The phloem is a central component of the plant’s complex vascular system that plays a vital role in moving photoassimilates from sites of primary acquisition to the heterotrophic tissues and organs of the plant. Indeed, as much as 50% to 80% of the CO2 photoassimilated in a mature leaf is transported out of the leaf in the phloem to satisfy the needs of the nonphotosynthetic organs of the plant (Kalt-Torres et al., 1987). In recent years, new data have shown that the phloem also plays a key role in moving information molecules that coordinate many facets of plant growth and development (Turgeon and Wolf, 2009). This Update will focus on phloem loading’s contribution to assimilate partitioning and its role in balancing photosynthetic activity with sink utilization of photoassimilates.
PHLOEM-LOADING MECHANISMS
The export of carbohydrate from photosynthesizing leaves (sources) provides the substrate for the growth and maintenance of nonphotosynthetic plant tissues (sinks), and the phloem is the delivery system for exported sugars. High concentrations of sugars, predominantly sucrose (Suc), in the sieve elements of source tissues raise turgor pressure, resulting in hydrostatic pressure-driven mass flow of sugars to the sieve elements of sink tissues, where sugars are unloaded and turgor pressure drops. Three different strategies for loading sugars into the phloem have been described (Rennie and Turgeon, 2009; Slewinski and Braun, 2010), which vary in the route that sugars take to enter the phloem and the energetics of accumulation. However, there is considerable flexibility in phloem loading across species (Rennie and Turgeon, 2009), and not all species strictly adhere to one strategy.
In species with symplastic continuity, sugars diffuse from the mesophyll to the phloem down a concentration gradient through numerous plasmodesmata. In some trees, no differential accumulation of sugars in the phloem is observed, and high sugar levels are measured in both the mesophyll and phloem. In this example, all the cells of the leaf have high turgor, and translocation out of the leaf via the phloem is driven by their collective contribution to pressure-driven flow (Rennie and Turgeon, 2009). In the case of symplastic phloem loading, Suc moves through plasmodesmata into the companion cells, where it is the substrate for raffinose and stachyose synthesis. These trisaccharides and tetrasaccharides are larger than Suc and are unable to diffuse back to the mesophyll through the plasmodesmata. This symplastic mechanism, termed polymer trapping, represents thermodynamically active accumulation of these raffinose sugars, because energy is used to create a high concentration in the phloem versus the surrounding mesophyll (Turgeon and Wolf, 2009).
In many plant species, Suc diffuses along a concentration gradient from mesophyll cells to the vein, where it enters the apoplast. It is then actively transported into phloem companion cell/sieve element complexes through transporters that couple Suc accumulation to the proton motive force across the plasma membrane (Sauer, 2007). Suc transporter proteins (SUTs) function as electrogenic Suc-H+ symporters with 1:1 stoichiometry (Bush, 1990; Kühn and Grof, 2010). The proton motive force used to transport Suc against its concentration gradient is large enough to drive the transport reaction several orders of magnitude away from equilibrium (Bush, 1993). Typically, apoplastic Suc is on the order of 20 mm while Suc in the phloem is close to 1 m. Apoplastic loaders generally have few plasmodesmata connecting the minor vein phloem to surrounding cells, although this is not a conserved anatomical feature of apoplastic loaders (Rennie and Turgeon, 2009).
COORDINATION OF SINK UTILIZATION AND PHOTOSYNTHESIS
Experimental manipulations of source supply, source activity, and sink strength have provided strong evidence for the hypothesis that photosynthesis and sink utilization of carbohydrates are tightly coordinated (Moorby, 1977; Paul and Foyer, 2001; Kaschuk et al., 2010). Generally, when sink activity is decreased by removing active sinks or introducing nutrient deficiency, carbohydrates accumulate in leaves and photosynthesis becomes inhibited (Moorby, 1977; Paul and Pellny, 2003). Similarly, when Suc export from source leaves is restricted, for example by cold girdling of petioles or down-regulation of Suc transporter abundance using recombinant DNA technology in species with apoplastic loading, photosynthesis is inhibited (Riesmeier et al., 1994; Krapp and Stitt, 1995; Bürkle et al., 1998; Zhang and Turgeon, 2009). In these cases of decreased sink demand or inhibited sugar transport, sugars accumulate in source leaves, enhancing the expression of genes involved in carbohydrate storage and utilization and suppressing photosynthetic gene expression and subsequent growth (Sheen, 1990; Paul and Pellny, 2003; Stitt et al., 2010). On the other hand, increased sink demand can enhance photosynthetic activity (Hodgkinson, 1974; Hall and Brady, 1977; Kaschuk et al., 2010). Partial defoliation of recently fully expanded leaves that have high photosynthetic rates resulted in increased photosynthesis in older leaves, including those that had started down the senescence pathway (Hodgkinson, 1974). In this example, the plant compensated for the initial loss of photosynthetic capacity by increasing photosynthetic rates in older leaves to maintain the same export capacity under constant sink demand. In complementary observations, photosynthetic rates in young leaves remained high for longer periods during fruit filling and the photosynthetic decline of older leaves was reversed in response to increased demand by the filling fruit (Hall and Brady, 1977). In soybean (Glycine max), increased sink demand due to N2 fixation, compared with nitrate-fed plants, resulted in higher rates of photosynthesis and delayed leaf senescence (Kaschuk et al., 2010).
Growth of plants at elevated atmospheric [CO2], which alters source supply, has provided further evidence for the coordination of source photosynthesis and sink demand. In C3 plants, high [CO2] directly stimulates photosynthesis, which leads to increased carbohydrate supply and respiratory metabolism in leaves, increased plant growth and biomass, and, in crops, increased economic yield (Ainsworth and Long, 2005; Leakey et al., 2009). Early studies of C3 plants grown in pots at elevated [CO2] demonstrated that stimulation of photosynthesis is limited by the sink’s capacity to use or store additional photoassimilate (Arp, 1991). Plants grown in small pots at elevated [CO2] showed initial increases in photosynthetic rates, which were followed by down-regulation of photosynthetic activity, presumably because of negative feedback by inadequate sink capacity. Significantly, these results suggest that source activity initially limited carbon partitioning and growth, but later, sink capacity limited photosynthesis. Recent experiments with field-grown plants provide additional evidence that the degree of stimulation of photosynthesis by elevated [CO2] is related to environmental, experimental, or genetic factors that determine sink strength (Leakey et al., 2009). For example, a single gene mutation in soybean that changed growth habit from indeterminate to determinate caused significant accumulation of nonstructural carbohydrates in leaves and eliminated any increase in photosynthesis by elevated [CO2] (Ainsworth et al., 2004). Conversely, fast-growing poplar (Populus spp.) trees, which export more than 90% of photosynthate during the day, are able to maintain maximal stimulation of photosynthesis at elevated [CO2] (Davey et al., 2006). Therefore, maintenance of stimulated photosynthesis at elevated [CO2] is directly related to the sinks’ capacity to utilize or store the additional carbohydrate (Leakey et al., 2009).
The sophisticated regulation of starch synthesis and degradation in Arabidopsis (Arabidopsis thaliana) provides further evidence for the coordination of carbon supply and carbon utilization (for review, see Smith and Stitt, 2007; Stitt et al., 2010). Starch accumulates in the light in order to support metabolism, assimilate export, and growth during the dark periods, at the end of which starch levels are minimal. The almost complete utilization of starch reserves by the end of the dark period is consistent across Arabidopsis accessions (Cross et al., 2006). Furthermore, Arabidopsis precisely adjusts the rates of starch synthesis and degradation across a range of photoperiods in order to avoid carbon starvation (Gibon et al., 2009). Thus, the products of photosynthesis are tightly tuned to plant demand. Interestingly, starch content at the end of the light period, which provides for carbon metabolism and growth at night, is negatively correlated with biomass accumulation across 94 Arabidopsis accessions (Sulpice et al., 2009). This suggests that plants with a more conservative starch-accumulating strategy incur less growth and that plants with higher growth rates have better carbon use efficiency. However, biomass accumulation in Arabidopsis is positively correlated with the amount of protein invested in primary metabolism (Sulpice et al., 2010). Thus, there is a clear connection between investment in photosynthetic machinery and plant growth in Arabidopsis.
Evidence for a specific role of carbon export and phloem loading in the coordination of photosynthesis with sink utilization comes from examination of apoplastic and symplastic species grown in low light and transferred to a high-light environment. Most species have higher photosynthetic capacity when grown in a high-light environment than in a low-light environment. However, not all species can fully acclimate to a change in light environment. Amiard et al. (2005) tested the hypothesis that species with symplastic loading would not fully acclimate to a transition from a low-light to a high-light environment, since carbohydrate export depends on anatomical characteristics that are fixed during development, such as frequency of plasmodesmata. Apoplastic loaders, on the other hand, were hypothesized to have greater flexibility, since carbohydrate export could be controlled by adjusting the numbers of membrane-bound transfer proteins (Amiard et al., 2005). Three species with apoplastic loading, pea (Pisum sativum), spinach (Spinacia oleracea), and Arabidopsis, all showed complete acclimation and up-regulation of photosynthetic capacity when transferred from low to high light (Adams et al., 2007). Two species with symplastic loading, Cucurbita pepo and Verbascum phoeniceum, accumulated excess starch in leaves and were unable to fully acclimate to the high-light environment (Amiard et al., 2005; Adams et al., 2007). The incomplete acclimation was consistent with a bottleneck in carbon export caused by an inability to increase vein and plasmodesmatal frequency in mature leaves (Amiard et al., 2005).
REGULATION OF PHLOEM LOADING
The large body of work briefly summarized above provides convincing evidence that assimilate partitioning plays a central role in balancing photosynthetic activity in the leaves with photoassimilate utilization and storage in sinks. A simple model for apoplastic phloem loaders that accounts for the integrated responses described above points to phloem loading as the key step in controlling this complex network. For example, assume that sink demand drops in response to an environmental or developmental change. As utilization decreases, Suc begins to build up in sink tissues and phloem. With a lower rate of unloading, the turgor pressure of sink phloem remains high and, consequently, the mass flow of assimilate from the leaves drops. With lower rates of export in the source phloem, carbohydrate builds up in the mesophyll and well-described pathways, including short-term increases in starch accumulation and Suc storage in the vacuole, followed by hexose-mediated changes in photosynthetic gene expression, lead to lower rates of photosynthesis (Sheen, 1990; Krapp and Stitt, 1995; Smith and Stitt, 2007). In contrast, accelerated use of assimilate by sink tissues (i.e. increased sink demand) would enhance the rate of phloem unloading, thus lowering the turgor of sink phloem and increasing mass flow because of the larger hydrostatic pressure difference between the source and sink phloem. Increased rates of efflux via the leaf phloem would stimulate phloem loading and lower mesophyll carbohydrate levels, which could stimulate photosynthetic activity (assuming no other rate-limiting steps). Because phloem unloading in sink tissues is generally through passive pathways, the key question in this model is how are dynamic changes in Suc concentrations in the leaf phloem communicated to the mesophyll? Remember, the proton-Suc symporter described above can transport Suc against a concentration gradient of 2 or 3 orders of magnitude. Thus, decreased rates of mass flow and the resulting accumulation of Suc in the leaf phloem would not alter mesophyll sugar levels, because the proton motive force that drives the symporter can load the phloem against ever-increasing Suc concentration differences. Therefore, phloem loading must be down-regulated in order for photoassimilates to back up into the mesophyll.
If phloem loading is a focal point for balancing photosynthetic activity in the leaves with utilization in the sinks, then Suc symporter activity must be dynamically regulated. There is clear evidence for transcriptional and posttranslational regulation of phloem loading (Vaughn et al., 2002) and emerging evidence for redox regulation (for review, see Slewinski and Braun, 2010). The first evidence suggesting that the redox status of the cell might be an important regulator of symporter function was based on increased transport activity of SUT1 expressed in yeast and Xenopus oocytes that were treated with redox agents (Krügel et al., 2008). Unfortunately, this result was later shown to be an artifact caused by the redox agents lowering the pH of the transport solutions, which subsequently increased transport activity (Krügel et al., 2008). Nevertheless, that study provided novel insight by demonstrating a redox effect on symporter localization and dimerization, which was also shown to be cell type specific. The formation of homomeric and heteromeric dimers between AtSUC2, AtSUT2, and AtSUC4 in yeast had previously been demonstrated using a novel split-ubiquitin system (Schulze et al., 2003). Krügel et al. (2008) showed that under oxidizing conditions in yeast, signals from SUT1-GFP chimeric proteins in the endoplasmic reticulum decreased, while localization into plasma membrane raft domains increased. Oxidizing conditions, which would be consistent with active photosynthesis and carbon fixation in the daytime, also prompted a shift from monomeric to dimeric complexes of SUT1. The impact of localization and dimerization on the functional activity of Suc symporters in plant cells is unknown but remains an area of keen interest.
Recently, novel posttranslational regulation of Suc transporters was suggested from a study identifying interactions between cytochrome b5 and Suc and sorbitol transporters (Fan et al., 2009). A split-ubiquitin system, coimmunoprecipitation, and bimolecular fluorescence were used to show that the apple (Malus domestica) Suc symporter MdSUT1 and the apple sorbitol transporter MdSOT6 both interact with the apple endoplasmic reticulum-localized cytochrome b5 protein encoded by MdCYB5. Coexpression of the transporters and cytochrome b5 in yeast cells increased transport activity by enhancing substrate affinity. Significantly, point mutations in the N terminus of each transporter eliminated the protein-protein interaction with the cytochrome and abolished the stimulation of transport activity. It will be very interesting to extend these observations to plant cells and determine if symporter activity is regulated by the presence or absence of cytochrome b5.
The first evidence for posttranslational modification of symporter activity was okadaic acid-dependent inhibition of proton-coupled Suc accumulation in plasma membrane vesicles isolated from phosphatase inhibitor-treated sugar beet (Beta vulgaris) leaves (Roblin et al., 1998). Subsequent work showed that okadaic acid-dependent loss of Suc symporter activity was the result of decreased symporter transcription and symporter degradation (Ransom-Hodgkins et al., 2003). Ransom-Hodgkins et al. (2003) also showed, using nuclear run-on experiments, that calphostin C, a kinase inhibitor, stimulated symporter transcriptional activity, resulting in increased symporter mRNA and protein abundance, and increased transport activity. Taken together, these data strongly suggest that phloem loading is regulated by a protein phosphorylation cascade that controls Suc symporter transcriptional activity, which ultimately determines symporter protein abundance and, therefore, phloem-loading capacity (Ransom-Hodgkins et al., 2003).
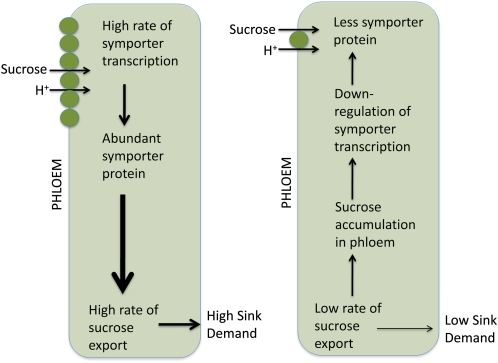
Analysis of the impact of a protein phosphorylation cascade on symporter expression and transport activity parallels research suggesting that Suc is a signal molecule that regulates phloem loading (Chiou and Bush, 1998; Vaughn et al., 2002; Ransom-Hodgkins et al., 2003). Initial speculation that SUT2 might function as a Suc sensor that impacts transport activity (Barker et al., 2000) was called into doubt because it lacked several characteristics associated with known Glc sensors in yeast (Barth et al., 2003; Eckardt, 2003); thus, any role as a sensor remained an open question. However, there is direct evidence that changes in Suc levels in the companion cells regulate transcriptional activity for the symporter, and because of the rapid turnover of symporter mRNA and protein (Vaughn et al., 2002), this dynamic regulation of transcription controls phloem loading. Moreover, calphostin C blocks Suc-dependent loss of symporter activity, thus linking Suc signaling to a protein phosphorylation signal cascade that regulates symporter transcription (Ransom-Hodgkins et al., 2003). These authors hypothesized that a change in Suc levels in the source phloem is the integrating signal that regulates phloem-loading capacity and, thereby, balances source photosynthetic activity with sink utilization (Fig. 1). Increased Suc levels in the leaf phloem will also impact turgor pressure, and it is possible that turgor is a player in this signaling mechanism, as has been suggested for assimilate export in developing seed coats (Patrick, 1994). It is important to note that this hypothesis is supported by experiments with apoplastic phloem loaders only and that regulation of the Suc symporter is not likely to play a similar role in symplastic loading plants.
Figure 1.
Phloem loading capacity is a function of Suc symporter abundance in the plasma membrane, which is directly proportional to the rate of symporter transcription. The transcription rate of the symporter in the phloem is regulated by a signaling cascade that is controlled by changes in Suc levels. Suc levels are a dynamic balance between loading activity and export, and rapid turnover of both symporter mRNA and protein allows for dynamic control. If sink demand is high, Suc levels are low and transcription is high. If sink demand drops, export slows and Suc builds up and down-regulates symporter transcription and abundance. As phloem-loading capacity drops, carbohydrate then builds up in the mesophyll and photosynthesis is down-regulated.
OPPORTUNITY TO ENHANCE PHOTOSYNTHESIS AND IMPROVE YIELDS
For crops that use apoplastic phloem loading, one approach to increase yields would be to uncouple phloem loading from the Suc-sensing system that regulates assimilate partitioning. The rationale behind this approach is 2-fold. First, constitutive expression of the Suc symporter would maintain constant rates of Suc removal from the mesophyll, especially under conditions in which sink demand is low and loading would be expected to drop. In the absence of carbohydrate accumulation in the mesophyll, photosynthetic rates would be expected to stay high. Second, recent papers note that sugar accumulation in the leaf is associated with the onset of senescence (Nooden et al., 1996; Wingler et al., 2006). If sugar accumulation is a trigger, then constitutive phloem-loading activity would keep sugar levels low and thereby delay senescence. Constitutive expression of the symporter would maintain leaf photosynthetic activity under conditions where decreased demand would normally decrease rates, and the delay in leaf senescence would contribute to a net increase in carbon fixation. Taken together, there should be a net increase in yield when integrated over a growing season.
To date, experiments have successfully altered Suc transport activity, yet improvements in yield have not been realized. An early experiment exploring the impact of altered Suc transport activity on growth used the cauliflower mosaic virus 35S promoter to express a spinach Suc symporter (SoSUT1) in transgenic potato (Solanum tuberosum; Leggewie et al., 2003). Although two transgenic lines showed higher rates of Suc uptake into leaf plasma membrane vesicles, no differences in 14CO2 assimilation or leaf translocation rates were observed between these lines and wild-type plants. There were also no differences in total tuber yield or tuber number. However, the 35S promoter drives expression in all cells, including the mesophyll, so the result was not surprising. Ectopic symporter activity in the mesophyll cells will compete for Suc released into the apoplast and result in a futile cycle of release and recapture by those cells. In another test of ectopic expression, Srivastava et al. (2009) expressed AtSUC2 in a Atsuc2 mutant background using exotic phloem-specific promoters. The promoters were the rolC promoter from Agrobacterium rhizogenes and a promoter element from Commelina yellow mottle virus. The commelina yellow mottle virus promoter construct restored nearly wild-type growth and carbon partitioning, but the weaker rolC transgenic plants were only partially restored to wild-type growth. While these results do not appear to support the notion that uncoupling the symporter from sink regulation will increase growth, the transgenic plants used in the experiments were grown in relatively low light at 100 to 150 μmol photons m−2 s−1. At this photon flux density, photosynthesis is generally light limited and sink demand is expected to exceed carbon assimilation. Thus, one would not expect to see any negative feedback on photosynthetic activity or any difference between the wild-type and transgenic plants in these experiments. Growing these transgenics in elevated [CO2] might provide a better test of the potential for enhancing phloem loading and alleviating negative feedback on photosynthesis.
Another approach for increasing yield would be to overexpress Suc transporters in sink cells, thereby enhancing sink demand and inducing an increase in photosynthesis and assimilate export. When a vicilin promoter was used to express a potato Suc symporter, StSUT1, in storage parenchyma cells of developing pea seeds, there was enhanced Suc influx into cotyledons and greater cotyledon growth rates (Rosche et al., 2002), demonstrating the feasibility of engineering specific cell types for enhanced Suc transport. In a related experiment, Weichert et al. (2010) overexpressed a barley (Hordeum vulgare) symporter in grain using the Horedin endosperm-specific promoter. While the authors observed increased seed protein levels, overall yield was up, but not at a statistically significant level except under controlled conditions. It is noteworthy that these authors reported significant changes in gene expression patterns associated with carbon and nitrogen metabolism, demonstrating that transgenic manipulation of Suc transport can have a significant impact on multiple metabolic pathways.
CONCLUSION
Growth and development in multicellular plants is a globally integrated process in which primary assimilation in source tissues is balanced by the metabolic needs of heterotrophic sinks. The complex coordination of source and sink activity between highly dispersed organs is mediated by dynamic regulatory processes in the plant’s vascular system. The regulatory system described here that controls phloem loading is likely to be only one of several systems that balance assimilation with utilization. Indeed, the recent demonstration of several complex signaling and control processes linked through the phloem (Turgeon and Wolf, 2009) suggests that many pathways involved with global regulation of plant growth have yet to be discovered. In a future world of elevated [CO2], enhancing the capacity for Suc export and carbon utilization is an important component of maximizing photosynthesis and yield.
References
- Adams WW, III, Watson AM, Mueh KE, Amiard V, Turgeon R, Ebbert V, Logan BA, Combs AF, Demmig-Adams B. (2007) Photosynthetic acclimation in the context of structural constraints to carbon export from leaves. Photosynth Res 94: 455–466 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP. (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165: 351–371 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Nelson R, Long SP. (2004) Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric For Meteorol 122: 85–94 [Google Scholar]
- Amiard V, Mueh KE, Demmig-Adams B, Ebbert V, Turgeon R, Adams WW., III (2005) Anatomical and photosynthetic acclimation to the light environment in species with differing mechanisms of phloem loading. Proc Natl Acad Sci USA 102: 12968–12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp WJ. (1991) Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14: 869–875 [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB. (2000) SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth I, Meyer S, Sauer N. (2003) PmSUC3: characterization of a SUT2/SUC3-type sucrose transporter from Plantago major. Plant Cell 15: 1375–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkle L, Hibberd JM, Quick WP, Kühn C, Hirner B, Frommer WB. (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR. (1990) Electrogenicity, pH-dependence, and stoichiometry of the proton-sucrose symport. Plant Physiol 93: 1590–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR. (1993) Proton-coupled sugar and amino acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol 44: 513–542 [Google Scholar]
- Chiou TJ, Bush DR. (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95: 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, Palacios N, Stitt M. (2006) Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol 142: 1574–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey PA, Olcer H, Zakhleniuk O, Bernacchi CJ, Calfapietra C, Long SP, Raines CA. (2006) Can fast-growing plantation trees escape biochemical down-regulation of photosynthesis when grown throughout their complete production cycle in the open air under elevated carbon dioxide? Plant Cell Environ 29: 1235–1244 [DOI] [PubMed] [Google Scholar]
- Eckardt NA. (2003) The function of SUT2/SUC3 sucrose transporters: the debate continues. Plant Cell 15: 1259–1262 [Google Scholar]
- Fan RC, Peng CC, Xu YH, Wang XF, Li Y, Shang Y, Du SY, Zhao R, Zhang XY, Zhang LY, et al. (2009) Apple sucrose transporter SUT1 and sorbitol transporter SOT6 interact with cytochrome b5 to regulate their affinity for substrate sugars. Plant Physiol 150: 1880–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Pyl ET, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M. (2009) Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ 32: 859–874 [DOI] [PubMed] [Google Scholar]
- Hall AJ, Brady CJ. (1977) Assimilate source-sink relationships in Capsicum annuum L. II Effects of fruiting and defloration on the photosynthetic capacity and senescence of the leaves. Aust J Plant Physiol 4: 771–783 [Google Scholar]
- Hodgkinson KC. (1974) Influence of partial defoliation on photosynthesis, photorespiration and transpiration by lucerne leaves of different ages. Aust J Plant Physiol 1: 561–578 [Google Scholar]
- Kalt-Torres W, Kerr PS, Usuda H, Huber SC. (1987) Diurnal changes in maize leaf photosynthesis. I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol 83: 283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschuk G, Hungria M, Leffelaar PA, Giller KE, Kuyper TW. (2010) Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max [L.] Merrill) dependent on N2 fixation or nitrate supply. Plant Biol (Stuttg) 12: 60–69 [DOI] [PubMed] [Google Scholar]
- Krapp A, Stitt M. (1995) An evaluation of direct and indirect mechanisms for the ‘sink-regulation’ of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195: 313–323 [Google Scholar]
- Krügel U, Veenhoff LM, Langbein J, Wiederhold E, Liesche J, Friedrich T, Grimm B, Martinoia E, Poolman B, Kühn C. (2008) Transport and sorting of the Solanum tuberosum sucrose transporter SUT1 is affected by posttranslational modification. Plant Cell 20: 2497–2513; erratum Krügel U, Veenhoff LM, Langbein J, Wiederhold E, Liesche J, Friedrich T, Grimm B, Martinoia E, Poolman B, Kühn C (2009) Plant Cell 21: 4059–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn C, Grof CP. (2010) Sucrose transporters of higher plants. Curr Opin Plant Biol 13: 288–298 [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60: 2859–2876 [DOI] [PubMed] [Google Scholar]
- Leggewie G, Kolbe A, Lemoine R, Roessner U, Lytovchenko A, Zuther E, Kehr J, Frommer WB, Riesmeier JW, Willmitzer L, et al. (2003) Overexpression of the sucrose transporter SoSUT1 in potato results in alterations in leaf carbon partitioning and in tuber metabolism but has little impact on tuber morphology. Planta 217: 158–167 [DOI] [PubMed] [Google Scholar]
- Moorby J. (1977) Integration and regulation of translocation within the whole plant. Symp Soc Exp Biol 31: 425–454 [PubMed] [Google Scholar]
- Nooden LD, Hillsberg JW, Schneider MJ. (1996) Induction of leaf senescence in Arabidopsis thaliana by long days through a light dosage effect. Physiol Plant 96: 491–495 [Google Scholar]
- Patrick JW. (1994) Turgor-dependent unloading of assimilates from coats of developing legume seed: assessment of the significance of the phenomenon in the whole plant. Physiol Plant 50: 645–654 [Google Scholar]
- Paul MJ, Foyer CH. (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383–1400 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Pellny TK. (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54: 539–547 [DOI] [PubMed] [Google Scholar]
- Ransom-Hodgkins WD, Vaughn MW, Bush DR. (2003) Protein phosphorylation plays a key role in sucrose-mediated transcriptional regulation of a phloem-specific proton-sucrose symporter. Planta 217: 483–489 [DOI] [PubMed] [Google Scholar]
- Rennie EA, Turgeon R. (2009) A comprehensive picture of phloem loading strategies. Proc Natl Acad Sci USA 106: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin G, Sakr S, Bonmort J, Delrot S. (1998) Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Lett 424: 165–168 [DOI] [PubMed] [Google Scholar]
- Rosche E, Blackmore D, Tegeder M, Richardson T, Schroeder H, Higgins TJV, Frommer WB, Offler CE, Patrick JW. (2002) Seed-specific overexpression of a potato sucrose transporter increases sucrose uptake and growth rates of developing pea cotyledons. Plant J 30: 165–175 [DOI] [PubMed] [Google Scholar]
- Sauer N. (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581: 2309–2317 [DOI] [PubMed] [Google Scholar]
- Schulze WX, Reinders A, Ward J, Lalonde S, Frommer WB. (2003) Interactions between co-expressed Arabidopsis sucrose transporters in the split-ubiquitin system. BMC Biochem 4: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. (1990) Metabolic repression of transcription in higher plants. Plant Cell 2: 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewinski TL, Braun DM. (2010) Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Sci 178: 341–349 [Google Scholar]
- Smith AM, Stitt M. (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Srivastava AC, Ganesan S, Ismail IO, Ayre BG. (2009) Effective carbon partitioning driven by exotic phloem-specific regulatory elements fused to the Arabidopsis thaliana AtSUC2 sucrose-proton symporter gene. BMC Plant Biol 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lunn J, Usadel B. (2010) Arabidopsis and primary photosynthetic metabolism: more than the icing on the cake. Plant J 61: 1067–1091 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl ET, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, et al. (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106: 10348–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Trenkamp S, Steinfath M, Usadel B, Gibon Y, Witucka-Wall H, Pyl ET, Tschoep H, Steinhauser MC, Guenther M, et al. (2010) Network analysis of enzyme activities and metabolite levels and their relationship to biomass in a large panel of Arabidopsis accessions. Plant Cell 22: 2872–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. (2009) Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Vaughn MW, Harrington GN, Bush DR. (2002) Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci USA 99: 10876–10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichert N, Saalbach I, Weichert H, Kohl S, Erban A, Kopka J, Hause B, Varshney A, Sreenivasulu N, Strickert M, et al. (2010) Increasing sucrose uptake capacity of wheat grains stimulates storage protein synthesis. Plant Physiol 152: 698–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Purdy S, MacLean JA, Pourtau N. (2006) The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot 57: 391–399 [DOI] [PubMed] [Google Scholar]
- Zhang CK, Turgeon R. (2009) Downregulating the sucrose transporter VpSUT1 in Verbascum phoeniceum does not inhibit phloem loading. Proc Natl Acad Sci USA 106: 18849–18854 [DOI] [PMC free article] [PubMed] [Google Scholar]