Abstract
The deciduous bark habit is widespread in the woody plant genus Eucalyptus. Species with deciduous bark seasonally shed a layer of dead bark, thereby maintaining smooth-bark surfaces on branches and stems as they age and increase in diameter. This has a significant cost in terms of fire protection, because smooth-barked species have thinner bark than rough-barked species that accumulate successive layers of dead bark. Eucalypts are closely associated with fire, suggesting that the smooth-bark habit must also provide a significant benefit. We suggest that this benefit is corticular photosynthesis. To test this, we quantified the contribution of corticular photosynthesis to wood production in smooth-barked branches of Eucalyptus miniata growing in tropical savanna in northern Australia. We covered branch sections with aluminum foil for 4 years to block corticular photosynthesis and then compared the oxygen and carbon stable isotope composition of foil-covered and uncovered branch sections. We developed theory to calculate the proportion of wood constructed from corticular photosynthate and the mean proportional refixation rate during corticular photosynthesis from the observed isotopic differences. Coverage with aluminum foil for 4 years increased wood δ13C by 0.5‰ (P = 0.002, n = 6) and wood δ18O by 0.5‰ (P = 0.02, n = 6). Based on these data, we estimated that 11% ± 3% of wood in the uncovered branch sections was constructed from corticular photosynthate, with a mean δ13C of −34.8‰, and that the mean proportional refixation rate during corticular photosynthesis was 0.71 ± 0.15. This demonstrates that corticular photosynthesis makes a significant contribution to the carbon economy of smooth-barked eucalypts.
Eucalyptus is a large genus of woody flowering plants containing more than 700 species. Most of these species only occur naturally in Australia, with a few species also found in Papua New Guinea, Indonesia, East Timor, and the Philippines. Eucalypts dominate the forests and woodlands of Australia. They also occur in arid shrublands, although typically not as canopy-dominant components. Eucalypts range in life form from shrubs to the tallest angiosperm trees in the world (Williams and Brooker, 1997).
A distinguishing characteristic of many eucalypt species is a deciduous bark, whereby an outer layer of dead bark tissue is seasonally shed to expose a smooth bark surface (Chattaway, 1953). These smooth-barked, decorticating species differ from the rough-barked species, in which the dead, outer bark persists and accumulates on the tree. The decorticating process acts to maintain smooth-bark surfaces as the stems and branches increase in diameter with increasing age. Some species are decorticating in the upper branches and stem but have persistent, rough bark on the lower stem. About half the eucalypt species are wholly smooth barked over both the main stem and branches, and about three-fourths have smooth bark over the canopy branches, including branches larger than about 8 cm diameter (Slee et al., 2006).
Woody plants that have smooth bark typically have a layer of green, chlorophyllous tissue just beneath the bark surface (Sprugel and Benecke, 1991; Pfanz et al., 2002). This photosynthetic tissue refixes respired CO2, reducing the CO2 efflux from the woody tissue in the presence of sunlight, thereby recycling part of the respired carbon that would have otherwise been lost from the plant to the atmosphere (Strain and Johnson, 1963; Benecke, 1985; Cernusak and Marshall, 2000; Pfanz and Aschan, 2000; Wittmann et al., 2006; McGuire et al., 2009). Net uptake of CO2 from the atmosphere typically does not occur in the branches and stems of woody plants; therefore, the process has been termed refixation, or corticular photosynthesis, because most of the photosynthetic tissue is located in the bark cortex (Sprugel and Benecke, 1991; Nilsen, 1995).
Although many eucalypts maintain smooth-bark surfaces by seasonally shedding a layer of dead bark, little research has been conducted into corticular photosynthesis in these trees (Tausz et al., 2005; Cernusak et al., 2006; Cerasoli et al., 2009; Eyles et al., 2009). Of particular interest from an ecological and evolutionary perspective is the extent to which corticular photosynthesis contributes toward the carbon economy of smooth-barked eucalypts. In this study, we estimated the contribution of corticular photosynthesis to wood production in branches of Eucalyptus miniata, a commonly occurring eucalypt in the mesic savannas of northern Australia (Brooker and Kleinig, 2004). E. miniata maintains smooth bark on its upper stem and branches, while the lower stem accumulates a thick layer of dead, fibrous bark in mature trees (Fig. 1A). A green, chlorophyllous layer of tissue is visible beneath the smooth bark surface in the upper stem and branches (Fig. 1B). We covered branch sections of mature E. miniata trees with aluminum foil for 4 years to exclude sunlight and thereby block corticular photosynthesis. We then compared the stable oxygen and carbon isotope composition of the wood formed beneath the foil with that of wood formed in adjacent, uncovered branch sections. We used these isotopic differences to estimate (1) the contribution of corticular photosynthesis to wood production, and (2) the proportional refixation rate during corticular photosynthesis.
Figure 1.
A, An individual of E. miniata growing in tropical savanna near Darwin, Northern Territory, Australia. Note the stocking of thick, fibrous bark at the base of the tree that abruptly gives way to smooth, white bark partway up the main stem. B, Closer view of the transition from rough to smooth bark on the main stem. The outermost surface of the smooth bark has been scraped away from a square section, revealing the green, chlorophyllous layer of photosynthetic tissue just beneath the smooth bark surface.
THEORY
If wood is constructed from photosynthate contributed by both leaf photosynthesis and corticular photosynthesis (refixation), a mass balance for the oxygen in the wood can be written as:
where Wo is the oxygen content of the total wood dry matter, Lo is the oxygen content of the wood dry matter constructed from leaf photosynthate, and Co is the oxygen content of the wood dry matter constructed from corticular photosynthate. A similar mass balance can be written for 18O:
where RW is the 18O/16O ratio of total wood dry matter, RL is the 18O/16O ratio of wood dry matter constructed from leaf photosynthate, and RC is the 18O/16O ratio of wood dry matter constructed from corticular photosynthate. Table I provides a summary of all symbols and abbreviations used in this paper. Equation 2 can then be divided through by RS, the 18O/16O ratio of source water (i.e. water absorbed from the soil by the roots). Next, applying the relationship (RX/RS) − 1 = Δ18OX, where RX is the 18O/16O ratio of component X and Δ18OX is the 18O enrichment above source water of component X, gives the following:
Table I. Symbols used in the text.
| Symbol | Definition |
| a | 13C/12C fractionation during CO2 diffusion in air |
| b | 13C/12C discrimination by photosynthetic enzymes in the bark |
| Cc | Carbon content of wood dry matter constructed from corticular photosynthate |
| Co | Oxygen content of wood dry matter constructed from corticular photosynthate |
| ca | CO2 concentration in air outside the woody tissue |
| ci | CO2 concentration inside the bark |
| D | Woody tissue respiration rate |
| g | Bark surface conductance to CO2 |
| Lo | Oxygen content of wood dry matter constructed from leaf photosynthate |
| P | Corticular photosynthesis rate |
| pex | Proportion of oxygen exchanging with local water during cellulose synthesis |
| px | Proportion of unenriched water in tissue where cellulose synthesis is occurring |
| RC | 18O/16O of wood dry matter constructed from corticular photosynthate |
| RL | 18O/16O of wood dry matter constructed from leaf photosynthate |
| RS | 18O/16O of source water (water absorbed by roots from the soil) |
| RW | 18O/16O of total wood dry matter |
| RX | 18O/16O of component X |
| R′a | 13C/12C of CO2 in external air |
| R′C | 13C/12C of corticular photosynthate |
| R′D | 13C/12C of CO2 respired by the woody tissue |
| Wc | Carbon content of total wood dry matter |
| Wo | Oxygen content of total wood dry matter |
| Δ13CC | 13C depletion of corticular photosynthate relative to respired CO2 |
| Δ13CD | 13C depletion of CO2 respired by woody tissues relative to CO2 in external air |
| Δ18OC | 18O enrichment of wood dry matter constructed from corticular photosynthate |
| Δ18OL | 18O enrichment of wood dry matter constructed from leaf photosynthate |
| Δ18OLW | 18O enrichment of leaf water |
| Δ18OW | 18O enrichment of total wood dry matter |
| Δ18OX | 18O enrichment of component X above source water |
| δ13Ca | δ13C of CO2 in external air |
| δ13CC | δ13C of wood constructed from corticular photosynthate |
| δ13CD | δ13C of CO2 respired by the woody tissue |
| δ13CL | δ13C of wood constructed from leaf photosynthate |
| δ13CW | δ13C of total wood dry matter |
| δ18OS | δ18O of source water (water absorbed by roots from the soil) |
| δ18OX | δ18O of component X |
| εcp | Difference between Δ18O of wood dry matter and Δ18O of cellulose |
| εwc | 18O/16O fractionation between organic oxygen and local water |
Subtracting Equation 1 from Equation 3 gives:
Substituting from Equation 1 and solving Equation 4 for Co/Wo, the proportion of total wood dry matter constructed from corticular photosynthate, gives:
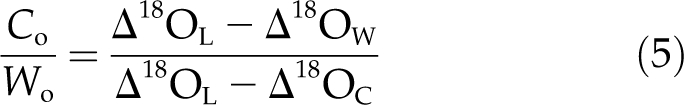 |
To a very close approximation, the Δ18O of any component X can be calculated as:
where δ18OX and δ18OS are δ18O values of component X and source water, respectively.
The 18O enrichment of wood dry matter constructed from leaf photosynthate (Δ18OL) can be described as (Barbour and Farquhar, 2000; Cernusak et al., 2005):
where Δ18OLW is the 18O enrichment of leaf water above source water, pex is the proportion of oxygen atoms exchanging with local water during the synthesis of wood cellulose, px is the proportion of unenriched source water at the site of wood synthesis, εwc is the equilibrium fractionation between organic oxygen and local water, and εcp is the Δ18O difference between wood cellulose and total wood dry matter. The Δ18OLW can range between approximately 0‰ and 30‰ and varies primarily as a function of relative humidity (Craig and Gordon, 1965; Dongmann et al., 1974; Farquhar et al., 2007). The combined term pexpx has been observed to be relatively constant in trees, having a value of about 0.4 (Roden et al., 2000; Cernusak et al., 2005, 2008). The εwc has a value of approximately 27‰ (Sternberg and DeNiro, 1983; Sternberg et al., 1984; Yakir and DeNiro, 1990). Finally, the εcp has been observed to be relatively constant for wood dry matter in trees, having a value of approximately −5‰ (Borella et al., 1999; Barbour et al., 2001; Cernusak et al., 2005).
It was previously observed that water in the bark of Eucalyptus globulus was not evaporatively enriched in 18O compared with xylem water (Cernusak et al., 2005). This result likely applies to bark water generally, consistent with low evaporation rates from bark surfaces (Cernusak and Marshall, 2000; Cernusak et al., 2001; Wittmann and Pfanz, 2008). Xylem water has also been shown to have the same 18O composition as water absorbed from soil by roots (Barbour, 2007). Therefore, in the case of corticular photosynthesis, the first term on the right side of Equation 7 should have a value of zero. Thus, for wood constructed from corticular photosynthate, Equation 7 becomes:
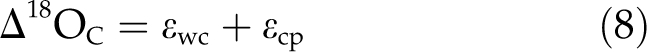 |
The derivation presented above suggests that the difference in 18O composition between wood dry matter constructed from leaf photosynthate and that constructed from corticular photosynthate should be determined by the magnitude of leaf water 18O enrichment. Thus, for a leaf water enrichment of 10‰, the predicted difference between Δ18OL and Δ18OC will be 6‰, and for a leaf water enrichment of 20‰, the predicted difference will be 12‰.
The carbon isotope signature of wood constructed from corticular photosynthate is also expected to differ from that of wood constructed from leaf photosynthate. During refixation in photosynthetic bark, photosynthetic enzymes discriminate against the heavier carbon isotope, 13C (Cernusak et al., 2001). Because the source of CO2 for refixation is primarily respired CO2, refixed photosynthate is expected to have a δ13C more negative than that of the respiratory CO2. The 13C depletion of refixed photosynthate relative to respired CO2 can be described as (Cernusak et al., 2001, 2009):
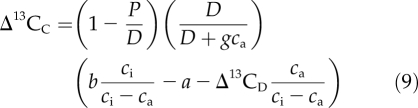 |
The Δ13CC is defined as (R′D/R′C) − 1, where R′D is the 13C/12C ratio of respired CO2 in the woody tissue and R′C is the 13C/12C ratio of refixed photosynthate. In Equation 9, P is the corticular photosynthesis rate (μmol CO2 m−2 s−1), D is the respiration rate (μmol CO2 m−2 s−1), g is the bark surface conductance to CO2 (mol m−2 s−1), ca is the external CO2 concentration (μmol mol−1), ci is the CO2 concentration inside the bark (μmol mol−1), b is the discrimination against 13C by photosynthetic enzymes in the bark (approximately 29‰ for Rubisco), and a is the 13C/12C fractionation during diffusion of CO2 in air (4.4‰). The Δ13CD is defined as (R′a/R′D) − 1, where R′a is the 13C/12C ratio of CO2 in air outside the branch or stem and R′D is the 13C/12C ratio of respired CO2.
The first term on the right side of Equation 9 describes the departure from unity of the proportional refixation rate, P/D. When P/D is small, the Δ13CC is large, and when P/D is large, the Δ13CC is small. The second term on the right side of Equation 9 accounts for the diffusion of CO2 from air outside the branch or stem into the bark. If there is no CO2 in the air outside the woody tissue, the term goes to unity. It is reduced from unity as gca increases, which describes the one-way diffusive flux of CO2 from the external air into the bark (Cernusak et al., 2009). The third term on the right side of Equation 9 describes 13C/12C fractionations associated with enzymatic discrimination, diffusional fractionation, and variation in the 13C/12C ratio of the respired CO2. A full derivation for Equation 9 is given in Part 3 of the Appendix of Cernusak et al. (2001).
We used Equation 5 to estimate the proportion of wood constructed from corticular photosynthate in the uncovered branch sections. In Equation 5, the Δ18OL was determined from the wood sampled from the foil-covered branch sections. Wood in these sections was assumed to have formed in the absence of any refixation and, therefore, to have been derived entirely from leaf photosynthate. The Δ18OW was determined from the uncovered branch sections, where wood was assumed to have been constructed from both leaf and corticular photosynthate. The Δ18OC was calculated from Equation 8, assuming εwc = 27‰ and εcp = −5‰. For calculations of Δ18OL and Δ18OW, the oxygen isotope composition of source water, δ18OS, was assumed to be −5‰. This is the amount-weighted mean δ18O of rainfall for Darwin between 1962 and 2002 (International Atomic Energy Agency; http://www-naweb.iaea.org), approximately 30 km from the study site. The Δ18OL and Δ18OW were then calculated according to Equation 6.
We then used the estimate of Co/Wo, the proportion of wood dry matter constructed from corticular photosynthate, calculated from Equation 5, to estimate the δ13C of wood constructed from corticular photosynthate, δ13CC. Following a derivation analogous to that given for Equation 5, but for δ13C, leads to the following:
where δ13CL is δ13C of wood constructed from leaf photosynthate, δ13CW is δ13C of wood constructed from both leaf and corticular photosynthate, Wc is the total wood carbon content, and Cc is the wood carbon content derived from corticular photosynthate. The Cc/Wc was assumed equal to Co/Wo calculated from Equation 5, δ13CL was determined from wood sampled from the foil-covered branch sections, and δ13CW was determined from wood sampled from the uncovered branch sections. The Δ13CC was then calculated as:
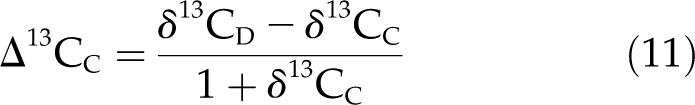 |
where δ13CD is the δ13C of respired CO2 in the woody tissue. We assumed that δ13CD had the same value as δ13CW.
Having estimated Δ13CC, we then solved Equation 9 for P, the corticular photosynthesis rate, in order to estimate P/D, the proportional refixation rate. This required estimates for D, g, ca, b, a, δ13CD, and δ13Ca. We assumed that D = 3 μmol CO2 m−2 s−1, based on previous measurements in E. miniata (Cernusak et al., 2006), g = 0.001 mol m−2 s−1 (Cernusak and Marshall, 2000; Cernusak et al., 2001; Ubierna et al., 2009b), ca = 380 μmol mol−1, b = 29‰, a = 4.4‰, δ13CD = δ13CW, and δ13Ca = −8‰. The Δ13CD for Equation 9 was then calculated as:
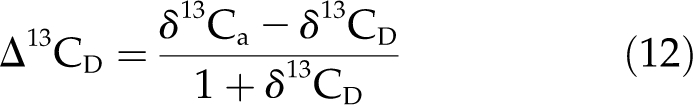 |
and ci was calculated as:
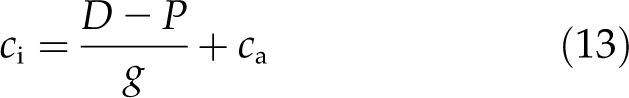 |
We conducted a sensitivity analysis to investigate the effect of variation in these assumed parameter values on the estimate of the proportional refixation rate, P/D.
The above calculations assumed that wood in the foil-covered branch sections was constructed exclusively from leaf-derived photosynthate. If corticular photosynthate was translocated into the foil-covered branch sections from the sun-exposed sections, this would have biased the calculations, such that we would have underestimated the contribution of corticular photosynthesis to wood production in sun-exposed branches. Further experimentation is required to determine the fate of corticular photosynthate and whether it is likely to be translocated from its source to other parts of the plant.
RESULTS
The isotopic composition of the outer 3 mm of wood and of the bark in foil-covered and uncovered branch sections is shown in Table II. Covering the branch sections with aluminum foil for 4 years resulted in relatively small, but consistent, shifts in both δ18O and δ13C of wood compared with the adjacent, uncovered branch sections. Wood δ18O was 0.5‰ higher in foil-covered compared with uncovered branch sections (P = 0.02, n = 6), with differences for individual branches ranging from 1.1‰ to 0.1‰. Wood δ13C was also 0.5‰ higher in foil-covered compared with uncovered branch sections (P = 0.002, n = 6), with differences for individual branches ranging from 0.7‰ to 0.3‰. The trend for bark δ13C was similar, with bark δ13C of foil-covered branch sections being 0.5‰ higher compared with that of uncovered sections (P = 0.001, n = 6). Bark δ18O was 0.3‰ higher in foil-covered compared with uncovered branch sections, but the difference was not statistically significant (P = 0.13, n = 6).
Table II. Oxygen and carbon stable isotope composition of branch sections covered with aluminum foil compared with adjacent sections on the same branch not covered with foil.
Values are given for wood and bark separately. Concentrations of nitrogen and carbon for the same samples are also shown. Values are means of six branches, with se given in parentheses.
| Tissue | δ18O | δ13C | [Nitrogen] | [Carbon] |
| ‰ | mg g−1 | |||
| Wood: foil covered | 21.8 (0.1) | −27.4 (0.2) | 1.7 (0.2) | 465 (4) |
| Wood: no foil | 21.3 (0.2) | −27.9 (0.2) | 1.9 (0.2) | 463 (4) |
| Bark: foil covered | 21.1 (0.3) | −28.0 (0.2) | 1.8 (0.1) | 449 (6) |
| Bark: no foil | 20.7 (0.2) | −28.5 (0.2) | 2.1 (0.2) | 442 (6) |
The nitrogen concentration of bark in foil-covered branch sections tended to be lower than that in uncovered sections (P = 0.06, n = 6), with a mean difference of 0.3 mg g−1 (Table II). The nitrogen concentration of wood in foil-covered branch sections was lower than that in uncovered sections by 0.2 mg g−1 (P = 0.008, n = 6). Carbon concentrations of both bark and wood were similar between foil-covered and uncovered branch sections (P = 0.27 and P = 0.68, respectively, n = 6).
Applying Equation 5, as described in “Materials and Methods,” resulted in an estimate for Co/Wo, the proportion of wood constructed from corticular photosynthate, of 0.11 ± 0.03 (mean ± se, n = 6). Thus, the change in δ18O of wood between foil-covered and uncovered branch sections indicated that corticular photosynthesis accounted for 11% of wood dry matter production. This estimate is sensitive to the assumed value for δ18OS, the δ18O of source water. If δ18OS were assumed to be −4‰ instead of −5‰, the mean estimate for Co/Wo would be 0.14, and if δ18OS were assumed to −6‰ instead of −5‰, the mean estimate for Co/Wo would be 0.09.
Applying estimates of Co/Wo derived from wood δ18O and the difference in wood δ13C between foil-covered and uncovered branch sections, in conjunction with Equation 9, resulted in a mean estimate for P/D, the proportional refixation rate, of 0.71 ± 0.15 (mean ± se, n = 6). This P/D corresponded to a discrimination during corticular photosynthesis, Δ13CC, of 7.2 ± 3.2‰ (mean ± se, n = 6). Assuming mean δ13CD of −27.9‰, this equates to a mean δ13CC of −34.8‰. Application of Equation 9 in this context requires a number of assumed parameter values. A sensitivity analysis of the effect of changing the assumed parameter values is shown in Table III. For a given δ13C difference between foil-covered and uncovered branch sections (δ13CL-δ13CW), the estimate of P/D is relatively sensitive to changes in Cc/Wc, b, and δ13CD and relatively insensitive to changes in D, g, ca, and a. The estimate of P/D is also sensitive to changes in δ13CL-δ13CW (Table III), but this was an observed parameter in our analysis rather than an assumed parameter.
Table III. A sensitivity analysis of the effect of changing assumed parameter values on predicted estimates of P/D, the proportional refixation rate during corticular photosynthesis.
Calculations were performed according to Equation 9. Parameters were varied one at a time, and all other parameters were fixed at the middle value when not under examination. The effect of a halving or a doubling of the middle value for each parameter is shown, except for δ13CD, which was varied by ±3‰. Symbols are as defined in Table I.
| Parameter | Range of Values | Predicted P/D |
| δ13CL − δ13CW (‰) | 0.25, 0.5, 1 | 0.94, 0.83, 0.62 |
| Cc/Wc (mol mol−1) | 0.05, 0.1, 0.2 | 0.59, 0.83, 0.95 |
| D (μmol m−2 s−1) | 1.5, 3, 6 | 0.86, 0.83, 0.82 |
| g (mmol m−2 s−1) | 0.5, 1, 2 | 0.82, 0.83, 0.86 |
| ca (μmol mol−1) | 190, 380, 760 | 0.82, 0.83, 0.86 |
| b (‰) | 15, 29, 58 | 0.45, 0.83, 0.99 |
| a (‰) | 2.2, 4.4, 8.8 | 0.85, 0.83, 0.80 |
| δ13CD (‰) | –25, –28, –31 | 0.70, 0.83, 0.95 |
DISCUSSION
Excluding sunlight from E. miniata branch sections by covering them with aluminum foil for 4 years resulted in increases in both δ18O and δ13C of underlying wood compared with that of adjacent, uncovered branch sections. The isotopic enrichments, although relatively small, were consistent among branches and statistically significant. The average increase in δ13C of wood was 0.5‰. This can be compared with a δ13C increase of 0.8‰ in wood of Pinus monticola branches following coverage with aluminum foil for one growing season (Cernusak et al., 2001). Additionally, three woody plant species native to California showed increases in δ13C of phloem sugars in branches and stems of 1‰ to 2‰ following light exclusion by aluminum foil in defoliated plants (Saveyn et al., 2010). We also observed an increase in the δ18O of branch wood of E. miniata of 0.5‰ in response to long-term light exclusion. To our knowledge, this is the first time that the effect of corticular photosynthesis on woody tissue δ18O has been quantified. Thus, our experiment clearly demonstrated the capacity of corticular photosynthesis to influence both the carbon and oxygen stable isotope composition of branch wood in E. miniata.
Based on the increase in wood dry matter δ18O of branch sections covered with aluminum foil and Equation 5, we estimated that 11% ± 3% of wood in uncovered branch sections was constructed from corticular photosynthate (Co/Wo = 0.11 ± 0.03). This estimate is sensitive to the assumed value of δ18OS, the δ18O of water absorbed by roots from the soil. We assumed a value for δ18OS of −5‰ based on measurements of the δ18O of rainfall in Darwin between 1962 and 2002 (International Atomic Energy Agency; http://www-naweb.iaea.org). This assumed δ18OS is also similar to measurements of xylem water δ18O recorded for mature canopy trees in the vicinity of our study site (Kelley, 2002). Changes to the estimate of Co/Wo in uncovered branch sections would be relatively small if the assumed value for δ18OS were shifted up or down by 1‰, as described above. Therefore, the mean estimate of Co/Wo in branches of E. miniata of 0.11 is reasonably well constrained.
The estimate of 11% for the contribution of corticular photosynthate to wood production in E. miniata branches is also consistent with a simple scaling of observed instantaneous refixation rates. At an irradiance of 1,000 μmol photons m−2 s−1, a proportional refixation rate during corticular photosynthesis (P/D) of 0.55 was previously observed in excised branches of E. miniata (Cernusak et al., 2006). On a 24-h basis, this refixation rate would scale to 0.275 if we assume that the estimated P/D took place for 10 h d−1 (Cernusak et al., 2006). If we then assume that branch carbon use efficiency is 0.6 (Gifford, 1994, 2003), such that branch respiration accounts for 40% of total branch carbon allocation, the scaled refixation rate as a proportion of total branch carbon allocation would be 0.11. This scaled refixation rate would be expected to be the same as Co/Wo if refixed photosynthate were not preferentially used for any one metabolic process over another. Thus, the δ18O-based estimate of Co/Wo is in very good agreement with an estimate based on a simple scaling of observed instantaneous refixation rates in E. miniata branches.
We employed the estimate of Co/Wo based on the δ18O measurements, along with the difference in wood δ13C between foil-covered and uncovered branch sections, to estimate the δ13C of wood constructed from corticular photosynthate, as described in Equation 10. Our mean estimate for this parameter was −34.8‰. We then used this value to parameterize Equation 9 and estimate the mean P/D during corticular photosynthesis in the uncovered branch sections. The resulting estimate was 0.71 ± 0.15. This application of Equation 9 required assumed values for several parameters. However, a sensitivity analysis showed that changing many of these parameters had little effect on the estimate of P/D (Table III); therefore, we suggest that 0.71 is a realistic value. This value is higher than the instantaneous P/D of 0.55 previously observed under unidirectional irradiance of 1,000 μmol photons m−2 s−1 (Cernusak et al., 2006). This is to be expected, as the instantaneous rate of 0.55 was based on gas-exchange measurements for whole branch sections. Thus, it represents an unweighted average of both the illuminated and shaded sides of the branch. The isotopic estimate based on wood δ13C, on the other hand, is a corticular photosynthesis-weighted average. The P/D on the illuminated side of the branch in this case will be more highly represented than that on the shaded side of the branch, because the corticular photosynthesis rate will be higher on the illuminated side than on the shaded side. Thus, the δ13C-based estimate of P/D would always be expected to be higher than that based on gas-exchange measurements, unless the gas-exchange measurements were made under isotropic illumination, such that all sides of the branch were evenly illuminated.
Most of the CO2 fixed during corticular photosynthesis is likely derived from within woody tissues themselves. The δ13C of this CO2 source, therefore, could potentially be affected by processes such as variation in the δ13C of CO2 produced by respiration within woody tissues (Damesin et al., 2005; Maunoury et al., 2007; Kodama et al., 2008) or uptake by roots of CO2 dissolved in soil water (Levy et al., 1999; Moore et al., 2008; Teskey et al., 2008; Ubierna et al., 2009a). The δ13C of internally supplied CO2 enters Equation 9 as δ13CD, which is used to calculate Δ13CC and Δ13CD. In our analysis, we assumed that internally supplied CO2 had the same δ13C as xylem wood, such that δ13CD was set equal to δ13CW. There is clearly some uncertainty in assigning this value to δ13CD. A shift of 3‰ in the assumed value of δ13CD caused a moderate shift in predicted P/D in the sensitivity analysis (Table III). Thus, a more refined understanding of the δ13C dynamics of the internal CO2 pool in woody tissues can contribute toward more robust δ13C-based estimates of P/D.
We observed small reductions in the nitrogen concentrations of branch sections covered with aluminum foil compared with adjacent, uncovered sections (Table II). A visual inspection of the foil-covered sections at harvest showed that there was no green tissue beneath the bark surface, in contrast to the uncovered sections. Coverage of stem sections with aluminum foil in other woody plant species caused significant reductions in stem chlorophyll concentrations (Bossard and Rejmanek, 1992; Saveyn et al., 2010). We suggest that coverage of the E. miniata branch sections with aluminum foil for 4 years would have led to the disassembly of the photosynthetic machinery in the underlying bark and wood that would otherwise have been associated with corticular photosynthesis. The small reductions in nitrogen concentration of 0.3 mg g−1 for bark and 0.2 mg g−1 for wood as a result of foil coverage suggest that the amount of nitrogen required for corticular photosynthesis is small, being only 10% to 15% of the nitrogen normally contained in the bark and outer 3 mm of wood. This suggests high nitrogen use efficiency for corticular photosynthesis. A high nitrogen use efficiency is consistent with the high CO2 concentrations found in woody tissues (Cernusak and Marshall, 2000; Teskey et al., 2008; Ubierna et al., 2009a), which would minimize photorespiration and maximize the efficiency of photosynthetic enzymes.
Eucalypts rose to prominence in Australia in close association with increasing aridity and increasing occurrence of fire during the Pleistocene (Barlow, 1981; Hill, 1994). They are generally well adapted to frequent fire. Adaptations include woody capsules that release seeds after fire, dormant buds that can promote rapid recovery of the canopy following scorching, lignotubers, and thick insulating bark (Barlow, 1981; Williams and Brooker, 1997). The last of these is particularly interesting in the context of corticular photosynthesis. For a given diameter of branch or stem wood, smooth-barked eucalypts with decorticating bark have thinner bark than rough-barked species that accumulate successive layers of dead bark (Gill and Ashton, 1968; Vines, 1968; Cernusak et al., 2006). To a first approximation, the temperature rise at the stem or branch cambium for a given heat input depends only on the bark thickness (Vines, 1968). It follows that trees with thicker bark should be better protected from thermal damage to the cambium during fire events. Why then would the decorticating bark habit be so widespread among eucalypts? We suggest that corticular photosynthesis provides an explanation. Smooth-barked species, wherein smooth bark is maintained by seasonally shedding an outer layer of bark, can maintain their capacity to refix respired CO2 as woody tissues increase in size with increasing age. In rough-barked species, on the other hand, the accumulation of successive layers of dead bark significantly reduces the amount of sunlight that can penetrate to living cells that could contain chloroplasts.
These considerations suggest that corticular photosynthesis should provide a significant benefit to smooth-barked tissues, because the maintenance of smooth bark carries a significant cost in terms of reduced protection from fire. We have demonstrated that corticular photosynthesis contributed 11% ± 3% of the carbon incorporated into wood in branches of E. miniata. We have also shown that the nitrogen allocation required to support corticular photosynthesis is apparently small, being only about 10% to 15% of the nitrogen present in the bark and outer wood. However, the most significant benefit of corticular photosynthesis likely derives from its water use efficiency. Because evaporation rates from smooth bark surfaces are very low, corticular photosynthesis proceeds with a minimum of water loss. It was estimated in branches of P. monticola that the water use efficiency of corticular photosynthesis was 50 times greater than the water use efficiency of leaf photosynthesis (Cernusak and Marshall, 2000). This fundamental difference in water use efficiency between leaves and bark results from the fact that leaves must expose moist tissues to the atmosphere in order to take up CO2, whereas bark primarily uses internally produced CO2. This advantage in terms of water use efficiency likely contributes to the drought tolerance of smooth-barked eucalypts. Drought tolerance is presumably one of the key features that led to the evolutionary success of eucalypts with the onset of increasing aridification in Australia during the Pleistocene (Barlow, 1981; Hill, 1994; Bowman, 2000).
Eucalypts typically have open canopies. Most species have isobilateral leaves that hang in a more or less vertical direction (Williams and Brooker, 1997). Thus, light penetration in eucalypt canopies is relatively high, and light interception by woody tissues is probably higher than in other woody plant taxa that tend to have higher leaf area indices. Thus, the characteristically open nature of most eucalypt canopies would maximize the contribution of corticular photosynthesis to the carbon economy of smooth-barked branches and stems (Tausz et al., 2005).
Some eucalypt species retain dead bark on the lower stem but have decorticating bark on the upper stem and branches. E. miniata is an excellent example of such a species (Fig. 1). This strategy would appear to provide the benefits of both fire protection by thick dead bark on the lower stem and maintenance of the capacity for corticular photosynthesis on the upper stem and branches. In the mesic savannas where E. miniata occurs, the frequent fires are typically surface fires that consume the grassy fuel layer but do not burn in the crowns of the overstory trees (Williams et al., 1999). Thus, the strategy of retaining dead bark on the lower stem and seasonally shedding bark from the upper stem and branches to maintain corticular photosynthesis may be particularly advantageous for a savanna tree such as E. miniata.
CONCLUSION
The deciduous bark habit is exceptionally widespread in the genus Eucalyptus. Species with decorticating bark have thinner bark than species that accumulate successive layers of rough, dead bark. Eucalypts are generally well adapted to coexisting with fire, but the prevalence of decorticating bark among eucalypts is counterintuitive in this context, because thin bark allows the cambium temperature to increase more during fire events than thick bark. Maintenance of smooth-bark surfaces by seasonally shedding a layer of dead bark, therefore, carries a cost in terms of reduced protection from fire, which suggests that it must also provide a benefit, given the close association between eucalypts and fire. We suggest that this benefit is the maintenance of a capacity for corticular photosynthesis as woody tissues increase in diameter with increasing age. Corticular photosynthesis provides an effective mechanism for recycling respired CO2 that would otherwise be lost from woody tissues to the atmosphere. We have demonstrated that corticular photosynthesis contributed 11% ± 3% of wood production in branches of mature E. miniata trees, based on isotopic shifts in branch wood following long-term light exclusion. Thus, corticular photosynthesis can make a significant contribution to the carbon economy of eucalypts that maintain smooth bark on their branches and stems by seasonally shedding a layer of dead bark. Corticular photosynthesis is particularly advantageous in terms of its water use efficiency and likely contributes to the drought tolerance of smooth-barked eucalypts.
MATERIALS AND METHODS
Our study site was located approximately 30 km southeast of Darwin, Northern Territory, Australia in a tropical savanna in the Howard River catchment (12°29.7′ S, 131°09.0′ E). The site has recently been described in detail (Hutley et al., 2000; O’Grady et al., 2000; Cernusak et al., 2006). In order to explore the effect of refixation on the isotopic composition of E. miniata branches, we covered branch sections with aluminum foil. The foil was expected to block all sunlight from reaching the bark beneath it. Therefore, the wood formed beneath the foil was expected to form in the absence of any photosynthetic refixation. The branch sections covered with foil were approximately 30 cm long. Branch diameters at the conclusion of the experiment ranged from 3.2 to 4.6 cm. The aluminum foil was secured to the bark with adhesive tape at the ends of the foil-covered sections. The foil was applied to branches at heights above the ground ranging from 6 to 10 m. The branches were accessed with a 16-m elevated work platform (cherry picker). Foil was applied to the branches in October 2004. The branches were harvested 4 years later, in September 2008. Foil was initially applied to 12 branches. When we returned 4 years later, we were able to relocate six of the foil-covered branches spread across four mature E. miniata individuals. We observed no evidence of fungal infection or insect attack on the foil-covered branch sections.
After the branches were harvested, a wood disc was taken from the center of the foil-covered section. Discs were also taken from the same branches, but approximately 30 cm away from each end of the foil-covered section to provide samples that had been exposed to sunlight over the 4-year period. The discs had a width of approximately 1 cm. The bark was removed from each disc, and the outermost circumference of wood (sapwood) was removed to a depth of approximately 3 mm. The wood and bark were oven dried at 70°C for several days and then ground to a fine powder for isotopic and elemental analyses.
The δ13C and total nitrogen and carbon concentrations of the bark and wood were determined on subsamples of approximately 3 mg. Analyses were carried out in an elemental analyzer (ECS 4010; Costech Analytical Technologies) linked via a continuous-flow interface to a stable isotope ratio mass spectrometer (Delta XP; Finnigan MAT). The δ18O of the wood and bark dry matter was determined on subsamples of approximately 1 mg, which was pyrolyzed in a high-temperature furnace (Thermoquest TC/EA; Finnigan MAT) linked via continuous-flow interface to an isotope ratio mass spectrometer. Isotopic and elemental analyses were carried out in the Stable Isotope Core Laboratory at Washington State University in Pullman, Washington. The precision of isotopic analyses, based on the sd of repeated measurements of working standards during the sample runs, was 0.2‰ for δ18O and 0.1‰ for δ13C. The δ18O and δ13C values have been expressed relative to the Vienna Standard Mean Ocean Water and PeeDee Belemnite international standards, respectively.
The stable isotope and elemental composition of wood and bark dry matter was compared between foil-covered and uncovered branch sections using paired t tests. Results were considered statistically significant at P < 0.05. Data for wood discs taken 30 cm from either end of the foil-covered section were averaged for the uncovered values.
References
- Barbour MM. (2007) Stable oxygen isotope composition of plant tissue: a review. Funct Plant Biol 34: 83–94 [DOI] [PubMed] [Google Scholar]
- Barbour MM, Andrews JT, Farquhar GD. (2001) Correlations between oxygen isotope ratios of wood constituents of Quercus and Pinus samples from around the world. Aust J Plant Physiol 28: 335–348 [Google Scholar]
- Barbour MM, Farquhar GD. (2000) Relative humidity- and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant Cell Environ 23: 473–485 [Google Scholar]
- Barlow BA. (1981) The Australian flora: its origin and evolution. Robertson R, Briggs BG, Eichler H, Pedley L, Ross JH, Symon DF, Wilson PG, , Flora of Australia, Vol 1, Introduction. Australian Government Publishing Service, Canberra, Australia, pp 25–75 [Google Scholar]
- Benecke U. (1985) Tree respiration in steepland stands of Nothofagus truncata and Pinus radiata, Nelson, New Zealand. Turner H, Tranquillini W, , Establishment and Tending of Subalpine Forest: Research and Management, Vol 270. Eidgenössische Anstalt für das Forstliche Versuchswesen, Berlin, pp 61–70 [Google Scholar]
- Borella S, Leuenberger M, Saurer M. (1999) Analysis of δ18O in tree rings: wood-cellulose comparison and method dependent sensitivity. J Geophys Res 104: 19267–19273 [Google Scholar]
- Bossard CC, Rejmanek M. (1992) Why have green stems? Funct Ecol 6: 197–205 [Google Scholar]
- Bowman DMJS. (2000) Australian Rainforests: Islands of Green in a Land of Fire. Cambridge University Press, Cambridge, UK [Google Scholar]
- Brooker MIH, Kleinig DA. (2004) Field Guide to Eucalypts: Northern Australia, Ed 2, Vol 3. Blooming Books, Melbourne, Australia [Google Scholar]
- Cerasoli S, McGuire MA, Faria J, Mourato M, Schmidt M, Pereira JS, Chaves MM, Teskey RO. (2009) CO2 efflux, CO2 concentration and photosynthetic refixation in stems of Eucalyptus globulus (Labill.). J Exp Bot 60: 99–105 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Farquhar GD, Pate JS. (2005) Environmental and physiological controls over oxygen and carbon isotope composition of Tasmanian blue gum, Eucalyptus globulus. Tree Physiol 25: 129–146 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Hutley LB, Beringer J, Tapper NJ. (2006) Stem and leaf gas exchange and their responses to fire in a north Australian tropical savanna. Plant Cell Environ 29: 632–646 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Marshall JD. (2000) Photosynthetic refixation in branches of western white pine. Funct Ecol 14: 300–311 [Google Scholar]
- Cernusak LA, Marshall JD, Comstock JP, Balster NJ. (2001) Carbon isotope discrimination in photosynthetic bark. Oecologia 128: 24–35 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Tcherkez G, Keitel C, Cornwell WK, Santiago LS, Knohl A, Barbour MM, Williams DG, Reich PB, Ellsworth DS, et al. (2009) Why are non-photosynthetic tissues generally 13C enriched compared to leaves in C3 plants? Review and synthesis of current hypotheses. Funct Plant Biol 36: 199–213 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Aranda J, Turner BL. (2008) Conifers, angiosperm trees, and lianas: growth, whole-plant water and nitrogen use efficiency, and stable isotope composition (δ13C and δ18O) of seedlings grown in a tropical environment. Plant Physiol 148: 642–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattaway MM. (1953) The anatomy of bark. I. The genus Eucalyptus. Aust J Bot 1: 402–438 [Google Scholar]
- Craig H, Gordon LI. (1965) Deuterium and oxygen-18 variations in the ocean and the marine atmosphere. Tongiorgi E, , Proceedings of a Conference on Stable Isotopes in Oceanographic Studies and Palaeotemperatures. Lischi and Figli, Pisa, Italy, pp 9–130 [Google Scholar]
- Damesin C, Barbaroux C, Berveiller D, Lelarge C, Chaves M, Maguas C, Maia R, Pontailler JY. (2005) The carbon isotope composition of CO2 respired by trunks: comparison of four sampling methods. Rapid Commun Mass Spectrom 19: 369–374 [DOI] [PubMed] [Google Scholar]
- Dongmann G, Nürnberg HW, Förstel H, Wagener K. (1974) On the enrichment of H218-O in the leaves of transpiring plants. Radiat Environ Biophys 11: 41–52 [DOI] [PubMed] [Google Scholar]
- Eyles A, Pinkard EA, O’Grady AP, Worledge D, Warren CR. (2009) Role of corticular photosynthesis following defoliation in Eucalyptus globulus. Plant Cell Environ 32: 1004–1014 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Cernusak LA, Barnes B. (2007) Heavy water fractionation during transpiration. Plant Physiol 143: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RM. (1994) The global carbon cycle: a viewpoint on the missing sink. Aust J Plant Physiol 21: 1–15 [Google Scholar]
- Gifford RM. (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30: 171–186 [DOI] [PubMed] [Google Scholar]
- Gill AM, Ashton DH. (1968) The role of bark type in relative tolerance to fire of three central Victorian eucalypts. Aust J Bot 16: 491–498 [Google Scholar]
- Hill RS. (1994) The history of selected Australian taxa. Hill RS, , History of the Australian Vegetation: Cretaceous to Recent; Cambridge University Press, Cambridge, UK, pp 390–419 [Google Scholar]
- Hutley LB, O’Grady AP, Eamus D. (2000) Evapotranspiration from eucalypt open-forest savanna of northern Australia. Funct Ecol 14: 183–194 [Google Scholar]
- Kelley G. (2002) Tree water use and soil water dynamics in savannas of northern Australia. PhD thesis. Northern Territory University, Darwin, Australia [Google Scholar]
- Kodama N, Barnard RL, Salmon Y, Weston C, Ferrio JP, Holst J, Werner RA, Saurer M, Rennenberg H, Buchmann N, et al. (2008) Temporal dynamics of the carbon isotope composition in a Pinus sylvestris stand: from newly assimilated organic carbon to respired carbon dioxide. Oecologia 156: 737–750 [DOI] [PubMed] [Google Scholar]
- Levy PE, Meir P, Allen SJ, Jarvis PG. (1999) The effect of aqueous transport of CO(2) in xylem sap on gas exchange in woody plants. Tree Physiol 19: 53–58 [DOI] [PubMed] [Google Scholar]
- Maunoury F, Berveiller D, Lelarge C, Pontailler JY, Vanbostal L, Damesin C. (2007) Seasonal, daily and diurnal variations in the stable carbon isotope composition of carbon dioxide respired by tree trunks in a deciduous oak forest. Oecologia 151: 268–279 [DOI] [PubMed] [Google Scholar]
- McGuire MA, Marshall JD, Teskey RO. (2009) Assimilation of xylem-transported 13C-labelled CO2 in leaves and branches of sycamore (Platanus occidentalis L.). J Exp Bot 60: 3809–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJP, Gonzalez-Meler MA, Taneva L, Pippen JS, Kim HS, Delucia EH. (2008) The effect of carbon dioxide enrichment on apparent stem respiration from Pinus taeda L. is confounded by high levels of soil carbon dioxide. Oecologia 158: 1–10 [DOI] [PubMed] [Google Scholar]
- Nilsen ET. (1995) Stem photosynthesis: extent, patterns, and role in plant carbon economy. Gartner B, , Plant Stems: Physiology and Functional Morphology. Academic Press, San Diego, pp 223–240 [Google Scholar]
- O’Grady AP, Chen X, Eamus D, Hutley LB. (2000) Composition, leaf area index and standing biomass of eucalypt open forests near Darwin in the Northern Territory, Australia. Aust J Bot 48: 629–638 [Google Scholar]
- Pfanz H, Aschan G. (2000) The existence of bark and stem photosynthesis in woody plants and its significance for the overall carbon gain: an eco-physiological and ecological approach. Prog Bot 62: 477–510 [Google Scholar]
- Pfanz H, Aschan G, Langenfeld-Heyser R, Wittmann C, Loose M. (2002) Ecology and ecophysiology of tree stems: corticular and wood photosynthesis. Naturwissenschaften 89: 147–162 [DOI] [PubMed] [Google Scholar]
- Roden JS, Lin GG, Ehleringer JR. (2000) A mechanistic model for interpretation of hydrogen and oxygen isotope ratios in tree-ring cellulose. Geochim Cosmochim Acta 64: 21–35 [Google Scholar]
- Saveyn A, Steppe K, Ubierna N, Dawson TE. (2010) Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants. Plant Cell Environ 33: 1949–1958 [DOI] [PubMed] [Google Scholar]
- Slee AV, Brooker MIH, Duffy SM, West JG. (2006) Euclid: Eucalypts of Australia, Ed 3 Centre for Plant Biodiversity Research, Canberra, Australia [Google Scholar]
- Sprugel DG, Benecke U. (1991) Measuring woody-tissue respiration and photosynthesis. Lassoie JP, Hinckley TM, , Techniques and Approaches in Forest Tree Ecophysiology. CRC Press, Boca Raton, FL, pp 329–355 [Google Scholar]
- Sternberg L, DeNiro M. (1983) Biogeochemical implications of the isotopic equilibrium fractionation factor between the oxygen atoms of acetone and water. Geochim Cosmochim Acta 47: 2271–2274 [Google Scholar]
- Sternberg LSL, DeNiro MJ, Keeley JE. (1984) Hydrogen, oxygen, and carbon isotope ratios of cellulose from submerged aquatic Crassulacean acid metabolism and non-Crassulacean acid metabolism plants. Plant Physiol 76: 68–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain BR, Johnson PL. (1963) Corticular photosynthesis and growth in Populus tremuloides. Ecology 44: 581–584 [Google Scholar]
- Tausz M, Warren CR, Adams MA. (2005) Is the bark of shining gum (Eucalyptus nitens) a sun or a shade leaf? Trees Struct Funct 19: 415–421 [Google Scholar]
- Teskey RO, Saveyn A, Steppe K, McGuire MA. (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177: 17–32 [DOI] [PubMed] [Google Scholar]
- Ubierna N, Kumar AS, Cernusak LA, Pangle RE, Gag PJ, Marshall JD. (2009a) Storage and transpiration have negligible effects on δ13C of stem CO2 efflux in large conifer trees. Tree Physiol 29: 1563–1574 [DOI] [PubMed] [Google Scholar]
- Ubierna N, Marshall JD, Cernusak LA. (2009b) A new method to measure carbon isotope composition of CO2 respired by trees: stem CO2 equilibration. Funct Ecol 23: 1050–1058 [Google Scholar]
- Vines RG. (1968) Heat transfer through bark and the resistance of trees to fire. Aust J Bot 16: 499–514 [Google Scholar]
- Williams JE, Brooker MIH. (1997) Eucalypts: an introduction. Williams JE, Woinarski JCZ, , Eucalypt Ecology: Individuals to Ecosystems. Cambridge University Press, Cambridge, UK, pp 1–15 [Google Scholar]
- Williams RJ, Cook GD, Gill AM, Moore PHR. (1999) Fire regime, fire intensity and tree survival in a tropical savanna in northern Australia. Aust J Ecol 24: 50–59 [Google Scholar]
- Wittmann C, Pfanz H. (2008) Antitranspirant functions of stem periderms and their influence on corticular photosynthesis under drought stress. Trees Struct Funct 22: 187–196 [Google Scholar]
- Wittmann C, Pfanz H, Loreto F, Centritto M, Pietrini F, Alessio G. (2006) Stem CO2 release under illumination: corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant Cell Environ 29: 1149–1158 [DOI] [PubMed] [Google Scholar]
- Yakir D, DeNiro MJ. (1990) Oxygen and hydrogen isotope fractionation during cellulose metabolism in Lemna gibba L. Plant Physiol 93: 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]



