Plants typically convert only 2% to 4% of the available energy in radiation into new plant growth. This low efficiency has provided an impetus for trying to genetically manipulate plants in order to achieve greater efficiencies. But to what extent can increased photosynthesis be expected to increase plant growth? This question is addressed by treating plant responses to elevated CO2 as an analog to increasing photosynthesis through plant breeding or genetic manipulations. For plants grown under optimal growth conditions and elevated CO2, photosynthetic rates can be more than 50% higher than for plants grown under normal CO2 concentrations. This reduces to 40% higher for plants grown under the average of optimal and suboptimal conditions, and over the course of a full day, average photosynthetic enhancements under elevated CO2 are estimated to be about 30%. The 30% enhancement in photosynthesis is reported to increase relative growth rate by only about 10%. This discrepancy is probably due to enhanced carbohydrate availability exceeding many plants’ ability to fully utilize it due to nutrient or inherent internal growth limitations. Consequently, growth responses to elevated CO2 increase with a plant’s sink capacity and nutrient status.
However, even a 10% enhancement in relative growth rate can translate into absolute growth enhancements of up to 50% during the exponential growth phase of plants. When space constraints and self-shading force an end to exponential growth, ongoing growth enhancements are likely to be closer to the enhancement of relative growth rate.
The growth response to elevated CO2 suggests that increases in photosynthesis almost invariably increase growth, but that the growth response is numerically much smaller than the initial photosynthetic enhancement. This lends partial support to the usefulness of breeding plants with greater photosynthetic capacity, but dramatic growth stimulation should not be expected. The usefulness of increasing photosynthetic capacity can be maximized through changes in management practices and manipulation of other genetic traits to optimize the conditions under which increased photosynthesis can lead to maximal growth increases.
Photosynthesis is a relatively inefficient process, with only a maximum of 8% to 10% of the energy in sunlight being converted to the chemical energy in reduced sugars (Long et al., 2006; Zhu et al. 2010). Further considering carbon losses from autotrophic respiration and limitations by other factors such as water and nutrient limitations, realized conversion efficiencies are typically just 2% to 4% of the energy received in sunlight (Long et al., 2006; Zhu et al. 2010). Therefore, it has been a long-standing aim to increase the photosynthesis of plants to achieve greater conversion efficiencies of available sunlight (Reynolds et al., 2000; Sinclair et al., 2004; Long et al., 2006; Zhu et al., 2010).
But to what extent can increased photosynthesis increase ultimate plant growth? Is growth controlled by photosynthesis, or are other plant or environmental factors more important in controlling growth? Is the rate of photosynthesis simply scaled up or down to provide an amount of carbon that is controlled by other growth-limiting processes? If photosynthesis controls growth, it can provide an impetus and rationale for enhancing photosynthesis, but if other factors are more important in controlling growth, then any emphasis on improving photosynthesis might lead to little ultimate growth increase.
There is a very useful analog for addressing this question. For C3 plants, increasing CO2 concentration enhances photosynthesis in much the same way as any engineering approaches might (Drake et al., 1997). Increasing CO2 concentrations, however, also modify stomatal conductance, which can become important under water-limited conditions. This constitutes an important difference between CO2- and plant breeding-mediated enhancements of photosynthesis. The following discussion, therefore, is restricted to conditions where CO2 responses are due to direct photosynthetic responses rather than involving changes in plant water balance.
PHOTOSYNTHETIC RESPONSE TO CO2 CONCENTRATION
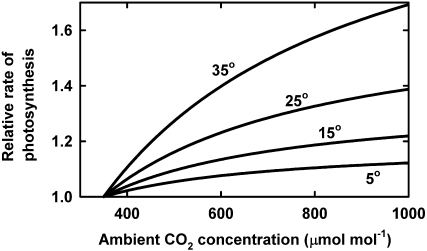
Leaf photosynthesis is readily observed to increase with increasing CO2 concentration (Drake et al., 1997), and these responses have been formalized through models of leaf photosynthesis (Farquhar et al., 1980; Farquhar and von Caemmerer, 1982; Medlyn et al., 2002). Using these models, coupled with assumptions about changes in stomatal conductance with changing CO2 concentration (Ball et al., 1987), it is possible to calculate the response of photosynthesis to increasing CO2 concentration (Fig. 1; Kirschbaum, 2004). Internal (air space and “wall”) resistances are ignored in these calculations, although they can substantially reduce chloroplast CO2 concentrations below average intercellular concentrations (Evans and von Caemmerer, 1996).
Figure 1.
Photosynthetic response to CO2 concentration, shown at four temperatures, based on calculations for RuBP regeneration-limited photosynthesis. All rates are expressed relative to the rates at 350 mmol mol–1. Calculations are based on the model of Farquhar et al. (1980) and Farquhar and von Caemmerer (1982) as described by Medlyn et al. (2002).
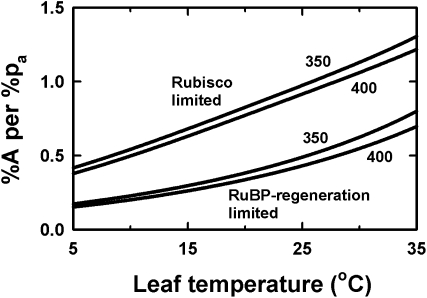
It is important to distinguish between the responses of Rubisco-limited photosynthetic rates, which respond more strongly to CO2, and ribulose 1,5-bisphosphate (RuBP) regeneration-limited rates, which respond less strongly (Fig. 2). The relative responsiveness to increases in CO2 concentration also gradually diminishes with increasing atmospheric CO2 (Fig. 2). Photosynthesis shifts from limitation by Rubisco kinetics at lower intercellular CO2 concentrations to RuBP regeneration-limited rates at higher concentration. At lower radiation levels, photosynthesis is also generally RuBP regeneration limited. Zhu et al. (2010) also argued that most plants still have amounts and kinetic properties of Rubisco that are better suited to preindustrial CO2 concentrations, so that even under current conditions, plants generally have excess Rubisco and are more likely to be RuBP regeneration limited. In the context of responses to elevated CO2, and over most conditions experienced by leaves, the RuBP regeneration-limited responsiveness to CO2 concentration, therefore, is likely to be the relevant response function.
Figure 2.
Photosynthetic response to increasing atmospheric CO2 concentration shown as a function of temperature and separately for photosynthesis limited by Rubisco or RuBP regeneration. This was calculated for base CO2 concentrations of 350 and 400 mmol mol–1 as shown. Data are expressed as percentage increase in net assimilation rate (%A) for a percentage increase in CO2 concentration (%pa). Based on the calculations of Medlyn et al. (2002).
These enhancements of photosynthesis are broadly consistent with experimental observations (Table I). Ellsworth et al. (2004) and Ainsworth and Long (2005) in their respective reviews of the literature found 40% and 29% enhancements of photosynthesis in free-air CO2 enrichment (FACE) experiments (Table I) at elevated CO2 concentrations of 500 to 600 mmol mol–1. Drake et al. (1997), in a review of potted plant experiments, found that photosynthesis was increased by 23% to 58% when plants were grown in elevated CO2 of about 700 mmol mol–1, depending on their nitrogen status and the size of pots they were grown in. Plants grown with inadequate nutrients or in small pots were likely to be affected by feedback inhibition, an issue that is further discussed below. For the plants that were least affected by these extra limitations, a 58% enhancement in photosynthesis was observed, which lies between the theoretical enhancements for RuBP regeneration-limited and Rubisco-limited rates at 25°C (Table I).
Table I. Comparison of theoretical enhancements in photosynthesis in response to elevated CO2 and experimentally observed enhancements.
| Observation | CO2 Enhancement |
Source | |
| RuBP Regeneration | Rubisco | ||
| % | |||
| Response of photosynthesis | This studya | ||
| 5°C | +9 | +25 | This study |
| 15°C | +16 | +48 | This study |
| 25°C | +28 | +78 | This study |
| 35°C | +50 | +117 | This study |
| Intercellular to ambient CO2 | −1 | Drake et al. (1997) | |
| Photosynthesis (large pots)b | +58 | Drake et al. (1997) | |
| Photosynthesis (small pots) | +28 | Drake et al. (1997) | |
| Photosynthesis (high nitrogen) | +57 | Drake et al. (1997) | |
| Photosynthesis (low nitrogen) | +23 | Drake et al. (1997) | |
| Acclimationc (large pots) | −7 | Drake et al. (1997) | |
| Acclimation (small pots) | −20 | Drake et al. (1997) | |
| Acclimation (high nitrogen) | −20 | Drake et al. (1997) | |
| Acclimation (low nitrogen) | −39 | Drake et al. (1997) | |
| Starch | +162 | Drake et al. (1997) | |
| Suc | +60 | Drake et al. (1997) | |
| Photosynthesis | +40 | Ellsworth et al. (2004) | |
| Acclimation | −7.5 | Ellsworth et al. (2004) | |
| Photosynthesis | +29 | Ainsworth and Long (2005) | |
| Acclimation (Vcmax) | −13 | Ainsworth and Long (2005) | |
| Acclimation (Jmax) | −5 | Ainsworth and Long (2005) | |
| Starch | +84 | Ainsworth and Long (2005) | |
The enhancement of rates between 700 and 350 mmol mol–1, based on simulations with the Farquhar photosynthesis model as described by Medlyn et al. (2002), using RuBP regeneration-limited or Rubisco-limited responsiveness as indicated. Drake et al. (1997) summarized the results of pot experiments, and Ellsworth et al. (2004) and Ainsworth and Long (2005) summarized the results of FACE experiments. The elevated CO2 concentration in pot experiments was usually around 700 mmol mol–1, whereas in FACE experiments, increased CO2 concentrations were usually only 500 to 600 mmol mol–1.
Large pots were defined as having a root volume greater than 10 L, and small pots had root volumes less than that.
Acclimation is defined as the rate of photosynthesis measured at a common CO2 concentration.
However, photosynthetic measurements are usually taken under saturating radiation levels and avoid measurements at cold temperatures. These are the conditions that most likely lead to Rubisco limitation and where the CO2 enhancement of photosynthesis is maximized (Fig. 2; Table I). For parts of the day with lower temperature or lower radiation, or for canopies where a proportion of leaves experience reduced light levels through self-shading, the enhancement of photosynthesis is likely to be less. Conversely, for plants experiencing times of high temperatures, the photosynthetic stimulation could be even greater than that measured under moderate temperatures.
For most experimental growing conditions, however, it seems likely that the actual enhancement of photosynthesis will be less than that measured under high irradiation and warm temperatures; therefore, it is likely to be less than the enhancements reported by Drake et al. (1997). The simple average of photosynthetic enhancements reported with large and small pots and high and low nitrogen reported by Drake et al. (1997) was 42%. If one assumes the average enhancement in photosynthesis over the day to be only about three-quarters of that measured under high radiation, the actual realized average enhancement of photosynthesis would be about 30%. Does a 30% photosynthetic enhancement lead to a 30% increase in growth?
GROWTH RESPONSES TO CO2 CONCENTRATION
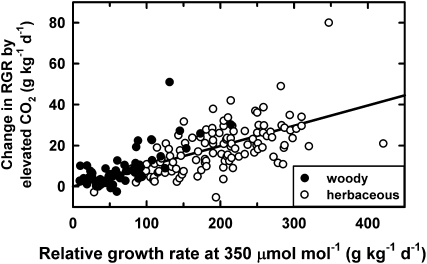
Poorter (1993) showed in an experiment with 10 species with contrasting growth rates, and Poorter and Navas (2003) showed for 179 experimental observations obtained from the literature, that the change in relative growth rate (ΔRGR) by elevated CO2 was a strong linear function of the relative growth rate of the same plants at 350 mmol mol–1 (Fig. 3). This meant that the relative growth enhancement (ΔRGR/RGR) was the same for different species with different inherent relative growth rates. This relative enhancement also constituted a relative increase of relative growth rate by only about 10%.
Figure 3.
Increase in relative growth rate (RGR) for plants grown in elevated CO2 expressed against the plant’s relative growth rate under normal atmospheric CO2. Data are shown for woody and herbaceous species. A linear relationship was fitted to the observations and forced through the origin. The slope of the relationship is 0.099 and implies an average 9.9% stimulation of relative growth rate by exposure to elevated CO2. Without forcing the line through the origin, the slope of the relationship would be 0.095. Data are redrawn from Poorter and Navas (2003).
This leads to the question of why a 30% increase in photosynthesis results in an increase in relative growth rate of only about 10%. Poorter (1993) analyzed the growth response to elevated CO2 of 10 species in greater detail and found that photosynthesis expressed on a leaf area basis was enhanced by 20%, but the enhancement was only 6.5% on a weight basis because leaves also tended to be heavier per unit leaf area. It appeared that the increased amounts of carbohydrates could not be fully utilized by plants. Much of the extra carbohydrate remained in the leaf as sugars or starch (Table I) and made leaves heavier per unit area (Poorter, 1993). This contributed to an ineffective transformation of photosynthetic carbon gain into new growth.
SINK LIMITATIONS AND DOWNWARD ACCLIMATION
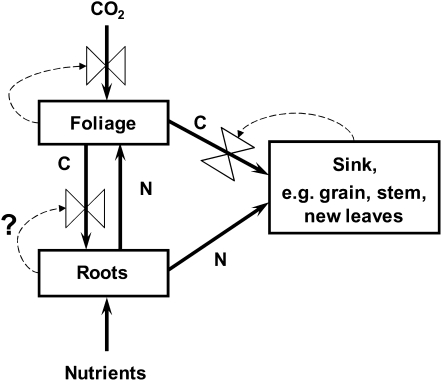
The reason for the much lower growth-rate enhancement than the enhancement of photosynthesis may be understood with reference to Figure 4. Extra carbon can only lead to extra growth if plants have a use for it, which may be for the growth of new foliage, roots, or other sinks such as developing seeds. If a plant’s capacity to utilize carbon is limited (sink limited), any increase in photosynthesis cannot be sustained and will be curtailed through feedback processes. Hence, the rate of photosynthesis measured under standard conditions, or the concentration of photosynthetic enzymes, is usually lower in plants grown under elevated CO2, here referred to as “downward acclimation.”
Figure 4.
Conceptual diagram of the interaction between foliage, roots, and potential carbon (C) and nitrogen (N) sources, sinks, and fluxes in the plant. Dashed lines show feedback control processes, which are only shown here for carbon fluxes. The feedback arrow from roots is designated by a question mark to indicate that this feedback process will operate in small pots but not in large pots or in the field.
Additional carbon can also only be converted into useful plant tissue if nutrients are available as well. This is illustrated here with respect to nitrogen, as that is commonly the most limiting nutrient in nature, but the same consideration applies with respect to any other plant nutrient. If plant nutrition is limited, then any additional carbon cannot be used productively, and a growth stimulation cannot be sustained despite an initial enhancement of photosynthetic carbon gain.
It has been argued that it is common in nature for plants to have excess carbon (Körner, 2003; Millard et al., 2007). Others (Long et al., 2006), on the other hand, have reasoned that this is contradicted by the fact that nearly all species show growth responses to elevated CO2 (Fig. 3). Therefore, it is probably better to consider growth not as being categorically limited by carbon or by specific other factors but as a continuum where the greater availability of one resource (carbon) shifts the plant toward greater limitation by other growth-limiting resources (Reynolds et al., 2000).
For instance, Thomas and Strain (1991) showed experimentally, and Arp (1991) showed from a literature review, that the frequently observed downward acclimation of photosynthesis for plants grown in elevated CO2 was strongly related to the size of the pots that plants were grown in. Downward acclimation was generally confined to studies that used pots with a volume of less than 10 L, whereas no consistent downward acclimation was reported from studies that used larger pot volumes (Arp, 1991) and only minor downward acclimation was typically observed in FACE experiments (Table I; Ellsworth et al., 2004; Ainsworth and Long, 2005).
This was also apparent in the data summarized by Drake et al. (1997). Their summarized data for plants grown in large pots showed a 58% photosynthetic enhancement at growth CO2 concentration and a slight reduction of 7% in photosynthetic rate measured at a common CO2 concentration (Table I). In contrast, plants grown in small pots showed a photosynthetic enhancement of only 28% and downward acclimation of 20%. With respect to pot size, the feedback effect is likely to operate through the availability of carbon sinks. Root growth can be curtailed by rooting volumes in small pots, leading to strong downward acclimation (Fig. 4), but root growth remains unrestricted in large pots or in the field, leading to minimal downward acclimation.
The effect of the source-sink balance on CO2 responsiveness was shown more directly by Lewis et al. (2002), who worked with Xanthium strumarium. They found a strong stimulation of photosynthesis during the initial vegetative growth phase but a much reduced stimulation during the plant’s flowering stage, when plants were thought to have been sink limited. The stronger CO2 stimulation was regained during the final fruiting stage, when developing seeds constituted a large potential sink to utilize any enhanced carbon fixation.
Similarly, Ainsworth et al. (2004) used a FACE facility to grow two soybean (Glycine max) cultivars that had been genetically modified to switch between determinate and indeterminate growth varieties. In one of the cultivars (Williams-dt1), the indeterminate variety showed a more sustained growth response to elevated CO2 than the determinate variety. For the other cultivar (Elf), the two varieties both displayed similarly sustained responsiveness to elevated CO2. This was interpreted to indicate that for cv Elf, even the determinate form had sufficient sink capacity to fully utilize increased amounts of carbohydrates, whereas for cv Williams-dt1, the switch to an indeterminate growth form substantially increased its ability to utilize an increased carbohydrate supply.
In further support, Bunce and Sicher (2003) demonstrated that reversible short-term down-regulation of photosynthesis in high-CO2-grown plants was related to the radiation receipt of the previous day and thus established a direct link to the plant’s carbohydrate balance. Such a direct link to photosynthesis was also seen by Küppers et al. (1986), who showed for eucalypts (Eucalyptus sp.) grown in the field that leaves that had experienced high radiation levels for half a day down-regulated their photosynthetic carbon gain in the afternoon compared with leaves that had received less radiation and thus had gained less carbohydrate.
A similar pattern was evident for the CO2 responsiveness of plants grown with different nitrogen supply rates, with reported downward acclimation of 20% for high-nitrogen-grown plants and 39% for those grown with low nitrogen supply (Table I). The role of carbohydrate supply is further supported by the reported increases in starch and Suc in high-CO2-grown plants (Table I). These summary data support the notion that there are generally no categorical differences between low- and high-CO2-grown plants, because even plants grown in small pots or with limited nitrogen still showed enhanced photosynthesis with elevated CO2, but the responsiveness to CO2 diminished when other factors became more limiting (such as the availability of nutrients or root sinks).
The evidence discussed above indicates that the feedback effects from a plant’s carbohydrate status is a common feature of life under current and future CO2 concentrations, but also that it does not generally lead to categorical differences. Lower sink strength tends to lower the responsiveness of plant growth to CO2 enrichment but does not make growth completely unresponsive. Similarly, even under conditions when plants have high sink strength, their growth response still does not appear to match the potential enhancement that might be predicted based on a consideration of their photosynthetic responses alone.
THE EXPONENTIAL GROWTH PHASE
A large number of research papers have summarized biomass enhancement ratios due to growing plants in elevated CO2 (Table II). Biomass enhancement ratios are numerically similar to initial photosynthetic responses. Enhancement ratios are generally less for slow-growing than for fast-growing plants, less for unfertilized than for fertilized plants, less for plants grown at low temperatures, but similar for herbaceous and woody species. CO2 responses reported for single-plant studies generally showed much greater responses than studies where plants were grown in entire swards (Table II). Biomass enhancement ratios for single plants were numerically also much greater than the enhancement in relative growth rates (compare with Table II; Fig. 3).
Table II. Growth enhancements in response to elevated CO2 reported in different reviews.
Wang (2007) primarily focused on the differences in CO2 responses between single-species populations and multiple-species communities that invariably showed lesser responses. Only the findings from single-species populations are shown here.
| Observation | CO2 Enhancement | Source |
| % | ||
| Fast-growing herbaceous plants | +59 | Poorter and Navas (2003) |
| Slow-growing herbaceous plants | +25 | Poorter and Navas (2003) |
| All herbaceous plants | +45 | Poorter and Navas (2003) |
| Woody plants | +48 | Poorter and Navas (2003) |
| Low-nutrient-grown plants | +25 | Poorter and Navas (2003) |
| Low-temperature-grown plants | +27 | Poorter and Navas (2003) |
| Herbaceous populations | +29 | Wang (2007) |
| Woody populations | +35 | Wang (2007) |
| Unfertilized populations | +10 | Wang (2007) |
| Heavily fertilized populations | +28 | Wang (2007) |
| Dry matter production | +20 | Ainsworth and Long (2005) |
| Grassland biomass | +12 | Lee at al. (2010) |
| Forest growth | +23 | Norby et al. (2005) |
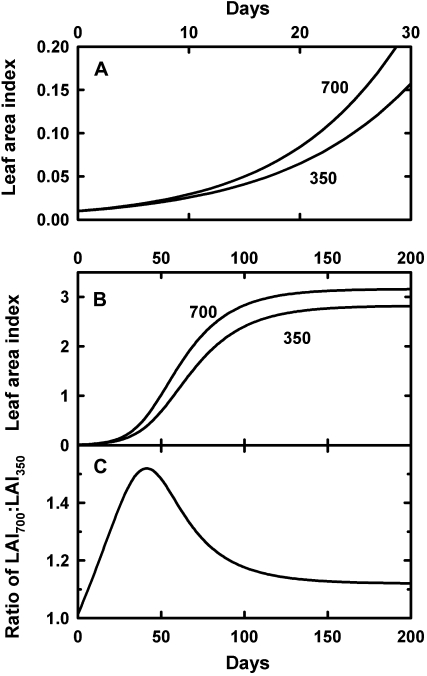
These particular differences between plant groups and growing conditions can be understood through consideration of the exponential growth of plants that can compound even moderate changes in relative growth rate into more substantial enhancements in biomass at intermediate growth stages (Figs. 5 and 6). Assuming that an individual plant grows with a relative growth rate according to the mean (150 g kg−1 d−1) of the values reported by Poorter and Navas (2003), as shown in Figure 3, one can obtain a growth curve as depicted in Figure 5, A and B. Growth increases exponentially at first, but as plants increase in size, self-shading develops and the initial exponential growth changes asymptotically into linear growth during the development of a closed canopy (Fig. 5B).
Figure 5.
Modeled response to doubling CO2 concentration based on exponential growth rate for the initial growth phase (A) and for a longer growth period (B) and the biomass enhancement (C), calculated as the ratio of the two curves shown in B. The two curves in A and B refer to growth at 350 and 700 mmol mol–1, respectively. Details of the model are given in Supplemental Appendix S1. LAI, Leaf area index.
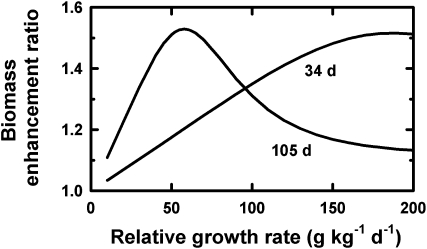
Figure 6.
Modeled biomass enhancement ratios in response to doubling CO2 concentration for experiments lasting 34 or 105 d, the median lengths of experiments on herbaceous and woody plant species, respectively (Poorter, 1993). Details of the model are given in Supplemental Appendix S1.
Based on the enhancement in relative growth rate deduced from Figure 3, it is assumed that the relative growth rate is increased by 10% (to 165 g kg−1 d−1) through elevated CO2, which results in the second curve in Figure 5, A and B. Plants start at the same initial leaf area at the start of the experiment, but thereafter, the biomass of plants under the two contrasting growth conditions progressively diverges until plants in both high and low CO2 are constrained by the same ultimate space limitations. With the simple assumptions used here for senescence and allocation of carbohydrate to other plant parts, high-CO2 plants retain a size and growth advantage even at the equilibrium size of their canopies.
If one plots the ratio of the sizes of the plants in elevated and normal CO2, it begins at 1 (i.e. the same size) at the start of the experiment, but then the sizes progressively diverge, with the greatest size ratio (under the assumptions used here) found after 42 d (Fig. 5C). The difference between high- and low-CO2-grown plants then diminishes again. Even though the relative growth rate is enhanced by only 10%, that can lead to a 50% biomass enhancement ratio at intermediate growth stages but only a slightly greater than 10% enhancement when the sward stage is reached.
Similarly, for a given length of an experiment (34 d) and for the same relative enhancement of relative growth rate, the observed biomass enhancement ratios increase with increasing relative growth rate to reach a peak for a relative growth rate of about 190 g kg−1 d−1 (Fig. 6). This explains why fast-growing plants have higher biomass enhancement ratios than slower growing plants (Table II) despite having the same relative increase in relative growth rate (as deduced from Fig. 3). It might also explain the lower biomass enhancement ratios in plants grown with lower nutrients (Table II), although no compilation has yet been done of the relative increase in relative growth rate for plants under different fertility conditions.
But why do woody plants show the same biomass enhancement ratios as herbaceous plants despite typically having lower inherent relative growth rates (Fig. 3)? This is due to experiments on herbaceous plants typically being run for only 30 to 40 d whereas experiments on woody plants are run for an average of over 100 d (Poorter, 1993), which then lead to similar observed biomass enhancement ratios for herbaceous and woody plants (Fig. 6).
This simple model can thus help us understand and reconcile the findings from different research groups and different experimental settings. Photosynthesis may be enhanced by 50% under optimal conditions (Drake et al., 1997), 40% over the average of optimal and suboptimal conditions (see the calculations above), and 30% over the whole day. Despite this 30% increase in photosynthesis, relative growth rate tends to be enhanced by only 10% (Poorter and Navas, 2003). During the exponential growth phase, however, a 10% enhancement in relative growth rate can lead to an absolute growth enhancements of 50% at intermediate growth stages, as seen in most reviews, such as by Poorter and Navas (2003). Once canopy closure occurs, benefits from exponential growth tend to diminish and revert back to scaling linearly with the enhancement of initial carbon gain (as seen in the data summarized by Ainsworth and Long, 2005; Norby et al., 2005; Wang, 2007; Lee et al., 2010; Table II).
Biomass enhancement ratios are thus a poor means of expressing the responsiveness of plants to elevated CO2, as the same relative enhancements of relative growth rate (as seen in Fig. 3 and used for the simulations here) can lead to very different biomass enhancement ratios by simply varying the length of an experiment (Figs. 5C and 6). It simply reflects the length of time over which the compounding effect during the exponential growth phase can act to amplify the actual underlying response to CO2.
SOME COMPLICATING ISSUES
Additional complications arise through plant-plant interactions. Wang (2007) showed that the growth response of mixed-species communities was less than the response of single-species populations. Hence, even if one can understand and anticipate the interactions that modify single-plant responses to elevated CO2, further difficulties are encountered in trying to apply those findings to plants growing under natural competition. The complications that arise in mixed species swards, however, do not affect the interpretation of results in single-species swards, such as for agricultural crops, where a deliberate manipulation of photosynthetic capacity might be implemented.
Increasing carbon supply is likely to also modify plant carbon-to-nutrient ratios, which will have their own potentially important consequences. A fairly direct consequence is the generally observed reduction in protein concentrations in food crops grown under elevated CO2 (Taub et al., 2008).
A subtler and more complex interaction operates via the effect of plant nutrient concentrations on pests and diseases. Stiling and Cornelissen (2007) conducted a meta-analysis of plant-herbivore interactions and found that plants grown under elevated CO2 usually had lower nutrient concentrations, which reduced the growth rate of herbivores feeding on that plant material. The herbivores tried to compensate for their reduced nutrient intake by consuming greater amounts of foliage, but even with that adjustment, herbivores did less well when feeding on plants grown in elevated CO2. One would have to assume that the same could be expected for plants with artificially increased photosynthetic capacity if that can be achieved without greater nitrogen investment in foliage.
CONCLUSION
I have attempted here to summarize the current knowledge from CO2 enrichment studies that can help us understand the extent to which increasing photosynthesis is likely to translate into increased growth. High-CO2 experiments provide a wealth of observations that can be useful in anticipating the potential benefits that could result from enhancing photosynthesis.
Increasing photosynthesis increases carbon availability for plants. Whether, or to what extent, that translates into increased growth depends on the nature of colimiting factors, especially nutrient availability. Any increase in carbon availability will exacerbate nutrient limitations. This interaction may range from a complete absence of any growth response to increasing photosynthesis in very infertile conditions to a strong enhancement under very fertile conditions. Most situations are likely to lie somewhere between these extremes.
A more subtle additional limitation lies in the limited growth capacity or number of growing points in a plant. Plants may have all the required external resources available but be unable to turn them into new growth because of a limitation of meristematic tissue (e.g. because of a deterministic growth pattern). If a plant’s growth is limited by genetic constraints, plant growth will respond to an increase in resource availability only up to the limit set by these genetic constraints. Extra carbon will then be unable to be utilized by plants.
Across the many high-CO2 experiments, growth enhancements are generally only modest, with an average 10% enhancement of relative growth rate. On the other hand, even a 10% enhancement in relative growth rate can translate into much more substantial absolute growth enhancements during the early exponential growth phase of plants.
The experience from high-CO2 experiments shows that enhancing photosynthesis generally increases growth. This would lend support to the usefulness of artificially increasing plant photosynthesis. High-CO2 experiments also show us, however, that growth responses are numerically only a fraction of the potential enhancement of photosynthesis, which correspondingly reduces the benefit gained from plant manipulations to increase photosynthetic rates.
This also indicates that the effectiveness with which photosynthetic gains can be translated into growth benefits is affected by other plant and environmental factors. Growth responses tend to be greater under conditions where plants have access to adequate nutrition and for plants with greater sink capacity, be that due to its growth stage (e.g. grain filling) or genetic features, such as having an indeterminate rather than determinate growth habit. Genetic manipulation of photosynthesis should thus consider appropriate crop management, or concurrently breed for other plant attributes to maximize the utility of any increase in photosynthetic capacity.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Appendix S1. Description of a simple model to analyze the exponential growth phase.
Supplementary Material
Acknowledgments
I thank Hendrik Poorter for access to the raw data for Figure 3 and for useful discussions of the underlying concepts of plant responses to elevated CO2 as well as John Evans, Roger Parfitt, Susanne von Caemmerer, Adrian Walcroft, and David Whitehead for useful comments on the manuscript.
References
- Ainsworth EA, Long SP. (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165: 351–371 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Nelson R, Long SP. (2004) Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric For Meteorol 122: 85–94 [Google Scholar]
- Arp WJ. (1991) Effects of source sink relations on photosynthetic acclimation to elevated carbon dioxide. Plant Cell Environ 14: 869–876 [Google Scholar]
- Ball JT, Woodrow IE, Berry JA. (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. Biggins J, , Progress in Photosynthesis Research. Martin-Nijhoff Publishers, Dordrecht, The Netherlands, pp 221–224 [Google Scholar]
- Bunce JA, Sicher RC. (2003) Daily irradiance and feedback inhibition of photosynthesis at elevated carbon dioxide concentrations in Brassica oleracea. Photosynthetica 41: 481–488 [Google Scholar]
- Drake BG, Gonzalez-Meler MA, Long SP. (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48: 609–639 [DOI] [PubMed] [Google Scholar]
- Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD. (2004) Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob Change Biol 10: 2121–2138 [Google Scholar]
- Evans JR, von Caemmerer S. (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S. (1982) Modelling of photosynthetic response to environmental conditions. Lange OL, Nobel PS, Osmond CB, Ziegler H, , Physiological Plant Ecology II: Water Relations and Carbon Assimilation. Encyclopedia of Plant Physiology, New Series Vol 12B; Springer-Verlag, Berlin, pp 549–588 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry J. (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Kirschbaum MUF. (2004) Direct and indirect climate change effects on photosynthesis and transpiration. Plant Biol (Stuttg) 6: 242–253 [DOI] [PubMed] [Google Scholar]
- Körner C. (2003) Carbon limitation in trees. J Ecol 91: 4–17 [Google Scholar]
- Küppers M, Wheeler A, Küppers BIL, Kirschbaum MUF, Farquhar GD. (1986) Carbon fixation in eucalypts in the field: analysis of diurnal variations in photosynthetic capacity. Oecologia 70: 273–282 [DOI] [PubMed] [Google Scholar]
- Lee M, Manning P, Rist J, Power SA, Marsh C. (2010) A global comparison of grassland biomass responses to CO2 and nitrogen enrichment. Philos Trans R Soc Lond B Biol Sci 365: 2047–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Wang XZ, Griffin KL, Tissue DT. (2002) Effects of age and ontogeny on photosynthetic responses of a determinate annual plant to elevated CO2 concentration. Plant Cell Environ 25: 359–368 [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29: 315–330 [DOI] [PubMed] [Google Scholar]
- Medlyn BE, Dreyer E, Ellsworth DE, Forstreuter M, Harley PC, Kirschbaum MUF, LeRoux X, Loustau D, Montpied P, Strassemeyer J, et al. (2002) Temperature response of parameters of a biochemically-based model of photosynthesis. II. A review of experimental data. Plant Cell Environ 25: 1167–1179 [Google Scholar]
- Millard P, Sommerkorn M, Grelet GA. (2007) Environmental change and carbon limitation in trees: a biochemical, ecophysiological and ecosystem appraisal. New Phytol 175: 11–28 [DOI] [PubMed] [Google Scholar]
- Norby RJ, DeLucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulemans R, et al. (2005) Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci USA 102: 18052–18056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H. (1993) Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 104/ 105: 77–97 [Google Scholar]
- Poorter H, Navas ML. (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol 157: 175–198 [DOI] [PubMed] [Google Scholar]
- Reynolds MP, van Ginkel M, Ribaut JM. (2000) Avenues for genetic modification of radiation use efficiency in wheat. J Exp Bot 51: 459–473 [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Purcell LC, Sneller CH. (2004) Crop transformation and the challenge to increase yield potential. Trends Plant Sci 9: 70–75 [DOI] [PubMed] [Google Scholar]
- Stiling P, Cornelissen T. (2007) How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Glob Change Biol 13: 1823–1842 [Google Scholar]
- Taub DR, Miller B, Allen H. (2008) Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Glob Change Biol 14: 565–575 [Google Scholar]
- Thomas RB, Strain BR. (1991) Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol 96: 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. (2007) Effects of species richness and elevated carbon dioxide on biomass accumulation: a synthesis using meta-analysis. Oecologia 152: 595–605 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.