Photorespiration has been a target for crop improvement ever since the energy losses associated with this pathway were identified in the 1970s. However, recent research highlights the importance of photorespiration as a recycling pathway for the products of ribulose-1,5-bisphosphate (RuBP) oxygenation and its intimate interconnection with primary metabolism. Nevertheless, reducing photorespiratory losses by installation of alternative salvage pathways in Arabidopsis (Arabidopsis thaliana) resulted in enhanced growth and biomass. Such approaches will probably also prove useful under field conditions, and in a future atmosphere containing higher CO2 concentrations combined with high temperature or limiting water.
PHOTORESPIRATION EVOLVED AS A METABOLITE RECYCLING PATHWAY
Photorespiration is an exceptional biochemical pathway as it starts with what might be considered an erroneous reaction: Rubisco fixes molecular oxygen (O2) instead of executing its intrinsic function in photosynthesis, fixation of carbon dioxide (CO2). CO2 uptake results in the formation of two molecules of 3-phosphoglycerate (3-PGA) that is used for biosynthetic reactions and the recycling of the acceptor molecule RuBP. During O2 fixation, one molecule of 3-PGA and one molecule of 2-phosphoglycolate (2-PG) are formed. The latter cannot be used by plants for biosynthetic reactions and it is a potent inhibitor of chloroplastic function (Anderson, 1971). The catalytic activity of Rubisco with O2 as a substrate is some 100-fold lower than with CO2 at equivalent concentrations of the two gases (Tcherkez et al., 2006); thus, at a first glance, O2 fixation does not cause a major problem. This was probably true during early evolutionary times when the atmosphere was essentially free of O2 and contained much higher amounts of CO2 than nowadays (Kasting and Ono, 2006). However, oxygenic photosynthesis was a great evolutionary success that resulted in a drastic change of the atmospheric composition. Essentially all CO2 was fixed from the atmosphere down to levels clearly below 0.1% on a molar basis. Much of this fixed carbon was not released back to the atmosphere by decomposition of organic matter, but removed from the biogeochemical carbon cycle by sedimentation and fossilization. Concomitantly, the light reactions of oxygenic photosynthesis, as the name implies, produced immense amounts of O2 by using water as the primary electron donor. After saturation of mineral deposits and the seawater, O2 started to accumulate in the atmosphere (Buick, 2008). Today, atmospheric O2 is roughly 500-fold more abundant than CO2. Fortunately, some other factors such as increased solubility of CO2 compared to O2 in the aqueous cytosol and the chloroplast stroma (Ku and Edwards, 1977) again favor CO2 fixation by Rubisco. All this ends up with approximately 25% oxygenase reaction under moderate growth conditions. This rate seems to be a balanced trade-off of atmospheric CO2 and O2 concentrations, resulting in acceptable rates of RuBP oxygenation at highest possible rates of carboxylation. However, the fraction of oxygenase reactions can significantly rise in warm and dry habitats. Under increasing temperatures, the affinity of Rubisco for CO2 decreases (Jordan and Ogren, 1984). Moreover, plants tend to close stomata to reduce transpiratory losses. The remaining level of CO2 inside the leaf is rapidly decreased and O2 is available in excess, resulting in high rates of RuBP oxygenation.
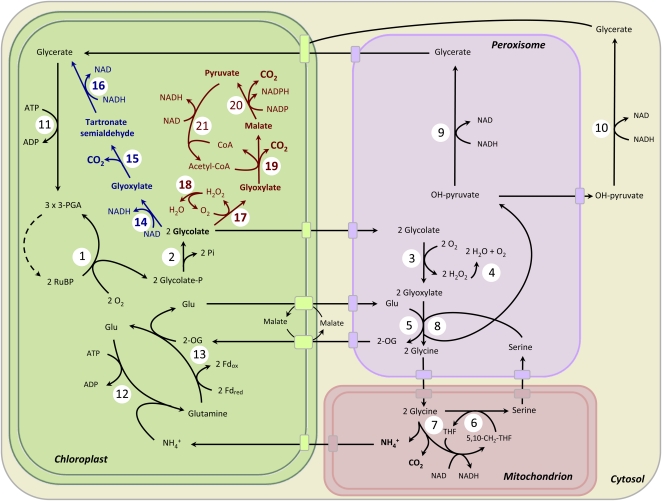
Eventually, the photorespiratory pathway evolved to make the best of this problematic situation. Its function is to convert 2-PG synthesized by the oxygenase activity of Rubisco back to 3-PGA recovering 75% of the carbon. It is seemingly difficult biochemically to synthesize a C3 compound or a C6 compound from a C2 compound such as 2-PG. Thus, photorespiration is made up from a complex series of reactions taking place mainly in the chloroplast, the peroxisome, and the mitochondrion (see Fig. 1; for review, see Maurino and Peterhansel, 2010): 2-PG is dephosphorylated to glycolate in the chloroplast and transported to the peroxisome where it is oxidized to glyoxylate. O2 is the electron donor in this reaction and the resulting hydrogen peroxide (H2O2) is detoxified by a peroxisomal catalase. Glyoxylate is transaminated to Gly that is transported to the mitochondrion. In a composite biochemical reaction, two molecules of Gly are converted to one molecule of Ser, the first C3 compound in the pathway, and the remaining carbon and nitrogen is released as CO2 and ammonia (NH3), respectively. This reaction is the reason for the negative reputation of photorespiration as a wasteful pathway, because molecules are released that had been fixed before in energy-consuming reactions. This may limit plant biomass production dependent on the degree of RuBP oxygenation. The Ser molecule resulting from this reaction is transported back to the peroxisome. The amine group is used to form a new Gly molecule from glyoxylate and the resulting hydroxypyruvate is reduced to glycerate. Finally, glycerate is phosphorylated in the chloroplast to form 3-PGA, which can be fed back to the Calvin cycle.
Figure 1.
The photorespiratory pathway (black) short circuited by the bacterial glycolate pathway (blue) and alternatively by the intracholorplastic glycolate oxidation pathway (red). Enzymes overexpressed for the full functioning of these pathways are highlighted in bold. 1, Rubisco; 2, 2-PG phosphatase; 3 and 17, GO; 4 and 18, catalase; 5, Glu-glyoxylate aminotransferase; 6, Gly decarboxylase; 7, Ser hydroxymethyl transferase; 8, Ser-glyoxylate aminotransferase; 9 and 10, hydroxypyruvate reductase; 11, glycerate kinase; 12, Gln synthetase; 13, Gln-oxoglutarate aminotransferase; 14, GlcDH; 15, glyoxylate carboligase; 16, tartronate semialdehyde reductase; 19, malate synthase; 20, NADP-malic enzyme; 21, pyruvate dehydrogenase. THF, Tetrahydrofolate; 5,10-CH2-THF, 5,10-methylenetetrahydrofolate. Modified from Maurino and Peterhansel (2010).
In this Update article, we discuss opportunities to improve plant performance by metabolic engineering of photorespiration. We summarize recent data about the vital importance of photorespiration, the existence of alternative detours to the core cycle, and the multiple overlaps of photorespiration with other pathways of primary plant metabolism. On this basis, we discuss how photorespiratory losses could be reduced and whether there is hope that this will enhance productivity of crop plants in the field.
PHOTORESPIRATION IS ESSENTIAL EVEN FOR PLANTS WITH LOW RATES OF RUBP OXYGENATION
The photorespiratory pathway, as described above (Fig. 1), has been mainly identified by mutational studies. Mutants in most enzymes of this pathway cannot survive in normal atmosphere with 21% O2 and 0.04% CO2; however, they can be rescued in low O2 or high CO2 conditions (Somerville, 2001; Boldt et al., 2005). It was generally assumed that such conditionally lethal phenotypes were due to the high carbon losses associated with the inability to recycle 2-PG from the oxygenase activity of Rubisco. Consequently, a minor role was expected for photorespiration in plants that express carbon-concentrating mechanisms resulting in low photorespiratory rates. C4 plants such as maize (Zea mays) and sugarcane (Saccharum officinarum) established a CO2 pump that enhances inorganic carbon concentration in the vicinity of Rubisco (Sage, 2004). CO2 is taken up in mesophyll cells by an oxygen-insensitive carboxylase and released by specific decarboxylases in bundle sheath cells around veins where Rubisco is located (Drincovich et al., 2010). This strongly reduces the oxygenation of RuBP. Genes and enzymes related to photorespiration are found in maize (Popov et al., 2003) and it remained an open question whether photorespiration in C4 plants is just an evolutionary relict. This question has been recently answered by characterization of a maize line with a transposon insertion in the gene encoding the major isoform of peroxisomal glycolate oxidase (GO; Zelitch et al., 2009). The mutant only survived at elevated CO2 and accumulated glycolate when grown at current ambient levels of CO2, typical attributes of a photorespiratory mutant of a C3 plant. Carbon losses are probably little as the rate of RuBP oxygenation is low in maize, thus, accumulation of photorespiratory intermediates is the most probable reason for the phenotype. 2-PG has been shown to inhibit enzymes of the Calvin cycle involved in RuBP regeneration (Anderson, 1971) and of starch breakdown (Kelly and Latzko, 1976), respectively, but we know quite little about whether this is the major reason for low photosynthesis in the photorespiratory mutants, how this exactly works, and whether other so-far-unknown inhibitory effects cause the observed growth retardation.
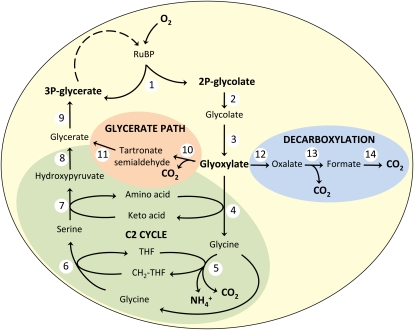
Independently, similar results were obtained from the characterization of mutants of the cyanobacterium Synechocystis (Eisenhut et al., 2008). Albeit based on a different biochemical principle, Synechocystis is also capable of concentrating CO2 near Rubisco and suppressing RuBP oxygenation (Price et al., 2008). Knockout of photorespiratory metabolism in this species was rather difficult as Synechocystis expresses at least three pathways for the metabolism of 2-PG (see Fig. 2; Eisenhut et al., 2008). The first pathway is similar to the photorespiratory cycle of higher plants described above, suggesting that this pathway has been transferred to eukaryotes concomitantly with photosynthesis during primary cyanobacterial endosymbiosis. The second pathway resembles bacterial glycolate metabolism (Pellicer et al., 1996). Here, glycolate is oxidized to glyoxylate by a glycolate dehydrogenase (GlcDH) that uses organic cofactors and does not produce H2O2 such as GO in plant peroxisomes. In a second step, two molecules of glyoxylate form one molecule of the 3C compound tartronic semialdehyde. During this reaction, CO2 is released similar to the higher plant pathway. The reaction product tartronic seminaldehyde is reduced to glycerate that is phosphorylated to 3-PGA, which can enter the Calvin cycle. Thus, the bacterial pathway is something like a short version of the higher plant pathway that does not require transamination. Knockout of both pathways resulted in Synechocystis cells that showed reduced growth, but were still able to survive at normal CO2 concentrations. However, by further mutagenesis, a third pathway was identified. Here, glycolate is completely oxidized to CO2 through four successive oxidative reactions with glyoxylate, oxalate, and formate as probable intermediates. Only if this pathway is also disrupted, Synechocystis requires enhanced CO2 for growth. From these data, we can learn two things: (1) Evidently, all organisms performing oxygenic photosynthesis require photorespiration to survive, because even low amounts of 2-PG synthesis are unacceptable for the cell when this compound or other intermediates of photorespiration accumulate, and (2) there is an unexpected plasticity in photorespiratory metabolism already in unicellular prokaryotic photosynthetic cells.
Figure 2.
Three pathways cooperate in the metabolism of 2-PG in Synechocystis: a plant-like C2 cycle, a bacterial-like glycerate path, and a decarboxylation pathway. 1, Rubisco; 2, 2-PG phosphatase; 3, GlcDH; 4, aminotransferase; 5, Gly decarboxylase; 6, Ser hydroxymethyl transferase; 7, aminotransferase; 8, hydroxypyruvate reductase; 9, glycerate kinase; 10, glyoxylate carboligase; 11, tartronate semialdehyde reductase; 12, hydroxyacid dehydrogenase; 13, oxalate decarboxylase; 14, formate dehydrogenase. THF, Tetrahydrofolate; CH2-THF, 5,10-methylenetetrahydrofolate. Modified from Eisenhut et al. (2008).
THE PHOTORESPIRATORY CYCLE IS MORE COMPLEX THAN ANTICIPATED
If photorespiration is so flexible in Synechocystis and if the higher plant pathway has been taken up by endosymbiosis, then we would expect that the alternative routes to metabolize glycolate have been transferred concomitantly. Homologs to the enzymes of the bacterial pathway are not found in the genome of Arabidopsis, with the exception of a GlcDH homolog that has been relocated to mitochondria (Bari et al., 2004; see below). For the components of the third Synechocystis pathway, also no genes have been readily identified in plant genomes. However biochemical data exist suggesting that such a pathway is present in higher plant chloroplasts, because isolated chloroplasts can form CO2 from both glycolate and glyoxylate (Zelitch, 1972; Goyal and Tolbert, 1996).
Despite limited evolutionary conservation of the Synechocystis alternative pathways, there is evidence for other alternative metabolic routes for photorespiratory intermediates in higher plants: (1) It has been reported that glyoxylate can spontaneously react to form formate and CO2 in peroxisomes in the presence of H2O2 (Wingler et al., 1999). The resulting formate can be used to form C1 compounds for the conversion of Ser to Gly in the mitochondrion. This reaction was only detectable in mutants lacking the core pathway, but might play a role in plants with low nitrogen supply as any transamination or nitrogen release reaction is circumvented in this alternative pathway. (2) In both monocots and dicots, knockout mutants in the peroxisomal hydroxypyruvate reductase (HYDROXYPYRUVATE REDUCTASE1 [HPR1]) are not highly affected by growth in air (Murray et al., 1989; Timm et al., 2008). In Arabidopsis, a cytoplasmic hydroxypyruvate reductase (HPR2) provides a bypass to the core cycle as the combined deletion of both isoforms results in air-sensitive plants with reduced photosynthetic performance (Timm et al., 2008). This cytosolic bypass might operate at higher rates in conditions where intraperoxisomal NADH levels are low. (3) The peroxisome and the chloroplast might not be the only organelles where glycolate can be oxidized, but this reaction might also take place in mitochondria. As mentioned above, Arabidopsis mitochondria contain a homolog to the bacterial GlcDH. This homolog is also found in unicellular eukaryotic green algae such as Chlamydomonas and seemingly the only glycolate-oxidizing enzyme in these cells (Nakamura et al., 2005). Algae of this group lack typical peroxisomes and, thus, do not possess a higher plant-type GO. Physiological analysis of Arabidopsis knockout mutants for the mitochondrial GlcDH homolog revealed that the enzyme contributes to photorespiration (Niessen et al., 2007). However, the enzymatic properties of the purified enzyme rather suggested that it is involved in d-lactate metabolism (Engqvist et al., 2009). All GlcDH enzymes accept both glycolate and d-lactate as substrates, but a much higher catalytic efficiency with d-lactate was reported for the Arabidopsis enzyme. So, the existence of mitochondrial glycolate oxidation in higher plants is still under debate.
REDUCING PHOTORESPIRATORY LOSSES ENHANCES GROWTH IN ARABIDOPSIS
Since carbon, nitrogen, and energy losses associated with photorespiration have been discovered, scientists tried to identify mutants with reduced photorespiration and higher photosynthesis and yield. However, despite significant efforts, all mutants in photorespiratory enzymes showed stunted growth and chlorosis most probably because 2-PG and other intermediates accumulated in their cells (see above). The suggested alternative pathways probably did not have the capacity to metabolize the huge amounts of 2-PG synthesized in a higher plant leaf. Zelitch (1992) described the selection for natural variation in photorespiration in a population of tobacco (Nicotiana tabacum) plants. Here, plants with low photorespiration showed higher photosynthesis and growth due to higher levels of peroxisomal catalase; however, the effect could not be stabilized in successive generations, but disappeared occasionally. Because of the limited success of such approaches, researchers started to use the carbon-concentrating mechanisms of C4 plants as blueprints to design novel pathways aiming to reduce 2-PG synthesis by Rubisco in transgenic plants. The complexity of the approach and the poor development of transgenic technology during these times are reasons why these attempts were not successful, although some promising physiological results were obtained (Matsuoka et al., 2001). These ideas have recently been revitalized by plant scientists with the hope to find solutions for the increased food demand of the fast-growing world population (Hibberd et al., 2008). Based on better technologies and the advance of our knowledge about plant metabolism, a new attempt toward transferring C4 properties to C3 plants with a focus on rice (Oryza sativa) is being made. However, still more information is necessary particularly about the establishment and regulation of C4 anatomy and C4-type chloroplast development. Moreover, it has to be considered that all carbon-concentrating mechanisms inevitably consume energy and are therefore most efficient when rates of RuBP oxygenation would be otherwise high. At low expected oxygenation rates and low availability of energy from sunlight, the energy input into a C4 cycle might rather reduce than increase photosynthesis.
In parallel to the described attempts aiming to reduce RuBP oxygenation, our labs started independent efforts that were based on another idea: If the oxygenase activity of Rubisco is inevitable, let’s make the best of the resulting 2-PG. Without knowing about the alternative pathways in Synechocystis (see above), the Peterhansel group started to establish the bacterial glycolate pathway in Arabidopsis chloroplasts (Fig. 1; Kebeish et al., 2007), whereas the Maurino/Flügge group designed a novel pathway that fully oxidized glycolate to CO2 in plant chloroplasts (Fig. 1; Maurino and Flügge, 2009). For the latter pathway, three new genes coding for GO, malate synthase, and catalase were overexpressed in Arabidopsis chloroplasts. Glycolate formed by the oxygenase activity of Rubisco is converted into glyoxylate by the novel plastidal GO and H2O2 is produced. In the next step, malate synthase generates malate by condensing a C2 unit from acetyl-CoA with glyoxylate. Malate is further decarboxylated to pyruvate by the chloroplastic NADP-malic enzyme, rendering NADPH and CO2. Finally, chloroplastic pyruvate dehydrogenase converts pyruvate into acetyl-CoA, yielding NADH and another molecule of CO2. The intraplastidic H2O2 produced in the GO reaction is detoxified by catalase. As a result of this cycle, one molecule of glycolate is converted by oxidation into two molecules of CO2, and reducing power in the form of NADPH and NADH is produced.
Both pathways have several possible advantages compared to the major photorespiratory pathway: (1) In both cases, CO2 is produced in chloroplasts and potentially enhances the CO2 concentration in the vicinity of Rubisco. (2) Both pathways avoid transamination reactions, by this ammonia release, and the energy costs for refixation. (3) In both pathways, additional reducing equivalents are produced in the chloroplast. This most probably provides an advantage at low-light availability when oxidized NAD(P) is still available. However, under high-light conditions, reducing power might be available in excess. One probably essential function of the major photorespiratory pathway under these conditions is the export of reducing equivalents from the chloroplast instead of the production of even more reducing equivalents (Kozaki and Takeba, 1996). Thus, the transgenic pathways would be counterproductive or run at low efficiency against a steep NADP(H) gradient under such conditions.
Overexpression of the bacterial pathway in Arabidopsis chloroplasts resulted in an increase in leaf biomass of 30% in average at the end of the growth period (Kebeish et al., 2007). Based on physiological data, CO2 was in fact concentrated in chloroplasts and the flux of metabolites through the major photorespiratory pathway was reduced. Unexpectedly, most of these effects were already obtained when the bacterial GlcDH alone was overexpressed. The two additional enzymes of the pathway catalyzing the conversion of glyoxylate to glycerate only slightly improved physiological parameters and growth. This provided additional evidence for a chloroplastic glycolate oxidation pathway in wild-type plants that is boosted by overexpression of GlcDH. Such interaction of endogenous and transgenic pathways was also observed for the second transgenic approach in which transgenic lines expressing the novel pathway produced more leaves, had higher fresh and dry weight, and displayed higher photosynthetic capacities (Maurino and Flügge, 2009). The successful establishment of this pathway and the improved growth of plants overexpressing the functional pathway provide independent evidence that diverting glycolate metabolism from the photorespiratory pathway in the chloroplast in fact can improve photosynthesis.
WHAT CAN WE EXPECT FOR CROPS IN THE FIELD?
Enhanced growth, as described above, was observed with transgenic Arabidopsis plants under optimal water and nitrogen supply that were grown under short days and limited light availability to extend the vegetative phase. Results obtained under such conditions might be of limited relevance for crops in the field that are exposed to multiple and varying stresses during their life cycle. However, field experiments under enhanced CO2 supply indicated that a suppression of photorespiration indeed resulted in higher growth and more yield (Long et al., 2006). Nevertheless, it was also observed that the improvements were less than predicted from studies in closed cabinets. Hence, how much do crops in the field suffer from a reduction in RuBP oxygenation? Problems with overreduction of the chloroplast have already been discussed above. Others have argued that photorespiration might participate in defense reactions by producing H2O2 (Taler et al., 2004) or that Gly and Ser produced during photorespiration are used by the plant for multiple other purposes (e.g. Madore and Grodzinski, 1984). An interesting additional argument comes from a recent study by Bloom et al. (2010) on nitrogen assimilation under conditions of low RuBP oxygenation (low O2 or high CO2). After prolonged exposure to such conditions, plants showed lower capacities to reduce nitrate (NO3−), whereas the assimilation of reduced ammonia (NH4+) was unaffected. The authors argued that this is caused by the lower availability of reducing equivalents in the cytosol (because less reducing equivalents are exported at lower photorespiratory flux), the interference of enhanced carbon availability with nitrite uptake into the chloroplast (because dissolved CO2 and nitrite might use the same transporters), or the competition of enhanced photosynthesis with nitrite reduction for reducing power in the chloroplast. Whereas the first proposed reason might also occur in crops overexpressing pathways for glycolate conversion in the chloroplast, interference with nitrite uptake or the availability of reducing power are not expected, because CO2 is produced inside the chloroplast and additional reducing equivalents are provided.
The field experiments under elevated CO2 mentioned above were not performed to test whether glycolate oxidation in the chloroplast might enhance yield, but because atmospheric CO2 concentrations will rise to levels double as high as today until the year 2100 (Intergovernmental Panel on Climate Change, 2007). Consequently, carboxylation efficiency of Rubisco will increase and this will result in increased biomass production by C3 crops (Long et al., 2006). RuBP oxygenation will be strongly reduced, posing the obvious question of whether reduction of photorespiratory losses is still a valuable target for improvement of future crops. Based on an evolutionary algorithm, Zhu et al. (2007) suggested that, already at current CO2 concentrations, a reduction in the amount of nitrogen invested into photorespiratory proteins and a simultaneous increase in the concentrations of photosynthetic enzymes could optimize the photosynthetic rate. The outcome would be probably even more dramatic at elevated CO2. Thus, reducing the plant’s investment into photorespiratory enzymes might be the most straightforward redesign of photorespiration that can result in enhanced productivity of future crops. However, additional knowledge about the regulation of photorespiratory gene expression is necessary to implement such approaches in novel crops.
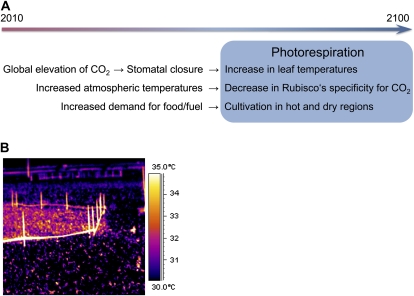
The calculations by Zhu et al. (2007) were performed for plants under standardized growth conditions and in the absence of stress with the exception of limited nitrogen availability. However, it is expected that climate change will impose additional stress on many agricultural production systems (Fig. 3A). In the future, we will probably have to produce food on less favorable grounds to nourish the increasing world population especially in developing countries. These are mostly hot and dry regions of the world where we expect high rates of photorespiration even with increased atmospheric CO2 levels. Furthermore, a decrease in Rubisco’s specificity for CO2 by about 10% can be anticipated if the average atmospheric temperature increases by 3°C as predicted (Jordan and Ogren, 1984; Intergovernmental Panel on Climate Change, 2007). This effect might be aggravated as plants tend to close stomata at elevated CO2, which will reduce transpirational leaf cooling (Ainsworth and Rogers, 2007). The resulting increased leaf temperatures are easily observable on infrared pictures of fields where a limited area has been exposed to elevated CO2 levels (Fig. 3B). Thus, photorespiratory losses will be significant in a future high CO2 atmosphere.
Figure 3.
Photorespiration in future agricultural production systems. A, Impact of climate change and the increased demands for food and fuel on photorespiratory losses. B, Thermal image of a soybean (Glycine max) field where a limited area has been exposed to elevated CO2 concentrations (courtesy of Andrew Leakey, reprinted with permission from Long et al., 2004).
References
- Ainsworth EA, Rogers A. (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 [DOI] [PubMed] [Google Scholar]
- Anderson LE. (1971) Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta 235: 237–244 [DOI] [PubMed] [Google Scholar]
- Bari R, Kebeish R, Kalamajka R, Rademacher T, Peterhänsel C. (2004) A glycolate dehydrogenase in the mitochondria of Arabidopsis thaliana. J Exp Bot 55: 623–630 [DOI] [PubMed] [Google Scholar]
- Boldt R, Edner C, Kolukisaoglu U, Hagemann M, Weckwerth W, Wienkoop S, Morgenthal K, Bauwe H. (2005) D-GLYCERATE 3-KINASE, the last unknown enzyme in the photorespiratory cycle in Arabidopsis, belongs to a novel kinase family. Plant Cell 17: 2413–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Burger M, Rubio Asensio JS, Cousins AB. (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328: 899–903 [DOI] [PubMed] [Google Scholar]
- Buick R. (2008) When did oxygenic photosynthesis evolve? Philos Trans R Soc Lond B Biol Sci 363: 2731–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drincovich MF, Lara M, Maurino VG, Andreo C. (2010) C4 decarboxylases. Different solutions for the same biochemical problem, the provision of CO2 in the bundle sheath cells. Raghavendra A, Sage RF, , C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Springer, Heidelberg, pp 277–300 [Google Scholar]
- Eisenhut M, Ruth W, Haimovich M, Bauwe H, Kaplan A, Hagemann M. (2008) The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc Natl Acad Sci USA 105: 17199–17204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist M, Drincovich MF, Flügge UI, Maurino VG. (2009) Two D-2-hydroxy-acid dehydrogenases in Arabidopsis thaliana with catalytic capacities to participate in the last reactions of the methylglyoxal and β-oxidation pathways. J Biol Chem 284: 25026–25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Tolbert NE. (1996) Association of glycolate oxidation with photosynthetic electron transport in plant and algal chloroplasts. Proc Natl Acad Sci USA 93: 3319–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd JM, Sheehy JE, Langdale JA. (2008) Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr Opin Plant Biol 11: 228–231 [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (2007) Climate change 2007: synthesis report. http://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_synthesis_report.htm (June 30, 2010)
- Jordan DB, Ogren WL. (1984) The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Planta 161: 308–313 [DOI] [PubMed] [Google Scholar]
- Kasting JF, Ono S. (2006) Palaeoclimates: the first two billion years. Philos Trans R Soc Lond B Biol Sci 361: 917–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebeish R, Niessen M, Thiruveedhi K, Bari R, Hirsch HJ, Rosenkranz R, Stäbler N, Schönfeld B, Kreuzaler F, Peterhänsel C. (2007) Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat Biotechnol 25: 593–599 [DOI] [PubMed] [Google Scholar]
- Kelly GJ, Latzko E. (1976) Inhibition of spinach-leaf phosphofructokinase by 2-phosphoglycollate. FEBS Lett 68: 55–58 [DOI] [PubMed] [Google Scholar]
- Kozaki A, Takeba G. (1996) Photorespiration protects C3 plants from photooxidation. Nature 384: 557–560 [Google Scholar]
- Ku SB, Edwards GE. (1977) Oxygen inhibition of photosynthesis. I. Temperature dependence and relation to O2/CO2 solubility ratio. Plant Physiol 59: 986–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR. (2006) Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312: 1918–1921 [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591–628 [DOI] [PubMed] [Google Scholar]
- Madore M, Grodzinski B. (1984) Effect of oxygen concentration on C-photoassimilate transport from leaves of Salvia splendens L. Plant Physiol 76: 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Furbank RT, Fukayama H, Miyao M. (2001) Molecular engineering of C4 photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 52: 297–314 [DOI] [PubMed] [Google Scholar]
- Maurino VG, Flügge UI, inventors August 27, 2009. Means for improving agrobiological traits in a plant by providing a plant cell comprising in its chloroplasts enzymatic activities for converting glycolate into malate. Patent Application No. WO2009103782 [Google Scholar]
- Maurino VG, Peterhansel C. (2010) Photorespiration: current status and approaches for metabolic engineering. Curr Opin Plant Biol 13: 249–256 [DOI] [PubMed] [Google Scholar]
- Murray AJS, Blackwell RD, Lea PJ. (1989) Metabolism of hydroxypyruvate in a mutant of barley lacking NADH-dependent hydroxypyruvate reductase, an important photorespiratory enzyme activity. Plant Physiol 91: 395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Kanakagiri S, Van K, He W, Spalding M. (2005) Disruption of the glycolate dehydrogenase gene in the high-CO2-requiring mutant HCR89 of Chlamydomonas reinhardtii. Can J Bot 83: 820–833 [Google Scholar]
- Niessen M, Thiruveedhi K, Rosenkranz R, Kebeish R, Hirsch HJ, Kreuzaler F, Peterhänsel C. (2007) Mitochondrial glycolate oxidation contributes to photorespiration in higher plants. J Exp Bot 58: 2709–2715 [DOI] [PubMed] [Google Scholar]
- Pellicer MT, Badía J, Aguilar J, Baldomà L. (1996) glc locus of Escherichia coli: characterization of genes encoding the subunits of glycolate oxidase and the glc regulator protein. J Bacteriol 178: 2051–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov VN, Dmitrieva EA, Eprintsev AT, Igamberdiev AU. (2003) Glycolate oxidase isoforms are distributed between the bundle sheath and mesophyll tissues of maize leaves. J Plant Physiol 160: 851–857 [DOI] [PubMed] [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. (2008) Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot 59: 1441–1461 [DOI] [PubMed] [Google Scholar]
- Sage RF. (2004) The evolution of C4 photosynthesis. New Phytol 161: 341–370 [DOI] [PubMed] [Google Scholar]
- Somerville CR. (2001) An early Arabidopsis demonstration: resolving a few issues concerning photorespiration. Plant Physiol 125: 20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taler D, Galperin M, Benjamin I, Cohen Y, Kenigsbuch D. (2004) Plant eR genes that encode photorespiratory enzymes confer resistance against disease. Plant Cell 16: 172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez GG, Farquhar GD, Andrews TJ. (2006) Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci USA 103: 7246–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm S, Nunes-Nesi A, Pärnik T, Morgenthal K, Wienkoop S, Keerberg O, Weckwerth W, Kleczkowski LA, Fernie AR, Bauwe H. (2008) A cytosolic pathway for the conversion of hydroxypyruvate to glycerate during photorespiration in Arabidopsis. Plant Cell 20: 2848–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Leegood RC. (1999) Photorespiratory metabolism of glyoxylate and formate in glycine-accumulating mutants of barley and Amaranthus edulis. Planta 207: 518–526 [Google Scholar]
- Zelitch I. (1972) The photooxidation of glyoxylate by envelope-free spinach chloroplasts and its relation to photorespiration. Arch Biochem Biophys 150: 698–707 [DOI] [PubMed] [Google Scholar]
- Zelitch I. (1992) Control of plant productivity by regulation of photorespiration. Bioscience 42: 510–516 [Google Scholar]
- Zelitch I, Schultes NP, Peterson RB, Brown P, Brutnell TP. (2009) High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiol 149: 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, de Sturler E, Long SP. (2007) Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol 145: 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]