It has long been recognized that photosynthesis and respiration in the plant cell must be intimately linked, given that they share carbon dioxide and oxygen as substrate and product or as product and substrate, respectively (for review, see Siedow and Day, 2000). While the core reaction schemes of the pathways of photosynthesis, respiration, and the associated process of photorespiration are well defined, it is only since the advent and widespread adoption of reverse genetic strategies that the high level of interaction between them has begun to be fully realized (Bauwe et al., 2010; Sweetlove et al., 2010). However, the exact contribution of each pathway to energy status is dependent on cell type, and fundamental questions such as the degree of inhibition of the tricarboxylic acid (TCA) cycle in the light remain somewhat controversial. Here, we will outline current understanding of the influence of mitochondrial function, focusing almost exclusively on the illuminated leaf of C3 plants and taking the majority of our case studies from tomato (Solanum lycopersicum) and Arabidopsis (Arabidopsis thaliana). We contend that, having achieved a more comprehensive understanding of the interaction between photosynthesis and respiration (and indeed also photorespiration), the manipulation of mitochondrial metabolism and machinery has recently emerged as a novel potential means to enhance photosynthesis.
THE NEED FOR COORDINATION OF CELLULAR ENERGY METABOLISM
Given that, as stated above, the pathways of photosynthesis and respiration almost catalyze opposite reactions, it follows that their relative activities must be carefully regulated within plant cells. The same is true for photorespiration, which, although being obligatorily linked to photosynthesis since its entrance reaction is the transfer of oxygen onto ribulose 1,5-bisphosphate by the action of Rubisco, it can also dramatically restrict photosynthesis if it is itself restricted under conditions in which its intermediate metabolites accumulate (Bauwe et al., 2010). While the coordination of these pathways has been presumed for decades, our understanding of the precise details of how this is achieved is currently fragmentary. In some plant tissues, such as roots, the complete reliance of mitochondrial oxidative phosphorylation to meet the energy demands of the cell greatly simplifies matters. However, recent demonstrations of photosynthesis occurring in germinating seeds and the fact that some enzymes of the photorespiratory pathway are expressed in a root-specific fashion (Borisjuk and Rolletschek, 2009; https://www.genevestigator.com) mean that such generalizations should not be blithely assumed. A similar caution needs to be taken regarding the assumed inhibition of the TCA cycle in the illuminated leaf. There is some conflict between in vitro enzyme measurements (Tovar-Méndez et al., 2003) and even detailed flux profiles (Tcherkez et al., 2005), with the results from transgenic plants described below in the section “Altering Photosynthetic Carbon Assimilation and Yield by Targeting the TCA Cycle.” What is undoubtedly sure is that the TCA cycle flux is reduced in the illuminated leaf when compared with that seen in the dark. However, it seems likely that this can be explained in part by the operation of different flux modes in the light (Sweetlove et al., 2010).
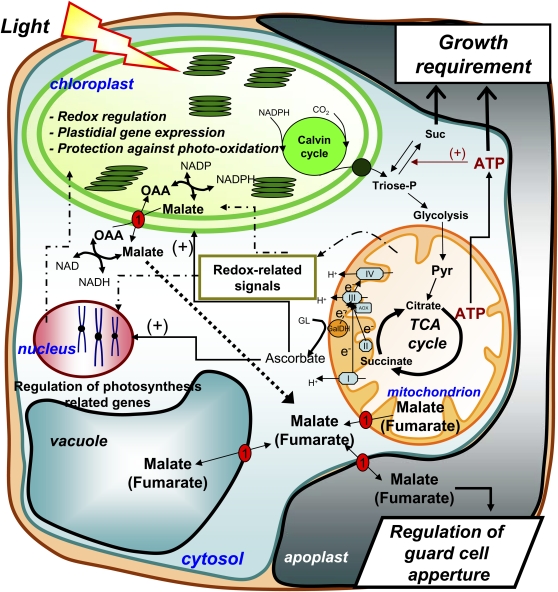
Photosynthesis directly provides substrate for mitochondrial reactions occurring in the illuminated leaf. In heterotrophic tissues and the darkened leaf, substrates from photosynthesis are provided indirectly via storage pools. While these facts have long been established, the converse impact of mitochondria on photosynthesis has only been demonstrated more recently. For this reason, together with the fact that this interaction probably involves signal transduction and redox changes alongside the changes in substrate provision and gene expression that link photosynthate production to its utilization, it is unlikely that our understanding of this interaction is complete. That said, considerable advances have been made in the last few decades, and the suggested putative associated regulatory linkages linking mitochondrial function and photosynthetic efficiency are presented in Figure 1. This Update gives a broad overview illustrated with a few case studies. One of the first roles proposed for the mitochondria during photosynthesis was that they supplied a large proportion of the ATP required to sustain the high rates of Suc synthesis (Krömer et al., 1993). Partial support that this is indeed true was determined by measuring decreased cytosolic levels of ATP following the inhibition of mitochondrial function. However, clear genetic evidence for such a role has yet to be provided, with conflicting results being documented for the cytoplasmic male sterile (CMSII) mutant of tobacco (Nicotiana sylvestris) and the Aco1 mutant of Solanum pennellii (described in detail below). Second, evidence has accumulated that certain reactions of the mitochondrial TCA cycle are crucial to support cytosolic nitrate accumulation (Nunes-Nesi et al., 2007b). However, the results of a recent study in Brassicus napus question the absolute requirement of current nitrate assimilation during photosynthesis (Gauthier et al., 2010). Given the lack of consensus in response to these hypotheses, it is perhaps not surprising that other roles have also been postulated. These include the regulation of metabolite distribution as a means to balance cellular redox status (Raghavendra and Padmasree, 2003; Scheibe et al., 2005), a signaling function coordinating energy metabolism (Nunes-Nesi et al., 2007b), buffering of metabolism by the photorespiratory process (Bauwe et al., 2010), and mitochondrially mediated retrograde signaling (Zarkovic et al., 2005). Given that the last two of these have recently been reviewed in the literature, we will not cover them in any detail here. However, the processes by which they are thought to operate are also indicated in Figure 1.
Figure 1.
Summary of major interactions and consequences of alterations on mitochondrial activity on photosynthesis. Plants obtain the energy they require for growth from ATP produced through mitochondrial respiration and photosynthesis. The export of excess NAD(P)H through the “malate valve” will allow the production of ATP during both photosynthesis and oxidative phosphorylation. The ATP is also needed for the conversion of triose phosphate to Suc. Additionally, the malate (and fumarate) produced by the TCA cycle is transported to the vacuole, where it is stored. By a yet unclear mechanism, the mitochondrial function triggers stomatal movement by controlling organic acid levels in both the vacuole and the apoplast, leading to a relative control of carbon dioxide assimilation. It is also hypothesized that an increased activity of l-galactono-1,4-lactone dehydrogenase, which catalyzes the conversion of l-galactono-1,4-lactone to ascorbate and is coupled to the mitochondrial electron transport chain, leads to an up-regulation of photosynthesis by an unclear mechanism involving the modulation of gene expression in both cytosol and chloroplast, redox regulation, or merely efficient removal of photosynthate to support growth requirements. The dotted arrow represents an unknown mechanism. AOX, Alternative oxidase; e-, electron; GalDH, l-galactono-1,4-lactone dehydrogenase; GL, l-galactono-1,4-lactone; OAA, oxaloacetic acid; Pyr, pyruvate; 1, dicarboxylate transporter; I, II, III, and IV, cytochrome pathway complex of the mitochondrial electron transport chain.
We will focus on the first two of these postulated processes, given their clear importance in explaining some of the results following genetic intervention (detailed below). The regulation of metabolite distribution as a means to balance cellular redox status is a long-established mechanism of metabolic and photosynthetic control based on the operation of the “so-called” malate valve, which effectively operates as an indirect export system for reducing equivalents. This functions due to the operation of the chloroplastic NADP-malate dehydrogenase, which is under the control of the ferredoxin-thioredoxin system and uses excess NADPH to convert oxaloacetic acid into malate in order to regenerate the electron acceptor NADP. Since this electron transfer to malate facilitates the continuous production of ATP, this shunt effectively operates as a redox regulator of photosynthesis (Backhausen et al., 1998). Thus far, we have only considered the chloroplast and cytosol in isolation. However, the effectiveness of this mechanism is directly dependent on the maintenance of a malate gradient from chloroplast to cytosol. Given the complex compartmentation of malate (Meyer et al., 2010), it thus follows that the interaction between mitochondrial, vacuolar, and peroxisomal malate pools, and even that of the apoplast with the cytosolic pool, will be important in facilitating or indeed limiting photosynthesis by allowing or halting the regeneration of NADP. A second, redox-related mechanism linking mitochondrial function to that of the plastid is that mediated by ascorbate. Much is known concerning the importance of ascorbate within photosynthesis, where it plays a multifaceted role, acting in the Mehler peroxidase reaction to regulate the redox state of photosynthetic electron carriers and as a cofactor for violaxanthin de-epoxidase, an enzyme involved in xanthophyll cycle-mediated photoprotection, as well as in controlling guard cell signaling and stomatal movement and the expression levels of both nuclear and chloroplastic components of the photosynthetic apparatus (Nunes-Nesi et al., 2005). Recently, it was identified that the terminal enzyme of ascorbate biosynthesis, l-galactono-1,4-lactone dehydrogenase, is coupled to the cytochrome pathway (Bartoli et al., 2000) and that, on inhibition of the TCA cycle, can be engaged as an alternative electron donor to the mitochondrial electron transport chain (Nunes-Nesi et al., 2005; Dinakar et al., 2010). Furthermore, in the same study, loading of photosynthetic cells with exogenously supplied ascorbate was demonstrated to result in an elevated net carbon dioxide assimilation rate (Nunes-Nesi et al., 2005). However, despite clear evidence of a bridging role of ascorbate between mitochondrial and plastidial functions, the exact mechanistic details underlying this remain to be fully elucidated.
HORSES FOR COURSES: CELL TYPE AND ENVIRONMENTAL VARIANCE IN THE CELLULAR DEPENDENCE OF THE MAJOR PATHWAYS OF ENERGY METABOLISM
Before moving on to describing how photosynthesis has been modified following the manipulation of mitochondrial processes, it is prudent to comment on another layer of complexity that will, in the future, need to be resolved in order to allow fine-tuning of photosynthesis. This is the diversity of cell types in plants. There are over 40 different cell types in a typical plant species, and the energy requirements of these differ substantially, as does the reliance on the different pathways of energy metabolism. These differences are governed by factors including, among others, the level of solar irradiance they are exposed to, the growth rate of the tissue, the bulk of the surrounding tissue, the substrate storage capacity of the cell, the relative health of the cell, and many other factors. As a result, energy metabolism is tailored to the specific demands of the cell and its environmental circumstances. The case of the inhibition of the TCA cycle in the illuminated leaf is described above. However, factors such as partial pressure of gasses in the environment, temperature, and a wide range of stresses also impact on the interplay between photosynthesis and respiration. In C4 and Crassulacean acid metabolism plants, additional mechanisms are in place that considerably alter the pattern of energy metabolism in leaves; however, these are largely outside the scope of this article. Since characteristics of C4 metabolism are found in the stems and petioles of C3 plants, it needs to be considered in the context of C3 metabolism (Hibberd and Quick, 2002). Recent work of the Hibberd laboratory has illustrated that C4 acid decarboxylases required for C4 photosynthesis are active in the midvein of the C3 species Arabidopsis and are important in sugar and amino acid metabolism (Brown et al., 2010). They were also able to reduce chlorophyll biosynthesis specifically in cells close to leaf veins of this species by using an enhancer trap system and showed that photosynthesis in these cells played an important role for the shikimate pathway and in leaf senescence as well as contributing to plant fitness (Janacek et al., 2009). It seems likely that further use of such cell type-specific modifications, as well as comparative deep sequencing such as that carried out by Bräutigam et al. (2011), will allow us to dramatically enhance our understanding of how diverse cells coordinate photosynthetic metabolism at a functional level.
ALTERING PHOTOSYNTHETIC CARBON ASSIMILATION AND YIELD BY TARGETING THE TCA CYCLE
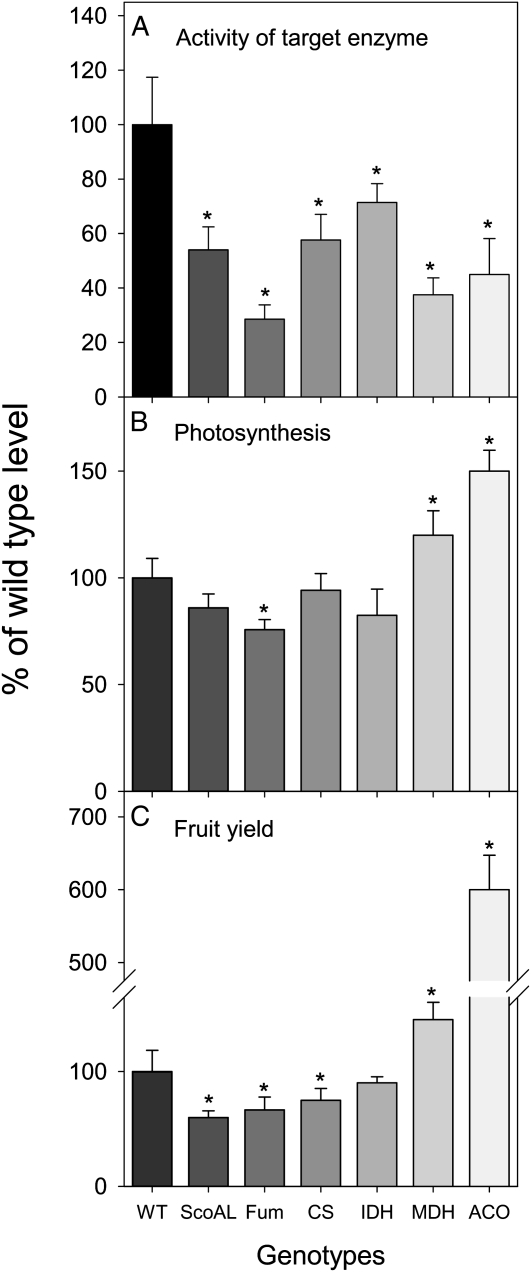
While reverse genetic approaches to modify the expression of constituent enzymes of the TCA cycle were adopted in the later 1980s (for review, see Fernie et al., 2004), early studies on this pathway concentrated on the importance of this pathway in plant fertility and the uptake of nutrients from the soil. In 2003, however, studies of the wild species tomato (Solanum pennellii) mutant Aco1, deficient in the expression of aconitase, demonstrated a 50% increase in the rate of carbon dioxide assimilation and a 6-fold increase in fruit yield (Carrari et al., 2003; Fig. 2). The increase in fruit yield, while greater than any other recorded following manipulation of enzymes of the TCA cycle, must be viewed with caution due to two peculiarities of S. pennellii. First, fruits of this species are considerably smaller than those of cultivated tomato (S. lycopersicum), and second, this species displays dramatically elevated activities of aconitase in comparison with the cultivated tomato (Steinhauser et al., 2010). Antisense inhibition of the mitochondrial malate dehydrogenase also enhanced the rate of carbon dioxide assimilation (Nunes-Nesi et al., 2005), although only by 20%. By contrast, the assimilation rates in lines deficient in fumarase were reduced to 75% of wild-type rates (Nunes-Nesi et al., 2007a), while tomato plants deficient in the expression of succinyl-CoA ligase, mitochondrial citrate synthase, and NAD-dependent isocitrate dehydrogenase (Studart-Guimarães et al., 2007; Sienkiewicz-Porzucek et al., 2008, 2010) exhibited no effect. While it is not possible to drawn a universal conclusion across the genotypes, since this relationship is additionally complicated by alterations in shoot-root partitioning of the genotypes (van der Merwe et al., 2009), the fruit yield of the genotypes at least partially reflects their leaf photosynthetic capabilities. Furthermore, it should be noted that succinyl-CoA ligase-deficient plants were greatly compromised with respect to fruit yield despite displaying essentially unaltered rates of carbon dioxide assimilation (Fig. 2C). Moreover, the effects of the various genetic manipulations are not greatly influenced by the degree of inhibition of the target enzyme. Accordingly, while the reduction in fumarase activity was the most dramatic and displayed the most detrimental effect on photosynthesis, the relationship between the degree of loss of activity in the transgenic lines did not correlate (either positively or negatively) with their carbon assimilation rates. Going beyond tomato plants, this statement is further supported by the lack of an observed growth phenotype of Arabidopsis knockout mutants for the NAD-dependent isocitrate dehydrogenase (Lemaitre et al., 2007) and by potato (Solanum tuberosum) plants exhibiting reduced expression of mitochondrial citrate synthase (Landschütze et al., 1995).
Figure 2.
Characteristics of tomato plants deficient in the enzymes of the TCA cycle. A, Activity of the target enzyme in the tomato transgenic lines. B, Assimilation rate at 1,000 μmol m−2 s−1. C, Fruit yield based on total dry weight accumulated in the fruits at the end of development. For each set of transgenics, one of the moderately inhibited lines was chosen. For full details, see the respective references. WT, Wild type (S. lycopersicum ‘Moneymaker’); ScoAL, succinyl-CoA ligase; Fum, fumarase; CS, citrate synthase; IDH, isocitrate dehydrogenase; MDH, malate dehydrogenase; ACO, aconitase. The lines used were as follows: succinyl-CoA ligase, RL40; fumarase, FL11; citrate synthase, CS22; isocitrate dehydrogenase, IDH4; malate dehydrogenase, AL21; aconitase, Aco-1. Asterisks indicate values that were determined by Student’s t test to be significantly different (P < 0.05) from the respective wild type.
Intriguingly, but perhaps not surprisingly, given the broad number of associations between respiration and photosynthesis and the centrality of the TCA cycle as a metabolic hub, it is clear that more than one mechanism underlies the changes in photosynthetic performance described above. In the case of fumarase, the carbon dioxide assimilation is decreased to 75% of that observed in wild-type plants, with fumarase antisense plants additionally displaying impaired stomatal opening (Nunes-Nesi et al., 2007a). Detailed physiological, biochemical, and gene expression studies have revealed that this is most likely mediated via the uptake of malate and/or fumarate by the guard cell from its surrounding mesophyll cells. Conversely, the enhanced rates of photosynthesis evidenced in the Aco1 and mitochondrial malate dehydrogenase plants appear to be regulated by changes in redox status, most likely relayed by ascorbate, the terminal biosynthetic enzyme of which is associated with the mitochondrial cytochrome pathway (Bartoli et al., 2000). However, the exact details concerning how photosynthesis is up-regulated in these plants are as yet unclear, largely due to the complications in dissecting the many possible functions of this enigmatic metabolite. Irrespective of the molecular mechanisms that confer the phenotypes described here, it is clear that genetic manipulation of certain steps of the TCA cycle could prove to be a worthy route to explore in an attempt to enhance photosynthesis and crop yield. To place this in a broader context, the maximal increase in assimilation documented for this approach, 50%, is slightly higher than that observed following the overexpression of a cyanobacterial Fru-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco (Miyagawa et al., 2001) and is similar in magnitude to that observed following the introduction of a chloroplastic photorespiratory bypass (Kebeish et al., 2007).
ALTERING PHOTOSYNTHETIC CARBON ASSIMILATION BY OTHER MITOCHONDRIAL MANIPULATIONS
It is not only the alteration of enzymes of the TCA cycle that causes an effect on the rate of photosynthesis but also the alteration of several members of the mitochondrial electron transport chain. Arguably, the clearest effects were observed following the biochemical and physiological identification of a T-DNA insertional mutant of Arabidopsis deficient in the expression of the uncoupling protein AtUCP1, which revealed a specific inhibition of photorespiration (Sweetlove et al., 2006). Uncoupling proteins are integral to the inner mitochondrial membrane and function to dissipate the mitochondrial gradient as heat. It has been postulated that this is especially important when the demand for oxidation of NADH is high and thus may potentiate high TCA cycle flux (Smith et al., 2004). Consistent with this suggestion, the ucp1 mutants displayed dramatically reduced rates of carbon dioxide assimilation linked to a reduced rate of photorespiratory Gly oxidation (Sweetlove et al., 2006). Intriguingly, similar uncoupling roles during photorespiration were also suggested for the alternative oxidase (Bartoli et al., 2005; Strodtkötter et al., 2009) and the internal NADH dehydrogenases (Escobar et al., 2004). Both of these protein systems could allow electron transport without proton translocation and therefore could fulfill the same role as the UCP, albeit by employing a different means to the same end. The fact that both nonphosphorylating bypasses and the UCP are required for the same function suggests that it is of very high importance for maintaining the energy balance of the cell. Evaluation of plants deficient in any of the components suggests that neither mechanism is completely effective in doing so. This is not surprising, since UCP will lead to a partial dissipation of the proton gradient but will not completely remove the thermodynamic constraints on electron flux (Sweetlove et al., 2006). Similarly, flux through the alternative pathway is never absolute, since the alternative oxidase is in competition for electrons with complex III of the cytochrome pathway. Effects on photosynthesis, however, are not restricted to the alternative pathway of respiration. Indeed, the tobacco mutant CMSII, which lacks functional complex I, also exhibited reduced photosynthesis during photorespiratory conditions via a mechanism highly similar to that observed in the Arabidopsis ucp1 mutants (Dutilleul et al., 2003). It is thus clear that components of the mitochondrial electron transport chain are essential for the proper maintenance of intracellular redox gradients (Fig. 1), to allow considerable rates of photorespiration and in turn efficient photosynthesis. From the perspective of enhancing photosynthesis, as yet, only alternative oxidase has a demonstrated role. Bartoli et al. (2005) demonstrated that the natural up-regulation of this protein during drought stress in wheat (Triticum aestivum) protected the plants against the loss of photosynthetic capacity compared with plants chemically inhibited in this activity. Whether a robust increase in photosynthetic capacity and plant performance in either ambient or stress conditions could be achieved by modifying any of these proteins by genetic means remains to be tested.
In summary, although the last few years have seen considerable advances in the understanding of the interaction between mitochondrial and extramitochondrial metabolism, and in the identification of some of the key players orchestrating these interactions, our knowledge remains far from complete. For example, we are still lacking the molecular identity of many of the transporter proteins required to mediate the operation of the major pathways of energy metabolism (Reumann and Weber, 2006). Our understanding of signal transduction cascades and of retrograde signaling from the mitochondria and/or chloroplast also remains fragmentary (Kleine et al., 2009). Moreover, the role of mitochondrial metabolism in C4 and Crassulacean acid metabolism plants as well as plants exhibiting mixed photosynthetic metabolism has yet to be determined. That said, nascent developments aimed at increasing the sophistication of approaches to gain high resolution of metabolism at both spatial and temporal levels are occurring (Janacek et al., 2009; Niittylae et al., 2009; Brown et al., 2010). Despite the fact that the manipulation of various steps of mitochondrial metabolism has been demonstrated to alter photosynthesis under ambient conditions, it is likely that these changes, by and large, will not be maintained under extreme environments, and producing plants to meet this aim remains a critical challenge.
References
- Backhausen JE, Emmerlich A, Holtgrefe S, Horton P, Nast G, Roggers JJM, Müller-Röber B, Scheibe R. (1998) Transgenic potato plants with altered levels of chloroplast NADP-malate dehydrogenase: interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta 207: 105–114 [Google Scholar]
- Bartoli CG, Gomez F, Gergoff G, Guiamét JJ, Puntarulo S. (2005) Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. J Exp Bot 56: 1269–1276 [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH. (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123: 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwe H, Hagemann M, Fernie AR. (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15: 330–336 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H. (2009) The oxygen status of the developing seed. New Phytol 182: 17–30 [DOI] [PubMed] [Google Scholar]
- Bräutigam A, Kajala K, Wullenweber J, Sommer M, Gagneul D, Weber KL, Carr KM, Gowik U, Maß J, Lercher MJ, et al. (2011) An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiol 155: 142–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NJ, Palmer BG, Stanley S, Hajaji H, Janacek SH, Astley HM, Parsley K, Kajala K, Quick WP, Trenkamp S, et al. (2010) C acid decarboxylases required for C photosynthesis are active in the mid-vein of the C species Arabidopsis thaliana, and are important in sugar and amino acid metabolism. Plant J 61: 122–133 [DOI] [PubMed] [Google Scholar]
- Carrari F, Nunes-Nesi A, Gibon Y, Lytovchenko A, Loureiro ME, Fernie AR. (2003) Reduced expression of aconitase results in an enhanced rate of photosynthesis and marked shifts in carbon partitioning in illuminated leaves of wild species tomato. Plant Physiol 133: 1322–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakar C, Abhaypratap V, Yearla SR, Raghavendra AS, Padmasree K. (2010) Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta 231: 461–474 [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Garmier M, Noctor G, Mathieu C, Chétrit P, Foyer CH, de Paepe R. (2003) Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell 15: 1212–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar MA, Franklin KA, Svensson AS, Salter MG, Whitelam GC, Rasmusson AG. (2004) Light regulation of the Arabidopsis respiratory chain: multiple discrete photoreceptor responses contribute to induction of type II NAD(P)H dehydrogenase genes. Plant Physiol 136: 2710–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Carrari F, Sweetlove LJ. (2004) Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol 7: 254–261 [DOI] [PubMed] [Google Scholar]
- Gauthier PPG, Bligny R, Gout E, Mahe A, Nogues S, Hodges M, Tcherkez GGB. (2010) In folio isotopic tracing demonstrates that nitrogen assimilation into glutamate is mostly independent from current carbon dioxide assimilation into glutamate in illuminated leaves of Brassica napus. New Phytol 185: 988–999 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Quick WP. (2002) Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415: 451–454 [DOI] [PubMed] [Google Scholar]
- Janacek SH, Trenkamp S, Palmer B, Brown NJ, Parsley K, Stanley S, Astley HM, Rolfe SA, Paul Quick W, Fernie AR, et al. (2009) Photosynthesis in cells around veins of the C(3) plant Arabidopsis thaliana is important for both the shikimate pathway and leaf senescence as well as contributing to plant fitness. Plant J 59: 329–343 [DOI] [PubMed] [Google Scholar]
- Kebeish R, Niessen M, Thiruveedhi K, Bari R, Hirsch HJ, Rosenkranz R, Stäbler N, Schönfeld B, Kreuzaler F, Peterhänsel C. (2007) Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat Biotechnol 25: 593–599 [DOI] [PubMed] [Google Scholar]
- Kleine T, Voigt C, Leister D. (2009) Plastid signalling to the nucleus: messengers still lost in the mists? Trends Genet 25: 185–192 [DOI] [PubMed] [Google Scholar]
- Krömer S, Malmberg G, Gärdestrom P. (1993) Mitochondrial contribution to photosynthetic metabolism. Plant Physiol 102: 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschütze V, Willmitzer L, Müller-Röber B. (1995) Inhibition of flower formation by antisense repression of mitochondrial citrate synthase in transgenic potato plants leads to a specific disintegration of the ovary tissues of flowers. EMBO J 14: 660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre T, Urbanczyk-Wochniak E, Flesch V, Bismuth E, Fernie AR, Hodges M. (2007) NAD-dependent isocitrate dehydrogenase mutants of Arabidopsis suggest the enzyme is not limiting for nitrogen assimilation. Plant Physiol 144: 1546–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, De Angeli A, Fernie AR, Martinoia E. (2010) Intra- and extra-cellular excretion of carboxylates. Trends Plant Sci 15: 40–47 [DOI] [PubMed] [Google Scholar]
- Miyagawa Y, Tamoi M, Shigeoka S. (2001) Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat Biotechnol 19: 965–969 [DOI] [PubMed] [Google Scholar]
- Niittylae T, Chaudhuri B, Sauer U, Frommer WB. (2009) Comparison of quantitative metabolite imaging tools and carbon-13 techniques for fluxomics. Methods Mol Biol 553: 355–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR. (2007a) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 50: 1093–1106 [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Lytovchenko A, Smith AM, Loureiro ME, Ratcliffe RG, Sweetlove LJ, Fernie AR. (2005) Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol 137: 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Sweetlove LJ, Fernie AR. (2007b) Operation and function of the tricarboxylic acid cycle in the illuminated leaf. Physiol Plant 129: 45–56 [Google Scholar]
- Raghavendra AS, Padmasree K. (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 8: 546–553 [DOI] [PubMed] [Google Scholar]
- Reumann S, Weber AP. (2006) Plant peroxisomes respire in the light: some gaps of the photorespiratory C2 cycle have become filled—others remain. Biochim Biophys Acta 1763: 1496–1510 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S. (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56: 1481–1489 [DOI] [PubMed] [Google Scholar]
- Siedow JN, Day D. (2000) Respiration and photorespiration. Buchanan BB, Gruissem W, Jones RL, , Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 676–728 [Google Scholar]
- Sienkiewicz-Porzucek A, Nunes-Nesi A, Sulpice R, Lisec J, Centeno DC, Carillo P, Leisse A, Urbanczyk-Wochniak E, Fernie AR. (2008) Mild reductions in mitochondrial citrate synthase activity result in a compromised nitrate assimilation and reduced leaf pigmentation but have no effect on photosynthetic performance or growth. Plant Physiol 147: 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienkiewicz-Porzucek A, Sulpice R, Osorio S, Krahnert I, Leisse A, Urbanczyk-Wochniak E, Hodges M, Fernie AR, Nunes-Nesi A. (2010) Mild reductions in mitochondrial NAD-dependent isocitrate dehydrogenase activity result in altered nitrate assimilation and pigmentation but do not impact growth. Mol Plant 3: 156–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AMO, Ratcliffe RG, Sweetlove LJ. (2004) Activation and function of mitochondrial uncoupling protein in plants. J Biol Chem 279: 51944–51952 [DOI] [PubMed] [Google Scholar]
- Steinhauser MC, Steinhauser D, Koehl K, Carrari F, Gibon Y, Fernie AR, Stitt M. (2010) Enzyme activity profiles during fruit development in tomato cultivars and Solanum pennellii. Plant Physiol 153: 80–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strodtkötter I, Padmasree K, Dinakar C, Speth B, Niazi PS, Wojtera J, Voss I, Do PT, Nunes-Nesi A, Fernie AR, et al. (2009) Induction of the AOX1D isoform of alternative oxidase in A. thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A. Mol Plant 2: 284–297 [DOI] [PubMed] [Google Scholar]
- Studart-Guimarães C, Fait A, Nunes-Nesi A, Carrari F, Usadel B, Fernie AR. (2007) Reduced expression of succinyl-coenzyme A ligase can be compensated for by up-regulation of the γ-aminobutyrate shunt in illuminated tomato leaves. Plant Physiol 145: 626–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Beard KFM, Nunes-Nesi A, Fernie AR, Ratcliffe RG. (2010) Not just a circle: flux modes in the plant TCA cycle. Trends Plant Sci 15: 462–470 [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Lytovchenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, Eickmeier I, Fernie AR. (2006) Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci USA 103: 19587–19592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez G, Cornic G, Bligny R, Gout E, Ghashghaie J. (2005) In vivo respiratory metabolism of illuminated leaves. Plant Physiol 138: 1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Méndez A, Miernyk JA, Randall DD. (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem 270: 1043–1049 [DOI] [PubMed] [Google Scholar]
- van der Merwe MJ, Osorio S, Moritz T, Nunes-Nesi A, Fernie AR. (2009) Decreased mitochondrial activities of malate dehydrogenase and fumarase in tomato lead to altered root growth and architecture via diverse mechanisms. Plant Physiol 149: 653–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkovic J, Anderson SL, Rhoads DM. (2005) A reporter gene system used to study developmental expression of alternative oxidase and isolate mitochondrial retrograde regulation mutants in Arabidopsis. Plant Mol Biol 57: 871–888 [DOI] [PubMed] [Google Scholar]