asterales (Asteraceae and Campanulaceae), and Dipsacales as well as within the Boraginaceae (Hendry, 1993). Fructans are believed to accumulate in the vacuole (Wiemken et al., 1986), although fructans and fructan degrading enzymes (fructan exohydrolases [FEHs]) have also been reported in the apoplast (Livingston and Henson, 1998; Van den Ende et al., 2005). To explain this observation, it was hypothesized that fructans can be transferred from the vacuole to the outer side of the plasma membrane by vesicle-mediated exocytosis (Valluru et al., 2008, and refs. therein), especially under stress. Fructans might protect plants against freezing/drought stresses (Valluru and Van den Ende, 2008) by stabilizing membranes (Vereyken et al., 2001; Hincha et al., 2002, 2003). Recent studies on transgenic plants carrying fructan biosynthetic genes (Parvanova et al., 2004; Li et al., 2007; Kawakami et al., 2008) suggest that the enhanced tolerance of these plants is associated with the presence of fructans. Their reduced lipid peroxidation levels indicate that fructans, similar to raffinose family oligosaccharides (Nishizawa et al., 2008), might directly act as reactive oxygen species scavengers, too (Van den Ende and Valluru, 2009; Bolouri-Moghaddam et al., 2010; Stoyanova et al., 2010).
While dicotyledonous species were believed to exclusively store inulin-type fructan consisting of linear β(2,1)-linked fructofuranosyl units, β(2,6) levan-type and mixed-type fructans predominate in monocots (Vijn and Smeekens, 1999). Different types of fructan biosynthetic enzymes (also termed fructosyltransferases [FTs]) have now been characterized that can readily explain the diversity of fructans in plants (Lasseur et al., 2006; Tamura et al., 2009). They can be classified in S-type FTs (using Suc as donor substrate) and F-type FTs (using fructans as donor substrate; Schroeven et al., 2009). Two different enzymes (Suc:Suc 1-fructosyltransferase [1-SST] and fructan:fructan 1-fructosyltransferase [1-FFT]) are required to synthesize the most common and best studied inulin-type fructans occurring in Asteracean species (Edelman and Jefford, 1968; Van den Ende and Van Laere, 2007). Depending on the species, a more complex cocktail of FTs (1-SST, 1-FFT, Suc:fructan 6-fructosyltransferase [6-SFT], and 6G-FFT) is needed within the monocots (Prud’homme et al., 2007; Yoshida et al., 2007). Structure-function relationships have been found explaining the evolutionary differences between vacuolar invertases and FTs on the one hand (Schroeven et al., 2008; Altenbach et al., 2009) and between different types of FTs on the other hand (Lasseur et al., 2009; Schroeven et al., 2009). Clearly, the capacity for fructan biosynthesis arose many times in the course of evolution, in bacteria, fungi, and higher plants (Ritsema et al., 2006; Altenbach and Ritsema, 2007). In plants, FTs evolved from vacuolar invertases (Wei and Chatterton, 2001; Schroeven et al., 2008), while FEHs probably evolved from cell wall invertases (Le Roy et al., 2007). Like other Suc splitting enzymes, S-type FTs can fulfill important roles in regulating source/sink balances in plants (Ji et al., 2010).
Pachysandra terminalis (Japanese spurge; Buxaceae) is a frost-hardy evergreen plant originating from Japan and China but became increasingly popular as a ground-cover plant in Europe and North America (Zhu and Beck, 1991; Zhou et al., 2005). Pachysandra can survive freezing temperatures up to −33°C (plants.usda.gov). Together with Sarcococca, Styloceras, Buxus, and Notobuxus, the genus Pachysandra belongs to the Buxaceae family within the basal eudicots (Hoot et al., 1999; von Balthazar et al., 2000; Anderson et al., 2005; Jiao and Li, 2009). Pachysandra and other members of the Buxaceae contain alkaloids with antibacterial, antiviral, and anticancer properties (Kinghorn et al., 2004; Devkota et al., 2008). Moreover, P. terminalis was found to be an effective vole repellent (Curtis et al., 2002).
To the best of our knowledge, so far the presence of fructans has not been reported in P. terminalis or any other species within the basal eudicots. To date, the presence of graminan- and levan-type fructans with predominant β(2,6) linkages was thought to be restricted to monocot plant species only. In this article, we demonstrate for the first time, to our knowledge, that cereal-type levans and graminans are present in P. terminalis, a representative of the primitive eudicots. We report on the purification and characterization of the main FT involved and on the cloning and heterologous expression of the cDNA encoding this enzyme, showing peculiar substrate specificity characteristics as a function of the incubation temperature and time.
RESULTS AND DISCUSSION
P. terminalis Accumulates Both Graminan- and Levan-Type Fructans
Linear β(2,1) inulin-type fructans with 1-kestotriose as backbone, as occurring, for example, in chicory roots (Fig. 1), are the most studied fructans and become more and more popular as prebiotics in food (Roberfroid and Delzenne, 1998; Roberfroid, 2007). Linear levan-type fructans (backbone: 6-kestotriose) accumulate in a few grass species, including Dactylis glomerata (Fig. 1). Graminan-type fructans, with 1&6-kestotetraose (also termed bifurcose) as backbone, are predominant in cereals such as wheat (Triticum aestivum in Fig. 1; Yoshida et al., 2007). As a result of a major screening of >100 plant species belonging to different families in the plant kingdom, by means of high-performance anion-exchange chromatography and pulsed-amperometric detection (HPAEC-PAD), the rhizomes of P. terminalis were found to contain a complicated mixture of so far unidentified oligo- and polysaccharides in Figure 1. When a neutral, carbohydrate-containing fraction of P. terminalis rhizomes was subjected to mild acid and enzymatic hydrolysis with chicory (Cichorium intybus) 1-FEH IIa (Van den Ende et al., 2001) and sugar beet (Beta vulgaris) 6-FEH (Van den Ende et al., 2003a), a massive production of Fru was observed (data not shown). A closer inspection of the oligosaccharides from P. terminalis on the HPAEC-PAD chromatograms revealed that it contained (1) a series of peaks corresponding to the graminan-type fructans as occurring in wheat and (2) another series with elution times identical to the linear levan-type fructans as accumulating in D. glomerata (Fig. 1). These findings already suggested that P. terminalis accumulates a mixture of both graminan- and levan-type fructans, as well as some 1-kestotriose. High degree of polymerization (DP) levan-type fructans occur both in Pachysandra and in Dactylis (arrows in Fig. 1). Hard proof of the putative fructan identities was generated by manually collecting individual compounds (a–m in Fig. 1) during elution from a preparative HPAEC-PAD column. Enzymatic and mild acid hydrolysis treatments indeed confirmed the unique presence of the same graminan- and levan-type fructan structures (Table I) as earlier described in wheat (Bancal et al., 1993; Van den Ende et al., 2003b; Yoshida et al., 2007). However, the levan to graminan ratio is higher in Pachysandra compared to wheat (Fig. 1) but lower than the one observed in D. glomerata (Maleux and Van den Ende, 2007) and in Phleum pratense (Tamura et al., 2009). At the end of a long cold period, rhizomes of P. terminalis accumulated up to 12.3% of fructans on a fresh weight basis. For comparison, commercial chicory root cultivars can accumulate up to 18% inulin-type fructans (Van Waes et al., 1998; Van den Ende and Van Laere, 2007).
Figure 1.
HPAEC-PAD chromatograms of the neutral carbohydrate fractions derived from P. terminalis, wheat, D. glomerata, and chicory. A reference with sugar standards is also included. G, Glc; F, Fru; S, Suc; R, raffinose; St, stachyose; 1K, 1-kestotriose; M, maltose; 6K, 6-kestotriose; nK, neokestose or 6G-kestotriose; N, 1,1-nystose; B, 1&6-kestotetraose or bifurcose; F2, inulobiose; F3, inulotriose; IX, inulin with a DP of X; LX, levan with a DP of X; F2, inulobiose; F3, levantriose. Peaks a to m from P. terminalis were manually collected and further characterized (see Table I).
Table I.
Characterization of fructans in P. terminalis
| Peak | 1-FEH-Generated Products | 6-FEH-Generated Products | Fru to Glc Ratio after Mild Acid Hydrolysis | Fructan Structure |
| a | Suc; Fru | None | 2 | 1-Kestotriose (1K) |
| b | Fru | None | No Glc | Inulobiose (F2) |
| c | None | Suc; Fru | 2 | 6-Kestotriose (6K) |
| d | 1K; Suc; Fru | None | 3 | 1,1-Kestotetraose/1,1-nystose (N) |
| e | 6K; Fru (low) | 1K; Fru (low) | 3 | 1&6-Kestotetraose/ bifurcose (B) |
| f | F2; Fru | None | No Glc | Inulotriose (F3 in Fig. 1) |
| g | 6K; Fru | None | 3 | 6,1-Kestotetraose (6,1K) |
| h | None | 6K; Suc; Fru | 3 | 6,6-Kestotetraose (6,6K) |
| i | B; 6,1K; Fru | N; Fru | 4 | 6&1,1-Kestopentaose + 1&6,1-kestopentaose |
| j | 6,6K; Fru | B; 1K; Fru | 4 | 1&6,6-Kestopentaose (1&6,6K) |
| k | None | 6,6K; 6K, Fru | 4 | 6,6,6-Kestopentaose (6,6,6K) |
| l | 6,6,6K; Fru | 1&6,6K; B; Fru | 5 | 1&6,6,6-Kestohexaose |
| m | None | 6,6,6K; 6,6K; 6K; Fru | 5 | 6,6,6,6-Kestohexaose |
Purification and Properties of a 6-SST/6-SFT Involved in Graminan and Levan Synthesis
The dominant character of graminan- and levan-type fructans in rhizomes of P. terminalis suggested the presence of a prominent enzymatic activity responsible for the formation of β(2,6) Fru-Fru linkages. Preliminary in vitro incubations with Suc as a single substrate indeed showed a prominent formation of 6-kestotriose compared to 1-kestotriose and 6G-kestotriose (neokestose) and the formation of 1&6-kestotetraose from Suc and 1-kestotriose (data not shown). Therefore, the enzyme was provisionally termed a 6-SST/6-SFT. The β(2,6)-forming enzyme was purified by monitoring its 6-SST activity (quantification of 6-kestotriose production from Suc by HPAEC-PAD). The results of a typical purification are presented in Table II. Although considerable activity losses occurred on concanavalin A, Mono S pH 4.5, and Mono S pH 4.0, a maximal purification of about 88-fold and a specific activity of 2.1 units mg−1 protein were found (Table II), similar to the purified 1-SST from chicory roots (Van den Ende et al., 1996a).
Table II. A typical purification of the 6-SST/6-SFT from P. terminalis rhizomes.
| Fraction | Protein | Activity | Recovery | Specific Activity | Purification |
| mg | units | % | units (mg protein)−1 | fold | |
| Crude extract | 162 | 3.9 | 100 | 0.024 | 1.0 |
| (NH4)2SO4 30%–80% | 151 | 3.7 | 95 | 0.025 | 1.0 |
| Concavanalin A | 5.3 | 2.5 | 64 | 0.48 | 20 |
| Mono S 4.5 | 0.92 | 1.5 | 38 | 1.6 | 67 |
| Mono S 4.0 | 0.17 | 0.35 | 8.8 | 2.1 | 88 |
Similar to all other plant FTs purified so far (Van den Ende and Van Laere, 2007), the retention on concanavalin A confirmed that this enzyme is also a glycoprotein. The molecular mass of the native protein was about 70 kD, as determined by gel filtration (data not shown). The most pure fraction (Mono S pH 4.0; Table II), resulted in 53- and 22-kD subunits after SDS-PAGE (Fig. 2). Such heterodimeric nature is a typical characteristic for FTs derived both from dicot and monocot species (Altenbach et al., 2004; Van den Ende and Van Laere, 2007), although a few monomeric FTs have also been reported (Koops and Jonker, 1994). Within GH32 plant enzymes, the large subunit contains the catalytic triad (Lammens et al., 2009), but the small subunit is also essential to preserve the biologic activity of FTs (Altenbach et al., 2004). The pH optimum of the 6-SST/6-SFT was between 5.0 and 5.5 (Fig. 3A), consistent with other Suc-splitting plant FTs (Van den Ende and Van Laere, 2007) and a presumptive vacuolar localization. The temperature optimum was close to 20°C (Fig. 3B), which is considerably lower than the 30°C to 35°C of the 1-kestotriose- and 6-kestotriose-forming enzymes in barley (Hordeum vulgare; Simmen et al., 1993) but comparable with the 20°C to 25°C of the 1-SST from Helianthus tuberosus (Koops and Jonker, 1996). The high activity at 0°C (40% of the maximum) might represent a specific adaptation to increase fructan accumulation at lower temperatures, as occurring within the Pooideae (Sandve and Fjellheim, 2010), including cereals (Yoshida et al., 2007) and other grasses (Hisano et al., 2004, 2008).
Figure 2.
SDS-PAGE (12%) of 3 μg native 6-SST/6-SFT enzyme from P. terminalis stained with Coomassie Brilliant Blue R 250. The size of the molecular mass markers in kilodaltons is indicated to the left.
Figure 3.
Effect of pH (A) and incubation temperature (B) on the 6-SST activity of the purified 6-SST/6-SFT enzyme from P. terminalis. Activities are expressed as a percentage of the maximal activity. Vertical bars indicate se for n = 3.
Mass Fingerprint of the Purified Pt 6-SST/6-SFT
The two subunits were excised and trypsinized in-gel, and the masses of ZipTip-eluted tryptic peptides were determined by quadrupole time-of-flight (Q-TOF) mass spectrometry. A theoretical tryptic digest of the cDNA-derived 6-SST/6-SFT sequence (obtained later; see Fig. 4) yielded 50 peptides (designated T1–T50 from N to C terminus). Most masses matched, within the acceptable mass measurement error of ±0.2 D, with one of the theoretical fragments (Supplemental Table S1). Two fragments, representing the N- and C-terminal parts of the enzyme, did not match with the masses of the theoretical fragments, demonstrating the existence of an alternative N-terminal (VPYPWSNAQLSWQR and YPWSNAQLSWQR) and C-terminal (IWEMNSAFIQPFH) processing in planta. To the best of our knowledge, such (differential) N- or C-terminal processing has not been reported before for plant FTs.
Figure 4.
Alignment of the P. terminalis 6-SST/6-SFT (FN870376), P. pyrifolia vacuolar invertase (the most identical GH32 member, BAF35859), barley 6-SFT (X83233), and P. pratense 6-SFT (BAH30252). Potential glycosylation sites are underlined. The three carboxylic acids that are crucial for enzyme catalysis (Lammens et al., 2009) are in bold and underlined. Boxes indicate motifs involved in donor and acceptor substrate specificity (Van den Ende et al., 2009). The arrow indicates the N terminus of the mature 6-SST/6-SFT protein. Consensus line: Asterisks indicate identical residues, colons indicate conserved substitutions, and periods indicate semiconserved substitutions.
Cloning a cDNA Encoding the Pt 6-SST/6-SFT and Phylogenetic Analysis
A full 6-SST/6-SFT cDNA from P. terminalis was obtained by a combination of reverse transcription (RT)-PCR, PCR, and 5′ and 3′ RACE RT-PCR. On the one hand, the Pt 6-SST/6-SFT cDNA-derived amino acid sequence (EMBL accession no. FN870376) is aligned (Fig. 4) with the most identical sequence available in the literature (65% identity with Pyrus pyrifolia vacuolar invertase; Yamada et al., 2007). On the other hand, it is compared to the functionally related 6-SFT enzymes from barley and P. pratense (Sprenger et al.,1995; Tamura et al., 2009). Five potential glycosylation sites [N-X-(S/T)] were detected (Fig. 4). The estimated molecular mass of the derived polypeptide, without taking into account putative glycosylations, was predicted at 60.7 kD. Its predicted pI was 5.2, which is typical for all vacuolar FTs and invertases (Van den Ende and Van Laere, 2007). The NWMNDPNG (or β-fructosidase) motif is changed into SWMSDPDG (Fig. 4). An altered β-fructosidase motif is typical for all FTs described so far (Altenbach et al., 2009; Van den Ende et al., 2009). Another characteristic of FTs is the presence of an altered W(A/G)W motif (Altenbach et al., 2009). Pt 6-SST/6-SFT is an exception since it contains a full WAW motif (Fig. 4). Intriguingly, in Pt 6-SST/6-SFT, an Asp/Gln couple (DEEQ) is observed instead of an Asp/Arg couple as observed in P. pyrifolia VI (DDDR) and in the P. pratense and barley 6-SFTs (DDER; Fig. 4). Perhaps the Asp/Gln couple can fulfill a similar role for stabilizing Suc (Lasseur et al., 2009).
An unrooted phylogenetic tree was constructed containing the Pt 6-SST/6-SFT cDNA-derived amino acid sequence (see arrow, No. 1 in Fig. 5) and many other vacuolar types of plant invertases and FTs. Three distinct groups can be clearly distinguished (Fig. 5). Groups I and II contain dicotyledonous enzymes, and group III contains monocotyledonous enzymes. Hitherto, it was believed that all dicot FTs (see 1-SSTs and 1-FFTs in subgroup IIb, Fig. 5) evolved from Type II vacuolar invertases (Van den Ende et al., 2002) by changes in their WMNDPNG and WGW motifs (Schroeven et al., 2008; Altenbach et al., 2009). Here, we demonstrate for the first time that an FT (Pt 6-SST/6-SFT; No. 1 in Fig. 5) shows higher identity to Type I vacuolar invertases (52%–65% identity to 2–12 in Fig. 5) than to Type II vacuolar invertases (51%–57% identity to 13–18 in Fig. 5), suggesting that the basal eudicot Pt 6-SST/6-SFT evolved from a Type I vacuolar invertase or, alternatively, from an ancestral vacuolar invertase before the separation of the two vacuolar invertase subgroups by duplication. Further sequencing of vacuolar invertases of lower plants and a number of basal eudicot species is necessary to discriminate between these two possibilities. However, care should be taken since gene losses might further complicate the interpretation of such phylogenetic analyses. For instance, the two only vacuolar invertases in the genome of Arabidopsis (Arabidopsis thaliana; Nos. 10 and 11 in Fig. 5) belong to Type I vacuolar invertases, suggesting that the group II gene was lost during evolution and the group I gene was duplicated in this species. Four subgroups (IIIa–IIId) can be differentiated within monocots. The subgroups IIIb and IIId contain vacuolar invertases. Subgroup IIIc harbors both vacuolar invertases and FTs from Asparagales. Subgroup IIIa contains mainly FTs, but the presence of a genuine invertase in this subgroup (No. 34 shows an intact WMNDPNG and WGW motif; Schroeven et al., 2008; Lasseur et al., 2009) cannot be excluded. This subgroup further splits up in enzymes that biosynthesize β(2,1) fructosyl linkages (1-SSTs) and those that make β(2,6) linkages (6-SFTs and a putative 6-FT). Taken together, the phylogenetic tree (Fig. 5) suggests that the capability to create fructans was gained at least four times throughout plant evolution: within the basal eudicots and within the Asterales, Poaceae, and Asparagales. In this scenario, it can be speculated that different FTs were created by a small number of mutations (Schroeven et al., 2008, 2009). It was recently speculated that this evolution occurred in two steps: first, the development of S-type FTs (1-SST and 6-SFT; still using Suc as a donor substrate) from vacuolar invertases followed by the development of F-type FTs (1-FFT and 6G-FFT; using fructan as donor substrate) from S-type FTs (Schroeven et al., 2009).
Figure 5.
Unrooted phylogenetic tree of vacuolar invertases and FTs. Three main groups can be discerned. First group (I): Type I vacuolar invertases and a single FT from (basal and core) eudicots. 1, P. terminalis 6-SST/6-SFT (FN870376); 2, Vitis vinifera INV(Q9S943); 3, sugar beet INV (AJ277455); 4, Daucus carota INV (Q42722); 5, chicory INV (AJ419971); 6, P. pyrifolia INV (BAF35859); 7, Citrus unshiu INV (AB074885); 8, Phaseolus vulgaris INV (O24509); 9, Vicia faba INV (Q43857); 10, Arabidopsis INV (AY039610); 11, Arabidopsis INV (AY046009); 12, Brassica oleracea INV (AF274298). Second group, subgroup IIa: Type II vacuolar invertases from core eudicots. 13, V. vinifera INV (QS944); 14, D. carota INV (X75352); 15, Lycopersicon esculentum INV (P29000); 16, Capsicum annuum INV (P93761); 17, Ipomoea batatas INV (AY037937); 18, I. batatas INV (AF017082). Second group, subgroup IIb: core eudicot fructan biosynthetic enzymes 1-SST and 1-FFT. 19, Cynara scolymus 1-SST (Y09662); 20, H. tuberosus 1-SST (AJ009757); 21, Taraxacum officinale 1-SST (AJ250634); 22, chicory 1-SST (U81520); 23, Lactuca sativa 1-SST (ABX90019); 24, Echinops ritro 1-FFT (AJ811624.1); 25, chicory 1-FFT (U84398); 26, Cynara scolymus 1-FFT (AJ000481); 27, H. tuberosus 1-FFT (AJ009756); 28, Viguiera discolor 1-FFT (AJ811625). Third group: subgroup IIIa, FTs and a putative INV from Poaceae. 29, Barley 6-SFT (X83233); 30, wheat 6-SFT (AB029887); 31, Lolium perenne putative 6-FT (AF494041); 32, Poa ampla 6-SFT (AF192394); 33, P. pratense 6-SFT (BAH30252); 34, L. perenne putative INV (AF481763); 35, L. perenne 6G-FFT (AF492836); 36, Festuca arundinacea 1-SST (AJ297369); 37, L. perenne 1-SST (AY245431); 38, wheat 1-SST (AB029888); 39, wheat 1-FFT (AB088409). Subgroup IIIb: monocot vacuolar invertases. 40, Oryza sativa INV; 41, L. perenne INV (AY082350); 42, wheat INV (AJ635225). Subgroup IIIc: FTs from Asparagales. 43, Allium cepa 6G-FFT (AY07838); 44, Asparagus officinalis 6G-FFT (AB084283); 45, A. officinalis INV (AF002656); 46, A. cepa INV (AJ006067); 47, A. cepa 1-SST (AJ006066); 48, Agave tequiliana 1-SST (DQ535031). Subgroup IIId, monocot vacuolar INV. 49, Tulipa gesneriana putative INV (X97642); 50, Zea mays INV (P49175); 51, O. sativa INV (AF276703). The P. terminalis 6-SST/6-SFT is indicated with an arrow. The bar indicates a distance value of 0.1.
Heterologous Expression of the Pt 6-SST/6-SFT cDNA in Pichia pastoris: Comparison of the Native and Heterologously Produced Enzymes
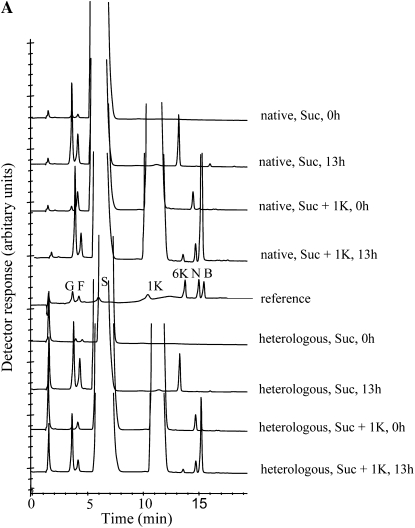
Heterologous expression in P. pastoris is considered as a reliable method to study the functionality within the GH32 family (Van den Ende et al., 2009). We are confident that the native 6-SST/6-SFT enzyme was >99% pure since the same enzyme preparation was used for enzyme crystallization and three-dimensional structure determination (W. Lammens, K. Le Roy, R. Vergauwen, A. Rabijns, A. Van Laere, S.V. Strelkov, and W. Van den Ende, unpublished data). Short-term incubations with the native and recombinant Pt 6-SST/6-SFT and Suc showed the production of Glc and 6-kestotriose (Fig. 6). When using 1-kestotriose as a single substrate (data not shown), or when combining Suc and 1-kestotriose, no 1,1-nystose was produced, showing that the (native and recombinant) enzymes lack 1-FFT activity. By combining Suc and 1-kestotriose as substrates, 1&6-kestotetraose (bifurcose) is the main fructan product formed by both the native and heterologous enzyme (Fig. 6). Only low amounts of 6-kestotriose were produced, indicating that 1-kestotriose is a more preferential acceptor substrate than Suc (Fig. 6). This was further corroborated by kinetic studies (both at 0°C and 30°C) on the recombinant enzymes at varying Suc concentrations (6-SST activity) and at a constant Suc concentration (150 mm) combined with varying 1-kestotriose concentrations (6-SFT activity). With Suc as single substrate, no saturation was observed, not even at 1.2 m Suc (data not shown). The Suc to 1-kestotriose transfer reaction was clearly saturable (Fig. 7; Supplemental Fig. S1). The apparent Km values for 1-kestotriose as an acceptor substrate were estimated at 35 mm (0°C) and 76 mm (30°C), respectively. These apparent Km values are surprisingly low compared to those derived for other plant FTs (Schroeven et al., 2008). Inhibition of 1&6-kestotetraose formation was observed at 0°C but not at 30°C (Fig. 7). The native enzyme shows only minor 1-SST side activities at 0°C but higher 1-SST side activities at 30°C (Supplemental Fig. S2), probably contributing to the difference in fructan patterns (graminan to levan ratio) generated from Suc as a single substrate (Supplemental Fig. S2). However, this temperature-dependent difference in the graminan to levan ratio was found to be less prominent for the recombinant enzyme (Fig. 8).
Figure 6.
In vitro fructan synthesis by the purified native and heterologous 6-SST/6-SFT (1 μg/80 μL) when incubated with 300 mm Suc and with 300 mm Suc + 300 mm 1-kestotriose. Incubation times, 0 and 13 h; incubation temperature, 0°C. A reference with sugar standards is included. G, Glc; F, Fru; S, Suc; 1K, 1-kestotriose; 6K, 6-kestotriose; N, 1,1-nystose; B, 1&6-kestotetraose or bifurcose.
Figure 7.
Substrate velocity plots for the production of 1&6-kestotetraose (abbreviated Bif) from 150 mm Suc and a range of 1-kestotriose concentrations. Reaction temperatures: 0°C (diamonds) and 30°C (squares). The corresponding linear Hanes plots are shown in Supplemental Figure S1. Vertical bars indicate se for n = 3.
Figure 8.
Long-term in vitro fructan synthesis by the native 6-SST/6-SFT at 0°C (A, 2.5 μg/ 80 μL) and at 30°C (B, 1 μg/80 μL) with 300 mm Suc. A reference with sugar standards, phlein, and the carbohydrate profile of P. terminalis are also included for comparison. G, Glc; F, Fru; S, Suc; 1K, 1-kestotriose; 6K, 6-kestotriose; N, 1,1-nystose; B, 1&6-kestotetraose or bifurcose; LX, levan with a DP of X.
Hydrolytic Activities of 6-SST/6-SFT: A Multifunctional Premature FT?
Evolutionary Considerations
Besides limited sucrolytic activities (Fig. 6), prolonged incubation times (substrate depletion) surprisingly demonstrated that the Pt 6-SST/6-SFT also showed intrinsic 6-FEH activities (Fig. 8). Typically, the hydrolase to transferase ratio increases with increasing temperature, as observed for the chicory 1-SST and 1-FFT (Van den Ende et al., 1996a, 1996b). Therefore, it seems reasonable to assume that the intrinsic 6-FEH activity of Pt 6-SST/6-SFT might increase at higher temperatures, explaining why higher DP levans do not accumulate at 30°C (Fig. 8B) compared to 0°C (Fig. 8A). This finding is in line with previous observations that some vacuolar invertases show intrinsic FEH activities (Van den Ende et al., 2003a; De Coninck et al., 2005; Ji et al., 2007), suggesting that perhaps many vacuolar invertases have such characteristics. This new point of view sheds a different light on some former data and conclusions. Indeed, leaves of many grasses, typically showing huge soluble invertase activities, also show high in vitro FEH activities, even during periods of active fructan synthesis (Lothier et al., 2007, and refs. therein). Therefore, it should be reinvestigated whether these prominent FEH activities represent genuine FEH activities rather than FEH side activities of vacuolar invertases. Perhaps these vacuolar invertase side activities are strong enough to play a role in planta. Whether genuine 6-FEH-type enzymes occur in the vacuoles of fructan plants is still not clear since the characterized 6-FEH from wheat is believed to be apoplastic (Van Riet et al., 2006).
We propose here that intrinsic FEH activities in vacuolar invertases can have important physiological and evolutionary implications. In Poaceae, it is believed that the fructan syndrome was initiated shortly after a global supercooling period at the Eocene-Oligocene boundary (Sandve and Fjellheim, 2010). When vacuolar invertase develops transferase capacity (by creating extra space and interaction capacity for binding the sugar acceptor substrate; Altenbach et al., 2009), the fructans that are produced need to be broken down at a later time point, consistent with the idea that fructans mainly act as reserve compounds. Therefore, coevolution of a cell wall invertase into specific FEHs (Le Roy et al., 2007) is needed as well as the recruitment of a vacuolar targeting signal to translocate the FEHs from the cell wall to the vacuole (Van den Ende et al., 2001). However, maturation of vacuolar invertases to genuine FTs as well as the subsequent development of specific FEHs probably require extended time periods throughout evolution. This means that there is probably an evolutionary window in which fructans are accumulating, while specific vacuolar FEHs are not yet available. We speculate that the Pt 6-SST/6-SFT, probably representing a premature or evolutionary young FT still containing many side activities (invertase, FEH, and 1-SST), might be involved in vacuolar fructan polymerization reactions as long as the Suc concentration is high enough. However, when the Suc concentration decreases, e.g. after defoliation or arrest of photosynthesis, the enzyme might play a role in fructan degradation as well. In chicory, in which the fructan syndrome developed a very long time ago (Fig. 5; Schroeven et al., 2009), a similar kind of Suc-based regulation is observed by means of very specific, Suc-inhibited 1-FEHs (Van den Ende et al., 2001). Such depolymerization reactions might provide hexoses for regrowth, contribute to osmotic adjustments, or adjust the DP of the fructans to create a sugar mix that is optimal for membrane and protein stabilization under stress (Livingston et al., 2009, and refs. therein).
It is well known that graminan- and levan-type (2,6 linkage forming) FT genes are induced by cold in grasses (del Viso et al., 2009; Tamura et al., 2009; del Viso et al., 2010). We demonstrate here that the β(2,6) linkage-forming Pt 6-SST/6-SFT enzyme shows an excellent activity even at lower temperatures (Fig. 3B) and a good levan polymerization rate at 0°C (Fig. 8A), and both might represent important physiological adaptations to lower temperatures. Particularly, the presence of high DP fructans with β(2,6) linkages, as occurring in Pachysandra (Fig. 1), might play an important role in these processes, as suggested before (Volaire et al., 1998). A recent extensive screening of 42 annual bluegrass ecotypes, accumulating levan-type fructans, highlighted a strong correlation between the concentrations of high DP fructans and adaptation to subfreezing temperatures (Dionne et al., 2009). Noteworthy, the results also revealed that other known cryoprotectants, such as Suc and Pro, were not closely correlated with variations in cold tolerance in this species. Combined with the results presented here, the data bring a new contribution to the current debate on the adaptive value of fructans for cold adaptation in grasses and fructan accumulating basal eudicots.
CONCLUSION
So far, graminan- and levan-type fructans with predominant β(2,6) linkages were believed to be absolutely restricted to monocot plant species. The discovery of cereal-type fructans in P. terminalis, a basal eudicot cold-tolerant species, changed this point of view and confirms the polyphyletic origin of fructan biosynthesis. Enzyme characterization of the native and recombinant versions of an important 6-SST/6-SFT type of FT involved in the synthesis of fructans in Pachysandra revealed some remarkable properties, consistent with the point of view that it represents a premature FT involved in the regulation of fructan levels in this species. The enzyme shows peculiar, temperature-dependent properties, urging deeper research into the side activities of vacuolar invertases and premature FTs of other fructan plants.
MATERIALS AND METHODS
Plant Material and Carbohydrate Analyses
Pachysandra terminalis (Buxaceae) was grown on a private field in the Leuven area (Belgium) and harvested in the winter period (February). Soluble sugars were extracted from its rhizomes and analyzed by analytical HPAEC-PAD (DX-300; Dionex) as described (Vergauwen et al., 2000). Individual fructans were collected manually using preparative HPAEC-PAD. For some compounds (e.g. peaks f and g, Fig. 1), repeated injections were necessary to collect enough material for further analysis. All the isolated fructans were subjected to enzymatic incubations with sugar beet (Beta vulgaris) 6-FEH and chicory (Cichorium intybus) 1-FEH IIa (10 units) in reaction mixtures buffered at pH 5.0 with 100 mm NaAc. After several time periods, the reaction was stopped by boiling for 5 min. All assays were analyzed by analytical HPAEC-PAD. In parallel, the isolated fructans were subjected to mild acid hydrolysis as described by Vanhaecke et al. (2006). The DP was determined by the Fru to Glc ratio (Table I).
Purification of the 6-SST/6-SFT and Q-TOF Mass Spectrometry
P. terminalis leaves and stems were removed. Rhizomes were washed with cold tap water, and 0.7 kg was homogenized in 0.9 liters of 50 mm NaAc buffer, pH 5.0, containing 1 mm phenylmethylsulfonylfluoride, 1 mm mercaptoethanol, 10 mm NaHSO3, and 0.1% (w/v) Polyclar AT. The homogenate was squeezed through cheesecloth.
Ammonium sulfate was added to a saturation of 30% and stirred on ice for 30 min. After centrifugation for 20 min at 40,000g, the pellet was discarded. Ammonium sulfate was further added to the supernatant up to a final concentration of 80% and stirred on ice for 30 min. This time, the supernatant was discarded after centrifugation for 20 min at 40,000g. The precipitate was collected and redissolved in 50 mm NaAc buffer (pH 5.0) supplemented with 1 mm CaCl2, 1 mm MnCl2, and 1 mm MgCl2. Undissolved material was spun down for 10 min at 40,000g. The supernatant was applied to a concavanalin A-Sepharose column equilibrated with 50 mm NaAc buffer (pH 5.0) supplemented with 1 mm CaCl2, 1 mm MnCl2, and 1 mm MgCl2. The column was washed with the same buffer. Bound proteins were eluted with 0.5 m methyl α-d-mannopyranoside in 50 mm NaAc buffer. The eluate was collected and proteins were loaded onto a Mono S (HR5/5; Pharmacia Uppsala) cation-exchange column equilibrated with 50 mm NaAc buffer, pH 4.5. Bound proteins were eluted using a linear gradient from 0.0 to 0.3 m NaCl in 30 min. Fractions of 1 mL were collected. The most active fractions were reapplied onto the Mono S column at pH 4.0 and eluted as described above. SDS-PAGE of the most active fraction occurred in 12.5% (w/v) polyacrylamide gel, and staining was performed with Coomassie Brilliant Blue R 250. The purification procedure was performed at 0°C to 4°C, and 0.02% NaN3 (w/v) was added to all buffers to prevent microbial growth.
The SDS-PAGE protein bands (Fig. 2) were subjected to mass spectrometric identification. The Coomassie Brilliant Blue-stained protein bands were excised, trypsinized, extracted, desalted, and analyzed on Q-TOF as earlier described (Van den Ende et al., 2001). Sequence information was derived from the tandem mass spectrometry data with the aid of MaxEnt 3 (deconvoluting and deisotoping of data) and PepSeq software of the Micromass BioLynx software package.
Enzyme Assays
During purification of the 6-SST/6-SFT enzyme, aliquots (10–50 μL) were incubated in 50 mm NaAc buffer at pH 5.0 supplemented with 300 mm Suc (final concentration) and 0.02% (w/v) NaN3. After 30 min incubation, the reaction was stopped by heating at 95°C for 5 min. Samples were diluted 3-fold with 0.02% (w/v) NaN3. From these, 25 μL was automatically injected onto the HPAEC-PAD column. Enzymatic activity is expressed in units, defined as the amount of enzyme producing 1 μmol of 6-kestotriose per min with 300 mm Suc as a single substrate. The same assay was used to determine temperature and pH optima of the enzyme. The 6-kestotriose standard was a generous gift of Dr. M. Iizuka (Kobe Shoin Women’s University, Japan) and was used as a standard. The 1&6-kestotetraose (bifurcose) is not commercially available. Small amounts were obtained from wheat (Triticum aestivum) crown tissue by preparative HPAEC-PAD. Phlein consists of levan-type fructans ranging from DP4 to DP12, a kind gift of Dr. J. Chatterton (Utah State University, Logan, UT).
Enzyme Kinetics
For the kinetical analyses, great care was taken to select time points in the linear region, ensuring that <10% of the original substrate was consumed. On the one hand, the recombinant 6-SST/6-SFT was incubated at 0°C and 30°C in 50 mm sodium acetate buffer (pH 5.0) containing 0.02% (w/v) NaN3 and different Suc concentrations ranging between 50 and 1200 mm. Similarly, the enzyme was incubated together with 150 mm Suc (donor substrate) and a range of 1-kestotriose concentrations (12.5–800 mm) as acceptor substrate. The incubation reactions were stopped by heating at 90°C for 5 min. The reaction products were analyzed by HPAEC-PAD as described (Vergauwen et al., 2000). By comparing the peak areas of 1&6-kestotetraose (6-SFT activity) and 6-kestotriose (6-SST activity) with known amounts of standard compounds, the products were quantified. In the case of 6-SFT activity, an apparent Km could be estimated based on the linear Hanes plots (Supplemental Fig. S1) as described before (Schroeven et al., 2008).
RNA Preparation, Cloning, Sequencing, and Phylogeny
Fresh rhizomes were washed, peeled, cut in small pieces, and mixed. Total RNA was isolated using the RNeasy plant mini kit (Qiagen). The peptide sequence YPWSNAQ (part of the N terminus; Supplemental Table S1) was used to create the sense primer PTN (5′-TAYCCNTGGWSNAAYGCNCA-3′), while part of the C terminus (WEMNSAF; Supplemental Table S1) was used to design the antisense primer PTC (5′-AANGCNSWRTTCATYTCCCA-3′). One-step RT-PCR with these primers (Access RT-PCR System; Promega) was performed. The RT reaction was performed at 48°C. PCR conditions were as follows: 94°C for 3 min, followed by 37 cycles of 94°C for 30 s, 50.5°C for 30 s, and 72°C for 2 min. Final extension was at 72°C for 10 min. A smear was detected after agarose gel electrophoresis. Therefore, a second nested PCR was performed on this reaction mixture using conserved GH32-specific primers HFQP (5′-GSWTWYCAYTTYCARCC-3′) and CTERMINV (5′-CCNGTNGCRTTGTTRAA-3′). PCR conditions were as follows: 94°C for 3 min, followed by 30 cycles of 94°C for 30 s, 48°C for 30 s, and 72°C for 2 min. Final extension was at 72°C for 10 min. A clear 1,500-bp band was detected. The PCR mixture was ligated in the TOPO-XL vector and transformed to Escherichia coli (TOPO-XL cloning kit; Invitrogen). Plasmids were extracted using the Qiaprep Spin Miniprep kit (Qiagen), and the insert was sequenced. Two specific forward primers PAF1 (5′-GTGTGGGCTTGGACTAAGGA-3′) and PAF2 (5′-AGCTTTGCTCAGGGTGGAAG-3′) were selected. In a first RT-PCR, PAF1 was combined with an oligo(dT)-based primer. The RT reaction was performed at 48°C. PCR conditions were as follows: 94°C for 3 min, followed by 37 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 2 min. Final extension was at 72°C for 10 min. Since multiple bands were still visible, a seminested PCR was set up with PAF2 and an oligo(dT) primer with identical PCR conditions except that an annealing temperature of 57°C was used and 30 cycles were accomplished. The obtained 500-bp PCR product was amplified, cloned in the TOPO-TA vector (TOPO-TA cloning kit; Invitrogen), and sequenced. To clone the 5′ part of the cDNA, the SMART PCR cDNA synthesis kit (Clontech) was used. Total RNA was isolated as described above, and single-stranded cDNA was produced using the Takara Bio PrimeScript reverse transcriptase. Further manipulations were as described in the manufacturer’s manual. The advantage 2 polymerase kit (Clontech) was used for DNA amplification. In the process, the 5′ SMART primer 5′-CAACGCAGAGTACGCGGG-3′ was combined with the specific 6-SST/6-SFT antisense primer PtR1 (5′-GGATAGGCGAGGCTCAACATCTC-3′). The obtained PCR product (approximately 750 bp) was cloned in the TOPO-TA vector and sequenced. The phylogenetic tree was created with TREEVIEW as described before (Van den Ende et al., 2005).
Heterologous Expression in Pichia pastoris
Because the N-terminal protein part was found (Supplemental Table S1), the exact mature protein encoding region could be selected. An RT-PCR was performed by using the primers PicPtF (5′-AGCTGCAGTTCCGTATCCATGGAGCAACGCT-3′) and PicPtR (5′-CATCTAGATCAACGATGGAAGGGCTGAATG-3′). The Pfu Proofreading polymerase (Promega) was used (annealing temperature 57°C for 35 cycles). Three independently amplified PCR fragments were first subcloned in the TOPO-XL vector and fully sequenced (TOPO-XL cloning kit; Invitrogen). After vector amplification in E. coli, the DNA fragment was cut out with PstI and XbaI and ligated in the pPICZα B vector (Invitrogen). The resulting expression plasmid pPt 6-SST/6-SFT contains the mature protein part in frame behind the α-factor signal sequence. Further handlings and transformation were as described by Van den Ende et al. (2006).
Sequence data from this article can be found in the EMBL data library under accession number FN870376.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Linear Hanes plots derived from the data presented in Figure 7.
Supplemental Figure S2. Long-term in vitro fructan synthesis by the purified native 6-SST/6-SFT at 0°C and at 30°C with 300 mm Suc.
Supplemental Table S1. Peptide masses of the native 6-SST/6-SFT from P. terminalis.
Supplementary Material
Acknowledgments
We thank Veerle Cammaer and Ingeborg Millet for technical assistance.
References
- Altenbach D, Nüesch E, Meyer AD, Boller T, Wiemken A. (2004) The large subunit determines catalytic specificity of barley sucrose:fructan 6-fructosyltransferase and fescue sucrose:sucrose 1-fructosyltransferase. FEBS Lett 567: 214–218 [DOI] [PubMed] [Google Scholar]
- Altenbach D, Ritsema T. (2007) Structure-function relations and evolution of fructosyltransferases. Shiomi N, Benkeblia N, Onodera S, , Recent Advances in Fructooligosaccharides Research. Research Signpost, Kerala, India, pp 135–156 [Google Scholar]
- Altenbach D, Rudiño-Pinera E, Olvera C, Boller T, Wiemken A, Ritsema T. (2009) An acceptor-substrate binding site determining glycosyl transfer emerges from mutant analysis of a plant vacuolar invertase and a fructosyltransferase. Plant Mol Biol 69: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CL, Bremer K, Friis EM. (2005) Dating phylogenetically basal eudicots using rbcL sequences and multiple fossil reference points. Am J Bot 92: 1737–1748 [DOI] [PubMed] [Google Scholar]
- Bancal P, Gibeaut DM, Carpita NC. (1993) Analytical methods for the determination of fructan structure and biosynthesis. Suzuki M, Chatterton NJ, , Science and Technology of Fructans. CRC Press, Boca Raton, FL, pp 83–118 [Google Scholar]
- Bolouri-Moghaddam MR, Le Roy K, Xiang L, Rolland F, Van den Ende W. (2010) Sugar signalling and antioxidant network connections in plant cells. FEBS J 277: 2022–2037 [DOI] [PubMed] [Google Scholar]
- Curtis PD, Rowland ED, Good GL. (2002) Developing a plant-based vole repellent: screening of ten candidate species. Crop Prot 21: 299–306 [Google Scholar]
- De Coninck B, Le Roy K, Francis I, Clerens S, Vergauwen R, Halliday A, Smith SM, Van Laere A, Van den Ende W. (2005) Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant Cell Environ 28: 432–443 [Google Scholar]
- del Viso F, Casabuono AC, Couto AS, Hopp HE, Puebla AF, Heinz RA, Heinz RA. (2010) Functional characterization of a sucrose:fructan 6-fructosyltransferase of the cold-resistant grass Bromus pictus by heterelogous expression in Pichia pastoris and Nicotiana tabacum and its involvement in freezing tolerance. J Plant Physiol (in press) [DOI] [PubMed] [Google Scholar]
- del Viso F, Puebla AF, Fusari CM, Casabuono AC, Couto AS, Pontis HG, Hopp HE, Heinz RA. (2009) Molecular characterization of a putative sucrose:fructan 6-fructosyltransferase (6-SFT) of the cold-resistant Patagonian grass Bromus pictus associated with fructan accumulation under low temperatures. Plant Cell Physiol 50: 489–503 [DOI] [PubMed] [Google Scholar]
- Devkota KP, Lenta BN, Fokou PA, Sewald N. (2008) Terpenoid alkaloids of the Buxaceae family with potential biological importance. Nat Prod Rep 25: 612–630 [DOI] [PubMed] [Google Scholar]
- Dionne J, Rochefort S, Huff DR, Desjardins Y, Bertrand A, Castonguay Y. (2009) Variability for freezing tolerance among 42 Ecotypes of green-type annual bluegrass. Crop Sci 50: 321–336 [Google Scholar]
- Edelman J, Jefford T. (1968) The mechanism of fructosan metabolism in higher plants as exemplified in Helianthus tuberosus. New Phytol 67: 517–531 [Google Scholar]
- Hendry G. (1993) Evolutionary origins and natural functions of fructans. A climatological, biogeographic and mechanistic appraisal. New Phytol 123: 3–14 [Google Scholar]
- Hincha DK, Zuther E, Hellwege EM, Heyer AG. (2002) Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology 12: 103–110 [DOI] [PubMed] [Google Scholar]
- Hincha DK, Zuther E, Heyer AG. (2003) The preservation of liposomes by raffinose family oligosaccharides during drying is mediated by effects on fusion and lipid phase transitions. Biochim Biophys Acta 1612: 172–177 [DOI] [PubMed] [Google Scholar]
- Hisano H, Kanazawa A, Kawakami A, Yoshida M, Shimamoto Y, Yamada T. (2004) Transgenic perennial ryegrass plants expressing wheat fructosyltransferase genes accumulate increased amounts of fructan and acquire increased tolerance on a cellular level to freezing. Plant Sci 167: 861–868 [Google Scholar]
- Hisano H, Kanazawa A, Yoshida M, Humphreys MO, Iizuka M, Kitamura K, Yamada T. (2008) Coordinated expression of functionally diverse fructosyltransferase genes is associated with fructan accumulation in response to low temperature in perennial ryegrass. New Phytol 178: 766–780 [DOI] [PubMed] [Google Scholar]
- Hoot SB, Magallon S, Crane PR. (1999) Phylogeny of basal eudicots based on three molecular data sets: atpB, rbcL, and 18S nuclear ribosomal DNA sequences. Ann Mo Bot Gard 86: 1–32 [Google Scholar]
- Ji X, Shiran B, Wan J, Lewis DC, Jenkins CL, Condon AG, Richards RA, Dolferus R. (2010) Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ 33: 926–942 [DOI] [PubMed] [Google Scholar]
- Ji X, Van den Ende W, Schroeven L, Clerens S, Geuten K, Cheng S, Bennett J. (2007) The rice genome encodes two vacuolar invertases with fructan exohydrolase activity but lacks the related fructan biosynthesis genes of the Pooideae. New Phytol 173: 50–62 [DOI] [PubMed] [Google Scholar]
- Jiao Z, Li J. (2009) Phylogenetics and biogeography of eastern Asian–North American disjunct genus Pachysandra (Buxaceae) inferred from nucleotide sequences. J Syst Evol 47: 191–201 [Google Scholar]
- Kawakami A, Sato Y, Yoshida M. (2008) Genetic engineering of rice capable of synthesizing fructans and enhancing chilling tolerance. J Exp Bot 59: 803–814 [DOI] [PubMed] [Google Scholar]
- Kinghorn AD, Su BN, Jang DS, Chang LC, Lee D, Gu JQ, Carcache-Blanco EJ, Pawlus AD, Lee SK, Park EJ, et al. (2004) Natural inhibitors of carcinogenesis. Planta Med 70: 691–705 [DOI] [PubMed] [Google Scholar]
- Koops AJ, Jonker HH. (1994) Purification and characterization of the enzymes of fructan biosynthesis in tubers of Helianthus tuberosus Colombia 1. Fructan-fructan fructosyl transferase. J Exp Bot 45: 1623–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koops AJ, Jonker HH. (1996) Purification and characterisation of the enzymes of fructan biosynthesis in tubers of Helianthus tuberosus Colombia 2. Purification of sucrose:sucrose 1-fructosyltransferase and reconstitution of fructan synthesis in vitro with purified sucrose:sucrose 1-fructosyltransferase and fructan:fructan 1-fructosyltransferase. Plant Physiol 110: 1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens W, Le Roy K, Schroeven L, Van Laere A, Rabijns A, Van den Ende W. (2009) Structural insights into GH32 and GH68 enzymes: functional implications. J Exp Bot 60: 727–740 [DOI] [PubMed] [Google Scholar]
- Lasseur B, Lothier J, Djoumad A, De Coninck B, Smeekens S, Van Laere A, Morvan-Bertrand A, Van den Ende W, Prud’homme MP. (2006) Molecular and functional characterization of a cDNA encoding fructan:fructan 6G-fructosyltransferase (6G-FFT)/fructan:fructan 1-fructosyltransferase (1-FFT) from perennial ryegrass (Lolium perenne L.). J Exp Bot 57: 2719–2734 [DOI] [PubMed] [Google Scholar]
- Lasseur B, Schroeven L, Lammens W, Le Roy K, Spangenberg G, Manduzio H, Vergauwen R, Lothier J, Prud’homme MP, Van den Ende W. (2009) Transforming a fructan:fructan 6G-fructosyltransferase from perennial ryegrass into a sucrose:sucrose 1-fructosyltransferase. Plant Physiol 149: 327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy K, Lammens W, Verhaest M, De Coninck B, Rabijns A, Van Laere A, Van den Ende W. (2007) Unraveling the difference between invertases and fructan exohydrolases: A single amino acid (Asp-239) substitution transforms Arabidopsis cell wall invertase1 into a fructan 1-exohydrolase. Plant Physiol 145: 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Yang AF, Zhang XC, Gao F, Zhang JR. (2007) Improving freezing tolerance of transgenic tobacco expressing sucrose:sucrose 1-fructosyltransferase gene from Lactuca sativa. Plant Cell Tissue Organ Cult 89: 37–48 [Google Scholar]
- Livingston DP, III, Henson CA. (1998) Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol 116: 403–408 [Google Scholar]
- Livingston DP, III, Hincha DK, Heyer AG. (2009) Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol Life Sci 66: 2007–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothier J, Lasseur B, Le Roy K, Van Laere A, Prud’homme MP, Barre P, Van den Ende W, Morvan-Bertrand A. (2007) Cloning, gene mapping, and functional analysis of a fructan 1-exohydrolase (1-FEH) from Lolium perenne implicated in fructan synthesis rather than in fructan mobilization. J Exp Bot 58: 1969–1983 [DOI] [PubMed] [Google Scholar]
- Maleux K, Van den Ende W. (2007) Levans in excised leaves of Dactylis glomerata: effects of light, sugars, temperature and senescence. J Plant Biol 50: 671–675 [Google Scholar]
- Nishizawa A, Yabuta Y, Shigeoka S. (2008) Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 147: 1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvanova D, Ivanov S, Konstantinova T, Karanov E, Atanassov A, Tsvetkov T, Alexieva V, Djilianov D. (2004) Transgenic tobacco plants accumulating osmolytes show reduced oxidative damage under freezing stress. Plant Physiol Biochem 42: 57–63 [DOI] [PubMed] [Google Scholar]
- Prud’homme MP, Morvan-Bertrand A, Lasseur B, Lothier J, Meuriot F, Decau ML, Noiraud-Romy N. (2007) Lolium perenne, backbone of sustainable development, source of fructans for grazing animals and potential source of novel enzymes. Shiomi N, Benkeblia N, Onodera S, , Recent Advances in Fructooligosaccharides Research. Research Signpost, Kerala, India, pp 231–258 [Google Scholar]
- Ritsema T, Hernández L, Verhaar A, Altenbach D, Boller T, Wiemken A, Smeekens S. (2006) Developing fructan-synthesizing capability in a plant invertase via mutations in the sucrose-binding box. Plant J 48: 228–237 [DOI] [PubMed] [Google Scholar]
- Roberfroid MB. (2007) Prebiotics: the concept revisited. J Nutr 137(3, Suppl 2) 830S–837S [DOI] [PubMed] [Google Scholar]
- Roberfroid MB, Delzenne NM. (1998) Dietary fructans. Annu Rev Nutr 18: 117–143 [DOI] [PubMed] [Google Scholar]
- Sandve SR, Fjellheim S. (2010) Did gene family expansions during the Eocene-Oligocene boundary climate cooling play a role in Pooideae adaptation to cool climates? Mol Ecol 19: 2075–2088 [DOI] [PubMed] [Google Scholar]
- Schroeven L, Lammens W, Kawakami A, Yoshida M, Van Laere A, Van den Ende W. (2009) Creating S-type characteristics in the F-type enzyme fructan:fructan 1-fructosyltransferase of Triticum aestivum L. J Exp Bot 60: 3687–3696 [DOI] [PubMed] [Google Scholar]
- Schroeven L, Lammens W, Van Laere A, Van den Ende W. (2008) Transforming wheat vacuolar invertase into a high affinity sucrose:sucrose 1-fructosyltransferase. New Phytol 180: 822–831 [DOI] [PubMed] [Google Scholar]
- Simmen U, Obenland D, Boller T, Wiemken A. (1993) Fructan synthesis in excised barley leaves (Identification of two sucrose:sucrose fructosyltransferases induced by light and their separation from constitutive invertases). Plant Physiol 101: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger N, Bortlik K, Brandt A, Boller T, Wiemken A. (1995) Purification, cloning, and functional expression of sucrose:fructan 6-fructosyltransferase, a key enzyme of fructan synthesis in barley. Proc Natl Acad Sci USA 92: 11652–11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanova S, Geuns J, Hideg E, Van den Ende W. (2010) The food additives inulin and stevioside counteract oxidative stress. Int J Food Sci Nutr (in press) [DOI] [PubMed] [Google Scholar]
- Tamura K, Kawakami A, Sanada Y, Tase K, Komatsu T, Yoshida M. (2009) Cloning and functional analysis of a fructosyltransferase cDNA for synthesis of highly polymerized levans in timothy (Phleum pratense L.). J Exp Bot 60: 893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valluru R, Lammens W, Claupein W, Van den Ende W. (2008) Freezing tolerance by vesicle-mediated fructan transport. Trends Plant Sci 13: 409–414 [DOI] [PubMed] [Google Scholar]
- Valluru R, Van den Ende W. (2008) Plant fructans in stress environments: emerging concepts and future prospects. J Exp Bot 59: 2905–2916 [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Clerens S, Vergauwen R, Boogaerts D, Le Roy K, Arckens L, Van Laere A. (2006) Cloning and functional analysis of a high DP 1-FFT from Echinops ritro. Comparison of the native and recombinant enzymes. J Exp Bot 57: 775–789 [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Clerens S, Vergauwen R, Van Riet L, Van Laere A, Yoshida M, Kawakami A. (2003b) Fructan 1-exohydrolases. β (2,1) trimmers during graminan biosynthesis in stems of wheat (Triticum aestivum L.)? Purification, characterization, mass mapping and cloning of two fructan 1-exohydrolase isoforms. Plant Physiol 131: 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W, De Coninck B, Clerens S, Vergauwen R, Van Laere A. (2003a) Unexpected presence of fructan 6-exohydrolases (6-FEHs) in non-fructan plants: characterization, cloning, mass mapping and functional analysis of a novel “cell-wall invertase-like” specific 6-FEH from sugar beet (Beta vulgaris L.). Plant J 36: 697–710 [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Lammens W, Van Laere A, Schroeven L, Le Roy K. (2009) Donor and acceptor substrate selectivity among plant glycoside hydrolase family 32 enzymes. FEBS J 276: 5788–5798 [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Michiels A, Le Roy K, Van Laere A. (2002) Cloning of a vacuolar invertase from Belgian endive leaves (Cichorium intybus). Physiol Plant 115: 504–512 [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Michiels A, Van Wonterghem D, Clerens SP, De Roover J, Van Laere AJ. (2001) Defoliation induces fructan 1-exohydrolase II in Witloof chicory roots. Cloning and purification of two isoforms, fructan 1-exohydrolase IIa and fructan 1-exohydrolase IIb. Mass fingerprint of the fructan 1-exohydrolase II enzymes. Plant Physiol 126: 1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W, Valluru R. (2009) Sucrose, sucrosyl oligosaccharides, and oxidative stress: scavenging and salvaging? J Exp Bot 60: 9–18 [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Van Laere A. (2007) Fructans in dicotyledonous plants: Occurrence and metabolism. Shiomi N, Benkeblia N, Onodera S, , Recent Advances in Fructooligosaccharides Research. Research Signpost, Kerala, India, pp 1–14 [Google Scholar]
- Van den Ende W, Van Wonterghem D, Dewil E, Verhaert P, De Loof A, Van Laere A. (1996a) Purification and characterization of 1-SST, the key enzyme initiating fructan biosynthesis in young chicory roots (Cichorium intybus L.). Physiol Plant 98: 455–466 [Google Scholar]
- Van den Ende W, Van Wonterghem D, Verhaert P, Dewil E, Van Laere A. (1996b) Purification and characterization of fructan:fructan fructosyl transferase from chicory (Cichorium intybus L) roots. Planta 199: 493–502 [Google Scholar]
- Van den Ende W, Yoshida M, Clerens S, Vergauwen R, Kawakami A. (2005) Cloning, characterization and functional analysis of novel 6-kestose exohydrolases (6-KEHs) from wheat (Triticum aestivum). New Phytol 166: 917–932 [DOI] [PubMed] [Google Scholar]
- Vanhaecke M, Van den Ende W, Van Laere A, Herdewijn P, Lescrinier E. (2006) Complete NMR characterization of lychnose from Stellaria media (L.) Vill. Carbohydr Res 341: 2744–2750 [DOI] [PubMed] [Google Scholar]
- Van Riet L, Nagaraj V, Van den Ende W, Clerens S, Wiemken A, Van Laere A. (2006) Purification, cloning and functional characterization of a fructan 6-exohydrolase from wheat (Triticum aestivum L.). J Exp Bot 57: 213–223 [DOI] [PubMed] [Google Scholar]
- Van Waes C, Baert J, Carlier L, Van Bockstaele E. (1998) A rapid determination of the total sugar content and the average inulin chain length in roots of chicory (Cichorium intybus L). J Sci Food Agric 76: 107–110 [Google Scholar]
- Vereyken IJ, Chupin V, Demel RA, Smeekens SCM, De Kruijff B. (2001) Fructans insert between the headgroups of phospholipids. Biochim Biophys Acta 1510: 307–320 [DOI] [PubMed] [Google Scholar]
- Vergauwen R, Van den Ende W, Van Laere A. (2000) The role of fructan in flowering of Campanula rapunculoides. J Exp Bot 51: 1261–1266 [PubMed] [Google Scholar]
- Vijn I, Smeekens S. (1999) Fructan: more than a reserve carbohydrate? Plant Physiol 120: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Thomas H, Lelièvre F. (1998) Survival and recovery of perennial forage grasses under prolonged Mediterranean drought. I. Growth, death, water relations and solute content in herbage and stubble. New Phytol 140: 439–449 [DOI] [PubMed] [Google Scholar]
- von Balthazar M, Endress PK, Qiu YL. (2000) Phylogenetic relationships in Buxaceae based on nuclear internal transcribed spacers and plastid ndhF sequences. Int J Plant Sci 161: 785–792 [Google Scholar]
- Wei JZ, Chatterton NJ. (2001) Fructan biosynthesis and fructosyltransferase evolution: expression of the 6-SFT (sucrose:fructan 6-fructosyltransferase) gene in crested wheatgrass (Agropyron cristatum). J Plant Physiol 158: 1203–1213 [Google Scholar]
- Wiemken A, Frehner M, Keller F, Wagner W. (1986) Fructan metabolism, enzymology and compartmentation. Curr Top Plant Biochem Physiol 5: 17–37 [Google Scholar]
- Yamada K, Kojima T, Bantog N, Shimoda T, Mori H, Shiratake K, Yamaki S. (2007) Cloning of two isoforms of soluble acid invertase of Japanese pear and their expression during fruit development. J Plant Physiol 164: 746–755 [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kawakami A, Van den Ende W. (2007) Graminan metabolism in cereals: wheat as a model system. Shiomi N, Benkeblia N, Onodera S, , Recent Advances in Fructooligosaccharides Research. Research Signpost, Kerala, India, pp 201–212 [Google Scholar]
- Zhou SP, Sauve RJ, Mmbaga MT. (2005) Adaptation of Pachysandra terminalis Sieb. & Zucc. to freezing temperatures by the accumulation of mRNA and cold-induced proteins. HortScience 40: 346–347 [Google Scholar]
- Zhu JJ, Beck E. (1991) Water relations of Pachysandra leaves during freezing and thawing. Plant Physiol 97: 1146–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.