Abstract
Oilseed plants like Arabidopsis (Arabidopsis thaliana) develop green photosynthetically active embryos. Upon seed maturation, the embryonic chloroplasts degenerate into a highly reduced plastid type called the eoplast. Upon germination, eoplasts redifferentiate into chloroplasts and other plastid types. Here, we describe seedling plastid development1 (spd1), an Arabidopsis seedling albino mutant capable of producing normal green vegetative tissues. Mutant seedlings also display defects in etioplast and amyloplast development. Precocious germination of spd1 embryos showed that the albino seedling phenotype of spd1 was dependent on the passage of developing embryos through the degreening and dehydration stages of seed maturation, suggesting that SPD1 is critical during eoplast development or early stages of eoplast redifferentiation. The SPD1 gene was found to encode a protein containing a putative chloroplast-targeting sequence in its amino terminus and also domains common to P-loop ATPases. Chloroplast localization of the SPD1 protein was confirmed by targeting assays in vivo and in vitro. Although the exact function of SPD1 remains to be defined, our findings reveal aspects of plastid development unique to embryo-derived cells.
During seed development in some plants, embryos contain photosynthetically active chloroplasts that can refix respiration-derived carbon dioxide (King et al., 1998) and improve carbon sequestration of photosynthate into seed storage products (Borisjuk et al., 2003; Ruuska et al., 2004; Goffman et al., 2005; Allen et al., 2009). Plastids are also responsible for numerous other essential metabolic activities, including the biosynthesis of nucleic acids, amino acids, and various lipids during all stages of plant development (Galili, 1995; Ohlrogge and Browse, 1995).
Chloroplasts develop in Arabidopsis (Arabidopsis thaliana) embryos as early as 48 h after fertilization and persist for approximately 10 d (Mansfield and Briarty, 1991). During seed maturation, the embryos become colorless as chloroplasts dedifferentiate to nonphotosynthetic plastids by losing their chlorophyll, internal membrane structures, and starch (Mansfield and Briarty, 1992). Upon germination, these dedifferentiated plastids, termed eoplasts, are converted into the various plastid types required for proper seedling development, such as chloroplasts in shoots and amyloplasts in the hypocotyls and root tips (Whatley, 1978; Saito et al., 1989).
Little is known about the molecular requirements for plastid development in embryo-derived cells. Studies of several mutants with seedling-specific chloroplast defects, which result in albino, pale-green, or yellow-green phenotypes, suggest that plastid development in embryo-derived cells may have different molecular requirements than needed for plastid biogenesis in adult leaf tissues (Ishizaki et al., 2005; Shimada et al., 2007; Chi et al., 2008). This report describes a mutant named seedling plastid development1 (spd1) in which plastid development is specifically affected during embryo maturation and seedling development in Arabidopsis.
RESULTS
spd1 Growth Phenotype
The spd1 mutant was isolated from a fast-neutron-mutagenized Arabidopsis seedling population in a screen to identify mutants with reduced hypocotyl gravitropism. The most striking phenotype of the spd1 mutation was observed in light-grown seedlings, where, in contrast to wild-type seedlings (Fig. 1A), the cotyledons and upper hypocotyl of spd1 appeared mostly albino but often contained small clusters of green cells (Fig. 1, B and C). The spd1 seedling albino phenotype was found to segregate as expected for a nuclear-recessive mutation (25.2% of 1,636 F2 plants exhibited the spd1 white-cotyledon phenotype; χ2 = 0.02). Reciprocal crosses to the parental ecotype (Columbia) produced green F1 seedlings that were indistinguishable from the wild type. There was no change in the spd1 phenotype after being backcrossed to the wild type five times, supporting the idea that the phenotype was due to a mutation at a single locus.
Figure 1.
Phenotypes of spd1 seedlings. A, Eight-day-old wild-type seedlings with green cotyledons. B, Eight-day-old spd1 seedlings with albino cotyledons and first true leaves that appear as small opposing spikes (see arrows). C, Eight-day-old spd1 seedlings from a segregating T1 line transformed with a construct carrying 35S::SPD1-YFP-HA. The green seedling (indicated with a black arrow) was confirmed by genomic PCR to be a transformant. Note patches of green cells in mostly albino spd1 cotyledons in B and C. D, Yellow pigmentation of etiolated cotyledons of a 4-d-old dark-grown wild-type seedling. E, Lack of pigmentation in etiolated cotyledons of a 4-d-old dark-grown spd1 seedling.
When grown on solid medium containing 2% Suc, about 95% of homozygous spd1 seedlings survived and began developing green tissues similar in appearance to the wild type (compare true leaves in Fig. 1, A–C). Once the initial true leaves formed, the seedlings could be transplanted to soil, and all subsequent growth was visually indistinguishable from the wild type (see rosette leaf chlorophyll levels in Table I). By contrast, when germinated on solid medium lacking a supplemental carbon source or in potting soil, fewer than 5% of spd1 seedlings produced green true leaves and survived into adulthood. Seedlings that survived in the absence of Suc invariably had green sectors in their cotyledons or hypocotyls, as seen in most mutants grown on Suc-containing medium (Fig. 1B; see below). We also occasionally observed spd1 seedlings that produced albino or partly albino first true leaves when growing on Suc-supplemented medium (Fig. 1B). When this occurred, those leaves were narrow and pointed and usually failed to grow beyond 2 mm in length. However, these mutants developed subsequent rosette leaves that were indistinguishable from those of wild-type plants. Growth under different fluence rates of white light (3–150 μmol m−2 s−1) had no obvious effect on the extent of the spd1 albino phenotype (data not shown), indicating that the seedling albinism was not due to photobleaching. In addition, the spd1 cotyledons grown in darkness lacked pigmentation (Fig. 1E), in contrast to the yellow cotyledons of the wild type (Fig. 1D), indicating that the plastid defect in the mutant seedling tissues was light independent. There were no differences in the morphology of etiolated spd1 and wild-type seedlings.
Table I. Chlorophyll content in spd1 and 5-week-old wild-type rosette leaves.
Chlorophyll (Chl) was measured per mg fresh weight. Data are shown with se. n = 5 for both.
| Leaf | μg Chl mg−1 | Chl a/b Ratio |
| Wild type | 1.150 ± 0.003 | 3.280 ± 0.004 |
| spd1 | 1.150 ± 0.006 | 3.250 ± 0.008 |
spd1 Cotyledon Variegation and Cell Size
When present, the green sectors in spd1 seedlings were more frequently found near the center of their cotyledons (Fig. 1, B and C). These green cells had a mean plan area 1,158% greater than did the white cells. The mean plan area of green spd1 cells was also 217% larger than green cotyledon mesophyll cells in wild-type seedlings. To test if this difference in the cell area was due to the number of plastids, the relationship between plastid number per mesophyll cell and the cell area for both wild-type and spd1 mesophyll cells was measured (Fig. 2). The number of chloroplasts per cell in green spd1 cotyledon cells was 226% greater than that in corresponding green wild-type cells. The increased plastid number showed a highly significant correlation with cell size. Regression analysis of the data showed a strong correlation between chloroplast number and cell size for wild-type and mutant data sets individually (r2 > 0.8) and a stronger correlation when the two data sets were combined (r2 = 0.911). Also, the plastid number observed in white spd1 cotyledon cells (Fig. 2) is similar to what was reported for wild-type embryo cells (Mansfield and Briarty, 1992), indicating that the spd1 mutation did not alter eoplast numbers in embryos. The slope of the linear relationship between cell size and chloroplast number obtained in our study is comparable with the one reported previously (Pyke and Leech, 1991).
Figure 2.
Relationship between cotyledon mesophyll cell plan area and plastid number in light-grown wild-type and spd1 seedlings. Plastid number and cell plan area were measured in 30 wild-type and mutant cells from at least 10 seedlings. The regression line shows the best fit for the combined data set. Mean cotyledon cell areas were 2,161 μm2 for the wild type, 4,702 μm2 for spd1 green cells, and 406 μm2 for spd1 albino cells. Plastids in albino cells were observed in spd1 plants expressing a plastid-targeted GFP reporter protein. Mean plastid number per cotyledon cell was 56 in the wild type, 127 in green spd1 cells, and 27 in albino spd1 cells.
The Seedling Plastids of spd1 Exhibit Structural Abnormalities
Ultrastructural analysis of light-grown spd1 mutant cotyledon cells revealed the presence of degenerate organelle-like structures in albino cells (Fig. 3B) as compared with wild-type chloroplasts (Fig. 3A). These structures, several of which were found in each albino cell examined, appear to be surrounded by a double-membrane envelope and were of roughly similar size to normal chloroplasts but had no organized thylakoid membrane system. Instead, they usually contained round or oblong membrane-bound internal vesicles. In green cells from spd1 cotyledons, chloroplasts contained organized thylakoid membranes and appeared indistinguishable from those of the wild type (Fig. 3C). No cells were observed to contain a mixture or intermediate forms of these two plastid types.
Figure 3.
Ultrastructure of plastids in spd1 and wild-type cotyledon mesophyll cells. A, Wild-type cotyledon chloroplast showing thylakoid membrane organization. B, Putative degenerate plastid in spd1 showing no clear internal membrane structure. C, spd1 “green” cell chloroplast showing thylakoid membrane organization. D, Wild-type etioplast with characteristic crystalline prolamellar body. E, spd1 etiolated cotyledon parenchyma cell containing several globular bodies but lacking clear etioplasts. Bars = 500 nm in A to D and 2 μm in E.
In order to test if the degenerate structures in albino spd1 cells were plastids, we crossed spd1 with plants producing GFP linked to a plastid-targeting transit peptide from the Arabidopsis Recombination Protein A (RECA; Köhler et al., 1997). In the obtained plants, the RECA-GFP was found localized to chloroplasts in green cotyledon cells of wild-type plants and to what were presumably mutant plastids in albino spd1 cotyledon cells (Supplemental Fig. S1). Furthermore, in the albino spd1 cells, the RECA-GFP accumulation colocalized with diamidino-2-phenylindole (DAPI) staining except in the nucleus, which did not contain GFP. These data indicate that the degenerate organelle-like structures found in the spd1 mutants are plastids that retained protein-import capabilities.
Plastid development was also affected in etiolated spd1 seedlings. In 5-d-old etiolated wild-type cotyledon mesophyll cells, we observed numerous etioplasts containing characteristic prolamellar bodies (Fig. 3D). By contrast, no clear etioplasts were detected in cotyledon mesophyll cells of etiolated spd1 seedlings. Additionally, each cell exhibited other structural abnormalities, such as the lack of normally developed vacuoles (Fig. 3E). These data suggest that cellular development, including plastid biogenesis, is disrupted during or before germination of the mutant.
The spd1 Phenotype Is Dependent on Embryo Maturation
The phenotype of spd1 seedlings indicated that the tissues exhibiting plastid development defects upon germination were limited to those established during embryo development (i.e. in the cotyledon and hypocotyl). Hence, we hypothesized that spd1 would affect plastid development or differentiation during some or all stages of embryogenesis. We initially tested this by manually dissecting embryos from wild-type and spd1 siliques to visually track chloroplast development. From 6 d post anthesis (DPA) until maturity at 16 DPA, wild-type and spd1 embryos were identical in appearance with respect to developmental rate, morphology, and color (Fig. 4). Ultrastructural analysis of cotyledon cells from mutant and wild-type embryos at 9 DPA showed that chloroplast size and thylakoid membrane organization were also similar (Supplemental Fig. S2). Other aspects of cellular ultrastructure were also the same, including the formation of apparent protein and lipid bodies at 15 DPA, when maturation and desiccation were ensuing (Supplemental Fig. S2). At 15 DPA, we could not identify plastids in either spd1 or wild-type embryos due to the abundance of lipid and protein storage bodies.
Figure 4.
Wild-type (WT) and spd1 embryo development. Green embryo development in spd1 was similar to the wild type. Representative embryos dissected from ovules between 5 and 16 DPA are shown.
Since chloroplast development was similar during embryogenesis in wild-type and spd1 embryos, we hypothesized that processes during or after seed maturation were important in producing the spd1 mutant phenotype. To test this, spd1 and wild-type embryos were dissected from siliques between 11 and 16 DPA and precociously germinated. The embryos were allowed to develop for 7 d on agar medium containing 2% Suc. Embryos dissected at 11 DPA rarely germinated, whereas those 12 DPA and older readily germinated. After 7 d, seedlings obtained from precociously germinated 12-DPA embryos had wild-type levels of green area (Fig. 5), and the cotyledons and hypocotyls appeared similar to the wild type. Seedlings grown from embryos collected between 12 and 16 DPA had progressively less green area and correspondingly less chlorophyll per seedling (Fig. 5). Embryos isolated at 16 DPA produced mostly white seedlings that appeared like spd1 seedlings grown from mature seeds. These results suggest that the spd1 phenotype may manifest itself during the latest stages of embryogenesis, when the embryonic chloroplasts are dedifferentiating to eoplasts.
Figure 5.
Precocious germination can bypass development of albino cotyledons in the spd1 seedlings. Data show the percentage cotyledon area that was green (y axis) 7 d after seedlings had been precociously germinated from embryos dissected from ovules between 12 and 16 DPA. Data represent means ± se from at least 14 cotyledons. WT, Wild type.
A Lack of Amyloplast Development Correlates with Reduced spd1 Hypocotyl Gravitropism
Because spd1 was initially identified in a visual screen for mutants with reduced hypocotyl gravitropism, we examined spd1 seedlings for defects in amyloplast development by staining for the presence of starch-containing amyloplasts. The hypocotyls of etiolated spd1 seedlings were found to be devoid of starch granules (Fig. 6B) compared with the wild type (Fig. 6A). As seen in Figure 6C, the gravity response of etiolated spd1 mutant hypocotyls was significantly less than in the wild type, which is consistent with previously described agravitropic starchless mutants in Arabidopsis (Kiss et al., 1997) and with the gravity perception response by plants being initiated by the repositioning of amyloplasts (Sack, 1997). In contrast, starch accumulation and gravitropism of spd1 roots appeared comparable with those in the wild type (Fig. 6, D–F). Amyloplast development and the gravitropic response of the inflorescence in spd1 plants were also similar to those in the wild type (data not shown), indicating that the gravity defect of the spd1 mutation did not affect a general aspect of tropic responses. These findings indicate that the seedling-specific gravity defect in spd1 hypocotyls results from a defect in hypocotyl amyloplast development.
Figure 6.
Amyloplast development and gravitropism in 4-d-old wild-type (WT) and spd1 seedlings. A, Wild-type hypocotyl endodermal cells displayed abundant starch grains. B, spd1 hypocotyl endodermal cells were devoid of starch grains. C, Gravitropism measured as degrees of curvature of hypocotyls after gravistimulation. D and E, Columella cell amyloplasts in the root cap of the wild type (D) had abundant starch grains, as did those in spd1 (E). F, Gravitropism measured as degrees of curvature of roots after gravistimulation. Data represent means ± se; n = 20 for hypocotyls, n = 10 for roots.
Identification of the SPD1 Locus
To identify the genetic lesion responsible for the spd1 phenotype, we crossed spd1 to the Landsberg erecta wild-type ecotype of Arabidopsis and were able to map the SPD1 locus to an interval of 13 annotated genes on chromosome 3. Sequencing of the candidate genes in spd1 plants revealed a lesion in exon 5 of the At3g10420 gene. The lesion consists of replacement of three bases (GTT) with two bases (AA; Fig. 7A), consistent with results for fast-neutron mutagenesis (Li and Zhang, 2002). This results in a frameshift, causing a change of the encoded amino acid at residue 420 from Leu to Lys that is immediately followed by a premature stop codon (Fig. 7). Successful complementation of the spd1 phenotype with two independent constructs containing the At3g10420 gene sequence (35S::SPD1-YFP-HA, shown in Fig. 1C, and 35S::SPD1-GFP-HIS6, data not shown) confirmed that the mutation in At3g10420 is responsible for the spd1 white-cotyledon phenotype. Hence, we named At3g10420 as SPD1.
Figure 7.
The spd1 mutation. A, The spd1 mutation is the result of a GTT deletion and AA insertion (boldface) in At3g10420. This results in a premature stop codon, leading to a protein that lacks the most C-terminal 263 amino acids. The top line represents the DNA sequence and the bottom line is the encoded amino acid. B, Predicted protein structure of SPD1. SPD1 has a predicted chloroplast-targeting sequence (black) and an ATP hydrolysis domain (gray). The spd1 mutation results in a premature stop codon at the location indicated. Bar at bottom = 74 amino acids.
SPD1 Is Related to an AAA+ ATPase Protein
The SPD1 gene is predicted to encode a protein of 648 amino acids. The SPD1 protein contains a region with significant sequence similarity to the Bacillus subtilis stage III sporulation protein AA (SpoIIIAA), an AAA+ ATPase (for ATPases associated with various cellular activities) that plays a role during spore formation (Illing and Errington, 1991). More specifically, residues 217 to 340 of SPD1 show 31.8% sequence identity (E value = 3.7e-13) with the catalytic domain of SpoIIIAA (Supplemental Fig. S3). This region contains two key motifs of P-loop ATPases: the Walker A motif at amino acids 220 to 227 (GxxxxGK[T/S], where x is any amino acid; Walker et al., 1982) and the Walker B motif at amino acids 294 to 299 (hhhhDE, where h is typically a hydrophobic amino acid; Walker et al., 1982). Database searches also revealed the presence of SPD1 homologs in rice (Oryza sativa) and grape (Vitis vinifera) that share 43% and 29% global sequence identity, respectively (Supplemental Fig. S4). Furthermore, the Arabidopsis genome was found to encode two proteins with predicted chloroplast localization, At1g33290 and At1g73170, which show global identities of 30.5% and 44% with SPD1, respectively, in the predicted amino acid sequence (Supplemental Fig. S5). These findings suggest that SPD1 is one member of a gene family of AAA+ ATPases important to plant development.
SPD1 Localizes to Plastids
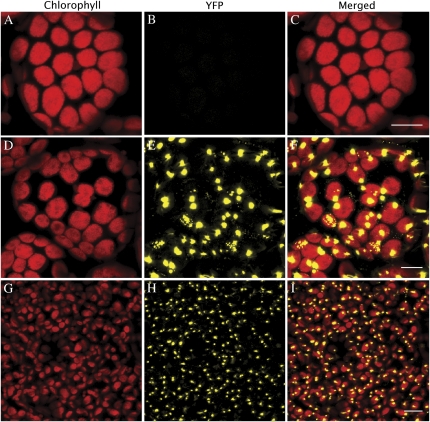
The SPD1 protein contains a predicted chloroplast-targeting sequence of 78 amino acids in its N terminus (Emanuelsson et al., 2000; Supplemental Fig. S5), consistent with a previous study that identified the protein in the chloroplast stroma proteome (Zybailov et al., 2008). We examined spd1 plants complemented with a construct encoding 35S::SPD1-YFP-HA or 35S::SPD1-GFP-HIS6 to confirm the plastid localization of SPD1. Confocal imaging of leaf mesophyll cells of the spd1;SPD1-YFP-HA plant showed an accumulation of the yellow fluorescent protein (YFP) signal in distinct foci that appear to be associated with the chloroplast envelope (Fig. 8; Supplemental Movie S1). Most chloroplasts were found to be associated with at least one SPD1-YFP-HA spot, but some chloroplasts were associated with multiple foci. Similar results were obtained in the mutant complemented with the GFP construct (spd1;SPD1-GFP-HIS6 in Supplemental Fig. S6) as well as in Nicotiana benthamiana mesophyll cells transiently producing the SPD1-GFP-HIS6 protein (Supplemental Fig. S7).
Figure 8.
In vivo cell imaging of SPD1-YFP-HA chloroplast localization. Leaf mesophyll cells in Arabidopsis plants transformed with a 35S::SPD1-YFP-HA construct showed the YFP signal localized to individual spots at or near the surface of the chloroplasts. Wild-type palisade mesophyll cells (A–C), 35S::SPD1-YFP-HA in spd1 palisade mesophyll cells (D–F), and 35S::SPD1-YFP-HA in spd1 cotyledon mesophyll cells (G–I) are shown. Bars = 10 μm.
To further confirm the chloroplast localization of the tagged SPD1, we isolated total protein and chloroplasts from spd1;SPD1-GFP-HIS6 leaves and subjected them to immunoblotting with an anti-His antibody (Fig. 9). The antibody against the His tag recognized a protein of approximately 110 kD in total protein extracts and isolated chloroplasts from complemented spd1 plants (Fig. 9A, lanes 3 and 4) but not in those from the wild type (Fig. 9A, lanes 1 and 2). The assay using a sodium carbonate wash of isolated chloroplasts revealed that SPD1-GFP-HIS6 was integrally associated with membranes, as were Tic110, Toc75, Toc159 (86-kD fragment; Fig. 9A, lane 7), and chlorophyll a/b-binding protein (Fig. 9B, lanes 2–4), but not the large subunit of Rubisco (Fig. 9B, lanes 2–4). SPD1-GFP-HIS6 was resistant to thermolysin, which digests proteins at the cytoplasmic surface of the outer envelope (Cline et al., 1984). Under the same conditions, the 86-kD fragment of Toc159 was completely digested to 52 kD, as shown by Agne et al. (2009), while two other envelope proteins (Toc75 and Tic110) remained intact (Fig. 9A, compare lanes 8 and 9). In the assay with trypsin, which can penetrate the outer but not the inner membrane of the chloroplast envelope (Jackson et al., 1998), the His-tagged SPD1 protein was partially digested (Fig. 9A, compare lanes 11 and 12). Under this condition, the 86-kD fragment of Toc159 was completely digested, whereas another outer envelope protein (Toc75) was partially degraded and an inner envelope protein (Tic110) remained intact (Fig. 9A, compare lanes 11 and 12). Susceptibility of SPD1-GFP-HIS6 to trypsin was further confirmed by a separate experiment, in which the His-tagged protein was completely digested whereas Toc75 was partially digested and Tic110 was fully resistant (Fig. 9C, compare lanes 1 and 2). These data suggest that the C-terminal tag of SPD1-GFP-HIS6 is located in either the outer membrane or the space between the outer and inner membranes of the chloroplast envelope. This result is consistent with the in vivo imaging data showing the GFP signal in the peripheral area of chloroplasts (Supplemental Fig. S6)
Figure 9.
Chloroplast localization of SPD1-GFP-HIS6. Chloroplasts (containing 12 μg of chlorophyll) isolated from the complemented plant (spd1;SPD1-GFP-HIS6) were lysed hypotonically and fractionated into supernatant (S1) and pellet. The pellet fraction was resuspended in 0.1 m Na2CO3 and again separated into supernatant (S2), which contains peripheral membrane proteins, and pellet (P), which contains integral membrane proteins. Chloroplasts (containing 12 μg of chlorophyll) were also treated with thermolysin (t-lysin) or trypsin (Trp) in the absence (−) or presence (+) of 1% Triton X-100 (Tx100). A, One-third of the resultant samples as well as proteins in total extracts (13 μg; t) or chloroplasts containing 4 μg of chlorophyll (chl) isolated from the wild type (wt) or the complemented plant were analyzed by 7.5% SDS-PAGE and immunoblotting with the anti-His antibody (top panel) or the mixture of antisera against Toc159, Tic110, and Toc75 (bottom panel). Signals corresponding to SPD1-GFP-HIS6, Tic110, the 86-kD fragment of Toc159, Toc75, and the 52-kD fragment of Toc159 are indicated at right as HIS, 110, 86, 75, and 52, respectively. B, Another one-third of S1, S2, and P fractions from the complemented chloroplasts as well as total chloroplasts containing 4 μg of chlorophyll were separated by 12% SDS-PAGE and analyzed by Coomassie Brilliant Blue staining. The large subunit of Rubisco (Rubisco LSU) and chlorophyll a/b-binding protein (CAB) are indicated at right. C, Immunoblots of chloroplasts (containing 10 μg of chlorophyll) from spd1;SPD1-GFP-HIS6 plants treated without or with trypsin (Trp) in the absence (−) or presence (+) of 1% Triton X-100 (Tx100), with antibodies against His tag (top), Tic110 (middle), or Toc75 (bottom).
We also performed an in vitro assay to examine chloroplast localization of nontagged SPD1. Although the predicted size of the translation product is 75 kD, the radiolabeled protein produced in vitro migrated around 100 kD (Fig. 10A, lane 1). The imported protein recovered in chloroplasts moved faster than the translation product (Fig. 10A, compare lanes 1 and 2), indicating the presence of a cleavable sequence. Similar to SPD1-GFP-HIS6 in the complemented spd1 plants, imported SPD1 was found resistant to thermolysin, which digested an outer envelope protein, digalactosyldiacylglycerol synthase 1 (DGD1), as shown by Froehlich et al. (2001; Fig. 10A, compare lanes 2 and 3), but was sensitive to trypsin (Fig. 10A, compare lanes 5 and 6). Interestingly, however, unlike the fusion protein in complemented spd1 plants, the nontagged SPD1 was mainly targeted to the soluble fraction of chloroplasts (Fig. 10B, compare lanes 2–4). Taken together, our data indicate that the nontagged SPD1 is targeted in vitro to the space between the outer and inner membranes of the chloroplast envelope.
Figure 10.
SPD1 is targeted to the chloroplast envelope with a shift in its mobility on SDS-PAGE. A, Radiolabeled precursor protein (tl; 10% of the translation product subjected to the assay) was incubated with isolated chloroplasts under import conditions. After reisolation, the chloroplasts were treated without or with thermolysin (t-lysin) or trypsin (Trp) in the absence (–) or presence (+) of 1% Triton X-100 (Tx100). B, After incubation, resultant chloroplasts containing imported proteins (imp) were reisolated and separated into S1, S2, and P fractions as described in the legend to Figure 9.
DISCUSSION
During Arabidopsis seed development, embryos contain photosynthetically functional chloroplasts until late stages of embryo maturation. The chloroplasts then dedifferentiate, forming basal-state plastids termed eoplasts that, upon germination, redifferentiate into various plastid types (Whatley, 1978). Little is known about eoplast formation or the earliest steps of plastid development from eoplasts during germination. In this report, we describe the spd1 mutant, which affects plastid development from Arabidopsis eoplasts. Although the spd1 mutation results in albino seedlings, developing embryos are visually indistinguishable from wild-type embryos and contain normal-looking chloroplasts (Fig. 4; Supplemental Fig. S2). Additionally, most other aspects of seed maturation appear to be properly established in spd1 seeds, as the germination rate of spd1 seedlings is similar to wild-type seedlings (data not shown). The spd1 albino phenotype could be rescued by precociously germinating embryos, but rescue became less effective as the mutant embryos progressed further into the degreening process (Fig. 5). Rescue of a similar phenotype under heterotrophic conditions has been observed in other mutants (Albrecht et al., 2008; Kim et al., 2009), suggesting the presence of multiple coordinators of plastid dedifferentiation whose attenuated function can be bypassed by precocious germination. In all, the spd1 phenotype is dependent on plastid-specific processes that occur during late embryo and seed maturation.
Several mutants have been found in Arabidopsis that exhibit seedling-specific albinism and thus may represent additional perturbations to eoplast formation or redifferentiation, including white cotyledon1 (Yamamoto et al., 2000), sigma factor6 (Ishizaki et al., 2005), snowy cotyledon1-1 (Albrecht et al., 2006; Ruppel and Hangarter, 2007), cyo1/sco2 (Shimada et al., 2007; Albrecht et al., 2008), and delayed greening1 (Chi et al., 2008). However, the phenotypes of these mutants appear to be specific for seedling chloroplast development, whereas the spd1 mutant described here also affects the development of other plastid types, including etioplasts and amyloplasts (Figs. 3 and 6). Since multiple mature plastid types are all impaired in the mutant, SPD1 appears to have a critical function prior to the steps leading to plastid specificity. The lack of starch-containing amyloplasts in the upper hypocotyl of spd1 (Fig. 6B) is reminiscent of reduced-starch and starchless mutants that exhibit gravitropism defects (Kiss et al., 1996, 1997). Root columella cells in spd1 seedlings were found to contain amyloplasts (Fig. 6E) and were gravitropic (Fig. 6F). However, it was previously reported that fully developed Arabidopsis embryos do not contain mature columella cells but rather initial cells (Scheres et al., 1994). Thus, the columella cells seen in germinating seedlings presumably represent postgermination secondary growth and maturation from the root apical meristem. Also, mesophyll cells in spd1 adult leaves are able to develop normal chloroplasts, unlike cotyledon mesophyll cells. Like the root columella, adult leaves are derived from postgermination secondary growth from the apical meristem. Thus, the spd1 plastid defect appears to be restricted to seedling tissues that are fully matured in the embryo. Overall, the stage-specific plastid developmental phenotypes of spd1 and other recently described seedling mutants suggest that plastid development from eoplasts has unique molecular requirements different than plastid development from proplastids derived from meristematic cells.
Our in vivo and in vitro data clearly indicate chloroplast localization of SPD1 (Figs. 8–10), in agreement with previously published proteomics data (Zybailov et al., 2008). Most nuclear-encoded proteins that are destined for the chloroplast are translated on cytosolic ribosomes with an N-terminal transit peptide and are posttranslationally imported into the chloroplast via the TOC-TIC apparatus (for translocons at the outer and inner envelope membranes of chloroplasts; Schnell et al., 1997), whereupon the target peptide is removed by stromal processing peptidase (Richter et al., 2005). Suborganellar sorting can be achieved with or without the use of a second signal peptide located C terminal to the SPP cut site. In our studies, the mobility of the SPD1-GFP-HIS6 protein recognized by the His tag antibody in complemented spd1 plants appears to correspond to that of SPD1-GFP-HIS6 with the predicted transit peptide still attached (110 kD) rather than the mature protein with the transit peptide removed (predicted to be 95 kD). However, our in vitro assay demonstrates that SPD1 migrates slower on SDS-PAGE than expected (Fig. 10). The in vitro import result also demonstrates the clear shift of mobility of the protein after import, supporting the presence of the predicted cleavable targeting sequence. Hence, the protein recognized by the anti-His antibody in the spd1;SPD1-GFP-HIS6 plant may be a processed form. Interestingly, SPD1-GFP-HIS6 in complemented plants was found to be integrally associated with the envelope membrane, although its primary sequence lacks any predicted transmembrane domains, whereas nontagged SPD1 was targeted to the space between the outer and inner membranes of the chloroplast envelope in vitro. The membrane-associated fusion protein in complemented plants may represent a nonfunctional form; the functional form in planta may be C-terminally truncated so that it cannot be detected on the confocal microscope or by the anti-His tag antibody. Alternatively, the SPD1 protein targeted to the intermembrane space of the chloroplast envelope in vitro may be an intermediate en route to either of the envelope membranes. Preparation of an antibody against the endogenous SPD1 and extensive in vitro import assays will be necessary to address the specific localization site.
The amino acid sequence of SPD1 contains residues similar to P-loop ATPase proteins and is likely a functional ATPase based on its annotation and sequence similarity with the B. subtilis SpoIIIAA protein (Supplemental Fig. S3). Members of the AAA+ ATPase superfamily are known to perform a variety of cellular roles related to protein unfolding, disassembly, and/or disaggregation in both prokaryotes and eukaryotes (Hanson and Whiteheart, 2005; Ammelburg et al., 2006). This gene family is highly abundant within the Arabidopsis genome (more than 170 genes identified; Winter and Hauser, 2006) and includes well-characterized plastid-localized members such as Hsp93 and FtsH, which play roles in organelle protein import (Li and Chiu, 2010) and PSII D1 protein turnover (Lindahl et al., 2000), respectively. The AAA+ ATPase superfamily also includes proteins that are anchored to the inner membrane of mitochondria and perform catalytic functions in the intermembrane space (Leonhard et al., 1996; Tatsuta and Langer, 2009). Based on these studies, we hypothesize that SPD1 may play a role in protein homeostasis within the intermembrane space of the chloroplast. This function may be performed at very distinct places, as opposed to an equal distribution throughout the compartment, given its spot-like localization (Fig. 8; Supplemental Figs. S6 and S7; Supplemental Movie S1).
In summary, the SPD1 gene described here encodes a member of the AAA+ ATPase superfamily involved in plastid development during early seedling growth. The spd1 mutant displays pleiotropic defects to chloroplast, etioplast, and amyloplast development in germinating seedlings. The mutant phenotype was found to require passage through late stages of seed maturation for it to become apparent, indicative of SPD1 serving a critical function associated with plastid interconversion to or from the eoplast during embryo maturation.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All plants used were of the Columbia ecotype of Arabidopsis (Arabidopsis thaliana). The spd1 mutant was isolated from fast-neutron-mutagenized M2 seeds (Lehle Seeds). spd1 seeds were sown on petri plates on medium containing half-strength Murashige and Skoog (MS) basal salt mixture (Sigma-Aldrich), 1% agar, and 2% Suc and then cold treated at 4°C for 72 h. The seeds were then moved to a growth room under 12 h light d−1 at 23°C. Light was provided by cool-white fluorescence bulbs (General Electric) at fluence rates of 100 to 150 μmol m−2 s−1. To grow spd1 plants to maturity, seedlings were transferred to water-soaked Metromix 360 (Sun Grow Horticulture) when adult leaves had developed sufficiently to sustain subsequent plant development. Plants were fertilized with K-Grow all-purpose plant food (Kmart) on a 2-week cycle.
spd1 Mutant Screen
Fast-neutron-mutagenized seeds were prepared as above with the following deviations during the screen. Cold-treated seeds were warmed to 23°C and then exposed to red light for 30 min to promote uniform germination. The petri plates were then oriented vertically and placed in darkness for 60 h. Seedlings were then moved to fresh agar medium and aligned parallel to each other. The seedlings were returned to a vertical orientation for 1 h to allow recovery from mechanical stimulation. The petri dishes were then reoriented by 90° so the seedlings were in the horizontal position. The gravitropic responses of hypocotyls and roots were recorded by time-lapse imaging with IR illumination and an IR-sensitive camera.
Chlorophyll Measurements
Chlorophyll was extracted from 5-week old rosette leaves in 80% acetone. Absorbance was measured at 663 and 646 nm. Total chlorophyll and chlorophyll a/b ratios were calculated according to Lichtenthaler and Wellburn (1983).
Precocious Germination Experiments
Wild-type and spd1 plants were selected based on similarity in appearance and time of bolting. Each day, flowers on primary inflorescences were tagged at anthesis. Siliques spanning a range of 5 to 16 DPA were excised and surface sterilized. Embryos were dissected from ovules collected from the middle of each silique. The isolated embryos were placed onto growth medium (half-strength MS basal salts, 1% agar, and 2% Suc) and allowed to grow under 12 h light d−1 at 100 to 150 μmol m−2 s−1 for 7 d. Cotyledons were then removed from the seedlings, and images were captured with a Nikon E800 microscope system (Nikon Instruments) using both bright-field and chlorophyll autofluorescence. The total area of green and white sectors was measured with ImageJ (Abramoff et al., 2004) and expressed as a percentage of the total cotyledon area for each cotyledon.
Identification and Cloning of SPD1
A mapping population was established from a cross between spd1 (Columbia ecotype) and the Landsberg erecta wild-type ecotype of Arabidopsis. Analysis of sequence polymorphisms in 400 F2 recombinant lines homozygous for spd1 placed the mutation on chromosome 3 in an approximately 45-kb region covering two overlapping bacterial artificial chromosomes (F14P13 and F13M14), with a total of 13 annotated genes contained within the interval. The Indiana Molecular Biology Institute sequenced annotated candidate genes contained within the interval.
For rescue of spd1, the full-length SPD1 gene sequence (At3g10420) was inserted into the cloning vector pDONR201 using the Gateway BP clonase reaction (Invitrogen) with gene-specific primers (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCAACAATGAGGGCGTTGAATTCGCG-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCATCCTTAAGGAAAGGAAG-3′). It was subsequently moved into the binary vectors pEarleyGate101 (SPD1-YFP-HA) and pEarleyGate103 (SPD1-GFP-HIS6; Earley et al., 2006) using the Gateway LR clonase reaction (Invitrogen) and then transformed into Agrobacterium tumefaciens (GV3101) using the Escherichia coli helper strain pRK2013. Arabidopsis plants (ecotype Columbia) were transformed by the floral dip method (Clough and Bent, 1998).
Transmission Electron Microscopy
Plant materials were cut into small pieces and placed into a 3% formaldehyde/gluteraldehyde solution in 0.1 m sodium cacodylate buffer, pH 7.4 (Electron Microscopy Sciences), and fixed overnight at 4°C. The fixed samples were washed and then postfixed in 2% OsO4 at 4°C overnight. The samples were then washed, dehydrated, and embedded in soft spurs resin (Electron Microscopy Sciences). Cotyledon pieces were sectioned using an automated ultramicrotome with a glass knife or a diamond knife (Pelco International). The sections were stained with a 2% uranyl acetate solution (Electron Microscopy Sciences) and lead citrate (Electron Microscopy Sciences) as described previously (Venable and Coggeshall, 1965). Stained sections were observed and imaged using a JEOL-1010 transmission electron microscope (JEOL USA).
Light Microscopy
For microscopy of deetiolated seedlings, seedlings were collected, vacuum infiltrated with a 2% Suc solution, and examined with a Nikon E800 microscope. Starch was stained by immersing seedlings in a solution of I2KI (5% KI, 10% I2) for 15 min and then destained in water for 20 min. To measure cell size and determine plastid numbers, cotyledons from light-grown seedlings were vacuum infiltrated with a 2% Suc solution and images of cells were captured from a Nikon E800 microscope using bright-field and chlorophyll fluorescence. Mesophyll cell areas were measured with ImageJ (Abramoff et al., 2004) from micrograph images of cells in the focal plane just below the epidermis.
To test for plastid protein uptake in spd1 cells, we mobilized a recA chloroplast-targeting peptide fused to S65TmGFP4 (Köhler et al., 1997) into A. tumefaciens strain GV3101 and transformed wild-type plants by floral dip transformation (Clough and Bent, 1998). Transformed plants were selected on 50 μg mL−1 kanamycin (Sigma-Aldrich) and crossed into the spd1 mutant background. Homozygous lines were subsequently identified and used to analyze GFP import into plastids using a Nikon E800 microscope system. DAPI (AnaSpec) staining was used to identify DNA-containing organelles.
Live cell imaging of SPD1-YFP-HA was accomplished using a Leica SP5 laser scanning inverted five-channel confocal microscope (Leica Microsystems) with a 63×/1.20 water objective (Leica Plan Apochromat). The tissues were infiltrated with and mounted in 200 mm mannitol. YFP and chlorophyll fluorescence were excited by the 514-nm argon laser with emission ranges of 525 to 600 nm and 650 to 720 nm for YFP and chlorophyll florescence, respectively.
Transformation in Nicotiana benthamiana leaves was done as described previously (Wroblewski et al., 2005). In short, prior to leaf infiltration, a single colony was inoculated in 5 mL of Luria-Bertani broth with 50 μg mL−1 kanamycin and grown overnight at 30°C. After 24 h, a 1:10 dilution of the culture was made in fresh Luria-Bertani broth and 50 μg mL−1 kanamycin and grown for 5 h at 30°C. The bacteria were harvested and resuspended to an optical density at 600 nm of 0.5. The bacterial suspension was infused into N. benthamiana leaves using a syringe. Inoculated leaf tissue was harvested after 48 h, and images were taken on a Leica SP5 laser scanning inverted five-channel confocal microscope.
Chloroplast Preparation, Immunoblotting, and Protein Import Assay
For analysis of SPD1-GFP-HIS6 protein, wild-type and spd1;SPD1-GFP-HIS6 Arabidopsis plants were grown on half-strength MS containing 0.8% phytoagar and 2% Suc at 24°C and 19 h light d−1 at 100 μmol m−2 s−1. Total protein from the plants was extracted, and its protein concentration was determined as described (Inoue et al., 2005), except that phenylmethylsulfonyl fluoride was not included in the extraction buffer. Isolation of chloroplasts was done as according to Chiu and Li (2008).
Fractionation of chloroplasts into soluble, peripheral, and integral membrane fractions (S1, S2, and P, respectively) was done according to Inoue et al. (2006). For thermolysin treatment, chloroplasts containing 12 μg of chlorophyll were resuspended in 100 μL of import buffer containing 1 mm CaCl2 and 2.4 μg of the protease and incubated for 30 min on ice. The reaction was quenched by adding 1 volume of import buffer containing 20 mm EDTA. For trypsin treatment, chloroplasts containing 12 μg of chlorophyll were incubated in 100 μL of import buffer containing 12 μg of trypsin for 30 min in the dark. The reaction was quenched by adding 1 volume of import buffer containing 60 μg of trypsin inhibitor. For the experiment shown in Figure 9C, chloroplasts containing 10 μg of chlorophyll were resuspended in 100 μL of import buffer with 10 μg of trypsin for 30 min in the dark. The reaction was quenched with 1 volume of import buffer containing 20 μg of trypsin inhibitor followed by reisolation of chloroplasts through a 40% Percoll cushion. Proteins in chloroplasts treated with detergent-containing buffer were recovered by TCA precipitation. All the proteases and trypsin inhibitor were purchased from Sigma-Aldrich.
For immunoblotting, detection was done using secondary antisera conjugated with alkaline phosphatase and the substrates 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Bio-Rad Laboratories). Antiserum against the His tag (His probe H-15; sc-803) was purchased from Santa Cruz Biotechnology. Antisera against pea (Pisum sativum) Toc75 (Tranel et al., 1995) and pea Tic110 (Jackson et al., 1998) were kind gifts from K. Keegstra (Michigan State University), and the anti-Toc159 antibody (Kikuchi et al., 2006) was kindly provided by M. Nakai (Osaka University). Import assay using chloroplasts isolated from pea seedlings was done according to Inoue et al. (2005).
Sequence Alignments
The SPD1 annotation was obtained from The Arabidopsis Information Resource (http://www.arabidopsis.org). The publicly available program TCOFFEE was used to produce sequence alignments. Outputs were designed in the BOXSHADE program (http://www.ch.embnet.org/).
Sequence data from this article can be found in the Arabidopsis Genome Initiative or the GenBank/EMBL databases under the following accession numbers: At3g10420 (SPD1), At1g73170, At1g33290, At3g46740 (Toc75), At1g06950 (Tic110), At4g02510 (Toc159), At3g11670 (DGD1), AC009400 (F14P13), AC011560 (F13M14), BAC7994 (Oryza sativa), XP_002271948.1 (Vitis vinifera), and NP_390232.1 (Bacillus subtilis SpoIIIAA).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Plastid DNA stained with DAPI and plastid-targeted RECA-GFP colocalize in albino spd1 and wild-type cotyledon mesophyll cells.
Supplemental Figure S2. Electron micrograph images of wild-type and spd1 embryonic cell features.
Supplemental Figure S3. Alignment of the ATPase domain from SPD1 to B. subtilis SpoIIIAA.
Supplemental Figure S4. Alignment of the SPD1 protein sequence to orthologs in rice and grape.
Supplemental Figure S5. Alignment of the SPD1 protein sequence to two related proteins in the Arabidopsis genome.
Supplemental Figure S6. Chloroplast localization of SPD1-GFP-HIS6 in Arabidopsis mesophyll cells.
Supplemental Figure S7. Chloroplast localization of SPD1-GFP-HIS6 in N. benthamiana mesophyll cells.
Supplemental Movie S1. SPD1-YFP-HA localizes to distinct foci associated with chloroplasts in N. benthamiana mesophyll cells.
Supplementary Material
References
- Abramoff MD, Magelhaes PJ, Ram SJ. (2004) Image processing with ImageJ. Biophotonics Int 11: 36–42 [Google Scholar]
- Agne B, Infanger S, Wang F, Hofstetter V, Rahim G, Martin M, Lee DW, Hwang I, Schnell D, Kessler F. (2009) A toc159 import receptor mutant, defective in hydrolysis of GTP, supports preprotein import into chloroplasts. J Biol Chem 284: 8670–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht V, Ingenfeld A, Apel K. (2006) Characterization of the snowy cotyledon 1 mutant of Arabidopsis thaliana: the impact of chloroplast elongation factor G on chloroplast development and plant vitality. Plant Mol Biol 60: 507–518 [DOI] [PubMed] [Google Scholar]
- Albrecht V, Ingenfeld A, Apel K. (2008) Snowy cotyledon 2: the identification of a zinc finger domain protein essential for chloroplast development in cotyledons but not in true leaves. Plant Mol Biol 66: 599–608 [DOI] [PubMed] [Google Scholar]
- Allen DK, Ohlrogge JB, Shachar-Hill Y. (2009) The role of light in soybean seed filling metabolism. Plant J 58: 220–234 [DOI] [PubMed] [Google Scholar]
- Ammelburg M, Frickey T, Lupas AN. (2006) Classification of AAA+ proteins. J Struct Biol 156: 2–11 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H, Walenta S, Panitz R, Wobus U, Weber H. (2003) Energy status and its control on embryogenesis of legumes: ATP distribution within Vicia faba embryos is developmentally regulated and correlated with photosynthetic capacity. Plant J 36: 318–329 [DOI] [PubMed] [Google Scholar]
- Chi W, Ma JF, Zhang DY, Guo JK, Chen F, Lu CM, Zhang L. (2008) The pentratricopeptide repeat protein DELAYED GREENING1 is involved in the regulation of early chloroplast development and chloroplast gene expression in Arabidopsis. Plant Physiol 147: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Li HM. (2008) Tic40 is important for reinsertion of proteins from the chloroplast stroma into the inner membrane. Plant J 56: 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Andrews J, Keegstra K. (1984) Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol 75: 675–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song KM, Pikaard CS. (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Froehlich JE, Benning C, Dörmann P. (2001) The digalactosyldiacylglycerol (DGDG) synthase DGD1 is inserted into the outer envelope membrane of chloroplasts in a manner independent of the general import pathway and does not depend on direct interaction with monogalactosyldiacylglycerol synthase for DGDG biosynthesis. J Biol Chem 276: 31806–31812 [DOI] [PubMed] [Google Scholar]
- Galili G. (1995) Regulation of lysine and threonine synthesis. Plant Cell 7: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffman FD, Alonso AP, Schwender J, Shachar-Hill Y, Ohlrogge JB. (2005) Light enables a very high efficiency of carbon storage in developing embryos of rapeseed. Plant Physiol 138: 2269–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Whiteheart SW. (2005) AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol 6: 519–529 [DOI] [PubMed] [Google Scholar]
- Illing N, Errington J. (1991) The spoIIIA operon of Bacillus subtilis defines a new temporal class of mother-cell-specific sporulation genes under the control of the sigma E form of RNA polymerase. Mol Microbiol 5: 1927–1940 [DOI] [PubMed] [Google Scholar]
- Inoue K, Baldwin AJ, Shipman RL, Matsui K, Theg SM, Ohme-Takagi M. (2005) Complete maturation of the plastid protein translocation channel requires a type I signal peptidase. J Cell Biol 171: 425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Furbee KJ, Uratsu S, Kato M, Dandekar AM, Ikoma Y. (2006) Catalytic activities and chloroplast import of carotenogenic enzymes from citrus. Physiol Plant 127: 561–570 [Google Scholar]
- Ishizaki Y, Tsunoyama Y, Hatano K, Ando K, Kato K, Shinmyo A, Kobori M, Takeba G, Nakahira Y, Shiina T. (2005) A nuclear-encoded sigma factor, Arabidopsis SIG6, recognizes sigma-70 type chloroplast promoters and regulates early chloroplast development in cotyledons. Plant J 42: 133–144 [DOI] [PubMed] [Google Scholar]
- Jackson DT, Froehlich JE, Keegstra K. (1998) The hydrophilic domain of Tic110, an inner envelope membrane component of the chloroplastic protein translocation apparatus, faces the stromal compartment. J Biol Chem 273: 16583–16588 [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Hirohashi T, Nakai M. (2006) Characterization of the preprotein translocon at the outer envelope membrane of chloroplasts by blue native PAGE. Plant Cell Physiol 47: 363–371 [DOI] [PubMed] [Google Scholar]
- Kim J, Rudella A, Ramirez Rodriguez V, Zybailov B, Olinares PDB, van Wijk KJ. (2009) Subunits of the plastid ClpPR protease complex have differential contributions to embryogenesis, plastid biogenesis, and plant development in Arabidopsis. Plant Cell 21: 1669–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SP, Badger MR, Furbank RT. (1998) CO2 refixation characteristics of developing canola seeds and silique wall. Aust J Plant Physiol 25: 377–386 [Google Scholar]
- Kiss JZ, Guisinger MM, Miller AJ, Stackhouse KS. (1997) Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol 38: 518–525 [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T. (1996) Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant 97: 237–244 [DOI] [PubMed] [Google Scholar]
- Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR. (1997) Exchange of protein molecules through connections between higher plant plastids. Science 276: 2039–2042 [DOI] [PubMed] [Google Scholar]
- Leonhard K, Herrmann JM, Stuart RA, Mannhaupt G, Neupert W, Langer T. (1996) AAA proteases with catalytic sites on opposite membrane surfaces comprise a proteolytic system for the ATP-dependent degradation of inner membrane proteins in mitochondria. EMBO J 15: 4218–4229 [PMC free article] [PubMed] [Google Scholar]
- Li HM, Chiu CC. (2010) Protein transport into chloroplasts. Annu Rev Plant Biol 61: 157–180 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y. (2002) Reverse genetics by fast neutron mutagenesis in higher plants. Funct Integr Genomics 2: 254–258 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11: 591–592 [Google Scholar]
- Lindahl M, Spetea C, Hundal T, Oppenheim AB, Adam Z, Andersson B. (2000) The thylakoid FtsH protease plays a role in the light-induced turnover of the photosystem II D1 protein. Plant Cell 12: 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield SG, Briarty LG. (1991) Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can J Bot 69: 461–476 [Google Scholar]
- Mansfield SG, Briarty LG. (1992) Cotyledon cell development in Arabidopsis thaliana during reserve deposition. Can J Bot 70: 151–164 [Google Scholar]
- Ohlrogge J, Browse J. (1995) Lipid biosynthesis. Plant Cell 7: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Leech RM. (1991) Rapid image analysis screening procedure for identifying chloroplast number mutants in mesophyll cells of Arabidopsis thaliana (L.) Heynh. Plant Physiol 96: 1193–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Zhong R, Lamppa G. (2005) Function of the stromal processing peptidase in the chloroplast import pathway. Physiol Plant 123: 362–368 [Google Scholar]
- Ruppel NJ, Hangarter RP. (2007) Mutations in a plastid-localized elongation factor G alter early stages of plastid development in Arabidopsis thaliana. BMC Plant Biol 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska SA, Schwender J, Ohlrogge JB. (2004) The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol 136: 2700–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD. (1997) Plastids and gravitropic sensing. Planta (Suppl 1) 203: S63–S68 [DOI] [PubMed] [Google Scholar]
- Saito GY, Chang YC, Walling LL, Thomson WW. (1989) A correlation in plastid development and cytoplasmic ultrastructure with nuclear gene expression during seed ripening in soybean. New Phytol 113: 459–469 [Google Scholar]
- Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P. (1994) Embryonic origin of the Arabidopsis primary root and meristem initials. Development 120: 2475–2487 [Google Scholar]
- Schnell DJ, Blobel G, Keegstra K, Kessler F, Ko K, Soll J. (1997) A consensus nomenclature for the protein-import components of the chloroplast envelope. Trends Cell Biol 7: 303–304 [DOI] [PubMed] [Google Scholar]
- Shimada H, Mochizuki M, Ogura K, Froehlich JE, Osteryoung KW, Shirano Y, Shibata D, Masuda S, Mori K, Takamiya KI. (2007) Arabidopsis cotyledon-specific chloroplast biogenesis factor CYO1 is a protein disulfide isomerase. Plant Cell 19: 3157–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T, Langer T. (2009) AAA proteases in mitochondria: diverse functions of membrane-bound proteolytic machines. Res Microbiol 160: 711–717 [DOI] [PubMed] [Google Scholar]
- Tranel PJ, Froehlich J, Goyal A, Keegstra K. (1995) A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway. EMBO J 14: 2436–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable JH, Coggeshall R. (1965) A simplified lead citrate stain for use in electron microscopy. J Cell Biol 25: 407–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ. (1982) Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J 1: 945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley JM. (1978) A suggested cycle of plastid developmental interrelationships. New Phytol 80: 489–502 [Google Scholar]
- Winter V, Hauser MT. (2006) Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci 11: 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski T, Tomczak A, Michelmore R. (2005) Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J 3: 259–273 [DOI] [PubMed] [Google Scholar]
- Yamamoto YY, Puente P, Deng XW. (2000) An Arabidopsis cotyledon-specific albino locus: a possible role in 16S rRNA maturation. Plant Cell Physiol 41: 68–76 [DOI] [PubMed] [Google Scholar]
- Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ. (2008) Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS ONE 3: e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.